Abstract
Sensitization of the heat-activated ion channel transient receptor potential vanilloid 1 (TRPV1) through lipids is a fundamental mechanism during inflammation-induced peripheral sensitization. Leukotriene B4 is a proinflammatory lipid mediator whose role in peripheral nociceptive sensitization is not well understood to date. Two major G-protein-coupled receptors for leukotriene B4 have been identified: the high-affinity receptor BLT1 and the low-affinity receptor BLT2. Transcriptional screening for the expression G-protein-coupled receptors in murine dorsal root ganglia showed that both receptors were among the highest expressed in dorsal root ganglia. Calcium imaging revealed a sensitization of TRPV1-mediated calcium increases in a relative narrow concentration range for leukotriene B4 (100–200 nm). Selective antagonists and neurons from knock-out mice demonstrated a BLT1-dependent sensitization of TRPV1-mediated calcium increases. Accordingly, leukotriene B4-induced thermal hyperalgesia was mediated through BLT1 and TRPV1 as shown using the respective knock-out mice. Importantly, higher leukotriene B4 concentrations (>0.5 μm) and BLT2 agonists abolished sensitization of the TRPV1-mediated calcium increases. Also, BLT2 activation inhibited protein kinase C- and protein kinase A-mediated sensitization processes through the phosphatase calcineurin. Consequently, a selective BLT2-receptor agonist increased thermal and mechanical withdrawal thresholds during zymosan-induced inflammation. In accordance with these data, immunohistochemical analysis showed that both leukotriene B4 receptors were expressed in peripheral sensory neurons. Thus, the data show that the two leukotriene B4 receptors have opposing roles in the sensitization of peripheral sensory neurons forming a self-restricting system.
Keywords: calcineurin, G protein-coupled receptor (GPCR), inflammation, leukotriene, pain
Introduction
Leukotriene B4 (LTB4)3 is well known as a proinflammatory lipid mediator, which causes the recruitment of neutrophils to the site of inflammation and enhances pain sensation (nociception) through the release of pronociceptive mediators by the recruited immune cells (1). LTB4 is generated through lipoxygenase 5 and leukotriene A4-hydrolase (2, 3) and acts mainly through two G-protein-coupled receptors: BLT1 and BLT2 whereby BLT1 has a higher affinity for LTB4 (pKd 9.2) than BLT2 (pKd 7.2) (4–7). The nociceptive effects of LTB4 are attributed mainly to the recruitment of neutrophils (1), causing a long-lasting (several hours) hyperalgesia (8). In contrast, LTB4 reduces the proresolving lipid 18S-resolvin LTB4-induced NF-κB activation, as well as neutrophil infiltration by antagonizing BLT1, thereby limiting inflammation in mice (9, 10).
Ion channels such as the transient receptor potential channels transduce physical (e.g. heat or cold) and chemical (e.g. endogenous lipids) stimuli in the peripheral nociceptive system (11–13). One member of this family, the transient receptor potential vanilloid 1 (TRPV1) ion channel is a key element in peripheral nociceptor sensitization and modulation during inflammation. TRPV1 activity can be sensitized by G-protein-coupled receptors, such as bradykinin or prostaglandin E2 receptors (13, 14), which induce TRPV1-phosphorylation by PKC or PKA, respectively (15, 16). Although activation of PKA and PKC leads to phosphorylation and sensitization of TRPV1, activation of the protein phosphatase 2B (calcineurin) causes dephosphorylation and desensitization of TRPV1 (17, 18). In regard to LTB4, BLT1 activation has been shown to increase nociception (19, 20), and it has been shown that at very high concentrations (EC50 = 11.7 μm), LTB4 may act directly on TRPV1 as a weak agonist (20).
Although the proinflammatory and pronociceptive roles of BLT1 have been studied more intensively, the relevance of the BLT2 receptor in inflammation and especially in nociception is not fully understood. For BLT2, both pro- and anti-inflammatory effects have been described. For example, BLT2 is expressed in colon cryptic cells appears to protect against dextran sodium sulfate-induced colitis, possibly by enhancing barrier function in epithelial cells of the colon (21). Similarly, in the mouse model of ovalbumin-induced allergic airway disease, BLT2 has a protective role (22). In contrast, BLT2-deficient mice have a reduced incidence and severity of disease in autoantibody-induced inflammatory arthritis, including protection from bone and cartilage loss (23).
Here, we describe that BLT1 and BLT2 are coexpressed in peripheral sensory neurons and that low concentrations of LTB4 sensitize TRPV1-mediated intracellular calcium increases through BLT1-induced PKC activation, whereas higher LTB4 concentrations desensitize TRPV1-mediated calcium increases through BLT2-induced calcineurin activation. Fittingly, in vivo selective activation of BLT2 caused strong antinociceptive effects on thermal hyperalgesia and mechanical allodynia.
Results
Low concentrations of LTB4 sensitize TRPV1-mediated calcium increases through BLT1
G-protein-coupled receptors play an important role in the modulation of the peripheral nociceptive system. To investigate which receptors are present in dorsal root ganglia, we performed a semiquantitative mRNA screen for 546 known G-protein-coupled receptors with RNA of DRGs from adult mice. The LTB4 receptors BLT1 and BLT2 were among the most abundant receptors with the 14th and 7th highest mRNA copy numbers, respectively (Table 1, GEO accession number GSE75476).
Table 1.
List of the 30 highest expressed mRNAs of GPCRs in murine DRGs
| Gene | Receptor name | Target copies/ng cDNAa | Ratio (target/Gapdh)b | |
|---|---|---|---|---|
| 1 | PTGER3 | EP3 receptor | 2658.4 | 25.8 |
| 2 | ADRA2C | Adrenoceptor α2C | 2603.7 | 22.1 |
| 3 | GPR88 | G-protein-coupled receptor 88 | 2000.8 | 25.9 |
| 4 | ADRA2A | Adrenoceptor α2A | 1828.4 | 15.4 |
| 5 | GPR153 | G-protein-coupled receptor 153 | 1790.7 | 16.2 |
| 6 | ADRB1 | β1-Adrenoceptor | 1613.9 | 13.6 |
| 7 | BLTR2 | BLT2 | 1257.5 | 11.6 |
| 8 | PTGER1 | EP1 receptor | 823.9 | 8.9 |
| 9 | OXTR | Oxytocin receptor | 812.6 | 7.3 |
| 10 | TAS1R1 | Taste receptor type 1 member 1 | 768.7 | 10.2 |
| 11 | NPFFR1 | Neuropeptide FF receptor 1 | 758.1 | 6.8 |
| 12 | FZD8 | Frizzled class receptor 8 | 758.1 | 10.8 |
| 13 | FZD5 | Frizzled class receptor 5 | 650.9 | 8.7 |
| 14 | BLTR | BLT1 | 642.0 | 5.9 |
| 15 | GPR62 | G-protein-coupled receptor 62 | 615.8 | 5.8 |
| 16 | ADRA1D | α1D-Adrenoceptor | 590.7 | 5.4 |
| 17 | CRHR2 | Corticotropin-releasing hormone receptor 2 | 547.4 | 7.4 |
| 18 | GPR26 | G-protein-coupled receptor 26 | 539.8 | 5.2 |
| 19 | GPR25 | G-protein-coupled receptor 25 | 525.1 | 4.8 |
| 20 | ADRB3 | β3-adrenoceptor | 510.7 | 4.3 |
| 21 | GPR137B | G-protein-coupled receptor 137B | 473.2 | 6.1 |
| 22 | GPR54 | Kisspeptin receptor | 453.9 | 4.3 |
| 23 | LGR6 | Leucine-rich repeat-containing GPCR6 | 450.8 | 4.9 |
| 24 | Vmnr2r54 | Vomeronasal receptor | 447.7 | 4.8 |
| 25 | FZD7 | Frizzled family receptor 7 | 435.4 | 5.8 |
| 26 | HTR6 | 5-Hydroxytryptamine receptor 6 | 429.4 | 4.3 |
| 27 | GPR135 | G-protein-coupled receptor 135 | 429.4 | 5.5 |
| 28 | DRD4 | Dopamine receptor D4 | 414.8 | 3.5 |
| 29 | P2Y4 | Pyrimidinergic receptor P2Y4 | 403.5 | 4.4 |
| 30 | DRD5 | Dopamine receptor D5 | 392.4 | 3.3 |
a Total RNA was isolated from DRGs of untreated adult mice and subjected to semiquantitative RT-PCR. The data are corrected for target copies/ng cDNA.
b The relative expression of GPCR genes expressed as ratio between copy number of target/ng cDNA and copy number of Gapdh/ng cDNA.
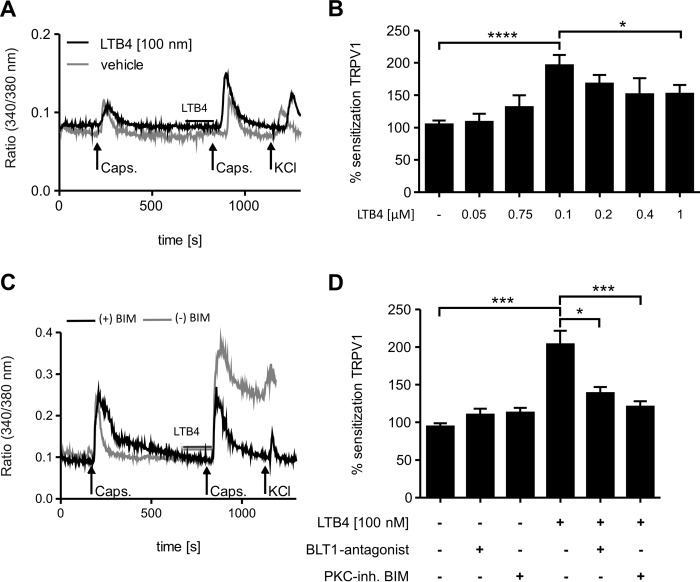
Previously it has been demonstrated in electrophysiological experiments with isolated membrane patches of sensory neurons that LTB4 acts at concentrations >10 μm as a weak agonist for TRPV1 (20). Therefore, we investigated in this study the impact of BLT1 and BLT2 activation on sensitization the ion channel TRPV1 in peripheral sensory neurons. To examine sensitization of TRPV1-mediated responses, calcium imaging experiments were performed with cultured DRG neurons from adult mice. Here, dorsal root ganglia neurons were first stimulated with capsaicin (200 nm) alone, followed by an incubation with LTB4 prior to a second capsaicin stimulation. Sensitization of TRPV1-mediated calcium increases by LTB4 was calculated as the ratio between the second peak (cells treated with LTB4 and capsaicin) and the first peak (cells stimulated with capsaicin alone). The second capsaicin stimulations was induced 10 min after the first to avoid a decrease of the second capsaicin response caused by desensitization of TRPV1 (24). In accordance with a previous report (20), no elevation of intracellular calcium concentrations was observed during the incubation of LTB4 (Fig. 1A). However, preincubation with LTB4 significantly enhanced the capsaicin-stimulated calcium increases reaching a maximum with 100 nm LTB4 (Fig. 1, A and B). Surprisingly, the sensitizing effect disappeared when LTB4 concentrations were further increased (Fig. 1B). To investigate the potential involvement of BLT1 in this sensitization process, we first tested the effect of LTB4 on dorsal root ganglia cultures from BLT1-deficient mice and observed that neither 0.1 nor 1 μm LTB4 was able to sensitize TRPV1-mediated calcium increases in the absence of BLT1 (data not shown). Also, incubation with the BLT1 antagonist U75302 abolished the sensitizing effect of 100 nm LTB4 in dorsal root ganglia cultures from wild-type mice (Fig. 1, C and D). Because BLT1 is known to activate PKC signaling, we hypothesized that LTB4 may sensitize TRPV1 through PKC activation. Fittingly, the PKC inhibitor bisindolylmaleimide (BIM) (Fig. 1, C and D) eliminated the sensitizing effect of LTB4. To test whether or not the sensitizing effect of LTB4 is restricted to TRPV1-expressing neurons, we tested TRPM8-positive neurons, which are an individual TRPV1-negative subgroup of nociceptors (25). Therefore, dorsal root ganglia neurons were stimulated twice with menthol (100 μm) and with 100 nm LTB4 or its vehicle prior to the second menthol stimulus. These two menthol stimuli were followed by a capsaicin stimulus (250 nm, 20 s) to ensure that menthol responders are TRPV1-negative. Interestingly, LTB4 was not able to sensitize TRPM8-mediated calcium increases (data not shown), suggesting that the sensitizing effect is limited to TRPV1-expressing neurons.
Figure 1.
LTB4 sensitizes TRPV1-mediated calcium influx through BLT1. A–D, calcium imaging from DRG culture from C57BL/6N (A and B) or BLT1 knock-out mice (C and D). A, capsaicin (200 nm) stimulates intracellular calcium increases in DRG neurons (black line). Incubation with LTB4 before a second stimulation sensitizes intracellular calcium increases (gray line). Neurons were identified by responses to KCl (50 mm). Shown is a representative trace. B, same as A except that different LTB4 concentrations were used. Sensitization represents the ratio of the second peak by the first peak. The data are presented as means ± S.E. (n = 32–86; one-way ANOVA; Kruskal-Wallis test; Dunn's post hoc test). *, p < 0.05; ****, p < 0.0001. C and D, same as A and B except that DRGs were incubated with the BLT1 antagonist U75302 (1 μm) or the PKC inhibitor BIM (1 μm) before LTB4 treatment. The data are shown as means ± S.E. (n = 45–118). DRGs of three animals were evaluated. Statistics were derived using one-way ANOVA (Kruskal-Wallis test, Dunn's post hoc test). *, p < 0.05; ***, p < 0.001.
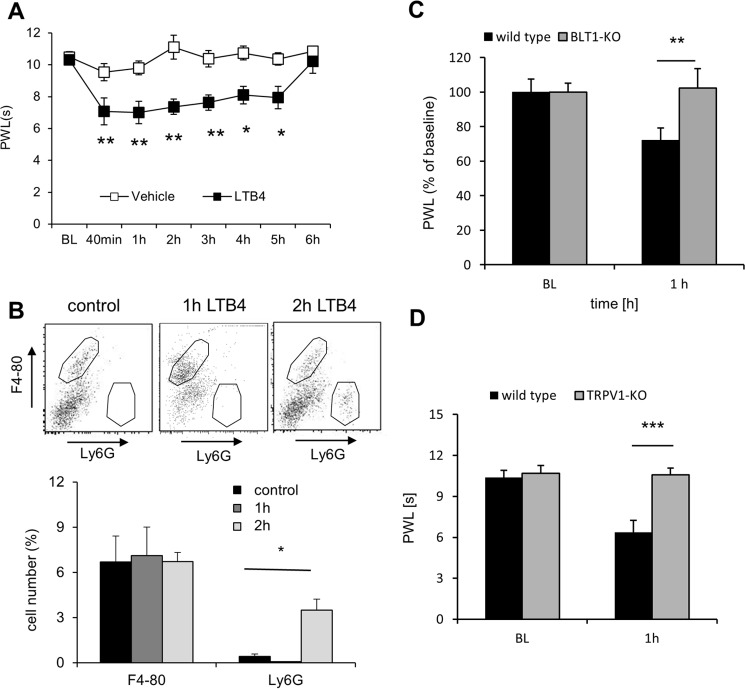
Next, we injected 20 ng of LTB4 in the hind paw of adult mice and determined the thermal withdrawal thresholds in these mice. This dose has previously been shown to be sufficient to evoke a maximal mechanical allodynia in mice (8, 26). Accordingly, we observed a long-lasting decrease of the paw withdrawal thresholds after LTB4 injection (Fig. 2A), which is known to depend on the recruitment of neutrophils (8). Indeed, we found increased neutrophil numbers in the paw 2 h after LTB4 injection (Fig. 2B), whereas the number of macrophages (F4–80+) was not altered. Importantly, 1 h after LTB4 injection, before neutrophil recruitment occurs (Fig. 2B), wild-type but not BLT1-deficient mice reacted to LTB4 injection with a decrease of thermal withdrawal thresholds (Fig. 2C). Likewise, genetic deletion of TRPV1 prevented LTB4-induced thermal hyperalgesia at this early time point (Fig. 2D). Taken together, our data show that LTB4 sensitizes TRPV1-mediated calcium increases in sensory neurons and subsequently thermal hyperalgesia through BLT1.
Figure 2.
BLT1 and TRPV1 mediate LTB4-induced thermal hyperalgesia. A, thermal withdrawal thresholds after injection of 10 μl of LTB4 (20 ng) or vehicle in one hind paw of wild-type mice. The data are shown as means ± S.E. (n = 6; two-way ANOVA; Bonferroni post correction). *, p < 0.05; **, p < 0.01. B, FACS determination of F4–80 (macrophages)- and Ly6G (neutrophils)-positive cells in the paw of untreated wild-type mice and 1 or 2 h after LTB4 injection. The data are shown as means ± S.E. (n = 4–6; one-way ANOVA; Bonferroni post hoc test). *, p < 0.05. C, thermal paw withdrawal latencies before and after injection of 10 μl of LTB4 (20 ng) in one hind paw of wild-type or BLT1 knock-out mice. The data are show as means ± S.E. (n = 7–8; two-way ANOVA; Bonferroni post correction). **, p < 0.01. D, thermal paw withdrawal latencies before and after injection of 10 μl LTB4 (20 ng) in one hind paw of wild-type or TRPV1 knock-out mice. The data are shown as means ± S.E. (n = 6; two-way ANOVA; Bonferroni post correction). ***, p < 0.001. PWL, paw withdrawal latency.
LTB4 desensitizes TRPV1-mediated calcium increases through BLT2
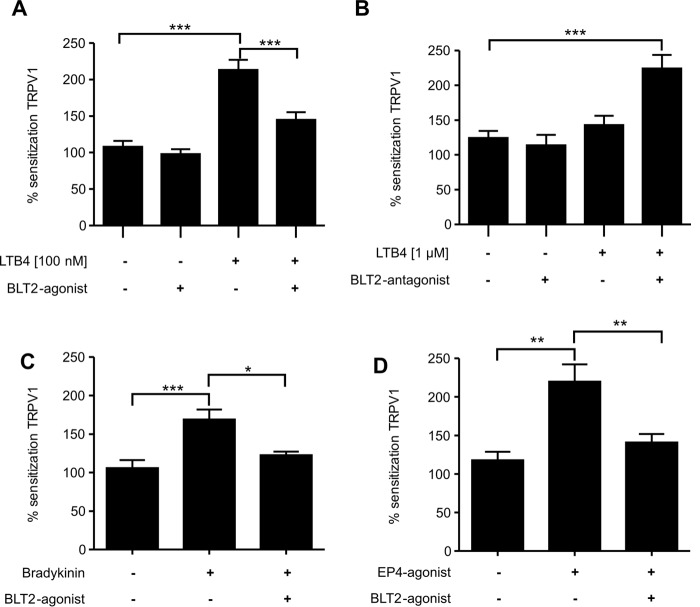
Because LTB4 sensitizes TRPV1-mediated calcium increases only in a very narrow concentration range and BLT1 has a higher affinity to LTB4 as compared with BLT2, we investigated whether or not activation of BLT2 is responsible for the decreased sensitization observed at higher LTB4 concentrations. The selective BLT2 agonist CAY10583 (6) alone had no effect on TRPV1-mediated calcium increases in DRG cultures but abolished the LTB4-induced sensitization of TRPV1-mediated calcium increases (Fig. 3A). Fittingly, blockage of BLT2 by the BLT2 antagonist LY255283 increased sensitization of the TRPV1-mediated calcium increases at high (1 μm) LTB4 concentrations (Fig. 3B). To investigate whether or not BLT2 activation inhibits sensitization produced by other mediators in addition to LTB4, we tested the effect of the BLT2 agonist on the sensitization induced by bradykinin and a prostaglandin E2 receptor 4 (EP4) agonist. Notably, bradykinin induces TRPV1 sensitization through the PLC/PKCϵ pathway, similar to the one used by BLT1 (16). Accordingly, the BLT2-agonist CAY10583 abolished bradykinin-induced TRPV1 sensitization (Fig. 3C). Next, we tested whether or not BLT2 activation also affects PKA-mediated TRPV1 sensitization. Therefore we used EP4 activation, which induces TRPV1 sensitization through the cAMP/PKA pathway (27). Incubation with the selective EP4 receptor agonist ONO-AE-329 (28) induced a robust sensitization of TRPV1-mediated calcium increases, which was abolished in presence of the BLT2 agonist (Fig. 3D).
Figure 3.
Activation of BLT2 causes desensitization of TRPV1-mediated calcium influx. Calcium imaging was performed on DRG cells as in Fig. 1 except that cells were incubated with agonists or antagonists 2 min before LTB4 application. A, LTB4-induced (100 nm) TRPV1 sensitization in absence and presence of the BLT-2 agonist CAY10583 (400 nm). The data are shown as means ± S.E. (n = 50–159; one-way ANOVA; Kruskal-Wallis test; Dunn's multiple comparisons test). ***, p < 0.001. B, BLT2 inhibition increases TRPV1 desensitization induced by high dose of LTB4 (1 μm). LTB4-induced (1 μm) TRPV1 desensitization in absence or presence of the BLT2 antagonist LY255283 (10 μm). The data are shown as means ± S.E. (n = 26–93; one-way ANOVA; Kruskal-Wallis test; Dunn's multiple comparisons test). ***, p < 0.001. C and D, BLT2 activation suppresses TRPV1 sensitization induced by different signaling pathways. Capsaicin-induced TRPV1 activation was sensitized using bradykinin (0.5 μm) (C) or the EP4 agonist ONO-AE-329 (500 nm) (D) in the absence or presence of the BLT2 agonist CAY10583 (400 nm). The data are shown as means ± S.E. (C, n = 47–87; D, n = 52–109; one-way ANOVA; Kruskal-Wallis test, Dunn's multiple comparisons test). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
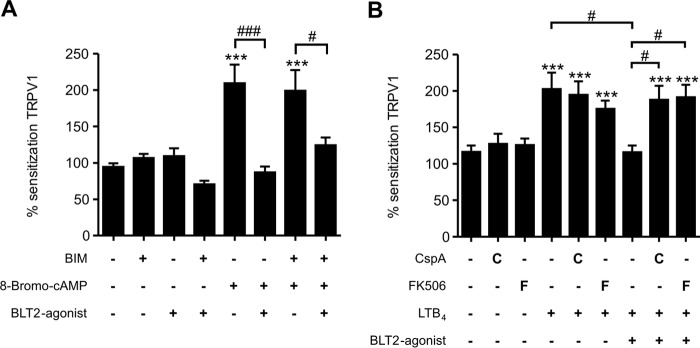
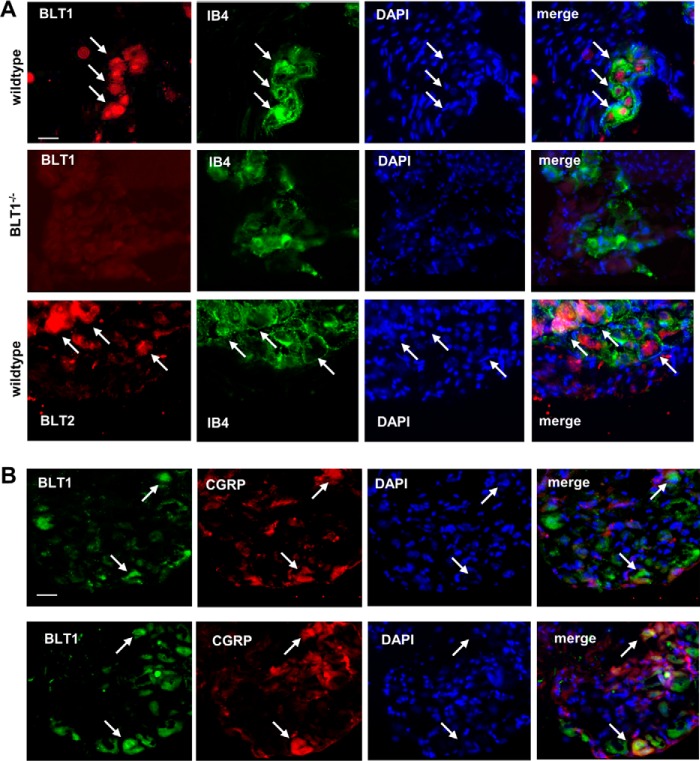
To investigate whether or not BLT2 desensitizes TRPV1-mediated calcium increases through the PKC pathway, we sensitized TRPV1 using 8-bromo-cAMP and found that PKC inhibition did not affect BLT2-induced desensitization of the TRPV1-mediated calcium increases (Fig. 4A). Notably, PKA- and PKC-mediated TRPV1 sensitization is based on TRPV1 phosphorylation (29), which can be reversed by protein phosphatase 2B (calcineurin) (17). Because of the unselective effect of BLT2 activation on PKA- and PKC-mediated TRPV1 sensitization, we hypothesized that the effect of BLT2 activation might be mediated by calcineurin. Therefore, we sensitized TRPV1-mediated calcium influx with a low LTB4 concentration (100 nm) in the absence or presence of the protein-phosphatase 2B inhibitors cyclosporin A and FK506 (tacrolimus) (30, 31). Both inhibitors were used at concentrations that had no effect on LTB4-induced sensitization of TRPV1-mediated calcium increases by itself. Both inhibitors completely abolished the BLT2-mediated desensitization (Fig. 4B), which strongly suggests that calcineurin mediates the BLT2-induced desensitization of the TRPV1-mediated calcium increase. Next, we tested whether or not BLT1 and BLT2 are expressed in sensory neurons in dorsal root ganglia. We found that BLT1 and BLT2 were expressed in CGRP- and IB4-positive cells, which represent peptidergic and non-peptidergic sensory neurons, respectively (Fig. 5, A and B).
Figure 4.
BLT2-mediated desensitization of TRPV1-mediated calcium influx is calcineurin dependent. A, BLT2 activation suppresses PKA-dependent TRPV1 sensitization by 8-bromo-cAMP independently of PKC. Capsaicin-induced TRPV1 activation was sensitized using 8-bromo-cAMP (20 μm) with or without preincubation with the PKC inhibitor BIM (1 μm) and/or the BLT2 agonist CAY10583 (400 nm). The data are shown as means ± S.E. (n = 24–73; one-way ANOVA; Kruskal-Wallis test; Dunn's multiple comparisons test). ***, p < 0.001 (comparison with vehicle); ###, p < 0.001 (comparison between conditions); #, p < 0.05 (comparison between conditions). B, capsaicin-induced TRPV1 activation was sensitized using LTB4 (100 nm) with or without preincubation with the BLT2 agonist CAY10583 (400 nm) and/or the calcineurin inhibitors cyclosporin A (CspA, 100 nm) and FK506 (10 nm). The data are shown as means ± S.E. (n = 53–123; one-way ANOVA; Kruskal-Wallis test; Dunn's multiple comparisons test). ***, p < 0.001 (comparison to vehicle); #, p < 0.05 (comparison between treatments).
Figure 5.
BLT1 and BLT2 are expressed in sensory neurons. A and B, representative images showing the colocalization of BLT1 and BLT2 with IB4 (A) and CGRP (B) in dorsal root ganglia. The white bar represents 30 μm. Arrows depict cells expressing LTB4 receptors.
BLT2 activation decreases zymosan-induced thermal hyperalgesia and mechanical allodynia
In the next step we tested whether or not BLT2 activation is able to decrease nociception. We found that intraplantar application of the BLT2 agonist CAY10583 significantly increased thermal (Fig. 6A) but not mechanical paw withdrawal latencies (Fig. 6B). In accordance with the proposed TRPV1-desensitizing mechanism, we observed no effect of the BLT2 agonist on thermal withdrawal latencies in TRPV1-deficient mice (data not shown).
Figure 6.
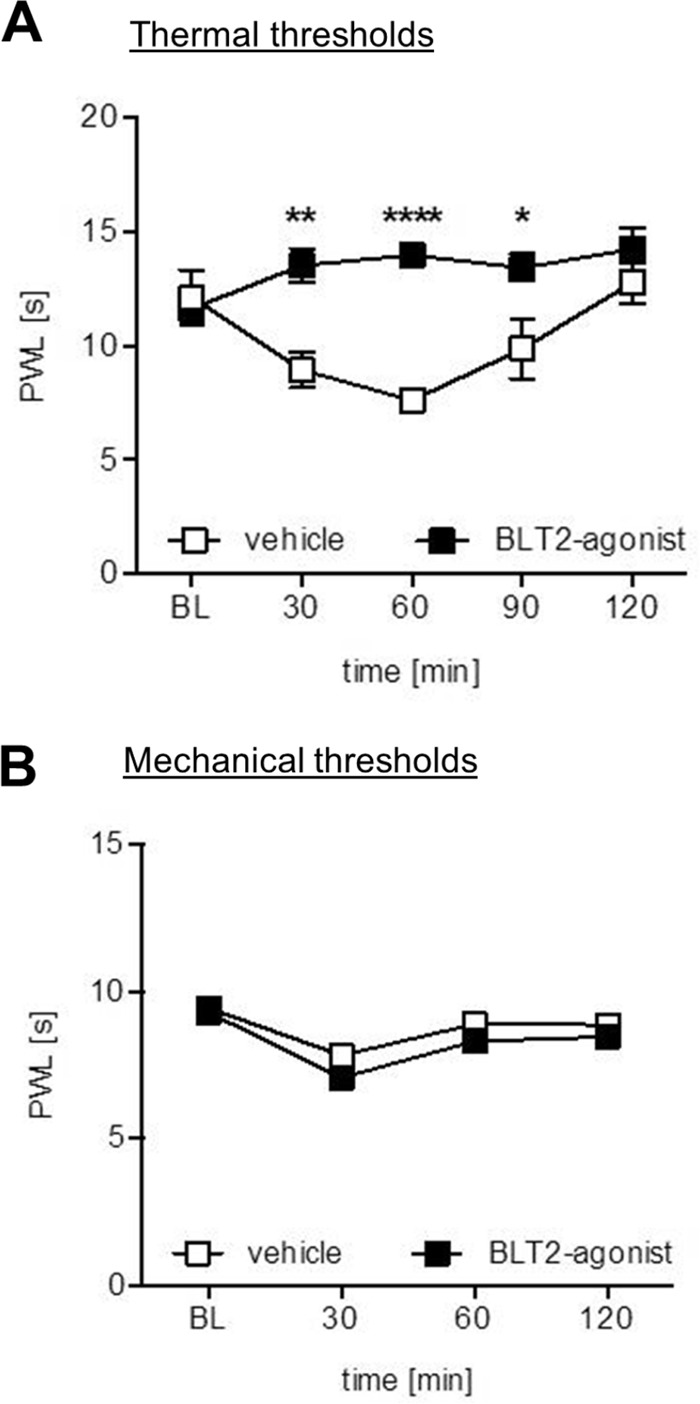
BLT2 activation decreases basal thermal hyperalgesia. A, thermal withdrawal thresholds after intraplantar injection of 10 μl of BLT2 agonist Cay10583 (5 μm) or vehicle (0.9% NaCl) in wild-type mice. The data are shown as means ± S.E. (n = 6; two-way ANOVA; post t test with Bonferroni correction). *, p < 0.05; **, p < 0.01; ***, p < 0.001. B, mechanical withdrawal thresholds after intraplantar injection of 10 μl of BLT2 agonist Cay10583 (5 μm) or vehicle (0.9% NaCl) in wild-type mice. The data are shown as means ± S.E. (n = 6; two-way ANOVA; post t test with Bonferroni correction). No significant differences were detected between treatments.
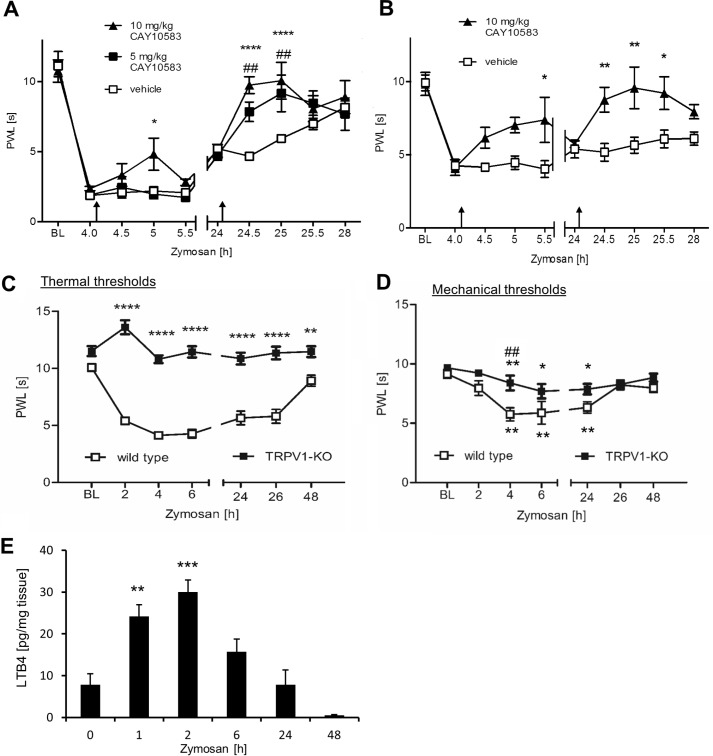
Next, the effect of the BLT2 agonist CAY10583 on nociceptive behavior during peripheral inflammation was tested. Zymosan injection decreases thermal and mechanical withdrawal thresholds within 2–4 h. Afterward paw withdrawal thresholds returned gradually over the following 48 h to baseline level (Fig. 7A). We administered CAY10583 intraperitoneally 4 and 24 h after the zymosan injection and found that 5 and 10 mg/kg of CAY10583 significantly reduced thermal (Fig. 7A) and mechanical paw withdrawal latencies (Fig. 7B). Notably, TRPV1-mediated mechanical pain during inflammation involves indirect mechanisms such as mechanical stimulation-induced release of mediators from epithelial cells that subsequently act on nociceptors also activating TRPV1 (14). Accordingly, TRPV1-deficient mice did not exhibit zymosan-induced thermal hyperalgesia and had a reduced mechanical allodynia (Fig. 7, C and D). In accordance with our data showing that the BLT2-agonist reduces sensitization of TRPV1-mediated calcium increases, injection of the BLT2-agonist had no significant effect on zymosan-induced mechanical allodynia in TRPV1 knock-out mice (data not shown). Finally, we determined the endogenous LTB4 concentrations in the paw at the different time points after zymosan injection via LC-MS/MS. We found significantly increased LTB4 level early (1 and 2 h) after zymosan injection, which went back to baseline level 24 h after zymosan injection (Fig. 7E). Thus, the slightly stronger antinociceptive effect of the BLT2 agonist may be based on the low level of endogenous LTB4 in the paw at this time point. Taken together, the data show that BLT2 activation is able to decrease with a high efficacy nociceptive behavior during inflammatory pain.
Figure 7.
BLT2 activation decreases zymosan-induced thermal hyperalgesia and mechanical allodynia. A, thermal thresholds after injection of 20 μl of zymosan (3 mg/ml) in one hind paw of wild-type mice. 100 μl of CAY10582 or vehicle was administered intraperitoneally at the indicated times (black arrows). The data are shows as means ± S.E. (n = 6; two-way ANOVA; Bonferroni post correction). *, p < 0.05 (comparison to vehicle); ****, p < 0.0001 (comparison to vehicle); ##, p < 0.01 (comparison between treatments). B, same as A except that mechanical paw withdrawal latencies were determined. The data are shown as means ± S.E. (n = 6; two-way ANOVA; Bonferroni post correction). *, p < 0.05; **, p < 0.01. C, thermal thresholds after injection of 20 μl of zymosan (3 mg/ml) in one hind paw of wild-type or TRPV1 knock-out mice. The data are shows as means ± S.E. (n = 6; two-way ANOVA; Bonferroni post correction). **, p < 0.01; ****, p < 0.0001. D, same as C except that mechanical thresholds were determined. Thresholds within the genotypes were compared with baseline levels using one-way ANOVA and Bonferroni post correction. *, p < 0.05; **, p < 0.001. Thresholds between the groups were compared using two-way ANOVA and Bonferroni post correction. ##, p < 0.001. E, LTB4 level in untreated or zymosan-injected paws of wild-type mice. The data are shown as means ± S.E. (n = 5; one-way ANOVA; Bonferroni post hoc test). **, p < 0.01; ***, p < 0.001. PWL, paw withdrawal latency.
Discussion
LTB4 is a common proinflammatory mediator that is released mainly by neutrophils at the site of inflammation. It is not only a powerful chemoattractant for neutrophils but has also reported to be able to sensitize nociceptors, thereby increasing nociception. In this regard it has been shown that C-polymodal nociceptors and C-mechanoheat nociceptors are sensitized by LTB4 (32). As consequence LTB4 lowers the average heat threshold from 45 °C by ∼10 °C and decreases mechanical thresholds by almost 80% (32). BLT2 activation increased thermal but not mechanical thresholds in naïve mice. In contrast BLT2 activation was able to increase both thermal and mechanical withdrawal latencies during zymosan-induced inflammation. The broader response during inflammation is not surprising, because sensitization of nociceptors during an inflammation and stimulation of non-neuronal cells allows certain afferents to acquire responsiveness to mechanical stimuli to which they are, under naïve conditions, insensitive (14, 33, 34).
Although most of the proinflammatory effects of LTB4 are based on BLT1 activation, the previously known pronociceptive effects of LTB4 were thought to be due to a weak agonism of TRPV1, which occurs at relative high concentrations (EC50 = 11.7 μm) (20). Here, we found that 100-fold lower LTB4 concentrations (100 nm) sensitize TRPV1-mediated calcium increases through BLT1 and that, more surprisingly, higher LTB4 concentrations (0.4–1 μm) desensitize the TRPV1-mediated calcium influx through BLT2. Because BLT2-induced desensitization is mediated by the phosphatase calcineurin, BLT2 activation can inhibit not only BLT1-mediated sensitization but also other sensitization pathways (EP4 agonist or bradykinin) mediated by either PKA or PKC. This property makes BLT2 an interesting target for the development of novel analgesics, which aim to inhibit TRPV1-mediated pain sensations. Because of the prominent role of TRPV1 in pain perception, various direct TRPV1 inhibitors have been developed over the last years as potential analgesics. Unfortunately, so far TRPV1 inhibitors are tainted by their side effects of inducing hyperthermia (35). Because BLT2 activation is only reversing the sensitizing effects of pronociceptive inhibitors without altering basal TRPV1 activity, BLT2 agonists would circumvent this side effect.
BLT2 is highly expressed in the small intestine, colon, spleen, liver, and pancreas, as well as on T-cells, mast cells, peripheral sensory neurons, and keratinocytes (5, 6, 36, 37). Most of its functions in these tissues and cells are to date unknown or not fully understood, and BLT2 knock-outs appear as phenotypically normal. Interestingly, it was previously shown that BLT2 expression in CD4 T-cells has a protective role in allergic airway inflammation reducing the pathophysiology of asthma (22). Likewise, BLT2 is expressed in colon cryptic cells and appears to protect against dextran sodium sulfate-induced colitis, possibly by enhancing barrier function in epithelial cells of the colon (21). Thus, selective activation of BLT2 by agonists might produce, in addition to analgesia, additional beneficial effects in certain pathophysiological settings.
The fact that LTB4 concentration-dependently induces opposing effects through its different receptors is not an unusual concept in neurobiology. In this regard, it was shown that long-term potentiation is inhibited by dopamine receptor 1 receptors (49, 50) but reduced by D2 receptors (51, 52). Similarly, the neurotransmitter GABA has both inhibitory and stimulating effects on short-term memory. Low doses of GABA strongly inhibit reinforced memory seemingly activating GABACreceptors, whereas higher doses enhance weakly reinforced memory via GABAA receptors (38). Finally, in midbrain dorsal periaqueductal gray neurons, anandamide can either facilitate or inhibit excitatory transmission through TRPV1 or cannabinoid receptor 1, respectively (39). Accordingly, anandamide induces both panicolytic and panicogenic effects in rats via its actions at cannabinoid receptor 1 and TRPV1, respectively (37).
In the nociceptive system, P2Y receptors (40) and serotonergic signaling (41) are examples for such antagonistic responses to the same mediator. So are P2Y1, P2Y12, P2Y13, and P2Y14 receptors expressed in neurons of mouse dorsal root ganglia (42) that can act either pronociceptively (P2Y1) or antinociceptively (P2Y12, P2Y13, and P2Y14) (40).
However, LTB4 receptors are expressed in the same sensory neurons and have opposing roles in the regulation of TRPV1-mediated calcium increases. In the above mentioned examples for self-restricting signaling pathways in neurons, they serve to limit the consequences of an excessive activation of the affected signal pathway. In case of anandamide it prevents overshooting anxiety and panicogenic behavior, whereas in case of dopamine and GABA receptors, it restricts long-term potentiation development and, subsequently, modulates short term memory. For P2Y receptors it is believed that under normal conditions their opposing effects are balanced and that ADP increases during an inflammation shift this balance toward the pronociceptive effects (40). Considering these findings, the antagonism of the two LTB4 receptors may serve to restrict the input of a single pronociceptive mediator in nociceptor sensitization. This effect would be especially important for mediators such as LTB4 that are synthesized temporarily in high amounts to allow neutrophil recruitment to inflammation sites. These peak LTB4 concentrations could produce through BLT1 an excessive sensitization of peripheral sensory neurons that is limited or even reversed by activation of BLT2.
Experimental procedures
Animals
BLT1 and TRPV1 knock-out mice on C57BL/6J background were supplied by The Jackson Laboratory. Wild-type mice were supplied by Janvier (Le Genest, France). All mice were compared with age- and sex-matched wild-type mice. The animals were cared for corresponding to International Association for the Study of Pain (IASP) guidelines. For all experiments the ethics guidelines for investigations in conscious animals were obeyed, and the procedures were approved by the local ethics committee.
Reagents
LTB4, CAY10583, U75302, and LY255283 were from Cayman Chemicals (Ann Arbor, MI). Capsaicin was from Sigma-Aldrich, and ONO-AE-329 was a kind gift from Prof. R.M. Nüsing (Hospital of the Goethe University, Frankfurt, Germany). BIM, 8-bromo-cAMP, and cyclosporin A were purchased from Biomol (Hamburg, Germany); zymosan A was from Sigma-Aldrich; and bradykinin was from Tocris (Bristol, UK). Anti-BLT1 and anti-BLT2 antibodies were from Hölzel Diagnostika (Köln, Germany), anti-TRPV1 antibody was from Alomone Labs, FITC-labeled IB4 was from Sigma-Aldrich, and anti-cGRP antibody was from Alpha Diagnostics International Inc.
GPCR expression analysis
Dorsal root ganglia were dissected from adult mice on both sides of the intervertebral foramina from the segments S1 up to C3 and frozen immediately. RNA isolation and transcription were performed by using the RNeasy kit (Qiagen) according to the manufacturer's instructions. A total of 10 μg of RNA was isolated from 10 mice, pooled, and used as template for semiquantitative RT-PCR of 546 murine GPCRs as described previously (43). Semiquantitative RT-PCR was done by using primers designed with the Roche's online tool (Table 2), and quantification and data analysis were performed by using the LightCycler 480 Probe Master System (Roche). 25 ng of cDNA/reaction were used to generate Ct values. 6 ng of genomic DNA was used with the same primer pair as standard to calculate gene copy number per nanogram RNA. The housekeeping gene (Gapdh) was used as a control for cDNA preparation and PCRs. To show the relative abundance of each GPCR, relative expression values were calculated by taking into account the copy number of Gapdh/ng and expressed as ratio between copy number of target gene and of Gapdh (Table 1).
Table 2.
List of primers for GPCRs named in Table I
| Gene | Forward primer | Reverse primer |
|---|---|---|
| PTGER3 | GAAGTCTTTCCTGCTGTGCAT | AGGAGCTGCCCCACTAAGTC |
| ADRA2C | aagcggtagagtacaacctgaag | agcggagatgagccacac |
| GPR88 | GCCACCGCAAGTCCACTA | CGATGCCCAGGTAGCAGT |
| ADRA2A | ggtgacactgacgctggtt | gcacgttgccaaatactgtg |
| GPR153 | CGGTTCTCCCACGATGAT | CCAGAGCTGCCAAGTCCT |
| ADRB1 | catcatgggtgtgttcacg | ggaaagccttcaccacgtt |
| BLTR2 | CGCCTGGGAATGAGACAC | GCCGCCAGCAGTAGAAAG |
| VIPR2 | cagtgtgaactgaaaagaagatgg | agctgtgcagccggtagt |
| OXTR | CATCACCTTCCGCTTCTACG | CCACCTGCAAGTATTTGACCA |
| TAS1R1 | gcttcctcatgctgggttc | tcccgaagaagctgtagagg |
| NPFFR1 | TGCTGCTGCTCATCGACTAC | AGAAGGCCAGCCAGTGTG |
| FZD8 | gtagcatgtacacgcccatc | gttcacacacagagcgacaag |
| FZD5 | TACTGGGTGCTCATGCTCAA | ATCCAGACTCCCGACGTG |
| BLTR | ctgctggtgctgaacttgg | gaggaaaaagggagcagtga |
| GPR62 | GGCGGTTCTCGTAGAGGTG | CTAAGTAGAAGGCATCTTGAAGGTC |
| ADRA1D | actgagtgcagctgtgttgc | gaaggcccagaagcctagaa |
| CRHR2 | AACACGACCTTGGACCAGAT | GGGGCACGGTCTCTCTACTA |
| GPR26 | gctgctggtgggcactat | caggaggcagagcagcac |
| GPR25 | CGAACCCTGGAGTCCTAGC | GGCACAACTCCACTTGGTC |
| ADRB3 | ctgctagcatcgagaccttgt | aagggttggtgacagctagg |
| GPR137B | GTGGCAGCCAACTCGTTC | AGACACACGGGGAAGCAGTA |
| GPR54 | agtgcgcaccaaggtctc | gcttgaagcaccaggaaca |
| LGR6 | TGTCAAGTCAGTCCTTCTGGTG | CAGGTAGAGCAGTGGGTTGAG |
| Vmn2r54 | ttacataaacagggtgatgagagg | agcaggaaaaacagggtgag |
| FZD7 | CGTCTTCAGCGTGCTCTACA | TCATAAAAGTAGCAGGCCAACA |
| HTR6 | acgtcggacctgatggtg | catacagcgcgttcagca |
| GPR135 | CCGTGCTCATCATGATTATCTTT | GCACCAGGAAGCAGTAGGG |
Primary mouse dorsal root ganglia cultures
As previously described (24, 44) murine dorsal root ganglia were dissected on both sides of the intervertebral foramina from the segments S1 up to C3 and immediately stored in ice-cold Hanks' balanced salt solution containing Ca2+ and Mg2+. Afterward, the dorsal root ganglia were incubated with collagenase/dispase (500 units/ml collagenase (Biochrom AG, Berlin, Germany); 2.5 units/ml dispase (Roche) in neurobasal medium containing penicillin (100 units/ml), and streptomycin (100 g/ml), (all from Invitrogen) at 37 °C for 75 min. Dorsal root ganglia were washed two times with neurobasal medium containing 10% FCS and incubated with 0.05% trypsin/EDTA for 10 min. Washes were repeated twice, and the tissues were dissociated with a 1-ml Gilson pipette in 300 μl of neurobasal medium with 10% FCS, penicillin (100 units/ml), and streptomycin (100 μg/ml). 25 μl of the cell suspension was seeded on poly-l-lysine-coated coverslips and incubated for 2 h, before the addition of 1 ml of neurobasal medium containing l-glutamine (2 mm), B-27 (all from Invitrogen), and gentamicin (50 μg/ml) (BioWhittaker Europe, Verviers, Belgium).
Calcium-imaging experiments
Experiments were done 24 h after DRG cell preparation. The cells were loaded with Fura-2-AM for 45 min with medium containing 5 μm Fura-2-AM, 0.002% pluronic F-127 (both Biotinum). For determination of TRPV1 sensitization, the cells were stimulated twice with 200 nm capsaicin in Hanks' balanced salt solution (145 mm NaCl, 1.25 mm CaCl2, 1 mm MgCl2, 5 mm KCl, 10 mm d-glucose, and 10 mm HEPES, adjusted to pH 7.3, all from Sigma-Aldrich). For calcium imaging a Leica calcium-imaging setup, composed of a Leica DMI 4000 microscope equipped with a DFC360 FX (CCD) camera, Fura-2 filters, and an N-Plan 10/0.25 Ph1 objective, was used as previously described (24, 44). For recordings and calculation, the LAS AF software (Leica) was used. Sensitization of TRPV1-mediated calcium increases by LTB4 is expressed as the ratio of the second peak (cells treated with LTB4 and capsaicin) by the first peak (cells stimulated with capsaicin alone). Treatments with inhibitors, agonists, or antagonists were performed for 2 min before the second capsaicin stimulation, except for BIM, which was incubated for 45 min prior the experiment.
Behavioral tests
The experimenter was unaware of the treatments or the genotypes during all behavioral experiments. Mechanical withdrawal thresholds of the plantar side of a hind paw were determined using a plantar aesthesiometer (Dynamic Plantar Aesthesiometer, Ugo Basile). A steel rod (20-mm diameter) was pushed against the paw with ascending force until a strong and immediate withdrawal occurred. Thermal withdrawal thresholds were determined as described previously (45). The paw withdrawal latency was taken to be the mean of at least four consecutive trials with at least 20 s in between (24, 46). Peripheral inflammation was induced by subcutaneous injection of 20 μl of 3 mg/ml zymosan into the plantar side of one hind paw (47). For animal experiments (but not for other experiments), the BLT2 agonist CAY10583 was suspended in a solution of 1% methylcellulose and 1% soybean lecithin (both from Sigma-Aldrich) to avoid high concentrations of DMSO.
FACS analysis and sorting
The cells were isolated from zymosan-induced edemas and prepared for FACS analysis as previously described (48). FACS analysis was performed on a FACS Canto flow cytometer (Becton Dickinson). The cells were stained with PE-labeled anti-F4/80 (Biolegend) and FITC-labeled anti-Ly6G (eBiosciences) antibodies.
Liquid chromatography-tandem mass spectrometry
LC-MS/MS analysis of LTB4 from paws was performed as described previously (26). Paws were prepared at different time points after zymosan injection paws. Standard stock solutions with 2500 ng/ml of LTB4 and internal standard with 25 mg/ml of LTB4-d4 were prepared in methanol. Working standards were obtained by further dilution with a final concentration range of 0.1-250 ng/ml. Sample treatment was performed using liquid-liquid extraction. Therefore, homogenized tissue was extracted twice with 600 μl of ethyl acetate. The combined organic phases were removed at a temperature of 45 °C under a gentle stream of nitrogen. The residues were reconstituted with 50 μl of methanol/water/butylated hydroxytoluene (50:50:0.1, v/v/v), centrifuged for 2 min at 10,000 × g, and stored in glass vials.
The LC-MS/MS system consisted of a QTrap 5500 (AB Sciex, Darmstadt, Germany) equipped with a Turbo-V source operating in negative electrospray ionization mode, an Agilent 1200 binary HPLC pump and degasser (Agilent, Waldbronn, Germany), and an HTC Pal autosampler (CTC Analytics, Zwingen, Switzerland). High-purity nitrogen for the mass spectrometer was produced by a NGM 22-LC-MS nitrogen generator (CMC Instruments, Eschborn, Germany). For the chromatographic separation a Gemini NX C18 column and precolumn was used (150 × 2-mm inner diameter, 5-μm particle size, and 110 Å pore size; Phenomenex, Aschaffenburg, Germany). A linear gradient was used at a flow rate of 0.5 ml/min with a total run time of 17.5 min. Mobile phase A consists of water:ammonia (100:0.05, v/v), and mobile phase B consists of acetonitrile:ammonia (100:0.05, v/v). The gradient changed from 85% A to 10% within 12 min. These conditions were held for 1 min. Then the mobile phase shifted back to 85% A within 0.5 min, and it was maintained for 4 min to re-equilibrate the column. 20 μl of the extracted samples were injected into the LC-MS/MS system. Quantification was performed with Analyst software version 1.5 (AB Sciex) using the internal standard method (isotope-dilution mass spectrometry). Ratios of analyte peak area and internal standard area (y axis) were plotted against concentration (x axis), and calibration curves were calculated by least-squares regression with 1/concentration2 weighting.
Immunohistology
DRG sections were fixed for 10 min in 4% paraformaldehyde in PBS, permeabilized for 10 min with 0.1% Triton in PBS, blocked with 3% BSA in PBS for 1 h at room temperature, and incubated with antibodies against BLT1 or BLT2 (Bioss) and CGRP (Abcam) or FITC-labeled lectin IB4 (Sigma-Aldrich) followed by a AF488 or Cy5-labeled secondary antibody (Sigma-Aldrich).
Statistics
All statistics were calculated with GraphPad Prism 6 software (GraphPad Software, Inc.). Gaussian distribution was tested with D'Agostino-Pearson normality test. Differences between two independent groups were tested with Student's t test in case of Gaussian distribution. To compare more than two groups in repeated measurements, a one-way ANOVA was performed. For nonparametric testing of more than two groups, the Kruskal-Wallis test with the Dunn's multiple comparisons test was done. Results from behavior experiments were tested with a two-way ANOVA with Bonferroni post-test correction.
Author contributions
All authors were involved in planning experiments and discussion and interpretation of the data and critically revised and approved the manuscript. S. Z., A. L., and M. S. performed calcium imaging experiments; M. S., K. K., E.-M. T., J. S., H. J., and N. D. performed behavioral experiments; S. T. and S. O. did the GPCR expression screen; S. P. and J. S. performed FACS analysis; S. P. and K. S. did MELC analyses; C. A. and N. F. did LTB4 measurements; G. G. and K. S. designed the experiments; and S. Z., S. P., and K. S. wrote the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB1039-TP04, SFB1039-TP08, SFB1039-TP09, SFB1039-Z01, and SCHO817. This research work was also supported by the research funding program Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz of the State of Hessen, Research Center for Translational Medicine and Pharmacology TMP, by funds from the Else Kröner-Fresenius-Foundation as part of the Else Kröner graduate school (to E.-M. T.), and by the Else Kröner-Fresenius-Foundation Research Training Group Translational Research Innovation-Pharma (to S. Z. and K. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- LTB4
- leukotriene B4
- BLT
- LTB4 receptor
- DRG
- dorsal root ganglia
- GPCR
- G-protein-coupled receptors
- TRPV
- transient receptor potential vanilloid receptor 1
- BIM
- bisindolylmaleimide
- ANOVA
- analysis of variance.
References
- 1. Yokomizo T. (2011) Leukotriene B4 receptors: novel roles in immunological regulations. Adv. Enzyme Regul. 51, 59–64 [DOI] [PubMed] [Google Scholar]
- 2. Horn T., Adel S., Schumann R., Sur S., Kakularam K. R., Polamarasetty A., Redanna P., Kuhn H., and Heydeck D. (2015) Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signaling. Prog. Lipid Res. 57, 13–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert N. C., Bartlett S. G., Waight M. T., Neau D. B., Boeglin W. E., Brash A. R., and Newcomer M. E. (2011) The structure of human 5-lipoxygenase. Science 331, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yokomizo T., Izumi T., Chang K., Takuwa Y., and Shimizu T. (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387, 620–624 [DOI] [PubMed] [Google Scholar]
- 5. Yokomizo T., Kato K., Terawaki K., Izumi T., and Shimizu T. (2000) A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192, 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iizuka Y., Yokomizo T., Terawaki K., Komine M., Tamaki K., and Shimizu T. (2005) Characterization of a mouse second leukotriene B4 receptor, mBLT2: BLT2-dependent ERK activation and cell migration of primary mouse keratinocytes. J. Biol. Chem. 280, 24816–24823 [DOI] [PubMed] [Google Scholar]
- 7. Huang W. W., Garcia-Zepeda E. A., Sauty A., Oettgen H. C., Rothenberg M. E., and Luster A. D. (1998) Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J. Exp. Med. 188, 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine J. D., Lau W., Kwiat G., and Goetzl E. J. (1984) Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science 225, 743–745 [DOI] [PubMed] [Google Scholar]
- 9. Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., and Serhan C. N. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 [DOI] [PubMed] [Google Scholar]
- 10. Oh S. F., Pillai P. S., Recchiuti A., Yang R., and Serhan C. N. (2011) Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hucho T., and Levine J. D. (2007) Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55, 365–376 [DOI] [PubMed] [Google Scholar]
- 12. Patapoutian A., Tate S., and Woolf C. J. (2009) Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 8, 55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sisignano M., Bennett D. L., Geisslinger G., and Scholich K. (2014) TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog. Lipid Res. 53, 93–107 [DOI] [PubMed] [Google Scholar]
- 14. Gold M. S., and Gebhart G. F. (2010) Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grace M., Birrell M. A., Dubuis E., Maher S. A., and Belvisi M. G. (2012) Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67, 891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cesare P., and McNaughton P. (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. U.S.A. 93, 15435–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohapatra D. P., and Nau C. (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 280, 13424–13432 [DOI] [PubMed] [Google Scholar]
- 18. Zhang X., Li L., and McNaughton P. A. (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59, 450–461 [DOI] [PubMed] [Google Scholar]
- 19. Asahara M., Ito N., Yokomizo T., Nakamura M., Shimizu T., and Yamada Y. (2015) The absence of the leukotriene B4 receptor BLT1 attenuates peripheral inflammation and spinal nociceptive processing following intraplantar formalin injury. Mol. Pain 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hwang S. W., Cho H., Kwak J., Lee S. Y., Kang C. J., Jung J., Cho S., Min K. H., Suh Y. G., Kim D., and Oh U. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 97, 6155–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iizuka Y., Okuno T., Saeki K., Uozaki H., Okada S., Misaka T., Sato T., Toh H., Fukayama M., Takeda N., Kita Y., Shimizu T., Nakamura M., and Yokomizo T. (2010) Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory colitis. FASEB J. 24, 4678–4690 [DOI] [PubMed] [Google Scholar]
- 22. Matsunaga Y., Fukuyama S., Okuno T., Sasaki F., Matsunobu T., Asai Y., Matsumoto K., Saeki K., Oike M., Sadamura Y., Machida K., Nakanishi Y., Kubo M., Yokomizo T., and Inoue H. (2013) Leukotriene B4 receptor BLT2 negatively regulates allergic airway eosinophilia. FASEB J. 27, 3306–3314 [DOI] [PubMed] [Google Scholar]
- 23. Mathis S. P., Jala V. R., Lee D. M., and Haribabu B. (2010) Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J. Immunol. 185, 3049–3056 [DOI] [PubMed] [Google Scholar]
- 24. Holland S., Coste O., Zhang D. D., Pierre S. C., Geisslinger G., and Scholich K. (2011) The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J. Biol. Chem. 286, 3671–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Story G. M., Peier A. M., Reeve A. J., Eid S. R., Mosbacher J., Hricik T. R., Earley T. J., Hergarden A. C., Andersson D. A., Hwang S. W., McIntyre P., Jegla T., Bevan S., and Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 [DOI] [PubMed] [Google Scholar]
- 26. Sisignano M., Angioni C., Ferreiros N., Schuh C. D., Suo J., Schreiber Y., Dawes J. M., Antunes-Martins A., Bennett D. L., McMahon S. B., Geisslinger G., and Scholich K. (2013) Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS One 8, e81228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Southall M. D., and Vasko M. R. (2001) Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J. Biol. Chem. 276, 16083–16091 [DOI] [PubMed] [Google Scholar]
- 28. Moriyama T., Higashi T., Togashi K., Iida T., Segi E., Sugimoto Y., Tominaga T., Narumiya S., and Tominaga M. (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winter Z., Buhala A., Ötvös F., Jósvay K., Vizler C., Dombi G., Szakonyi G., and Oláh Z. (2013) Functionally important amino acid residues in the transient receptor potential vanilloid 1 (TRPV1) ion channel: an overview of the current mutational data. Mol. Pain 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lizanecz E., Bagi Z., Pásztor E. T., Papp Z., Edes I., Kedei N., Blumberg P. M., and Tóth A. (2006) Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Mol. Pharmacol. 69, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 31. Sieber M., and Baumgrass R. (2009) Novel inhibitors of the calcineurin/NFATc hub: alternatives to CsA and FK506? Cell Commun. Signal. 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin H. A., Basbaum A. I., Goetzl E. J., and Levine J. D. (1988) Leukotriene B4 decreases the mechanical and thermal thresholds of C-fiber nociceptors in the hairy skin of the rat. J. Neurophysiol. 60, 438–445 [DOI] [PubMed] [Google Scholar]
- 33. Schmidt R., Schmelz M., Forster C., Ringkamp M., Torebjörk E., and Handwerker H. (1995) Novel classes of responsive and unresponsive C nociceptors in human skin. J. Neurosci. 15, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaible H. G., and Schmidt R. F. (1983) Responses of fine medial articular nerve afferents to passive movements of knee joints. J. Neurophysiol. 49, 1118–1126 [DOI] [PubMed] [Google Scholar]
- 35. Brederson J. D., Kym P. R., and Szallasi A. (2013) Targeting TRP channels for pain relief. Eur. J. Pharmacol. 716, 61–76 [DOI] [PubMed] [Google Scholar]
- 36. Lundeen K. A., Sun B., Karlsson L., and Fourie A. M. (2006) Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J. Immunol. 177, 3439–3447 [DOI] [PubMed] [Google Scholar]
- 37. Casarotto P. C., Terzian A. L., Aguiar D. C., Zangrossi H., Guimarães F. S., Wotjak C. T., and Moreira F. A. (2012) Opposing roles for cannabinoid receptor type-1 (CB(1)) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology 37, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gibbs M. E., and Johnston G. A. (2005) Opposing roles for GABAA and GABAC receptors in short-term memory formation in young chicks. Neuroscience 131, 567–576 [DOI] [PubMed] [Google Scholar]
- 39. Kawahara H., Drew G. M., Christie M. J., and Vaughan C. W. (2011) Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey. Br. J. Pharmacol. 163, 1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puchalowicz K., Tarnowski M., Baranowska-Bosiacka I., Chlubek D., and Dziedziejko V. (2014) P2X and P2Y receptors-role in the pathophysiology of the nervous system. Int. J. Mol. Sci. 15, 23672–23704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Viguier F., Michot B., Hamon M., and Bourgoin S. (2013) Multiple roles of serotonin in pain control mechanisms: implications of 5-HT(7) and other 5-HT receptor types. Eur. J. Pharmacol. 716, 8–16 [DOI] [PubMed] [Google Scholar]
- 42. Malin S. A., and Molliver D. C. (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol. Pain 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang S., Iring A., Strilic B., Albarrán Juárez J., Kaur H., Troidl K., Tonack S., Burbiel J. C., Müller C. E., Fleming I., Lundberg J. O., Wettschureck N., and Offermanns S. (2015) P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125, 3077–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sisignano M., Park C. K., Angioni C., Zhang D. D., von Hehn C., Cobos E. J., Ghasemlou N., Xu Z. Z., Kumaran V., Lu R., Grant A., Fischer M. J., Schmidtko A., Reeh P., Ji R. R., et al. (2012) 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 32, 6364–6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hargreaves K., Dubner R., Brown F., Flores C., and Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 46. Coste O., Pierre S., Marian C., Brenneis C., Angioni C., Schmidt H., Popp L., Geisslinger G., and Scholich K. (2008) Antinociceptive activity of the S1P-receptor agonist FTY720. J. Cell. Mol. Med. 12, 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meller S. T., and Gebhart G. F. (1997) Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur. J. Pain 1, 43–52 [DOI] [PubMed] [Google Scholar]
- 48. Suo J., Linke B., Meyer dos Santos S., Pierre S., Stegner D., Zhang D. D., Denis C. V., Geisslinger G., Nieswandt B., and Scholich K. (2014) Neutrophils mediate edema formation but not mechanical allodynia during zymosan-induced inflammation. J. Leukocyte Biol. 96, 133–142 [DOI] [PubMed] [Google Scholar]
- 49. Calabresi P., Gubellini P., Centonze D., Picconi B., Bernardi G., Chergui K., Svenningsson P., Fienberg A. A., and Greengard P. (2000) Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 20, 8443–8451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kerr J. N., and Wickens J. R. (2001) Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 85, 117–124 [DOI] [PubMed] [Google Scholar]
- 51. Calabresi P., Saiardi A., Pisani A., Baik J. H., Centonze D., Mercuri N. B., Bernardi G., and Borrelli E. (1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J. Neurosci. 17, 4536–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamoto Y., Nakanishi H., Takai N., Shimazoe T., Watanabe S., and Kita H. (1999) Expression of N-methyl-d-aspartate receptor-dependent long-term potentiation in the neostriatal neurons in an in vitro slice after ethanol withdrawal of the rat. Neuroscience 91, 59–68 [DOI] [PubMed] [Google Scholar]