Abstract
Social bonds, especially attachment relationships, are crucial to our health and happiness. However, what we know about the neural substrates of these bonds is almost exclusively limited to rodent models and correlational experiments in humans. Here, we used socially monogamous non-human primates, titi monkeys (Callicebus cupreus) to experimentally examine changes in regional and global cerebral glucose metabolism during the formation and maintenance of pair bonds. Baseline positron emission tomography (PET) scans were taken of thirteen unpaired male titi monkeys. Seven males were then experimentally paired with females, scanned and compared, after one week, to six age-matched control males. Five of the six control males were then also paired and scanned after one week. Scans were repeated on all males after four months of pairing. PET scans were coregistered with structural magnetic resonance imaging (MRI), and region of interest (ROI) analysis was carried out. A primary finding was that paired males showed a significant increase in FDG uptake in whole brain following one week of pairing, which is maintained out to four months. Dopaminergic, “motivational” areas and those involved in social behavior showed the greatest change in glucose uptake. In contrast, control areas changed only marginally more than GCGM. These findings confirm the large effects of social bonds on global cerebral glucose metabolism. They also suggest that more studies should examine how social manipulations affect whole brain FDG uptake, as opposed to assuming that it does not change across condition.
Keywords: pair bond, imaging, PET, global cerebral glucose metabolism
Introduction
Our ability to form and maintain social relationships is crucial to our health, psychological well-being, and reproductive fitness (Uchino et al., 1996, Uchino, 2006). These relationships start with our initial attachment to our caregivers, and expand past our family circle with friendships as juveniles and adolescents. As adults, we may maintain multiple strong, selective social bonds including romantic attachments, friendships, and bonds with both our parents and our children.
The neural basis of relationships has primarily been studied in the context of parent-offspring or adult romantic relationships (Carter, 1998, Bartels and Zeki, 2000, Rilling et al., 2001, Bartels and Zeki, 2004, Acevedo et al., 2012). Adult romantic relationships, or pair bonds, can be conceptualized in the same way as offspring to parent attachments (Hazan and Shaver, 1987, Mason and Mendoza, 1998). As such, they involve a preference for the attachment partner, distress upon separation, and the ability of the attachment partner to buffer the subject against stress (Bowlby, 1969, Ainsworth et al., 1978). In non-human animals, these characteristics of a pair bond have only been demonstrated in a limited number of species, all of which display social monogamy, a social system in which a (usually male-female) pair shares a territory and reproduces together, generally with the aggressive exclusion of other non-related adults of their species (Kleiman, 1977, Fuentes, 1999, Diaz-Munoz and Bales, 2016). Prairie voles (Microtus ochrogaster) and titi monkeys (Callicebus cupreus) are two of these species.
Prairie voles have contributed most of our knowledge on the neurobiology of pair bonding (Gobrogge and Wang, 2015). In brief, pair bond formation is associated with the release of the peptide hormones oxytocin (OT) and vasopressin (AVP) from the hypothalamus (Winslow et al., 1993, Cho et al., 1999, Liu and Wang, 2003, Ross et al., 2009). OT and AVP are crucial for pair-bond formation in both sexes (Winslow et al., 1993, Williams et al., 1994, Cho et al., 1999, Lim et al., 2004, Lim and Young, 2004, Ross et al., 2009, Johnson et al., 2015), presumably because of their association with the formation of social memories (Engelmann et al., 1996, Bielsky et al., 2004). In prairie voles (but not polygamous voles such as meadow voles), the receptors for OT and AVP (V1a) are co-localized with dopamine D2 receptors in areas of the reward system such as the nucleus accumbens and ventral pallidum (Insel and Shapiro, 1992, Insel et al., 1994). Co-activation of OT or AVP (i.e. for social memory) and D2 (i.e. for Motivational) results in pair bond formation (Aragona et al., 2003, Aragona and Wang, 2009). Following this formation, dopamine D1 receptors are up-regulated along with aggression towards outsiders, a.k.a. mate-guarding (Aragona et al., 2006). Imaging studies of humans, in which subjects are thinking about the object of their romantic love, concur in implicating the reward system (Bartels and Zeki, 2000, Xu et al., 2011, Acevedo et al., 2012), and other hypothalamic areas (Acevedo et al., 2012).
Prairie voles are the only system in which the neurobiology of pair bonding has been studied in detail (Carter et al., 1995, Gobrogge and Wang, 2015). While they make an excellent model due to their clearly monogamous social system, some aspects of these findings have not held up in a wider taxonomic context. For instance, repeats within the vole promoter region of the gene for the AVP V1a receptor were found to predict the degree of pair bonding (Hammock and Young, 2005, Hammock et al., 2005). However, this pattern was not found across a wider sample of mammalian species (Fink et al., 2006). For this reason, our knowledge of the neurobiology of pair bonds should not be limited solely to one rodent model (Phillips et al., 2014). In this study, we utilized the titi monkey, a monogamous primate.
Titi monkeys, like prairie voles, live in nuclear family groups in nature. They duet, maintain small territories which they defend against intrusion, and the male is the main carrier of the infants (Mason, 1966, 1968, Mendoza and Mason, 1986b). Adult pairs display many characteristics of a pair bond, including preference for their partner (Carp et al., 2016), distress upon separation (Mendoza and Mason, 1986a), and the ability of the partner to buffer stress (Mendoza et al., 2000). In a previous study, we showed that males that had been in long-term pairs, vs. males that were not paired, showed many differences in regional cerebral glucose metabolism (rCGM) using positron emission tomography (PET) with [18F]-fluorodeoxyglucose (FDG)(Bales et al., 2007). We found FDG uptake differences between paired and lone males in the regions of the nucleus accumbens, ventral pallidum, medial preoptic area, medial amygdala, and supraoptic nucleus of the hypothalamus; while not finding differences in the central amygdala or periaqueductal grey. In that study, like many other studies, rCGM data were normalized to global cerebral glucose metabolism (GCGM), and were in units of proportion of whole brain FDG uptake (i.e. rCGM was divided by GCGM). However, because GCGM (the denominator of the equation) was higher in long-term paired males, this normalization caused it to appear that rCGM was lower in paired males in many areas when compared to lone males.
This previous study (Bales et al., 2007) was also cross-sectional, with paired males older than lone males. Thus, age was confounded with pairing status. The goal of the current study was to examine the neurobiology of pair bonding longitudinally over formation in titi monkey males, compared with age-matched controls when possible. As in our previous study, we used PET scans with conscious uptake of FDG. We hypothesized that after males were paired we would see increased global cerebral glucose metabolism (higher whole brain FDG uptake), with higher than average FDG uptake in regions of interest identified as either “reward” areas or “social memory” areas. We also examined areas that produce OT and AVP, as well as the prefrontal cortex which has recently been implicated in several studies of more “universal” or “altruistic” love (Mathur et al., 2010).
Experimental Procedures
All experimental procedures were approved by the Animal Care and Use Committee of the University of California, Davis, and complied with National Institutes of Health ethical guidelines as set forth in the Guide for Lab Animal Care.
Subjects
Subjects were 13 captive-born and reproductively naïve adult male titi monkeys (Callicebus cupreus) housed at the California National Primate Research Center (CNPRC) in Davis, CA. Subjects were removed from their natal group and housed alone for greater than one month prior to the beginning of the study. Animals were fed twice daily (0830 and 1330 h) a diet consisting of New World monkey chow, rice cereal, banana, apples, raisins, and baby carrots and water was available ad libitum. Further details of husbandry and training are available elsewhere (Tardif et al., 2006), with caging identical to that described in (Carp et al., 2016).
Experimental Design and Pair Formation
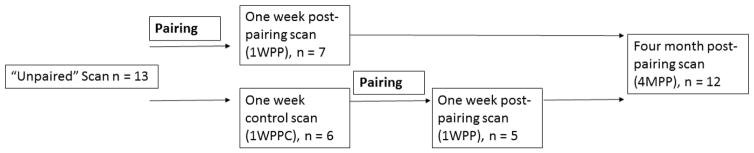
All 13 males (mean age 4.18 years, range 2–5.6 years) underwent a baseline “unpaired” positron emission tomography (PET) scan and blood draw (one male was removed afterwards from the study due to health reasons). After the unpaired scan, six males (mean age 4.32 years) were paired with females and six males served as their singly-housed age-matched controls (see experimental design in Figure 1). Newly-paired subjects and age-matched controls underwent another PET scan seven days after pair formation (experimental mean age 4.34 years, control mean age 4.17 years). After serving as age-matched controls, five of the six age-matched control males (mean age 4.80 years) were paired with females and scanned seven days after pairing. All 12 paired males underwent a final PET scan approximately four months after being paired with a female (mean = 108.43 days, range = 91–119 days). The decision to pair all males following the one week timepoint, rather than leave them as control males until the last time point, was made for ethical reasons to avoid a long period alone for these males, and for practical reasons (for breeding) as this is the only laboratory breeding colony of titi monkeys in the world.
Figure 1.
Experimental design of the project. At baseline (“Unpaired”), experimental and control groups were not paired and did not differ on any demographic or outcome measures. The experimental group was then paired, and outcome measures collected at one week post-pairing (1WPP). The control group was still housed alone at this point (1WPPC). The control group was then also paired and provided a one week post-pairing timepoint as well. All males were rescanned at four months post-pairing (4MPP).
The choice of single housing as a baseline for this experiment was made for several reasons. There are few potential housing partners available for adult male titi monkeys, which cannot be housed with unrelated adult males due to aggression. We were also trying to avoid the presence of a current attachment bond in the adult males, which would happen if they were still in the family group with their natal attachment figure, their father. Therefore, males were housed singly for the unpaired control condition. At all times they had visual, auditory, and olfactory contact with other titi monkeys and were therefore still engaged in social communication. However, this choice of control should be taken into account in the interpretation of the results.
We also chose to conduct our social conditions sequentially rather than in a counter-balanced fashion. This was in part because we did not know if any effects caused by pairing would be reversible if pairs were split up, or if pairing would induce permanent changes.
For pair formation, one male and one female were introduced into their new home cage that was novel for both animals. The male was released into the cage first, immediately followed by the female. The male and female did not have visual access to each other prior to pairing.
PET Scanning with FDG
Subjects and female pair-mates were relocated to a metabolism room at CNPRC 48 hours prior to their positron emission tomography (PET) scan. As in our previous study (Bales et al, 2007), animals were relocated prior to the scan in order to reduce the possible effect of novel housing on brain metabolism. Animals were fasted 6–12 h prior to the scan, with water available throughout the pre-scan period. On the day of the PET scan, the male was caught and removed from the cage. The subject was manually restrained while he received a bolus injection of [18F]-fluorodeoxyglucose (FDG, PETNET Solutions, Sacramento, CA) (mean = 1.979 mCi IV, SE = 0.04, administered in a volume of <2 ml) into the saphenous vein. Each subject was returned to their cage for 30 min of conscious uptake either with their pair-mate or alone, depending on the condition.
After the FDG uptake period, subjects were anesthetized with ketamine (25 mg/kg IM) and administered medetomidine (0.05 mg/kg IM). After the animal was sedated, a blood sample was collected from the femoral vein into a 3 ml heparin-coated tube and a sample of cerebrospinal fluid (CSF) was collected and put on ice. An endotracheal tube was placed and a catheter was placed in the saphenous vein in order to administer IV fluids (lactated ringers solution, 10 ml/kg/hr). Atipamazole was used to reverse medetomidine, and anesthesia was maintained with isoflurane (1–2%), while the male was positioned on the scanner bed feet first and the brain of the animal was positioned in the center of the scanner. PET imaging was performed on a microPET P4 scanner (Siemens Preclinical Solutions, Knoxville, TN). Image acquisition began a mean of 69.81 minutes (SE=1.41) post-FDG administration, and static PET scans were acquired for 60 minutes. Anesthesia was maintained throughout the scan. Animals were maintained in metabolism cages for 24 h after scanning, at which time radiation was decayed to background levels and animals were returned to their home cages.
MRI Scanning
Structural magnetic resonance imaging (MRI) scans were conducted in a GE Signa LX 9.1 scanner (General Electric Corporation, Milwaukee, WI) with a 1.5 T field strength and a 3″ surface coil. Each male was fasted 8–12 h before the procedure. At the start of the procedure, the male was sedated with ketamine (10 mg/kg IM) and medazolam (0.1 mg/kg IM), and an endotracheal tube was placed. A catheter was also placed in the saphenous vein in order to administer fluids as necessary. Anesthesia was maintained with isoflurane (1–2%) while the male was positioned in the MRI scanner. Each scan lasted approximately 20 min and consisted of a 3D SPGR pulse sequence in a coronal plane. Images of the entire brain were collected using the following parameters: echo time TE=7.9 ms, repetition time TR=22.0 ms, flip angle=30.0°, field of view=8 cm, number of excitations=3, matrix=256×256, and slice thickness=1 mm. As a precautionary measure, the male’s EtCO2, oxygen saturation, heart rate and blood pressure were monitored throughout.
PET and MRI Coregistration, Quantification of FDG Uptake
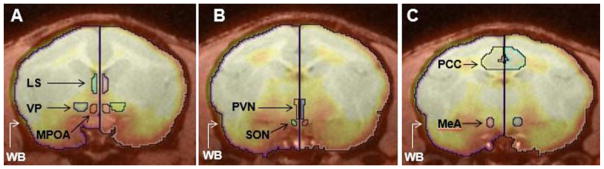
The following regions of interest (ROIs) were examined: nucleus accumbens, medial amygdala, periaquaductal gray (PAG), cerebellum, putamen, caudate, prefrontal cortex, lateral septum, ventral pallidum, medial preoptic area (MPOA), supraoptic nucleus of the hypothalamus (SON), paraventricular nucleus of the hypothalamus (PVN), and posterior cingulate cortex (PCC) (Figure 2).
Figure 2.
Positron emission tomography (PET) image of a titi monkey brain co-registered with magnetic resonance imaging (MRI) with regions of interest outlined in Inveon Research Workplace (IRW) software. Panel A shows the coronal view of the lateral septum (LS), ventral pallidum (VP), and medial preoptic area (MPOA). Panel B shows the coronal view of the paraventricular nucleus of the hypothalamus (PVN) and the supraoptic nuclues of the hypothalamus (SON). Panel C shows the coronal view of the posterior cingulate cortex (PCC) and medial amygdala (MeA). All three panels show the outline of the Whole Brain (WB).
ROI structures including whole brain were drawn on each subject’s MRI image using Siemen’s Inveon Research Workplace software (IRW, Siemens Healthcare, USA). Static PET images were reconstructed with a 3DRP reconstruction protocol. Because the size of the head is similar in all subjects, and comparisons were made across the same structure in different animals, the PET data were not corrected for photon attenuation or scatter. Unlike rhesus macaque monkeys that have large heads, thick skulls and need transmission scans, titi monkeys have much smaller heads, thin skulls and therefore scatter and attenuation are similar to levels encountered in rodent studies and considered to be small effects. Correcting for attenuation or scatter would require an extra transmission scan, on another day and this would be logistically very difficult to acquire since we are measuring conscious uptake due to the unique social circumstances of each test day. MRI images were co-registered with PET scan images using the automatic rigid registration algorithm in IRW and checked visually for acceptable registration accuracy. Mean FDG activity for the PET images were determined by applying ROIs defined on the MRI images to the PET images in IRW.
Typically, ROI mean FDG activities (in units of microcuries per cubic centimeter) are normalized by dividing by whole brain mean FDG activity (i.e. rCGM/GCGM). The resulting proportions of whole brain FDG uptake are used for statistical analysis, as was done in our previous study (Bales et al., 2007). In the current study, we were interested in changes in the “numerator” region of interest FDG uptake (rCGM) as well as the “denominator” whole brain FDG uptake (GCGM). Therefore, we corrected FDG uptake by the injected dose; this was done by dividing the ROI activity data by the injected dose and multiplying by 100%. This normalizes the FDG activity data for the amount injected into the subject, and is a standardized unit to use for statistical analysis. Data are presented in percent injected dose per gram of tissue (%ID/g) for rCGM regions of interest and for whole brain GCGM.
Blood sampling, timing and hormone analysis
Blood and CSF samples were collected after animals were sedated for the PET scan following the FDG uptake period, and placed on ice. In order to examine whether capture and sedation affected hormone concentrations, we collected timing data. We recorded what times blood and CSF samples were collected, and when animals were captured prior to their blood draw. Blood samples (n=43) were collected a mean of 5.32 min (SE=0.88) after capture and sedation of the subjects, and CSF samples (n=35) were collected a mean of 10.52 min (SE=0.37) after capture and sedation. While the veterinarians collected as many CSF samples as possible, sometimes they were unable to get a sample due to the small size of the animals (adults weigh approximately 1.0 – 1.5 kg). Blood samples in heparin-containing tubes were centrifuged at 3000 RPM for 15 minutes at 4° C. Plasma and CSF samples were stored at −70° C until assay. Plasma samples were assayed for oxytocin (OT), vasopressin (AVP), cortisol, insulin and glucose. CSF samples were assayed for AVP and OT. There were no statistically significant relationships (p>0.05) between the amount of time taken to collect the blood or CSF sample (“disturbance time”) and the hormone concentration of the sample.
AVP and OT concentrations were estimated in duplicate using commercial enzyme immunoassay kits (Enzo Life Sciences, Farmingdale, NY) previously validated for titi monkeys. Assay sensitivity was determined to be 2.34 pg/ml for AVP and 15.55 pg/ml for OT. Intra- and inter-assay coefficients of variation (CV) were 3.36% and 14.34% respectively for AVP, and 10.62% and 12.78%, respectively for OT. All CSF samples (n=35) were assayed for AVP. Thirty CSF samples were assayed for OT, because there were 5 samples that did not have sufficient volume to measure both hormones.
Plasma cortisol concentrations were estimated in duplicate using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA), previously validated for titi monkeys (Mendoza, unpublished data) and has been used many times to analyze cortisol in this species (Hoffman et al., 1995, Bales et al., 2007, Jarcho et al., 2011, Laugero et al., 2011, Ragen et al., 2013). Prior to assay, samples were diluted 1:4 in PBS gel buffer. Assay procedures were modified with the addition of 0.5 and 2.35 μg/dl concentrations of standards along with the provided range of 1.0–49 μg/dl. Assay sensitivity has been determined to be 0.261 μg/dl. Intra- and inter-assay CV were 3.20% and 6.26%, respectively.
Plasma insulin concentrations were measured in duplicate using commercial ELISA kits (Ultra Sensitive Rat Insulin, Crystal Chem Inc., Downers Grove, IL) as per manufacturer’s instructions for the wide range assay (0.1–12.8 ng/mL). This assay was validated for titi monkeys by assessing parallelism. All but one sample fell within the range of the assay, the lowest sample was set to the lowest assay standard (0.1 ng/ml). The antibody used in this insulin assay has high cross-reactivity with other species. Intra- and inter-assay CVs were 5.17% and 12.86%, respectively. Plasma glucose concentrations were measured in duplicate using commercial glucose colorimetric assay kits (Caymen Chemical Company, Ann Arbor, MI) as per manufacturer’s instructions. This assay was validated for titi monkeys by assessing parallelism. All samples were in detectable range of the assay. Intra- and inter-assay CVs were 2.28% and 3.70%, respectively.
Data Analysis
In order to limit Type I error, we first grouped our ROIs into hypothesis-driven factors. These included:
Factor 1: Motivational areas; Nucleus accumbens, caudate, putamen, ventral pallidum
Factor 2: Social areas; Medial amygdala, lateral septum, posterior cingulate cortex, medial preoptic area
Factor 3: Control areas; Periaqueductal gray, cerebellum
Factor 4: Areas that produce OT and AVP; Paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus
Factor 5: Prefrontal cortex (PFC)
While there were many possible ways to make these groupings, we chose these on the following principles:
Dopaminergic areas of the dorsal and ventral striatum were grouped together in Factor 1 and labeled Motivational areas. All of these areas have been previously implicated in either rodent (Resendez et al., 2016) or human (Acevedo et al., 2012) studies of pair bonding/romantic love, or both.
Areas involved in social recognition and social memory. The medial amygdala and lateral septum are both involved in these processes (Engelmann and Landgraf, 1994, Noack et al., 2015). The posterior cingulate has been implicated in sexual fidelity and space use in monogamous voles (Ophir et al., 2008). The medial preoptic area, while better known for its role in maternal memory (Dobolyi et al., 2014, Stolzenberg and Champagne, 2016), has also been implicated in oxytocinergic regulation of social recognition (Popik and van Ree, 1991). All of these areas were also previously implicated in our cross-sectional study of pair bonding in male titi monkeys (Bales et al., 2007).
“Control” areas. We chose the periaqueductal gray as one of our control areas that was not expected to change with pair bonding because: a) there was no difference between pair bonded and non-bonded males in this area in our cross-sectional study (Bales et al., 2007); and b) in a direct comparison of romantic love and maternal love in humans, the periaqueductal gray was activated by maternal but not romantic love (Bartels and Zeki, 2004). We chose the cerebellum as a second control area, again due to the lack of a known association with pair bonding.
We grouped the areas of the brain that produce OT and AVP peptide, the PVN and SON.
Finally, we felt that the PFC did not group naturally with any of the other factors. However, the size of the medial PFC varies in rodent species with differing social systems (monogamous species having a smaller PFC) (Kingsbury et al., 2012). The PFC has also shown plasticity in primates with regard to other types of social behavior (Kozorovitskiy et al., 2006).
To examine the effects of pairing between the experimental and control groups over time, we carried out growth curve analyses and added group as an external variable to test for differences in intercept and slope between groups. Specifically, the model that we used can be expressed as
where Yit is the observed score on subject i at measurement t, yi0 is the initial level score of subject i, yis is the slope, or the individual change over time, Bt is the set of coefficients that define the shape of the curve, and eit is the error score of subject i at measurement t. Sources of individual differences as well as the effects of a grouping variable on the level and slope can be included in the model as
where the level and slope scores now have fixed group intercepts (γ0 and γs), and the regression coefficients (γ0x and γsx), represent the effect of the observed variable X on the level and slope. This model follows the same formulation as hierarchical, multilevel, or random-effects models (Laird and Ware, 1982, Bryk and Raudenbush, 1992, McArdle et al., 2002, Ferrer et al., 2004).
In our analyses, we considered two sets of coefficients Bt to denote the shape of the curve. In the first set, we used Bt = [0,1,2] to represent a linear model of change across the three measurement occasions. In the second set, we used Bt = [0,1,2], including a latent basis for determining nonlinearity in the changes across the three occasions. Finally, to facilitate the interpretation of parameters, group was centered-coded (−1 = control; +1 = experimental).
Results
Results from these analyses are presented in Figure 1 and Table 1 for the key neural variables (representing regional cerebral glucose metabolism) in our study. For example, for factor 1 (Motivational areas) in Table 1, the results of the linear model indicate an average intercept (scores at baseline; y0 = .106), and an average slope (ys =.013), representing linear changes per measurement occasions. Both coefficients were reliably different from zero. The group effect on the intercept was not statistically significant (γ0x = −.005) indicating that both groups did not differ at baseline. However, the group effect on the slope was different from zero (γsx = .016), indicating that the experimental group had a larger slope than the control group. Finally, about 54% of the variance in this factor 1 was explained by the time and group effects.
Table 1.
Parameter Estimates from Curve Models with Group Differences for Factors
| Parameters | Factor 1: Motivational Areas | Factor 2: Social Areas | Factor 3: Control Areas | Factor 4: PVN and SON | Factor 5: PFC |
|---|---|---|---|---|---|
| Linear Model | |||||
| Intercept | .106 (.005)* | .091 (.005)* | .102 (.006)* | .076 (.005)* | .111 (.006)* |
| Time slopea | .013 (.006)* | .012 (.005)* | .011 (.006)* | .009 (.004)* | .012 (.006)* |
| Group* Intercept | −.004 (.005)n | −.003 (.005)n | −.006 (.006)n | −.001 (.005)n | −.005 (.006)n |
| Group* Slope | .016 (.006)* | .012 (.005)* | .013 (.006)* | .008 (.004)* | .015 (.006)* |
| Residual Variance | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* |
| −2LL | 172.5 | 186.3 | 179.4 | 188.9 | 173.0 |
| BIC | 154.5 | 168.3 | 158.9 | 171.0 | 155.0 |
| % Varianced | .536 | .521 | .483 | .326 | .328 |
|
| |||||
| Nonlinear Model | |||||
| Intercept | .106 (.006)* | .090 (.005)* | .102 (.006)* | .075 (.006)* | .111 (.006)* |
| Time slopeb | .025 (.012)n | .023 (.010)* | .022 (.011)n | .016 (.008)* | .022 (.011)n |
| Latent basisc | .593 (.151)* | .624 (.150)* | .621 (.157)* | .851 (.234)* | .676 (.200)* |
| Group* Intercept | −.006 (.006)n | −.005 (.005)n | −.007 (.006)n | −.003 (.006)n | −.007 (.007)n |
| Group* Slope | .032 (.012)* | .025 (.010)* | .027 (.011)* | .017 (.008)* | .032 (.011)* |
| Residual Variance | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* | <.001 (<.001)* |
| −2LL | 172.9 | 186.9 | 179.9 | 190.9 | 173.9 |
| BIC | 149.9 | 163.9 | 156.8 | 167.8 | 150.9 |
| % Varianced | .597 | .625 | .618 | .479 | .512 |
Note. Entries are parameter estimates and standard errors (in parentheses).
parameter estimate with p < .05.
parameter estimate with p > .05.
Intercept = scores at baseline.
changes per measurement occasion.
total changes from baseline to four months post pairing.
percentage of changes from the baseline to 7-days post pairing.
Explained variance due to time and group effects.
Number of subjects = 13; number of observations = 43. Group code (−1 = control; +1 = experimental). Factor 1 includes Motivational areas (NAcc, VP, Caud, Put); Factor 2 includes social areas (MeA, LS, MPOA, PCC); Factor 3 includes control areas (PAG, Cere); Factor 4 includes OT and AVP producing nuclei (PVN, SON); Factor 5 is the prefrontal cortex (PFC).
The results from the nonlinear model are similar but consider an additional parameter. These results indicate an average intercept (scores at baseline; y0 = .106) an average slope (ys =.025), representing the total change from baseline to the last occasion, and a latent basis (B2 = .593), indicating that about 59% of all the changes took place from baseline to one week post-pairing. As was the case for the linear model, the group effect on the intercept was not statistically significant (γ0x = −.006) indicating that both groups did not differ at baseline. As before, the group effect on the slope was different from zero (γsx = .032), indicating that the experimental group showed more total changes than the control group. This model including nonlinear time and group effects explained about 60% of the variance in factor 1.
Table 1 shows that for all five neural factors, the groups do not differ at baseline in both linear and nonlinear models. The group effect on the slope is significantly different from zero for all five factors in both linear and non-linear models, indicating that for all five factors the experimental group changed more than the control group. The latent basis for all five factors varies from .593 for factor 1, to .814 for factor 4. Thus for all five factors, a majority of the change in the experimental group occurred in the first week post-pairing. For the PVN and SON, over 81% of the change occurred in the first week post-pairing.
Values in Table 1 are based on data normalized for injected dose and are in units of % injected dose/gram (%ID/g), rather than on data normalized for GCGM (i.e. rCGM/GCGM) where units would be proportion of whole brain FDG uptake (proportion of GCGM). In Table 2 we show that for GCGM, the group effect on the slope was different from zero (γsx =.014), indicating that experimental animals had a greater change in GCGM than the control group. The % variance explained by the linear model is 42.3%.
Table 2.
Parameter Estimates from Curve Models with Group Differences for Whole Brain
| Parameters | Whole Brain |
|---|---|
| Linear Model | |
| Intercept | .106 (.006)* |
| Time slopea | .012 (.006)* |
| Group* Intercept | −.004 (.006)n |
| Group* Slope | .014 (.006)* |
| Residual Variance | <.001 (<.001)* |
| −2LL | 176.9 |
| BIC | 158.9 |
| % Varianced | .423 |
|
| |
| Nonlinear Model | |
| Intercept | .106 (.006)* |
| Time slopeb | .023 (.011)* |
| Latent basisc | .631 (.168)* |
| Group* Intercept | −.006 (.006)n |
| Group* Slope | .029 (.011)* |
| Residual Variance | <.001 (<.001)* |
| −2LL | 177.4 |
| BIC | 154.3 |
| % Varianced | .578 |
Note. Entries are parameter estimates and standard errors (in parentheses).
parameter estimate with p < .05.
parameter estimate with p > .05.
Intercept = scores at baseline.
changes per measurement occasion.
total changes from baseline to the four month post-pairing timepoint.
percentage of changes from the baseline to 7-days post pairing.
Explained variance due to time and group effects.
Number of subjects = 13; number of observations = 43. Group code (−1 = control; +1 = experimental).
We were also interested in how much of the change in rCGM for each factor can be explained by the changes in GCGM. For a linear model, Factors 1 (Motivational areas) and 2 (Social areas) explained approximately 10% more variance than GCGM (53.6% and 52.1%, respectively, vs. 42.3%). Factor 3 (control areas) explain only slightly more variation (6%) than GCGM at 48.3%. Factors 4 and 5 both explain less variance than explained by the change in GCGM. For the non-linear model, most of the relationships are similar. However, with GCGM explaining 57.8% of variance in a non-linear model, Factor 1 does not improve the model with 59.7% explained.
Table 3 shows the models for the hormonal variables: Plasma insulin, glucose, cortisol, OT, and AVP; as well as CSF OT and AVP. Baselines did not differ between groups for any of the hormones examined. No changes in hormonal concentrations were significantly different between the experimental group and the control group. Plasma insulin displayed a significant negative slope over time (γsx =−.785).
Table 3.
Parameter Estimates from Curve Models with Group Differences for Hormonal Variables
| Parameters | CSF OT | Plasma OT | CSF AVP | Plasma AVP | Plasma Insulin | Plasma Glucose | Plasma Cortisol |
|---|---|---|---|---|---|---|---|
| Linear Model | |||||||
| Intercept | 48.7 (5.75)* | 711.3 (41.4)* | 3.55 (.664)* | 291.9 (18.6)* | 2.31 (.623)* | 93.4 (5.39)* | 90.9 (6.32)* |
| Time slopea | 4.57 (4.29)n | −10.2 (22.3)n | −.092 (.611)n | 11.6 (14.9)n | −.785 (.280)* | 1.62 (2.47)n | −5.88 (3.24)n |
| Group* Intercept | .056 (5.75)n | −54.6 (41.4)n | −1.11 (.664)n | −9.29 (18.7)n | −.588 (.623)n | .038 (5.39)n | −9.99 (6.31)n |
| Group* Slope | −.168 (4.29)n | 18.4 (22.3)n | .996 (.611)n | 13.1 (14.9)n | .370 (.280)n | −3.43 (2.47)n | 2.99 (3.24)n |
| Residual Variance | 279 (110)* | 11593 (3340)* | 2.94 (1.46)* | 5235 (1516)* | .744 (.304)* | 146.9 (42.0)* | 147.4 (60.1)* |
| −2LL | 213.6 | 472.8 | 122.7 | 431.9 | 108.5 | 310.7 | 320.4 |
| BIC | 231.6 | 490.8 | 143.2 | 449.9 | 129.0 | 328.6 | 340.9 |
| % Varianced | .114 | .032 | .309 | .060 | .596 | .071 | .375 |
Note. Entries are parameter estimates and standard errors (in parentheses).
parameter estimate with p < .05.
parameter estimate with p > .05.
Intercept = scores at baseline.
changes per measurement occasion.
Explained variance due to time and group effects.
Number of subjects = 13; number of observations = 43 for plasma hormones; 30 for CSF OT and 35 for CSF AVP. Group code (−1 = control; +1 = experimental).
Discussion
These results confirm, in a longitudinal model with an age-matched control group, that titi monkey males show an increase in whole brain neural FDG uptake within one week of pairing. Within the brain, these increases are more marked in areas rich in dopamine receptors (“Motivationalal”) and areas involved in the formation of social memories. These increases are still evident, and even exaggerated, after four months post-pairing.
This study focused primarily on hypothesis-driven examination of forebrain neural areas suggested by studies in prairie voles and our own previous cross-sectional study. Measurement of FDG uptake is not specific as far as what type of peptide release or receptor activation is occurring in each area, although the assumption was that similar peptides would be involved as are in voles (primarily AVP and OT). Our finding of increased rCGM in areas of the mesolimbocortical system (NAcc, VP) strongly suggests the involvement of dopamine and opioids in this process. The formation of a pair bond is likely to be highly motivated both because of the reward value involved with mating (Pfaus et al., 2012) as well as the social reward (Northcutt and Lonstein, 2009). However, based on our previous findings that dopamine D1 receptors did not change in the NAcc and VP with pair-bonding in male titi monkeys, it is more likely that D2 receptors were involved in these areas (Hostetler et al., 2016). Also likely involved are μ and κ opioid receptors, which are involved in pair bond regulation in both prairie voles (Resendez et al., 2012, Resendez et al., 2013) and titi monkeys (Ragen et al., 2013, Ragen et al., 2015b). Opioid receptors in titi monkeys are found in primarily the same areas as in other primates, throughout the forebrain including many of the areas we included in factors 1 and 2 (Ragen et al., 2015a).
This study did not support the hypothesis that sustained activation of OT and AVP producing areas occurs during pair bond formation and maintenance in male titi monkeys, although it does not eliminate the possibility that FDG uptake in these areas (SON and PVN) is increased on a shorter timescale (for instance, within 48 hours of pairing as seen in (Bales et al., 2007)). Indeed, the non-linear model showed that over 80% of the change in these areas occurred in the first week post-pairing, which was substantially higher than for other factors. The question of whether areas with OT and V1a receptors, rather than peptide, are involved is a more complicated one. We recently published the OT and V1a receptor distribution of titi monkeys. In contrast to the more common biomedical model, the rhesus monkey (Macaca mulatta) (Freeman et al., 2014a), titi monkeys display OT receptors in more forebrain areas, including the lateral septum (Freeman et al., 2014b). However, given that V1a receptors are much more widespread than OT receptors even in titi monkeys, their involvement is very likely. In particular, the titi monkey has V1a rather than OT receptors in the NAcc, which was implicated in this study.
This study did not find any changes in plasma hormone concentrations of AVP, OT, cortisol, glucose or insulin in response to pair formation or maintenance from blood samples collected on PET scan days. Similarly, there were no changes in CSF AVP or OT in response to pairing. However, the many events that happened on blood sampling day (i.e. housing in new cage and room, captured twice, sedation) could easily have obscured sensitive hormonal changes. Ideally, “baseline blood samples” are collected when animals are housed in their home cages and animals can be captured quickly and blood can be collected before the stress of capture affects the hormonal concentrations (Mendoza, 2017). Timing of handling and blood sampling in this study was optimized for the PET procedure rather than measurement of hormones. We did find that subjects showed a decrease in plasma insulin concentrations over time, perhaps as a response to habituation to the procedures.
A major finding of this study was the long-term increase in brain glucose metabolism due to pairing. While it might be thought at first that this was due to an impoverished social state prior to pairing, we do not believe this to be the case; all males had visual, olfactory and auditory access to multiple other groups of titi monkeys. An associated shift was not seen in plasma glucose concentrations with pairing in this sample. Most studies of FDG uptake use data that are normalized by using proportions of whole brain activity, which is calculated by dividing the regional cerebral glucose metabolism (rCGM) by the GCGM. Thus, in many cases group differences in GCGM are not examined, reported and are assumed to not exist. Exceptions include aging (Kochunov et al., 2009), Alzheimer’s disease (Cunnane et al., 2011), exercise (Kemppainen et al., 2005), and traumatic brain injury (Soustiel et al., 2005), all of which result in a reduction in GCGM. To our knowledge, establishment of a pair bond may be one of the only non-pharmacological manipulations to lead to long-term increases in whole brain FDG uptake in an adult.
There are still many unanswered questions in the neurobiology of social bonds in titi monkeys and other primate species. It is unclear if whole brain FDG uptake would increase further more than four months post pairing, and whether this increase would be sustained indefinitely. The neurochemicals which are responsible for the higher rCGM in some regions remain to be confirmed. Chemically specific PET ligands are available for some of these (such as dopamine D1 and D2 receptors); however, there is still no OT receptor PET ligand (Smith et al., 2016), and no easily available V1a receptor PET ligand. Finally, the neural basis of other aspects of the pair-bond, such as separation distress and mate-guarding (or “jealousy”), also remain to be considered.
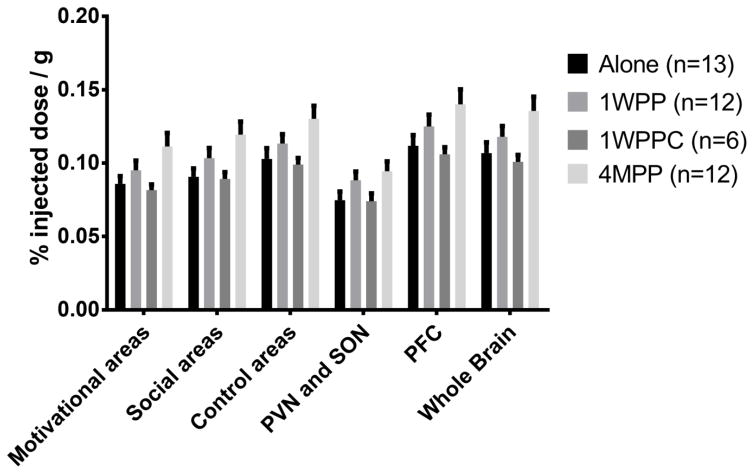
Figure 3.
FDG uptake, in units of % injected dose/g, for factors including Motivational areas included in Factor 1 (NAcc, VP, Caud, Put); social areas included in Factor 2 (MeA, LS, MPOA, PCC); control areas included in Factor 3 (PAG, Cere); PVN and SON (Factor 4); PFC (Factor 5), during Alone PET scan (Alone), one week post-pairing scan (1WPP), one week post-pairing control scan (1WPPC), and four months post-pairing scan (4MPP). All factors showed a significant effect of experimental group in a linear model. However, Factors 1 and 2 showed greater change than GCGM.
Highlights.
Socially monogamous male titi monkeys were followed longitudinally during pair bond formation.
Paired males displayed an increase in global cerebral glucose metabolism until at least four months post pairing.
Areas involved in motivation and in social memory displayed higher increases than the whole brain increases.
Acknowledgments
Thanks to the many members of the Bales lab who assisted with data collection, including Thomas Schaefer, Sarah Carp, and Carlos Almeida; Dr. Laurie Brignolo, Dr. Angela Colagross-Schouten, Dr. Kari Christe, Dr. Laura Summers, and the veterinary staff for veterinary care and assistance with the PET scans; Jaleh Janatpour and Kevin Theis for animal care; Vanessa Bakula, Sarah Grisso, Deborah Kent, and Research Services at CNPRC; Thanks to Michelle Connell, Jennifer Fung and Charles Smith in CMGI for assistance with PET scanning and protocol development. All research in the current study was compliant with animal care regulations and national laws.
Funding Sources
This work was supported by the National Institutes of Health (grant numbers HD053555 and P51OD01107), and the Good Nature Institute. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. SCAN. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment. Hillsdale, NJ: Erlebaum; 1978. [Google Scholar]
- Aragona BJ, Liu Y, Curtis T, Stephan FK, Wang ZX. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang ZX. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Frontiers in Behavioral Neuroscience. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. New York: Basic Books, Inc; 1969. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Newbury Park CA: Sage Publications; 1992. [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus) American Journal of Primatology. 2016;78:326–339. doi: 10.1002/ajp.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz SL, Bales KL. “Monogamy” in Primates: Variability, Trends, and Synthesis. Introduction to Special Issue on Primate Monogamy. American Journal of Primatology. 2016;78:283–287. doi: 10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Grattan DR, Stolzenberg DS. Preoptic inputs and mechanisms that regulate maternal responsiveness. Journal of Neuroendocrinology. 2014;26:627–640. doi: 10.1111/jne.12185. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiology & Behavior. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: Focus on learning and memory. Neuroscience and Biobehavioral Reviews. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Hamagami F, McArdle JJ. Modeling latent growth curves with incomplete data using different types of structural equation modeling and multilevel software. Structural Equation Modeling. 2004;11:452–483. [Google Scholar]
- Fink S, Excoffier L, Heckel G. Mammalian monogamy is not controlled by a single gene. Proceedings of the National Academy of Sciences. 2006;103:10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014a;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014b;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. American Anthropologist. 1999;100:890–907. [Google Scholar]
- Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Hormones and Behavior. 2015;76:91–105. doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes, Brain, and Behavior. 2005;4:289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: Evidence for paternal attachment. Developmental Psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Hinde K, Maninger N, Mendoza SP, Mason WA, Rowland DJ, Wang GB, Cherry SR, Bales KL. Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus) American Journal of Primatology. 2016 doi: 10.1002/ajp.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, Brain, and Behavior. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activaton across forebrain nuclei in male prairie voles. Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J. High intensity exercise decreases global brain glucose uptake in humans. Journal of Physiology. 2005;568(1):323–332. doi: 10.1113/jphysiol.2005.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Gleason ED, Ophir AG, Phelps SM, Young LJ, Marler CA. Monogamous and promiscuous rodent species exhibit discrete variation in the size of the medial prefrontal cortex. Brain, behavior, and evolution. 2012;80:4–14. doi: 10.1159/000339247. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Ramage AE, Lancaster JL, Robin DA, Narayana S, Coyle T, Royall DR, Fox P. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. NeuroImage. 2009;45:17–28. doi: 10.1016/j.neuroimage.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature Neuroscience. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Laugero KD, Smilowitz JT, German JB, Jarcho MR, Mendoza SP, Bales KL. Plasma omega 3 polyunsaturated fatty acid status and monounsaturated fatty acids are altered by chronic social stress and predict endocrine responses to acute stress in titi monkeys. Prostaglandins, Leukotreines, and Essential Fatty Acids. 2011;84:71–78. doi: 10.1016/j.plefa.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. Journal of Neuroendocrinology. 2004;16:325–332. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Mason WA. Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Studies in Zoology. 1966;13:23–28. [Google Scholar]
- Mason WA. Use of space by Callicebus groups. In: Jay PC, editor. Primates: Studies in Adaptation and Variability. New York: Holt, Rinehart, and Wilson; 1968. pp. 200–216. [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic Motivational. NeuroImage. 2010;51:1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the lifespan. Developmental Psychology. 2002;38:115–142. [PubMed] [Google Scholar]
- Mendoza SP. Hormones, Brain, and Behavior. 3 2017. Social stress: concepts, assumptions, and animal models. [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: studies in non-human primates. In: Moberg GP, Mench JA, editors. Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. New York: CABI Publishing; 2000. pp. 227–247. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & Behavior. 1986a;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parenting within a monogamous society. In: Else JG, Lee PC, editors. Primate Ontogeny, Cognition, and Social Behaviour. Cambridge: Cambridge University Press; 1986b. pp. 255–266. [Google Scholar]
- Noack J, Murau R, Engelmann M. Consequences of temporary inhibition of the medial amygdala on social recognition memory performance in mice. Frontiers in Neuroscience. 2015;9:152. doi: 10.3389/fnins.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proceedings of the National Academy of Sciences. 2008;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Kippen TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, Parada M. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Archives of Sexual Behavior. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ’t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. Why primate models matter. American Journal of Primatology. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. European Neuropsychopharmacology. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Freeman SM, Laredo SA, Mendoza SP, Bales KL. μ and κ opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): implications for social behavior and endocrine functioning. Neuroscience. 2015a;290:421–434. doi: 10.1016/j.neuroscience.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus) Neuroscience. 2015b;287:32–42. doi: 10.1016/j.neuroscience.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38:2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ. μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. Journal of Neuroscience. 2013;33:9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, Porter-Stransky KS, Nevarez N, McLean JW, Kunmuench MA, Murphy AZ, Mathews TA, Aragona BJ. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016;5:e15325. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. K-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. The Journal of Neuroscience. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, O’Brien DJ, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biological Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Barnhart TE, Abbott DH, Ahlers EO, Kukis DL, Bales KL, Goodman MM, Young LJ. Initial investigation of three selective and potent small molecule oxytocin receptor PET ligands. Bioorganic & Medicinal Chemistry Letters. 2016 doi: 10.1016/j.bmcl.2016.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustiel JF, Glenn TC, Shik V, Boscardin J, Mahamid e, Zaaroor M. Monitoring of cerebral blood flow and metabolism in traumatic brain injury. Journal of Neurotrauma. 2005;22:955–965. doi: 10.1089/neu.2005.22.955. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Hormones and Behavior. 2016;77:204–210. doi: 10.1016/j.yhbeh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller E, Abbott D, Schultz-Darken N, Mendoza S, Mason W, Bourgeois S, Ruiz J. Preparing New World monkeys for laboratory research. ILARJournal. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin centrally administered facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Xu S, Aron A, Brown LL, Cao G, Feng T, Weng X. Reward and Motivational systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Human Brain Mapping. 2011;32:249–257. doi: 10.1002/hbm.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]