This study investigated the combined effect of drought and heat wave on physiological responses of tomato seedlings. We found that the negative impacts of drought stress were exacerbated by heat wave through stomatal and biochemical limitations. Additionally, the recovery of leaf gas exchange in the combination of drought and heat wave was delayed more than in other treatments. Our results suggest that future climates characterized by drought and extreme heat may have substantial impacts on tomato productivity and survival.
Keywords: heat stress, photosynthesis, recovery, stomatal conductance, water deficit, water relations
Abstract
Heat waves in combination with drought are predicted to occur more frequently with climate warming, yet their interactive effects on crop carbon and water balance are still poorly understood. Hence, research on the capacity of crops to withstand and recover from the combined stress is urgently needed. This study investigated the effects of drought and heat wave on a crop species as well as the recovery from the combined stress. Seedlings were grown in growth chambers under two soil water conditions (i.e. well watered and drought stress) at ambient temperature (26 °C) for 10 days. Afterwards, half of the seedlings were exposed to a 7-day 42 °C heat wave. All the drought-stressed seedlings were then rehydrated upon relief of the heat wave. Leaf gas exchange, the maximum carboxylation capacity (Vcmax), plant growth, relative chlorophyll content and leaf water potential were examined during the experimental period. The heat wave reduced leaf gas exchange rates, Vcmax and relative chlorophyll content, while it had no impacts on leaf water potential. In contrast, drought stress led to greater reductions in leaf gas exchange rates, growth and water potential than heat wave alone. Seedlings underwent a greater degree of stress in the combination of drought and heat wave than under the single drought treatment. The recovery of leaf gas exchange from drought stress lagged behind the water potential recovery and was delayed by heat wave. Our results show that drought stress had a predominant role in determining plant physiological responses and the negative impacts of drought stress were exacerbated by heat wave. The greater stress in the combination of drought and heat wave translated into the slower recovery of leaf gas exchange. Therefore, drought combined with heat wave may induce greater risks on crops under future climates.
Introduction
Weather extremes, such as heat waves and severe droughts, are expected to increase in frequency and intensity under climate change (Reichstein et al. 2013; IPCC 2014). Plants appear to be more sensitive to weather extremes than to gradual changes in weather mean conditions, and therefore weather extremes may have more pronounced impacts on plants (Medvigy et al. 2010; De Boeck et al. 2011; Bauweraerts et al. 2013; Anderegg et al. 2015; Siebers et al. 2015). Despite the future co-occurrence of drought and heat waves, we still lack of evidence as to what degree the interactive effects of drought and extreme heat would affect the physiological responses of crops (De Boeck et al. 2011; Teskey et al. 2014). In particular, there is still limited information on how crops would recover from the combined stress of drought and heat wave. Tomato is recognized as the 4th most valuable agricultural product worldwide (O’Carrigana et al. 2014). Hence, experimentally quantifying the tolerance of tomato to the combined extremes and the following recovery from stress can provide insights into better predicting tomato physiology and productivity in the future, thereby understanding how tomato would cope with climate change.
Plant carbon and water balance are both affected by drought and heat alone or in combination (Hartmann et al. 2013; Mitchell et al. 2013; Will et al. 2013; Zhao et al. 2013; Duan et al. 2014). On one hand, as the soil dries, plants close their stomata to reduce transpiration and water loss in order to avoid the failure of xylem water transport, while the consequent stomatal closure is accompanied with inhibition in growth and a reduction in carbon assimilation (McDowell et al. 2008; Woodruff et al. 2015). On the other hand, as air temperature and associated vapor pressure deficit (VPD) increase, plants can up-regulate transpiration for leaf cooling to prevent excessive heat damage (Ameye et al. 2012; Teskey et al. 2014). Nevertheless, larger evapotranspiration demands could induce soil water deficit and/or have negative feedbacks on stomatal conductance, thereby leading to stomatal closure and reduced photosynthesis (Duursma et al. 2014). In addition, once the thermal threshold is exceeded, non-stomatal limitations, such as degradation of chlorophyll, reduced activity of Rubisco activase and damage of photosystem II (PSII) could occur, further reducing carbon assimilation (e.g. Cunningham and Read 2006; Rennenberg et al. 2006; Haldimann et al. 2008; Hamilton III et al. 2008; Bauweraerts et al. 2014; Teskey et al. 2014; Wujeska-Klause et al. 2015).
Drought and extreme heat stress are usually thought to be highly interrelated. Each stress can exacerbate the stress severity of the other (Hamerlynck et al. 2000; Arnone et al. 2008; De Boeck et al. 2011; Ameye et al. 2012; Carmo-Silva et al. 2012; Bauweraerts et al. 2013; Teskey et al. 2014; Lobell et al. 2015; Ruehr et al. 2016). Drought stress is usually increased by heat (Zhao et al. 2013; Duan et al. 2014), while stomatal closure due to short term drought stress can prevent leaf cooling under heat and therefore push plants towards the thermal threshold (Teskey et al. 2014). Hence, the combined stress of drought and extreme heat would have more pronounced negative impacts on plants than the single factor. For instance, it has been demonstrated that photosynthesis in a herbaceous community was reduced to a greater extent in the combination of drought and heat wave than single drought or heat wave treatment (De Boeck et al. 2011). However, it has also been recognized that prolonged drought stress can improve the biochemical thermotolerance of plants (Chaves et al. 2002).
Unlike investigation on the resistance of plants to drought and heat wave, the recovery of plant physiological processes from the combination of the two stresses is far less studied. The recovery rate of leaf physiological responses may vary depending on the severity of previous stress and the legacy effects on plants (Anderegg et al. 2015; Mitchell et al. 2016). For example, one recent study has shown that recovery of photosynthesis in the combination of drought and heat stress was much slower than that in the single drought treatment even three weeks after the release of stress (Ruehr et al. 2016), mainly due to the irreversible photoinhibition under the combined stress. Additionally, information on the coordination of gas exchange and water relations during recovery from combined extreme stress remains scarce. Whether their relationships would be modified by the combined stress is still unknown. Consequently, more efforts are needed to investigate the capacity of plants to regulate carbon and water relations during the recovery following multiple stresses, providing more insights into implications for plant survival and productivity in the future real world.
This study aimed to examine the leaf physiology and growth of cherry tomato (Solanum lycopersicum) seedlings during the periods of combined stress of drought and heat wave and recovery following stress. Leaf gas exchange, plant growth, relative chlorophyll content, leaf water relations and the maximum carboxylation capacity (Vcmax) were measured or estimated during the course of the experiment. The key research question is how plant carbon and water relations are coordinated during the recovery from the combined stress of drought and heat wave, which is crucial for tomato management strategies under climate change. We hypothesized that: (i) single heat wave treatment would lead to lower steady state leaf gas exchange rates, decreased water use efficiency, lower Vcmax, reduced plant growth and more negative leaf water potential compared with ambient temperature control; (ii) heat wave would exacerbate drought stress by reducing steady state leaf gas exchange rates, Vcmax, plant growth, leaf water potential, chlorophyll content to a greater extent in the combination of drought and heat wave treatment than in the single drought treatment; (iii) the coordination of leaf gas exchange and leaf water potential during recovery from drought would be shifted between ambient temperature and heat wave treatments, i.e. the recovery of leaf physiology from drought would be slower in the combination of drought and heat wave than in the single drought treatment.
Methods
Plant materials and growth conditions
Seeds of cherry tomato (Solanum lycopersicum; produced by Beijing Fangxuanyuan Seed Co, Ltd.) were sown and germinated in seed raising tube stocks in a poly-tunnel under ambient environmental conditions for one month. Healthy seedlings with similar heights (15–20 cm) were then transplanted into 3.7-L plastic pots filled with soil (one seedling per pot) and were grown for another month. Twenty four potted seedlings were then randomly assigned into two illuminated growth chambers (GS-1, Wuhan Ruihua Co, Ltd, China) (twelve seedlings per growth chamber) with a 26 °C/19 °C day/night temperature cycle and 15 h/9 h day/night photoperiod, 60% relative humidity (RH), and 250 µmol m−2 s−1 photosynthetic photon flux density (PPFD). The seedlings were well irrigated daily and fertilized weekly with a commercial liquid fertilizer (Soluble nutrient fertilizer, Demaisie Crop Science Co, Ltd, China). Seedlings were rotated within and between growth chambers every week to minimise potential effects of growth chambers on plant performance.
Experimental design
Following 2 weeks of additional growth in the growth chambers, half of the seedlings (i.e. six seedlings) in each growth chamber were randomly assigned to two water treatments (i.e. well watered and drought treatments) on Day 0. The drought treatment consisted of one cycle of drought and recovery. Seedlings in the drought treatment in both growth chambers were treated under moderate drought stress until they were rehydrated on Day 20. During that time, the heat wave was imposed on one of the growth chambers for a period of 7 days. Specifically, from Day 10, one growth chamber was subjected to heat wave treatment (i.e. 42 °C/35 °C day/night temperature cycle), while the other one was maintained at ambient temperature treatment (i.e. 26 °C/19 °C day/night temperature cycle) (see Fig. 1A). The temperature in the heated growth chamber returned to ambient level on Day 17. Thus, this experiment was a factorial of 2 water × 2 temperature treatments, with six seedlings in each treatment. The four treatments in this experiment were: (1) Ambient temperature plus well watered (AW); (2) Ambient temperature plus drought (AD); (3) Heat wave plus well watered (HW); and (4) Heat wave plus drought (HD). Details of water and heat wave treatments are described below.
Figure 1.
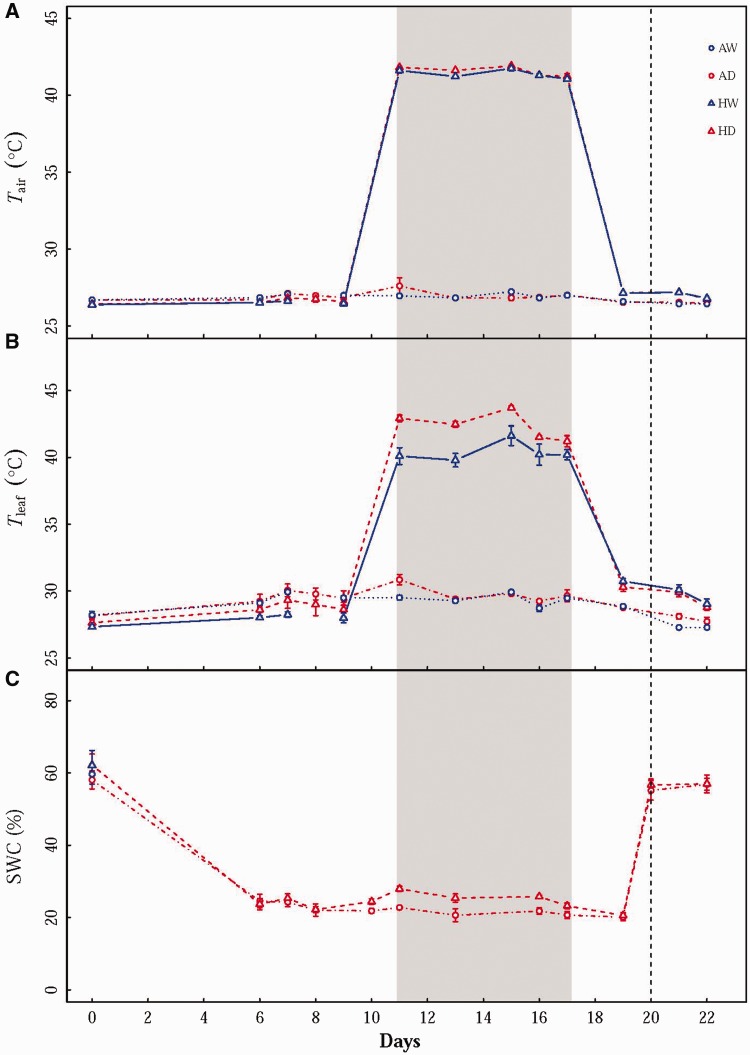
Measured (A) daytime air temperature (Tair), (B) leaf temperature (Tleaf) and (C) soil water content (SWC) at 8 cm depth throughout the experiment. Values are means ± SE (n = 4–6). The grey area represents the period during which a 7-day 42 °C heat wave was applied. The vertical line represents the day when droughted seedlings were rehydrated. Treatments: AW–Ambient temperature plus well watered; AD–Ambient temperature plus drought; HW–Heat wave plus well watered; HD–Heat wave plus drought.
Water treatment
Seedlings in AW and HW treatments were maintained well watered throughout the experiment, while seedlings in AD and HD treatments were subjected to a target moderate drought stress and followed by rehydration. Given that measuring stomatal conductance (gs) is non-destructive, and that gs is a good indicator of plant and leaf water stress, we controlled this variable in order to establish the target drought stress. Water was then withheld in seedlings in the drought treatment until gs declined to the range of 0–0.05 mol m−2 s−1 (see Duan et al. 2014). By controlling gs in this range, we established standardised drought stress across temperature treatments, ensuring that seedlings in AD and HD were subjected to similar drought stress throughout the experiment. When gs followed into the range of 0–0.05 mol m−2 s−1, every pot in the drought treatment was weighed in the afternoon (between 1600 h and 1700 h) each day to determine gravitational water loss and the corresponding soil volumetric water content (SWC) (measured by a hand-set TDR with a depth of 8 cm; TZS-I, Zhejiang Topu Co. Ltd. China). About 50–100 ml water was then added to each pot to maintain the target SWC through the cycle of drought stress (25 % on average from Day 6 to Day 19) (see Fig. 1C). Note that the SWC in the well watered treatments was about 60 % on average. All seedlings in AD and HD were then rehydrated to well watered conditions on Day 20.
Heat wave treatment
On Days 10 and 11, the temperature in the heated growth chamber was raised from 26 °C to 42 °C in 4 steps, i.e. 30 °C, 34 °C, 38 °C, 42 °C and was maintained at each step for 4 h to allow plants to acclimate to the temperature changes. The temperature was then maintained at 42 °C/35 °C day/night before it declined back to 26 °C/19 °C day/night on Day 17. In this case, plants in HW and HD were subjected to a 7-day of + 16 °C heat stress treatment.
Leaf gas exchange measurements
Leaf gas exchange measurements were taken on recently, fully expanded leaves from four seedlings per treatment (n = 4) using a portable open path gas exchange system (Licor-6400, Li-Cor, Lincoln, NE, USA) supplying photosynthetic photon flux density (PPFD) by red-blue light source (6400-02B). Leaf photosynthesis under saturating light (Asat, µmol m−2 s−1), stomatal conductance (gs, mol m−2 s−1), transpiration (E, mmol m−2 s−1), leaf-to-air VPD (VpdL, kPa), air temperature (Tair) and leaf temperature (Tleaf) were measured at mid-day (between 0930 h and 1400 h) throughout the experiment, at PPFD of 1200 µmol m−2 s−1, [CO2] of 400 µmol mol−1 and mid-day growth temperatures. Besides, the ratio of intercellular to atmospheric [CO2] (Ci/Ca) and the instantaneous water use efficiency (WUEi, μmol CO2·mol−1 H2O; determined by Asat/gs) were also calculated. Leaf dark respiration (Rd, µmol m−2 s−1) was measured during the day after Asat measurements at zero PPFD, [CO2] of 400 µmol mol−1 and mid-day growth temperatures.
Leaf gas exchange responses to short-term temperature increases
On Days 10 and 11, when the growth chamber was heated along the temperature steps, the responses of leaf gas exchange to leaf temperature were simultaneously measured at PPFD of 1200 μmol m−2 s−1, [CO2] of 400 μmol mol−1 and a series of growth temperatures (26 °C, 30 °C, 34 °C, 38 °C and 42 °C). Four seedlings per water treatment (n = 4) were randomly chosen for the measurements.
Estimation of vcmax
The Vcmax (µmol m−2 s−1) was estimated using the ‘one-point method’ which was described in De Kauwe et al. (2016).
| (Eqn 1) |
where Asat is the leaf photosynthesis under saturating light (µmol m−2 s−1); Ci is the intercellular CO2 concentration (µmol mol−1); Γ* is the CO2 compensation point in the absence of mitochondrial respiration (µmol mol−1); Km is the Michaelis–Menten constant, which is determined by
| (Eqn 2) |
where Kc is the Michaelis constant for CO2 (µmol mol−1); Ko is the Michaelis constant for O2 (mmol mol−1); Oi is the intercellular concentrations of O2. The determination of Γ*, Kc, Ko and Oi can be estimated according to Bernacchi et al. (2001).
Growth measurements
Height (H, cm) was measured from the stem base to the highest shoot tip and basal diameter (D, cm) was measured at 1-cm height. D2H (cm3) was calculated as D2 times H, to estimate the aboveground growth (Kubiske et al. 2006).
Leaf water potential measurements
Leaf water potential (Ψl, MPa) measurements were taken on one leaf from each of the four seedlings per treatment (n = 4) during the daytime throughout the experiment using a Scholander-type pressure chamber (PMS instruments, Corvallis, Oregon USA). The leaves were cut with a blade, wrapped in moist paper towel and measured for Ψl immediately after collection.
Chlorophyll measurements
The non-destructive chlorophyll measurements were conducted using a portable chlorophyll meter (SPAD-502, Konica Minolta Optics Inc, Osaka, Japan). The SPAD values can reflect the relative chlorophyll content. Three leaves from each of six seedlings per treatments (n = 6) were measured.
Statistical analyses
Statistical analyses were performed with R 3.2.2 (R Core Team, 2015). The parameters including Asat, gs, E, WUEi, Vcmax, Tair, Tleaf, Ψl, SPAD value and growth during the course of the experiment were analysed using a linear mixed-effects model (package ‘nlme’ in R), with water treatment (well-watered vs. drought), temperature treatment (ambient temperature vs. heat wave), and days as categorical fixed effects. Seedling number was treated as a random effect in all analyses. As we showed the data of SWC only in drought treatment, we analysed SWC with temperature and days as fixed effects and seedling number as a random effect. At each time step, the main and interactive effects of water and temperature on parameters were analysed using two-way ANOVA and followed by one-way ANOVA when interactive effects were significant. During the period of recovery from drought (i.e. the recovery after 0 h, 1 h, 2 h, 8 h and 24 h), Asat, gs, E, andΨl were analysed using a linear mixed-effects model, with temperature and days as fixed effects and seedling number as a random effect. Subsequently, Student’s t-tests were used to compare means between AD and HD treatments. The homoscedasticity and normality were checked prior to all the statistic analyses. In all cases, the results were considered significant if P ≤ 0.05.
Results
Temperature and soil water conditions
The air temperature was elevated by about 16 °C during the 7-d heat wave period (i.e. from Day 10 to Day 17) and returned to ambient temperature level afterwards. The leaf temperature varied in concert with air temperature throughout the experiment (Fig. 1B), but the leaf temperature in HW treatment was elevated to a lesser degree during the heat wave period than that in HD treatment. After the heat wave, leaf temperature was unable to go back to the target temperature, about 1 °C higher than the ambient temperature treatments. The SWC declined from 60 % at the beginning to about 20 % on Day 6 and was maintained at this level until seedlings were rehydrated on Day 20 (Fig. 1C). During the heat wave period, the SWC in HD treatment was not lower than that in AD treatment, indicating that soil water conditions were similar between temperature treatments.
Growth
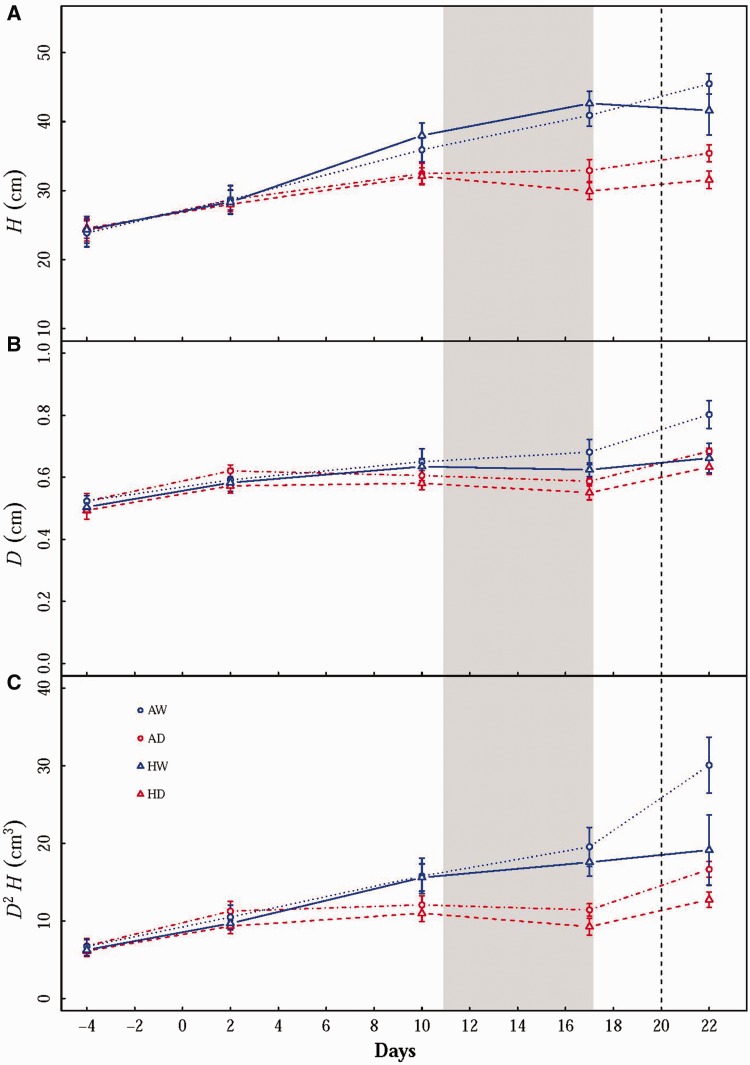
The growth parameters of S. lycopersicum seedlings were substantially reduced by drought stress during the experiment (Fig. 2, Table 1). At the end of the heat wave period (i.e. Day 17), growth parameters were not changed by the heat wave treatments. Hence, drought had a primary impact on seedling growth. At the end of the experiment (i.e. Day 22), heat wave had remarkably reduced seedling D2H at both water conditions (Two-way ANOVA: P = 0.017) (Fig. 2C).
Figure 2.
(A) Height (H), (B) basal diameter (D) and (C) estimated stem growth (D2H) of Solanum lycopersicum seedlings throughout the experiment. Values are means ± SE (n = 6). The grey area represents the period during which a 7-day 42 °C heat wave was applied. The vertical line represents the day when droughted seedlings were rehydrated. Treatments: AW–Ambient temperature plus well watered; AD– Ambient temperature plus drought; HW–Heat wave plus well watered; HD–Heat wave plus drought.
Table 1.
Summary of linear mixed model analysis of effects of water, temperature treatment and time on measured parameters of solanum lycopersicum seedlings over the entire experimental period. Significant values are shown in bold (P < 0.05).
| Parameter | Water | Temp | Days | Water:Temp | Water:Days | Temp: Days | Water: Temp:Days | |
|---|---|---|---|---|---|---|---|---|
| Tair | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 96 | 12 | 96 | 96 | 96 | |
| P | 0.2938 | <0.0001 | <0.0001 | 0.0196 | 0.0020 | <0.0001 | 0.0213 | |
| Tleaf | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 96 | 12 | 96 | 96 | 96 | |
| P | 0.0059 | <0.0001 | <0.0001 | 0.1424 | <0.0001 | <0.0001 | 0.0008 | |
| H | numDF | 1 | 1 | 4 | 1 | 4 | 4 | 4 |
| denDF | 20 | 20 | 80 | 20 | 80 | 80 | 80 | |
| P | 0.3966 | 0.8369 | <0.0001 | 0.8145 | <0.0001 | 0.0001 | 0.0085 | |
| D | numDF | 1 | 1 | 4 | 1 | 4 | 4 | 4 |
| denDF | 20 | 20 | 80 | 20 | 80 | 80 | 80 | |
| P | 0.0874 | 0.0769 | <0.0001 | 0.8437 | <0.0001 | 0.0001 | 0.0026 | |
| D2H | numDF | 1 | 1 | 4 | 1 | 4 | 4 | 4 |
| denDF | 20 | 20 | 80 | 20 | 80 | 80 | 80 | |
| P | 0.0001 | 0.3519 | <0.0001 | 0.4035 | <0.0001 | 0.0001 | 0.0073 | |
| Asat | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 95 | 12 | 95 | 95 | 95 | |
| P | <0.0001 | 0.0001 | <0.0001 | 0.0057 | <0.0001 | <0.0001 | 0.0014 | |
| gs | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 95 | 12 | 95 | 95 | 95 | |
| P | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| E | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 95 | 12 | 95 | 95 | 95 | |
| P | <0.0001 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | |
| WUEi | numDF | 1 | 1 | 8 | 1 | 8 | 8 | 8 |
| denDF | 12 | 12 | 96 | 12 | 96 | 96 | 96 | |
| P | 0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Vcmax | numDF | 1 | 1 | 9 | 1 | 9 | 9 | 9 |
| denDF | 13 | 13 | 105 | 13 | 105 | 105 | 105 | |
| P | <0.0001 | 0.1608 | <0.0001 | 0.9778 | <0.0001 | <0.0001 | <0.0001 | |
| SPAD | numDF | 1 | 1 | 5 | 1 | 5 | 5 | 5 |
| denDF | 20 | 20 | 100 | 20 | 100 | 100 | 100 | |
| P | 0.1949 | 0.0475 | <0.0001 | 0.0722 | 0.0242 | <0.0001 | 0.0217 | |
| Ψl | numDF | 1 | 1 | 7 | 1 | 7 | 7 | 7 |
| denDF | 13 | 13 | 83 | 13 | 83 | 83 | 83 | |
| P | <0.0001 | 0.0926 | <0.0001 | 0.3584 | <0.0001 | 0.2181 | 0.7756 |
Numerator and denominator df are the numerator and denominator degrees of freedom for the F-tests.
Time course of leaf physiological responses
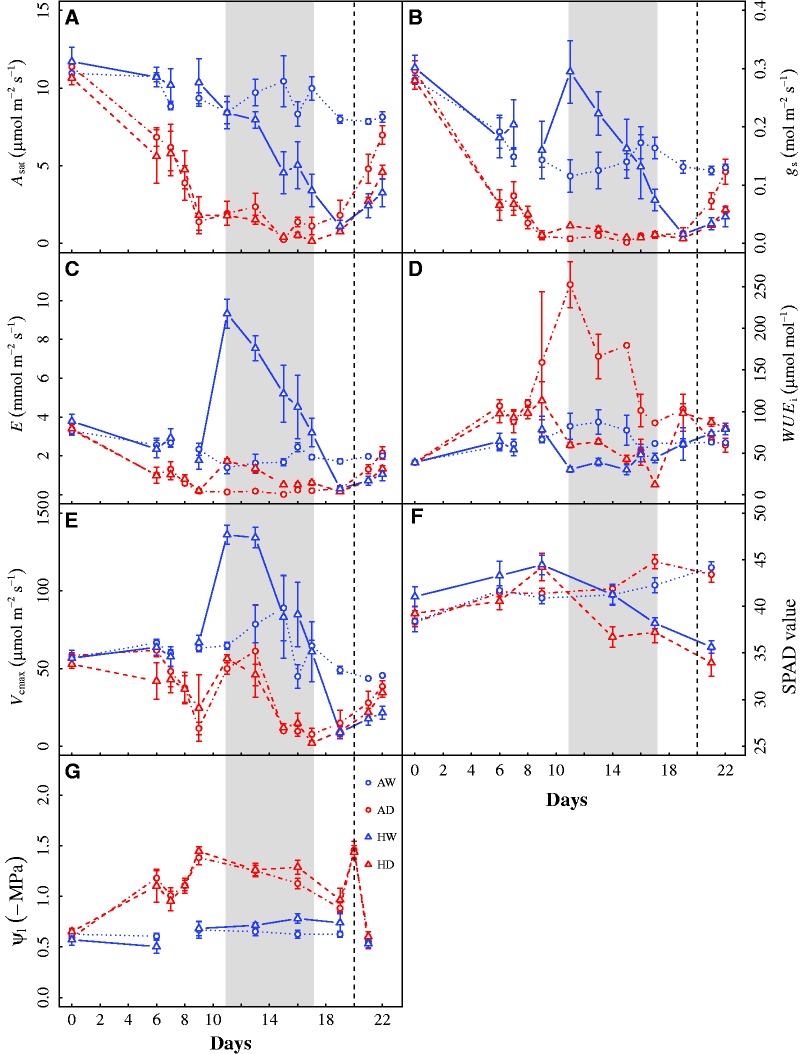
Asat of S. lycopersicum seedlings in AW control did not change too much over time, while Asat in HW treatment declined substantially as heat wave progressed, recovering to only 39 % of AW control in the end (Fig. 3A, Table 1). These results reflect that the heat wave had significant negative impacts on photosynthesis. Asat was reduced in the drought treatments compared with the well watered treatments at both temperatures, maintaining lower than 10 % of pre-drought values until the seedlings were rehydrated. During the pre-heat wave and heat wave period (i.e. from Day 0 to Day 17), Asat in droughted seedlings did not differ between temperature treatments, indicating that photosynthesis in response to drought was not altered by heat wave. By contrast, the recovery of Asat from drought was slower in HD treatment than AD treatment (t-test: P < 0.05). For instance, after two days rehydration, Asat in AD treatment almost returned to the value of the control, while that in HD treatment was only 71 % of Asat in AD treatment.
Figure 3.
(A) Leaf photosynthesis under saturating light (Asat), (B) stomatal conductance (gs), (C) transpiration (E), (D) instantaneous water use efficiency (WUEi), (E) the estimated maximum carboxylation capacity (Vcmax), (F) SPAD value and (G) leaf water potential (Ψl) of Solanum lycopersicum seedlings throughout the experiment. Values are means ± SE (n = 4–6). The grey area represents the period during which a 7-day 42 °C heat wave was applied. The vertical line represents the day when droughted seedlings were rehydrated. Treatments: AW–Ambient temperature plus well watered; AD– Ambient temperature plus drought; HW–Heat wave plus well watered; HD–Heat wave plus drought.
For the entire experimental period, gs appeared to be one of the dominant factors in determining Asat responses (Fig. 3B, [see Supporting Information—Fig. S1]). During the 7-day heat wave, gs in HW treatment was initially increased by 81 % compared with the pre-heat wave value, but declined sharply as heat wave prolonged. At the end of the experiment, full recovery of gs in HW treatment was not observed. gs were similar between AD and HD treatments prior to rehydration, but gs in AD treatment recovered faster from drought than that in HD treatment (t-test: P < 0.05). The time course responses of E were similar to those of gs in all treatments (Fig. 3C, Table 1).
WUEi was significantly enhanced in the drought treatments compared with the well watered treatments at both temperatures over the entire experimental period (Fig. 3D, Table 1). During the 7-day heat wave, WUEi in HW and HD treatments were both substantially reduced compared with the ambient temperature treatments, suggesting that the heat wave had large negative impacts on WUEi.
Vcmax in AD and HD treatments declined sharply compared with the well watered treatments when the target drought stress was achieved (Fig. 3E, Table 1). Vcmax of droughted plants was maintained at relatively low values until plants were rehydrated, afterwards exhibiting substantial recovery. Over the entire experimental period, Vcmax did not significantly differ between AD and HD treatments, suggesting that the heat wave had minimal effects on Vcmax when the stomata were almost closed. However, Vcmax in HW treatment showed large variations (Fig. 3E). It was increased to nearly 200% of Vcmax in AW control at the onset of the heat wave (i.e. Day 11), while it had continuous declines as the heat wave was prolonged. Although Vcmax in HW treatment was recovered to some extent after the release of heat wave, it could not return to the control value even at the end of the experiment.
SPAD value (i.e. relative chlorophyll content) did not change too much in all treatments prior to the heat wave, while it had a pronounced reduction in the heat wave treatments (i.e. HW and HD) compared with the ambient temperature treatments (i.e. AW and AD), maintaining only 80% of ambient temperature values in the end (Fig. 3F, Table 1).
Ψl was generally more negative in the drought treatments (i.e. AD and HD treatments; −1.3 MPa on average) than the well watered treatments (i.e. AW and HW treatments; -0.65 MPa on average) (Fig. 3G, Table 1), reflecting the moderate drought stress on seedlings. Yet, heat wave did not lead to overall reduction in Ψl than did the ambient temperature over the course of the entire experiment, except for Day 16 (Two-way ANOVA: P = 0.011).
Leaf gas exchange as function of leaf temperature
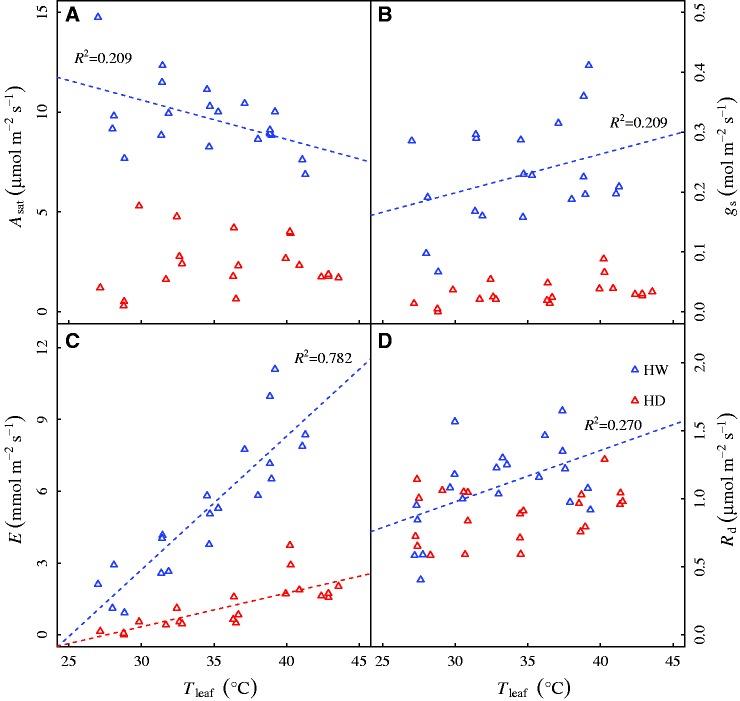
Asat, gs and Rd of S. lycopersicum seedlings had weak but significant linear relationships with leaf temperature, only in HW treatment (Fig. 4). By contrast, E of both well watered and drought seedlings had pronounced positive relationships with leaf temperature (Fig. 4C), indicating that short-term increases in temperature had greater impacts on E than other gas exchange traits.
Figure 4.
(A) Leaf photosynthesis under saturating light (Asat), (B) stomatal conductance (gs), (C) transpiration (E) and (D) dark respiration (Rd) of Solanum lycopersicum seedlings grown under well-watered and drought conditions as function of leaf temperature during the period of short-term temperature increases. Data points are raw data of the measured variables at growth temperature of 26 °C, 30 °C, 34 °C, 38 °C and 42 °C. Data are fitted with linear functions (y = ax + b) and only significant relationships are showed. The correlation coefficients (R2) are also given. Treatments: HW–Heat wave plus well watered; HD–Heat wave plus drought.
Physiological recovery from drought
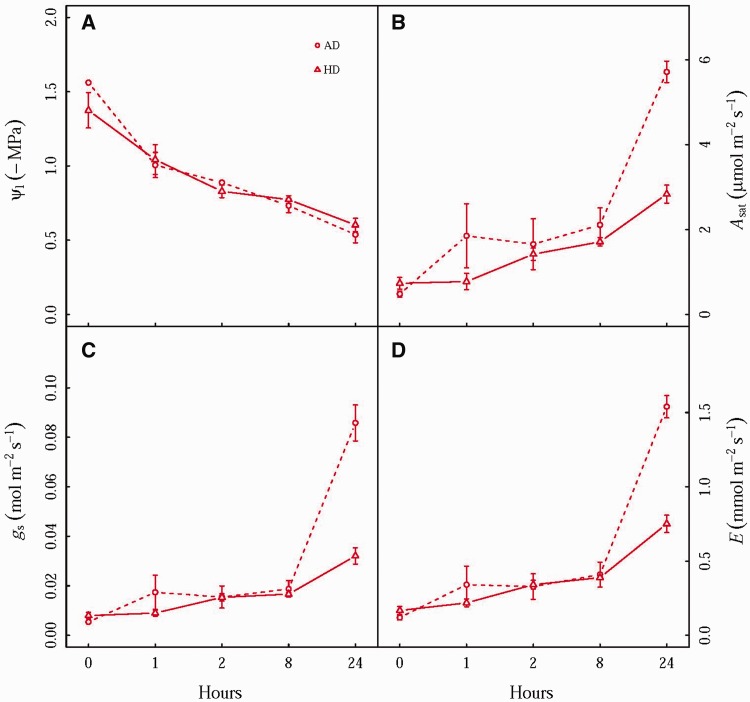
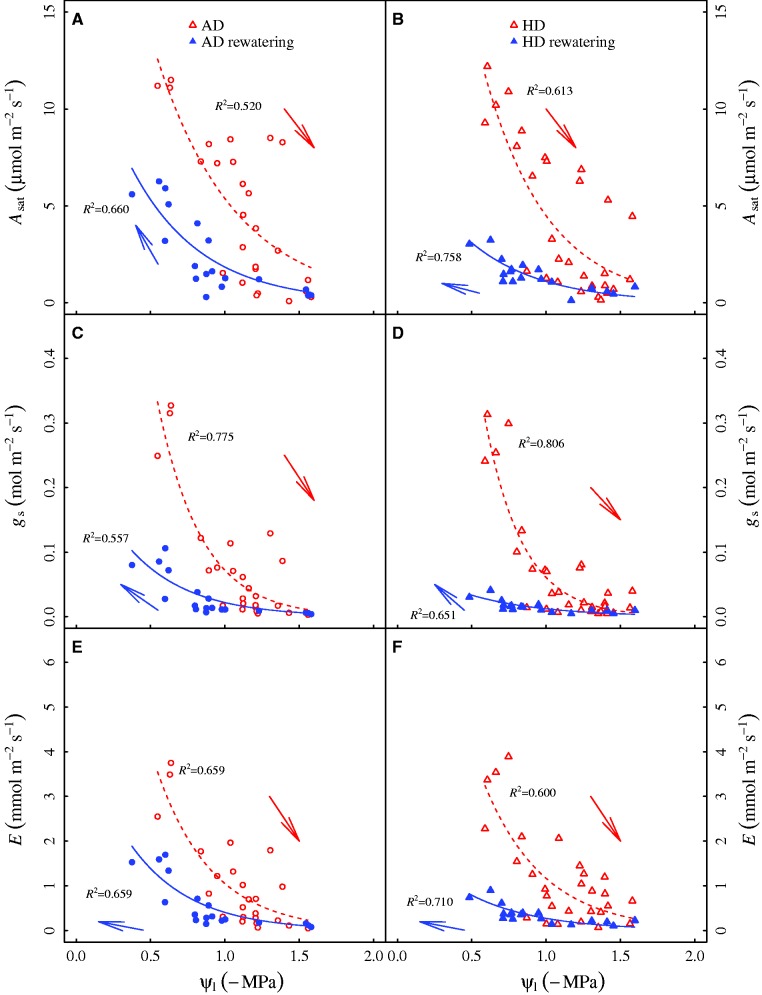
The leaf water potential had a fast recovery from drought, returning to well watered control values in about 8 h after rehydration, similarly in AD and HD treatments (Fig. 5A, Table 2). Nevertheless, leaf gas exchange was not fully recovered even after 24 h rehydration (Fig. 5B–D, Table 2), reflecting the hysteresis in the response of leaf gas exchange to leaf water potential. More interestingly, after 24 h recovery, Asat, gs and E in AD was 210 %, 260 % and 214 % higher than those in HD treatment, respectively, suggesting that heat wave delayed the recovery of leaf gas exchange. These results are explained in Fig. 6, which shows leaf gas exchange as a function of leaf water potential during drought stress and the 24-h recovery from drought. As leaf water potential declined (i.e. to more negative values), gas exchange exhibited similar reductions between AD and HD treatment. After rehydration, however, seedlings in AD treatment exhibited sharper recovery of leaf gas exchange, particularly when leaf water potential was higher than -1 MPa. Generally, these results suggest that despite the fast recovery of leaf water potential, the recovery of leaf gas exchange lagged, to an even greater degree in HD treatment than AD treatment, suggesting the coordination of leaf gas exchange and leaf water potential was shifted between AD and HD treatments.
Figure 5.
The recovery following drought of (A) leaf water potential (Ψl), (B) leaf photosynthesis under saturating light (Asat), (C) stomatal conductance (gs) and (D) transpiration (E) of Solanum lycopersicum seedlings grown under ambient temperature and 7-d 42 °C heat wave treatments. Values are means ± SE (n = 4). Hours represent 0, 1, 2, 8, 24 h after the recovery from drought. Treatments: AD–Ambient temperature plus drought; HD–Heat wave plus drought.
Table 2.
Summary of linear mixed model analysis of effects of temperature treatment and recovery time on measured parameters of solanum lycopersicum seedlings during the 24-h recovery from drought. Here, only values in AD and HD treatment were analysed to examine whether initial temperature treatment modifies the recovery of physiological parameters of seedlings from drought. Hours represent 0, 1, 2, 8 and 24 h after rehydration. Significant values are shown in bold (P < 0.05).
| Parameter | Temp | Hours | Temp:Hours | |
|---|---|---|---|---|
| Ψl | numDF | 1 | 4 | 4 |
| denDF | 7 | 24 | 24 | |
| P | 0.7914 | <0.0001 | 0.2376 | |
| Asat | numDF | 1 | 4 | 4 |
| denDF | 7 | 24 | 24 | |
| P | 0.6936 | <0.0001 | <0.0001 | |
| gs | numDF | 1 | 4 | 4 |
| denDF | 7 | 24 | 24 | |
| P | 0.5246 | <0.0001 | <0.0001 | |
| E | numDF | 1 | 4 | 4 |
| denDF | 7 | 24 | 24 | |
| P | 0.5429 | <0.0001 | <0.0001 |
Numerator and denominator df are the numerator and denominator degrees of freedom for the F-tests.
Figure 6.
Leaf photosynthesis under saturating light (Asat), stomatal conductance (gs) and transpiration (E) of Solanum lycopersicum seedlings grown under ambient temperature (A, C and E) and 7-d 42 °C heat wave treatments (B, D and F) as function of leaf water potential (Ψl) during the periods of drought stress and the following recovery. Data points are raw data of the measured variables. Data are fitted with exponential functions: y = a × e (-bx) (P < 0.01 for all parameters). The correlation coefficients (R2) are also given. Red and open symbols represent values during the drought stress, while blue and closed symbols represent values during the recovery from drought. Treatments: AD–Ambient temperature plus drought; HD–Heat wave plus drought.
Discussion
This study investigated the individual and interactive effects of drought and heat wave on leaf physiology and growth of S. lycopersicum seedlings, and how leaf physiology recovered from the combined stress. Our results showed that the 7-day heat wave treatment (i.e. HW), as predicted in the first hypothesis, reduced growth, leaf gas exchange rates, Vcmax and water use efficiency compared with ambient temperature control (i.e. AW), while it did not have significant impacts on leaf water potentials. Compared with well watered conditions, drought stress led to stomatal closure, lower Vcmax, reduced growth and more negative leaf water potentials, similarly in AD and HD treatments. However, the observed lower relative chlorophyll content in HD treatment can partially support our second hypothesis that heat wave would exacerbate drought stress. Our results also demonstrated that recovery of leaf gas exchange lagged behind water potential recovery and leaf gas exchange exhibited much slower post-drought recovery in HD treatment than AD treatment, which agrees with our third hypothesis.
Plant physiological responses during and post single heat wave treatment
The 7-day + 16 °C single factor heat wave (i.e. 42 °C) had negative impacts on the growth of S. lycopersicum seedlings, which is consistent with previous studies (e.g. Bauweraerts et al. 2014; Ruehr et al. 2016). Under high soil water availability, despite the initial sharp rise in leaf stomatal conductance and transpiration at the onset of the heat wave, photosynthesis declined gradually in parallel with stomatal conductance as heat wave progressed, maintaining a relatively low leaf level water use efficiency. Even 2 days after the relief of a heat wave, photosynthesis and stomatal conductance exhibited continued declines. These results indicate that the effect of heat wave on plant performance depends not only on the duration of heat exposure but also on the heat stress legacy (Teskey et al. 2014; Mitchell et al. 2016). Apparently, the reduction in photosynthesis in the present study was partly attributed to stomatal closure. It is noted that leaf water potentials did not significantly differ between ambient temperature and heat wave treatments or among pre-, during and post heat wave period. This is not surprising because we well watered the plants in HW treatment daily to maintain the high soil water availability. The observed stomatal closure associated with heat wave is thereby not likely to be induced by soil water deficit. However, the close negative correlation of stomatal conductance with leaf-to-air VPD (Linear regression; gs =-0.15 × VPD + 0.77; R2= 0.76) illustrated that the high VPD associated with high temperature could have inhibited stomatal conductance. It has been demonstrated that the gradient in water potential between guard cells and epidermal cells rather than bulk leaf water potential, can affect stomatal responses to high VPD (Bunce, 1997). Abscisic acid (ABA) may also be involved in this process (McAdam & Brodribb, 2015).
In addition to stomatal limitation, the non-stomatal limitation could also play the role in determining photosynthesis in response to the heat wave. During the 7-day heat wave period, leaf senescence and reduction in chlorophyll content were observed in well watered plants, which is supported by other studies (Marchand et al. 2006; Wang et al. 2015). For example, Wang et al. (2015) found that tomato grown under 42 °C for 24 h had significantly lower chlorophyll content than that grown under 25 °C. In the present study, the very high leaf temperature (i.e. about 40 °C) could have caused leaf damage, thereby limiting photosynthesis. There is also evidence that Vcmax was substantially reduced in HW treatment compared with AW control towards the end of the heat stress, indicating that effects of non-stomatal limitation on photosynthesis were progressively enhanced. Together, it is suggested that the non-stomatal limitation can be a co-limiting factor affecting photosynthesis responses under heat stress and its contribution may vary depending on the duration of exposure to stress. The partial recovery of photosynthesis, stomatal conductance and Vcmax in the end indicated that the 42 °C heat stress had remarkably negative impacts on S. lycopersicum seedlings even after the heat stress was relieved. It is worth for further studies to quantify the relative contribution of stomatal and non-stomatal limitation on photosynthesis under stress and the following recovery, for better modeling plant responses to future climate change (Zhou et al. 2013).
Plant physiological responses in combination of drought and heat wave
Drought stress in the present study appeared to have substantially negative impacts on plant growth, leaf gas exchange, Vcmax and leaf water relations, generating a greater degree of reduction in the above traits than well watered treatments, under either ambient temperature or heat wave treatment. In agreement with other studies (Zhou et al. 2013, 2014), the results also showed that the reduction in photosynthesis due to water stress was influenced by a combination of stomatal and non-stomatal limitations. Alongside current evidence (Carmo-Silva et al. 2012; Bauweraerts et al. 2013, 2014; Duan et al. 2013; Ruehr et al. 2016), our results confirmed that water availability has a dominant role in determining plant physiological responses. Drought and heat stress are usually linked and heat stress has been found to exacerbate the negative impacts of drought on plant physiology (Duan et al. 2014; Teskey et al. 2014; Adams et al. 2015). The high VPD associated with heat stress often accelerates evapotranspiration, thereby aggravating soil water depletion. In the present study, to minimize the potential confounding effects of heat wave on soil water conditions, we maintained soil water content through the drought period, which is evident in the similar values of soil water content and leaf water potentials between the two temperature treatments. Since leaf stomata were almost completely closed, the difference of leaf gas exchange was not observed between AD and HD treatment. However, the reduced chlorophyll content in HD treatment compared with AD treatment reflected the fact that the combined stress of drought and heat wave resulted in greater leaf damage than drought alone (De Boeck et al. 2011). Therefore, the above results demonstrated that the combined negative effects of drought and heat wave on plant physiological responses were much larger than single drought stress effect.
Post-drought recovery under ambient temperature and heat wave
The present study has added into the current uncertain knowledge of how plant physiology recovers from the combined stress of drought and heat wave. More importantly, the present study examined a much less studied aspect of how leaf gas exchange and water relations are coordinated during the recovery from weather extremes. After rehydration, leaf water potential recovered at a higher rate than leaf gas exchange traits in both AD and HD treatments, indicating that the recovery of leaf gas exchange was decoupled with that of leaf water potential (see Brodribb and McAdam 2013; Martorell et al. 2014). Thus, the slower recovery of leaf photosynthesis and stomatal conductance was likely to be explained by non-hydraulic factors other than leaf water potential. ABA has been suggested as a contributor in regulating stoma re-opening during recovery from drought (Brodribb and McAdam 2013). Future determination of ABA will be helpful to understand plant stomatal behaviour during drought stress and the following recovery.
More interestingly, recovery of leaf gas exchange was much slower in HD treatment than AD treatment, which suggests that previous imposed heat wave delayed the post-drought recovery of leaf gas exchange. This finding is in line with the recent study on black locust tree seedlings (Ruehr et al. 2016), reflecting that the recovery of leaf gas exchange largely depends on the degree of previous drought stress. Particularly, the slower recovery of photosynthesis in HD treatment than in AD treatment can be attributed to stomatal and non-stomatal limitations (i.e. reduced chlorophyll content). Further studies are required to determine the detailed physiological processes and contributors during the recovery of plant from weather extremes. Altogether, our study demonstrated that heat wave largely affected the recovery of leaf gas exchange from drought in S. lycopersicum seedlings and highlighted the importance of studies on the interactive effects of drought and heat wave on plant recovery.
Conclusions
This study provides new insights into how tomato seedlings recover from the combined stress of drought and heat wave. Heat wave and drought both had significant negative impacts on photosynthetic responses of S. lycopersicum seedlings through stomatal and non-stomatal limitations. Heat wave in combination with drought had greater negative effects on plants than single drought stress. Leaf gas exchange of seedlings grown in the combination of drought and heat wave exhibited slower post-stress recovery and its recovery was decoupled with water potential recovery. Therefore, our study demonstrated that drought and heat wave in combination had significant negative effects on plant growth and leaf physiology during and post stress. Stomatal and non-stomatal limitations to photosynthesis need to be considered for more accurately predicting crop responses to environmental stresses. Results from our growth chamber study may not be easily extrapolated to field studies. However, our study confirms that drought and heat wave are interactively linked and more field studies are needed to uncover the particular physiological mechanisms of how crops respond to cyclic stress of drought and heat wave, which will provide more reliable predictions of crop responses under future climates characterized by more frequent weather extremes.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (31600483; 31570444; 31360175) and Jiangxi Provincial Department of Education (KJLD12097; GJJ14744; GJJ151097) and Gan-Po 555 Talent Project.
Contributions by the Authors
H.D., J.W. and H.F. conceived the experiment, H.D., X.Y. and Z.X. conducted the experiment, H.D., J.W., G.H, S.Z., W.L. and Y.L. analysed the results, H.D. wrote the manuscript with input from all of the other authors.
Conflicts of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article —
Figure S1. Leaf photosynthesis under saturating light (Asat) as a function of stomatal conductance (gs) of Solanum lycopersicum seedlings throughout the experiment. Data points are raw data of the measured variables. Data are fitted with exponential saturation functions: y = a × (1−e(-bx)). The correlation coefficient (R2) in each treatment is 0.402 (AW), 0.744 (HW), 0.936 (AD) and 0.926 (HD), respectively. The fitted functions are not significant differences among treatments. Treatments: AW–Ambient temperature plus well watered; AD–Ambient temperature plus drought; HW–Heat wave plus well watered; HD–Heat wave plus drought.
Figure S2. (A) Leaf-to-air vapour deficit (VpdL) and (B) ratio of intercellular to atmospheric [CO2] (Ci/Ca) of Solanum lycopersicum seedlings throughout the experiment. Values are means ± SE (n = 4–6). The grey area represents the period during which a 7-day 42 °C heat wave was applied. The vertical line represents the day when droughted seedlings were rehydrated. Treatments: AW–Ambient temperature plus well watered; AD– Ambient temperature plus drought; HW–Heat wave plus well watered; HD–Heat wave plus drought.
Table S1. The raw data of leaf gas exchange and estimated Vcmax in this paper.
Supplementary Material
Literature Cited
- Adams HD, Collins AD, Briggs SP, Vennetier M, Dickman LT, Sevanto SA, Garcia-Forner N, Powers HH, McDowell NG. 2015. Experimental drought and heat can delay phenological development and reduce foliar and shoot growth in semiarid trees. Global Change Biology 21:4210–4220. [DOI] [PubMed] [Google Scholar]
- Ameye M, Wertin TM, Bauweraerts I, McGuire MA, Teskey RO, Steppe K. 2012. The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytologist 196:448–461. [DOI] [PubMed] [Google Scholar]
- Anderegg WRL, Schwalm C, Biondi F, Camarero JJ, Koch G, Litvak M, Oqle K, Shaw JD, Shevliakova E, Williams AP, Wolf A, Ziaco E, Pacala S. 2015. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349:528–532. [DOI] [PubMed] [Google Scholar]
- Arnone JA, 3rd, Verburg PS, Johnson DW, Larsen JD, Jasoni RL, Lucchesi AJ, Batts CM, von Naqy C, Coulombe WG, Schorran DE, Buck PE, Braswell BH, Coleman JS, Sherry RA, Wallace LL, Luo Y, Schimel DS. 2008. Prolonged suppression of ecosystem carbon dioxide uptake after an anomalously warm year. Nature 455:383–386. [DOI] [PubMed] [Google Scholar]
- Bauweraerts I, Wertin TM, Ameye M, McGuire MA, Teskey RO, Steppe K. 2013. The effect of heat waves, elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Global Change Biology 19:517–528. [DOI] [PubMed] [Google Scholar]
- Bauweraerts I, Mannaerts TB, Wertin TM, McGuire MA, Teskey RO, Steppe K. 2014. Elevated [CO2] and growth temperature have a small positive effect on photosynthetic thermotolerance of Pinus taeda seedlings. Trees 28:1515–1526. [Google Scholar]
- Bernacchi C, Singsaas E, Pimentel C, Portis A, Jr, Long S. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24:253–259. [Google Scholar]
- Brodribb TJ, McAdam SA. 2013. Abscisic acid mediates a divergence in the drought response of two conifers. Plant Physiology 162:1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce JA. 1997. Does transpiration control stomatal responses to water vapour pressure deficit? Plant, Cell and Environment 20:131–135. [Google Scholar]
- Carmo-Silva AE, Gore MA, Andrade-Sanchez P, French AN, Hunsaker DJ, Salvucci ME. 2012. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environmental and Experimental Botany 83:1–11. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SC, Read J. 2006. Foliar temperature tolerance of temperate and tropical evergreen rain forest trees of Australia. Tree Physiology 26:1435–1443. [DOI] [PubMed] [Google Scholar]
- De Boeck HJ, Dreesen FE, Janssens IA, Nijs I. 2011. Whole‐system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytologist 189:806–817. [DOI] [PubMed] [Google Scholar]
- De Kauwe MG, Lin YS, Wright IJ, Medlyn BE, Crous KY, Ellsworth DS, Maire V, Prentice IC, Atkin OK, Rogers A, Niinemets Ü, Serbin SP, Meir P, Uddling J, Togashi HF, Tarvainen L, Weerasinghe LK, Evans BJ, Yoko Ishida F, Domingues TF. 2016. A test of the ‘one-point method’ for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytologist 210:1130–1144. [DOI] [PubMed] [Google Scholar]
- Duan H, Amthor JS, Duursma RA, O’Grady AP, Choat B, Tissue DT. 2013. Carbon dynamics of eucalypt seedlings exposed to progressive drought in elevated [CO2] and elevated temperature. Tree Physiology 33:779–792. [DOI] [PubMed] [Google Scholar]
- Duan H, Duursma RA, Huang G, Smith RA, Choat B, O’Grady AP, Tissue DT. 2014. Elevated [CO2] did not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell and Environment 37:1598–1613. [DOI] [PubMed] [Google Scholar]
- Duursma RA, Barton CV, Lin YS, Medlyn BE, Eamus D, Tissue DT, Ellsworth DS, McMurtrie RE. 2014. The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agricultural and Forest Meteorology 189:2–10. [Google Scholar]
- Haldimann P, Gallé A, Feller U. 2008. Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiology 28:785–795. [DOI] [PubMed] [Google Scholar]
- Hamerlynck EP, Huxman TE, Loik ME, Smith SD. 2000. Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of the Mojave Desert evergreen shrub, Larrea tridentata. Plant Ecology 148:183–193. [Google Scholar]
- Hamilton III EW, Heckathorn SA, Joshi P, Wang D, Barua D. 2008. Interactive effects of elevated CO2 and growth temperature on the tolerance of photosynthesis to acute heat stress in C3 and C4 species. Journal of Integrative Plant Biology 5:1375–1387. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Ziegler W, Kolle O, Trumbore S. 2013. Thirst beats hunger-declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytologist 200:340–349. [DOI] [PubMed] [Google Scholar]
- IPCC. 2014. IPCC fifth assessment synthesis report. Climate change 2014: Longer report. Stockholm, Sweden.
- Kubiske ME, Quinn VS, Heilman WE, Mcdonald EP, Marquardt PE, Teclaw RM, Friend AL, Karnosky DF. 2006. Interannual climatic variation mediates elevated CO2 and O3 effects on forest growth. Global Change Biology 12:1054–1068. [Google Scholar]
- Lobell DB, Hammer GL, Chenu K, Zheng B, McLean G, Chapman SC. 2015. The shifting influence of drought and heat stress for crops in northeast Australia. Global Change Biology 21:4115–4127. [DOI] [PubMed] [Google Scholar]
- Marchand FL, Verlinden M, Kockelbergh F, Graae BJ, Beyens L, Nijs I. 2006. Disentangling effects of an experimentally imposed extreme temperature event and naturally associated desiccation on Arctic tundra. Functional Ecology 20:917–928. [Google Scholar]
- Martorell S, Diaz-Espejo A, Medrano H, Ball MC, Choat B. 2014. Rapid hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant Cell and Environment 37:617–626. [DOI] [PubMed] [Google Scholar]
- McAdam SA, Brodribb TJ. 2015. The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiology 167:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA. 2008. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought?. New Phytologist 178:719–739. [DOI] [PubMed] [Google Scholar]
- Medvigy D, Wofsy SC, Munger JW, Moorcroft PR. 2010. Responses of terrestrial ecosystems and carbon budgets to current and future environmental variability. Proceedings of the National Academy of Sciences 107:8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ, O'Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA. 2013. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist 197:862–872. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, O'Grady AP, Pinkard EA, Brodribb TJ, Arndt SK, Blackman CJ, Duursma RA, Fensham RJ, Hilbert DW, Nitschke CR, Norris J, Roxburgh SH, Ruthrof KX, Tissue DT. 2016. An eco-climatic framework for evaluating the resilience of vegetation to water deficit. Global Change Biology 22:1677–1689. [DOI] [PubMed] [Google Scholar]
- O’Carrigana A, Hinde E, Lu N, Xu X, Duan H, Huang G, Mak M, Bellotti B, Chen Z. 2014. Effects of light irradiance on stomatal regulation and growth of tomato. Environmental and Experimental Botany 98:65–73. [Google Scholar]
- R Core Team 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reichstein M, Bahn M, Ciais P, Frank D, Mahecha MD, Seneviratne SI, Zscheischler J, Beer C, Buchmann N, Frank DC, Papale D, Rammiq A, Smith P, Thonicke K, van der Vslde M, Vicca S, Walz A, Wattenbach M. 2013. Climate extremes and the carbon cycle. Nature 500:287–295. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Loreto F, Polle A, Brilli F, Fares S, Beniwal RS, Gessler A. 2006. Physiological responses of forest trees to heat and drought. Plant Biology 8:556–571. [DOI] [PubMed] [Google Scholar]
- Ruehr NK, Gast A, Weber C, Daub B, Arneth A. 2016. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiology 36:164–178. [DOI] [PubMed] [Google Scholar]
- Siebers MH, Yendrek CR, Drag D, Locke AM, Rios Acosta L, Leakey ADB, Ainsworth EA, Bernaccni CJ, Ort DR. 2015. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Global Change Biology 21:3114–3125. [DOI] [PubMed] [Google Scholar]
- Teskey R, Wertin T, Bauweraerts I, Ameye M, McGuire MA, Steppe K. 2014. Responses of tree species to heat waves and extreme heat events. Plant Cell and Environment 38:1699–1712. [DOI] [PubMed] [Google Scholar]
- Wang G, Kong F, Zhang S, Meng X, Wang Y, Meng Q. 2015. A tomato chloroplast-targeted DnaJ protein protects Rubisco activity under heat stress. Journal of Experimental Botany 66:3027–3040. [DOI] [PubMed] [Google Scholar]
- Will RE, Wilson SM, Zou CB, Hennessey TC. 2013. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytologist 200:366–374. [DOI] [PubMed] [Google Scholar]
- Woodruff DR, Meinzer FC, Marias DE, Sevanto S, Jenkins MW, McDowell NG. 2015. Linking nonstructural carbohydrate dynamics to gas exchange and leaf hydraulic behavior in Pinus edulis and Juniperus monosperma. New Phytologist 206:411–421. [DOI] [PubMed] [Google Scholar]
- Wujeska-Klause A, Bossinge G, Tausz M. 2015. Responses to heatwaves of gas exchange, chlorophyll fluorescence and antioxidants ascorbic acid and glutathione in congeneric pairs of Acacia and Eucalyptus species from relatively cooler and warmer climates. Trees 29:1929–1941. [Google Scholar]
- Zhao J, Hartmann H, Trumbore S, Ziegler W, Zhang Y. 2013. High temperature causes negative whole-plant carbon balance under mild drought. New Phytologist 200:330–339. [DOI] [PubMed] [Google Scholar]
- Zhou S, Duursma RA, Medlyn BE, Kelly JW, Prentice IC. 2013. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agricultural and Forest Meteorology 182:204–214. [Google Scholar]
- Zhou S, Medlyn B, Sabaté S, Sperlich D, Prentice IC. 2014. Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiology 34:1035–1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.