Abstract
Pancreatic cancer is one of the most lethal cancer types. Enhancer of zeste homolog 2 (EZH2) is an oncogenic protein overexpressed in pancreatic cancer, and EZH2 could be a potential therapeutic target for the treatment of pancreatic cancer. Although significant progress has been made toward understanding the function and deregulation of EZH2 in cancer cells, the posttranslational regulation of EZH2 in cancer cells is still unclear. F-box and WD repeat domain-containing 7 (FBW7) acts as a tumor suppressor by targeting multiple oncoprotein substrates for ubiquitination and degradation. Here we demonstrate that EZH2 is a bona fide substrate of FBW7 in pancreatic cancer cells. We provide evidence that the activated CDK5 kinase is involved in the EZH2 phosphorylation that is required for FBW7-mediated degradation. We further show that FBW7 suppresses EZH2 activity and inhibits tumor migration and invasion via degradation of EZH2 in pancreatic cancer cells. Furthermore, immunohistochemistry analysis revealed that expression of EZH2 protein negatively correlates with FBW7 protein levels in a cohort of human pancreatic cancer specimens. Collectively, our findings demonstrate that FBW7 is a novel E3 ligase of EZH2 that regulates the EZH2 protein level in pancreatic cancer and represents a viable strategy for effective treatment of pancreatic cancer.
Keywords: cell invasion, cell migration, E3 ubiquitin ligase, pancreatic cancer, ubiquitylation (ubiquitination)
Introduction
Pancreatic cancer is one of the most lethal cancer types (1). The most common type of pancreatic cancer is adenocarcinoma (accounting for 95%), which originates from the exocrine part of the pancreas and is classified as pancreatic ductal adenocarcinoma (PDAC).3 PDAC is insensitive to both chemo- and radiotherapy (1). The prognosis for pancreatic cancer remains poor, with only 4.4% having a 5-year survival rate, making it the fourth leading cause of all cancer deaths in China (2). Thus, there is an immediate need to discover novel therapeutic targets for pancreatic cancer.
Aberrations of epigenetic regulation play an important role in the initiation and progression of cancers; therefore, the biochemical mediators of these processes could serve as novel and efficacious therapeutic targets (3–5). Enhancer of zeste homolog 2 (EZH2), a histone methyltransferase, interacts with other Polycomb-group (PcG) proteins such as SUZ12 and embryonic ectodomain development (EED), to form a protein complex called Polycomb repressive complex 2 (PRC2) (6). In many types of cancer cells, EZH2 epigenetically represses tumor suppressor gene expression through trimethylating H3K27 in order to mediate cell proliferation, invasion, and migration (7, 8). Moreover, EZH2 is highly expressed in many types of solid tumors, such as pancreatic cancer, and its higher expression is associated with a poor outcome (8, 9). It has been shown previously that EZH2 expression is regulated by the RB-E2F1 pathway (10) or by sex hormones such as androgens (11). Moreover, EZH2 is also regulated by microRNAs such as miR101 (12). Although significant progress has been made toward understanding the function and deregulation of EZH2 in cancer cells, the posttranslational regulation of EZH2 in cancer cells is still unclear.
F-box and WD repeat domain-containing 7 (FBW7), the substrate recognition component of the Skp1·Cul1·F-box (SCF) ubiquitin ligase complex, binds to phosphorylated substrates within conserved phosphodegron motifs called Cdc4 phosphodegrons (CPDs) (13). FBW7 functions as a tumor suppressor by targeting multiple oncoprotein substrates for ubiquitination and degradation, including cyclin E, c-Myc, c-Jun, Notch-1, and SREBP1 (14–19). Dysregulation of FBW7 has been proposed to drive the progression of pancreatic cancer (20).
In this study, we demonstrated that FBW7 acted as an E3 ligase of EZH2 in pancreatic cancer cells and found that the activated CDK5 kinase was involved in the EZH2 phosphorylation that was required for FBW7-mediated EZH2 degradation. Furthermore, we showed that EZH2 and FBW7 protein levels are negatively correlated in pancreatic cancer patient specimens. Taken together, these results are contributing to a better understanding of the regulatory mechanism of EZH2 in human pancreatic cancer and may also shed new light on developing novel therapeutic strategies for pancreatic cancer.
Results
FBW7 interacts with EZH2 in pancreatic cancer cells
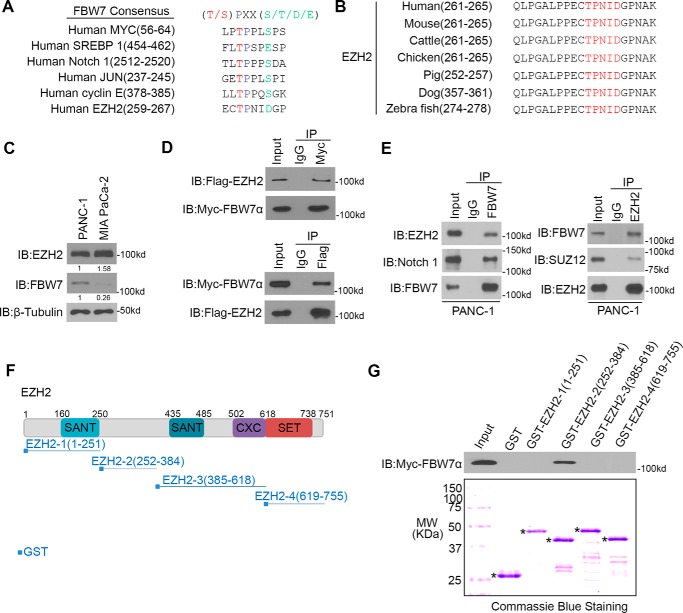
The FBW7-binding consensus motif (T/S)PXX(S/T/D/E) has been identified in several FBW7 substrates, including cyclin E, c-Myc, c- Jun, Notch-1, and SREBP1 (14–19). We performed a protein motif search and discovered that EZH2 harbors one perfectly matched (261TPNID265) FBW7- binding motif (Fig. 1, A and B). Then we examined the basal protein levels of EZH2 and FBW7 in PANC-1 and MIA PaCa2 cells (Fig. 1C). We found that there was a higher expression level of EZH2 but a lower expression level of FBW7 in MIA PaCa-2 cells compared with PANC-1 cells, which indicates that EZH2 might negatively correlate with FBW7 expression in these two cell lines.
Figure 1.
FBW7 interacts with EZH2 in pancreatic cancer cells. A, comparison of FBW7 binding sites in EZH2 with the FBW7-binding consensus motif (Cdc4- phosphodegron) defined in the known FBW7 substrates. B, schematic of conserved CPD motifs in EZH2 in different species. C, Western blotting analysis of WCLs of PANC-1 and MIA PaCa-2 cells. The immunoblots (IB) are representative of results from three independent experiments. D, Western blotting analysis of co-immunoprecipitation of ectopically expressed FLAG-EZH2 and Myc-FBW7α in 293T cells (n = 3). E, Western blotting analysis of co-immunoprecipitation of endogenous FBW7 and EZH2 proteins in PANC-1 cells (n = 3). F, schematic depicting a set of GST-EZH2 recombinant protein constructs. G, Western blotting analysis of FBW7 proteins in PANC-1 WCL pulled down by GST or GST-EZH2 recombinant proteins (n = 3). The bottom panel shows Coomassie Blue staining of GST and GST-EZH2 recombinant protein input. Asterisks indicate the proteins with the correct molecular weight (MW).
This observation prompted us to investigate whether FBW7 functions as an E3 ubiquitin ligase of EZH2. Because substrate binding is a key event for E3 ligase-mediated ubiquitination and subsequent proteasome degradation, we first examined the interaction of FBW7 with EZH2 by using coimmunoprecipitation (co-IP) assay. Ectopically expressed FLAG-EZH2 protein was coimmunoprecipitated by Myc-FBW7. A similar result was obtained in a reciprocal co-IP experiment using FLAG antibody (Fig. 1D). Furthermore, the interaction between endogenous EZH2 and FBW7 in PANC-1 cells was confirmed by reciprocal co-IP assays (Fig. 1E). To test which fragment of EZH2 is involved in FBW7 binding, we generated four GST-EZH2 recombinant proteins (Fig. 1F) (21, 22). GST pulldown assays showed that FBW7 specifically interacted with GST-EZH2-2 (252–384) but not the other three truncation mutants of EZH2 or GST alone in the lysates of PANC-1 cells (Fig. 1G). These data suggest that FBW7 interacts with EZH2 in pancreatic cancer cells.
EZH2 is a substrate of FBW7 in pancreatic cancer cells
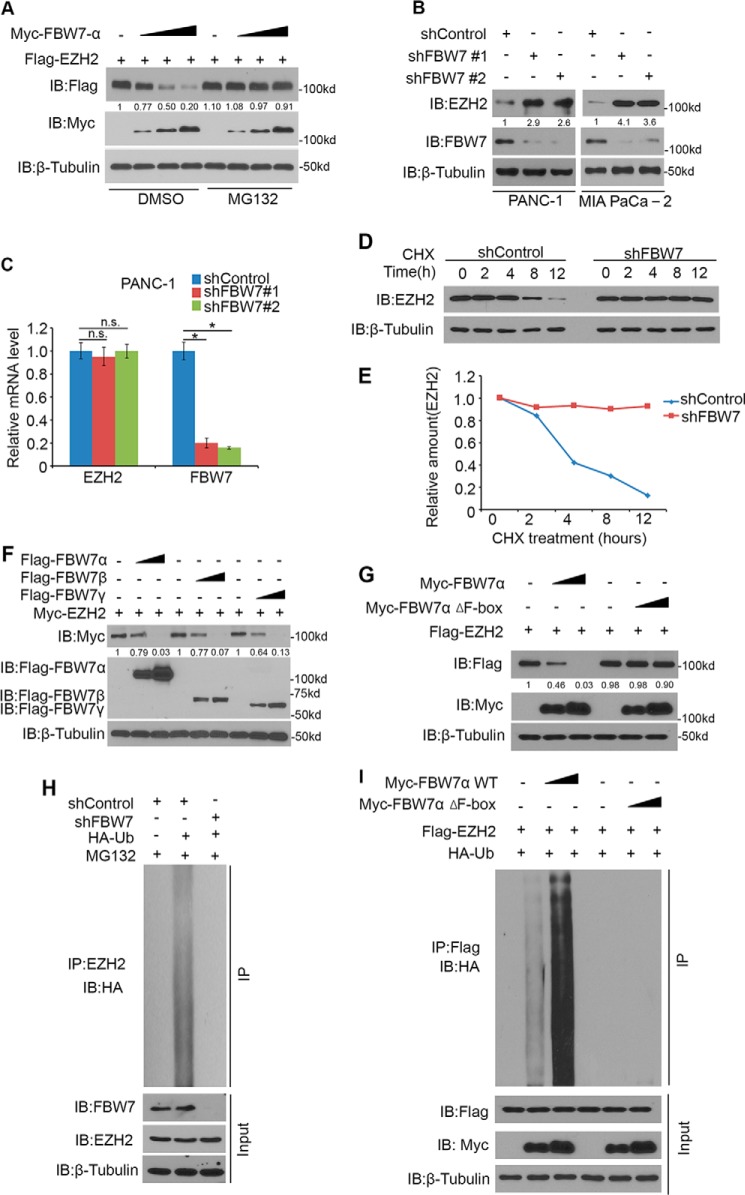
FBW7α is the dominant isoform of FBW7 (23). To investigate whether FBW7 functions as an E3 ubiquitin ligase of EZH2 in pancreatic cancer, we co-transfected EZH2 with increasing amounts of FBW7α in PANC-1 pancreatic cancer cells. Ectopically expressed EZH2 was down-regulated by co-expression of FBW7, and this effect was completely blocked by treatment with the proteasome inhibitor MG132 (Fig. 2A), which indicates that FBW7 decreased EZH2 protein levels via the proteasome pathway. To test the effect of FBW7 on endogenous EZH2 in pancreatic cancer cells, we performed knockdown of endogenous FBW7 by two independent FBW7-specific shRNAs, which increased EZH2 protein levels (Fig. 2B) and had no effect on EZH2 mRNA levels (Fig. 2C) in both PANC-1 and MIA PaCa-2 pancreatic cancer cells. Importantly, knockdown of FBW7 prolonged the half-life of endogenous EZH2 protein in PANC-1 cells (Fig. 2, D and E). Furthermore, PANC-1 cells were transfected with three isoforms (α, β, and γ) of FBW7 individually, resulting in a marked reduction in the protein level of EZH2 in a dose-dependent manner (Fig. 2F). FBW7 is the substrate-binding subunit of the SCFFBW7 ubiquitin ligase complex, and the F-box domain is essential for its E3 ligase activity (24). As shown in Fig. 2G, wild-type FBW7α, but not the FBW7α Δ F-box mutant, promoted EZH2 degradation. Furthermore, we found that knockdown of endogenous FBW7 in PANC-1 cells decreased the polyubiquitination of endogenous EZH2 (Fig. 2H). Meanwhile, EZH2 was polyubiquitinated by co-expression of wild-type FBW7α but not the enzymatically dead mutant (FBW7α Δ F-box) in a dose-dependent manner (Fig. 2I). Together, these data demonstrate that FBW7 promotes EZH2 protein ubiquitination and proteasome degradation in pancreatic cancer cells.
Figure 2.
EZH2 is a substrate of FBW7 in pancreatic cancer cells. A, Western blotting analysis of WCLs of PANC-1 cells transfected with the indicated constructs. Cells were treated with or without 20 μm MG132 for 8 h before harvest. Data are representative of multiple experiments (n = 3). IB, immunoblot. B, PANC-1 and MIA PaCa-2 cells were transfected with control or two independent FBW7-specific shRNAs. 48 h after transfection, cells were harvested for Western blotting analysis. Data are representative of multiple experiments (n = 3). C, PANC-1 cells were transfected with control or shRNAs as indicated. 48 h after transfection, cells were harvested for RT-qPCR analysis of EZH2 and FBW7 mRNAs. Data are mean ± S.D. from experiments with three replicates. *, p < 0.05 compared with the shControl group; n.s., not significant. D and E, PANC-1 cells were transfected with control and FBW7-specific shRNAs. After 48 h, cells were treated with 50 μg/μl cycloheximide (CHX). At different time points, cells were harvested for Western blotting analysis. At each time point, the intensity of EZH2 was normalized to the intensity of β-tubulin (loading control) first and then to the value at the 0-h time point. F, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis. Data are representative of multiple experiments (n = 3). G, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis. Data are representative of multiple experiments (n = 3). H, PANC-1 cells were transfected with the indicated plasmids for 48 h, followed by treatment with 20 μm MG132 for 8 h. Immunoprecipitated EZH2 proteins were analyzed by WB for ubiquitination. I, PANC-1 cells were transfected with the indicated plasmids for 16 h, followed by treatment with 20 μm MG132 for 8 h. Immunoprecipitated FLAG-EZH2 proteins were analyzed by WB for ubiquitination.
FBW7-mediated degradation of EZH2 is Thr261-dependent
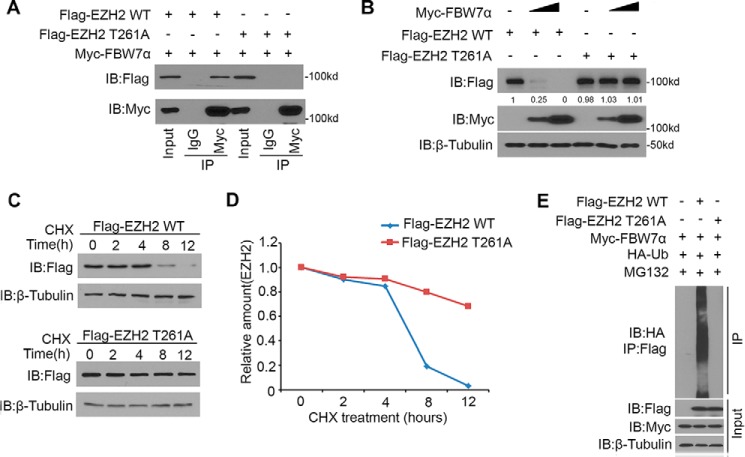
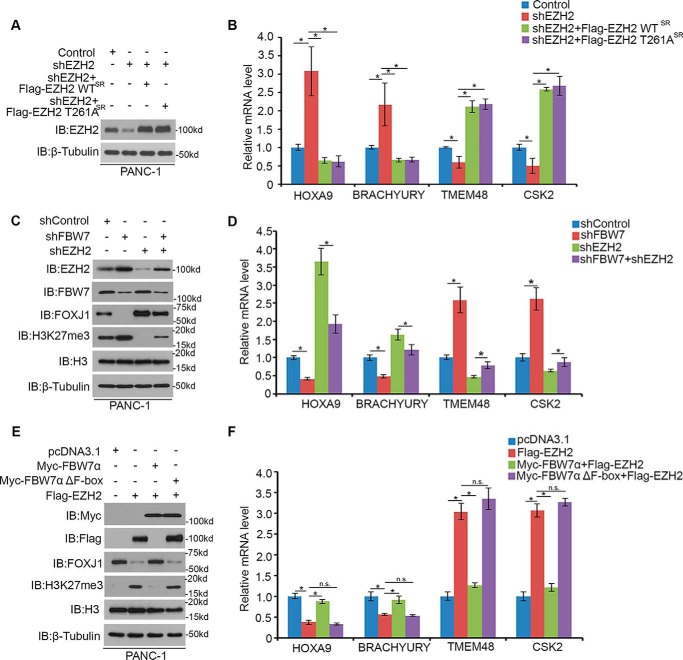
FBW7 often recognizes phosphorylated Ser/Thr residues in the CPD motif. We demonstrated that EZH2 harbored one perfectly matched (261TPNID265) FBW7-binding motif (Fig. 1B). Therefore, we speculated that the regulation of EZH2 by FBW7 might be mediated by phosphorylation of Thr261 in its CPD. We generated one mutant of EZH2 (T261A) to determine how FBW7 regulates degradation of this mutant. Co-IP assays demonstrated that EZH2-WT, but not the T261A mutant, was coimmunoprecipitated by Myc-FBW7 (Fig. 3A). These data indicated that Thr261 of EZH2 was required for EZH2-FBW7 interaction. Moreover, overexpression of FBW7 decreased the protein level of EZH2-WT but had little effect on the level of the EZH2-T261A mutant (Fig. 3B). Furthermore, the EZH2-T261A mutant prolonged the half-life of EZH2 protein (Fig. 3, C and D). To further determine the importance of Thr261 of EZH2 on FBW7-mediated degradation, EZH2 WT or EZH2 T261A mutant was co-transfected with FBW7α in PANC-1 cells. Ubiquitination assays demonstrated that FBW7α enhanced polyubiquitination of EZH2-WT but not the T261A mutant (Fig. 3E). These results suggest that FBW7-mediated degradation of EZH2 is Thr261-dependent.
Figure 3.
FBW7-mediated degradation of EZH2 is Thr261-dependent. A, Western blotting analysis of co-immunoprecipitation of ectopically expressed FLAG-EZH2 WT and FLAG-EZH2 T261A and Myc-FBW7α in 293T cells. Data are representative of multiple experiments (n = 3). IB, immunoblot. B, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis. Data are representative of multiple experiments (n = 3). C and D, PANC-1 cells were transfected with the indicated plasmids. After 24 h, cells were treated with 50 μg/μl CHX. At different time points, cells were harvested for Western blotting analysis. E, PANC-1 cells were transfected with the indicated plasmids for 16 h, followed by treatment with 20 μm MG132 for 8 h. Immunoprecipitated FLAG-EZH2 proteins were analyzed by WB for ubiquitination.
Activated CDK5 induces degradation of EZH2
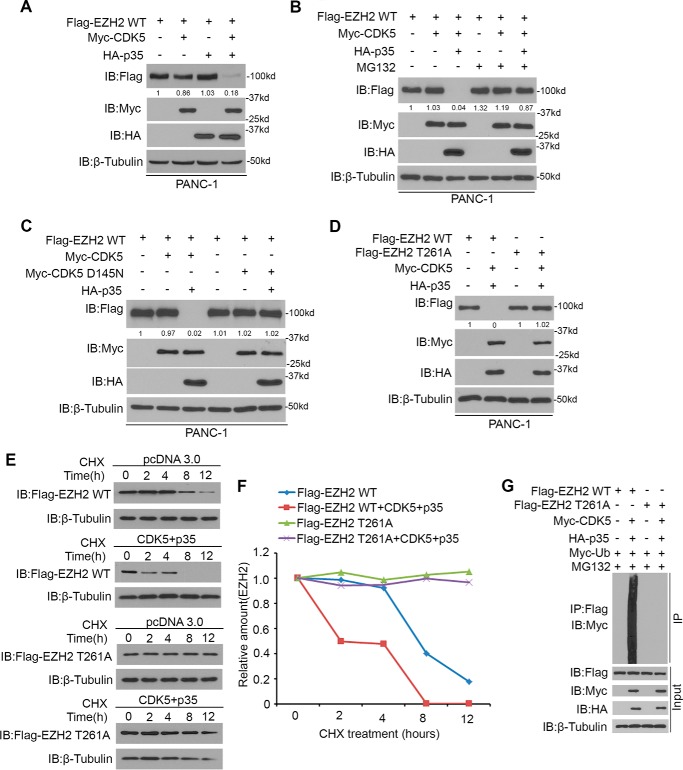
Phosphorylation of the first Ser/Thr in the CPD motif of the FBW7 target proteins by corresponding kinase, including GSK3β (25), CDK1/2 (26), or CDK5 (27), is required for FBW7-mediated degradation. To determine which kinase is involved in the phosphorylation of Thr261, we first checked the amino acid sequence in EZH2 and found that Thr261 in EZH2 did not match the general CDK1/2 consensus motif (S/TPXK/R) (26). Of note, knockdown of the endogenous GSK3β by shRNA or the treatment with a selective GSK3β inhibitor, LiCl, could not abrogate the degradation process of EZH2 induced by FBW7 (endogenous c-myc as a positive control) (supplemental Fig. 1, A and B). These results indicated that GSK3β was not likely to be the kinase involved in FBW7-mediated degradation of EZH2. We speculated that the Thr261 of EZH2 might be phosphorylated by CDK5 and then recognized by FBW7. To test whether CDK5 induces degradation of EZH2, overexpression of CDK5 or p35, an activator of CDK5, could not decrease the protein level of wild-type EZH2, but co-transfection with CDK5 and p35 resulted in down-regulation of wild-type EZH2, and this process was blocked by the proteasome inhibitor MG132 (Fig. 4, A and B). Furthermore, the dominant-negative mutant of CDK5 (CDK5 D145N) (27) could not decrease the protein level of EZH2 compared with wild-type CDK5 (Fig. 4C). Importantly, co-overexpression of CDK5 and p35 only led to down-regulation of wild-type EZH2, shortening the half-life and promoting polyubiquitination of wild-type EZH2 but not the EZH2 T261A mutant (Fig. 4, D–G). Together, these results suggest that activated CDK5 induces degradation of wild-type EZH2 and, at least, is involved in phosphorylation of Thr261 in EZH2.
Figure 4.
Activated CDK5 induces degradation of EZH2. A, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis. Data are representative of multiple experiments (n = 3). IB, immunoblot. B, Western blotting analysis of WCLs of PANC-1 cells transfected with the indicated constructs. Cells were treated with 20 μm MG132 for 8 h before harvest or left untreated. Data are representative of multiple experiments (n = 3). C and D, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis. Data are representative of multiple experiments (n = 3). E and F, PANC-1 cells were transfected with the indicated plasmids. After 24 h, cells were treated with 50 μg/μl CHX. At different time points, cells were harvested for Western blotting analysis. G, PANC-1 cells were transfected with the indicated plasmids for 16 h, followed by treatment with 20 μm MG132 for 8 h. Immunoprecipitated FLAG-EZH2 proteins were analyzed by WB for ubiquitination.
FBW7 suppresses EZH2 activity in pancreatic cancer cells
To evaluate the biological consequences of FBW7-mediated EZH2 degradation, we examined the effect of FBW7 on the transactivation of EZH2 target genes. It is known that EZH2 represses gene expression in a Polycomb-dependent way, and such target genes include FOXJ1, HOXA9, and BRACHYURY (6). In addition to the canonical Polycomb-dependent gene repression function, EZH2 also gains a Polycomb-independent gene activation function and promotes the gene expression of TMEM48 and CSK2 (28). As expected, knockdown of EZH2 by specific shRNAs increased the protein expression of FOXJ1, HOXA9, and BRACHYURY and down-regulated TMEM48 and CSK2 expression in PANC-1 cells. Intriguingly, the effect of EZH2 appears to be specific because restored expression of shRNA-resistant EZH2 by EZH2-WT or EZH2 T261A reversed the EZH2 knockdown-induced effect in its target genes, whereas knockdown of FBW7 was shown to restore the role of EZH2 in these genes activities (Fig. 5, A–D). To further test the role of FBW7 in EZH2 activity, we transfected EZH2 alone and co-expressed wild-type FBW7α or the enzymatically dead mutant (FBW7α Δ F-box) together with EZH2 in PANC-1 cells. It was consistent with the finding that overexpression of EZH2 decreased the protein level of FOXJ1, repressed HOXA9 and BRACHYURY expression, and activated TMEM48 and CSK2 expression (Fig. 5, E and F). Importantly, co-expression of FBW7α abrogated the effect of EZH2. In contrast, overexpression of FBW7α Δ F-box had no obvious effect on the suppression of EZH2 activity. Thus, these data demonstrate that FBW7-mediated degradation of EZH2 negatively impacts its transactivation function in pancreatic cancer cells.
Figure 5.
FBW7 suppresses EZH2 activity in pancreatic cancer cells. A, PANC-1 cells were transfected with the indicated constructs. 48 h after transfection, cells were harvested for Western blotting analysis. IB, immunoblot. B, PANC-1 cells were transfected with the indicated constructs. 48 h after transfection, cells were harvested for RT-qPCR analysis of HOXA9, BRACHYURY, TMEM48, and CSK2 mRNAs. Data are mean ± S.D. from experiments with three replicates. *, p < 0.05 compared with the shControl group; n.s., not significant. C and D, PANC-1 cells were transfected with control or shRNAs as indicated. 48 h after transfection, cells were harvested for Western blotting analysis and RT-qPCR analysis. Data are mean ± S.D. from experiments with three replicates. *, p < 0.05 compared with the control group. E and F, PANC-1 cells were transfected with the indicated plasmids for 24 h, followed by Western blotting analysis and RT-qPCR analysis. Data are mean ± S.D. from experiments with three replicates. *, p < 0.05 compared with the control group.
FBW7 inhibits tumor migration and invasion via degradation of EZH2 in pancreatic cancer cells
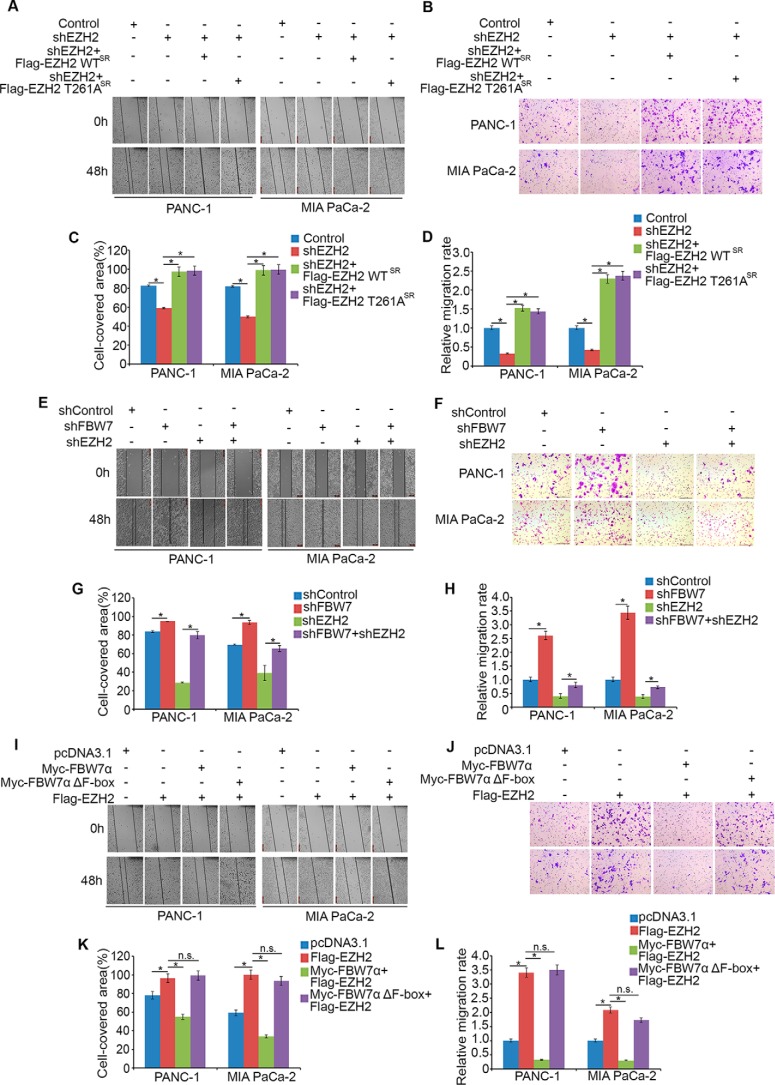
It is previously reported that EZH2 knockdown in pancreatic cancer cell lines inhibited cell migration and invasion but not proliferation (8, 29). We performed a cell proliferation assay after knocking down EZH2 and rescuing EZH2 by shRNA-resistant EZH2 wild-type or EZH2 T261A plasmids in PANC-1 and MIA PaCa-2 cells within 48 h (supplemental Fig. 1, C and D). The data also demonstrated that EZH2 had a subtle effect on altering cell proliferation in pancreatic cancer. To elucidate the biological function of FBW7 in mediating EZH2 degradation, we used a wound healing assay and transwell invasion assay to examine the effect on cell migration and invasion in pancreatic cancer cells. We observed that knockdown of EZH2 reduced migration and invasion activities in both PANC-1 and MIA PaCa-2 cells (Fig. 6, A–F), whereas restored expression of shRNA-resistant EZH2 by EZH2-WT or EZH2 T261A reversed the EZH2 knockdown-induced effect (Fig. 6, A–D). Moreover, knockdown of FBW7 alone increased migration and invasion activities, and FBW7 blocked the effect of EZH2 on cell migration and invasion when we knocked down both FBW7 and EZH2 (Fig. 6, E and H). Furthermore, we demonstrated that overexpression of EZH2 alone increased migration and invasion activity, and co-expression of wild-type FBW7α, but not the enzymatically dead mutant FBW7α Δ F-box, with EZH2 abrogated the effect of overexpression of EZH2 alone in both PANC-1 and MIA PaCa-2 cells (Fig. 6, I–L). Thus, these results indicate that FBW7 inhibits tumor cell migration via degradation of EZH2 in pancreatic cancer cells.
Figure 6.
FBW7 inhibits tumor migration and invasion via degradation of EZH2 in pancreatic cancer cells. A, E, and I, PANC-1 cells and MIA PaCa-2 cells were transfected with the indicated constructs and cultured to confluence on 6-well plates. The cell layer was scratched with a 200-μl pipette tip. For each sample, at least three scratched fields were photographed immediately (0 h) or 48 h after scratching. Photographs of representative images were taken at ×100 magnification. C, G, and K, quantification of cell migration by ImageJ after scratching of PANC-1 and MIA PaCa-2 cells. *, p < 0.05; n.s., not significant. B, D, F, H, J, and L, PANC-1 and MIA PaCa-2 cells were transfected with the indicated constructs. 48 h after transfection, cells were used for Matrigel invasion assays. Representative images of invasion assay are shown in B, F, and J, and the quantification results are shown in D, H, and L, respectively. Scale bars = 200 μm. Data are mean ± S.D. from experiments with three replicates. *, p < 0.05.
EZH2 and FBW7 protein levels negatively correlate in human pancreatic cancer specimens
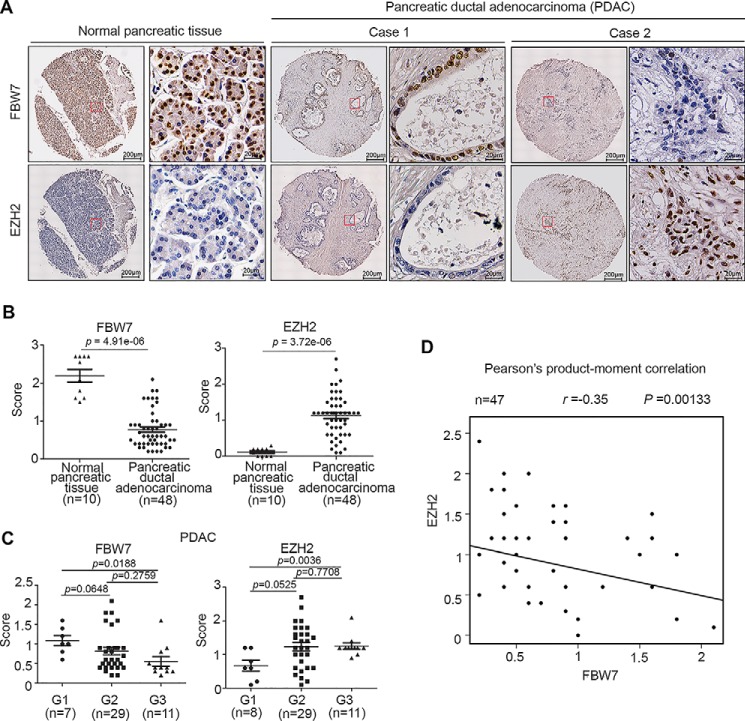
It has been shown previously that EZH2 expression is up-regulated in pancreatic cancer tissues and that it correlates with a poor prognosis (8). However, FBW7 is down-regulated in pancreatic cancer tissues, and low expression of FBW7 is associated with high malignancy and a poor prognosis in pancreatic cancer cases (20). Thus, to investigate the clinical relevance of FBW7 and EZH2, we examined the expression of these proteins by immunohistochemistry in human pancreatic cancer specimens obtained from a cohort of patients (n = 10 normal pancreatic tissue specimens, n = 47 PDAC tissue specimens). Immunohistochemistry staining was evaluated by measuring both the percentage of positive cells and staining intensity. Examples of both strong and weak staining of FBW7 and EZH2 proteins staining are shown in Fig. 7A. PDAC tissues had lower expression of FBW7 (p = 4.91e−06) but a higher level of EZH2 (p = 3.72e−06) compared with adjacent normal tissues (Fig. 7B). It was also shown that well differentiated tumors (G1) had higher FBW7 expression (p = 0.0188) compared with poorly differentiated tumors (G3). In contrast, well differentiated tumors had lower EZH2 expression (p = 0.0036) compared with poorly differentiated tumor (Fig. 7C). Moreover, the statistical analysis indicated that EZH2 was negatively correlated with FBW7 expression in this cohort (Pearson's product-moment correlation r = −0.35, p = 0.00133) (Fig. 7D). These data suggest that EZH2 and FBW7 protein levels are negatively correlated in human pancreatic cancer specimens.
Figure 7.
EZH2 and FBW7 protein levels negatively correlate in pancreatic cancer patient specimens. A, representative images of immunohistochemistry analysis of EZH2 and FBW7 protein expression on tissue microarray (n = 10 normal pancreatic tissue specimens, n = 47 PDAC tissue specimens) tissue sections. B, box plots of EZH2 and FBW7 protein expression based on their staining index in normal pancreatic tissues and PDAC specimens. C, box plots of EZH2 and FBW7 protein expression based on their IS in PDAC specimens at different clinical stages. G1, G2, and G3 represent well differentiated, moderately differentiated, and poorly differentiated tumors, respectively. D, correlation analysis of the IS of expression levels of EZH2 and FBW7 proteins in human pancreatic cancer specimens (n = 47).
Discussion
Epigenetic silencing of tumor suppressor genes mediated by aberrant DNA methylation and histone modification leads to uncontrolled proliferation in human cancer (9). EZH2 is a catalytic subunit of polycomb repressive complex 2 (PRC2), which represses tumor suppressor genes via trimethylation of lysine 27 of histone 3 (H3K27) (30). EZH2 is an oncogenic protein overexpressed in pancreatic cancer. Aberrant nuclear accumulation of EZH2 enhances tumorigenesis, promotes liver metastasis, and facilitates cancer stem cell maintenance in pancreatic cancer cells (31). Inhibition of EZH2 sensitizes pancreatic cancer to chemotherapy (32). Although significant progress has been made toward understanding the function and deregulation of EZH2 in cancer cells, there is little study of the modification of EZH2 after translation. In this study, we provide evidence that FBW7 interacted with EZH2 in pancreatic cancer cells and downregulated EZH2 via ubiquitination and degradation, which indicated that FBW7 might play a significant role in the modification of EZH2.
FBW7, the substrate recognition component of the SCF ubiquitin ligase complex, functions as a tumor suppressor by targeting multiple oncoprotein substrates for ubiquitination and degradation. FBW7 substrates have a consensus FBW7-binding motif, (T/S)PXX(S/T/D/E), that mediates FBW7 recognition. Through motif analysis, we identified a perfectly matched FBW7-binding motif (261TPNID265) in EZH2. It is reported that up-regulation of EZH2 is correlated with a poor prognosis in pancreatic cancer cases (8). Moreover, low expression of FBW7 is relevant to a poor prognosis in pancreatic cancer (20). Thus, our study not only identifies EZH2 as a novel ubiquitination and degradation substrate of FBW7, and FBW7-mediated degradation of EZH2 negatively impacts its transactivation function and inhibits tumor migration in pancreatic cancer cells, but also demonstrates that EZH2 and FBW7 protein levels were negatively correlated in human pancreatic cancer specimens. To this end, these results suggest that down-regulation of FBW7, inducing high EZH2 protein levels, to some extent promoted tumor progression in pancreatic cancer.
Phosphorylation of the first Ser/Thr in the CPD motif of the FBW7 target proteins is essential for FBW7-mediated degradation. We provide evidence that FBW7-mediated degradation of EZH2 is Thr261-dependent. The majority of FBW7 substrates, such as c-jun and c-myc, are phosphorylated by GSK3β within their CPD motifs for recognition and subsequent ubiquitination by FBW7. Recently, Ko et al. (33) reported that GSK3β could interact with EZH2 and phosphorylate EZH2 at Ser363 and Thr367, which down-regulated H3K27 trimethylation and EZH2 oncogenic functions in breast cancer cells. Importantly, there was no change in EZH2 protein level after treatment with the inhibitor of GSK3β or knockdown of endogenous GSK3β (33). Our results also demonstrated that knockdown of endogenous GSK3β or treatment of cells with LiCl could not abrogate the degradation process of EZH2 induced by FBW7. In addition, CDK1 and CDK2 could phosphorylate EZH2 at Thr350 and Thr416 (34), but these two sites do not match the FBW7-binding motif. In other words, phosphorylation of EZH2 at Thr261 might require another kinase. Maskey et al. (27) reported that CDK5 phosphorylated NDE1 within a specific FBW7 phosphodegron and induced down-regulation of NDE1. An important aspect of our findings is that activation of CDK5 via p35 induces degradation of wild-type EZH2 but not the EZH2 T261A mutant. Thus, our results demonstrate that CDK5 acts as the kinase that phosphorylates EZH2 at Thr261 and reduces its protein stability.
In summary, we identify that FBW7 is an E3 ubiquitin ligase of EZH2 and that its low expression contributes to an aberrant accumulation of EZH2 in pancreatic cancer. We discover that FBW7-mediated degradation of EZH2 is Thr261-dependent. Activated CDK5 phosphorylates EZH2 and promotes EZH2 degradation. These findings have revealed the important function of FBW7 in the regulation of the EZH2 protein level in pancreatic cancer, which might represent a viable strategy for the effective treatment of pancreatic cancer.
Experimental procedures
Cell lines, cell culture, and treatment
The pancreatic cancer cell lines PANC-1 and MIA PaCa- 2 were purchased from the Chinese Academy of Science Cell Bank and cultured in a 5% CO2, 37 °C, and 95% humidity incubator. PANC-1 and MIA PaCa-2 cells were cultured in DMEM supplemented with 10% fetal bovine serum (Thermo Fisher Scientific). Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the protocol of the manufacturer.
Plasmids and reagents
FLAG-EZH2 was cloned into the pcDNA3.0 vector. HA-p35, Myc- CDK5, and Myc-FBW7 were cloned into the pCMV vector. GST-EZH2 was cloned into the pGEX-4T-1 vector. Myc-FBW7 Δ F-box and Myc-CDK5 D145N were generated using the KOD-Plus mutagenesis kit (Toyobo). The antibodies used were FBW7 (Bethyl, 301-720A-1), EZH2 (Cell Signaling Technology, 5246S), c-myc (Santa Cruz Biotechnology, SC-40), anti-FLAG (Sigma-Aldrich, F-3165), and anti-HA (Covance, MMS-101R).
Immunoprecipitation and Western blotting
Briefly, cells were harvested and lysed by IP buffer (150 mm NaCl, 1% Nonidet P-40, 50 mm Tris-HCl (pH 7.5), 0.5% sodium deoxycholate, and 1% protease inhibitor cocktails, Sigma-Aldrich). Cell lysates were centrifuged for 15 min, and the supernatant was incubated with the indicated antibodies and protein G beads (Thermo Fisher Scientific) at 4 °C overnight. The beads were washed five times with IP buffer, and the precipitated proteins were subjected to further analysis. For Western blotting, cells were lysed by radioimmune precipitation assay buffer. Protein for each sample was separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were immunoblotted with specific primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies and visualized by SuperSignal West Pico stable peroxide solution (Thermo Scientific).
RNA isolation, reverse transcription, and real-time PCR
Total RNA was isolated from PDAC cells using TRIzol reagent (Thermo Fisher Scientific). Reverse transcription was performed using SuperScript® II reverse transcriptase (Thermo Fisher Scientific). RT-PCR was carried out on a QTX detection system (Bio-Rad). All reactions were performed in triplicate. The primers used were as follows: EZH2, 5′-CCCTGACCTCTGTCTTACTTGTGGA-3′ (forward) and 5′-ACGTCAGATGGTGCCAGCAATA-3′ (reverse); FBW7, 5′-CCACTGGGCTTGTACCATGTT-3′ (forward) and 5′-CAGATGTAATTCGGCGTCGTT-3′ (reverse); HOXA9, 5′-TTGGAGGAAATGAATGCTGA-3′ (forward) and 5′-TGGTCAGTAGGCCTTGA GGT-3′ (reverse); BRACHYURY, 5′-AGGTGGGGAAGTTTCCTTCT-3′ (forward) and 5′-GCAAATGAGGTCCTTTTGGT-3′ (reverse); TMEM48, 5′-AGGTCGCGGGACATACTGT-3′ (forward) and 5′-TGCAGATGGGTAGAAATAGCACT-3′ (reverse); CSK2, 5′-TTCGACGAACACTACGAGTACC-3′ (forward) and 5′-GGACACCAAGTCTCCTC CAC-3′ (reverse); and GAPDH, 5′-ACCCACTCCTCCACCTTTGAC-3′ (forward) and 5′-TGTTGCTGTAGCCAAATTCGTT-3′ (reverse). GAPDH was used as an internal reference.
RNA interference
Lentivirus-based control and gene-specific shRNAs were purchased from Sigma-Aldrich. Transfections were performed according to the protocol of the manufacturer (Sigma-Aldrich). After infection, puromycin (0.5 μg/ml) was used to select stably transduced cells. shRNA sequences were as follows: shFBW7-1, CCGGATGGGTTTCTACGGCACATTACTCGAGTAATGTGCCGTAGAAACCCATTTTTTG; shFBW7-2, CCGGCCAATTGTGTAGACGATATACCTCGAGGTATATCGTCTACACAATTGGTTTTTG; shEZH2-1, CCGGGCTAGGTTAATTGGGACCAAACTCGAGTTTGGTCCCAATTAACCTAGCTTTTTG; and shEZH2-2, CCGGCCAACACAAGTCATCCCATTACTCGAGTAATGGGATGACTTGTGTTGGTTTTTG.
In vitro migration assay
Cells were transfected with the indicated plasmids and cultured to confluence on 6-well plates. The cell layer was scratched with a 200-μl pipette tip, and detached cells were removed. For each sample, at least three scratched fields were photographed immediately. Cell migration was evaluated by measuring the cell-covered area.
In vitro invasion assay
The in vitro cell invasion assay was performed using a BioCoat Matrigel invasion chamber (BD Biosciences) according to the protocol of the manufacturer. PANC-1 and MIA PaCa-2 cells were cultured in the insert for 24 h. Cells were fixed in methanol for 15 min and then stained with 1 mg/ml crystal violet for 20 min. At least five fields for each group were photographed after staining, and invaded cells were counted.
Tissue microarray and immunohistochemistry
The tissue microarray slides were purchased from Biomax US (lot no. PA1001a). The immunohistochemistry staining was evaluated by two independent pathologists who were blinded to the clinical details. Based on the percentage of positive cells and the staining intensity (35), the final immunoreactivity score (IS) for each case was calculated as follows: staining percentage × intensity.
Statistical analysis
All data are expressed as means ± S.D. For experiments with only two groups, Student's t test was used for statistical comparisons. For analysis of correlation between EZH2 and FBW7 protein expression in human pancreatic cancer specimens, Pearson's product-moment correlation was used. p < 0.05 was considered statistically significant.
Author contributions
H. W. and D. W. conceived the study. X. J., P. F., C. Y., J. X., W. Z., and S. Z. performed the experiments. T. L., X. J., D. W., and H. W. wrote the paper.
Supplementary Material
This work was supported in part by grants from the National Natural Science Foundation of China (31560320 to D. W.), the Natural Science Foundation of Jiangxi Province (20142BAB215049 to D. W.), and the Scientific Research Training Program for Young Talents of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figure S1 and Table S1.
- PDAC
- pancreatic ductal adenocarcinoma
- H3K27
- lysine 27 of histone 3
- SCF
- Skp1·Cul1·F-box
- CPD
- Cdc4 phosphodegron
- IP
- immunoprecipitation
- IS
- immunoreactivity score
- WCL
- whole-cell lysate
- CHX
- cycloheximide
- WB
- Western blotting
- RT-qPCR
- quantitative real-time PCR.
References
- 1. The Lancet Oncology (2014) Pancreatic cancer in the spotlight. Lancet Oncol. 15, 241. [DOI] [PubMed] [Google Scholar]
- 2. Long J., Luo G. P., Xiao Z. W., Liu Z. Q., Guo M., Liu L., Liu C., Xu J., Gao Y. T., Zheng Y., Wu C., Ni Q. X., Li M., and Yu X. (2014) Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China Cancer Lett. 346, 273–277 [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez-Paredes M., and Esteller M. (2011) Cancer epigenetics reaches mainstream oncology. Nat. Med. 17, 330–339 [DOI] [PubMed] [Google Scholar]
- 4. Chi P., Allis C. D., and Wang G. G. (2010) Covalent histone modifications: miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. Cancer 10, 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tzatsos A., Paskaleva P., Ferrari F., Deshpande V., Stoykova S., Contino G., Wong K. K., Lan F., Trojer P., Park P. J., and Bardeesy N. (2013) KDM2B promotes pancreatic cancer via Polycomb-dependent and -independent transcriptional programs. J. Clin. Invest. 123, 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S., Bohrer L. R., Rai A. N., Pan Y., Gan L., Zhou X., Bagchi A., Simon J. A., and Huang H. (2010) Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat. Cell Biol. 12, 1108–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simon J. A., and Kingston R. E. (2009) Mechanisms of polycomb gene silencing: knowns and unknowns. Nat. Rev. Mol. Cell Biol. 10, 697–708 [DOI] [PubMed] [Google Scholar]
- 8. Han T., Jiao F., Hu H., Yuan C., Wang L., Jin Z. L., Song W. F., and Wang L. W. (2016) EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget 7, 11194–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ougolkov A. V., Bilim V. N., and Billadeau D. D. (2008) Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin. Cancer Res. 14, 6790–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bracken A. P., Pasini D., Capra M., Prosperini E., Colli E., and Helin K. (2003) EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22, 5323–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohrer L. R., Chen S., Hallstrom T. C., and Huang H. (2010) Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology 151, 5136–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varambally S., Cao Q., Mani R. S., Shankar S., Wang X., Ateeq B., Laxman B., Cao X., Jing X., Ramnarayanan K., Brenner J. C., Yu J., Kim J. H., Han B., Tan P., et al. (2008) Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strohmaier H., Spruck C. H., Kaiser P., Won K. A., Sangfelt O., and Reed S. I. (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 [DOI] [PubMed] [Google Scholar]
- 14. Welcker M., Orian A., Jin J., Grim J. E., Grim J. A., Harper J. W., Eisenman R. N., and Clurman B. E. (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. U.S.A. 101, 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yada M., Hatakeyama S., Kamura T., Nishiyama M., Tsunematsu R., Imaki H., Ishida N., Okumura F., Nakayama K., and Nakayama K. I. (2004) Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nateri A. S., Riera-Sans L., Da Costa C., and Behrens A. (2004) The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 17. Sundqvist A., Bengoechea-Alonso M. T., Ye X., Lukiyanchuk V., Jin J., Harper J. W., and Ericsson J. (2005) Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 1, 379–391 [DOI] [PubMed] [Google Scholar]
- 18. Gupta-Rossi N., Le Bail O., Gonen H., Brou C., Logeat F., Six E., Ciechanover A., and Israël A. (2001) Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J. Biol. Chem. 276, 34371–34378 [DOI] [PubMed] [Google Scholar]
- 19. Zhang W., and Koepp D. M. (2006) Fbw7 isoform interaction contributes to cyclin E proteolysis. Mol. Cancer Res. 4, 935–943 [DOI] [PubMed] [Google Scholar]
- 20. Ji S., Qin Y., Shi S., Liu X., Hu H., Zhou H., Gao J., Zhang B., Xu W., Liu J., Liang D., Liu L., Liu C., Long J., Zhou H., et al. (2015) ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 25, 561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaneko S., Li G., Son J., Xu C. F., Margueron R., Neubert T. A., and Reinberg D. (2010) Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 24, 2615–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D., Ding L., Wang L., Zhao Y., Sun Z., Karnes R. J., Zhang J., and Huang H. (2015) LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 6, 41045–41055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Welcker M., and Clurman B. E. (2008) FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8, 83–93 [DOI] [PubMed] [Google Scholar]
- 24. Kumar Y., Kapoor I., Khan K., Thacker G., Khan M. P., Shukla N., Kanaujiya J. K., Sanyal S., Chattopadhyay N., and Trivedi A. K. (2015) E3 ubiquitin ligase Fbw7 Negatively regulates osteoblast differentiation by targeting Runx2 for degradation. J. Biol. Chem. 290, 30975–30987 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Koo J., Wu X., Mao Z., Khuri F. R., and Sun S. Y. (2015) Rictor undergoes glycogen synthase kinase 3 (GSK3)-dependent, FBXW7-mediated ubiquitination and proteasomal degradation. J. Biol. Chem. 290, 14120–14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakajima T., Kitagawa K., Ohhata T., Sakai S., Uchida C., Shibata K., Minegishi N., Yumimoto K., Nakayama K. I., Masumoto K., Katou F., Niida H., and Kitagawa M. (2015) Regulation of GATA-binding protein 2 levels via ubiquitin-dependent degradation by Fbw7: involvement of cyclin B-cyclin-dependent kinase 1-mediated phosphorylation of THR176 in GATA-binding protein 2. J. Biol. Chem. 290, 10368–10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maskey D., Marlin M. C., Kim S., Kim S., Ong E. C., Li G., and Tsiokas L. (2015) Cell cycle-dependent ubiquitylation and destruction of NDE1 by CDK5-FBW7 regulates ciliary length. EMBO J. 34, 2424–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu C., Jin X., Yang J., Yang Y., He Y., Ding L., Pan Y., Chen S., Jiang J., and Huang H. (2016) Inhibition of EZH2 by chemo- and radiotherapy agents and small molecule inhibitors induces cell death in castration-resistant prostate cancer. Oncotarget 7, 3440–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu L., Xu Z., Zhong L., Wang H., Jiang S., Long Q., Xu J., and Guo J. (2016) Enhancer of zeste homolog 2 (EZH2) promotes tumour cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma. BJU Int. 117, 351–362 [DOI] [PubMed] [Google Scholar]
- 30. Reijm E. A., Timmermans A. M., Look M. P., Meijer-van Gelder M. E., Stobbe C. K., van Deurzen C. H., Martens J. W., Sleijfer S., Foekens J. A., Berns P. M., and Jansen M. P. (2014) High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol. 25, 2185–2190 [DOI] [PubMed] [Google Scholar]
- 31. Chen Y., Xie D., Yin Li W., Man Cheung C., Yao H., Chan C. Y., Chan C. Y., Xu F. P., Liu Y. H., Sung J. J., and Kung H. F. (2010) RNAi targeting EZH2 inhibits tumor growth and liver metastasis of pancreatic cancer in vivo. Cancer Lett. 297, 109–116 [DOI] [PubMed] [Google Scholar]
- 32. Cao Q., Yu J., Dhanasekaran S. M., Kim J. H., Mani R. S., Tomlins S. A., Mehra R., Laxman B., Cao X., Kleer C. G., Varambally S., and Chinnaiyan A. M. (2008) Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27, 7274–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko H. W., Lee H. H., Huo L., Xia W., Yang C. C., Hsu J. L., Li L. Y., Lai C. C., Chan L. C., Cheng C. C., Labaff A. M., Liao H. W., Lim S. O., Li C. W., Wei Y., et al. (2016) GSK3β inactivation promotes the oncogenic functions of EZH2 and enhances methylation of H3K27 in human breast cancers. Oncotarget 7, 57131–57144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei Y., Chen Y. H., Li L. Y., Lang J., Yeh S. P., Shi B., Yang C. C., Yang J. Y., Lin C. Y., Lai C. C., and Hung M. C. (2011) CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 13, 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin X., Tian S., and Li P. (2016) Histone acetyltransferase 1 promotes cells proliferation and induces cisplatin resistance in hepatocellular carcinoma. Oncol. Res., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.