Abstract
Cancer immunotherapy can induce long lasting responses in patients with metastatic cancers of a wide range of histologies. Broadening the clinical applicability of these treatments requires an improved understanding of the mechanisms limiting cancer immunotherapy. The interactions between the immune system and cancer cells are continuous, dynamic and evolving from the initial establishment of a cancer cell to the development of metastatic disease, which is dependent on immune evasion. As the molecular mechanisms of resistance to immunotherapy are being elucidated, actionable strategies to prevent or treat them may be derived to improve clinical outcomes for patients.
Introduction
Metastatic cancers remain an incurable disease for the great majority of patients, as the intrinsic genomic instability common to all cancers facilitates the escape from cytotoxic or targeted therapies. The recent breakthroughs in the understanding of tumor immune-biology and the development of newer generation of cancer immunotherapies have opened a brand new chapter in the war against cancer. This change in landscape is based on the discovery of cancer immune checkpoints and the success of checkpoint inhibitors, as well as the advances in technology to generate genetically modified immune cells (Miller and Sadelain, 2015). The focus of treatment has shifted from the tumor itself to the host’s immune system, to mobilize immune cells to recognize and eventually eliminate the cancer cells. A hallmark of immunotherapy is the durability of responses, likely due to the memory of the adaptive immune system, which translates into long-term survival for a subset of patients.
The early efforts to harness the immune system in cancer control pioneered by Dr. William B. Coley in the 1890’s (Coley, 1910) were overlooked due to the lack of consistency in response and were soon overwhelmed by the development of more effective treatments such as radiotherapy and chemotherapy. However, investigations persisted to unravel and elucidate the interactions between the immune system and cancer cells. The concept of cancer immunosurveillance, which was proposed by Paul Ehrlich (Ehrlich, 1956) and enriched by Burnet and Thomas (Burnet, 1971) in the 1950’s, stated that the emergence of malignant cells is a frequent event but is suppressed by the host’s natural immunity, that cancer develops when this immunity is weakened, and that lymphocytes are responsible for this process. Finally, the cancer immune-editing concept was elucidated by Schreiber et al in 2002 (Dunn et al., 2002), recognizing a dual role of the host’s immunity, both as an extrinsic tumor suppressor and a facilitator of tumor growth and progression, acting across three sequential phases, elimination, equilibrium and escape, through constant interactions between tumor cells, immune cells and the tumor microenvironment. Importantly, host immune responses and tumor genomics are tightly related, as illustrated by the notion that neoantigens arising from genomic mutations may shape immune responses (Schumacher and Schreiber, 2015), however these responses may prove ineffective against a heterogeneous and evolving tumor microenvironment.
The process of T cell activation involves antigen presentation by the major histocompatibility complex (MHC) molecules on the antigen presenting cells (APC) to the corresponding T cell receptor (TCR) on naïve T cells. The interaction of costimulatory molecules CD28 and B7 is required for full activation, which is tightly regulated by inhibitory checkpoints to avoid collateral damage and autoimmunity. The CTLA-4 receptor on activated effector T cells and regulatory T cells (Treg) was discovered in the 1980’s (Brunet et al., 1987). Seminal work by James Allison and colleagues showed that CTLA-4 competes with CD28 for B7 ligands and inhibits proliferation and IL-2 secretion by T cells (Krummel and Allison, 1995), and CTLA-4 blocking antibodies could treat tumors in immune competent animal models (Leach et al., 1996). Subsequent clinical testing resulted in the approval of ipilimumab for treatment of advanced melanoma in 2011, the first in class CTLA-4 checkpoint inhibitor approved by the US Food and Drug Administration (FDA) (Hodi et al., 2010; Robert et al., 2011). Pooled data from clinical trials of ipilimumab confirmed durable clinical responses with a plateau in the survival curve beginning around year 3, lasting 10 years or more in a subset of approximately 21% of patients (Schadendorf et al., 2015). In 2015, ipilimumab was also approved by the FDA as adjuvant therapy for locally advanced melanoma. Due to enhanced immune responses, possibly during early stages of T cell activation, significant immune-related toxicities have been observed but most can be managed by systemic steroid therapy.
Another checkpoint receptor expressed by activated T cells, programed death 1 (PD-1), was cloned in 1992 (Ishida et al., 1992), and subsequently its ligand PD-L1 was characterized (Dong et al., 1999; Freeman et al., 2000). PD-L1 expression can be constitutive or induced in many tumors to evade immune attack. Since PD-L1 expression can be induced by IFNγ, which is expressed during an active anti-tumor immune response, it has been referred to as a mechanism of adaptive immune resistance (Table 1). Antibodies blocking the PD-1/L1 inhibitory axis can unleash activated tumor-reactive T cells and have been shown in clinical trials to induce durable anti-tumor responses in increasing numbers of tumor histologies, including the tumor types that are not traditionally considered “immunotherapy sensitive” (Okazaki et al., 2013; Zou et al., 2016). This led to the approval of two anti-PD1 antibodies (pembrolizumab and nivolumab) and one anti-PD-L1 antibody (atezolimumab) for the treatment of advanced melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), head and neck squamous carcinoma (HNSCC), Hodgkin’s lymphoma, and bladder cancer. Currently there are over ten anti-PD-1/PD-L1 antibodies in various stage of clinical testing in many different tumor types. Interestingly, there have been thousands of patients receiving PD-1 blockade therapy thus far, with similar immune related toxicities as observed for anti-CTLA-4 but with generally lower frequency, possibly since the PD-1/L1 checkpoint may act later in the T cell response resulting in a more restricted T cell reactivity towards tumor cells, with the majority of patients tolerating treatment well (Larkin et al., 2015c). Due to the non-overlapping mechanism of action of anti-CTLA4 and anti-PD1 antibodies (Das et al., 2015; Gubin et al., 2014), clinical testing of the combination of these two classes of checkpoint inhibitors showed improved clinical response (up to 60%) in melanoma at the expense of significantly increased frequency of toxicities (Larkin et al., 2015b). The combination of CTLA4 and PD-1/L1 checkpoint blockade has been approved as front line therapy for advanced melanoma patients, and is being tested in other tumor types with different dose levels and intervals of anti-CTLA4 to reduce toxicity.
Table 1.
Terminology for different resistance mechanisms to immunotherapy
| Term | Description |
|---|---|
| Primary resistance | A clinical scenario where a cancer does not respond to an immunotherapy strategy. The mechanistic basis of lack of response to immunotherapy may include adaptive immune resistance |
| Adaptive immune resistance | A mechanism of resistance where a cancer is recognized by the immune system but it protects itself by adapting to the immune attack. Given the evolving nature of the immune/cancer cell interaction, this could clinically manifest as primary resistance, mixed responses or acquired resistance |
| Acquired resistance | A clinical scenario in which a cancer initially responded to immunotherapy but after a period of time it relapsed and progressed |
Cell-based immunotherapy was pioneered by many investigators including Alex Fefer, Phil Greenberg, Zelig Eshhar, Steven Rosenberg and colleagues in the 1980’s, inspired by the correlation of the number of tumor infiltrating lymphocytes (TIL) and survival in some cancers. This process required TILs to be isolated from the patient’s surgical specimen, expanded in vitro and re-infused back to the lymphocyte-depleted patient. In these studies, sufficient TILs could not be isolated or expanded from tumors of approximately 50–60% of patients, which limited the number of patients who could be treated. For patients who could be treated with the expanded TILs, the reported response rate was 50% for melanoma, including 20% complete responses, and 95% of these complete responders had more than 5 years of survival (Rosenberg et al., 2011). This approach, however, requires large surgical samples, experienced academic centers, and tumors enriched with anti-tumor T cells, which is a rare event for most tumor types. The recent advance of gene transfer technologies and T cell engineering has enabled more versatile approaches including adoptive cell transfer (ACT) of the patient’s peripheral T cells that are genetically modified to target cancer specific antigens, via physiological TCR or chimeric antigen receptors (CAR) (Sadelain, 2016; Yang and Rosenberg, 2016). TCR are usually cloned from TILs that are reactive to specific cancer antigens having no or very limited expression in normal adult tissue but are widely expressed by cancer cells. Such TCR recognize tumor antigen presented in the context of major histocompatibility complex (MHC). Clinical success has been documented (Yee et al., 2015). The TCR approach allows intracellular antigen target but is MHC restricted, and can be subject to treatment failure for tumors that have down-regulated their MHC surface expression. CAR technology was first developed by Eshhar et al, by genetically engineering T cells with chimeric genes linking single chain antibodies (scFv) targeting tumor cell surface antigens to intracellular signaling adaptors for TCR – in the first iteration to the T cell specific activating ζ chain of the CD3 complex. Subsequent modification with co-stimulatory molecules CD28 (second generation) and 4-1BB (third generation) has enabled the expansion of T cells while retaining function upon repeated antigen exposure. CAR T cell does not require MHC restriction and can be engineered to enhance T cell function. Recent clinical success with CD19 targeting CAR to treat CD19+ B cell malignancy has shown great success, with a remarkable 90% complete remission (CR) in a cohort of 30 patients with relapsed or refractory pediatric acute lymphoblastic leukemia (ALL), and two thirds of these patients remained in remission after 6 months (Maude et al., 2014). The biggest challenge facing the field of ACT is the identification of target tumor antigens that are not expressed by normal tissues, both to maximize specificity and efficacy, and to minimize toxicity (Fesnak et al., 2016). A commonly seen toxicity in ACT therapy is cytokine release syndrome which can be life-threatening, and requires prompt management with steroids and IL-6 receptor antibody (tocilizumab).
Despite the unprecedented durable response rates observed with cancer immunotherapies, the majority of patients do not benefit from the treatment (primary resistance) and some responders relapse after a period of response (acquired resistance). Several common cancer types have shown very low frequency of response (breast, prostate, colon) and heterogeneous responses have been seen even between distinct tumors within the same patient (Figure 1). For the purposes of this review article, we categorized primary, adaptive and acquired resistance as described in Table 1, in keeping with the most typical conceptualization for practicing clinicians. However, in considering resistance mechanisms to immune-based therapies, it is important to remember that the immune response is dynamic and constantly evolving in each patient, either as a result of the patient’s own environmental and genetic factors or as a result of treatment interventions, including surgery, chemotherapy, radiation therapy and immunotherapy. Anti-tumor immune responses that are ongoing throughout the course of a patient’s disease may be affected by many of these factors, and the establishment of resistance mechanisms relevant to immunotherapeutic failure may pre-date immunotherapy challenge. Without recourse to detailed immune and tumor characterization, these resistance mechanisms can be divided clinically into those that prevent a patient ever from responding to an immunotherapy or those that facilitate relapse after an initial response. Thus, although resistance to immunotherapies may manifest at different times, in many cases similar or overlapping mechanisms enable tumor cells to evade anti-tumor immune responses. We discuss known resistance mechanisms and provide rationale for combination therapies to overcome resistance.
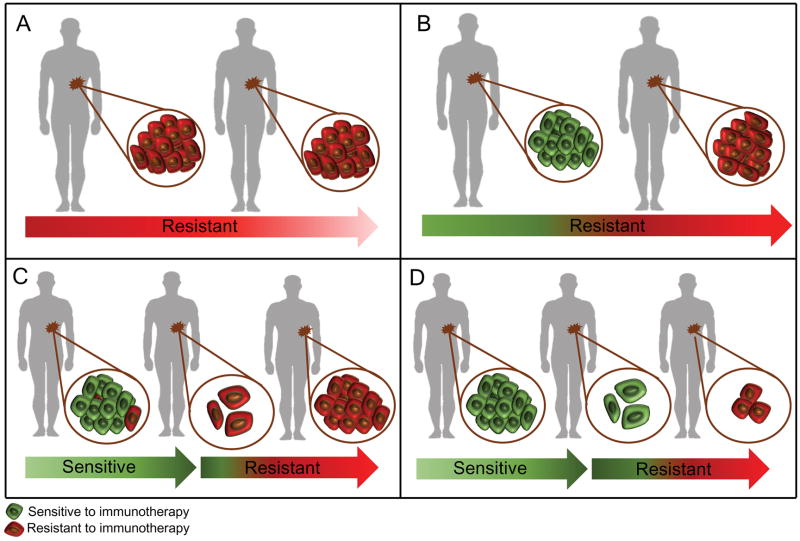
Figure 1. Clinical scenarios of primary, adaptive and acquired resistance to immunotherapy.
A) Patient’s tumor is resistant to immunotherapy with no active immune response. B) Patient’s tumor is resistant to immunotherapy; active anti-tumor immune response but turned off by checkpoints or other adaptive resistance mechanisms. C) Patient has an initial response to immunotherapy but later progressed – heterogeneous population and selection of resistant clones that were present before treatment started. D) Patient has an initial response to immunotherapy but later progressed, true acquired resistance during the immunotherapy.
Primary and Adaptive Resistance to Immunotherapy
Patients who have primary resistance to checkpoint inhibitors do not respond to the initial therapy. Ongoing studies indicate that both tumor cell-intrinsic and tumor cell-extrinsic factors contribute to the resistance mechanisms (Table 2). The most straightforward reason why a tumor would not respond to immune checkpoint therapy or ACT is lack of recognition by T cells because of absence of tumor antigens (Gubin et al., 2014). Alternatively, cancer cells may have tumor antigens but develop mechanisms to avoid presenting them on the surface restricted by MHC, either due to alterations in the antigen presenting machinery such as proteasome subunits or transporters associated with antigen processing (TAP), beta-2-microglobulin (B2M) or MHC itself (Marincola et al., 2000; Sucker et al., 2014). B2M is required for HLA class I folding and transport to the cell surface, and its genetic deficiency would lead to lack of CD8 T cell recognition (Figures 2 and 3).
Table 2.
Mechanisms of primary and adaptive resistance to immunotherapy
| Mechanism | Examples | |
|---|---|---|
| Tumor cell-intrinsic | Absence of antigenic proteins | Low mutational burden Lack of viral antigens Lack of cancer-testis antigens Overlapping surface proteins |
| Absence of antigen presentation | Deletion in TAP transporters Deletion in B2M Silenced HLA |
|
| Genetic T cell exclusion | MAPK oncogenic signaling Stabilized b-catenin Mesenchymal transcriptome Oncogenic PD-L1 expression |
|
| Insensibility to T cells | Mutations in interferon gamma pathway signaling | |
| Tumor cell-extrinsic | Absence of T cells | Lack of T cells with tumor antigen-specific TCRs |
| Inhibitory immune checkpoints | VISTA, LAG-3, TIM-3 | |
| Immunosuppressive cells | TAMs, Tregs |
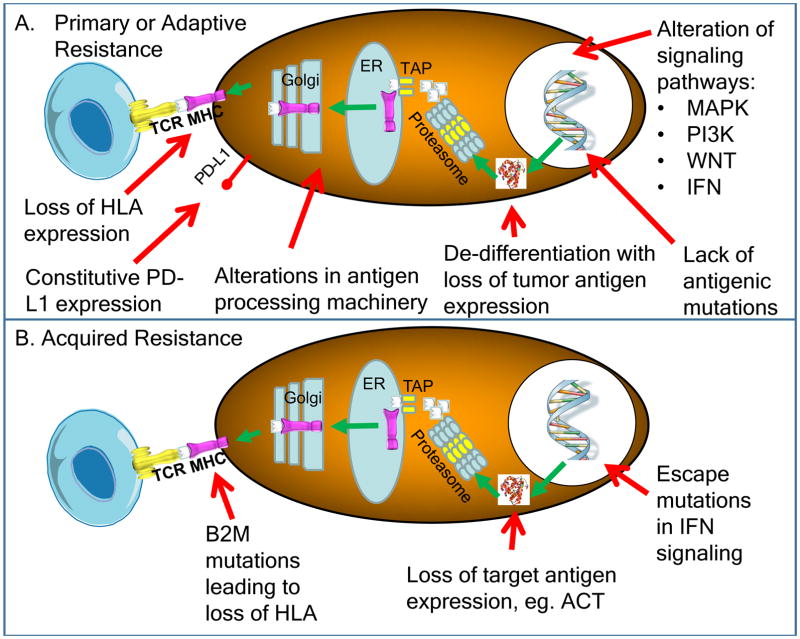
Figure 2. Known intrinsic mechanisms of resistance to immunotherapy.
A) Intrinsic factors that lead to primary or adaptive resistance including lack of antigenic mutations, loss of tumor antigen expression, loss of HLA expression, alterations in antigen processing machinery, alterations of several signaling pathways (MAPK, PI3K, WNT, IFN) and constitutive PD-L1 expression. B) Intrinsic factors that are associated with acquired resistance of cancer, including loss of target antigen, HLA, altered interferon signaling, as well as loss of T cell functionality.
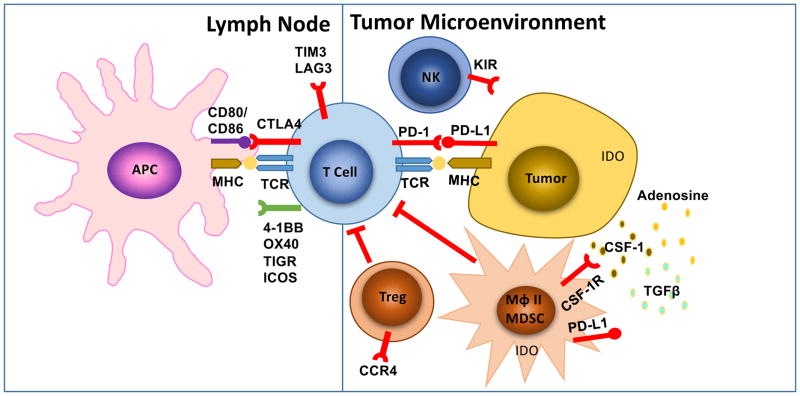
Figure 3. Known extrinsic mechanisms of resistance to immunotherapy.
This includes CTLA-4, PD1 and other immune checkpoints, T cell exhaustion and phenotype change, immune suppressive cell populations (Tregs, MDSC, type II macrophages), cytokine and metabolite release in the tumor microenvironment (CSF-1, tryptophan metabolites, TGFβ, adenosine). LN: lymph node; TME: tumor microenvironment; APC: antigen presenting cells; MHC: major histocompatibility complex; TCR: T cell receptor; TLR: toll like receptor; Treg: regulatory T cell; MDSC: myeloid-derived suppressor cell; Mϕ II: type II macrophage.
Tumor Cell-Intrinsic Factors for Primary and Adaptive Resistance
Tumor cell-intrinsic factors that contribute to immunotherapy resistance include expression or repression of certain genes and pathways in tumor cells that prevent immune cell infiltration or function within the tumor microenvironment. These mechanisms may exist at the time of initial presentation, which highlight primary resistance mechanisms, or these mechanisms may evolve later, which highlight adaptive resistance mechanisms. Multiple tumor-intrinsic mechanisms have recently been identified and include: 1) signaling through the mitogen-activated protein kinase (MAPK) pathway and/or loss of PTEN expression, which enhances PI3K signaling; 2) expression of WNT/β-catenin signaling pathway; 3) loss of interferon-gamma (IFNγ) signaling pathways; and 4) lack of T cell responses due to loss of tumor antigen expression.
Oncogenic signaling through the MAPK pathway results in the production of VEGF and IL-8, among many other secreted proteins, which have known inhibitory effects on T cell recruitment and function (Liu et al., 2013). Similarly, loss of PTEN, which enhances PI3K signaling and is a common phenomenon across several cancers, including 30% of melanomas, was found to be associated with resistance to immune checkpoint therapy (Peng et al., 2016). PTEN loss in tumors of the Cancer Genome Atlas (TCGA) melanoma dataset correlated with significantly decreased gene expression of IFNγ, granzyme B, and CD8+ T cell infiltration; importantly, the frequency of PTEN deletions and mutations was higher in non-T cell–inflamed tumors as compared to T cell–inflamed tumors. In a murine model, PTEN-knockout tumors were less susceptible to adoptive cell therapy than PTEN-expressing tumors.
The potential of oncogenic signaling pathways to induce T cell exclusion from cancers has also been described through the stabilization of β-catenin resulting in constitutive WNT signaling (Spranger et al., 2015). In a murine model, tumors with elevated β-catenin lacked a subset of DCs known as CD103+ DCs, due to decreased expression of CCL4, a chemokine that attracts CD103+ DCs. In addition, murine tumors lacking β-catenin responded effectively to immune checkpoint therapy whilst β-catenin-positive tumors did not. Non-T-cell-inflamed human melanoma tumors, which lacked T cells and CD103+ DCs in the tumor microenvironment, had significantly higher expression of tumor intrinsic β-catenin signaling genes.
Cancer cells that constitutively express immunosuppressive cell surface ligands like PD-L1 may actively inhibit antitumor T cell responses. A genetic amplification of a locus in chromosome 9 that contains the genes for the two ligands of PD-1 - PD-L1 and PD-L2 - and the interferon gamma receptor signaling molecule Janus kinase 2 (JAK2) is termed the PDJ amplicon (Ansell et al., 2015b; Green et al., 2010; Rooney et al., 2015). PDJ is amplified in the malignant Reed-Sternberg cells in Hodgkin’s disease, and anti-PD-1 therapy results in objective responses in over 80% of patients with chemotherapy-refractory Hodgkin’s disease (Ansell et al., 2015a). Other mechanisms that have been described to lead to constitutive PD-L1 expression by cancer cells include PTEN deletions or PI3K/AKT mutations (Lastwika et al., 2016; Parsa et al., 2007), EGFR mutations (Akbay et al., 2013); MYC overexpression (Casey et al., 2016), CDK5 disruption (Dorand et al., 2016), and an increase in PD-L1 transcripts stabilized by truncation of the 3-untranslated region (UTR) of this gene (Kataoka et al., 2016). It is currently unclear if constitutive PD-L1 expression resulting from these oncogenic signaling processes results in decreased or increased likelihood of responding to anti-PD-1/L1 therapy, but it may indeed result in lack of response to other cancer immunotherapy strategies by actively inhibiting antitumor T cells.
The interferon-gamma pathway is emerging as a key player in primary, adaptive and acquired resistance to checkpoint blockade therapy (Gao et al., 2016; Pardoll, 2012; Ribas, 2015; Shin et al., 2016; Zaretsky et al., 2016). It has both favorable and detrimental effects on antitumor immune responses. Interferon-gamma produced by tumor-specific T cells that have recognized their cognate antigen on cancer cells or antigen presenting cells induces an effective antitumor immune response through: 1) enhanced tumor antigen presentation that occurs as a result of increased expression of proteins, such as MHC molecules, involved in antigen presentation; 2) recruitment of other immune cells; and 3) direct anti-proliferative and pro-apoptotic effects on tumor cells (Platanias, 2005). But continuous interferon-gamma exposure can lead to immunoediting of cancer cells resulting in immune escape (Benci et al., 2016; Shankaran et al., 2001). One mechanism by which cancer cells could escape the effects of interferon gamma is by downregulating or mutating molecules involved in the interferon gamma signaling pathway, which goes through the interferon gamma receptor chains, JAK1/2 and the signal transducer and activators of transcription (STATs) (Darnell et al., 1994). In cell line and animal models, mutations or epigenetic silencing of molecules in the interferon receptor signaling pathway results in loss of the anti-tumor effects of interferon gamma (Dunn et al., 2005; Kaplan et al., 1998). Analysis of tumors in patients who did not respond to therapy with the anti-CTLA-4 antibody ipilimumab revealed an enriched frequency of mutations in the interferon gamma pathway genes interferon gamma receptor 1 and 2 (IFNGR1/2), JAK2 and interferon regulatory factor 1 (IRF1) (Gao et al., 2016). Any of these mutations would prevent signaling in response to interferon gamma and give an advantage to the tumor cells escaping from T cells, thereby resulting in primary resistance to anti-CTLA-4 therapy. Mutations in this pathway would additionally result in lack of PD-L1 expression upon interferon gamma exposure, thereby resulting in cancer cells that would be genetically negative for inducible PD-L1 expression. In such a scenario, blocking PD-L1 or PD-1 with therapeutic antibodies would not be useful, and these would be patients who are primary resistant to anti-PD-1 therapy (Shin and Ribas, 2015; Shin et al., 2016).
An additional cancer cell-intrinsic mechanism of primary resistance to immunotherapy is expression of a certain set of genes that were found to be enriched in tumors from patients who did not respond to anti-PD-1 therapy, which was termed innate anti-PD-1 resistance signature or IPRES (Hugo et al., 2016). These genes that lead to lack of response are related to mesenchymal transformation, stemness and wound healing, and are preferentially expressed by cancers that seldom respond to PD-1 blockade therapy, such as pancreatic cancer.
Epigenetic modification of the DNA in cancer cells may lead to changes in gene expression of immune related genes, which can impact antigen processing, presentation and immune evasion (Karpf and Jones, 2002; Kim and Bae, 2011). Therefore, demethylating agents may enable re-expression of immune related genes, with potential for therapeutic impact, especially in the setting of combination treatment with immunotherapy. (Héninger et al., 2015). Histone deacetylase (HDAC) inhibitors led to increased expression of MHC and tumor-associated antigens, which synergized with adoptive cell transfer therapy to improve anti-tumor responses in a murine melanoma model (Vo et al., 2009a; Vo et al., 2009b). Similarly, in a lymphoma model, hypomethylating agents were found to increase CD80 expression on tumor cells, with concomitant increase in tumor-infiltrating CD8+ T cells (Wang et al., 2013). These pre-clinical data indicate the potential to reverse the epigenetic changes in cancer cells, which may enable enhanced immune recognition and response to immunotherapy.
Tumor Cell-Extrinsic Factors for Primary and Adaptive Resistance
Tumor cell-extrinsic mechanisms that lead to primary and/or adaptive resistance involve components other than tumor cells within the tumor microenvironment, including regulatory T cells (Tregs), myeloid derived suppressor cells (MDSCs), M2 macrophages and other inhibitory immune checkpoints, which may all contribute to inhibition of anti-tumor immune responses.
Regulatory T cells (Tregs), which can be identified by expression of the FoxP3 transcription factor, have a central role in maintaining self-tolerance (Rudensky, 2011). The existence of suppressor T cells that could downregulate immune responses of antigen-specific T cells was first identified nearly four decades ago in thymectomized, lethally irradiated, bone marrow reconstituted mice (Gershon and Kondo, 1970). Tregs are known to suppress effector T cell (Teff) responses by secretion of certain inhibitory cytokine such as IL-10, IL-35 and TGF-β or by direct cell contact (Oida et al., 2003; Sakaguchi et al., 2008; Sundstedt et al., 2003). Published data indicate that many human tumors are infiltrated by Tregs (Chaudhary and Elkord, 2016; Ormandy et al., 2005; Woo et al., 2002). A vast number of murine studies have shown that the depletion of Treg cells from the tumor microenvironment can enhance or restore anti-tumor immunity (Linehan and Goedegebuure, 2005; Viehl et al., 2006). In murine models, response to anti-CTLA-4 therapy was shown to be associated with an increase in the ratio of Teff to Tregs (Quezada et al., 2006). This shift in the ratio of Teff to Tregs was found to be a result of both an increase in Teff and depletion of Tregs in a murine tumor model (Simpson et al., 2013a). These data suggest that tumors for which immunotherapy is unable to increase Teff and/or deplete Tregs to increase the ratio of Teff to Treg are likely to be resistant to treatment, either initially or during the relapsed disease setting. However, it is possible that tumor-infiltrating Tregs may coexist with other immune cells, indicating a potentially immune-responsive tumor. A retrospective study of patients treated with anti-CTLA-4 reported that a high baseline expression of FoxP3+ Tregs in the tumor was associated with better clinical outcomes (Hamid et al., 2011). Additional studies are ongoing to determine the impact of tumor-infiltrating Tregs on clinical outcomes to multiple immunotherapy strategies.
Myeloid-derived suppressor cells (MDSCs) have emerged as major regulators of immune responses in various pathological conditions including cancer. MDSCs were initially defined in murine models and were characterized by the expression of CD11b (CR3A or integrin αM) and Gr-1 markers (Bronte et al., 1998; Talmadge and Gabrilovich, 2013). Human MDSCs express markers such as CD11b+and CD33+, but are mostly negative for HLA-DR and lineage specific antigens (Lin) including CD3, CD19 and CD57. Monocytic MDSCs are HLA-DR-, CD11b+, CD33+ and CD14+; granulocytic MDSC are HLA-DR-, CD11b+, CD33+, CD15+; however, mature monocytes express HLA-DR (Wesolowski et al., 2013). MDSCs have been implicated in promoting angiogenesis, tumor cell invasion, and metastases (Yang et al., 2004; Yang et al., 2008). Furthermore, clinical findings have shown that the presence of MDSCs correlates with reduced survival in human cancers including breast cancer and colorectal cancer (Solito et al., 2011). Reports suggest that the presence of MDSCs in the tumor microenvironment correlates with decreased efficacy of immunotherapies, including immune checkpoint therapy (Meyer et al., 2014), adoptive T cell therapy (Kodumudi et al., 2012) and DC vaccination (Laborde et al., 2014). Therefore, eradicating or reprogramming MDSCs could enhance clinical responses to immunotherapy. Indeed, in melanoma, breast cancer and head & neck murine tumor models, selective inactivation of macrophage PI3Kγ synergized with immune checkpoint inhibitors to promote tumor regression and increase survival (De Henau et al., 2016; Kaneda et al., 2016). In one study, the investigators demonstrated that mice lacking PI3Kγ or tumor-bearing mice treated with PI3Kγ inhibitors (TG100-115 or IPI-549) had reduced tumor growth, which was associated with enhanced expression of pro-inflammatory cytokines and inhibition of immune-suppressive factors in the tumors (Kaneda et al., 2016). Moreover, genes and proteins associated with immune activation were upregulated in macrophages that were treated with PI3Kγ inhibitors or those from mice lacking PI3Kγ. These data established PI3Kγ as a molecular switch that regulates macrophage function. The investigators also demonstrated that a PI3Kγ inhibitor (TG100-115) plus anti-PD-1 led to improved tumor rejection and survival of tumor-bearing mice (Kaneda et al., 2016). In a second study, tumor-bearing mice treated with triple-combination therapy, a PI3Kγ inhibitor (IPI-549) plus anti-CTLA-4 and anti-PD-1, had improved tumor regression and long-term survival as compared to dual therapy with anti-CTLA-4 plus anti-PD-1 (De Henau et al., 2016). These pre-clinical studies highlight inhibitors of PI3Kγ as a therapeutic potential for combination strategies with immune checkpoint therapy in cancer patients.
Tumor-associated macrophages (TAMs) are another subset of cells that seem to affect responses to immunotherapy. TAMs include both M1 macrophages, which are involved in promoting anti-tumor immunity, and the M2 macrophages, which possess pro-tumorigenic properties (Chanmee et al., 2014). M1 and M2 macrophages can be distinguished based on the differential expression of transcription factors and surface molecules and the disparities in their cytokine profile and metabolism (Biswas and Mantovani, 2010; Hu et al., 2016). Clinical studies have shown an association between higher frequencies of TAMs and poor prognosis in human cancers (Hu et al., 2016). In a chemically induced mouse model of lung adenocarcinoma, depletion of TAMs reduced tumor growth as a result of down-regulation of M2/TAM recruitment, possibly due to the inactivation of CCL2/CCR2 signaling (Fritz et al., 2014). Likewise, depletion of M2 macrophages in various murine tumor models including cutaneous T cell lymphoma (Wu et al., 2014), colon cancer, lung cancer, breast cancer (Luo et al., 2006) and melanoma (Ries et al., 2014; Ruffell et al., 2014; Tham et al., 2015) have shown similar results. Several reports have discussed the role of macrophages in mediating therapeutic resistance in cancer (De Palma and Lewis, 2013; Ruffell et al., 2014; Ruffell and Coussens, 2015). Reports suggest that macrophages can directly suppress T cell responses through programmed death-ligand 1 (PD-L1) in hepatocellular carcinoma (Kuang et al., 2009) and B7-H4 in ovarian carcinoma (Kryczek et al., 2006). To overcome the potential resistance mechanism of macrophages, investigators tested blockade of CSF-1R, a receptor for macrophage-colony stimulating growth factor, in a murine model of pancreatic cancer and demonstrated decreased frequencies of TAMs, with subsequent increase in interferon production and restrained tumor progression. Importantly, neither PD-1 nor CTLA-4 blockade could significantly reduce tumor growth in the murine model, which was similar to findings from single agent studies in patients with pancreatic cancer (Le et al., 2013; Zhu et al., 2014). However, CSF1R blockade in combination with either an antibody against PD-1 or CTLA-4, in addition to gemcitabine, led to improved tumor regression (Zhu et al., 2014). These data suggest that CSF-1R blockade induced reduction of TAMs, which enabled response to immune checkpoint therapy. Similarly, in a melanoma model, CSF-1R inhibitor was shown to synergize with ACT therapy (Mok et al., 2014). Several early phase clinical trials are underway to testing the combination of CSF-1R inhibition with checkpoint inhibitors.
The immune response is dynamic and signals that enhance anti-tumor immune responses also tend to turn on inhibitory genes and pathways in order to tightly regulate the immune response. For example, initial T cell activation, via T-cell receptor signaling and CD28 co-stimulation, eventually leads to increased expression of the inhibitory CTLA-4 immune checkpoint (Leach et al., 1996). Similarly, effector T cell responses such as increased IFNγ production leads to increased expression of the PD-L1 protein on multiple cell types, including tumor cells, T cells and macrophages, which can engage the PD-1 receptor on T cells to suppress anti-tumor immunity (Chen, 2004; Dong et al., 2002). Apart from this, IFNγ may additionally promote the expression of immunosuppressive molecules such as indolaimine-2, 3-deoxygenase (IDO), a tryptophan-metabolizing enzyme that can contribute to peripheral tolerance and can have a direct negative effect on effector T-cell function (Gajewski et al., 2013). Similarly, carcinoembryonic antigen cell adhesion molecule-1 (CEACAM1), seems to be another inhibitory molecule that is induced by IFNγ (Takahashi et al., 1993), (Gray-Owen and Blumberg, 2006). Therapeutic antibodies blocking CEACAM1 (Ortenberg et al., 2012) and TIM-3 have resulted in enhanced anti-tumor immune responses (Pardoll, 2012; Sakuishi et al., 2010). A recent study in an immunocompetent mouse model of lung adenocarcinoma demonstrated that recurrent tumors after anti-PD-1 treatment were due to increased expression of TIM-3 on T cells. Notably, anti-PD-1 plus anti-TIM-3 led to improved responses in the tumor bearing mice. Similarly, two lung cancer patients who developed recurrent disease after anti-PD-1 treatment were found to have increased TIM-3 expression on T cells (Koyama et al., 2016). Immune suppressive cytokines are often released by tumor or macrophages for local suppression of anti-tumor immune responses. Transforming growth factor-β (TGFβ) is a cytokine that plays important roles in angiogenesis and immunosuppression by stimulating Tregs (Lebrun, 2012). Increased level of TGFβ is associated with poor prognosis in multiple different tumor types (Lin and Zhao, 2015; Massague, 2008). Preclinical models have shown synergy combining TGF-β receptor kinase inhibitor I with anti-CTLA-4 and inhibited tumor growth in a melanoma model (BRAFV600EPTEN−/−) (Hanks et al., 2014) or fractionated radiation therapy by enhance T cell priming (Vanpouille-Box et al., 2015). Adenosine was shown to inhibit T cell proliferation and cytotoxic function via the A2A receptor on T cells (Zhang et al., 2004) as well as promote metastasis via the A2B receptor on tumor cells (Mittal et al., 2016). In addition, CD73 is the enzyme that dephosphorylates adenosine monophosphate (AMP) to form adenosine, thus also suppressing immune function and promoting tumor cell metastasis (Stagg et al., 2010), as well as stimulates angiogenesis (Allard et al., 2014). High expression of CD73 is associated with poor prognosis in different cancer types (Leclerc et al., 2016; Loi et al., 2013; Turcotte et al., 2015). CD73 is also a potential biomarker for anti-PD-1 therapy, with high expression limiting anti-PD-1 efficacy, which can be rescued by concomitant A2A blockade (Beavis et al., 2015).
Specific chemokines and chemokine receptors are important for trafficking of MDSCs and Tregs to the tumor. For example, tumors secret ligands CCL5, CCL7, and CXCL8, bind to their receptors CCR1 or CXCR2 expressed on subtypes of MDSCs (Highfill et al., 2014), and attract MDSCs in the tumor microenvironment. Inhibitors of these chemokine receptors could abrogate immune evasion and improve antitumor T cell responses. CCR4 is highly expressed by Tregs in the blood and tumors (Sugiyama et al., 2013) and anti-CCR4 inhibits Treg recruitment as well as promotes antibody-dependent cell-mediated cytotoxicity (ADCC), further reducing the Treg population (Chang et al., 2012). CXCR4 is a receptor for the chemokine CXCL12 has been shown to promote an immunosuppressive tumor microenvironment through several mechanisms including Treg localization (Gil et al., 2014).
Acquired Resistance to Immunotherapy
A hallmark of cancer immunotherapy has been the induction of long lasting tumor responses. However, with higher activity and broader use of immunotherapies the denominator of patients with a tumor response has increased and the chances of finding patients who responded for a period of time and then progressed, termed acquired resistance, increases. It is becoming clear that approximately one fourth to one third of patients with metastatic melanoma who have objective responses to checkpoint blockade therapy with anti-CTLA-4 or anti-PD-1 will relapse over time even despite receiving continued therapy (Schachter J, 2016). The potential mechanisms of relapse include loss of T cell function, lack of T cell recognition by downregulation of tumor antigen presentation, and development of escape mutation variants in the cancer (Figure 2 and 3). There is evidence for each of these mechanisms can lead to acquired resistance to checkpoint inhibitor therapy or ACT.
If the antitumor T cells change their functional phenotype and stop exerting their cytotoxic activity, then a patient who responded to immunotherapy may develop a tumor relapse even if everything else continues to be the same. Acquired resistance to TCR engineered ACT is rather frequent, where the high initial antitumor response is followed by a high frequency of tumor relapses within months. This has been evident with the ACT of T cells expressing TCRs to melanosomal antigens (MART-1, gp100) and to cancer testis antigens (NY ESO-1) (Chodon et al., 2014; Morgan et al., 2006; Robbins et al., 2011). By studying how the TCR transgenic T cells change their functionality after ACT to humans, it has been reported that the initial highly cytolytic profile when administered shifts over time to a Th2-type cytokine release and lack of cytotoxic functions in late time points when recovered from patients at the time of tumor relapse (Ma et al., 2013; Ma et al., 2011).
Already in the 1990s it was well documented that some patients who initially responded to cancer immunotherapies with IL-2 or TIL ACT may develop acquired resistance through loss of the shared component of all HLA class I molecules, B2M, which led to absence of surface expression of HLA class I (D’Urso et al., 1991; Restifo et al., 1996). B2M is required for HLA class I folding and transport to the cell surface, and its genetic deficiency would lead to lack of CD8 T cell recognition. This mechanism of acquired resistance has also been documented in a case of late acquired resistance to anti-PD-1 therapy, where the resistant cells had a new and homozygous truncating mutation in B2M leading to lack of surface expression of HLA class I (Zaretsky et al., 2016). In two other cases of tumor relapse there were copy number neutral loss-of-function mutations in JAK1 or JAK2 concurrent with loss of heterozygosity due to deletion of the wild-type allele, which were absent in the baseline biopsies. These mutations allowed the cancer cells to escape from the anti-proliferative effects of interferon gamma (Zaretsky et al., 2016). Additional evidence of loss of antigen presenting machinery leading to acquired resistance to cancer immunotherapy is provided by a case of a patient with metastatic colorectal carcinoma who responded to TIL ACT. The therapeutic TIL recognized mutated KRAS G12D presented by HLA-C*08:02 resulting in an objective tumor response for 9 months, followed by an isolated relapse in a lesion that had lost HLA-C*08:02 in chromosome 6 (Tran et al., 2016). Therefore, acquired resistance to anti-PD-1 therapy and ACT could be mediated through genetic mechanisms that altered antigen presenting machinery and interferon gamma signaling.
As antitumor T cells are specific for cancer cells that express their cognate antigen, it is possible that cancers may develop acquired resistance through decreased expression or mutations in these tumor antigens. Data suggests that antitumor T cells turned on by checkpoint blockade therapy primarily recognize mutational neoantigens (Schumacher and Schreiber, 2015; van Rooij et al., 2013). Therefore, genetic deletions, mutations or epigenetic changes that would lead to loss of expression of these mutational neoantigens presented by MHC molecules may result in acquired resistance to checkpoint blockade therapy. However, thus far there has not been evidence of such mechanisms in the clinic. CAR T cells are also antigen-specific, but they rely on the whole protein expression on the cancer cell surface. In some cases of patients with acute lymphoblastic leukemia who responded initially to CD19 CAR T cell ACT it has been documented that the epitope in the CD19 protein sequence that is recognized by the CAR can be selectively deleted at progression (Ruella et al., 2016), and that preexisting alternatively spliced CD19 isoforms may predispose to acquired resistance (Sotillo et al., 2015). Therefore, there is evidence from the clinic that loss of the target of the antitumor T cells can result in progression to cancer immunotherapy.
This yin and yang of the immune response that results in immune editing and eventually immune escape is clearly a factor as we administer immunotherapeutic agents and attempt to drive anti-tumor immune responses, which may encounter a multitude of inhibitory pathways, either during initial treatment or at the time of relapsed disease. Additional inhibitory immune checkpoints that are often expressed in the tumor microenvironment include LAG-3, TIGIT, VISTA and many more that are being identified in ongoing studies (Topalian et al., 2015). Several clinical trials are currently underway to test antibodies against these inhibitory pathways, both as monotherapy and combination therapy strategies (Anderson et al., 2016; Sharma and Allison, 2015). To date, the combination of anti-CTLA-4 (ipilimumab) plus anti-PD-1 (nivolumab) has demonstrated improved clinical outcomes as compared to monotherapy and this combination was recently FDA-approved for patients with metastatic melanoma (Larkin et al., 2015a). We will need data from ongoing and future clinical trials to determine whether combination therapies targeting other inhibitory pathways, either as doublets or triplets in concurrent or sequential treatment strategies, will effectively overcome the resistance mechanisms that act to regulate immune responses and provide additional clinical benefit.
Monitoring Resistance Mechanisms
There are significant efforts underway to identify reliable predictive biomarkers of response and resistance to checkpoint inhibitors in baseline tumor biopsies in patients on immune checkpoint blockade. To date, the best predictive biomarkers identified include total tumor mutational load (Roszik et al., 2016; Snyder et al., 2014) as well as markers of an effective immune infiltrate within a tumor signifying a “hot” tumor microenvironment, typified by increased number of CD8+ cytotoxic T lymphocytes in proximity to programmed death receptor ligand-1 (PD-L1) positive cells (Taube et al., 2014; Tumeh et al., 2014). Mutational load is highly relevant, as tumors with a higher mutational load exhibit higher levels of neoantigens capable of inducing anti-tumor immune responses – translating into a higher likelihood of response to immune checkpoint blockade across several cancer types (Rizvi et al., 2015; Snyder et al., 2014; Van Allen et al., 2015). In addition to genomic markers and immune regulatory gene expression profiles (Hugo et al., 2016), immune markers in pre-treatment biopsies including the density and distribution of CD8+ T lymphocytes, PD-L1 expression, and T cell clonality (Taube et al., 2014; Tumeh et al., 2014) have also been associated with differential responses to immune checkpoint blockade, although significant limitations exist when each of these biomarkers is assessed in isolation. Integrative approaches incorporating analysis of several of these features have also been developed such as the cancer immunogram – which incorporates analysis of 7 distinct features within the tumor microenvironment: tumor sensitivity to immune effectors, tumor foreignness, general immune status, immune cell infiltration, absence of checkpoint molecule expression, absence of soluble inhibitors such as interleukin-1 and interleukin-6, and absence of inhibitory tumor metabolism (Blank et al., 2016). These efforts are critical and will ultimately contribute to more personalized treatment strategies for cancer immunotherapy.
An emerging strategy in elucidating mechanisms of response and resistance to immune checkpoint blockade involves the assessment of longitudinal tumor samples throughout the course of treatment. This approach is powerful, as it transcends conventional analysis of static time points and seeks to identify superior predictive biomarkers by assessing dynamic responses to cancer treatment. Such an approach has been employed to better understand response and resistance to immune checkpoint blockade (Chen et al., 2016; Hugo et al., 2016; Madore et al., 2015; Tumeh et al., 2014), and has yielded important information that would not have been elucidated through analysis of static unpaired biopsies. A key example is in a recent report describing immune markers in longitudinal tumor samples of patients on immune checkpoint blockade, demonstrating that while pre-treatment markers were largely non-predictive, immune markers in early on-treatment samples were highly predictive of treatment response (Chen et al., 2016). In addition to this, resistance mechanisms were identified via pairwise comparison of gene expression profiles in pre- to on-treatment tumor samples of responders versus non-responders, including defects in interferon signaling as well as antigen processing and presentation (Chen et al., 2016). This approach is currently under-utilized but is gaining traction in light of advantages over assessment of static baseline biomarkers (Figure 4), as well as an increasing need to better understand responses to a growing number of immunotherapeutic approaches. However nuances exist with regard to immune monitoring in the tumor microenvironment (Wargo et al., 2016), and an appreciation of the importance of concurrent monitoring in the peripheral blood is growing – though the ideal assays to perform are still being elucidated.
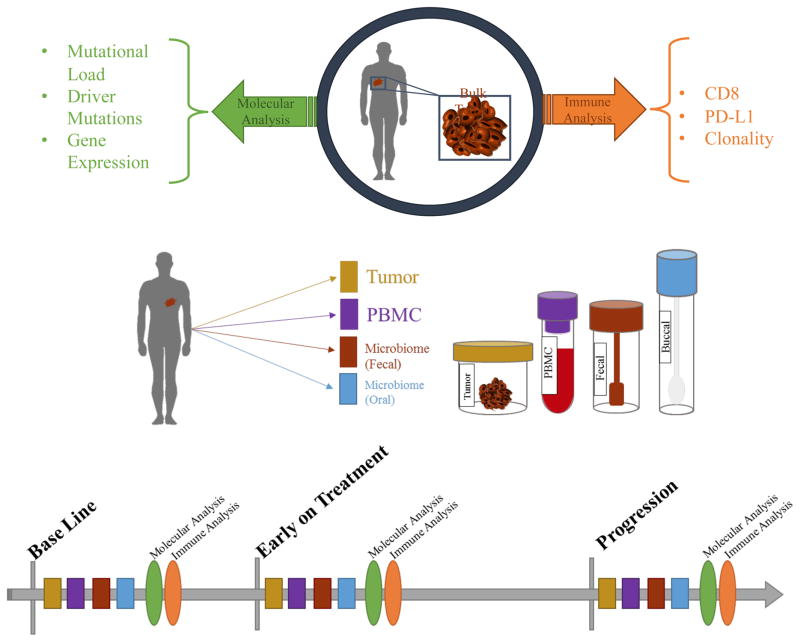
Figure 4. Schema for analysis of baseline and longitudinal tumor, blood, and other samples.
A) Baseline assessment of the tumor microenvironment typically involves molecular analysis for mutational load, driver mutations and gene expression, with immune profiling including analysis of CD8+ T cells, PD-L1 expression, and T cell clonality. B) Longitudinal evaluation of fresh serial human specimens (tumor, blood, serum, and microbiome) during treatment (at pre-treatment, early on-treatment, and progression time points) allows for deep analysis to unveil potential mechanisms of therapeutic resistance.
Overcoming Resistance to Immunotherapy
Based on insights gained (Hugo et al., 2016; Snyder et al., 2014; Van Allen et al., 2015), efforts are currently underway to derive actionable strategies to combat therapeutic resistance to immunotherapy. This includes fundamental efforts to transform immunologically “cold” tumors into “hot” tumors through the use of several approaches (Corrales et al., 2015; Holmgaard et al., 2013; Tang et al., 2016), and also involves tactics to either enhance endogenous T cell function (Gubin et al., 2014; Hodi et al., 2010; Miller et al., 2002; Redmond et al., 2007; Ribas et al., 2015; Weber et al., 2015) or to adoptively transfer antigen-specific T lymphocytes via ex vivo expansion of tumor-infiltrating lymphocytes (Rosenberg et al., 2011) or via administration of antigen-specific engineered T cells (via transduction with chimeric antigen receptors or T cell receptors) (Beatty et al., 2014; Kalos et al., 2011).
Though some of these approaches involve treatment with drugs as monotherapy (including monoclonal antibodies), the majority of contemporary approaches focus on combination strategies in an effort to overcome resistance associated with treatment with single-pronged efforts (Hicklin et al., 1998; Moon et al., 2014; Ninomiya et al., 2015). A prime example of enhanced efficacy with combination therapy is the use of combined therapy with blocking antibodies against 2 key immune checkpoints – CTLA-4 and PD-1, which results in significantly higher response rates to therapy and improved survival in patients with metastatic melanoma (Larkin et al., 2015b; Postow et al., 2015; Wolchok et al., 2013). The rationale for this combination approach is several fold, as blocking several checkpoints on anergized tumor-specific T cells has been shown to be more efficacious (Berrien-Elliott et al., 2013; Curran et al., 2010; Redmond et al., 2014; Spranger et al., 2014) and CTLA-4 blockade may itself facilitate the conversion of a tumor microenvironment from “cold” to “hot.” (Simpson et al., 2013b). Indeed, each of these checkpoint inhibitors has been shown to have both overlapping and unique effects on tumor-specific T cells (Gubin et al., 2014), substantiating the use of these in combination. Numerous other strategies combining immune modulation of the tumor microenvironment with immune checkpoint inhibitor therapy are currently being tested in clinical trials (Puzanov et al., 2016) (NCT02263508, NCT02626000; NCT02565992, NCT02043665; NCT02501473). Vaccine strategies against identified neoantigen epitopes are also being combined with immunotherapeutic approaches – though mature data are not available regarding efficacy.
Another combination strategy with strong clinical and pre-clinical rationale involves the use of molecularly targeted therapy in conjunction with immunotherapy. The most extensively studied cancer type treated with this strategy is melanoma, though the concept is now being widely extended across solid and liquid tumors. The rationale for combining these treatments is that treatment with molecularly targeted therapy can have a substantial effect on anti-tumor immunity with potential synergy when used with immunotherapy (Homet Moreno et al., 2016; Hu-Lieskovan et al., 2015; Koya et al., 2012). Perhaps most illustrative of this is oncogenic BRAF in melanoma. Though treatment with BRAF-targeted therapy alone provides limited durable disease control (Chapman et al.; Hauschild et al.), it is associated with favorable effects in the tumor microenvironment – with increased antigen (Boni et al., 2010) and HLA expression (Bradley et al., 2015), increased T cell infiltrate, and reduced immunosuppressive cytokines (Frederick et al., 2013; Wilmott et al., 2012), with T cell function (Comin-Anduix et al., 2010). Thus treatment with molecularly targeted therapy may indeed help convert a “cold” microenvironment to a “hot” one, with resultant increased expression of PD-L1 via the phenomenon of adaptive resistance (Taube et al., 2012) – further supporting a multi-modality treatment approach. Emerging strategies to enhance responses to immunotherapy are being developed based on novel insights into T cell and overall immune function. Examples of this include insights into metabolic reprogramming of T cells to enhance therapeutic responses (Buck et al., 2016; Chang and Pearce, 2016) and via modulation of the gut microbiome to augment responses to cancer immunotherapy (Sivan et al., 2015; Vetizou et al., 2015).
Complexities exist with validating these combination strategies, as the extent of possible combinations far outnumbers the human and technical resources available. There is an urgent need to test these combinations in appropriate pre-clinical models and expedite clinical translation through novel approaches to clinical trial design. In addition, we need to have a deep understanding of the kinetics of the immune response to each of these agents in isolation as well as in combination in order to narrow the search space of biologically promising and optimal combination strategies. Immune responses to targeted agents may be short-lived (Cooper et al., 2014), thus proper timing and sequence of therapy must be strongly considered.
Conclusions
Great advances occurred in the field of cancer immunotherapy due to elegant research work conducted to elucidate the mechanisms that regulate anti-tumor T cell responses, with eventual translation of these concepts to the clinic. This has allowed the rational design and clinical development of treatment strategies that may result in tumor regression and long-term survival for patients with metastatic cancer. However, the benefit, to date, has been limited to a minority of patients with certain cancer types. In addition, as a result of more successful immunotherapy treatments we now have a significant subset of patients who initially respond but eventually relapse. Bringing clinical benefit to the majority of patients requires a complete understanding of the mechanisms that would lead to an effective anti-tumor response and the different tumor cell-intrinsic and extrinsic factors that would result in primary, adaptive and acquired resistance to immunotherapy. Elucidation of these mechanisms will reveal important clues to the next steps that need to be taken to potentially overcome resistance to immunotherapy.
Table 3.
Examples of combination therapies being developed to overcome resistance to cancer immunotherapy
| Broad approach | Specific approach | Examples in clinical testing |
|---|---|---|
| Combination checkpoint blockade | Anti-PD-1/L1 plus anti-CTLA4 | Durvalumab+tremelimumab Nivolumab+ipilimumab Pembrolizumab+ipilimumab |
| Anti-PD-1 plus anti-PD-L1 | MEDI0680 + durvalumab PDR001 + FAZ053 |
|
| Anti-PD-1/L1 plus anti-TIM 3 | Nivolumab + TSR022 PDR001 + MBG453 |
|
| Anti-PD-1/L1 plus anti-LAG 3 | Nivolumab + BMS 986016 PDR001 + LAG525 Pembrolizumab + IMP321 REGN2810 + REGN3767 |
|
| Checkpoint blockade plus Immune-stimulatory agents | Anti-PD-1/L1 plus anti-41BB/CD137 | Avelumab + utomilumab Nivolumab + urelumab Pembrolizumab + utomilumab |
| Anti-CTLA4 plus anti-OX40 Anti-PD-1/L1 plus anti-OX40 Anti-CTLA4 plus Anti-PD-1/L1 plus anti-OX40 Anti-41BB/CD137 plus anti-OX40 |
Atezolimumab + MOXR0916 +/− bevacizumab Avelumab + PF-04518600 Durvalumab + MEDI0562 Pembrolizumab + GSK3174998 Tremelimumab + durvalumab + MEDI6469 Tremelimumab + MEDI0562 Utomilumab + PF-04518600 |
|
| Anti-CTLA4 plus anti-CD40 Anti-PD-1/L1 plus anti-CD40 |
Atezolimumab + RO7009789 Tremelimumab + CP870893 |
|
| Anti-PD-1/L1 plus anti-GITR | Nivolumab + BMS986156 PDR001 + GWN323 |
|
| Anti-PD-1/L1 plus anti-ICOS | Nivolumab + JTX-2011 | |
| Checkpoint blockade plus metabolic modulators | Anti-CTLA-4 plus IDO inhibitors Anti-PD-1/L1 plus IDO inhibitors |
Atezolizumab + GDC0919 Ipilimumab + epacadostat Ipilimumab + indoximid Nivolumab + BMS986205 Pembrolizumab+ epacadostat |
| Anti-PD-1/L1 plus A2AR inhibitors or anti-CD73 | Atezolizumab + CPI-444 Durvalumab + MEDI9447 PDR001+ PBF509 |
|
| Checkpoint blockade plus other immune modulators | Anti-PD-1/L1 plus TGFβ inhibitors | Nivolumab + LY2157299 PDR001 + NIS793 |
| Anti-PD-1/L1 plus CXCR4 inhibitors | Nivolumab + ulocuplumab Durvalumab + LY2510924 |
|
| Anti-PD-1/L1 plus CCR4 inhibitors | Nivolumab + mogamulizumab | |
| Anti-PD-1/L1 plus anti-CD27 | Nivolumab + varlilumab Atezolizumab + varlilumab |
|
| Anti-PD-1/L1 plus CD122-Biased Cytokine | Nivolumab + NKTR-214 | |
| Anti-PD-1/L1 plus yeast-derived soluble β-glucan | Pembrolizumab + Imprime PGG | |
| Anti-PD-1/L1 plus anti- TRAIL-DR5 | Nivolumab + DS-8273a | |
| Anti-PD-1/L1 plus Glutaminase Inhibitor | Nivolumab + CB839 | |
| Anti-PD-1/L1 plus IAP inhibitor | PDR001 + LCL161 | |
| Checkpoint blockade plus Macrophage inhibitors | Anti-CTLA4 plus CSF1R inhibitors Anti-PD-1/L1 plus CSF1R inhibitors |
Durvalumab + Pexidartinib (PLX3397) Durvalumab + LY3022855 Nivolumab + FPA008 Pembrolizumab + Pexidartinib PDR001 + BLZ945 Tremelimumab + LY3022855 |
| Checkpoint blockade plus injectable therapies | Anti-CTLA-4 plus oncolytic viruses Anti-PD-1/L1 plus oncolytic viruses |
Ipilimumab + Talimogene Laherparepvec Nivolumab + Talimogene Laherparepvec Pembrolizumab + DNX2401 Pembrolizumab + Talimogene Laherparepvec |
| Anti-CTLA4 plus TLR agonists Anti-PD-1/L1 plus TLR agonists |
Ipilimumab + MGN1703 Pembrolizumab + CMP001 Pembrolizumab + SD101 Tremelimumab + PF-3512676 |
|
| Checkpoint blockade plus cancer vaccines | Anti-CTLA4 plus DC vaccine Anti-PD-1/L1 plus DC vaccine Anti-PD-1/L1 plus peptide vaccine Anti-PD-1/L1 plus neoantigen vaccine |
Durvalumab + ADXS11-001 Durvalumab + TPIV200/huFR-1 Ipilimumab + GVAX Nivolumab + GVAX + CRS207 Nivolumab + CIMAvax Nivolumab+ CV301 Nivolumab + NEO-PV-01 Nivolumab + Viagenpumatucel-L (HS-110) Pembrolizumab + ADXS31-142 Durvalumab +/− tremelimumab + IMCgp100 |
| Checkpoint blockade plus adoptive cell transfer (ACT) | Anti-CTLA4 plus ACT Anti-PD-1/L1 plus ACT Anti-PD-1/L1 plus anti-CD137 plus ACT |
Atezolimuamb + KTE-C19 Ipilimumab + NYESO TCR ACT Nivolumab + NYESO TCR ACT Nivolumab + urelumab + TIL ACT Pembrolizumab + TIL ACT Ipilimumab + modified CD8 T cell ACT Pembrolizumab + modified CD8 T cell ACT |
| Checkpoint blockade plus targeted therapies | Anti-CTLA4 plus BRAF+MEK inhibitors Anti-CTLA4 plus VEGF inhibitors Anti-PD-1/L1 plus BRAF+MEK inhibitors Anti-PD-1/L1 plus EGFR inhibitors Anti-PD-1/L1 plus VEGF inhibitors Anti-PD-1/L1 plus PI3K delta inhibitor |
Atezolizumab + bevacizumab vs sunitinib Atezolizumab + trametinib Atezolizumab + vemurafenib +/− cobimetinib Durvalumab + ensartinib (ALK inhibitor) Durvalumab + gefitinib Durvalumab + trametinib +/− dabrafenib Ipilimumab + bevacizumab Ipilimumab + dabrafenib +/− trametinib Ipilimumab +vemurafenib Nivolumab + sunitinib or pazopanib Nivolumab + trametinib +/− dabrafenib PDR001 + sorafenib Pembrolizumab + dabrafenib + trametinib Pembrolizumab + lenalidomide Pembrolizumab + nintedarnib Pidilizumab + lenalidomide Tremelimumab + sunitinib Nivolumab + SYM004 |
| Anti-PD-1/L1 plus PARP inhibitors | Atezolizumab + Veliparib Durvalumab + olaparib BGB-A317 + BGB-290 |
|
| Anti-PD-1/L1 plus mTOR inhibitor | PDR001 + everolimus | |
| Anti-PD-1/L1 plus pan RAF inhibitor | PDR001 + LXH254 | |
| Anti-PD-1/L1 plus glutaminase inhibitor | Nivolumab + CB839 | |
| Checkpoint blockade plus radiation therapy (RT) | Anti-CTLA4 plus RT Anti-PD-1/L1 plus RT Anti-CTLA4 plus Anti-PD-1/L1 plus RT |
Atezolizumab + stereotactic radiation therapy Pembrolizumab + cisplatin/radiotherapy Pembrolizumab + sterotactic body radiotherapy Pembrolizumab + hypofractionated radiotherapy |
| Checkpoint blockade plus chemotherapy | Anti-CTLA4 plus chemotherapy Anti-PD-1/L1 plus chemotherapy Anti-CTLA4 plus Anti-PD-1/L1 plus chemotherapy |
Atezolizumab + carboplatin/paclitaxel Atezolizumab + carboplatin/gemcitabine Durvalumab + paclitaxel Ipilimumab +carboplatin/paclitaxel Ipilimumab +dacarbazine Nivolumab + platinum doublets Pembrolizumab + carbo/paclitaxel or carbo/pemetrexed |
| Checkpoint blockade plus epigenetic modifications | Anti-PD-1/L1 plus histone deacetylase inhibitors Anti-PD-1/L1 plus hypomethylating agents |
Azacitidine + entinostat followed by nivolumab Atezolizumab + azacitidine Nivolumab + RRX001 Pembrolizumab + CC486 Pembrolizumab + CC486 + romidepsin Pembrolizumab + romidepsin Pembrolizumab + vorinostat + tamoxifen PDR001 + panobinostat |
| Checkpoint blockade plus NK activation | Anti-CTLA4 plus anti-KIR Anti-PD-1/L1 plus anti-KIR |
Ipilimumab + lirilumab Nivolumab + lirilumab |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer discovery. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. International Journal of Cancer. 2014;134:1466–1473. doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New England Journal of Medicine. 2015a;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015b;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer immunology research. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis PA, Slaney CY, Milenkovski N, Henderson MA, Loi S, Stagg J, Kershaw MH, Darcy PK. CD73: A potential biomarker for anti-PD-1 therapy. Oncoimmunology. 2015;4:e1046675. doi: 10.1080/2162402X.2015.1046675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554. e1512. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrien-Elliott MM, Jackson SR, Meyer JM, Rouskey CJ, Nguyen TL, Yagita H, Greenberg PD, DiPaolo RJ, Teague RM. Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer research. 2013;73:605–616. doi: 10.1158/0008-5472.CAN-12-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer research. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz T, Liu S, Whittington M, Deng W, Li F, et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8+ T-cell Recognition of Melanoma. Cancer immunology research. 2015;3:602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. Journal of immunology (Baltimore, Md : 1950) 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. Immunological surveillance in neoplasia. Transplantation reviews. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gutgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nature immunology. 2016;17:364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DK, Sui J, Geng S, Muvaffak A, Bai M, Fuhlbrigge RC, Lo A, Yammanuru A, Hubbard L, Sheehan J. Humanization of an anti-CCR4 antibody that kills cutaneous T-cell lymphoma cells and abrogates suppression by T-regulatory cells. Molecular cancer therapeutics. 2012;11:2451–2461. doi: 10.1158/1535-7163.MCT-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines. 2016:4. doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nature reviews Immunology. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer discovery. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, Ng C, Avramis E, Seja E, Villanueva A, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2457–2465. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley WB. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proceedings of the Royal Society of Medicine. 1910;3:1–48. [PMC free article] [PubMed] [Google Scholar]
- Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, Escuin-Ordinas H, Chmielowski B, Koya RC, Ribas A. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:6040–6048. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer immunology research. 2014;2:643–654. doi: 10.1158/2326-6066.CIR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell reports. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. The Journal of clinical investigation. 1991;87:284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. Journal of immunology. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, et al. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353:399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, et al. A critical function for type I interferons in cancer immunoediting. Nature immunology. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Collected papers in four volumes including a complete bibliography. London: Pergamon Press; 1956. [Google Scholar]
- Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature reviews Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JM, Tennis MA, Orlicky DJ, Lin H, Ju C, Redente EF, Choo KS, Staab TA, Bouchard RJ, Merrick DT, et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Frontiers in immunology. 2014;5:587. doi: 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167:397–404. e399. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- Gil M, Komorowski MP, Seshadri M, Rokita H, McGray AJ, Opyrchal M, Odunsi KO, Kozbor D. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. Journal of immunology. 2014;193:5327–5337. doi: 10.4049/jimmunol.1400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. Journal of translational medicine. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Holtzhausen A, Evans K, Heid M, Blobe GC. Combinatorial TGF-{beta} signaling blockade and anti-CTLA-4 antibody immunotherapy in a murine BRAFV600E-PTEN-/-transgenic model of melanoma. Paper presented at: ASCO Annual Meeting Proceedings.2014. [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Héninger E, Krueger TE, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Frontiers in immunology. 2015;6:29. doi: 10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. The Journal of clinical investigation. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science translational medicine. 2014;6:237ra267–237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of experimental medicine. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2016;5:e1052212. doi: 10.1080/2162402X.2015.1052212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li X, Zhang C, Yang Y, Jiang J, Wu C. Tumor-associated macrophages in cancers. Clinical and Translational Oncology. 2016;18:251–258. doi: 10.1007/s12094-015-1373-0. [DOI] [PubMed] [Google Scholar]
- Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Science translational medicine. 2015;7:279ra241. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH, Wurster AL, Grusby MJ. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. The Journal of experimental medicine. 1998;188:1191–1196. doi: 10.1084/jem.188.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf AR, Jones DA. Reactivating the expression of methylation silenced genes in human cancer. Oncogene. 2002;21:5496–5503. doi: 10.1038/sj.onc.1205602. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, Maeda T, Nagata Y, Kitanaka A, Mizuno S, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534:402–406. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. American journal of translational research. 2011;3:166–179. [PMC free article] [PubMed] [Google Scholar]
- Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. Journal of immunology (Baltimore, Md : 1950) 2012;189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, Minasyan A, Graham NA, Graeber TG, Chodon T, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer research. 2012;72:3928–3937. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of experimental medicine. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of experimental medicine. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. The Journal of experimental medicine. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer Vaccines in the World of Immune Suppressive Monocytes (CD14(+)HLA-DR(lo/neg) Cells): The Gateway to Improved Responses. Frontiers in immunology. 2014;5:147. doi: 10.3389/fimmu.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New England Journal of Medicine. 2015a;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015b;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, Wolchok JD. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA oncology. 2015c;1:433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer research. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. Journal of immunotherapy. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Lebrun J-J. The dual role of TGF in human cancer: from tumor suppression to cancer metastasis. ISRN molecular biology. 2012;2012 doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J. CD73 expression is an independent prognostic factor in prostate cancer. Clinical Cancer Research. 2016;22:158–166. doi: 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-beta in cancer. Cancer biology & medicine. 2015;12:385–393. doi: 10.7497/j.issn.2095-3941.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunologic research. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, Chen JQ, Li HS, Watowich SS, Yang Y, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proceedings of the National Academy of Sciences. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. The Journal of clinical investigation. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, Avramis E, Cochran AJ, Witte ON, Baltimore D, et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer discovery. 2013;3:418–429. doi: 10.1158/2159-8290.CD-12-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T, Koya RC, Liu CC, Kwong GA, Radu CG, et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nature medicine. 2011;17:738–743. doi: 10.1038/nm.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment cell & melanoma research. 2015;28:245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Advances in immunology. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer immunology, immunotherapy : CII. 2014;63:247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Jacques FAP, Sadelain M. The Journey from Discoveries in Fundamental Immunology to Cancer Immunotherapy. Cancer cell. 2015;27:439–449. doi: 10.1016/j.ccell.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, Lynch DH. 4–1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. Journal of immunology. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- Mittal D, Sinha D, Barkauskas D, Young A, Kalimutho M, Stannard K, Caramia F, Haibe-Kains B, Stagg J, Khanna KK. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer research, canres. 2016 doi: 10.1158/0008-5472.CAN-16-0544. 0544.2016. [DOI] [PubMed] [Google Scholar]
- Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, Graeber TG, West BL, Bollag G, Ribas A. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer research. 2014;74:153–161. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM, Ramos CA. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. Journal of immunology (Baltimore, Md : 1950) 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature immunology. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer research. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- Ortenberg R, Sapir Y, Raz L, Hershkovitz L, Ben Arav A, Sapoznik S, Barshack I, Avivi C, Berkun Y, Besser MJ, et al. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Molecular cancer therapeutics. 2012;11:1300–1310. doi: 10.1158/1535-7163.MCT-11-0526. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature reviews Immunology. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. The Journal of clinical investigation. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. Journal of immunology. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer immunology research. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. Journal of the National Cancer Institute. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer discovery. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The lancet oncology. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszik J, Haydu LE, Hess KR, Oba J, Joon AY, Siroy AE, Karpinets TV, Stingo FC, Baladandayuthapani V, Tetzlaff MT, et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC medicine. 2016;14:168. doi: 10.1186/s12916-016-0705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AY. Regulatory T cells and Foxp3. Immunological reviews. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. The Journal of clinical investigation. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M. Chimeric antigen receptors: driving immunology towards synthetic biology. Curr Opin Immunol. 2016;41:68–76. doi: 10.1016/j.coi.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter JRA, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE-006. Paper presented at: J Clin Oncol. 2016;34:2016. (suppl; abstr 9504) [Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen T-T, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. Journal of clinical oncology. 2015 doi: 10.1200/JCO.2014.56.2736. JCO. 2014.2056. 2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35. doi: 10.1016/j.coi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. Primary Resistance to PD-1 Blockade Mediated by JAK(1/2) Mutations. Cancer discovery. 2016 doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013a;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013b:210. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer discovery. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. Journal for ImmunoTherapy of Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proceedings of the National Academy of Sciences. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucker A, Zhao F, Real B, Heeke C, Bielefeld N, Mabetaen S, Horn S, Moll I, Maltaner R, Horn PA, et al. Genetic evolution of T-cell resistance in the course of melanoma progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:6593–6604. doi: 10.1158/1078-0432.CCR-14-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y. Anti-CCR4 mAb selectively depletes effector-type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstedt A, O’Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. Journal of immunology (Baltimore, Md : 1950) 2003;170:1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Okai Y, Paxton RJ, Hefta LJ, Shively JE. Differential regulation of carcinoembryonic antigen and biliary glycoprotein by gamma-interferon. Cancer research. 1993;53:1612–1619. [PubMed] [Google Scholar]
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature reviews Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DY, Ellis RA, Lovat PE. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front Oncol. 2016;6:236. doi: 10.3389/fonc.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham M, Khoo K, Yeo KP, Kato M, Prevost-Blondel A, Angeli V, Abastado JP. Macrophage depletion reduces postsurgical tumor recurrence and metastatic growth in a spontaneous murine model of melanoma. Oncotarget. 2015;6:22857–22868. doi: 10.18632/oncotarget.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. The New England journal of medicine. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer research. 2015;75:4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:e439–442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer research. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Annals of surgical oncology. 2006;13:1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, et al. Enhanced Antitumor Activity Induced by Adoptive T-Cell Transfer and Adjunctive Use of the Histone Deacetylase Inhibitor LAQ824. Cancer research. 2009a;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer research. 2009b;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Mei ZY, Zhou JH, Yao YS, Li YH, Xu YH, Li JX, Gao XN, Zhou MH, Jiang MM, et al. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PloS one. 2013;8:e62924. doi: 10.1371/journal.pone.0062924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo JA, Reddy SM, Reuben A, Sharma P. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol. 2016;41:23–31. doi: 10.1016/j.coi.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The lancet oncology. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. Journal for immunotherapy of cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. Journal of immunology (Baltimore, Md : 1950) 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- Wu X, Schulte BC, Zhou Y, Haribhai D, Mackinnon AC, Plaza JA, Williams CB, Hwang ST. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. The Journal of investigative dermatology. 2014;134:2814–2822. doi: 10.1038/jid.2014.206. [DOI] [PubMed] [Google Scholar]
- Yang JC, Rosenberg SA. Chapter Seven - Adoptive T-Cell Therapy for Cancer. In: Robert DS, editor. Advances in immunology. Academic Press; 2016. pp. 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Lizee G, Schueneman AJ. Endogenous T-Cell Therapy: Clinical Experience. Cancer journal. 2015;21:492–500. doi: 10.1097/PPO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England journal of medicine. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Conrad DM, Butler JJ, Zhao C, Blay J, Hoskin DW. Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: role of cyclic adenosine 3′, 5′-monophosphate and phosphatases. The Journal of Immunology. 2004;173:932–944. doi: 10.4049/jimmunol.173.2.932. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer research. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv324–328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]