Synopsis
Melanocytic nevi represent benign melanocytic tumors of the skin which usually remain stable in size and behavior or disappear during life. Infrequently, melanocytic nevi undergo malignant transformation to melanoma. Nevertheless, a considerable percentage of cutaneous melanomas arise from melanocytic nevi, and the presence of large numbers of melanocytic nevi represents an independent risk factor for melanoma development. Identification of oncogenic somatic mutations in melanomas and melanocytic nevi highlights the molecular connection between cutaneous melanomas and their benign precursor lesions. The properties of melanocytic nevi can be explained by the concept of oncogene-induced senescence, a phenomenon which restricts the continued growth of cells expressing oncogenes. Understanding molecular and cellular mechanisms underlying oncogene-induced senescence should help identify pathways underlying melanoma development, leading to the development of new strategies for melanoma prevention and early detection.
Keywords: melanocyte, melanoma, nevi, oncogene, senescence
CONTENT
Melanocytic nevi
A melanocytic nevus is a benign clonal proliferation of melanocytes, the pigment-producing cells of the epidermis, hair follicle, and uveal tract of the eye (Argenziano et al., 2007). Melanocytes are normally interspersed as single cells among keratinocytes in human skin, resting atop the basement membrane. In melanocytic nevi, they are present in greater concentrations, either singly or in adherent “nests” or clusters of three of more melanocytic cells. The clinical appearances of melanocytic nevi are heterogeneous, associated in part with when during life the nevus is acquired and likely due as well to the specific differentiation state of the cell of origin and, as we will see, their acquired genetic mutations. They can be considered in two major groups, congenital and acquired melanocytic nevi.
A congenital nevus is present in 1 to 3 % of neonates at birth and shortly thereafter (Price et al., 2010). They are categorized according to their size (small <1.5 cm; medium 1.5–20 cm; large >20 cm-40 cm, giant>40cm) (Schaffer, 2015). Congenital nevi tend to have a globular pattern on dermoscopy and terminal hair follicles. Histologically, congenital nevi consist of big melanocytes which are fusiform, epithelioid, balloonized, or neuroid in shape tracking down from large nests between collagen bundles along cutaneous appendages, vessels and nerves (Argenziano et al., 2007). They extend deep into the reticular dermis and the subcutis. It is postulated that these nevi are a result of clonal proliferation of a melanoblast during embryogenesis.
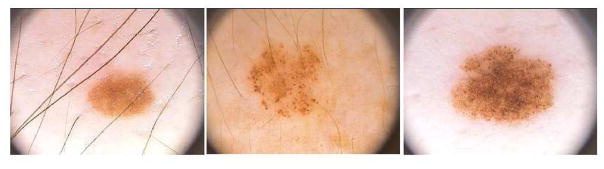
Acquired nevi appear early in childhood, after the first year of life, and increase in number with age, peaking during the 3rd or 4th decade of life (Bataille et al., 1998, Grulich et al., 1996). Heredity and environment (UV radiation) are predisposing factors. Clinically, they are usually smaller (<6 mm in diameter), flat, superficial and horizontally oriented lesions than congenital nevi. Dermatoscopically, they can have a reticular, globular, or homogeneous appearance, alone in combination (Figure 1). The distinct patterns probably correspond to different arrangments of the nested an adherent lesional melanocytes. Histologically, melanocytes in acquired nevi usually do not involve the reticular dermis or the subcutis and the melanocytes are monomorphous and small with an oval shape.
Figure 1.
Dermatoscopic patterns of acquired melanocytic nevi. (Left) Acquired melanocytic nevus with reticular, or net-like, pattern visible through homogeneous brown pigmentation. (Center) Acquired nevus with distinct globular dermatoscopic pattern. (Right) Acquired nevus with combined reticular and globular dermatoscopic pattern.
Most melanocytic nevi either disappear or remain stable during life with fewer than 5% undergoing detectable change when closely monitored (Haenssle et al., 2006, Kittler et al., 2000, Robinson and Nickoloff, 2004). However, it is estimated that 25–50% of cutaneous melanomas arise from melanocytic nevi as precursor lesions (Bevona et al., 2003, Sagebiel, 1993). A recent meta-analysis by Lin et al of 13 studies involving over 4000 cases revealed that 32% of melanomas are in fact associated with a nevus (Lin et al., 2014). Risk of malignant transformation is associated with increased size of congenital nevi (Egan et al., 1998), and risk of cutaneous melanoma correlates with number of total and clinically dysplastic nevi (Tucker et al., 1997) (Newton-Bishop et al., 2010).
In contrast to their malignant counterparts, most melanocytic nevi are benign tumors that initially proliferate but remain stable or disappear during life. However, despite these differences in behavior, nevi and melanomas share somatic mutations in common. A high proportion of large and giant congenital nevi have activating mutations at codon 61 of NRAS, one of the 3 major isoforms of the RAS family of GTPase proteins involved in cell growth, survival, and differentiation (Bauer et al., 2006) (Roh et al., 2015). Small and medium sized congenital melanocytic nevi, as well as many acquired melanocytic nevi, contain a key mutation in the BRAF gene, resulting in substitution of glutamic acid for valine at position V600 of the protein within the kinase domain in exon 15 (Pollock et al., 2003). This mutation results in constitutively active BRAFV600E (Wellbrock et al., 2004). BRAF is a serine-threonine kinase that is activated by the RAS family of proteins which, when activated, triggers the MAPK signaling cascade. Up to 80% of benign nevi carry the BRAFV600E mutation (Pollock et al., 2003). Both mutations are found at high frequency in cutaneous melanomas, with the BRAFV600E mutation being detected in about 60–70 % of malignant melanomas (Curtin et al., 2005). Initial correlation of somatic mutations in nevi with dermatoscopic pattern suggests that BRAF mutations may be most closely associated with globular, rather than reticular, melanocytic nevi (Marchetti et al., 2014). Despite activation of the MAPK pathway, which mediates a potent proliferative signal, benign nevi lose all proliferative activity. The paradox of stable melanocytic proliferations exhibiting oncogenic mutations at high frequency led to the suggestion that melanocytic nevi represent the outcome of oncogene-induced senescence in the skin (Bennett, 2003).
Cellular senescence
Most normal mammalian cells are unable to keep growing indefinitely. After 40–70 cell divisions in culture, the cell enters a state of senescence. In this state, basic metabolic processes occur, but the cell neither dies or divides, a phenomenon initially characterized by Hayflick with human fibroblasts (Shay and Wright, 2000, Hayflick and Moorhead, 1961).
Senescent cells display many phenotypic changes which, individually, cannot definitively indicate senescence, but can be used in combination to determine whether a cell or population of cells has undergone senescence. A major indicator of senescence is the cessation of cell division, combined with a resistance to undergo apoptosis. Another commonly accepted marker of senescent cells is the expression of β-galactosidase that is detectable at pH 6, referred to as senescence-associated β-galactosidase (SA β-gal) (Dimri et al., 1995). Several other phenotypic changes are associated with senescence. Senescent cells often show a striking morphological change—the cell flattens and may adopt a more dendritic shape (Campisi and d’Adda di Fagagna, 2007). Finally, a senescence-activated secretory phenotype, which is marked by an increased expression of secreted proteins, including cytokines, is activated (Coppe et al., 2008).
In addition to these cell-wide changes, several changes are also apparent on the molecular level in senescent cells. These cells show punctate condensation of their chromatin known as senescence-associated heterochromatic foci (SAHF). In addition to these chromatin changes, and perhaps in some cases due to these changes, there are a large number of genes that show expression changes after a cell enters senescence. Some of these genes are directly involved in the senescence process, such as the cell cycle regulators p16INK4A and p21WAF1, which are induced in senescence and maintain the tumor suppressor retinoblastoma protein (Rb) in its hypophosphorylated, and active, state (Campisi and d’Adda di Fagagna, 2007).
Another variant of senescence, premature senescence, has been characterized. Premature senescence refers to senescence that occurs without any detectable telomere dysfunction and can be induced by various stimuli. These stimuli include environmental stress in vitro, such as suboptimal nutrient levels or oxygen tension, and oncogene activation. The phenomenon of oncogene-induced senescence was first described as a proliferative cell cycle arrest in primary human and mouse fibroblasts following the retroviral introduction of oncogenic HRASG12V. Forced expression of HRASG12V in these cells results in cellular changes indistinguishable from the phenotype observed upon replicative senescence, and is associated with increases in the expression of p16, p53, and p21. Oncogene-induced senescence is absolutely dependent upon p16 and p53 in rodent cells, but their loss is not sufficient to abrogate this effect in human cells (Serrano et al., 1997). Several mechanisms of oncogene-induced senescence induction and escape are listed in Table 1.
Table 1.
Mechanisms of Oncogene-Induced Senescence Induction and Escape
| Mechanism | Epigenetic or Signaling Consequence | Effect on Senescence |
|---|---|---|
| BRAFV600E mutation | Constitutive activation of MAPK signaling | Senescence induction |
| NRAS activating mutations | Constitutive activation of MAPK signaling | Senescence induction |
| p53 suppression | Abrogated DNA Damage checkpoints, cell cycle changes | Suppress oncogene-induced senescence |
| RB suppression | Cell cycle changes | Suppress oncogene-induced senescence |
| SETDB1 overexpression | Chromatin remodeling | Suppress oncogene-induced senescence |
| PI3K pathway activation | Cell cycle changes | Suppress oncogene-induced senescence |
| Atg7 depletion | Increased oxidative stress | Suppress oncogene-induced senescence |
| BMI-1 activation | Repression of CDKN2A | Suppress oncogene-induced senescence |
| EZH2 activation | H3K27me3 trimethylation | Suppress oncogene-induced senescence |
| NF-κβ signaling (non-canonical) | RB stabilization | Suppress oncogene-induced senescence |
| CBX4 | Chromatin remodeling | Suppress oncogene-induced senescence |
| CBX7 | Repression of Ink4b/ARF/Ink4a | Suppress oncogene-induced senescence |
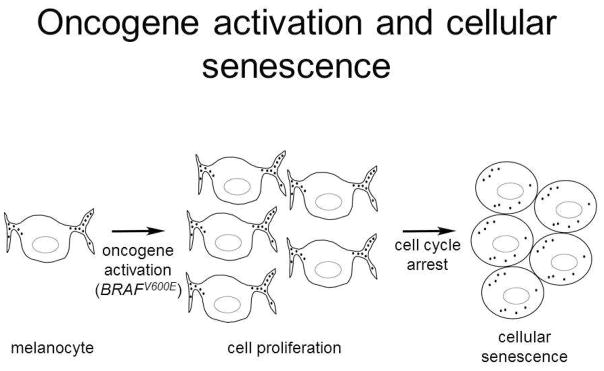
In humans, the concept of oncogene induced senescence (OIS) is well illustrated in the pathogenesis of melanocytic nevi. The forced expression of BRAFV600E in primary human melanocytes leads to a transient cellular proliferation followed by a growth arrest and induction of p16 and SA β-gal expression as senescence markers, similar to the changes reported upon expression of HRASG12V in fibroblasts (Serrano et al., 1997) (Michaloglou et al., 2005). A model for the transient proliferation, followed by growth arrest and senescence, of melanocytes upon oncogene activation to form a nevus is presented in Figure 2.
Figure 2.
Model for the development of a melanocytic nevus following activation of BRAF with acquisition of BRAFV600E somatic mutation. Acquisition of the activating mutation (most likely from an unrepaired oxidative modification to guanine) results in transient melanocyte proliferation driven by MAP kinase signaling (center). Subsequently a senescence response is induced and growth arrest occurs, leaving a clonal population of oncogene-harboring, melanocytic cells forming the nevus.
Melanocytic nevi in vivo express senescence markers as well, leading to the suggestion that oncogene-induced senescence in human melanocytes represents a barrier to tumor progression in melanoma development (Gray-Schopfer et al., 2006) (Michaloglou et al., 2005) Oncogene induced senescence has also been shown to occur in model systems. Patton et al. (Patton et al., 2005) showed that overexpression of BRAFV600E in transgenic zebrafish led to the development of fish nevi and in mice, expression of BRAFV600E in melanocytes produced nevi with biochemical evidence of senescence (Goel et al., 2009). A concept of how acquisition of an activating NRAS mutation in a melanocyte precursor, or melanoblast, during embryonic development might lead to the formation of a congenital melanocytic nevus is depicted in Figure 3.
Figure 3.
Concept for the development of large congenital melanocytic nevus following activation of NRAS following Q61 mutation in melanoblast. Depicted is a mouse Dct-lacZ embryo (Hornyak et al., 2001) where individual melanoblasts are stained blue. Melanoblasts migrate from the neural tube, at the “back” of the embryo, across to the ventral surface. Acquisition of a somatic activating mutation at codon 61 of NRAS in a single melanoblast may lead to massive proliferation of clonal progeny before oncogene-induced senescence results in growth arrest.
Adapted from Hornyak TJ, Hayes DH, Chiu LY, et al. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev 2001;101:47–59; with permission.
Maintenance and loss of senescence in nevi and melanoma - role of genetics and epigenetics
Understanding the mechanisms underlying the breakdown of the senescence barrier, leading to further tumor progression, may lead to advances in maintaining or reimposing it as a therapeutic strategy. The mechanisms that these cells develop to escape oncogene-induced senescence are largely unknown but have been explored in vitro as well as in vivo. Table 1 summarizes the effects of many of the proteins and mechanisms on oncogene-induced senescence to be discussed. Studies in zebrafish have shown that oncogene-induced senescence can be reversed in BRAFV600E expressing melanocytic nevus cells through experimental suppression of p53 (Patton et al., 2005). Suppression of RB function as well as loss of the phosphatase and tensin homolog (PTEN) and overexpression of SETDB1 have similarly been shown to abrogate oncogene induced senescence experimentally (Sage et al., 2000) (Dankort et al., 2009) (Ceol et al., 2011). Vredeveld et al. recently demonstrated that PI3K pathway activation acts as a crucial step in melanomagenesis in vitro by terminating BRAFV600E induced senescence (Vredeveld et al., 2012).
Essential autophagy gene autophagy-related-7, Atg7, has also been shown to promote the development of melanoma in BRAFV600E mutant Pten-null mice by overcoming senescence. In the same study, its loss increased oxidative stress, induced senescence, and improved the response to treatment with BRAF inhibitor dabrafenib (Xie et al, 2015).
Melanoma therapies targeting the OIS pathway are currently under investigation. In addition to the focus on BRAF inhibitors, vemurafenib and trametenib, which are clinically efficacious (Chapman et al., 2011) (Flaherty et al., 2012), there have also been initiatives to test these highly-selective BRAF inhibitors in combination with inhibitors of related pathways in melanomagenesis also relevant to senescence such as PIK3CA and Atg7 (Liu et al., 2013) (Xie et al., 2015).
Polycomb group (PcG) proteins have been implicated as mediators of cellular senescence. PcG proteins are members of distinct macromolecular complexes, the major ones being Polycomb Repressive Complex (PRC) 1 and PRC2, which modify and interact with histones to maintain a cell’s epigenetic state. The polycomb protein Bmi-1 was implicated in cellular senescence by virtue of its activity as an epigenetic repressor of the Cdkn2a locus, encoding the p16Ink4a tumor suppressor, in murine cells. In human cells, overexpression of BMI-1 extended the time to replicative senescence which was associated with repression of p16 expression (Dimri et al., 1995). EZH2 is the catalytic component of the Polycomb Repressive Complex 2 (PRC2), also consisting of other key members such as SUZ12 and EED, which have not been shown to be catalytic, but play structural roles. EZH2 functions as a histone methyltransferase, adding a third methyl group to the ε-amino group of lysine 27 on histone 3, generating the H3K27me3 modification which is associated with gene repression in genome-wide studies. In both replicatively and prematurely senescent cells, a reduction in the expression of EZH2 is associated with reduction in the H3K27me3 histone mark and upregulation of p16 expression at the Cdkn2a/CDKN2A locus (Bracken et al., 2007). A partial explanation for the reduction of the H3K27me3 mark in senescent cells was provided when it was established that during RAS-RAF activation, the H3K27me3 demethylase JMJD3 was induced and recruited to the p16 promoter to counter PcG protein-mediated repression of Cdkn2a and cause the p16-dependent growth arrest characteristic of oncogene-induced senescence (Agger et al., 2009).
Increased expression of EZH2 is commonly observed in cancer (Kleer et al., 2003). The observation that EZH2 expression might progressively increase during melanoma progression, together with the suppression of the senescent state occurring during that process, suggested that there might be a relationship between EZH2 overexpression in melanoma cells and the suppression of the senescent state, thereby promoting melanoma development and tumorigenicity.
To confirm that EZH2 expression is associated with melanoma progression, we obtained specimens of benign human melanocytic nevi and metastatic melanoma tumors, and observed, on a cell-by-cell basis, a marked increase in the percentage of melanocytic cells expressing EZH2. To test the hypothesis that the induction of EZH2 expression between nevus and melanoma cells was responsible for the loss of senescence, and tumor progression, we utilized RNA interference to suppress the expression of EZH2 in melanoma cells with NRAS activating mutations. Suppression of EZH2 in these cells led to the re-appearance of senescence markers such as SA β-gal, SAHF, and H3K9me3 foci, and was associated as well with other typical senescence changes such a G1 cell cycle arrest and an increase in cell and nuclear size. Stable suppression of EZH2 expression was also found to inhibit tumor cell colony formation in soft agar and in vivo tumor xenograft growth in immunodeficient mice (Fan et al., 2011).
Mechanistically, the induction of senescence in melanoma cells upon loss of EZH2 could not be attributed to an induction in p16 expression or activity, since all of the cells tested either did not express p16 or expressed a mutated form (Figure 4). Instead, we detected a p53-independent induction of p21 expression in most of the EZH2-suppressed melanoma cells, an induction that was found to be responsible for a significant proportion of the senescence effect. Nevertheless, the CDKN1A gene encoding p21 was not found to be a direct target of EZH2, exhibiting neither significant occupancy by EZH2 nor a high concentration of H3K27me3 modifications, as is typical for direct PRC2 interaction sites, following chromatin immunoprecipitation studies. Hence the direct targets of EZH2 in melanoma genomes responsible for the repression of p21/CDKN1A in these cells, and the suppression of the senescent state that promotes tumor progression, remain to be described. However, EZH2 expression has recently been found to be regulated by non-canonical NF-κβ signaling through stabilization of Rb via p21 and p53 (Iannetti et al., 2014) (De Donatis et al., 2015). This may be an explanation for the p53-independent induction of p21 that is seen in EZH2-suppressed melanoma cells. In addition, non-canonical NF-κβ signaling was shown to prevent oncogene-induced senescence. Continued activation of the non-canonical NF-κβ pathway in the context of EZH2 suppression would lead to an increase of p21 in an unsuccessful attempt by the cell to regulate EZH2 expression and bypass senescence. The roles of Polycomb group proteins in melanocyte and melanoma function have been summarized (Tiffen et al., 2015) (Huang and Hornyak, 2015).
Figure 4.
Model for the bypass of senescence in melanoma cells induced by EZH2 and/or non-canonical NF-κB signaling. Senescence in melanocytes can be mediated by either the tumor suppressors p16 or p53, but p16 function is invariably inactivated in most cutaneous melanomas whereas p53 gene sequence and function usually remains intact. EZH2 induction in melanoma cells results in senescence bypass by suppressing the expression of pro-senescent p21 downstream of p53-p21 activation. EZH2 expression is dependent upon non-canonical NF-κB signaling through the p52 and RelB subunits.
Additional recent studies have extended findings about the role of PcG proteins in senescence. A major role of Cbx4, a PRC1 component, is to suppress senescence of epidermal stem cells through its chromodomain and interactions with the H3 tail domain. Cbx4 expression seems to be important in these cells to fine tune the transition of epidermal stem cells between the quiescent and active states (Luis et al., 2011). Cbx7, another PRC1 component, mediates senescence by binding to and repressing the classic PcG protein target Ink4b/ARF/Ink4a. Interestingly, its ability to bind to the locus and inhibit senescence is dependent not only upon its ability to recognize and bind H3K27me3, but also to bind ANRIL, a non-coding RNA transcribed from the locus which bridges the interactions between H3K27me3 and PRC1 (Yap et al., 2010). Ras-induced senescence in mouse embryonic fibroblasts is associated with dramatic changes in both H3K4me3 and H3K27me3 trimethylation that alter expression of critical components of the Bmp2-Smad1 signaling pathway, enforcing senescence through the induction of a senescence-associated secretory phenotype (Kaneda et al., 2011) Though each of these studies were performed using primary cells, it is likely that genome-wide chromatin immunoprecipitation studies with cancer cells will also reveal important and unrecognized Polycomb-regulated genes responsible for the suppression of senescence in these cells.
SUMMARY/DISCUSSION
Melanocytic nevi most likely represent the outcome of clonal proliferation of single melanocytes that acquire highly-specific, somatic mutations which escape DNA repair and cause them to proliferate to a variable extent in the skin. These particular mutations, such as mutations at BRAFV600E and at codon 61 in NRAS, likely function in a highly cell type-specific context to drive proliferation, since benign tumors arising from other cell types do not feature identical mutations at high frequency. Concomitantly, expression of the oncogene induces a senescence response in the proliferating melanocytes which stops cell growth and presents a barrier to further malignant transformation. Infrequently, nevi undergo transformation to malignant melanoma. This transition is associated not only with further genetic changes, such as loss or mutation of the CDKN2A locus encoding the p16 tumor suppressor, but also with epigenetic changes, such as chromatin-level silencing of tumor suppressor gene expression that is also a factor in tumor progression. Polycomb proteins, including proteins comprising Polycomb Repressive Complex 2 (PRC2), have important roles in cancer development, The members of this complex have been found to be overexpressed in a variety of cancers including melanoma. EZH2 expression increases progressively from being undetectable in normal melanocytes to low in nevi and high in melanoma, with an increase seen between primary and metastatic lesion. EZH2 depletion from melanoma cell lines has been shown to decrease proliferation, increase the percentage of cells with a senescent phenotype, and decrease the volume of tumors generated with a xenograft experiment. In addition, several mutations of EZH2 have been identified that increase the H3K27Me3 activity of the PRC2 complex in lymphoma. Growing evidence suggests that the epigenetic regulation catalyzed by PRC2 may play a role in regulating senescence. Senescence is a barrier to uncontrolled proliferation, but can be bypassed. Reversing senescence bypass and rendering proliferating tumor cells dormant as a result may be a useful adjunct to targeted therapies and immunotherapies that are currently utilized for advanced melanoma and remain under investigation for other cutaneous malignancies.
Key Points.
Melanocytic nevi share oncogenic molecular mutations with melanomas
Oncogene-induced senescence explains in part why most nevi are stable and do not undergo progression to malignant melanoma
Determining mechanisms which reverse melanoma cells to a senescent phenotype could lead to the identification of therapeutic adjuncts in advanced melanoma
Acknowledgments
This work was support in part by Merit Review Award 1I01BX002582 from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development Service; and by NIH Award R01 AR064810, National Institutes of Health, U.S. Department of Health and Human Services. The contents do not represent the views of the U.S. Department of Veterans Affairs, the U.S. Department of Health and Human Services, or the United States Government.
Abbreviations and Acronyms
- BRAF
A member of the RAF family of serine/threonine-specific kinases which is frequently mutated in human melanoma and is a molecular target for therapy
- OIS
oncogene-induced senescence
- NRAS
member of the RAS family of GTPases (small GTPase proteins) which mediate growth factor receptor signaling and are critical for cell proliferation, survival, and differentiation. Activating mutations in NRAS proto-oncogene, particularly at codon 61, are also common in human melanoma
- BRAFV600E
The V600E mutation results in an amino acid substitution of valine (V) to glutamic acid (E) at position 600 of BRAF.
- MAP kinase (MAPK) signaling pathway:
Responsible for relaying extracellular signals from cell membrane to nucleus. Dysregulation of this pathway due to activating mutations in BRAF, RAS and other genes leads to increased signaling activity leading to cell proliferation, invasion, metastasis, migration, survival and angiogenesis.
- Senescence-associated β-galactosidase- (SA β-gal)
β-galactosidase activity that is detectable at pH 6.0 in cells undergoing replicative or induced senescence that us absent in proliferating cells. This is the most commonly used biomarker for senescence.
- Senescence-associated heterochromatic foci (SAHF)
Domains of condensed chromatin, or heterochromatin, that often form in senescent human cells. They play a role in repressing proliferation-promoting genes and their detection can help identify senescent cells.
- p16 (also p16INK4A)
tumor suppressor protein that functions as a cyclin-dependent kinase inhibitor and is encoded by the CDKN2A gene. Plays a significant role in cell cycle regulation; tumor suppressor implicated in the prevention of melanoma and many other cancers. It is one of the genes associated with hereditary melanoma and plays a role in cell senescence.
- p21WAF1
cyclin-dependent kinase inhibitor that mediates p53-dependent cell cycle arrest and likely plays a role as a tumor suppressor. This protein also inhibits apoptosis and may promote cell proliferation in some tumors.
- p53
mutation of this tumor suppressor gene is common in melanoma, moreso in many other cancers. In normal cells, p53 plays a role in cell cycle arrest and DNA repair or apoptosis, and can mediate cellular senescence.
- HRASG12V
oncogenic Ras protein that is frequently mutated in cancers. When the amino acid glycine G replaced with amino acid valine V at codon 12, it becomes permanently activated within the cell (proto-oncogene), leading to uncontrolled cell division and tumor formation.
- RB
tumor suppressor gene which normally arrests cells in the G1 or G1/S phase of the cell cycle by acting as a transcriptional repressor. Loss or inactivation can lead to uncontrolled cell proliferation
- Phosphatase and tensin homolog (PTEN)
tumor suppressor gene which is frequently lost/inactivated in melanoma. The PTEN protein is a phosphatase which negatively regulates the PI3K/Akt pathway and influences cell adhesion, migration, and invasion.
- SETDB1
Histone methyltransferase which is overexpressed in melanoma and accelerates its onset in zebrafish melanoma models harboring the BRAF V600E mutation
- PI3K-AKT pathway
activation of this pathway is one of the most significant signaling pathways in melanoma. It plays a role in melanoma initiation and resistance to therapeutics.
- Atg7
Essential autophagy gene, autophagy-related-7, promotes melanoma by limiting oxidative stress and overcoming senescence; inhibition may be of therapeutic value.
- PRC
polycomb repressive complexes 1 and 2 (PRC1 and PRC2) which are protein complexes associated with chromatin condensation and transcriptional repression (epigenetic modifications) PRC1 catalyzes the ubiquitylation of histone H2A and PRC2 methylates H3K27.
- Bmi-1
polycomb complex protein, member of PRC1, which regulates cell cycle inhibitor genes (p16). Its overexpression may promote tumor invasion and metastasis.
- EZH2 (Enhancer of zeste homolog 2)
epigenetic modifier and catalytic component of the polycomb repressive complex 2 (PRC2), which is thought to promote growth and metastasis of melanoma. Increased expression is associated with uncontrolled proliferation in melanoma.
- SUZ12
component of the PRC2 complex
- EED (embryonic ectoderm development)
component of PRC2
- JMJD3
Histone demethylase which promotes melanoma progression and metastasis through regulation of NF-kappa B and BMP signaling
- NF-kappaB
inducible transcription factor which regulates the expression of genes involved in the immune response and is involved in the regulation of apoptosis, angiogenesis, and tumor cell invasion. Its activity is upregulated in human melanoma and many other cancers
- Cbx4-PRC1
associated protein which protects epidermal stem cells from senescence by maintaining them in their undifferentiated state.
- ANRIL
antisense non-coding RNA which is involved in chromatin remodeling, transcription, posttranscriptional processing. It is often abnormally expressed in cancer.
- BMP (Bone Morphogenic Protein)
member of the transforming growth factor-beta (TGF-beta) superfamily which are involved in proliferation, apoptosis, differentiation, chemotaxis, and angiogenesis. It has been shown to have potent anti-tumor activity in the skin. In melanoma, it is overexpressed and is thought to promote cell invasion and migration.
- SMAD1
gene which encodes a protein involved in the downstream signaling pathway of bone morphogenic protein (BMP)
- BMP-Smad1
this signal and its regulation by epigenetic alterations are significant in Ras-induced senescence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGGER K, CLOOS PA, RUDKJAER L, WILLIAMS K, ANDERSEN G, CHRISTENSEN J, HELIN K. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev. 2009;23:1171–6. doi: 10.1101/gad.510809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARGENZIANO G, ZALAUDEK I, FERRARA G, HOFMANN-WELLENHOF R, SOYER HP. Proposal of a new classification system for melanocytic naevi. British Journal of Dermatology. 2007;157:217–227. doi: 10.1111/j.1365-2133.2007.07972.x. [DOI] [PubMed] [Google Scholar]
- BATAILLE V, GRULICH A, SASIENI P, SWERDLOW A, NEWTON BISHOP J, MCCARTHY W, HERSEY P, CUZICK J. The association between naevi and melanoma in populations with different levels of sun exposure: a joint case-control study of melanoma in the UK and Australia. Br J Cancer. 1998;77:505–10. doi: 10.1038/bjc.1998.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER J, CURTIN JA, PINKEL D, BASTIAN BC. Congenital Melanocytic Nevi Frequently Harbor NRAS Mutations but no BRAF Mutations. J Invest Dermatol. 2006;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- BENNETT DC. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22:3063–9. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- BEVONA C, GOGGINS W, QUINN T, FULLERTON J, TSAO H. Cutaneous melanomas associated with nevi. Arch Dermatol. 2003;139:1620–4. doi: 10.1001/archderm.139.12.1620. discussion 1624. [DOI] [PubMed] [Google Scholar]
- BRACKEN AP, KLEINE-KOHLBRECHER D, DIETRICH N, PASINI D, GARGIULO G, BEEKMAN C, THEILGAARD-MONCH K, MINUCCI S, PORSE BT, MARINE JC, HANSEN KH, HELIN K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPISI J, D’ADDA DI FAGAGNA F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- CEOL CJ, HOUVRAS Y, JANE-VALBUENA J, BILODEAU S, ORLANDO DA, BATTISTI V, FRITSCH L, LIN WM, HOLLMANN TJ, FERRE F, BOURQUE C, BURKE CJ, TURNER L, UONG A, JOHNSON LA, BEROUKHIM R, MERMEL CH, LODA M, AIT-SI-ALI S, GARRAWAY LA, YOUNG RA, ZON LI. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–7. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN PB, HAUSCHILD A, ROBERT C, HAANEN JB, ASCIERTO P, LARKIN J, DUMMER R, GARBE C, TESTORI A, MAIO M, HOGG D, LORIGAN P, LEBBE C, JOUARY T, SCHADENDORF D, RIBAS A, O’DAY SJ, SOSMAN JA, KIRKWOOD JM, EGGERMONT AM, DRENO B, NOLOP K, LI J, NELSON B, HOU J, LEE RJ, FLAHERTY KT, MCARTHUR GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPPE JP, PATIL CK, RODIER F, SUN Y, MUNOZ DP, GOLDSTEIN J, NELSON PS, DESPREZ PY, CAMPISI J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIN JA, FRIDLYAND J, KAGESHITA T, PATEL HN, BUSAM KJ, KUTZNER H, CHO KH, AIBA S, BROCKER EB, LEBOIT PE, PINKEL D, BASTIAN BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- DANKORT D, CURLEY DP, CARTLIDGE RA, NELSON B, KARNEZIS AN, DAMSKY WE, JR, YOU MJ, DEPINHO RA, MCMAHON M, BOSENBERG M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DONATIS GM, PAPE EL, PIERRON A, CHELI Y, HOFMAN V, HOFMAN P, ALLEGRA M, ZAHAF K, BAHADORAN P, ROCCHI S, BERTOLOTTO C, BALLOTTI R, PASSERON T. NF-kB2 induces senescence bypass in melanoma via a direct transcriptional activation of EZH2. Oncogene. 2015 doi: 10.1038/onc.2015.468. [DOI] [PubMed] [Google Scholar]
- DIMRI GP, LEE X, BASILE G, ACOSTA M, SCOTT G, ROSKELLEY C, MEDRANO EE, LINSKENS M, RUBELJ I, PEREIRA-SMITH O, PEACOCKE M, CAMPISI J. A Biomarker that Identifies Senescent Human Cells in Culture and in Aging Skin in vivo. 1995 doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN CL, OLIVERIA SA, ELENITSAS R, HANSON J, HALPERN AC. Cutaneous melanoma risk and phenotypic changes in large congenital nevi: a follow-up study of 46 patients. J Am Acad Dermatol. 1998;39:923–32. doi: 10.1016/s0190-9622(98)70264-6. [DOI] [PubMed] [Google Scholar]
- FAN T, JIANG S, CHUNG N, ALIKHAN A, NI C, LEE CC, HORNYAK TJ. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer Res. 2011;9:418–29. doi: 10.1158/1541-7786.MCR-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAHERTY KT, INFANTE JR, DAUD A, GONZALEZ R, KEFFORD RF, SOSMAN J, HAMID O, SCHUCHTER L, CEBON J, IBRAHIM N, KUDCHADKAR R, BURRIS HA, 3RD, FALCHOOK G, ALGAZI A, LEWIS K, LONG GV, PUZANOV I, LEBOWITZ P, SINGH A, LITTLE S, SUN P, ALLRED A, OUELLET D, KIM KB, PATEL K, WEBER J. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEL VK, IBRAHIM N, JIANG G, SINGHAL M, FEE S, FLOTTE T, WESTMORELAND S, HALUSKA FS, HINDS PW, HALUSKA FG. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28:2289–98. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY-SCHOPFER VC, CHEONG SC, CHONG H, CHOW J, MOSS T, ABDEL-MALEK ZA, MARAIS R, WYNFORD-THOMAS D, BENNETT DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULICH AE, BATAILLE V, SWERDLOW AJ, NEWTON-BISHOP JA, CUZICK J, HERSEY P, MCCARTHY WH. Naevi and pigmentary characteristics as risk factors for melanoma in a high-risk population: a case-control study in New South Wales, Australia. Int J Cancer. 1996;67:485–91. doi: 10.1002/(SICI)1097-0215(19960807)67:4<485::AID-IJC4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- HAENSSLE HA, KRUEGER U, VENTE C, THOMS KM, BERTSCH HP, ZUTT M, ROSENBERGER A, NEUMANN C, EMMERT S. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980–5. doi: 10.1038/sj.jid.5700119. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L, MOORHEAD PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HORNYAK TJ, HAYES DH, CHIU LY, ZIFF EB. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev. 2001;101:47–59. doi: 10.1016/s0925-4773(00)00569-4. [DOI] [PubMed] [Google Scholar]
- HUANG JM, HORNYAK TJ. Polycomb group proteins--epigenetic repressors with emerging roles in melanocytes and melanoma. Pigment Cell Melanoma Res. 2015;28:330–9. doi: 10.1111/pcmr.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IANNETTI A, LEDOUX AC, TUDHOPE SJ, SELLIER H, ZHAO B, MOWLA S, MOORE A, HUMMERICH H, GEWURZ BE, COCKELL SJ, JAT PS, WILLMORE E, PERKINS ND. Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10:e1004642. doi: 10.1371/journal.pgen.1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA A, FUJITA T, ANAI M, YAMAMOTO S, NAGAE G, MORIKAWA M, TSUJI S, OSHIMA M, MIYAZONO K, ABURATANI H. Activation of Bmp2-Smad1 signal and its regulation by coordinated alteration of H3K27 trimethylation in Ras-induced senescence. PLoS Genet. 2011;7:e1002359. doi: 10.1371/journal.pgen.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITTLER H, PEHAMBERGER H, WOLFF K, BINDER M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43:467–76. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- KLEER CG, CAO Q, VARAMBALLY S, SHEN R, OTA I, TOMLINS SA, GHOSH D, SEWALT RGAB, OTTE AP, HAYES DF, SABEL MS, LIVANT D, WEISS SJ, RUBIN MA, CHINNAIYAN AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C, PENG W, XU C, LOU Y, ZHANG M, WARGO JA, CHEN JQ, LI HS, WATOWICH SS, YANG Y, TOMPERS FREDERICK D, COOPER ZA, MBOFUNG RM, WHITTINGTON M, FLAHERTY KT, WOODMAN SE, DAVIES MA, RADVANYI LG, OVERWIJK WW, LIZEE G, HWU P. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUIS NM, MOREY L, MEJETTA S, PASCUAL G, JANICH P, KUEBLER B, COZUTTO L, ROMA G, NASCIMENTO E, FRYE M, DI CROCE L, BENITAH SA. Regulation of human epidermal stem cell proliferation and senescence requires polycomb-dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–46. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- MARCHETTI MA, KIURU MH, BUSAM KJ, MARGHOOB AA, SCOPE A, DUSZA SW, CORDOVA MA, FONSECA M, WU X, HALPERN AC. Melanocytic naevi with globular and reticular dermoscopic patterns display distinct BRAF V600E expression profiles and histopathological patterns. Br J Dermatol. 2014;171:1060–5. doi: 10.1111/bjd.13260. [DOI] [PubMed] [Google Scholar]
- MICHALOGLOU C, VREDEVELD LC, SOENGAS MS, DENOYELLE C, KUILMAN T, VAN DER HORST CM, MAJOOR DM, SHAY JW, MOOI WJ, PEEPER DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- NEWTON-BISHOP JA, CHANG YM, ILES MM, TAYLOR JC, BAKKER B, CHAN M, LEAKE S, KARPAVICIUS B, HAYNES S, FITZGIBBON E, ELLIOTT F, KANETSKY PA, HARLAND M, BARRETT JH, BISHOP DT. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol Biomarkers Prev. 2010;19:2043–54. doi: 10.1158/1055-9965.EPI-10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTON EE, WIDLUND HR, KUTOK JL, KOPANI KR, AMATRUDA JF, MURPHEY RD, BERGHMANS S, MAYHALL EA, TRAVER D, FLETCHER CD, ASTER JC, GRANTER SR, LOOK AT, LEE C, FISHER DE, ZON LI. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–54. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- POLLOCK PM, HARPER UL, HANSEN KS, YUDT LM, STARK M, ROBBINS CM, MOSES TY, HOSTETTER G, WAGNER U, KAKAREKA J, SALEM G, POHIDA T, HEENAN P, DURAY P, KALLIONIEMI O, HAYWARD NK, TRENT JM, MELTZER PS. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- ROBINSON JK, NICKOLOFF BJ. Digital epiluminescence microscopy monitoring of high-risk patients. Arch Dermatol. 2004;140:49–56. doi: 10.1001/archderm.140.1.49. [DOI] [PubMed] [Google Scholar]
- ROH MR, ELIADES P, GUPTA S, TSAO H. Genetics of melanocytic nevi. Pigment Cell & Melanoma Research. 2015;28:661–672. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGE J, MULLIGAN GJ, ATTARDI LD, MILLER A, CHEN S, WILLIAMS B, THEODOROU E, JACKS T. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–50. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGEBIEL RW. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: effect of tumor thickness. J Invest Dermatol. 1993;100:322S–325S. doi: 10.1111/1523-1747.ep12470218. [DOI] [PubMed] [Google Scholar]
- SERRANO M, LIN AW, MCCURRACH ME, BEACH D, LOWE SW. Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- SHAY JW, WRIGHT WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–6. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- TIFFEN J, GALLAGHER SJ, HERSEY P. EZH2: an emerging role in melanoma biology and strategies for targeted therapy. Pigment Cell Melanoma Res. 2015;28:21–30. doi: 10.1111/pcmr.12280. [DOI] [PubMed] [Google Scholar]
- TUCKER MA, HALPERN A, HOLLY EA, HARTGE P, ELDER DE, SAGEBIEL RW, GUERRY DT, CLARK WH., JR Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997;277:1439–44. [PubMed] [Google Scholar]
- VREDEVELD LC, POSSIK PA, SMIT MA, MEISSL K, MICHALOGLOU C, HORLINGS HM, AJOUAOU A, KORTMAN PC, DANKORT D, MCMAHON M, MOOI WJ, PEEPER DS. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–69. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLBROCK C, OGILVIE L, HEDLEY D, KARASARIDES M, MARTIN J, NICULESCU-DUVAZ D, SPRINGER CJ, MARAIS R. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- XIE X, KOH JY, PRICE S, WHITE E, MEHNERT JM. Atg7 Overcomes Senescence and Promotes Growth of BrafV600E-Driven Melanoma. Cancer Discov. 2015;5:410–23. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAP KL, LI S, MUNOZ-CABELLO AM, RAGUZ S, ZENG L, MUJTABA S, GIL J, WALSH MJ, ZHOU MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–74. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]