Abstract
The chemical potentialities of metabolites far exceed metabolic requirements. The required potentialities are realized mostly through enzymatic catalysis. The rest are realized spontaneously through organic reactions that (i) occur wherever appropriate reactants come together, (ii) are so typical that many have proper names (e.g. Michael addition, Amadori rearrangement, and Pictet-Spengler reaction), and (iii) often have damaging consequences. There are many more causes of non-enzymatic damage to metabolites than reactive oxygen species and free radical processes (the “usual suspects”). Endogenous damage accumulation in non-renewable macromolecules and spontaneously polymerized material is sufficient to account for aging and differentiates aging from wear-and-tear of inanimate objects by deriving it from metabolism, the essential attribute of life.
Keywords: aging, enzyme, enzyme mechanism, metabolism, substrate specificity, damage, mechanism, metabolite, side reaction, spontaneous chemistry, metabolite damage, side-product, aging
Introduction
The idea that aging results from the gradual accumulation of molecular damage is deeply rooted in the aging research field (1–3), although it can appear in verbal disguises so different as to seem conceptually independent. However, damage is implicit to DNA in the somatic mutation theory of aging (4), to the extracellular matrix proteins in the crosslinking theory (5), and to phospholipids in the membrane theory (6). The free-radical theory implies that reactive oxygen species (ROS)4 are responsible for damage (7), and the carbonyl-stress theory blames free carbonyls for it (8, 9). With regard to the last two theories, the former, which is based on the ideas of D. Harman (10), celebrated its 60th anniversary last year and remains the most influential in the “damage field,” and the latter (9) is its extension insofar as it attributes the origin of many of the most noxious molecular species to the free-radical oxidation of metabolites initially devoid of highly reactive carbonyl moieties (11). Being traceable back to serendipitous findings, these concepts as of today remain empirical.
The dark side of metabolism
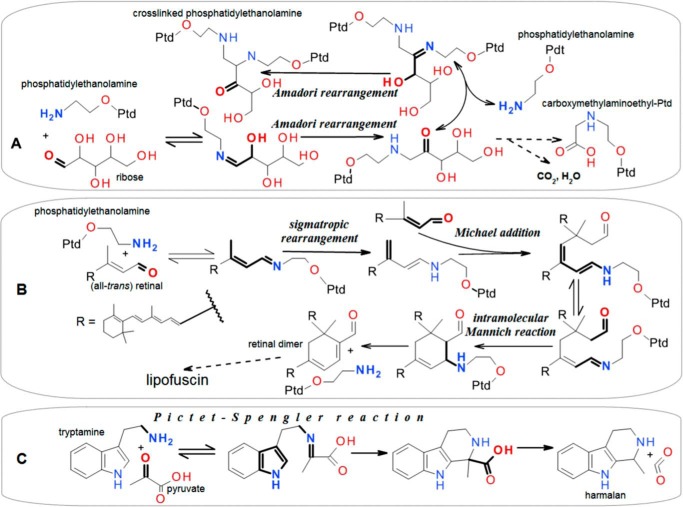
A theoretical approach to molecular damage may be derived from comparing a metabolic map (e.g. IUBMB-Nicholson Metabolic Maps, Minimaps, & Animaps website) or a database of metabolic pathways (e.g. Refs. 12 and 13) with a comprehensive manual of organic chemistry (e.g. Ref. 14). It may be inferred that in many cases of inevitable but reversible imine (Schiff base) formation between amines and carbonyl compounds, the structures of the interacting molecules provide for the migration of the double bond of the imine moiety away from it. This will convert the unstable C=N (imine) bond into the stable C–N bond and thus create a sink for the initial reactants. The migration mechanism may involve the so-called Amadori rearrangement when an aldose reacts with the amino group of, for example, ethanolamine, an amino acid, or a nucleic base (Fig. 1A). Another double-bond migration mechanism is a sigmatropic rearrangement, such as when ethanolamine reacts with retinal (Fig. 1B). Notably, the products may be able to bind other molecules, e.g. by imine formation (Fig. 1A) or by the Michael addition mechanism (Fig. 1B), providing for still further spontaneous transformations, such as involving another chemical classic, the Mannich reaction (Fig. 1B). Another case of transformation of the unstable C=N bond into the stable C–N bond is when an arylethylamine interacts with an aldehyde or ketone via the Pictet-Spengler reaction (Fig. 1C), which is widely used in organic synthesis to obtain polycyclic compounds (15).
Figure 1.
Examples of non-enzymatic synthesis of complex compounds from simpler metabolites via Schiff base formation followed by double-to-single carbon-nitrogen bond conversion. The starting reactive moieties are highlighted with thick bonds and bold characters. Any other metabolically relevant structure or atom may replace a non-highlighted part, including phosphatidyl (indicated by Ptd in A and B). A, ribose exemplifies any monosaccharide. The amino group may be on a phospholipid (23), an amino acid (24), or a nucleic base (25), either free or included in a biopolymer, which may become cross-linked by such modification. B, see “Physiological implications of endogenous chemical damage” for comments. See Refs. 26 and 27. C, tryptamine exemplifies any indoleamine, and pyruvate represents any carbonyl-containing compound, including saccharides. With catecholamines instead of indoleamines, the Pictet-Spengler reaction yields tetrahydroisoquinolines, whose neurotoxicity is implicated in brain aging (16, 17). Figures were drawn using MarvinSketch 16.5.2.0 (ChemAxon).
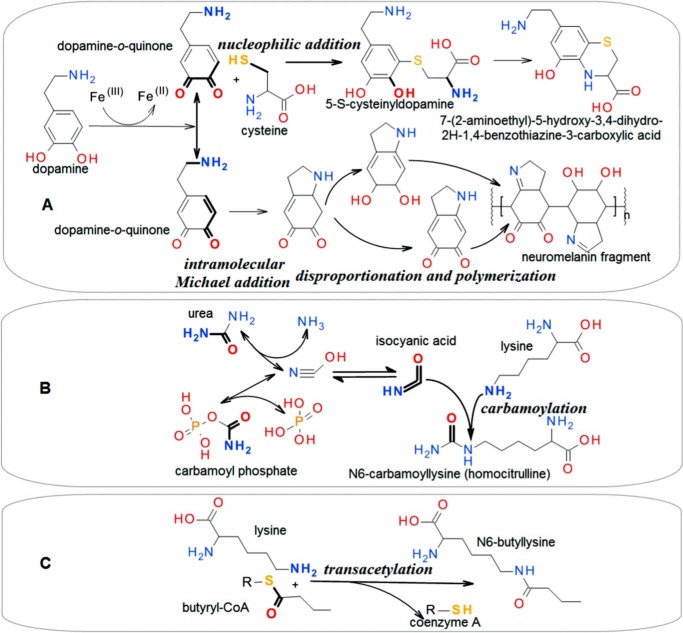
Looking at a metabolic map, one can notice many such inherently reactive metabolite pairs. However, few if any of the pair members are connected in the map by arrows marked with enzyme classification (EC) numbers. In some cases, the members are confined to different tissues or compartments; however, they often also occur in the same place and are thus doomed to react. That is how cytotoxic Pictet-Spengler products can be formed from biogenic amines and aldehydes (16, 17). The same is true for 5-S-cysteinyl dopamine, which is formed in the brain from dopamine-derived quinones via nucleophilic thiol addition (18) (Fig. 2A), and for many other compounds such as those in Figs. 1 and 2 and in the chemical damage database CD-MINE (19).
Figure 2.
Examples of non-enzymatic synthesis of complex compounds from simpler metabolites without Schiff base involvement. See Fig. 1 for general comments. A, dopamine exemplifies any catecholamine. Quinone formation is possible without oxygen involvement, being driven by ferric iron reduction (28). Cysteine may be a free amino acid or a part of glutathione or protein. 5-S-Cysteinyl dopamine is mostly protein-bound, whereas the free cysteine gives rise to cytotoxic dihydrobenzothiazine derivatives (18, 29). B, carbamylation of any amino-group bearer is possible. Only the homocitrulline level in human skin has been shown so far to increase with age (30). C, high reactivity of thioesters is long acknowledged in chemistry. Their ability to acylate cellular proteins non-enzymatically is reported in several recent publications (20, 31, 32).
Because of metabolic requirements, many inherently reactive moieties become even more reactive when they are attached to specific molecules known as coenzymes. Examples are the acyl-CoA species (Fig. 2B), which spontaneously acylate primary amines (20, 21). Similarly, acyl phosphates react non-enzymatically with amines (22).
Figs. 1 and 2 show that, in a metabolic system, not only spontaneous decay and degradation reactions, such as hydrolysis, oxidation, and racemization, but also spontaneous multistage synthetic processes take place (23–32). Can the products formed in this way be regarded as metabolites sensu stricto? They are not generated by enzymes, are not used purposefully, and are often hazardous. One way to view them is as damaged metabolites (33, 34). For example, 5-S-cysteinyldopamine is a damaged form of cysteine or dopamine. A related way to conceptualize this phenomenon is to view it as a sort of “underside” of metabolism or “parametabolism” (35, 36). In this view, 5-S-cysteinyldopamine and tetrahydroisoquinolines are the parametabolic products of catecholamine metabolism. A conceptually similar but more general approach is to regard such unwanted products as a manifestation of the imperfectness of metabolism and its components, which together produce deleterious effects at all levels of biological organization. The totality of such effects has been described as the “deleteriome,” which expands with age and represents the biological age of an organism (1).
A complementary point of view comes from the notion that life must have developed from the physicochemical world. It is true that molecules whose properties are useful for biological functions were selected for the metabolism of living organisms. However, the properties of such molecules are not limited to those required for life. For example, only the cyclic forms of sugars, such as glucose, are used by biological systems and thus have steric compatibility with the active centers of sugar-metabolizing enzymes. However, free sugars in solution consist of an equilibrium mixture of cyclic and linear forms. The linear carbonyl form can react with free amine groups to form Schiff bases, and then, via double-bond migration (Fig. 1), the damaged metabolites resulting from such interactions can leak into the parametabolic mess beneath the metabolic order and add to the deleteriome.
One way to increasing the deleteriome is by the spontaneous polymerization of damaged metabolites, such as catecholamine-derived quinones (Fig. 2A). In reality, such polymerization occurs in a milieu abundant in proteins, which are included in the resulting agglomerates, wherein they become covalently modified and misfolded and thus made prone to aggregation. Altogether, this leads to the accumulation of polymers of (damaged) metabolites associated with protein aggregates in the form of lipofuscin (37), neuromelanin (38), and other forms often referred to as waste.
Countermeasures
Several reasons may be envisioned for the need to control parametabolic short circuits, leaks, and waste heaps. The first is to reduce the loss of resources caused by fluxes into unwanted products. In this case, investment in measures to counter the losses is constrained by the need to avoid consuming more resources than are saved.
A second reason follows from the ability of some damaged metabolites to interfere with legitimate metabolic processes by replacing normal metabolites in their interactions with enzymes, transporters, or receptors whose specificity is imperfect. Such cases may warrant extra investment in countermeasures because the products are actively harmful as opposed to merely useless.
A third reason is because some damage-prone metabolites are present in cells not only as free entities but also as monomers of macromolecules whose turnover rate is low, making the elimination rates of damaged metabolites incorporated in macromolecular structures lower than those of their free counterparts. In such cases, parametabolic products may eventually reach higher steady-state levels than are achievable when all reactant species are free. The amounts of macromolecules modified in this way may increase enough to produce significant effects.
An extreme of the above possibility is observed when the turnover rate of a macromolecule is so low that its half-life is comparable with that of a cell containing the macromolecule or the organism itself (39, 40). In such a case, the level of modified macromolecular species will gradually increase during the whole lifespan of their host and unavoidably compromise vital functions. If this is not aging, then what is?
The most straightforward way to cope with such damage is to dilute it by increasing the normal biomass and/or to ensure the turnover of the sum of the normal and abnormal biomass during cell turnover (41). This mechanism operates by default in populations of unicellular organisms and in metazoans whose cell populations are all renewable, e.g. the coelenterate Hydra vulgaris, which exhibits no manifestations of aging (42). However, this does not eliminate the need to cope with the loss of resources via parametabolic products whose turnover rate is high or with the adverse effects of the steady-state levels of damaged metabolites. For this, mechanisms that have been termed “metabolite damage pre-emption” and “metabolite repair” (33, 43) have evolved. The latter are often coupled to ATP hydrolysis and/or consumption of reducing equivalents and thus are resource-intensive.
Strategies employed by cells to cope with parametabolic damage, including those of the metazoans that benefit from their non-renewable cell populations, are shown in Table 1 (41–55).
Table 1.
Mechanisms that help cells to cope with the endogenous chemical damage
| Option | Examples | Costs and limitations |
|---|---|---|
| Dilution | Any growing cell population (41) | Increases in biomass/size/amount must be unlimited. This is incompatible with biological strategies that rely on fixed-size non-renewable structures, such as those present in the brain and endoskeleton. |
| Total turnover | Hydra vulgaris (42) | Available resources are spent for de novo synthesis of turning-over material. This is incompatible with benefits afforded by the presence of non-renewable cells. |
| Uneven partition of damage | Damage segregation upon division in Escherichia coli and damage bias to mother cell upon budding in yeast (44) | Continuing cell proliferation is required to maintain tolerable proportions of relatively more and less damage-loaded cells. |
| Pre-emptiona | Glyoxalases (Glo) I and II in concert with glutathione convert reactive methylglyoxal (MGO) to inert d-lactate (45). | Resources are spent for the synthesis and turnover of pre-emption enzymes. Notably, Glo I is among the 5% most abundant proteins in many human tissues (PaxDb 1855892), and so is triose phosphate isomerase (PaxDb 1843191), whose ability to mitigate MGO production is limited by the diffusion rates of its substrates (45). |
| Directeda overflow | Accumulation of hazardous intermediates of riboflavin biosynthesis is limited by diverting them to an alternative pathway (46). | The level of the diverted metabolite must not fall below that required for basal metabolic functions. |
| Trapping | Methylglyoxal is trapped by the dipeptide carnosine (47). | Carnosine is a “suicide” trapping agent; therefore, resources are spent for its regeneration. |
| Repaira | Amadori products and methylglyoxal adducts are removed from proteins and free amino acids by fructosamine-3-kinase (48) and DJ-1/Park7-type deglycases (49), respectively. | Resources are required for the synthesis and turnover of DJ-1, which is among the 5% most abundant proteins in human tissues (PaxDb 1852305). Fructosamine-3-kinase consumes ATP. |
| SIRT3 sirtuin removes acyl groups from proteins damaged non-enzymatically by acyl-CoAs (21). | NAD is consumed in the deacetylation reaction. | |
| DNA repair | DNA repair mechanisms are so complex and resource-intensive that their affordable repertoire critically depends on available resources (50). | |
| Clearance | Ubiquitin-proteasomal system (51) | Ubiquitination consumes ATP. |
| Chaperone-mediated autophagy (52) | These mechanisms are thought to reallocate cell resources by their degradation and reuse according to changing demands and, as such, may produce no waste if an undamaged material is processed. Under stable conditions, they ensure the total turnover of cell constituents and, thus, the maintenance of a tolerable stationary level of damage outside of autophagosomes and lysosomes. However, some forms of damage, such as shown in Figs. 1 and 2, can resist degradation and accumulate with time in the form of lipofuscin. | |
| Microautophagy (53) | ||
| Macroautophagy (3) | ||
| Excretion | For the passage of water-soluble metabolites across cell membranes, transporters such as ATP-binding cassette (ABC) transporters (54) or solute carrier (SLC) proteins are required (55). The same must be true for hydrophilic damaged metabolites and the products of their trapping and of damage preemption. | ABC transporters consume ATP. Many SLCs depend on transmembrane gradients generated by energy-consuming mechanisms. Among about 400 known human SLC genes, three-quarters are not yet associated with a known transported substrate (55). Some of these orphan SLCs may be involved in the excretion of endogenous chemical damage products. |
| Disposal | Catecholamine-derived quinones polymerize spontaneously to yield neuromelanin (38). | Immediate damage by highly reactive monomers is reduced at the expense of delayed adverse effects of increasing space occupied by relatively inert but bulky polymers. |
a Other examples may be found in Ref. 34.
Other important aspects of counteracting the forces that produce chemical damage to living bodies are discussed in the next section in relation to the disposable soma theory of aging.
Evolutionary implications of endogenous chemical damage
One conclusion from the above is that many metabolites prone to adverse interactions, including such pivotal molecules as carbohydrates, urea, and acyl-CoAs, were seamlessly incorporated into metabolism at early stages of evolution under conditions that made the accumulation of their parametabolic products negligible because of turnover and dilution. Many known non-enzymatic interactions were at work prior to the advent of aerobic forms of life. For example, methylglyoxal, which participates in certain metabolic pathways in bacteria, performs no function in other prokaryotes. Nevertheless, it is generated in them and in eukaryotes as a by-product of glycolysis. In either case, it can interact spontaneously with nucleophiles, including those incorporated in proteins and nucleic acids, all this without the involvement of molecular oxygen and/or ROS (56).
Although the range of parametabolic reactions enormously expanded with the advent of metabolic use of molecular oxygen and its derivatives, the unwanted consequences of metabolism were never, and are not today, limited to ROS-related reactions (11, 57). The examples in Figs. 1 and 2 and in Table 1 are deliberately chosen as cases where the initiation of damage by ROS or nitric oxide and its propagation by free radicals are not essential. These examples still suggest that the deleteriome of a system where these processes take place will continuously increase, although its density may be maintained and even decreased by dilution due to growth and proliferation. ROS can contribute to the final picture quite appreciably; however, they are neither necessary nor sufficient to make a system age.
The significance of non-enzymatic processes changed dramatically when it emerged in the course of evolution that the presence of non-renewable cells (e.g. in the brain) and extracellular matrix components (e.g. in the endoskeleton) could have adaptive value for populations to the extent that their benefits outweighed the adverse effects of damage accumulation in individual bodies. Thus, the advent of non-renewable structures as means of protection from exogenous hazards was associated with the actuation of the hidden endogenous damage potential, which previously could be neutralized by dilution and turnover (36) and pre-emption (34).
An influential evolutionary concept directly relevant to endogenous chemical damage is the antagonistic pleiotropy theory of aging (58). Antagonistic pleiotropy refers to the ability of a mutation to produce positive effects on some vital function at the expense of negative effects on another function. Specifically with regard to aging, it was suggested that if a mutation that offers some advantage early in life confers a disadvantage at ages so advanced that they are virtually unachievable in the wild, the mutation must be supported by natural selection because, at the population level, its later-acting disadvantageous effects are outweighed by the earlier advantageous ones. As a result, the vitality of aged organisms must be compromised by the late disadvantageous effects of the genes whose early advantageous effects increase the chance for organisms to survive to the ages of manifestation of their disadvantageous effects.
Some of the concerns about the antagonistic pleiotropy theory include the actual identity of genes that produce early beneficial and late detrimental effects and of mechanisms that switch the effects from beneficial to detrimental (59). However, the above view on the causes of damage to metabolites and macromolecules changes the entire dispute. Any gene whose protein product, such as an enzyme, is involved in the production of a vital metabolite capable of unwanted interactions fits the concept of antagonistic pleiotropy insofar as it produces the direct beneficial effects on vitality through the metabolic functions and the adverse pleiotropic effects through the excessive chemical potentialities of its metabolic product. The adverse effects gradually increase with the accumulation of the results of unwanted interactions, such as the agglomerates of their products and/or damaged slowly turning-over proteins and nucleic acids. That is, the adverse pleiotropic effects are not late-acting, as they are commonly thought to be, but are cumulative (36). Another corollary is that there are no, and there never were, genes that are completely free of adverse pleiotropic effects. Antagonistic pleiotropy responsible for aging results from gene activity in the context of the whole system of interacting genes and their products rather than from any of its specific components taken separately at different points in the lifespan. Therefore, the diversity of damage forms will always exceed the number of protecting mechanisms, and, for non-renewable systems, the consequent cumulative damage will necessarily increase, manifesting as aging.
A common misconception worth mentioning is that damage results from “stresses,” such as oxidative stress (60) and carbonyl stress (2, 9). Stress is thought of as a condition wherein the effects of damaging factors surpass the ability of the system to protect itself from damage. However, damage occurs regardless of stresses, which merely modify the rate and composition of damage accumulation. For instance, in mammals under conditions assumed as basal, the gaseous products of lipid peroxidation are still exhaled (61) and damaged nucleosides are excreted (62).
It is possible, in principle, to slow down damage accumulation by supplementing pre-emption, repair, and elimination of damage to metabolites with repair of damage suffered by non-renewable proteins (48, 63) and DNA (64). However, increased investment into self-maintenance increases its total cost at the expense of other functions essential for survival of a population. This is what the “disposable soma theory of aging” is about: protection from damage that causes aging is limited by the need to allocate a part of the available resources to reproduction (65).
An important but largely overlooked question related to this theory is the quantitation of the energetic cost of damage to metabolites and macromolecules and its repair or pre-emption (including the costs of producing the enzymes that do nothing except protect from damage), particularly relative to the costs of other “maintenance” processes such as macromolecule turnover and sustaining chemical and ion gradients (66). A part of the problem is that the overall costs of maintenance are poorly understood and handled as a “black box” in current metabolic models rather than being partitioned among biochemical processes (67, 68). Another challenge is that good kinetic data for spontaneous reactions under in vivo conditions are very often lacking, making it hard to estimate the rates of formation of damaged metabolites. Special measurements under conditions that mimic those in vivo, as in Ref. 69, are required for that. Nevertheless, there is reason to think that parametabolism contributes significantly to maintenance costs, as, for instance, in the case of S-adenosylmethionine, which suffers relatively high rates of spontaneous racemization as well as cleavage and hydrolysis (70) and is energetically expensive to repair or replace (34).
However, it is still unclear whether the above total costs amount to, say, 50, 10, or 1% of the total energy flux. The lowest of these estimates would make the cost of coping with endogenous damage almost irrelevant, thus making the disposable soma theory less relevant, too.
Both the antagonistic pleiotropy and the disposable soma theories in their classic forms are based on considerations derived from evolutionary theory, population genetics, and physiological and ecological tradeoffs. These theories either treat the chemical constituents of living organisms as if they impose no constraints on “molding of senescence by natural selection” (71), or admit such constraints but treat them as resulting from unfortunate “frozen accidents” that were missed by selection. Why selection missed them is then explained in terms of tradeoffs or other balances, which is circular reasoning. This approach is comforting in that accidental flaws (and by inference aging) are more likely to be amenable to elimination than are essential features. However, “ignorance of the law excuses not.” By the laws of chemistry, carbonyls react with primary amines. This is not suggested by evolutionary theory and cannot be eliminated by natural selection. Water's capability of adding to carbon-carbon double bonds will be realized upon whatever opportunity, such as interacting with NAD(P)H to form NAD(P)H hydrate. Shifting the equilibrium between dysfunctional NAD(P)H hydrate and functional NAD(P)H toward the latter is possible at the expense of ATP hydrolysis by the repair enzyme NAD(P)H-hydrate dehydratase (72).
Another important aspect of chemical damage to biochemical systems is that any biochemical means devised by evolution to cope with such damage expands the range of possible interactions, including adverse ones. Thus, superoxide dismutase (SOD), which disposes of harmful superoxide, yields hydrogen peroxide, which is prone to forming hydroxyl radical via the Fenton or iron-catalyzed Haber-Weiss reaction. Therefore, SOD must be coupled with catalase or peroxidase. Increasing Cu,Zn-SOD expression may result in increased oxidative damage, and in some experimental settings, mimics phenotypes observed in Down's syndrome patients (73). Cu,Zn-SOD itself is inherently prone to aggregation (74), so that the mutant forms that are responsible for the hereditary amyotrophic lateral sclerosis merely exaggerate an existing tendency. From this perspective, it is no wonder that the incidence of the sporadic adult-onset forms of this disease is above zero.
Physiological implications of endogenous chemical damage
A good case for applying the ideas discussed above to a specific situation is provided by bisretinyls, the major constituents of lipofuscin that accumulate in the pigmented epithelium of the eye. Bisretinyls, such as retinal dimer (Fig. 1B), are byproducts of visual cycle biochemistry (75). 11-cis-retinal bound to the ϵ-amino group of lysine 296 of opsin in the outer segments of photoreceptor cells is converted to all-trans-retinal upon accepting a quantum of light. Although the imine moiety of retinal bound to opsin is formed in a non-enzymatic reversible manner, retinal is kept in its place by non-covalent interactions of its 11-cis configuration with properly arranged side chains of the other amino acids of rhodopsin. The all-trans-retinal does not fit opsin structure and is expelled from it. On the one hand, this is associated with opsin conformation changes triggering signal transduction via G-proteins. On the other hand, liberation from opsin enables retinal to form Schiff bases with amino groups, including those of phosphatidylethanolamine in photoreceptor cell membranes. To avoid this, all-trans-retinol dehydrogenase consumes NADPH to reduce all-trans-retinal to the less noxious all-trans-retinol. Retinol is transferred to pigmented epithelium cells, where it is converted to 11-cis-retinal. The latter enters receptor cells and forms the Schiff base with lysine 296 of opsin there.
Without delving into important details and conflicting views, it is sufficient in the present context to point out that the functional demands of light perception ensure that the aldehyde retinal is constantly present in a free form in an environment rich in ethanolamine moieties. The result is that the reversible Schiff base formation from an ethanolamine moiety and retinal can be followed by the irreversible addition of another retinal to the product, and after a series of further rearrangements, by the formation of retinyl dimer (Fig. 1B) and a host of related compounds accumulating in photoreceptor membranes, which are constantly shed off to be phagocytized by pigmented epithelium cells. The poorly degradable retinal dimer and related products form lipofuscin deposits in pigmented cells and thus increase the risk of macular degeneration, the most common form of age-related vision loss.
Several lessons follow from the above case. First, damage accumulation results from normal functions, and the pathways of damage formation may become clear only after the molecular details of normal functions become known. Second, damage manifests itself in a functionally significant manner at ages rarely achievable in the wild under the conditions in which the species in question evolved. Therefore, there was no selection pressure toward the prevention of accumulation of this sort of damage. However, there was pressure toward preventing any immediate damage, even at the expense of later adverse consequences. In fact, lipofuscin accumulation in pigmented epithelium is a consequence of clearing of photoreceptor cell membranes from damage caused by retinal liberated in the course of light perception. Third, via a series of transitions through rapidly turning-over cell constituents, damage finally accrues as a slowly turning-over material in the non-renewable component of a functional system where the deposits of damaged metabolites accumulate. Fourth, the pathways from metabolite damage to the accumulation of agglomerates of damaged metabolites and, further on, to age-associated functional decline may be deciphered at a resolution approaching specific chemical interactions between metabolites, which leaves no place for speculations regarding the causes of aging.
Lipofuscin abundance in steroid hormone-producing organs, such as testes, ovaries, and renal cortex, highlights other important aspects of molecular damage and its evolutionary origin (or neglect?). The latest evolutionary extensions of steroidogenic pathways yield products destined for secretion as hormones even though their chemical properties make them pseudo-substrates for some upstream steroidogenic enzymes. By binding to the active centers of these enzymes, the end products of steroidogenesis, such as testosterone (76) or cortisol (77), initiate one-electron oxygen reduction but do not react with the resulting ROS, which are released and damage everything in their vicinity, starting with the enzymes themselves.
The maximal steroidogenic capacities of testes, ovaries, and adrenal cortex markedly decrease during aging; however, their actual productivities decrease much less (78). A likely explanation of this discrepancy may be the observation that the negative feedback regulation of steroidogenic tissues is mediated by central catecholaminergic neurons, whose functions become compromised with aging (79). Catecholamines and their metabolites and parametabolites are prone to Pictet-Spengler-type interactions yielding products implicated in age-associated parkinsonism (17), and are subject to oxidation yielding neurotoxins (80), including quinones (81). These quinones in turn polymerize (Fig. 2A), leading to neuromelanin accumulation in catecholaminergic neurons present in brain structures whose deterioration is implicated in emotional and cognitive disorders (82, 83). The production of certain steroids may actually increase with aging in some animal species (78, 84), probably due to the release from inhibition mediated by catecholaminergic mechanisms, which may overcompensate for the decreased maximal steroidogenic capacity.
Altogether, such relationships may generate species-specific patterns of age-associated physiological changes, giving the appearance of a programmed process. However, such patterns are a sort of quasi-program formed as a by-product of genuine programmed (genetically determined) functions whose operation generates noxious parametabolic products (35). As a result, the functions of even the renewable components of a system will be compromised during aging by their milieu, which becomes progressively non-optimal because of quasi-programmed changes in the non-renewable components.
Concluding comments
Recent advances in the metabolomics of aging (85–90) defined patterns of age- and disease-specific changes in the levels of metabolites, i.e. low-molecular-weight, enzymatically generated compounds used in biological systems. Such compounds have specific places in metabolic maps, databases, and models of metabolism (12, 13, 91). These resources, however, omit the many cellular compounds that, like those in Figs. 1 and 2, are formed via chemical side reactions. The recently launched chemical damage database project CD-MINE (19) sets out to fill this gap.
Notably, spontaneous chemical reactions between metabolites are often labeled with proper names, such as Schiff, Pictet-Spengler, Amadori, Mannich, or Michael, just because they are typical and will take place wherever the respective reactants come together. Thus, from the chemical point of view, a metabolic system cannot but be plagued with numerous short circuits, leaks, and other adverse concomitants of metabolism.
Unwanted reactions of this sort give rise to diverse damage products that increase in number and abundance with age and are adjusted (with regard to both composition and rate of increase with age) by interventions that affect lifespan (88). These reactions in their entirety are sufficient to cause what is generally termed aging.
Logically, any pursuit of what aging is must end with an explanation (the explanans) that resides outside of what has to be explained (the explanandum). In this Minireview, we root out the cause of aging in the unwanted, “surplus-to-metabolic-requirements” chemical properties of the constituents of biological systems. This approach – unlike current evolutionary theories of aging – takes the explanans out of its explanandum. An interface between the two is metabolism – the quintessential attribute of life making biological aging different from the wear and tear observed in inanimate things. Abrogation of many of the alleged individual causes of aging (e.g. damage to DNA (92) or damage by free radicals) will only modify, but will not stop aging. As to the endogenous chemical damage, to abrogate it is the same as to abrogate metabolism, i.e. life itself.
This work was supported by National Institutes of Health Grants AG021518, AG047745, GM0625204, and GM061603 (to V. N. G.) and by National Science Foundation (NSF) Award MCB 1611711 (to A. D. H.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase.
References
- 1. Gladyshev V. N. (2016) Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 15, 594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yin D., and Chen K. (2005) The essential mechanisms of aging: irreparable damage accumulation of biochemical side-reactions. Exp. Gerontol. 40, 455–465 [DOI] [PubMed] [Google Scholar]
- 3. Kaushik S., and Cuervo A. M. (2015) Proteostasis and aging. Nat. Med. 21, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 4. Kennedy S. R., Loeb L. A., and Herr A. J. (2012) Somatic mutations in aging, cancer and neurodegeneration. Mech. Ageing Dev. 133, 118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjorksten J., and Tenhu H. (1990) The crosslinking theory of aging: added evidence. Exp. Gerontol. 25, 91–95 [DOI] [PubMed] [Google Scholar]
- 6. Pamplona R. (2008) Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim. Biophys. Acta 1777, 1249–1262 [DOI] [PubMed] [Google Scholar]
- 7. Finkel T., and Holbrook N. J. (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 [DOI] [PubMed] [Google Scholar]
- 8. Semchyshyn H. M. (2014) Reactive carbonyl species in vivo: generation and dual biological effects. ScientificWorldJournal 2014, 417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyons T. J., and Jenkins A. J. (1997) Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: a carbonyl stress hypothesis. Diabetes Rev. (Alex.) 5, 365–391 [PMC free article] [PubMed] [Google Scholar]
- 10. Harman D. (1956) Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 [DOI] [PubMed] [Google Scholar]
- 11. Gladyshev V. N. (2014) The free radical theory of aging is dead: long live the damage theory! Antioxid. Redox Signal. 20, 727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caspi R., Billington R., Ferrer L., Foerster H., Fulcher C. A., Keseler I. M., Kothari A., Krummenacker M., Latendresse M., Mueller L. A., Ong Q., Paley S., Subhraveti P., Weaver D. S., and Karp P. D. (2016) The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 44, D471–D480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wishart D. S., Mandal R., Stanislaus A., and Ramirez-Gaona M. (2016) Cancer metabolomics and the human metabolome database. Metabolites 6, E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMurry J. (2015) Organic Chemistry with Biological Applications, Cengage Stamford CT [Google Scholar]
- 15. Stöckigt J., Antonchick A. P., Wu F., and Waldmann H. (2011) The Pictet-Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. Engl. 50, 8538–8564 [DOI] [PubMed] [Google Scholar]
- 16. Herraiz T., and Galisteo J. (2014) Naturally-occurring tetrahydro-β-carboline alkaloids derived from tryptophan are oxidized to bioactive β-carboline alkaloids by heme peroxidases. Biochem. Biophys. Res. Commun. 451, 42–47 [DOI] [PubMed] [Google Scholar]
- 17. Deng Y., Zhang Y., Duan J., Xiong Y., and Qing H. (2012) An overview of endogenous catechol-isoquinolines and their related enzymes: possible biomarkers for Parkinson's disease. Curr. Translat. Geriatr. Exp. Gerontol. Rep. 1, 59–67 [Google Scholar]
- 18. Jameson G. N. L., Zhang J., Jameson R. F., and Linert W. (2004) Kinetic evidence that cysteine reacts with dopaminoquinone via reversible adduct formation to yield 5-cysteinyl-dopamine: an important precursor of neuromelanin. Org. Biomol. Chem. 2, 777–782 [DOI] [PubMed] [Google Scholar]
- 19. Lerma-Ortiz C., Jeffryes J. G., Cooper A. J. L., Niehaus T. D., Thamm A. M. K., Frelin O., Aunins T., Fiehn O., de Crécy-Lagard V., Henry C. S., and Hanson A. D. (2016) 'Nothing of chemistry disappears in biology': the top 30 damage-prone endogenous metabolites. Biochem. Soc. Trans. 44, 961–971 [DOI] [PubMed] [Google Scholar]
- 20. Simic Z., Weiwad M., Schierhorn A., Steegborn C., and Schutkowski M. (2015) The α-amino group of protein lysine residues is highly susceptible to nonenzymatic acylation by several physiological acyl-CoA thioesters. Chembiochem 16, 2337–2347 [DOI] [PubMed] [Google Scholar]
- 21. Weinert B. T., Moustafa T., Iesmantavicius V., Zechner R., and Choudhary C. (2015) Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34, 2620–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfe A. (2016) Bacterial protein acetylation: new discoveries unanswered questions. Curr. Genet. 62, 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solís-Calero C., Ortega-Castro J., Frau J., and Muñoz F. (2015) Nonenzymatic reactions above phospholipid surfaces of biological membranes: reactivity of phospholipids and their oxidation derivatives. Oxid. Med. Cell. Longev. 2015, 319505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., and Aldini G. (2013) Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 47, 3–27 [DOI] [PubMed] [Google Scholar]
- 25. Barea F., and Bonatto D. (2008) Relationships among carbohydrate intermediate metabolites and DNA damage and repair in yeast from a systems biology perspective. Mutat. Res. 642, 43–56 [DOI] [PubMed] [Google Scholar]
- 26. Fishkin N. E., Sparrow J. R., Allikmets R., and Nakanishi K. (2005) Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate. Proc. Natl. Acad. Sci. U.S.A. 102, 7091–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X., Chen J., Liu Z., Li J., Yao K., and Wu Y. (2016) Potential therapeutic agents against retinal diseases caused by aberrant metabolism of retinoids: agents against aberrant retinoid metabolism. Invest. Ophthalmol. Vis. Sci. 57, 1017–1030 [DOI] [PubMed] [Google Scholar]
- 28. El-Ayaan U., Herlinger E., Jameson R. F., and Linert W. (1997) Anaerobic oxidation of dopamine by iron(III). J. Chem. Soc. Dalton Trans. 16, 2813–2818 [Google Scholar]
- 29. Shen X.-M., and Dryhurst G. (1997) Further insights into the oxidation chemistry of norepinephrine and epinephrine in the presence of cysteine. Bioorg. Chem. 25, 130–153 [Google Scholar]
- 30. Gorisse L., Pietrement C., Vuiblet V., Schmelzer C. E. H., Köhler M., Duca L., Debelle L., Fornès P., Jaisson S., and Gillery P. (2016) Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. U.S.A. 113, 1191–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wagner G. R., and Hirschey M. D. (2014) Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell 54, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baeza J., Smallegan M. J., and Denu J. M. (2015) Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem. Biol. 10, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanson A. D., Henry C. S., Fiehn O., and de Crécy-Lagard V. (2016) Metabolite damage and metabolite damage control in plants. Annu. Rev. Plant Biol. 67, 131–152 [DOI] [PubMed] [Google Scholar]
- 34. Linster C. L., Van Schaftingen E., and Hanson A. D. (2013) Metabolite damage and its repair or pre-emption. Nat. Chem. Biol. 9, 72–80 [DOI] [PubMed] [Google Scholar]
- 35. Golubev A. G. (1996) The other side of metabolism: a review. Biochemistry (Mosc.) 61, 1443–1460 [Google Scholar]
- 36. Golubev A. (2009) How could the Gompertz-Makeham law evolve? J. Theor. Biol. 258, 1–17 [DOI] [PubMed] [Google Scholar]
- 37. Seehafer S. S., and Pearce D. A. (2006) You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol. Aging 27, 576–588 [DOI] [PubMed] [Google Scholar]
- 38. Zucca F. A., Basso E., Cupaioli F. A., Ferrari E., Sulzer D., Casella L., and Zecca L. (2014) Neuromelanin of the human substantia nigra: an update. Neurotox. Res. 25, 13–23 [DOI] [PubMed] [Google Scholar]
- 39. Toyama B. H., and Hetzer M. W. (2013) Protein homeostasis: live long, won't prosper. Nat. Rev. Mol. Cell Biol. 14, 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toyama B. H., Savas J. N., Park S. K., Harris M. S., Ingolia N. T., Yates J. R. 3rd, and Hetzer M. W. (2013) Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stroikin Y., Dalen H., Brunk U. T., and Terman A. (2005) Testing the “garbage” accumulation theory of ageing: mitotic activity protects cells from death induced by inhibition of autophagy. Biogerontology 6, 39–47 [DOI] [PubMed] [Google Scholar]
- 42. Schaible R., Scheuerlein A., Dańko M. J., Gampe J., Martínez D. E., and Vaupel J. W. (2015) Constant mortality and fertility over age in Hydra. Proc. Natl. Acad. Sci. U.S.A. 112, 15701–15706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niehaus T. D., Gerdes S., Hodge-Hanson K., Zhukov A., Cooper A. J. L., ElBadawi-Sidhu M., Fiehn O., Downs D. M., and Hanson A. D. (2015) Genomic and experimental evidence for multiple metabolic functions in the RidA/YjgF/YER057c/UK114 (Rid) protein family. BMC Genomics 16, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lade S. J., Coelho M., Tolić I. M., and Gross T. (2015) Fusion leads to effective segregation of damage during cell division: an analytical treatment. J. Theor. Biol. 378, 47–55 [DOI] [PubMed] [Google Scholar]
- 45. Rabbani N., Xue M., and Thornalley P. J. (2016) Methylglyoxal-induced dicarbonyl stress in aging and disease: first steps towards glyoxalase 1-based treatments. Clin. Sci. 130, 1677–1696 [DOI] [PubMed] [Google Scholar]
- 46. Frelin O., Huang L., Hasnain G., Jeffryes J. G., Ziemak M. J., Rocca J. R., Wang B., Rice J., Roje S., Yurgel S. N., Gregory J. F. 3rd, Edison A. S., Henry C. S., de Crécy-Lagard V., and Hanson A. D. (2015) A directed-overflow and damage-control N-glycosidase in riboflavin biosynthesis. Biochem. J. 466, 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hipkiss A. R., Baye E., and de Courten B. (2016) Carnosine and the processes of ageing. Maturitas 93, 28–33 [DOI] [PubMed] [Google Scholar]
- 48. Van Schaftingen E., Collard F., Wiame E., and Veiga-da-Cunha M. (2012) Enzymatic repair of Amadori products. Amino Acids 42, 1143–1150 [DOI] [PubMed] [Google Scholar]
- 49. Richarme G., Mihoub M., Dairou J., Bui L. C., Leger T., and Lamouri A. (2015) Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 290, 1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang A. D., and Agrawal A. F. (2012) DNA repair pathway choice is influenced by the health of Drosophila melanogaster. Genetics 192, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vilchez D., Saez I., and Dillin A. (2014) The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 5, 5659. [DOI] [PubMed] [Google Scholar]
- 52. Tasset I., and Cuervo A. M. (2016) Role of chaperone-mediated autophagy in metabolism. FEBS J. 283, 2403–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li W. W., Li J., and Bao J. K. (2012) Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 69, 1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paumi C. M., Chuk M., Snider J., Stagljar I., and Michaelis S. (2009) ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol. Mol. Biol. Rev. 73, 577–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. César-Razquin A., Snijder B., Frappier-Brinton T., Isserlin R., Gyimesi G., Bai X., Reithmeier R. A., Hepworth D., Hediger M. A., Edwards A. M., and Superti-Furga G. (2015) A call for systematic research on solute carriers. Cell 162, 478–487 [DOI] [PubMed] [Google Scholar]
- 56. Kalapos M. P. (2008) The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 171, 251–271 [DOI] [PubMed] [Google Scholar]
- 57. Lapointe J., and Hekimi S. (2010) When a theory of aging ages badly. Cell. Mol. Life Sci. 67, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams G. C. (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 [Google Scholar]
- 59. Leroi A. M., Bartke A., De Benedictis G., Franceschi C., Gartner A., Gonos E. S., Fedei M. E., Kivisild T., Lee S., Kartaf-Ozer N., Schumacher M., Sikora E., Slagboom E., Tatar M., Yashin A. I., et al. (2005) What evidence is there for the existence of individual genes with antagonistic pleiotropic effects? Mech. Ageing Dev. 126, 421–429 [DOI] [PubMed] [Google Scholar]
- 60. Piedrafita G., Keller M. A., and Ralser M. (2015) The impact of non-enzymatic reactions and enzyme promiscuity on cellular metabolism during (oxidative) stress conditions. Biomolecules 5, 2101–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., and Ratcliffe N. M. (2014) A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 [DOI] [PubMed] [Google Scholar]
- 62. Schneider M., Thoss G., Hübner-Parajsz C., Kientsch-Engel R., Stahl P., and Pischetsrieder M. (2004) Determination of glycated nucleobases in human urine by a new monoclonal antibody specific for N2-carboxyethyl-2′-deoxyguanosine. Chem. Res. Toxicol. 17, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 63. Vanhooren V., Navarrete Santos A., Voutetakis K., Petropoulos I., Libert C., Simm A., Gonos E. S., and Friguet B. (2015) Protein modification and maintenance systems as biomarkers of ageing. Mech. Ageing Dev. 151, 71–84 [DOI] [PubMed] [Google Scholar]
- 64. Stingele J., and Jentsch S. (2015) DNA-protein crosslink repair. Nat. Rev. Mol. Cell Biol. 16, 455–460 [DOI] [PubMed] [Google Scholar]
- 65. Kirkwood T. B., and Austad S. N. (2000) Why do we age? Nature 408, 233–238 [DOI] [PubMed] [Google Scholar]
- 66. Seaver S. M. D., Henry C. S., and Hanson A. D. (2012) Frontiers in metabolic reconstruction and modeling of plant genomes. J. Exp. Bot. 63, 2247–2258 [DOI] [PubMed] [Google Scholar]
- 67. Cheung C. Y. M., Williams T. C. R., Poolman M. G., Fell D. A., Ratcliffe R. G., and Sweetlove L. J. (2013) A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant J. 75, 1050–1061 [DOI] [PubMed] [Google Scholar]
- 68. Bordbar A., Feist A. M., Usaite-Black R., Woodcock J., Palsson B. O., and Famili I. (2011) A multi-tissue type genome-scale metabolic network for analysis of whole-body systems physiology. BMC Syst. Biol. 5, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weber A. L. (2004) Kinetics of organic transformations under mild aqueous conditions: implications for the origin of life and its metabolism. Orig. Life Evol. Biosph. 34, 473–495 [DOI] [PubMed] [Google Scholar]
- 70. Hoffman J. L. (1986) Chromatographic analysis of the chiral and covalent instability of S-adenosyl-l-methionine. Biochemistry 25, 4444–4449 [DOI] [PubMed] [Google Scholar]
- 71. Hamilton W. D. (1966) The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45 [DOI] [PubMed] [Google Scholar]
- 72. Niehaus T. D., Richardson L. G. L., Gidda S. K., ElBadawi-Sidhu M., Meissen J. K., Mullen R. T., Fiehn O., and Hanson A. D. (2014) Plants utilize a highly conserved system for repair of NADH and NADPH hydrates. Plant Physiol. 165, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lei X. G., Zhu J.-H., Cheng W.-H., Bao Y., Ho Y.-S., Reddi A. R., Holmgren A., and Arnér E. S. J. (2016) Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol. Rev. 96, 307–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lang L., Zetterström P., Brännström T., Marklund S. L., Danielsson J., and Oliveberg M. (2015) SOD1 aggregation in ALS mice shows simplistic test tube behavior. Proc. Natl. Acad. Sci. U.S.A. 112, 9878–9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kiser P. D., Golczak M., and Palczewski K. (2014) Chemistry of the retinoid (visual) cycle. Chem. Rev. 114, 194–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Quinn P. G., and Payne A. H. (1985) Steroid product-induced, oxygen-mediated damage of microsomal cenjchrome-P-450 enzymes in Leydig-cell cultures: relationship to desensitization. J. Biol. Chem. 260, 2092–2099 [PubMed] [Google Scholar]
- 77. Hornsby P. J. (1989) Steroid and xenobiotic effects on the adrenal cortex: mediation by oxidative and other mechanisms. Free Rad. Biol. Med. 6, 103–115 [DOI] [PubMed] [Google Scholar]
- 78. Zaidi S. K., Shen W.-J., and Azhar S. (2012) Impact of aging on steroid hormone biosynthesis and secretion. Open Longev. Sci. 6, 1–30 [Google Scholar]
- 79. Dilman V. M., Revskoy S. Y., and Golubev A. G. (1986) Neuroendocrine-ontogenetic mechanism of aging: toward an integrated theory of aging. Int. Rev. Neurobiol. 28, 89–156 [DOI] [PubMed] [Google Scholar]
- 80. Neuhaus J. F. G., Baris O. R., Hess S., Moser N., Schröder H., Chinta S. J., Andersen J. K., Kloppenburg P., and Wiesner R. J. (2014) Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain 137, 354–365 [DOI] [PubMed] [Google Scholar]
- 81. Bisaglia M., Filograna R., Beltramini M., and Bubacco L. (2014) Are dopamine derivatives implicated in the pathogenesis of Parkinson's disease? Ageing Res. Rev. 13, 107–114 [DOI] [PubMed] [Google Scholar]
- 82. Wakamatsu K., Tabuchi K., Ojika M., Zucca F. A., Zecca L., and Ito S. (2015) Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 135, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clewett D. V., Lee T.-H., Greening S., Ponzio A., Margalit E., and Mather M. (2016) Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 37, 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lo M. J., Kau M. M., Cho W. L., and Wang P. S. (2000) Aging effects on the secretion of corticosterone in male rats. J. Investig. Med. 48, 335–342 [PubMed] [Google Scholar]
- 85. Laye M. J., Tran V., Jones D. P., Kapahi P., and Promislow D. E. L. (2015) The effects of age and dietary restriction on the tissue-specific metabolome of Drosophila. Aging Cell 14, 797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jové M., Portero-Otín M., Naudí A., Ferrer I., and Pamplona R. (2014) Metabolomics of human brain aging and age-related neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 73, 640–657 [DOI] [PubMed] [Google Scholar]
- 87. Copes N., Edwards C., Chaput D., Saifee M., Barjuca I., Nelson D., Paraggio A., Saad P., Lipps D., Stevens S. M. Jr., and Bradshaw P. C. (2015) Metabolome and proteome changes with aging in Caenorhabditis elegans. Exp. Gerontol. 72, 67–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Avanesov A. S., Ma S., Pierce K. A., Yim S. H., Lee B. C., Clish C. B., and Gladyshev V. N. (2014) Age- and diet-associated metabolome remodeling characterizes the aging process driven by damage accumulation. eLife 3, e02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma S., Yim S. H., Lee S.-G., Kim E. B., Lee S.-R., Chang K.-T., Buffenstein R., Lewis K. N., Park T. J., Miller R. A., Clish C. B., and Gladyshev V. N. (2015) Organization of the mammalian metabolome according to organ function, lineage specialization, and longevity. Cell Metabolism 22, 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gebauer J., Gentsch C., Mansfeld J., Schmeißer K., Waschina S., Brandes S., Klimmasch L., Zamboni N., Zarse K., Schuster S., Ristow M., Schäuble S., and Kaleta C. (2016) A genome-scale database and reconstruction of Caenorhabditis elegans metabolism. Cell Systems 2, 312–322 [DOI] [PubMed] [Google Scholar]
- 91. Bohler A., Wu G., Kutmon M., Pradhana L. A., Coort S. L., Hanspers K., Haw R., Pico A. R., and Evelo C. T. (2016) Reactome from a WikiPathways perspective. PLoS Comput. Biol. 12, e1004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kaya A., Lobanov A. V., and Gladyshev V. N. (2015) Evidence that mutation accumulation does not cause aging in Saccharomyces cerevisiae. Aging Cell 14, 366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]