Abstract
Background
The primary aim of this study was to compare survival from neoadjuvant chemoradiotherapy plus surgery (NCRS) versus neoadjuvant chemotherapy plus surgery (NCS) for the treatment of esophageal or junctional adenocarcinoma. The secondary aims were to compare pathological effects, short-term mortality and morbidity, and to evaluate the effect of lymph node harvest upon survival in both treatment groups.
Methods
Data were collected from 10 European centers from 2001 to 2012. Six hundred and eight patients with stage II or III oesophageal or oesophago-gastric junctional adenocarcinoma were included; 301 in the NCRS group and 307 in the NCS group. Propensity score matching and Cox regression analyses were used to compensate for differences in baseline characteristics.
Results
NCRS resulted in significant pathological benefits with more ypT0 (26.7% versus 5%; P < 0.001), more ypN0 (63.3% versus 32.1%; P < 0.001), and reduced R1/2 resection margins (7.7% versus 21.8%; P < 0.001). Analysis of short-term outcomes showed no statistically significant differences in 30-day or 90-day mortality, but increased incidence of anastomotic leak (23.1% versus 6.8%; P < 0.001) in NCRS patients.
There were no statistically significant differences between the groups in 3-year overall survival (57.9% versus 53.4%; Hazard Ratio (HR)= 0.89, 95%C.I. 0.67-1.17, P = 0.391) nor disease-free survival (52.9% versus 48.9%; HR = 0.90, 95%C.I. 0.69-1.18, P = 0.443). The pattern of recurrence was also similar (P = 0.660). There was a higher lymph node harvest in the NCS group (27 versus 14; P < 0.001), which was significantly associated with a lower recurrence rate and improved disease free survival within the NCS group.
Conclusion
The survival differences between NCRS and NCS maybe modest, if present at all, for the treatment of locally advanced esophageal or junctional adenocarcinoma. Future large-scale randomized trials must control and monitor indicators of the quality of surgery, as the extent of lymphadenectomy appears to influence prognosis in patients treated with NCS, from this large multi-center European study.
Keywords: esophageal neoplasm, neoadjuvant therapy, chemotherapy, radiotherapy
Key messages
The study suggests that the survival benefit of neoadjuvant chemoradiotherapy over neoadjuvant chemotherapy maybe modest, if present at al, for the management of oesophageal adenocarcinoma. Locoregional control of oesophageal adenocarcinoma can either be achieved through neoadjuvant chemoradiotherapy or chemotherapy with extended lymphadenectomy.
Introduction
Multimodality treatment of esophageal cancer is the standard of care in Western centers, although surgery remains the primary curative modality. Two neoadjuvant approaches have been adopted. The first is neoadjuvant chemoradiotherapy, based in recent years on the CROSS regimen which resulted in a 5-year survival advantage of 14% in comparison with surgery alone [1, 2]. An alternative option is perioperative or preoperative chemotherapy using the MAGIC or OEO2 protocol, which showed, respectively, 5-year survival improvements of 13% and 6% compared with surgery alone [3, 4]. The maximum benefit in the CROSS-trial was observed in squamous cell carcinoma, with highly significant (HR = 0.48; 95%C.I. 0.28–0.83; P = 0.009) benefit compared with surgery alone, in comparison with adenocarcinoma, where the benefit was more modest (HR = 0.73; 95%C.I.0.55–0.98; P = 0.037), but the benefit of neoadjuvant chemoradiotherapy was consistent across subgroups, without any significant interaction identified [1, 2]. Moreover, two small underpowered randomized trials comprising 119 and 75 patients with esophageal adenocarcinoma did not show a significant difference in survival between neoadjuvant chemoradiotherapy plus surgery versus neoadjuvant chemotherapy plus surgery [5, 6]. The recently reported NeoRES trial in a mixed cohort of 181 patients with esophageal adenocarcinoma and squamous cell carcinoma, showed pathological benefits without any changes in survival associated with the addition of radiotherapy to neoadjuvant chemotherapy [7].
Therefore, the optimal multimodality treatment for esophageal adenocarcinoma remains undetermined and is the subject of investigation in the more recently initiated Neo-AEGIS trial, which randomizes patients (n = 574) with adenocarcinoma of the esophagus or esophago-gastric junction to the CROSS or MAGIC regimens, and is likely to be reported in 2021 [8].
The primary aim of the present retrospective multicenter European study was to compare survival from neoadjuvant chemoradiotherapy plus surgery (NCRS) versus neoadjuvant chemotherapy plus surgery (NCS) for the treatment of adenocarcinoma of the esophagus or esophago-gastric junction. The secondary aims were to compare pathological effects, short-term mortality and morbidity and to evaluate the effect of lymph node harvest upon survival in both treatment groups. The current retrospective study described herein was aimed to reach a sample size similar to the ongoing Neo-AEGIS trial [8].
Methods
Datasets
Consecutive patient data were retrieved from 10 prospectively maintained surgical European single-center databases; (i) Erasmus MC—University Medical Centre, Rotterdam, Netherlands; (ii) Academic Medical Centre, Amsterdam, Netherlands; (iii) VU Medical Centre, Amsterdam, Netherlands; (iv) Catharina Hospital, Eindhoven, Netherlands; (v) University Medical Centre, Groningen, Netherlands; (vi) Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands; (vii) Rijnstate Hospital, Arnhem, Netherlands; (viii) St James’s Hospital, Dublin, Ireland; (ix) Imperial College London, UK; and (x) Oxford University Hospitals, Oxford, UK. The datasets have been externally validated and are maintained as part of their respective countries national cancer audits. All patients with adenocarcinoma of the NCRS-arm within the CROSS-trial were included [1, 2]. Management plans and allocation of neoadjuvant therapy were decided upon at multi-disciplinary tumor boards at all centers participating in this study. The study period was from 2001 to 2012 with patient follow-up until December 2015.
Inclusion criteria
The study included patients with stage II or III esophageal or esophago-gastric junctional (Siewert type I and II) adenocarcinoma treated with neoadjuvant chemoradiotherapy plus surgery (CROSS regimen; NCRS group) [1, 2] or peri-/preoperative chemotherapy plus surgery (mainly MAGIC, OEO2 or OEO5 regimens; NCS group) (supplementary Appendix A, available at Annals of Oncology online) [3, 4, 9].
Exclusion criteria
The study did not include (i) patients with esophageal squamous cell carcinoma; (ii) patients with Siewert type III adenocarcinoma; and (iii) patients treated with definitive chemoradiotherapy. Furthermore, patients who underwent exploratory surgery but did not undergo surgical resection of the tumor due to tumor progression were excluded, as data were not routinely collected as part of the datasets included.
Clinical staging and follow-up
The approach to clinical pretreatment staging used a combination of endoscopic ultrasound (EUS), computerized tomography (CT) and on demand CT-positron emission tomography (CT-PET). EUS was used in 98.3% of patients in the NCRS group and 90.2% of patients in the NCS group. In all centers, after surgery patients were reviewed every 3 months during the first year. In the second year, follow-up took place every 6 months, and annually thereafter until 5 years. In cases of suspected recurrence, thoraco-abdominal CT, PET-CT, and/or upper gastrointestinal endoscopy were performed. Histological, cytological, or unequivocal radiological proof was required before a diagnosis of recurrence was made. The first site of recurrence was used to define whether loco-regional, distant, or mixed relapse had occurred. Median follow-up was 33.5 months (range 0.03–177.8 months), with 46 patients having follow-up of less than 3 years during the study period; 29 (15.8%) in the NCS group and 17 (11.8%) in the NCRS group.
Outcomes
The primary outcome measure was 3-year overall survival. Secondary outcomes included: 3-year disease-free survival; pattern of recurrence (within 3-years); pathological T-stage and N-stage (TNM7) [10]; tumor regression grade (TRG) as reported by Chirieac et al. [11]; 30-day and 90-day mortality; and 30-day morbidity, specifically anastomotic and chyle leak, pulmonary and cardiac complications, and reoperation. The time for overall survival was defined from date of surgery to date of death or date of last follow-up. The time for disease-free survival was defined from date of surgery and the earliest occurrence of disease progression resulting from loco-regional recurrence or distant dissemination, or death from any cause [1, 12].
Statistical analysis
Data are presented as prevalence (percentage), median (range), and for survival as median (95% confidence interval). Continuous variables are expressed as mean ± standard deviation or median (range) and categorical variables as percentage. A Mann–Whitney test was used for intergroup comparisons of continuous variables, whereas a χ2 test or Fisher’s exact test was used to compare categorical data. Overall and disease-free survivals were estimated using the Kaplan–Meier method. The log rank test was used to compare survival curves. Missing data were at random and, therefore, only available data were analyzed, in multivariate regression analysis listwise deletion was used.
Propensity matching
In order to reduce the effects of potential confounding factors in the comparisons of short and mid-term outcomes between groups, a propensity score (PS) was calculated to create well-balanced groups. The PS was estimated using a multivariable logistic regression model, with the treatment groups as the dependent variables and potential confounders as covariates. The following confounders were included in the propensity matching: age ≥70 years; male gender; American Society of Anesthesiologists (ASA) grade; clinical tumour (cT) stage; and clinical nodal (cN) positivity or negativity. All patients in the NCS group were matched 1:1 to patients in the NCRS group according to the propensity score using the global optimum method [13].
Cox regression analysis
Year of surgery and age as continuous variables were not included in the PS matching, as this would have further reduced the dataset dramatically to maintain a good level of matching (fewer than 10 patients per group, and neither demonstrated multivariate associations with endpoints). Therefore, overall- and disease-free survivals were also compared between groups using a multivariable Cox regression model. In this model, adjustment was performed for the same characteristics as in the PS approach, with the addition of year of surgery and age as continuous variable.
Risk-adjusted cumulative sum (RA-CUSUM) curve analysis for the effect of lymph node harvest on survival
RA-CUSUM analysis was used to determine a lymph node harvest threshold that affected overall survival in each of NCRS and NCS groups [14]. The threshold was defined as the minimum lymph node harvest for an alteration in overall survival relationships. Risk prediction models for overall survival were created using regression models. Potential risk factors included in the models were: age, male gender, ASA grade, and clinical T and N stages. The risk prediction models were used to calculate the predicted probability of survival in each case. For the CUSUM curve, the sum of all events was compared with the expected sum of events according to the risk-adjustment model, using the CUSUM equation Si = Si-1+ (Σi- ΣR); S0 = 0: Si is the cumulative sum, Σ i the sum of events at procedure number i, and ΣR the sum of expected events at procedure number i. The clinical impact of the threshold was determined by comparing the survival and recurrence rates before and after the change-point in overall survival. To ascertain whether the change-points observed in the CUSUM curves were reliable, we bootstrapped each curve with 1000 iterations to identify the confidence level (CL) of the change point. We computed the CUSUM values at the change point (n = 1000). We hypothesized that a reliable change point would have a CUSUM value that was greater than at least 95% of the simulated CUSUM values (CL > 95%).
CUSUM curves were computed using Excel (Excel for Mac 2011, version 14.1.4, Microsoft Corporation, Redmond, WA, USA). For the remaining statistical analysis, SPSS software was used (Statistical Package for the Social Sciences software, Version 22, SPSS, Chicago, IL, USA).
Results
Over the 12-year study period 608 patients were included: 301 in the NCRS group and 307 in the NCS group. The NCRS group consisted of patients from the centers, which participated in the CROSS-trial [1, 2] and St James’s Hospital, Dublin, whereas NCS patients were provided by Imperial College in London, Oxford University Hospitals in Oxford and St James’s Hospital in Dublin. During the study period, no patients with oesophageal adenocarcinoma from Imperial College London or Oxford University hospital received neoadjuvant chemoradiotherapy. Within the Dutch cohort, during the study period, less than 3% of patients could not undergo radiotherapy (e.g. due to history of radiotherapy) or had lymph nodes outside the maximum radiation field, received neoadjuvant chemotherapy, and were excluded from the study. From the NCS group, the number of patients receiving MAGIC/ECF regime was 51 (16.6%), OEO2/CF 138 (45%), OEO5/ECX was 87 (28.3%), EOX 21 (6.8%), and other regimes 10 (3.3%). After propensity matching, 442 patients were included in the analysis; 221 in the NCRS group and 221 in the NCS group.
Comparison of patient demographics and treatment strategies (Table 1)
Analysis of patient demographics before matching, showed a significantly lower median age and greater numbers of patients with ASA I in NCRS versus NCS group. After propensity matching, there were no significant differences between the groups in age, patients aged 70 years or older, distribution of patients by ASA grade, WHO performance status and clinical T and N stages. After matching, there were significantly more transhiatal resections (61.5% versus 0.5%; P < 0.001) and significantly fewer transthoracic resections (36.7% versus 94.6%; P < 0.001) for NCRS versus NCS.
Table 1.
Comparative analysis of patient demographics and surgical techniques from unmatched and propensity-matched groups
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| NCRS (n=301) (%) | NCS (n=307) (%) | P value | NCRS (n=221) (%) | NCS (n=221) (%) | P value | |
| Age (median (range)) | 61.3 (19–83) | 63.9 (30–82) | 0 .013 | 62 (19–83) | 63.3 (30–82) | 0 .936 |
| Age ≥ 70 | 66 (21.9) | 88 (28.7) | 0 .056 | 50 (22.6) | 50 (22.6) | >0 .999 |
| Male | 258 (85.7) | 252 (82.1) | 0 .224 | 192 (86.9) | 192 (86.9) | >0 .999 |
| ASA a | ||||||
| I | 54 (19.4) | 18 (5.9) | <0 .001 | 16 (7.2) | 16 (7.2) | >0 .999 |
| II | 190 (68.1) | 214 (69.7) | 173 (78.3) | 173 (78.3) | ||
| III | 35 (12.5) | 74 (24.1) | 32 (14.5) | 32 (14.5) | ||
| IV | 0 (0) | 1 (0 .3) | 0 (0) | 0 (0) | ||
| WHO performance status a | ||||||
| 0 | 252 (83.7) | 113 (89) | 0 .293 | 181 (81.9) | 61 (88.4) | 0 .381 |
| 1 | 47 (15.6) | 14 (11) | 38 (17.2) | 8 (11.6) | ||
| 2 | 2 (0 .7) | 0 (0) | 2 (0 .9) | 0 (0) | ||
| cT stagea | ||||||
| 1 | 10 (3.4) | 6 (2) | 0 .120 | 3 (1.4) | 3 (1.4) | >0 .999 |
| 2 | 43 (14.4) | 36 (11.7) | 23 (10 .4) | 23 (10 .4) | ||
| 3 | 236 (79.2) | 248 (80 .8) | 187 (84.6) | 187 (84.6) | ||
| 4 | 9 (3) | 17 (5.5) | 8 (3.6) | 8 (3.6) | ||
| cN stage a | ||||||
| Negative | 108 (35.9) | 93 (30 .3) | 0 .17 | 69 (31.2) | 69 (31.2) | >0 .999 |
| Positive | 193 (64.1) | 214 (69.7) | 152 (68.8) | 152 (68.8) | ||
| Operation | ||||||
| Transhiatal | 168 (55.8) | 1 (0 .3) | <0 .0001 | 136 (61.5) | 1 (0 .5) | <0 .001 |
| Transthoracic | 127 (42.2) | 284 (92.5) | 81 (36.7) | 209 (94.6) | ||
| 3-Stage | 6 (2) | 22 (7.2) | 4 (1.8) | 11 (5) | ||
NCRS, neoadjuvant chemoradiotherapy and surgery; NCS, neoadjuvant chemotherapy and surgery; ASA, American Society of Anesthesiologists classification.
Missing data.
Comparison of tumor pathology and short-term outcomes (Tables 2 and 3)
Both before and after matching, utilization of chemoradiotherapy was associated with significantly more down-staging. This is reflected in the matched comparison by significantly increased incidence of ypT0 (26.7% versus 5%; P < 0.001), ypN0 (63.3% versus 32.1%; P < 0.001) in the NCRS group compared with the NCS group. Neoadjuvant chemoradiotherapy was also associated with a significant reduction in the incidence of R1/2 resection margins (7.7% versus 21.8%; P < 0.001). The NCRS group had a significantly lower median number of harvested lymph nodes (14 versus 27; P < 0.001) and positive lymph nodes (0 versus 2; P < 0.001) in comparison with the NCS group. After matching, analysis of short-term outcomes showed no significant differences in 30-day mortality (4.1% versus 1.4%, P = 0.140) or 90-day mortality (5.9% versus 2.3%) and morbidity apart from an increased incidence of anastomotic leak (23.1% versus 6.8%; P < 0.001) in the NCRS group.
Table 2.
Comparative analysis of tumor pathology from unmatched and propensity-matched groups
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| NCRS (n= 301) (%) | NCS (n=307) (%) | P value | NCRS (n=221) (%) | NCS (n=221) (%) | P value | |
| Tumour location a | ||||||
| Proximal | 1 (0.3) | 0 (0) | 0.141 | 1 (0.5) | 0 (0) | 0.329 |
| Middle | 13 (4.4) | 6 (2) | 9 (4.1) | 5 (2.3) | ||
| Distal/EGJ | 284 (95.3) | 300 (98) | 210 (95.5) | 216 (97.7) | ||
| pT stage a | ||||||
| 0 | 85 (28.2) | 16 (5.2) | <0.001 | 59 (26.7) | 11 (5) | <0.001 |
| I | 45 (15) | 27 (8.8) | 32 (14.5) | 19 (8.6) | ||
| 2 | 61 (20.3) | 76 (24.8) | 49 (22.2) | 58 (26.2) | ||
| 3 | 108 (35.9) | 174 (56.9) | 79 (35.7) | 125 (56.6) | ||
| 4 | 2 (0.7) | 13 (4.2) | 2 (0.9) | 8 (3.6) | ||
| pN stage | ||||||
| 0 | 189 (62.8) | 98 (31.9) | <0.001 | 140 (63.3) | 71 (32.1) | <0.001 |
| 1 | 82 (27.2) | 92 (30) | 65 (29.4) | 76 (34.4) | ||
| 2 | 21 (7) | 57 (18.6) | 11 (5) | 41 (18.6) | ||
| 3 | 9 (3) | 60 (19.5) | 5 (2.3) | 33 (14.9) | ||
| Mandard TRG a | ||||||
| 1 | 85 (28.5) | 14 (5.4) | <0.001 | 59 (27.1) | 11 (5.6) | <0.001 |
| 2 | 73 (24.5) | 19 (7.4) | 58 (26.6) | 12 (6.1) | ||
| 3 | 80 (26.8) | 46 (17.8) | 62 (28.4) | 32 (16.3) | ||
| 4/5 | 60 (20.1) | 178 (68.9) | 39 (17.9) | 141 (71.9) | ||
| Resection margin a | ||||||
| R0 | 278 (92.4) | 220 (77.5) | <0.001 | 204 (92.3) | 165 (78.2) | <0.001 |
| R1/2 | 23 (7.6) | 64 (22.5) | 17 (7.7) | 46 (21.8) | ||
| LN harvest (median | ||||||
| (range)) | 15 (0–53) | 31 (0–129) | <0.001 | 14 (0–52) | 27 (0–129) | <0.001 |
| Total | 0 (0–28) | 2 (0–44) | <0.001 | 0 (0–9) | 2 (0–33) | <0.001 |
| Positive | ||||||
NCRS, neoadjuvant chemoradiotherapy and surgery; NCS, neoadjuvant chemotherapy and surgery; EGJ, oesophago-gastric junction; TRG, tumour regression grade.
Missing data.
Comparison of survival and recurrence, propensity matched (Table 3 and Figure 1)
Unmatched survival analysis suggested that NCRS was associated with a small improvement in 3-year overall survival (57.8% versus 49.8%; HR 0.79, 95%C.I. 0.63–1.00; P = 0.052) (Figure 1A: log rank test P = 0.047). There was no significant difference in 3-year disease-free survival between unmatched groups (52.8% versus 46.9%; HR 0.85; 95%C.I. 0.68–1.07; P = 0.163). After matching, observed differences between the groups in 3-year overall (57.9% versus 53.4%; HR 0.89, 95%C.I. 0.67–1.17, P = 0.391) (Figure 1B) or disease-free survival (52.9% versus 48.9%; HR 0.90, 95%C.I. 0.69–1.18, P = 0.443) were small and statistically non-significant. Following matching, there were no significant differences between the groups in the pattern of recurrence (P = 0.660) (Table 3).
Figure 1.
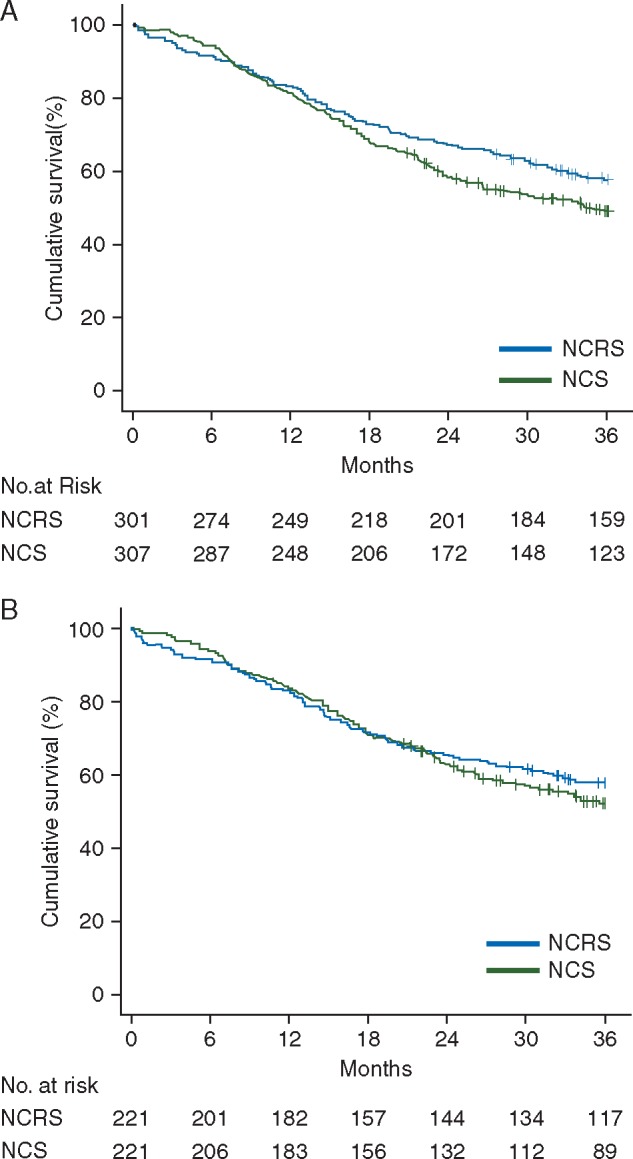
(A) Unmatched Kaplan–Meier survival analysis showing a significant (P = 0.047) improvement in overall survival with neoadjuvant chemoradiotherapy plus surgery (NCRS, n = 301) compared with neoadjuvant chemotherapy plus surgery (NCS, n = 307) for esophageal adenocarcinoma. (B) Propensity-matched Kaplan–Meier survival analysis showing no significant difference (P = 0.391) in overall survival between neoadjuvant chemoradiotherapy plus surgery (NCRS, n = 221) and neoadjuvant chemotherapy plus surgery (NCS, n = 221) for esophageal adenocarcinoma.
Table 3.
Comparative analysis of short-term outcomes and three-year recurrence from unmatched and propensity-matched groups.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| NCRS (n=301) (%) | NCS (n=307) (%) | P value | NCRS (n=221) (%) | NCS (n=221) (%) | P value | |
| 30-day mortality | 9 (3) | 5 (1.6) | 0.263 | 9 (4.1) | 3 (1.4) | 0.140 |
| 90-day mortality | 15 (5) | 7 (2.3) | 0.074 | 13 (5.9) | 5 (2.3) | 0.090 |
| Anastomotic leak a | 61 (20.4) | 15 (5.6) | <0.001 | 51 (23.1) | 13 (6.8) | <0.001 |
| Pulmonary complications a | 135 (44.9) | 103 (38.9) | 0.15 | 101 (45.7) | 72 (38.3) | 0.134 |
| Cardiac complications a | 58 (19.3) | 56 (21.1) | 0.581 | 43 (19.5) | 36 (19.1) | >0.999 |
| Chyle leak a | 22 (7.3) | 24 (9.1) | 0.448 | 17 (7.7) | 13 (6.9) | 0.850 |
| Reoperation | 27 (9.1) | 20 (6.5) | 0.108 | 22 (10.2) | 14 (6.5) | 0.050 |
| Recurrence | ||||||
| Locoregional | 15 (5.0) | 19 (6.2) | 0.542 | 10 (4.5) | 14 (6.3) | 0.660 |
| Distant | 73 (24.3) | 70 (22.8) | 56 (25.3) | 60 (27.1) | ||
| Mixed | 30 (10.0) | 22 (7.2) | 19 (8.6) | 14 (6.3) | ||
NCRS, neoadjuvant chemoradiotherapy and surgery; NCS, neoadjuvant chemotherapy and surgery.
Missing data.
Comparison of survival and recurrence, Cox regression (Table 4)
Cox regression analysis including year of treatment and age, as continuous variables did not show significant differences between NCRS and NCS in 3-year overall (HR 0.86; 95%C.I. 0.66–1.11; P = 0.232) and 3-year disease-free survival (HR 0.91; 95%C.I. 0.71–1.16; P = 0.459) (Table 4).
Table 4.
Cox regression analysis for overall survival, with correction for year of treatment and age as continuous variables
| Hazard ratio | 95% Confidence interval |
|||
|---|---|---|---|---|
| Lower | Upper | P value | ||
| Neoadjuvant therapy | ||||
| NCS | 1.00 | 0.66 | 1.11 | 0.232 |
| NCRS | 0.86 | |||
| Age | 1.01 | 1.00 | 1.02 | 0.195 |
| Gender | ||||
| Male | 1.00 | 0.62 | 1.21 | 0.402 |
| Female | 0.87 | |||
| ASA | 0.013 | |||
| I | 1.00 | 0.70 | 1.56 | 0.839 |
| II | 1.04 | 1.01 | 2.53 | 0.045 |
| III and IV | 1.60 | |||
| cT stage | 0.038 | |||
| 1 | 1.00 | 0.21 | 1.05 | 0.065 |
| 2 | 0.47 | 0.39 | 1.64 | 0.541 |
| 3 and 4 | 0.80 | |||
| cN stage | ||||
| cN0 | 1.00 | 1.17 | 2.05 | 0.002 |
| cNpositive | 1.55 | |||
| Year of surgery | 0.98 | 0.93 | 1.02 | 0.306 |
NCRS, neoadjuvant chemoradiotherapy and surgery; NCS, neoadjuvant chemotherapy and surgery.
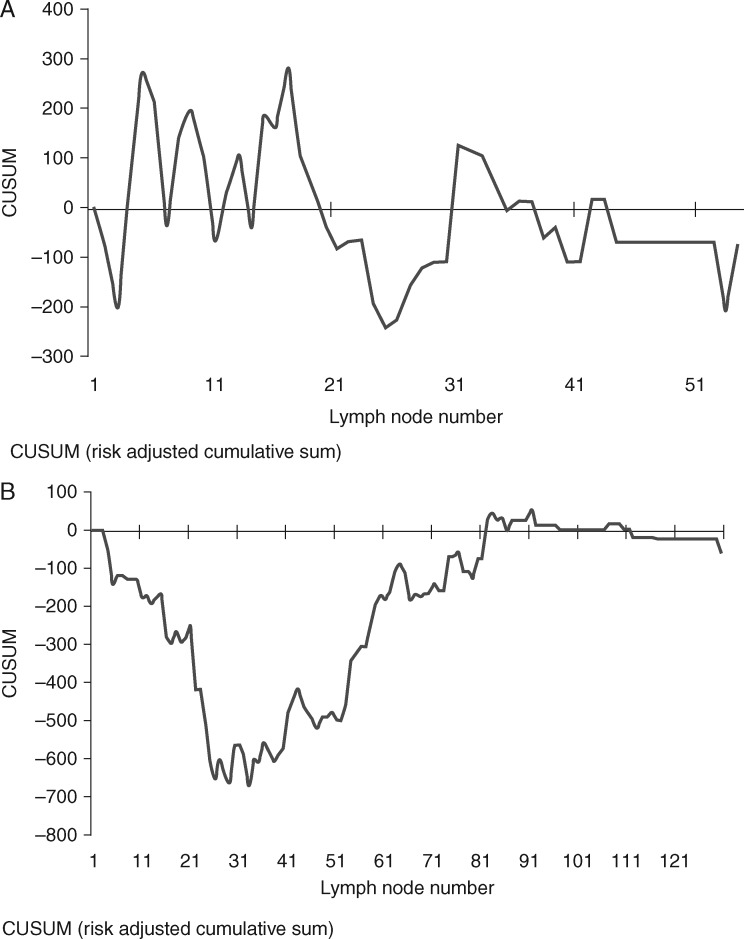
RA-CUSUM analysis of lymph node harvest (Figure 2)
In the NCRS group, RA-CUSUM analysis showed that lymph node harvest did not affect survival or recurrence with no identifiable change-point in the RA-CUSUM curve (Figure 2A).
Figure 2.
(A) RA-CUSUM analysis of lymph node harvest versus overall survival in chemoradiotherapy plus surgery group (NCRS); lymph node harvest does not affect survival with no discernable pattern to this CUSUM curve. (B) RA-CUSUM analysis of lymph node harvest versus overall survival in chemotherapy plus surgery group (NCS); change point as illustrated by the plateau of curve at 22–52 lymph nodes. Above 52 lymph nodes there were significant improvements in disease-free survival (22–36 months; P = 0.028), and overall recurrence (47.1%–15.9%; P < 0.001) and non-significant improvement in overall survival (27–38 months; P = 0.171).
In the NCS group, lymph node harvest significantly influenced survival and recurrence. The mean change-point in overall survival was seen to lie between 22 and 52 lymph nodes (confidence level 95.4%) (Figure 2B). At a lymph node harvest threshold of 52 lymph nodes, there were significant improvements in disease-free survival (22–36 months; P = 0.028), and overall recurrence (47.1%–15.9%; P < 0.001). However, the improvement in overall survival remained non-significant (27–38 months; P = 0.171).
Discussion
The present study showed no significant differences in overall or disease-free 3-year survival or pattern of recurrence between NCRS and NCS groups after propensity matching and Cox regression analysis. This is despite neoadjuvant chemoradiotherapy conferring significant pathological benefits in terms of tumor and nodal down staging and tumor regression grade. Lymph node harvest uniquely in the NCS group was shown to be significantly associated with disease-free survival and recurrence with an optimal threshold between 22 and 52 lymph nodes removed. Although the incidence of anastomotic leak was higher in the NCRS group, in-hospital mortality and other major postoperative complications were similar.
This study is the largest available analysis that compares NCRS with NCS for the treatment of esophageal and junctional adenocarcinoma. Despite higher rates of ypT0, ypN0, and R0 in the NCRS group, only small non-significant survival differences in overall and disease-free 3-year survival were evident. This might be partially explained by a non-significant increase in postoperative mortality rate in the NCRS group. This apparent paradox of significant down-staging at primary and nodal sites, yet no survival benefit, was also evident in three small underpowered randomized trials [5–7]. Taken together, these results suggest that survival differences between NCRS and NCS may be relatively modest, if present at all, and suggest that a large sample size is required for prospective RCTs that compare these modalities.
One intriguing element of the analysis is that the extent of lymphadenectomy may have impacted on disease-free survival and cancer recurrence exclusively in the NCS group. The absence of an association between lymph node harvest and survival in the NCRS group is consistent with the analysis of patients from the CROSS trial and further non-randomized data, which found that the total number of resected nodes was associated with survival in the surgery-alone group but not in the NCRS group [15, 16]. These results suggest that regional control of esophageal adenocarcinoma is essential and might either be achieved through chemotherapy with radical lymphadenectomy or neoadjuvant chemoradiotherapy with limited lymphadenectomy.
It was not possible to match for surgical technique as only one patient had transhiatal resection in the NCS group. At the centers where both transhiatal and transthoracic esophagectomies were performed, no significant difference in survival between the two techniques has been reported [17]. The difference in technique may be responsible for the observed differences in lymph node harvest between the NCRS and NCS groups, in line with results from randomized controlled trials [17–19]. Moreover, in the CROSS-trial lymph node, retrieval after neoadjuvant chemoradiotherapy appeared to be lower than after surgery alone (14 versus 18 lymph nodes, respectively), even when using the same surgical technique [15]. Other authors have also reported that chemoradiotherapy reduces lymph node harvest from within the radiotherapy field, e.g. in rectal cancer [20, 21]. The median number of lymph nodes retrieved in the NCRS group was 14. Consequently, in the present study, it was not possible to examine the added value of radical lymphadenectomy to neoadjuvant chemoradiotherapy on survival for esophageal adenocarcinoma, and this aspect will be of great interest in future trials such as Neo-AEGIS. The analysis in our study indicates that the quality of surgery, using lymph node retrieval as a proxy for quality and extent of lymphadenectomy, remains an important prognostic factor affecting the outcome from multimodality treatment of esophageal adenocarcinoma. We have previously shown that assurance of surgical quality within randomized controlled trials for the treatment of esophago-gastric cancer is an important aspect of study design and can affect variation in lymph node harvest and mortality [22]. Nevertheless, a recent analysis of the MAGIC trial has also shown that the presence of lymph node metastases after chemotherapy is an independent predictor of overall survival, although authors did not include lymph node count in the multivariate analysis [23].
Analysis of short-term outcomes showed no significant difference between NCRS and NCS apart from an increased incidence of anastomotic leak in the NCRS group. There was a non-significant increase in 30-day (4.1% versus 1.4%; P = 0.140) and 90-day mortality (5.9% versus 2.3%; P = 0.090) in the NCRS group. The centers involved in this study were high volume units, with all procedures performed by high volume surgeons, thus minimizing the effect of surgeon and hospital volume on short-term outcomes [24–26]. A significantly higher proportion of patients in the NCRS group had transhiatal resection with cervical esophageal anastomosis, which is known to be associated with a higher leak rate than thoracic anastomosis [27]. Theoretically, radiotherapy might affect perfusion of the gastric tube and thus anastomotic healing; this notwithstanding, no differences in anastomotic leak were found between the NCRS and surgery alone groups of the CROSS-trial. This effect is currently being further explored in an ongoing Dutch randomized trial comparing cervical with thoracic anastomosis after neoadjuvant chemoradiation [28].
There are limitations that must be considered in interpreting the results of this analysis, foremost its design as a retrospective, observational study. The propensity-matched analysis controlled for important factors that can influence long-term survival and cancer recurrence. However, both ASA-classification and cN status are subjective parameters in their clinical application that might have influenced the matching process. There may have been a small degree of selection bias within the Dutch cohort during the study period as less than 3% of patients could not undergo radiotherapy (e.g. due to history of radiotherapy) or had suspected lymph nodes outside the maximum radiation field, therefore, underwent neoadjuvant chemotherapy, and were excluded from the study. However, after matching 84.6% and 68.8% of patients in both groups had cT3 and cN positive staging, respectively, which is representative of the esophageal cancer population in Europe. Nevertheless, it was not possible to examine the benefits of NCS and NCRS separately for early and advanced disease because of the sample size of matched patients. Furthermore, there are inevitably other confounding variables including heterogeneity in surgical approach and type of chemotherapy used in the NCS group that may have varied between the groups. Moreover, the propensity matching reduced the sample size, resulting in less statistical power compared with the recently initiated Neo-AEGIS trial, and especially impeded correction for year of treatment and age as continuous variables. To overcome this limitation, data were also analyzed using Cox regression analysis. During the study period, there was some variation in the definition of complications, with the international consensus only recently published [29]. However, anastomotic leak was defined similarly in all centers with clinical or radiological evidence of leak and postoperative contrast evaluation of the anastomosis was standard of care in all participating centers. Unfortunately, data on toxicity of chemoradiotherapy and chemotherapy were not available in all participating centers. Patients were selected based on whether they underwent surgical resection (and not on whether they were planned to undergo NCS or NCRS), which impeded an intention-to-treat analysis. Therefore, patients not surgically resected due to disease progression, complete clinical response or patient physiological status were not included in this study. In the CROSS trial, 10% of patients in the multimodality arm did not undergo surgical resection due to toxicity or tumour progression, whereas in the MAGIC trial and OEO2 trial this was 17% and 14%, respectively [3, 4]. Finally, follow-up was not sufficient to compare long-term (≥5-year) survival between NCRS [2] and NCS, further emphasizing the need for publication of the long-term results from the MAGIC-trial.
In conclusion, this multi-center European study suggests that any prognostic differences between neoadjuvant chemoradiotherapy plus surgery and neoadjuvant chemotherapy plus surgery for the treatment of locally advanced esophageal and junctional adenocarcinoma are likely to be small. Our study suggests that loco-regional tumor control is of great importance, and can either be achieved through neoadjuvant chemotherapy with extended lymphadenectomy or concurrent chemoradiotherapy with limited lymphadenectomy. The benefit, if any, of extended lymphadenectomy after neoadjuvant chemoradiotherapy has not been addressed by this study and remains unclear. Therefore, future randomized trials evaluating multimodality treatment of esophageal adenocarcinoma must not only comprise an adequate sample size, but must also control and monitor quality of surgery during the trial. We would like to emphasize that this retrospective study does not provide a definitive answer to the unsolved question of the comparative benefits on NCS and NCRS in esophageal adenocarcinoma but supports the importance of the ongoing NeoAEGIS trial and its surgical quality measures.
Funding
S.R.M. is funded by the National Institute of Health Research (NIHR) (NIHR-CTF-2015-04-09). J.M.F. is funded by the NIHR Oxford Biomedical Research Centre. P.B. is funded by the NIHR Imperial Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Van Hagen P, Hulshof MC, van Lanschot JJ. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro J, van Lanschot JJ, Hulshof MC. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D, Allum WH, Stenning SP. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 4. Allum WH, Stenning SP, Bancewicz J. et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009; 27: 5062–5067. [DOI] [PubMed] [Google Scholar]
- 5. Burmeister BH, Thomas JM, Burmeister EA. et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomized phase II trial. Eur J Cancer 2011; 47: 354–360. [DOI] [PubMed] [Google Scholar]
- 6. Stahl M, Walz MK, Stuschke M. et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009; 27: 851–856. [DOI] [PubMed] [Google Scholar]
- 7. Klevebro F, Alexandersson von Dobeln G, Wang N. et al. A randomized clinical trial of neoadjuvant chemotherapy versus chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016; 27: 660–667. [DOI] [PubMed] [Google Scholar]
- 8. Reynolds JV. Randomised clinical trial of neoadjuvant and adjuvant chemotherapy (MAGIC Regimen) vs. neoadjuvant chemoradiation (CROSS protocol) in adenocarcinoma of the oesophagus and oesophago-gastric junction. https://clinicaltrials.gov/ct2/show/NCT01726452 (24 May 2016, date last accessed).
- 9. Alderson D, Langley RE, Nankivell MG. et al. Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072). J Clin Oncol 2015; 33: Abstr 4002. [Google Scholar]
- 10. Sobin LH, Gospodarowicz MK, Wittekind C.. TNM Classification of Malignant Tumours, 7th edition New York: John Wiley & Sons; 2009. [Google Scholar]
- 11. Chirieac LR, Swisher SG, Ajani JA. et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005; 103: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 12. Hudis CA, Barlow WE, Costantino JP. et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25: 2127–2132. [DOI] [PubMed] [Google Scholar]
- 13. D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 14. Grigg OA, Farewell VT, Spiegelhalter DJ.. Use of risk-adjusted CUSUM and RSPRT charts for monitoring in medical contexts. Stat Methods Med Res 2003; 12(2): 147–170. [DOI] [PubMed] [Google Scholar]
- 15. Talsma AK, Shapiro J, Looman CWN. et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg 2014; 260: 786–793. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro J, van Klaveren D, Lagarde SM. et al. Prediction of survival in patients with oesophageal or junctional cancer receiving neoadjuvant chemoradiotherapy and surgery. Br J Surg 2016; 103: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 17. Omloo JM, Lagarde SM, Hulscher JB. et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007; 246: 992–1000. [DOI] [PubMed] [Google Scholar]
- 18. Chu KM, Law SY, Fok M, Wong J.. A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg 1997; 174: 320–324. [DOI] [PubMed] [Google Scholar]
- 19. Boshier PR, Anderson O, Hanna GB.. Transthoracic versus transhiatal esophagectomy for the treatment of esophago-gastric cancer: a meta-analysis. Ann Surg 2011; 254: 894–906. [DOI] [PubMed] [Google Scholar]
- 20. Taflampas P, Christodoulakis M, Gourtsoyianni S. et al. The effect of preoperative chemoradiotherapy on lymph node harvest after total mesorectal excision for rectal cancer. Dis Colon Rectum 2009; 52: 1470–1474. [DOI] [PubMed] [Google Scholar]
- 21. Lykke J, Roikjaer O, Jess P. et al. Tumour stage and preoperative chemoradiotherapy influence the lymph node yield in stages I–III rectal cancer: results from a prospective nationwide cohort study. Colorectal Dis 2014; 16: O144–O149. [DOI] [PubMed] [Google Scholar]
- 22. Markar SR, Wiggins T, Ni M. et al. Assessment of the quality of surgery within randomized controlled trials for the treatment of gastro-esophageal cancer: a systematic review. Lancet Oncol 2015; 16: e23–e31. [DOI] [PubMed] [Google Scholar]
- 23. Smyth EC, Fassan M, Cunningham D. et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol 2016; 34: 2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markar SR, Karthikesalingam A, Thrumurthy S. et al. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000–2011. J Gastrointest Surg 2012; 16: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 25. Finks JF, Osborne NH, Birkmeyer JD.. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011; 364: 2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mamidanna R, Ni Z, Anderson O. et al. Surgeon volume and cancer esophagectomy, gastrectomy and pancreatectomy: a population-based study in England. Ann Surg 2016; 263(4): 727–32. [DOI] [PubMed] [Google Scholar]
- 27. Markar SR, Arya S, Karthikesalingam A. et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013; 20: 4274–4281. [DOI] [PubMed] [Google Scholar]
- 28. Rosman C. Minimally invasive esophageal resection for esophageal carcinoma: Restoring continuity of the gastro-intestinal tract at the level of the thorax or at the neck. www.trialregister.nl/trialreg/admin/rctview.asp?TC=4333 (5 May 2016, date last accessed).
- 29. Low DE, Alderson D, Cecconello I. et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015; 262: 286–294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.