Abstract
Nuclear actin-related proteins play vital roles in transcriptional regulation; however, their biological roles remain elusive. Here, we characterize Alp5, fission yeast homolog of Arp4/BAF53. The temperature-sensitive mutant alp5-1134 contains a single amino acid substitution in the conserved C-terminal domain (S402N) and displays mitotic phenotypes, including chromosome condensation and missegregation. Alp5 forms a complex with Mst1-HAT (histone acetyltransferase). Consistently, inhibition of histone deacetylases (HDACs), by either addition of a specific inhibitor or a mutation in HDAC-encoding clr6+ gene, rescues alp5-1134. Immunoblotting with specific antibodies against acetylated histones shows that Alp5 is required for histone H4 acetylation at lysines 5, 8, and 12, but not histone H3 lysines 9 or 14, and furthermore Clr6 plays an opposing role. Mitotic arrest is ascribable to activation of the Mad2/Bub1 spindle checkpoint, in which both proteins localize to the mitotic kinetochores in alp5-1134. Intriguingly, alp5-1134 displays transcriptional desilencing at the core centromere without altering the overall chromatin structure, which also is suppressed by a simultaneous mutation in clr6+. This result shows that Alp5 is essential for histone H4 acetylation, and its crucial role lies in the establishment of bipolar attachment of the kinetochore to the spindle and transcriptional silencing at the centromere.
INTRODUCTION
The actin-related proteins (ARPs) comprise a conserved protein family and are classified into at least eight subfamilies (Goodson and Hawse, 2002). These ARPs consist of two functional groups, depending upon their subcellular localization, cytoplasmic or nuclear. Unlike the conventional actin, ARPs do not form polymers in the cell; instead, these proteins are generally found in large multisubunit complexes and play regulatory roles toward the complex function. Nuclear ARPs are components of two distinct families of chromatin remodeling enzyme complexes, histone acetyltransferases (HATs) and SWI/SNF-related family ATPases (Olave et al., 2002). These two enzymes play pivotal roles in chromatin function via either acetylating the lysine residues of histones or altering the mobility and spacing of the nucleosome arrays in an ATP-dependent manner, respectively (Workman and Kingston, 1998; Sterner and Berger, 2000; Roth et al., 2001). In general, these modifications induce conformational alterations of the chromatin, thereby stimulating transcriptional accessibility. Because nucleosome-based chromatin structures lie at the heart of DNA-mediated cellular activities, other aspects of chromatin functions besides gene expression, such as DNA repair, DNA replication, and chromosome segregation, also seem to be regulated by these chromatin remodeling complexes (Ikura et al., 2000; Bird et al., 2002), although the precise mechanisms underlying these functions are not fully understood yet.
Among the nuclear ARPs so far identified, budding yeast Arp4 and its human homolog BAF53 are of vital importance in understanding the cellular role of nuclear ARPs for the following reasons. First, Arp4 is the only ARP essential for cell viability (Harata et al., 1994). Second, Arp4 forms distinct complexes in the nucleus with Esa1-HAT, Ino80, and Swr1 ATPases (Allard et al., 1999; Shen et al., 2000, 2003; Kobor et al., 2004; Mizuguchi et al., 2004). BAF53 was originally identified as one of the Brg1-associated factors (BAFs), in which Brg1 is a SWI/SNF family ATPase (Wang et al., 1996a,b; Zhao et al., 1998), and later found to also be a component of the Tip60 complex (Ikura et al., 2000). Esa1 and Tip60 are the catalytic subunits of the nucleosome acetyltransferase of histone H4 (NuA4-HAT) complex, which acetylates histone H4 (and H2A to some extent) (Allard et al., 1999; Galarneau et al., 2000; Doyon et al., 2004). Although a number of studies have attempted to dissect an essential function for Arp4 and shown that it plays a role in transcriptional regulation and DNA repair (Jiang and Stillman, 1996; Bird et al., 2002; Harata et al., 2002; Gorzer et al., 2003), the reason for its essentiality or the critical function in which Arp4 is involved remains to be established.
Fission yeast is one of the ideal systems in which to study the molecular mechanisms underlying chromatin structure and transcriptional regulation, such as higher order architecture and epigenetic gene silencing. Fission yeast centromeres are composed of three subdomains, a nonrepetitive central region (cnt), and two flanking heterochromatic repeated structures (imr and otr). Unlike budding yeast, these centromeric regions display transcriptional silencing in addition to the ability of forming the kinetochore (Allshire et al., 1994, 1995). It is known that lesions in histone modification, including inhibition or mutations in histone deacetylases (HDACs), fail to repress transcription at imr and otr, which are accompanied by defects in chromosome segregation (Allshire et al., 1995; Ekwall et al., 1997; Grewal et al., 1998). On the other hand, our knowledge with regards to the silencing mechanisms at the core cnt region remains limited. Some mutations in structural components of the kinetochore (e.g., the CENP-A homolog Cnp1, Sim4, and Mal2) are known to result in desilencing phenotypes specifically at the cnt region (Jin et al., 2002; Pidoux et al., 2003); however, the regulatory factors acting on this central region have not been identified so far.
In this study, we show that Alp5, the fission yeast homolog of Arp4/BAF53 is essential for cell division. This protein is required for in vivo histone H4 acetylation and plays a pivotal role in mitotic progression, particularly in faithful chromosome segregation. We present evidence that Alp5 is critical for the establishment of a stable attachment of the kinetochore to the mitotic spindle and transcription silencing at the core centromere region.
MATERIALS AND METHODS
Strains, Media, and Genetic Methods
Strains used in this study are listed in Table 1. The standard methods were followed as described previously (Moreno et al., 1991).
Table 1.
Strains
| Strains | Genotypes | Derivations |
|---|---|---|
| 972 | h− | Our stock |
| 513 | h−leu1 ura4 | Our stock |
| DH1134 | h−leu1 alp5-1134 | Our stock |
| FY648 | h+leu1 ade6-210 ura4+-DS/E otr1 :: ura4+ | Robin Allshire |
| FY336 | h−leu1 ade6-210 ura4+-DS/E cnt :: ura4+ | Robin Allshire |
| FY3223 | h+leu1 ade6 ura4 ade6-210 clr3-735 cen1R :: ura4+ | Robin Allshire |
| HU369 | h−leu1 ade6-210 ura4 clr3 :: kanR | Karl Ekwall |
| SPG1002 | h+leu1 ura4 ade6-210 clr6-1 cnt1 :: ura4+ | Yota Murakami |
| SPS41 | mat msmto leu1 ade6-210 clr6-1 | Shiv Grewal |
| AM11 | h−leu1/leu1 ura4/ura4 his7/his7 ade6-M210/ade6-M216 alp5+/alp5 :: ura4+ | This study |
| AM24 | h−leu1/leu1 ura4/ura4 his7/his7 ade6-M210/ade6-M216 alp5+/alp5+-myc-kan | This study |
| AM36 | h−leu1 ura4 ade6-210 alp5-1134 containing Ch16 | This study |
| AM44 | h−leu1 alp5 :: ura4+ containing p(AL-alp5+) | This study |
| AM49 | h+leu1 ura4 his2 mst1+-13myc-kanR | This study |
| AM50 | h+leu1 ura4 his2 mad2 :: kanR alp5-1134 | This study |
| AM53 | h−leu1 phd1(hos2) :: LEU2 alp5-1134 | This study |
| AM57 | h−leu1 ura4-DS/E otr1 :: ura4+alp5-1134 | This study |
| AM68 | h+leu1 ura4 his2 alp5-1134 ura4-DS/E cnt1 :: ura4+ | This study |
| AM115 | h+leu1 ura4 clr3 :: kanR alp5-1134 | This study |
| AM117 | h+leu1 his2 cdc13+-GFP-LEU2 alp5-1134 | This study |
| AM121 | h−leu1 ura4 mad2+-GFP-kanRalp5-1134 | This study |
| AM123 | h+leu1 ura4 his2 bub1+-GFP-kanR alp5-1134 | This study |
| AM147 | h+leu1 ura4 his2 mad3 :: ura4+alp5-1134 | This study |
| AM149 | h+leu1 ura4 his2 ade6 clr6-1alp5-1134 | This study |
| AM170 | h+leu1 ura4 his2 bub1 :: ura4+alp5-1134 | This study |
| AM173 | h−leu1 ura4 bub1+-RFP-kanRmad2+-GFP-kanRalp5-1134 | This study |
| AM184 | h+leu1 ura4 his2 mad1 :: ura4+alp5-1134 | This study |
| AM185 | h+leu1 ura4 his2 bub3 :: ura4+alp5-1134 | This study |
| AM196 | h90 leu1 ura4 ade6-210 clr3 :: kanRcen1R :: ura4+ | This study |
| AM197 | h−leu1 ura4 nuf2+-CFP-kanRbub1+-GFP-kanR | This study |
All the strains listed in this table contain leu1-32. ura4 used is ura4-D18 unless otherwise stated.
Gene Disruption and Construction of N-Terminally or C-Terminally Tagged Strains
Polymerase chain reaction (PCR)-mediated long oligonucleotide methods were used (Bähler et al., 1998). G418-resistnace marker gene kanr and ura4+ genes were used for selectable markers. For tagging with red fluorescent protein (RFP), a fast folding RFP was used (provided by Drs. Elmar Schiebel, Paterson Institute, Manchester, United Kingdom; and Michael Knop, EMBL, Heidelberg, Germany).
Immunochemistry
For indirect immunofluorescence microscopy, cells were fixed with methanol and the following antibodies were used as primary antibodies: mouse monoclonal anti-α-tubulin antibody (TAT-1 1/50; provided by Dr. Keith Gull, Oxford University, Oxford, United Kingdom) and affinity-purified rabbit polyclonal anti-Alp5 antibody (1/100). Cy3-conjugated goat anti-rabbit (C2306; Sigma-Aldrich, St. Louis, MO) or anti-mouse IgG (C2128; Sigma-Aldrich), fluorescein-linked sheep anti-mouse IgG (F0261; Amersham Biosciences, Piscataway, NJ) or Cy5-conjugated anti-rabbit IgG antibody (111-175-003; Jackson ImmunoResearch Laboratories, West Grove, PA) was used for secondary antibodies. For immunoprecipitation, rabbit polyclonal anti-myc antibody (9E10; Babco, Richmond, CA) was used as primary antibody. Two milligrams of total protein extracts was used. For immunoblotting, mouse monoclonal anti-myc antibody or rabbit polyclonal anti-Alp5 antibody was used. For immunoblotting against acid-extracted histones, the following antibodies were used: anti-histone H4 (07-108; Upstate Biotechnology, Lake Placid, NY), anti-acetyl histone H4 (Lys5, 07-327; Upstate Biotechnology), anti-acetyl histone H4 (Lys8, 07-328; Upstate Biotechnology), anti-acetyl histone H4 (Lys12, 07-323; Upstate Biotechnology), anti-acetyl histone H4 (Lys16, 07-329; Upstate Biotechnology), anti-hyperacetylated histone H4 (Penta, 06-946; Upstate Biotechnology), histone H3 (acetyl K9, ChIP grade, ab4441; AbCam, Cambridge, MA), and anti-acetyl histone H3 antibody (Lys14, 07-353; Upstate Biotechnology).
Microscopy
Immunofluorescence microscopy was viewed with a Zeiss Axioplan equipped with a chilled video charge-coupled device camera (C4742-95; Hamamatsu Photonics, Bridgewater, NJ) and the PC computer containing kinetic image AQM software (Kinetic Imaging, Nottingham, United Kingdom) and processed by use of Adobe Photoshop (version 6.0).
Histone Preparations
Standard methods (Pidoux et al., 2004) were followed. Briefly cell pellets from exponentially growing culture (1 × 108) were disrupted with glass beads. The recovered lysate was centrifuged at ∼19,000 × g for 10 min at 4°C. The pellet was resuspended in 0.5 ml of 0.4 M sulfuric acid and incubated for 1 h on ice. The extract was then centrifuged. The acid extraction was repeated once. The pooled supernatants (1 ml total) were precipitated overnight in glass at -20°C with 12 volumes of ice-cold acetone. The precipitate was collected by centrifugation. The pellet was air dried and resuspended in 100 μl of 4 M urea.
Micrococcal Nuclease (MNase) Digestion
The MNase digestion was performed using three centromeric probes corresponding to cnt, imr, and otr regions described previously (Takahashi et al., 1992). The 2.8-kb HindIII/EcoRI fragment derived from a pKT110 plasmid was used as a probe to detect the cnt region.
Supplementary Data
Supplementary data for this article are available.
RESULTS
alp5+ Is Required for Mitotic Progression and Accurate Chromosome Segregation
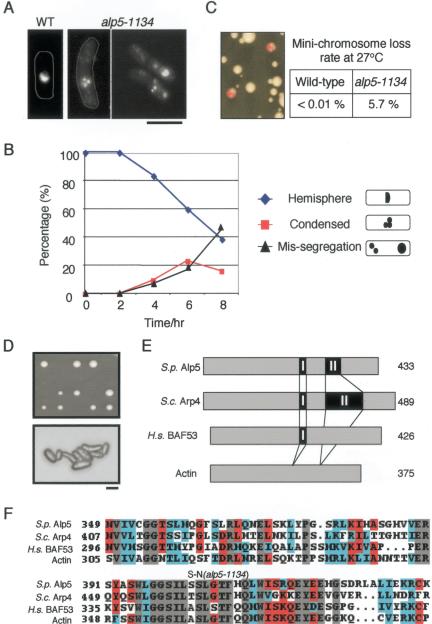
The alp5-1134 mutant was isolated through screening for temperature-sensitive (ts) mutants with growth polarity defects (Radcliffe et al., 1998). At the restrictive temperature, alp5-1134 cells divide two to three times, followed by mitotic delay with bent cell morphology. As shown in Figure 1, A and B, an accumulation of mitotic cells with condensed chromosomes is observed, which consisted of discrete bodies instead of interphase hemispherical shape (Figure 1A, left), followed by chromosome missegregation. Even at its permissive temperature, the alp5-1134 mutant displayed a high percentage of minichromosome loss (Figure 1C). These results show that Alp5 is required for mitotic progression and plays a role in ensuring a high fidelity of sister chromatid segregation.
Figure 1.
alp5+ is required for accurate chromosome segregation and encodes a conserved actin-related Arp4/BAF53 homolog. (A) Chromosome condensation in alp5-1134 cells. alp5-1134 mutant cells displaying condensed chromosomes (4,6-diamidino-2-phenylindole [DAPI], 36°C for 6 h) are shown. Wild-type control that contains interphase hemispherical chromosomes also is shown (left). Cell morphology was marked with a thin white line along the cell surface. Bar, 10 μm. (B) Changes of chromosome structures. On shift of the alp5-1134 culture from 26 to 36°C, samples were collected every 2-h interval and stained with DAPI. Percentage of three types of chromosomes is plotted, interphase hemispherical (diamonds in blue); condensed (squares in red); missegregated (and also often decondensed, triangles in black). (C) Loss of minichromosomes. alp5-1134 mutant cells containing minichromosomes (Ch16) (Niwa et al., 1989), which had been grown in minimal medium without adenine (selective conditions for minichromosomes), were plated on rich media plates and incubated at 27°C for 4 d. Colonies that lost minichromosomes are red (often sectored). More than 104 colonies were counted, and a frequency of minichromosome loss was calculated. (D) Gene disruption. A diploid strain heterozygous for alp5 (alp5+/alp5::ura4+) was sporulated and tetrad analysis was performed. Plates were incubated at 30°C for 4 d (top). Microscopic observation of lethal spores shows that spores germinated, divided a few times, and arrested in bent cell morphology (bottom). Bar, 10 μm. (E) Schematic structural comparison between Alp5 and actin-related proteins. (F) Amino acid sequences of the region in which a point mutation occurs in alp5-1134. Amino acid sequences, which are identical in all the four members (Alp5, budding yeast Arp4, fission yeast conventional actin and human BAF53), are shown in gray boxes, whereas residues identical in the three members are shown in red boxes. Conserved amino acid residues including homologous amino acids are shown in blue boxes. The position of a point mutation (S402N) found in alp5-1134 also is shown.
alp5+ Encodes an Essential Actin-related Protein Most Homologous to Mammalian BAF53 and Budding Yeast Arp4
The alp5+ gene was cloned by complementation from a fission yeast genomic library. Nucleotide sequencing showed that the alp5+ gene (SPBP23A10.08) encodes an actin-related protein consisting of 433 amino acid residues, most homologous to budding yeast Arp4 (33% identity and 50% similarity) and mammalian BAF53 (32% identity and 49% similarity) (see Supplementary Figure S1 for the fission yeast actin-related protein family). Gene disruption showed that, like ARP4 (Harata et al., 1994), the alp5+ gene is essential for cell viability (Figure 1D, top). On microscopic examination of tetrad plates, alp5::ura4+ spores could germinate and undergo some cell divisions (bottom). These cells displayed bent morphology, similar to the alp5-1134 mutant cells that are incubated at the restrictive temperature.
A close structural comparison between Alp5 and conventional actin indicated that Alp5 contains two internal insertions, insertions I (230-250) and II (300-330), which also are seen in Arp4 (Figure 1E). It is of note that BAF53 contains insertion I but not II. Nucleotide sequencing of DNA fragments prepared from the alp5-1134 mutant showed that alp5-1134 contains a single nucleotide exchange at 1208 from G to A (A for initiator ATG is denoted as +1), which leads to a point mutation at amino acid residue 402 from serine to asparagine (S402N). Amino acid comparison around the mutation site indicated that this region is highly conserved among the actin-related protein family, including conventional actin, although S402 itself is not invariant (Figure 1F). Structural prediction of the Alp5 protein based upon the three-dimensional (3-D) structure of actin (Kabsch et al., 1990) showed that S402 resides inside the α-helix chain, which is embedded in the conserved protein folds (Supplementary Figure S2). Together, Alp5 is a highly conserved Arp4/BAF53 homolog and the ts alp5-1134 mutation is derived from a single amino acid replacement at the conserved C-terminal region.
Alp5 Localizes to the Nucleus and Forms a Complex with the Mst1 Histone Acetyltransferase
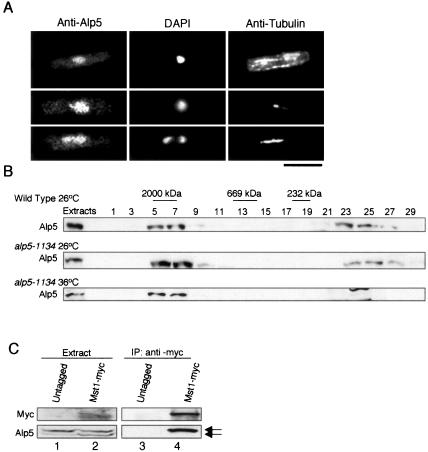
Because Arp4 and BAF53 are nuclear proteins (Olave et al., 2002), we expected that Alp5 also would localize to the nucleus. Epitope-tagging methods (Bähler et al., 1998) did not work for Alp5, because both N and C termini seem to be essential for Alp5 function, i.e., we could not succeed in making epitope-tagged haploid strains at either terminus. Consequently, rabbit polyclonal anti-Alp5 antibody was prepared (see Materials and Methods and Supplementary Figure S3). Immunofluorescence microscopy using purified anti-Alp5 sera showed that Alp5 localizes to the nucleus during the whole cell cycle (Figure 2A).
Figure 2.
Alp5 localizes to the nucleus and forms a complex with the Mst1-HAT. (A) The cellular localization of Alp5. Immunofluorescence microscopy was performed in exponentially growing wild-type cells upon fixation with methanol. Cells were stained with anti-Alp5 antibody (first panels), 4,6-diamidino-2-phenylindole (DAPI) (second panels), and anti-α-tubulin antibody (third panels). Representative images during the cell cycle, interphase (top), early mitosis (middle), and anaphase B (bottom), are shown. Bar, 10 μm. (B) Gel filtration chromatography. Soluble cell extracts were prepared from wild type grown at 26°C (top) or alp5-1134 grown either at 26°C (middle) or 36°C (bottom) and loaded onto Superose 6 columns. Each fraction together with total extracts (20 μg, shown as Extract) was run on SDS-PAGE, and immunoblotting was performed with anti-Alp5 antibody. Positions of size markers corresponding to 2000, 669, and 232 kDa also are shown. (C) Interaction between Alp5 and Mst1. Immunoprecipitation was performed using protein extracts prepared from an Mst1-myc strain with anti-myc antibody, followed by immunoblotting with anti-myc and anti-Alp5 antibodies (lane 4). As a negative control protein extracts also were prepared from an untagged strain (lane 3). Twenty micrograms of extracts before immunoprecipitation (lanes 1 and 2) also was run. Two Alp5 bands are shown with arrows.
We next examined the native size of Alp5 in the cell through gel filtration. As shown in Figure 2B (top), Alp5 existed as a large complex (∼2000 kDa). In addition, Alp5 also was found in smaller fractions (fraction numbers 23-25), which corresponded to either monomer or dimer size of Alp5 (predicated molecular mass of Alp5 is 48.7 kDa). To characterize the molecular defects resulting from the alp5-1134 mutation, we performed gel filtration analysis with this mutant strain. As shown in Figure 2B (middle and bottom), it was evident that the size of the complex containing the mutant Alp5-1134 protein was not noticeably altered when incubated at either the permissive or the restrictive temperature. This suggested that Alp5 forms a complex with other proteins in the cell and that the ts alp5-1134 mutant is not defective in this complex formation per se.
In both budding yeast and vertebrates, Arp4 and BAF53 form a large complex (∼2000 kDa) with MYST (MOZ, Ybf2/Sas3, Sas2, and Tip60)-type HATs (Allard et al., 1999; Galarneau et al., 2000). Fission yeast contains two MYST-type HATs, called Mst1 and Mst2, and the interaction between Alp5 and Mst1 was examined using a C-terminally myc-tagged strain (Mst1-myc). As shown in Figure 2C, Alp5 forms a complex with Mst1. It is of note that under this condition, two bands were detected in the extracts with the anti-Alp5 antibody, and only the upper band coprecipitated with Mst1. Currently, the nature of these two anti-Alp5-reacting bands is being investigated. In any case, these results showed that like its homologs, Alp5 is a nuclear protein and a component of the MYST-type HAT complex.
The alp5-1134 Mutation Is Resistant to and Partially Suppressed by an HDAC Inhibitor
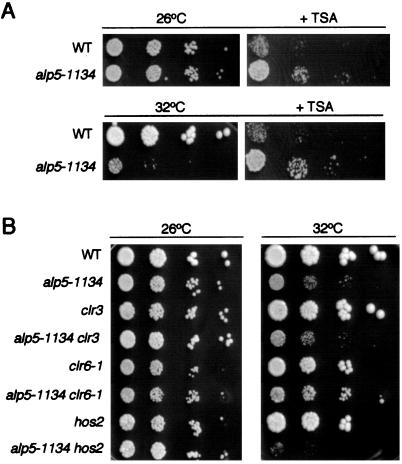
The interaction between Alp5 and Mst1-HAT prompted us to address the role of Alp5 in histone acetylation. To this end, we examined the growth characteristics of the alp5-1134 mutant in the presence of trichostatin A (TSA), which is an inhibitor of classes I and II HDACs (Yoshida et al., 1990; Verdin et al., 2003). As shown in Figure 3A, alp5-1134 cells displayed higher resistance to TSA compared with the wild-type cells at 26°C (top plates). It also was found that the addition of TSA in the media rescues the temperature sensitivity of alp5-1134. At the semirestrictive temperature of 32°C at which alp5-1134 cells could not form colonies efficiently, a substantial recovery of growth was observed in the presence of this drug (bottom plates). Suppression was, nonetheless, incomplete, because there were no colonies formed at 36°C (Minoda and Toda, unpublished data). This result suggested that Alp5 may play a role in histone acetylation in vivo and that the level of histone acetylation may be decreased in the alp5-1134 mutant.
Figure 3.
Suppression of alp5-1134 by either addition of the HDAC inhibitor or a mutation in HDAC-encoding clr6+ gene. (A) Suppression of alp5-1134 by TSA. Wild-type or alp5-1134 cells were spotted on rich plates in the absence (left) or presence of TSA (50 μg/ml) (5 × 103 cells in the far-left spots for each plate and then diluted 10-fold in each subsequent spot rightwards). Plates were incubated at either 26°C (top plates) or 32°C (bottom plates) and incubated for 4 d. (B) Suppression of alp5-1134 by clr6-1. Various strains indicated were spotted in a similar manner as in A.
Clr6 HDAC Acts Antagonistically with Alp5
Given the substantial suppression of the temperature-sensitivity of alp5-1134 by TSA addition, we sought to dissect the HDAC(s) genetically, which act in an opposed manner with Alp5. Fission yeast contains six open reading frames encoding HDACs, in which Clr6 and Hos2/Phd1/Hda1 belong to class I (Grewal et al., 1998; Kim et al., 1998; Olsson et al., 1998), Clr3 to class II HDAC (Thon et al., 1994; Grewal et al., 1998), and Hst2, Hst4, and Sir2 are class III HDACs (Freeman-Cook et al., 1999; Shankaranarayana et al., 2003). Homology search against the budding yeast database shows that Clr6, Hos2, and Clr3 are most homologous to budding yeast Rpd3, Hos2, and Hda1, respectively, and Hst2, Hst4, and Sir2 are designated according to the names of budding yeast HDACs that show highest homology (Table 2).
Table 2.
HDACs in yeast
| Gene products
|
||
|---|---|---|
| S. pombe | S. cerevisiae | Classes |
| Clr6 | Rpd3 | I |
| Hos2* | Hos2 | I |
| Hos1 | I | |
| Hos3 | I | |
| Clr3 | Hda1 | II |
| Sir2 | Sir2 | III |
| Hst2 | Hst2 | III |
| Hst4 | Hst4 | III |
| Hst1 | III | |
| Hst3 | III | |
S. pombe Hos2 is also called Phd1 or Hda1 (Kim et al., 1998; Olsson et al., 1998).
Double mutants between alp5-1134 and each deletion of hos2+ and clr3+ or a ts clr6-1 allele (Grewal et al., 1998) were constructed, and temperature-sensitivity was examined at various temperatures. As shown in Figure 3B, the growth defect of alp5-1134 at 32°C was rescued significantly by clr6-1, which was also the case with TSA treatment. This suppression was partial, because the double mutant was not capable of forming colonies at 36°C (Minoda and Toda, unpublished data). On the other hand, growth of the double mutant with the hos2 deletion was worse than that of the single alp5-1134 mutant, whereas the growth properties of alp5-1134clr3 cells were indistinguishable from the alp5-1134 single mutant (Figure 3B). In summary, the results presented here show that Alp5 is involved in histone acetylation and acts antagonistically with Clr6, but not with Hos2 or Clr3.
Alp5 Is Required for the Global Acetylation of the Histone H4 N-Terminal Tail and Counteracts with Clr6
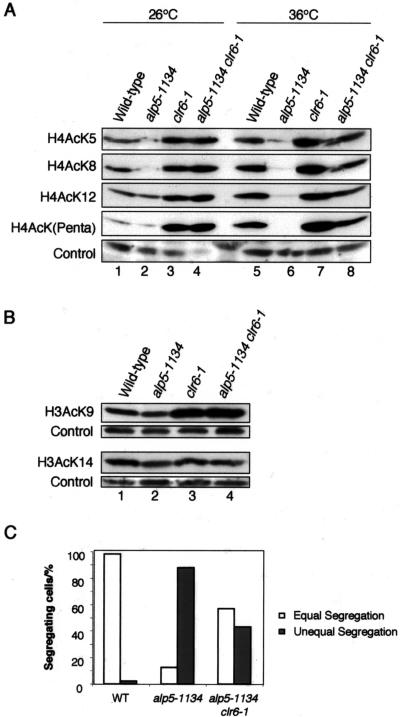
Although Arp4 and BAF53 are shown to be a component of the NuA4 complex that acetylates histone H4, how these actin-related proteins are involved in histone acetylation activities remains largely elusive (Doyon and Côté, 2004). The N-terminal tail of histone H4 is acetylated at lysine 5, 8, 12, and 16 in all eukaryotes (Strahl and Allis, 2000). To determine whether Alp5 regulates the global level of histone H4 acetylation in fission yeast, histones were acid extracted from the wild-type, alp5-1134, clr6-1, or alp5-1134clr6-1 cells (Figure 3B) and immunoblotted with the antibodies that recognize acetylated lysine residues of histone H4 tail. The antibodies used were anti-H4 AcK5, anti-H4 AcK8, anti-H4 AcK12, and anti-H4 hyperacetylated pentaAcK. It was found that in the alp5-1134 mutant, the level of histone H4 tail acetylation was dramatically decreased in all cases (Figure 4, lanes 1, 2, 5, and 6), which was particularly evident with the cultures incubated at 36°C (lane 6). This result indicated that Alp5 plays an essential role in the acetylation of histone H4 N-terminal tail in vivo.
Figure 4.
Acetylation levels of histone H3 and H4 tails in alp5-1134, clr6-1, and alp5-1134clr6-1 mutants and suppression of mitotic defects in the alp5-1134clr6-1 double mutant. (A) Acetylation of histone H4 tail. Histones were acid-extracted from a wild-type (lanes 1 and 5), alp5-1134 (lanes 2 and 6), clr6-1 (lanes 3 and 7), or alp5-1134clr6-1 strain (lanes 4 and 8) that were incubated either at 26°C (lanes 1-4) or 36°C for 12 h (lanes 5-8). Ten micrograms of each fraction was run on SDS-PAGE and immunoblotted with antibodies specific for acetylated histone H4. Antibodies used are anti-acetyl-histone H4 (Lys5, H4AcK5), anti-acetyl-histone H4 (Lys8, H4AcK8), anti-acetyl-histone H4 (Lys12 H4AcK12), and anti-hyperacetyl-histone H4 (Penta). Signals from nonspecific bands are shown as loading controls (bottom). (B) Histone H3 acetylation. Histone samples prepared in A at 36°C were immunoblotted with anti-acetyl-histone H3 antibody specific for Lys9 (H3AcK9, top) or Lys14 (H3AcK14, third panel). Strains used are wild type (lane 1), alp5-1134 (lane 2), clr6-1 (lane 3), or alp5-1134clr6-1 cells (lane 4). Ten micrograms of each fraction was run. Signals from nonspecific bands also are shown (control). (C) Partial suppression of chromosome missegregation in the alp5-1134clr6-1 mutant. Wild-type, alp5-1134, and alp5-1134clr6-1 cells were incubated at 36°C for 8 h, and the percentage of cells displaying equal or unequal chromosome segregation was counted among binucleated cells.
Consistent with previously reported results (Bjerling et al., 2002; Nakayama et al., 2003), all the lysine residues on the histone H4 tail examined were hyperacetylated in the clr6-1 mutant incubated at 36°C (Figure 4, lane 7). Also in this mutant, hyperacetylation was clearly observed even at 26°C (lane 3). Intriguingly, in the alp5-1134clr6-1 double mutant, the level of H4 tail acetylation became mutually compromised at 36°C and almost comparable with those of the wild-type cells (lanes 5 and 8). This reciprocal compensation for levels of H4 tail acetylation in the double mutant is in complete line with the results of genetic suppression presented earlier (Figure 3). The levels of histone H3 acetylation at K9 and K14 also were examined. As reported previously (Bjerling et al., 2002; Nakayama et al., 2003), we also found that Clr6 is required for deacetylation of H3 K9 (Figure 4B, top, lanes 1 and 3). However, in sharp contrast to histone H4 acetylation, Alp5 does not seem to be involved in the acetylation of this site, because the acetylation level of H3 K9 in the single alp5-1134 mutant was indistinguishable from that of wild-type (lanes 1 and 2), and furthermore the level of acetylation in the alp5-1134clr6-1 double mutant was not altered compared with that in the single clr6-1 mutant (lanes 3 and 4). On the other hand, the acetylation level of H3 K14 was not affected in any of the mutants examined (Figure 4B, third panel). These results show that Alp5 and Clr6 play an antagonistic role in histone acetylation and deacetylation specifically on histone H4 tail.
Given the suppression of both temperature-sensitivity and histone H4 tail acetylation in the alp5-1134clr6-1 double mutant, we asked whether mitotic phenotypes resulting from the alp5-1134 mutation also was rescued by clr6-1. It was indeed the case. After 8-h incubation at the restrictive temperature (at 36°C), the percentage of cells that displayed chromosome missegregation among binucleated cells was greatly reduced in the alp5-1134clr6-1 double mutant compared with that in alp5-1134 (50 vs. 90%, respectively; Figure 4C). Together, Alp5 is essential for histone H4 tail acetylation and mitotic progression, in which Clr6 HDAC acts antagonistically.
Alp5 Is Required for the Attachment of the Kinetochore to the Mitotic Spindle
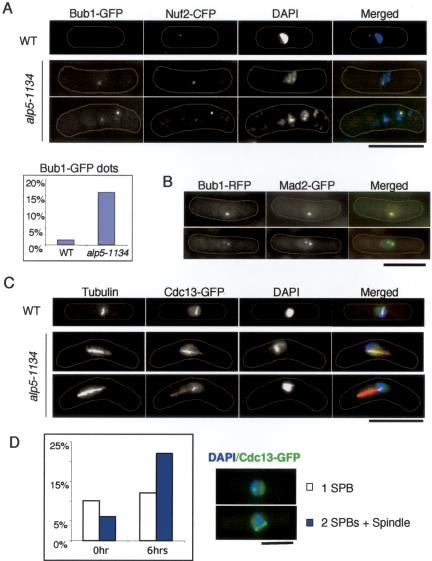
Because alp5-1134 shows mitotic arrest as described above, we asked whether the mitotic checkpoint is activated in this mutant. The attachment of the kinetochore to the spindle is monitored by a spindle assembly checkpoint, the activation of which leads to the localization of the checkpoint proteins (Mads and Bubs) to the mitotic kinetochore that is not attached to the spindle in a bipolar manner (Cleveland et al., 2003). In both yeast and animal cells, Bub1 and Mad2 are recruited to unattached kinetochores (Bernard et al., 1998; Waters et al., 1998; Garcia et al., 2001; Skoufias et al., 2001; Ikui et al., 2002; Toyoda et al., 2002). We found that Bub1-GFP localized to the kinetochores in the mitotically arrested alp5-1134 cells, which was confirmed by its colocalization with the kinetochore marker Nuf2-CFP (Nabetani et al., 2001) (Figure 5A). In addition to Bub1, Mad2 also colocalized to the kinetochores (Figure 5B).
Figure 5.
Activation of the spindle assembly checkpoint in alp5-1134 mutants. (A) Bub1 at the kinetochores. A wild-type or alp5-1134 strain containing Bub1-GFP and Nuf2-CFP was incubated at 36°C for 6 h. GFP and cyan fluorescent protein (CFP) signals were observed under fluorescence microscopy. Representative images of interphase wild-type cell (top rows) and mitotic alp5-1134 cells (lower two rows) are shown. Merged images (GFP in green, CFP in red, and 4,6-diamidino-2-phenylindole [DAPI] in blue) are shown in the right-most panels. Cell morphology was marked with thin white lines along the cell surface. Quantification data also are shown. (B) Colocalization of Bub1 and Mad2. An alp5-1134 strain containing Bub1-RFP and Mad2-GFP were incubated and processed as in A. Merged images are shown in the right panel (Bub1-RFP in red and Mad2-GFP in green). (C) Accumulation of Cdc13 cyclin at SPBs and mitotic spindles. Wild-type or alp5-1134 mutant cells containing Cdc13-GFP were incubated at 36°C for 6 h, fixed with methanol, and processed for immunofluorescence microscopy with anti-α-tubulin antibody. Representative pictures of mitotically arrested wild-type (top) or alp5-1134 cell (bottom two panels) are shown. Merged images (tubulin in red, Cdc13-GFP in green, and DAPI in blue) are shown in the right-most panels. (D) Percentage of cells containing Cdc13 at spindle pole bodies (SPBs) and spindles. Representative examples are shown in the right panels (Cdc13-GFP in green and DAPI in blue). Open columns show wild type, whereas blue columns show alp5-1134. Bar, 10 μm.
The activation of the spindle checkpoint results in the inhibition of the anaphase promoting complex/cyclosome (APC/C), in which B-type cyclin and securin are the major substrates. We found that Cdc13 (B-type cyclin in fission yeast) localized to spindles in mitotic alp5-1134 mutant cells (Figure 5, C and D), which is reminiscent of the mitotic localization of Cdc13 before APC/C activation (Tatebe and Yanagida, 2000; Decottignies et al., 2001). This indicated that the APC/C activity remains low in alp5-1134 cells. It should be noted that ∼50% of mitotic alp5-1134 cells displayed chromosome biorientation defects, in which chromosomes seemed to attach to the spindle in a mono-oriented manner (evident in Figure 5C, bottom). Consistent with an antagonistic relation between Alp5 and Clr6, in alp5-1134clr6-1 double mutants, mitotic arrest phenotypes were abolished and mono-oriented spindles were no longer observed at the restrictive temperature (Minoda and Toda, unpublished data). These results indicate that Alp5 is required for the bipolar attachment of the kinetochore to the spindle.
Mitotic Arrest in alp5-1134 Is Dependent upon the Spindle Checkpoint
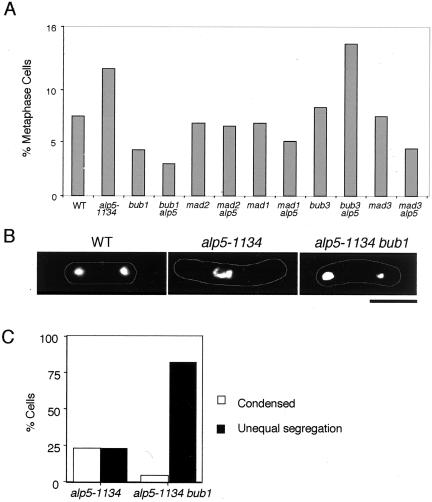
Having established the kinetochore localization of the spindle checkpoint proteins in the mitotically arrested alp5-1134 mutants, we next examined the dependency of this arrest upon the spindle checkpoint pathway. For this purpose, a series of double mutants between alp5-1134 and deletions of genes encoding the components of this checkpoint were constructed, and mitotic arrest phenotypes (e.g., chromosome condensation and spindle staining) were then examined. As shown in Figure 6A, all the double mutants except for alp5-1134bub3 abolished the mitotic arrest.
Figure 6.
Dependency of mitotic arrest phenotypes in alp5-1134 upon spindle assembly checkpoint. (A) Frequency of mitotic cells in alp5-1134 combined with various gene deletions in the spindle checkpoint pathway. Strains indicated were incubated at 36°C for 6 h, and the percentage of mitotic cells (based upon anti-tubulin staining) was counted. At least 300 cells were counted in each preparation. (B) Chromosome missegregation in alp5-1134bub1 double mutants. Representative examples for mitotic cells that display equal chromosome segregation (wild type, left), condensed chromosomes (alp5-1134, middle), and unequal chromosome segregation (alp5-1134bub1, right) are shown. Each strain was incubated at 36°C for 6 h and stained with DAPI. Bar, 10 μm. (C) Percentage of cells displaying condensed chromosomes or chromosome missegregation. At least 300 cells were counted.
To examine the phenotypic consequences of the lack of the spindle checkpoint in the alp5-1134 mutant, immunofluorescence microscopy of the alp5-1134bub1 double mutant was performed. The double mutant incubated at 36°C for 6 h showed a dramatic increase in the number of cells displaying chromosome missegregation (Figure 6, B and C). These double mutant cells contained decondensed chromosomes with interphase microtubules, consistent with the abolishment of the mitotic arrest. These results showed that the spindle checkpoint pathway functions to prevent the lethal chromosome missegregation in the alp5-1134 mutant.
Alp5 Plays a Role in Transcriptional Silencing Specifically at the Core Centromeres
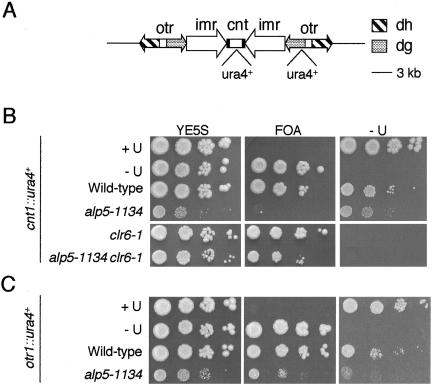
Activation of the spindle checkpoint and the appearance of mono-oriented spindles suggested that in alp5-1134 cells chromosome biorientation, a process essential for bipolar microtubule attachment (Tanaka, 2002), fails to be established. As a first step to address whether structural and functional integrity of kinetochores and centromeres is maintained in this mutant, the effect of the alp5-1134 mutation toward centromere gene silencing was examined. As mentioned above, fission yeast centromeres comprise three structural subdomains, a central cnt region, and two flanking repeated imr and otr structures (Figure 7A), and transcription is repressed at all these three sites as in animal centromeres (Allshire et al., 1994, 1995). We tested a centromere-desilencing phenotype by using standard colony assays, in which the ura4+ marker gene is integrated into individual centromeric sites (Allshire et al., 1995).
Figure 7.
Desilencing at the core centromere in the alp5-1134 mutant. (A) Schematic diagram showing the centromeric region of chromosome I. The position of the integrated ura4+ marker gene is shown. (B) Loss of silencing at the cnt region. Six strains (control Ura+ and Ura- strains, and wild-type, alp5-1134, clr6-1, and alp5-1134clr6-1 strains containing the ura4+ marker gene integrated at the core cnt region) were spotted in a serial dilution on plates containing rich media, rich media containing 5′FOA (100 μg/ml), or minimal plates lacking uracil and were incubated at 32°C for 4 d. (C) Normal silencing at the otr region. Four strains (control Ura+ and Ura- strains, and wild-type and alp5-1134 strains containing the ura4+ marker gene integrated at the otr region) were spotted in a manner similar as in B.
To our surprise we found that at 32°C the ura4+ gene integrated at cnt is desilenced in the alp5-1134 mutant, because no growth was observed on 5′-fluoroorotic acid (FOA)-containing plates, whereas cells grew on plates lacking uracil (Figure 7B, row 4). This desilencing phenotype of alp5-1134 also was dependent upon the presence of the functional Clr6 HADC, because desilencing was no longer observed in double mutants (row 6). In contrast, at the otr region, growth properties of alp5-1134 were similar to that of the wild-type cells, although overall growth was compromised at this semirestrictive temperature (Figure 7C). Thus, Alp5 is an essential factor for maintaining transcriptional silencing at the core domain, but it is not required for its repression at the flanking heterochromatin region.
Chromatin Structures at the Core Centromere Are Maintained in alp5-1134
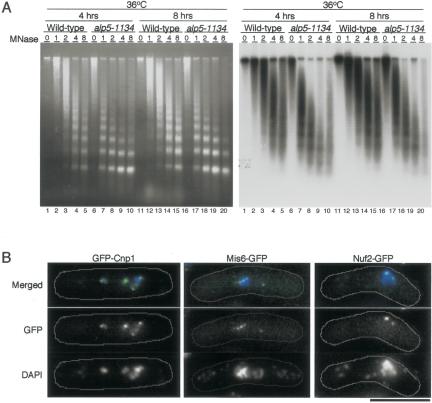
It is known that the core centromere regions comprise unique chromatin structures, in which MNase digestion gives smeared patterns instead of the regular nucleosomal ladders (Polizzi and Clarke, 1991; Takahashi et al., 1992). Several kinetochore proteins, such as Cnp1, Mal2, Mis6, and Sim4, are required for the establishment and the maintenance of these unique structures (Saitoh et al., 1997; Takahashi et al., 2000; Jin et al., 2002; Pidoux et al., 2003). Intriguingly, mutations of these kinetochore components lead to specific desilencing phenotypes at the cnt region (Jin et al., 2002; Pidoux et al., 2003), which also occurs in the alp5-1134 mutant. Given this parallelism, we next examined the chromatin structures at the centromeres by using MNase. The result showed that no gross differences were observed, i.e., similar smeared patterns were detected at the restrictive temperature (36°C; Figure 8A), although in alp5-1134, the periodic ladder patterns were slightly more apparent after 4 h (lanes 7-10).
Figure 8.
Overall chromatin structure in the core centromere region is maintained in the alp5-1134 mutant. (A) Chromatin structures at the centromere regions. Nuclear chromatin fractions prepared from wild-type (972) or the alp5-1134 mutant grown at either 36°C for 4 h or 8 h were digested with MNase for 0, 1, 2, 4, and 8 min, and Southern hybridization was performed using the core cnt probe. Patterns of ethidium bromide staining (left) and Southern hybridization (right) are shown. (B) Correct localization of kinetochore components in alp5-1134. Three alp5-1134 mutant strains containing GFP-Cnp1, Mis6-GFP, and Nuf2-GFP, respectively, were incubated at 36°C for 6 h, and GFP signals were observed under fluorescence microscopy. GFP (in green), 4,6-diamidino-2-phenylindole (DAPI) (in blue), and merged images are shown. Cell morphology was marked with thin white lines along the cell surface. Bar, 10 μm.
We also examined the cellular localization of Cnp1, Mis6, and Nuf2 in alp5-1134. All these three proteins seemed to localize to the kinetochore region normally (Figure 8B). Therefore, Alp5 is required for transcriptional silencing at the core centromere; however, unlike kinetochore components, Alp5 function is dispensable for the maintenance of the core kinetochore structure itself. Together, Alp5 is required for the establishment of bipolar attachment of the kinetochore to the spindle and plays a regulatory role in transcriptional silencing at the core centromere region.
DISCUSSION
In this study, we have presented findings on the in vivo function of fission yeast Alp5, a homolog of Arp4/BAF53. We show that Alp5 plays an essential role in the mitotic progression. The ts alp5-1134 mutant activates a spindle assembly checkpoint, and in its absence the level of chromosome missegregation becomes lethal for the mutant. We also have shown that Alp5 plays a crucial role in the centromere function, in particular gene silencing at the core centromere region. The requirement of Alp5 for transcriptional repression at the centromere is unexpected, because the role of Arp4/BAF53 has been considered as global gene expression or signal-dependent transcriptional stimulation (Jiang and Stillman, 1996; Zhao et al., 1998; Harata et al., 2002; Rando et al., 2002). Our work, therefore, sheds a novel light onto the centromere/kinetochore function of actin-related Alp5.
Requirement of Alp5 for Global Histone H4 Acetylation
Our work has highlighted two main roles of Alp5. One is its involvement in the global histone H4 acetylation, and the other for centromere/kinetochore integrity. In the alp5-1134 mutant, the global level of histone H4 tail acetylation (at lysines 5, 8, and 12) is decreased dramatically. Consistent with this, Alp5 interacts with the MYST-type HAT Mst1, which is a homolog of the budding yeast Esa1 and human Tip60. Esa1 and Tip60 are the catalytic subunits of the NuA4 complexes, which are required for histone H4 acetylation (Doyon and Côté, 2004). In spite of many previous analyses, the requirement of these conserved actin-related proteins for maintaining the global level of H4 acetylation remains elusive (Olave et al., 2002). Instead, in vitro assays show that in both budding yeast and human systems, Arp4 and BAF53 are not required for HAT activity toward the nucleosomes as substrates (Boudreault et al., 2003; Doyon et al., 2004). Thus, our result is the first in vivo demonstration that Alp5 plays an indispensable role in H4 acetylation reaction.
The question then arises as to how this actin-related protein is involved in histone H4 acetylation in the cell. In the ts alp5-1134 mutants, the size of the Alp5-containing complex remains the same as the wild type. This implies that Alp5 is not required for the assembly of the complex, in which overall complex structure would be maintained in the absence of Alp5 function. It is conceivable that Alp5 may have a regulatory function toward the catalytic activity of Mst1-HAT, such as targeting of the complex to the chromatin at specific sites. Further detailed in vivo analysis combined with in vitro biochemistry would be necessary to address this issue.
We also have shown that TSA treatment rescues alp5-1134 significantly. From the genetic analysis of the HDAC mutants, Clr6 was found to play an antagonistic function to Alp5. In particular, the determination of histone H4 acetylation levels by using antibodies specific for acetylated H4 lysine uncovered that, as reported previously (Bjerling et al., 2002; Nakayama et al., 2003), Clr6 is an HDAC directly deacetylating histone H4 tails (at 5, 8, and 12), whereas the Alp5-HAT acetylates these sites in a converse manner. This result is in line with the previous reports, given that Clr6 is a homolog of budding yeast Rpd3, which is responsible for the deacetylation of histone H4 at 5, 8, and 12 lysines (Bernstein et al., 2000; Suka et al., 2001; Robyr et al., 2002). It should be noted that Clr6 also plays a critical role in genome stability and chromosome segregation (Nakayama et al., 2003), supporting the notion that the coordinated regulation of Alp5-HAT and Clr6-HDAC is vital for the cell.
Kinetochore-Spindle Attachment and Alp5 Function
The prolonged localization of Mad2 to the mitotic kinetochore highly suggests that in the alp5-1134 mutant, the kinetochore fails to attach to the spindle. One possible role of Alp5 in the kinetochore-spindle attachment could stem from the regulatory function that ensures gene silencing at the cnt core centromere. In this scenario, it is plausible that in the alp5-1134 mutant, impairment in the kinetochore structure and function results in transcriptionally active cnt, leading to an inefficient capture of the kinetochore by the spindle. Condensed chromosomes observed in alp5-1134 also might arise from somehow compromised chromatin structures, although this also could be explained by the mitotic arrest derived from spindle checkpoint activation with low APC/C activity in this mutant. Another possible explanation for the unattached kinetochores in the mutant is that expression of some genes, which are essential for the kinetochore-spindle interaction, is down-regulated. This also would lead to a failure to establish of bipolar microtubule attachment. We have found that both interphase microtubules and mitotic spindles look more stable in the alp5-1134 mutant than in wild-type cells and that the alp5-1134 mutant is indeed resistant to microtubule-destabilizing drugs (Minoda and Toda, unpublished data). This clearly contrasts to other mitotic mutants, which result in the activation of the spindle checkpoint and display hypersensitivity to these drugs (Sato et al., 2003). Thus, Alp5 may be involved in the kinetochore-spindle attachment in an indirect manner via transcriptional regulation of genes involved in microtubule dynamics. Further analysis of the mechanisms underlying the requirement of Alp5 for bipolar microtubule attachment is needed to clarify this point.
The Role of Alp5 in the Core Centromere
The finding that Alp5 is essential for maintaining the silenced state at the core centromere region is novel and unpredicted. Mutations in the known kinetochore components such as mal2, mis6, mis12, and sim4 display transcriptional desilencing at cnt; however, in these mutants, the spindle checkpoint pathway remains inert (Pidoux et al., 2003). It is postulated that when the centromere/kinetochore structures are disrupted physically, the checkpoint signaling is no longer sensed, thereby these mutants being checkpoint insensitive (Cleveland et al., 2003). In the case of alp5-1134, on the other hand, consistent with the activation of the spindle checkpoint, MNase digestion experiments indicate that characteristic smeared patterns of the core centromeric chromatin are mostly maintained and all three kinetochore proteins, Cnp1, Mis6, and Nuf2, together with Mad2 and Bub1, localize to the centromere/kinetochore region. Alp5 is therefore a novel regulatory factor, the mutation of which results in the impairment of core centromere function, including transcriptional desilencing and checkpoint activation without disrupting the core kinetochore structure.
Heterochromatin regions, including the centromeres, are known to contain hypoacetylayed histone H4 in general (Grunstein, 1997), which also is shown to be the case in fission yeast (Mellone et al., 2003). Consequently, further down-regulation of histone H4 acetylation at cnt by the alp5-1134 mutation would not be predicted to lead to transcriptional silencing defects. The role for Alp5 in maintaining silencing at cnt could be, therefore, independent of its histone acetylation function. However, we have shown that in the double mutant between alp5-1134 and clr6-1 transcriptional silencing at cnt is restored, suggesting the importance of histone H4 acetylation for silencing at cnt. We envisage that balanced equilibrium between histone acetylation and deacetylation may be crucial for transcriptional silencing at the core centromere. Alternatively Alp5-dependent histone H4 acetylation might be linked to chromatin architecture, such as via chromatin remodeling reactions, which represses transcriptional accessibility at the core centromere region.
Recently, it was reported that Arp6 homolog in fission yeast is specifically required for telomere silencing, but not for centromere (Ueno et al., 2004). Given this distinct requirement of two ARPs (Alp5/Arp4 and Arp6) for silencing at two heterochromatin regions, i.e., centromere and telomere, respectively, it is tempting to speculate that individual nuclear ARPs might play a positive role in gene silencing at the heterochromatins in a location-specific manner. Understanding the mechanism underlying the harmonious regulation at the centromeres regarding chromatin structures, transcriptional silencing, and spindle checkpoint signaling is the next crucial question to be addressed, and further study of Alp5 and other nuclear ARPs would be the key to answer this issue.
Supplementary Material
Acknowledgments
We thank Drs. Robin Allshire, Tony Carr, Karl Ekwall, Shiv Grewal, Kevin Hardwick, Michael Knop, Jean-Paul Javerzat, Jonathan Millar, Yota Murakami, Julie Promisel Cooper, Da-Qiao Ding, Keith Gull, Yasushi Hiraoka, Hiromi Maekawa, Osami Niwa, Elmar Schiebel, Mizuki Shimanuki, Mitsuhiro Yanagida, and Minoru Yoshida for providing strains and materials used in this study. We thank Dr. Paul Bates and Biomolecular Modeling Laboratory for helping construct the 3-D structure of the Alp5 and Alp5-1134 proteins. We thank Drs. Alison Pidoux and Creighton T. Tuzon for advice on histone preparations and chromatin immunoprecipitation and Dr. Satoshi Katayama for cooperative help and discussion. We thank Dr. Jesper Q. Svejstrup for critical reading of the manuscript and useful suggestions. This work is supported by the Cancer Research UK (to T.T.) and the Human Frontier Science Program research grant (to T.T.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-06-0519. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0519.
Abbreviations used: ARP, actin-related protein; APC/C, anaphase promoting complex/cyclosome; 5′FOA, 5′-fluoroorotic acid; HAT, histone acetyl transferase; HDAC, histone deacetylase; MNase, micrococcal nuclease; MYST, MOZ, Ybf2/Sas3, Sas2, and Tip60; NuA4, nucleosome acetyltransferase of histone H4; TSA, trichostatin A; ts, temperature-sensitive.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Allard, S., Utley, R. T., Savard, J., Clarke, A., Grant, P., Brandl, C. J., Pillus, L., Workman, J. L., and Cote, J. (1999). NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18, 5108-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire, R. C., Javerzat, J. P., Redhead, N. J., and Cranston, G. (1994). Position effect variegation at fission yeast centromeres. Cell 76, 157-169. [DOI] [PubMed] [Google Scholar]
- Allshire, R. C., Nimmo, E. R., Ekwall, K., Javerzat, J. P., and Cranston, G. (1995). Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9, 218-233. [DOI] [PubMed] [Google Scholar]
- Bähler, J., Wu, J., Longtine, M. S., Shah, N. G., McKenzie III, A., Steever, A. B., Wach, A., Philippsen, P., and Pringle, J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. [DOI] [PubMed] [Google Scholar]
- Bernard, P., Hardwick, K., and Javerzat, J.-P. (1998). Fission yeast Bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 143, 1775-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, B. E., Tong, J. K., and Schreiber, S. L. (2000). Genomewide studies of histone deacetylase function in yeast. Proc. Natl. Acad. Sci. USA 97, 13708-13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, A. W., Yu, D. Y., Pray-Grant, M. G., Qiu, Q., Harmon, K. E., Megee, P. C., Grant, P. A., Smith, M. M., and Christman, M. F. (2002). Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419, 411-415. [DOI] [PubMed] [Google Scholar]
- Bjerling, P., Silverstein, R. A., Thon, G., Caudy, A., Grewal, S., and Ekwall, K. (2002). Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and In vivo specificity. Mol. Cell. Biol. 22, 2170-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault, A. A., Cronier, D., Selleck, W., Lacoste, N., Utley, R. T., Allard, S., Savard, J., Lane, W. S., Tan, S., and Cote, J. (2003). Yeast Enhancer of Poly-comb defines global Esa1-dependent acetylation of chromatin. Genes Dev. 17, 1415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D. W., Mao, Y., and Sullivan, K. F. (2003). Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407-421. [DOI] [PubMed] [Google Scholar]
- Decottignies, A., Zarzov, P., and Nurse, P. (2001). In vivo localisation of fission yeast cyclin-dependent protein kinase cdc2p and cyclin B during mitosis and meiosis. J. Cell Sci. 114, 2627-2649. [DOI] [PubMed] [Google Scholar]
- Doyon, Y., and Côté, J. (2004). The highly conserved and multifunctional NuA4 HAT complex. Curr. Opin. Genet. Dev, 14, 147-154. [DOI] [PubMed] [Google Scholar]
- Doyon, Y., Selleck, W., Lane, W. S., Tan, S., and Côté, J. (2004). Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 24, 1884-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall, K., Olsson, T., Turner, B. M., Cranston, G., and Allshire, R. C. (1997). Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91, 1021-1032. [DOI] [PubMed] [Google Scholar]
- Freeman-Cook, L. L., Sherman, J. M., Brachmann, C. B., Allshire, R. C., Boeke, J. D., and Pillus, L. (1999). The Schizosaccharomyces pombe hst4+ gene is a SIR2 homologue with silencing and centromeric functions. Mol. Biol. Cell 10, 3171-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarneau, L., Nourani, A., Boudreault, A.A., Zhang, Y., Heliot, L., Allard, S., Savard, J., Lane, W. S., Stillman, D. J., and Cote, J. (2000). Multiple links between the NuA4 histone acetyltransferase complex and epigenetic control of transcription. Mol. Cell 5, 927-937. [DOI] [PubMed] [Google Scholar]
- Garcia, M. A., Vardy, L., Koonrugsa, N., and Toda, T. (2001). Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 20, 3389-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson, H. V., and Hawse, W. F. (2002). Molecular evolution of the actin family. J. Cell Sci. 115, 2619-2622. [DOI] [PubMed] [Google Scholar]
- Gorzer, I., Schuller, C., Heidenreich, E., Krupanska, L., Kuchler, K., and Wintersberger, U. (2003). The nuclear actin-related protein Act3p/Arp4p of Saccharomyces cerevisiae is involved in transcription regulation of stress genes. Mol. Microbiol. 50, 1155-1171. [DOI] [PubMed] [Google Scholar]
- Grewal, S.I.S., Bonaduce, M. J., and Klar, A.J.S. (1998). Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150, 563-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein, M. (1997). Histone acetylation in chromatin structure and transcription. Nature 389, 349-352. [DOI] [PubMed] [Google Scholar]
- Harata, M., Karwan, A., and Wintersberger, U. (1994). An essential gene of Saccharomyces cerevisiae coding for an actin-related protein. Proc. Natl. Acad. Sci. USA 91, 8258-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata, M., Zhang, Y., Stillman, D. J., Matsui, D., Oma, Y., Nishimori, K., and Mochizuki, R. (2002). Correlation between chromatin association and transcriptional regulation for the Act3p/Arp4 nuclear actin-related protein of Saccharomyces cerevisiae. Nucleic Acids Res. 30, 1743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikui, A. E., Furuya, K., Yanagida, M., and Matsumoto, T. (2002). Control of localization of a spindle checkpoint protein, Mad2, in fission yeast. J. Cell Sci. 115, 1603-1610. [DOI] [PubMed] [Google Scholar]
- Ikura, T., Ogryzko, V. V., Grigoriev, M., Groisman, R., Wang, J., Horikoshi, M., Scully, R., Qin, J., and Nakatani, Y. (2000). Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102, 463-473. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. W., and Stillman, D. J. (1996). Epigenetic effects on yeast transcription caused by mutations in an actin-related protein present in the nucleus. Genes Dev. 10, 604-619. [DOI] [PubMed] [Google Scholar]
- Jin, Q.-W., Pidoux, A. L., Decker, C., Allshire, R. C., and Fleig, U. (2002). The Mal2p protein Is an essential component of the fission yeast centromere. Mol. Cell. Biol. 22, 7168-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch, W., Mannherz, H. G., Suck, D., Pai, E. F., and Holmes, K. C. (1990). Atomic structure of the actin: DNase I complex. Nature 347, 37-44. [DOI] [PubMed] [Google Scholar]
- Kim, Y. B., Honda, A., Yoshida, M., and Horinouchi, S. (1998). phd1+, a histone deacetylase gene of Schizosaccharomyces pombe, is required for the meiotic cell cycle and resistance to torichostatin A. FEBS Lett. 436, 193-196. [DOI] [PubMed] [Google Scholar]
- Kobor, M. S., Venkatasubrahmanyam, S., Meneghini, M. D., Gin, J. W., Jennings, J. L., Link, A. J., Madhani, H. D., and Rine, J. (2004). A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone Variant H2A.Z into euchromatin. PLoS Biol. 2, 587-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone, B. G., Ball, L., Suka, N., Grunstein, M. R., Partridge, J. F., and Allshire, R. C. (2003). Centromere silencing and function in fission yeast is governed by the amino terminus of Histone H3. Curr. Biol. 13, 1748-1757. [DOI] [PubMed] [Google Scholar]
- Mizuguchi, G., Shen, X., Landry, J., Wu, W. H., Sen, S., and Wu, C. (2004). ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303, 343-348. [DOI] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analyses of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 773-782. [DOI] [PubMed] [Google Scholar]
- Nabetani, A., Koujin, T., Tsutsumi, C., Haraguchi, T., and Hiraoka, Y. (2001). A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma 110, 322-334. [DOI] [PubMed] [Google Scholar]
- Nakayama, J., Xiao, G., Noma, K., Malikzay, A., Bjerling, P., Ekwall, K., Kobayashi, R., and Grewal, S.I.S. (2003). Alp13, an MRG family protein, is a component of fission yeast Clr6 histone deacetylase required for genomic integrity. EMBO J. 22, 2776-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, O., Matsumoto, T., Chikashige, Y., and Yanagida, M. (1989). Characterization of Schizosaccharomyces pombe minichromosome deletion derivatives and a functional allocation of their centromere. EMBO J. 8, 3045-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave, I. A., Reck-Peterson, S. L., and Crabtree, G. R. (2002). Nuclear actin and actin-related proteins in chromatin remodeling. Annu. Rev. Biochem. 71, 755-781. [DOI] [PubMed] [Google Scholar]
- Olsson, T. G., Ekwall, K., Allshire, R. C., Sunnerhagen, P., Partridge, J. F., and Richardson, W. A. (1998). Genetic characterization of hda1+, a putative fission yeast histone deacetylase gene. Nucleic Acids. res. 26, 3247-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux, A., Mellone, B., and Allshire, R. (2004). Analysis of chromatin in fission yeast. Methods 33, 252-259. [DOI] [PubMed] [Google Scholar]
- Pidoux, A. L., Richardson, W., and Allshire, R. C. (2003). Sim 4, a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J. Cell Biol. 161, 295-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi, C., and Clarke, L. (1991). The chromatin structure of centromeres from fission yeast: differentiation of the central core that correlates with function. J. Cell Biol. 112, 191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe, P., Hirata, D., Childs, D., Vardy, L., and Toda, T. (1998). Identification of novel temperature-sensitive lethal alleles in essential β-tubulin and nonessential α2-tubulin genes as fission yeast polarity mutants. Mol. Biol. Cell 9, 1757-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando, O. J., Zhao, K., Janmey, A., and Crabtree, G. R. (2002). Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc. Natl. Acad. Sci. USA 99, 2824-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyr, D., Suka, Y., Xenarios, I., Kurdistani, S. K., Wang, A., Suka, N., and Grunstein, M. (2002). Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109, 437-446. [DOI] [PubMed] [Google Scholar]
- Roth, S. Y., Denu, J. M., and Allis, C. D. (2001). Histone acetyltransferases. Annu. Rev. Biochem. 70, 81-120. [DOI] [PubMed] [Google Scholar]
- Saitoh, S., Takahashi, K., and Yanagida, M. (1997). Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell 90, 131-143. [DOI] [PubMed] [Google Scholar]
- Sato, M., Vardy, L., Koonrugsa, N., Tournier, S., Millar, J.B.A., and Toda, T. (2003). Deletion of Mia1/Alp7 activates Mad2-dependent spindle assembly checkpoint in fission yeast. Nat. Cell Biol. 5, 764-766. [DOI] [PubMed] [Google Scholar]
- Shankaranarayana, G. D., Motamedi, M. R., Moazed, D., and Grewal, S. I. (2003). Sir2 regulates histone h3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr. Biol. 13, 1240-1246. [DOI] [PubMed] [Google Scholar]
- Shen, X., Mizuguchi, G., Hamiche, A., and Wu, C. (2000). A chromatin remodelling complex involved in transcription and DNA processing. Nature 406, 541-544. [DOI] [PubMed] [Google Scholar]
- Shen, X., Ranallo, R., Choi, E., and Wu, C. (2003). Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol. Cell 12, 147-155. [DOI] [PubMed] [Google Scholar]
- Skoufias, D. A., Andreassen, P. R., Lacroix, F. B., Wilson, L., and Margolis, R. L. (2001). Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA 98, 4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner, D. E., and Berger, S. L. (2000). Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64, 435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl, B. D., and Allis, D. (2000). The language of covalent histone modifications. Nature [Lond.] 403, 41-45. [DOI] [PubMed] [Google Scholar]
- Suka, N., Suka, Y., Carmen, A. A., Wu, J., and Grunstein, M. (2001). Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8, 473-479. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Chen, E. S., and Yanagida, M. (2000). Requirement of Mis6 centromere connector for localizaing a CENP-A-like protein in fission yeast. Science 288, 2215-2219. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Murakami, S., Chikashige, Y., Funabiki, H., Niwa, O., and Yanagida, M. (1992). A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell 3, 819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. U. (2002). Bi-orientating chromosomes on the mitotic spindles. Curr. Opin. Cell Biol. 14, 365-371. [DOI] [PubMed] [Google Scholar]
- Tatebe, H., and Yanagida, M. (2000). Cut8, essential for anaphase, controls localization of 26S proteasome, facilitating destruction of cyclin and Cut2. Curr. Biol. 10, 1329-1338. [DOI] [PubMed] [Google Scholar]
- Thon, G., Cohen, A., and Klar, A. J. (1994). Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138, 29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda, Y., Furuya, K., Goshima, G., Nagao, K., Takahashi, K., and Yanagida, M. (2002). Requirement of chromatid cohesion proteins Rad21/Scc1 and Mis4/Scc2 for normal spindle-kinetochore interaction in fission yeast. Curr. Biol. 12, 347-358. [DOI] [PubMed] [Google Scholar]
- Ueno, M., Murase, T., Kibe, T., Ohashi, N., Tomita, K., Murakami, Y., Uritani, M., Ushimaru, T., and Harata, M. (2004). Fission yeast Arp6 is required for telomere silencing, but functions independently of Swi6. Nucleic Acids Res. 32, 736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin, E., Dequiedt, F., and Kasler, H. G. (2003). Class II histone deacetylases: versatile regulators. Trends Genet. 19, 286-293. [DOI] [PubMed] [Google Scholar]
- Wang, W., Cote, J., Xue, Y., Zhou, S., Khavari, P. A., Biggar, S. R., Muchardt, C., Kalpana, G. V., Goff, S. P., Yaniv, M., Workman, J. L., and Crabtree, G. R. (1996a). Purification and biochemical heterogeneity of the mammalian SWISNF complex. EMBO J. 15, 5370-5382. [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Xue, Y., Zhou, S., Kuo, A., Cairns, B. R., and Crabtree, G. R. (1996b). Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev. 10, 2117-2130. [DOI] [PubMed] [Google Scholar]
- Waters, J. C., Chen, R.-H., Murray, A. W., and Salmon, E. D. (1998). Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman, J. L., and Kingston, R. E. (1998). Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67, 545-579. [DOI] [PubMed] [Google Scholar]
- Yoshida, M., Kijima, M., Akita, M., and Beppu, T. (1990). Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174-17179. [PubMed] [Google Scholar]
- Zhao, K., Wang, W., Rando, O. J., Xue, Y., Swiderek, K., Kuo, A., and Crabtree, G. R. (1998). Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95, 625-636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.