Abstract
Context
Long-term adherence to pharmaceutical treatment for multiple sclerosis (MS) is poor. A focus on patient preferences when determining the patient’s therapeutic plan may improve this experience.
Objective
To identify factors important to patients with MS when evaluating their options for pharmaceutical agents that deliver disease-modifying therapy.
Design
Stated-choice experiment to a sample of patients with MS from privately and publicly insured enrollees in a regional health plan. The experiment presented each respondent with a set of 8 drug choices for MS, asking them to select their preferred disease-modifying agent (DMA). Each respondent was randomized to 1 of 6 possible sets of 8 drug choices, for a total of 48 drug pairings in the experiment. Each choice included 2 hypothetical DMAs and a “no drug” option. Drug attributes included dosage type and modality, efficacy, relapse risk, and drug side effects.
Results
The “no drug” alternative was a stronger substitute than the alternative drug when the focal drug characteristics changed, and the most important drivers of choice were type of side effects and risk of severe relapse.
Discussion
The heterogeneity of our sample and the inclusion of a “no drug” alternative in the DMA choice scenarios make this study an important contribution to this body of literature. The importance of the “no drug” alternative in our results is consistent with poor long-term adherence to DMAs.
Conclusion
Patient-centered MS therapy using DMAs should include discussion of side effects and relapse risk.
INTRODUCTION
Multiple sclerosis (MS) is a chronic demyelinating neuroinflammatory disease of the central nervous system, often diagnosed in young adulthood, affecting more than 400,000 patients in the US and estimated to affect 2.3 million worldwide.1,2 Relapsing-remitting MS is the most common type, affecting 85% of patients with MS, and is characterized by a pattern of short neurologic flares (days to months) followed by relative stability (months to years).1 Most of the pharmaceutical agents providing disease-modifying therapy available for patients with MS target relapsing-remitting MS. Patients have been shown to have better outcomes with early initiation of and good adherence to disease-modifying agents (DMAs).3 However, patients with relapsing-remitting MS tend to have high discontinuation rates for DMAs.4 A better understanding of patient preferences for DMA characteristics may improve the match between patient and therapy, enhancing adherence and improving outcomes.
Because of this, there is a growing body of literature using stated-choice experiments (also called choice-based conjoint analysis) to assess patient preferences by studying patterns of DMA choice when patients are presented hypothetical choice scenarios. In one of the earliest studies, Johnson et al5 drew a sample of patients from Web site users and clinical trial participants (n = 651), a population focused on patients who had experienced DMAs administered through infusion. They presented a survey that focused on progression of disease, relapse frequency, and the probability of severe adverse events, finding that delays in disability progression were primary drivers of DMA choice. Wicks et al6 also drew their sample from a Web site (n = 319), administering a survey that presented oral DMAs varying across a number of side effect and adverse event characteristics, frequency of administration, delay of disease progression, frequency of relapse, and change in magnetic resonance imaging results. They found that severe side effects and the risk of liver toxicity were the most important characteristics driving choice.
In a small sample of patients (n = 50) drawn from the patient population of a tertiary care center, Wilson et al7 asked the patients to sort a set of 16 cards describing DMA alternatives in the order of their preference. The alternatives varied by delay of disease progression and magnetic resonance imaging changes, risk of relapse, symptom improvement, adverse events, and mode of administration. They found strong preferences for delay in disease progression and symptom improvement, and a preference for oral medications. In an extension of this work, Wilson and colleagues8 administered a stated-choice experiment with DMAs varying across similar dimensions to the patients (n = 291) of the tertiary care clinic. Lack of severe side effects, symptom improvement, and delay of disease progression were found to be the most important DMA characteristics, with oral administration also highlighted as an important characteristic.
Utz et al9 administered a simplified experiment to patients identified through a tertiary care clinic (n = 156), in which each scenario paired an oral medication against an injection. Treatment frequency and frequency of gastrointestinal side effects were the only variable DMA characteristics. In contrast to more complex choice scenarios, they found that treatment frequency was the most important characteristic, followed by mode of administration.
Our objective was to use a stated-choice experiment to identify patient preferences influencing DMA selection. The fact that respondents in the studies described earlier were forced to select a DMA alternative in each of these experiments is an important feature that distinguishes the experimental setting from a real-world situation. We know that DMA adherence is poor, with almost 50% of all patients stopping their DMA regimen at some point.1 Therefore, we included a “no drug” alternative in our design. In addition, these previous surveys focus on probabilities of relapse and magnitude of symptom improvement. We added additional specificity to these attributes and broadened the scope of side effects typically included in these experiments.
METHODS
Patient Sample
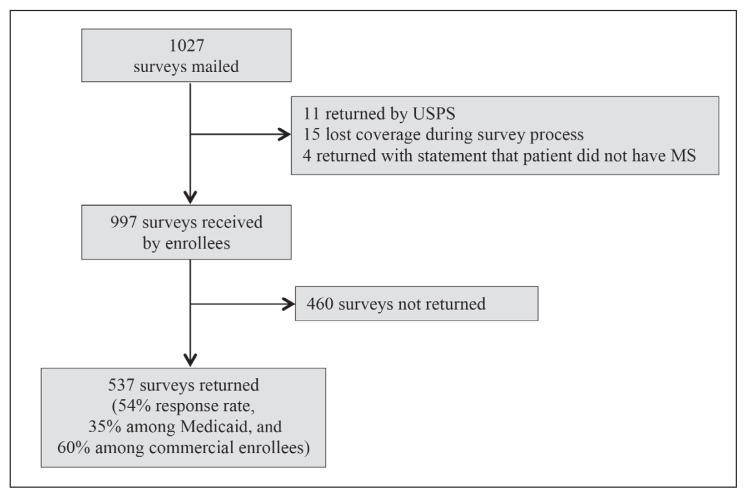
The patient sample was drawn from the enrollment files of a regional health plan in the Midwest. We identified patients who had 1 inpatient or 2 outpatient claims with an MS diagnosis indication (International Classification of Diseases, Ninth Revision [ICD-9] Code 340). We stratified the sample by the type of insurance coverage: commercial (employment-based), managed Medicare, and managed Medicaid (including those dually eligible for Medicare and Medicaid). We mailed 1027 surveys, including a small margin for undeliverable surveys, targeting 1000 surveys received by patients for a projected sample size of 600 (Figure 1). Adjusting for surveys returned by the postal service (n = 11), patients who lost health plan coverage during follow-up (n = 15), and surveys returned with an indication of no MS diagnosis (n = 4), we estimate that 997 surveys were actually received by patients with MS. We received 537 valid responses, for an overall response rate of 54.0%. A subanalysis of response rates indicated that the response in the Medicaid population was significantly lower than the balance of the population (34.5% vs 60.2%, respectively).
Figure 1.
Survey response rates.
MS = multiple sclerosis; USPS = US Postal Service.
Patient characteristics from the enrollment files and American Community Survey neighborhood characteristics from the patients’ residential area are shown in Table 1 for the respondents and nonrespondents. Neighborhood characteristics were matched at the most granular level available in the American Community Survey, either block group (race/ethnicity) or census tract (education, income). The respondent group was slightly older and more female, and tended to be healthier than nonrespondents were. Because response rates were low for the Medicaid enrollees, Table 1 also contains summary statistics for the population excluding this coverage group. The differences between respondents and nonrespondents are similar in this subgroup to the differences seen in the total population, other than a slight mitigation of the differences in the highest health risk category. In addition, a subanalysis excluding Medicaid enrollees produced almost identical results to those presented later.
Table 1.
Summary of patient characteristics
| Characteristic | Respondents | Nonrespondents |
|---|---|---|
| All enrollees | ||
| Population size, no. | 537 | 509 |
| Response rate, % | 51.3 | NA |
| Age, mean years (SD) | 56.6 (12.2) | 54.0 (13.7) |
| Female, no. (%) | 416 (77.5) | 322 (70.5) |
| Health status, no. (%) | ||
| Low risk or better | 7 (1.5) | 6 (1.5) |
| Medium risk | 220 (46.3) | 151 (37.5) |
| High risk | 153 (32.2) | 136 (33.8) |
| Very high risk | 95 (20.0) | 110 (27.3) |
| Neighborhood characteristics, mean % (SD) | ||
| Population nonwhite, non-Hispanic | 87.5 (16.5) | 83.8 (20.0) |
| Adults with no college education | 35.8 (15.1) | 36.0 (15.5) |
| Population with income under federal poverty limit | 10.0 (8.2) | 11.0 (8.9) |
| Excluding Medicaid enrollees | ||
| Population size, no. | 455 | 301 |
| Response rate, % | 60.2 | NA |
| Age, mean years (SD) | 56.7 (11.8) | 53.9 (12.3) |
| Female, no. (%) | 344 (75.6) | 205 (68.1) |
| Health status, no. (%) | ||
| Low risk or better | 7 (1.7) | 5 (1.9) |
| Medium risk | 196 (48.2) | 109 (40.5) |
| High risk | 127 (31.2) | 100 (37.2) |
| Very high risk | 77 (18.9) | 55 (20.5) |
| Neighborhood characteristics, mean % (SD) | ||
| Population nonwhite, non-Hispanic | 88.7 (14.8) | 86.8 (16.5) |
| Adults with no college education | 35.5 (15.0) | 35.0 (15.3) |
| Population with income under federal poverty limit | 9.2 (7.0) | 9.7 (7.5) |
NA = not applicable; SD = standard deviation.
Survey Design and Administration
Each respondent was given eight hypothetical scenarios and asked to make a choice about their preferred DMA option in each scenario. Each of the scenarios described two hypothetical DMAs and gave the respondent the option of choosing Drug A, Drug B, or neither drug. To our knowledge, this “no drug” option is unique in the literature, allowing us to explore the patient’s tendency to drop all therapy rather than switch to an alternative DMA.
The DMA attributes and levels of each attribute were determined on the basis of literature review and input from experts in the field. The DMAs varied across four attributes as summarized in Table 2: 1) dosage modality and frequency, 2) efficacy of treatment, 3) possible severity of relapse, and 4) common side effects. Dosage modality included oral medication, injection (no distinction between intramuscular or subcutaneous), and infusion. Dosage frequency varied for oral and injectable DMAs. Unlike the previous literature, the efficacy of the DMA was described by the domain of symptoms that improved, rather than by the strength of improvement7,8 or by the delay in progression of disease.6 The types of improvement included mental and emotional symptoms (impaired cognition or memory, depression), physical feeling (pain, fatigue, tingling, numbness), physical functioning (impaired mobility, coordination, bladder/bowel control, or vision), and combinations of these domains. Although relapse risk is typically included in these experiments as a probability or time interval between relapse,5–8 we distinguished DMAs by the probable severity of relapse rather than probability of relapse. Common side effects included cardiopulmonary symptoms, skin reactions, flulike symptoms, and neurologic symptoms; this fourth attribute included a level of “few side effects” as a reference value.
Table 2.
Disease-modifying agent attributes and levels
| Attribute level | Text description |
|---|---|
| Dosage | |
| Oral 1x/d | This drug is taken at home as a pill once a day. |
| Oral 2x/d | This drug is taken at home as a pill twice a day. |
| Inject 1/wk | This drug is taken at home by injection once a week. |
| Inject 1x/d | This drug is taken at home by injection once a day. |
| Infusion 1x/mo | This drug is given by infusion in an outpatient setting once a month. |
| Efficacy | |
| Mental/emotional | This drug works best improving how you feel mentally and emotionally. You could expect an improved ability to think or remember things, and reduced depression. |
| Physical feeling | This drug works best improving how you feel physically. You could expect reduced fatigue, less pain, and less numbness or tingling. |
| Physical function | This drug works best improving how you can do things physically. You could expect better coordination, bladder or bowel control, and vision. You could also expect more ability to walk and perform activities of daily living. |
| Mental/emotional and physical feeling | This drug works best improving both how you feel mentally and emotionally, and how you feel physically. You could expect an improved ability to think or remember things, and reduced depression. You also could expect reduced fatigue, less pain, and less numbness or tingling. |
| Mental/emotional and physical function | This drug works best improving both how you feel mentally and emotionally, and how you can do things physically. You could expect an improved ability to think or remember things, and reduced depression. You could expect better coordination, bladder or bowel control, and vision. You could also expect more ability to walk and perform activities of daily living. |
| Physical feeling and function | This drug works best improving both how you feel physically, and how you can do things physically. You could expect reduced fatigue, less pain, and less numbness or tingling. You could expect better coordination, bladder or bowel control, and vision. You could also expect more ability to walk and perform activities of daily living. |
| Helps all domains | This drug works well in all three areas, improving how you feel mentally and emotionally, how you feel physically, and how you can do things physically. You could expect an improved ability to think or remember things, and reduced depression. You could expect reduced fatigue, less pain, and less numbness or tingling. You could expect better coordination, bladder or bowel control, and vision. You could also expect more ability to walk and perform activities of daily living. |
| Relapse | |
| Mild | Relapses when taking this drug tend to be mild. They can be managed at home with an oral steroid. |
| Moderate | Relapses when taking this drug tend to be moderate. They can be managed in an outpatient setting with an intravenous steroid. |
| Severe | Relapses when taking this drug tend to be severe. You would have to check into a hospital for treatment. |
| Side effects | |
| Not significant | Most people experience few side effects with this drug. |
| Cardiopulmonary | The most common side effects with this drug affect your heart and lungs. They may include chest pain, shortness of breath, high blood pressure, or a heart rate that races, is too slow, or is uneven. |
| Skin reaction | The most common side effects with this drug are skin reactions. They may include flushing, redness, itching, or a rash. |
| Flulike symptoms | The most common side effects with this drug are flulike symptoms. They may include fatigue, nausea, vomiting, diarrhea, muscle aches, or abdominal pain. |
| Neurologic | The most common side effects with this drug affect your nervous system. They may include vision changes, changes in thinking or memory, depression, numbness in hands or feet, or a weakness on one side of the body. |
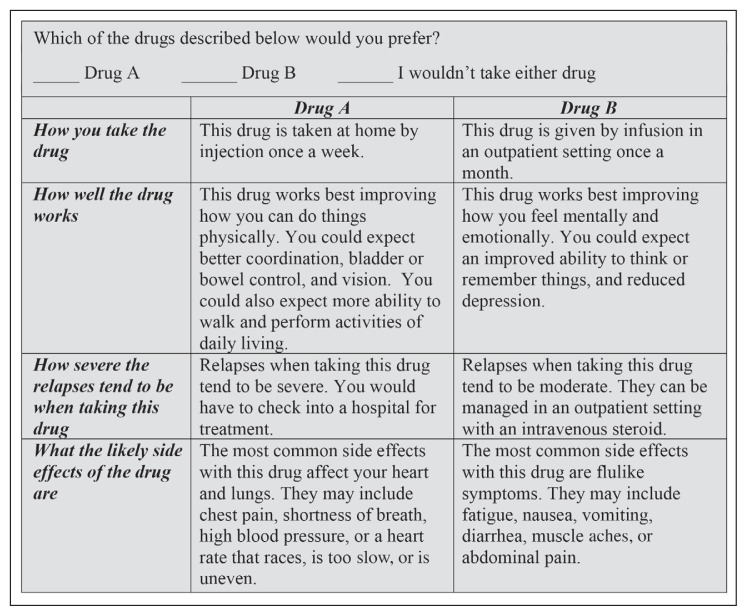
We used the AlgDesign10 package in R to create 48 scenarios of 2 drugs with a “no drug” alternative, blocked in 6 groups of 8. Each survey recipient was randomly assigned to 1 of the 6 blocks of 8 scenarios. Figure 2 shows a sample scenario as displayed for the survey recipient. The surveys were mailed under the health plan’s letterhead beginning in October 2014, with reminder postcards sent after approximately 3 weeks, and a second full survey mailing approximately 3 weeks after that. We received our last response in April 2015. All survey materials and the research protocol for this study were examined by Minneapolis-based Medica Research Institute’s external institutional review board, which provided a determination that the research was exempt from review under 45 Code of Federal Regulations (CFR) § 46.101(b)(2) and 45 CFR § 46.101(b)(4).
Figure 2.
Sample of disease-modifying agent choice on survey.
Econometric Framework
Each scenario had 3 response options (Drug A, Drug B, and neither drug), so we used a multinomial probit model for our analysis. Because the 8 scenarios resulted in repeated observations for each respondent, we also included random effects to account for the unobserved time-invariant characteristics of the respondents. The model was estimated using Roodman’s cmp package11 in Stata 12 (StataCorp LP, College Station, TX).
The parameters that result from the multinomial probit estimation indicate which factors have a statistically significant influence on choice, but they do not provide an intuitive understanding of the choice process. To provide more meaningful information, we used the estimated model to compute the marginal effects of changes in DMA attributes. We defined a marginal effect as the average change in the probability distribution of outcomes when a focal attribute of Drug A changes, with all other Drug A attributes and all Drug B attributes held constant at the scenarios’ actual levels. For example, the marginal effect moving from oral medication taken once per day to an injection taken once per week was computed by first projecting the population’s average choice probability with Drug A dosage modality set to “oral 1x/d” (with 1x indicating once) for all scenarios for each respondent, holding all other DMA characteristics at their actual value. Next we estimated the population average choice probability with Drug A dosage modality set to “inject 1x/wk,” with all other DMA characteristics at their actual value. The marginal effects were computed as the percentage-point changes in these average probabilities. Note that the 3 marginal effects always sum to 0 (subject to rounding error), because they capture a shifting of probabilities among the possible outcomes.
RESULTS
Survey Responses
Of the respondents, 92% provided a valid response for all 8 scenarios. The option “neither drug” was selected 44% of the time. As expected in this randomized design, the remainder was nearly evenly split between selecting Drug A and Drug B.
Current DMA regimen is summarized in Table 3. Forty-eight percent of the respondents were not receiving DMAs at the time of the survey; 25% of the respondents indicated that they had no experience with a DMA. Of the 537 respondents, 124 (23%) said they had chosen to discontinue a DMA against the advice of their physician. Side effects were listed as the dominant reason for this discontinuation (57% of those who had discontinued a DMA), with cost a distant second reason (13%).
Table 3.
Current disease-modifying agent (DMA) received by respondents
| Medication | Number (%) |
|---|---|
| Interferon beta-1a | 67 (12.5) |
| Interferon beta-1b | 20 (3.7) |
| Glatiramer acetate | 105 (19.6) |
| Fingolimod hydrochloride | 15 (2.8) |
| Teriflunomide | 15 (2.8) |
| Dimethyl fumarate | 44 (8.2) |
| Natalizumab | 13 (2.4) |
| Not currently receiving DMA | 258 (48.0) |
Forty-nine percent of the respondents indicated that they currently had no difficulty with walking or needed only occasional assistance, whereas 17% indicated they needed a wheelchair or scooter most of the time. Twelve percent of the respondents indicated they needed help with transferring, 33% had difficulty with speech or swallowing, and 18% needed help with activities of daily living.
Preferences for Disease-Modifying Agent Attributes
The marginal effects computed from the choice model are displayed in Table 4. Consistent with the respondents’ reported reasons for prior discontinuation of DMAs, side effect risk caused one of the largest impacts on choices. The probability of choosing Drug A declined dramatically if cardiopulmonary side effects (28.2 percentage points) or neurologic side effects (30.3 percentage points) were common. Skin-related side effects were the best-tolerated symptoms (12.9 percentage-point decline).
Table 4.
Marginal effects (standard errors) of changing Drug A characteristics
| Variable | ΔP: Drug A selected | ΔP: Drug B selected | ΔP: No drug selected |
|---|---|---|---|
| Marginal effect of changing dosage modality from oral 1x/d to: | |||
| Oral 2x/d | −0.013 (0.025) | 0.003 (0.005) | 0.011 (0.020) |
| Injection 1x/wk | −0.080 (0.021)a | 0.015 (0.004)a | 0.065 (0.017)a |
| Injection 1x/d | −0.076 (0.025)a | 0.014 (0.005)a | 0.061 (0.020)a |
| Infusion 1x/mo | −0.097 (0.023)a | 0.018 (0.004)a | 0.079 (0.019)a |
| Marginal effect of changing efficacy domain from improvement mentally/emotionally to: | |||
| How you feel physically | 0.027 (0.029) | −0.004 (0.005) | −0.023 (0.024) |
| How you function physically | 0.077 (0.025)a | −0.013 (0.004)a | −0.064 (0.021)a |
| Mentally/emotionally and how you feel physically | 0.067 (0.029)b | −0.011 (0.005)b | −0.056 (0.024)b |
| Mentally/emotionally and how you function physically | 0.116 (0.029)a | −0.021 (0.005)a | −0.096 (0.024)a |
| How you feel and function physically | 0.068 (0.028)b | −0.011 (0.005)b | −0.056 (0.024)b |
| All three domains | 0.174 (0.030)a | −0.033 (0.006)a | −0.141 (0.025)a |
| Marginal effect of changing from risk of mild relapse to: | |||
| Risk of moderate relapse | −0.113 (0.020)a | 0.022 (0.004)a | 0.090 (0.016)a |
| Risk of severe relapse | −0.251 (0.019)a | 0.043 (0.004)a | 0.207 (0.016)a |
| Marginal effect of changing from relatively few side effects to: | |||
| Risk of cardiopulmonary side effects | −0.282 (0.029)a | 0.054 (0.006)a | 0.228 (0.024)a |
| Risk of skin-related side effects | −0.129 (0.025)a | 0.028 (0.005)a | 0.100 (0.020)a |
| Risk of flulike symptoms | −0.194 (0.025)a | 0.040 (0.005)a | 0.154 (0.020)a |
| Risk of neurologic side effects | −0.303 (0.023)a | 0.056 (0.005)a | 0.247 (0.019)a |
p < 0.01.
p < 0.05.
Δ = change.
Respondents were also highly sensitive to the type of relapse risk. If Drug A had a risk of relapse requiring hospitalization, the probability of selecting that drug declined by 25.1 percentage points, relative to the probability when the risk was of a mild relapse treatable at home.
We found a preference for oral medications, with the probability of selecting Drug A declining by 7.6 to 9.7 percentage points across nonoral delivery modalities. However, the impact of dosage frequency did not drive a statistically significant difference in choice patterns. Respondents expressed a clear preference for DMAs that improved physical function (mobility and coordination), relative to DMAs that improved mental and emotional symptoms, or physical symptoms such as pain, numbness, or tingling.
Across all attributes, respondents were predicted to shift choices between the affected Drug A and the “no drug” option more than to shift to Drug B. For example, the 28.2 percentage-point decline in probability of selecting Drug A when cardiopulmonary symptoms are common was balanced by a 22.8 percentage-point increase in the probability of choosing no DMA, and only a 5.4 percentage-point increase in the probability of choosing Drug B.
DISCUSSION
We drew our sample from privately and publicly insured patients, providing a significantly more heterogeneous sample than in prior studies. Previous studies have drawn samples from those receiving care at tertiary care centers,7–9 from users of a Web site,5,6 or from participants in a clinical trial.5 Perhaps because of this difference, our patient population had significantly different DMA experience than in some of the previous literature. For example, Wilson et al7 recruited their population from patients receiving care at a tertiary care center, with 17% currently not receiving a DMA. We found a much higher fraction (48%) not currently receiving a DMA. Utz et al9 also recruited from a tertiary care center and found that 15% had never received a DMA, compared with our finding of 25%.
In addition to the sample source, our population may differ because of our reliance on self-report to exclude patients with primary progressive MS, because the ICD-9 diagnosis coding scheme does not distinguish among types of MS. Because current DMAs are ineffective for primary progressive MS, researchers with access to clinical data are typically careful to exclude patients with primary progressive MS before stated-choice experiments assessing patient preferences for DMAs.
Comparison of results across stated-choice experiments is difficult because of the wide variety of attribute configurations and presentation of results. However, several themes do emerge. Side effects, especially severe side effects or severe adverse events such as liver toxicity, are consistently shown to be an important driver of preferences.6–8 Where type of side effect is specified, the categories of cardiopulmonary or neurologic side effects that we found to be important are typically not included.6–9 Many studies include relapse risk as a DMA attribute, but risk is typically expressed as frequency of or time until relapse,5–8 with the result that this characteristic is of moderate importance to DMA choice. In contrast, by expressing relapse risk by intensity of treatment needed for the relapse, we have identified a critically important DMA attribute.
Others have found a preference for oral medications over injections and/or infusions,7–9 but frequency of administration was found to be meaningful only when the mode of administration was held fixed across scenarios and frequency varied independently.6,9 Because we combined frequency and mode into a single attribute, we also found that the importance of frequency was not highlighted.
We believe our study is unique in its assessment of preferences for the type of symptom targeted by the DMAs. Prior studies had looked at delay of progression of the disease5–8 or expressed improvements in terms of strength (mild, moderate, substantial).7,8 In contrast, we expressed improvements as targeting mental and emotional symptoms (cognition, depression), physical feelings (numbness, pain), or physical function (mobility, coordination). We found a meaningful preference for physical function as a target of the DMA’s efficacy.
Our study is also unique in the inclusion of an option of “no drug.” In our broad cross-section of patients with MS, we found a high rate of nonpharmaceutical treatment (48%) and that 23% had voluntarily stopped treatment in the past against physician advice. This indicates that no treatment is an important option for the patients. Indeed, we found that this “no drug” option was a stronger substitute for Drug A than the alternative pharmaceutical treatment, when Drug A’s characteristics were varied. In determining the preferred treatment path, supported by the advice of their physician, patients weigh the benefits of the expected efficacy of a DMA against the risk of side effects and severity of potential relapse. Although many patients find the efficacy of the DMA is well worth the potential for side effects or relapse, it is important to recognize that some patients may not.
Stated-choice experiments are an important tool in exploring patient preferences, but they have limitations. It is important to remember that they present only hypothetical situations to the patient in a way that may not resemble the real world. In addition, to reduce the cognitive challenge of the survey, these hypothetical comparisons are necessarily simplified and clinical endpoints are not described in the detail presented in the real world. Finally, the decision about DMA use is just one factor in a patient’s overall treatment path. The constrained choices presented to these participants, in the absence of physician advice and outside the context of other treatment modalities, provide only preliminary evidence in our effort to inform patient-centric care.
CONCLUSION
We found the attributes driving the greatest change in drug choice were type of side effect and severity of relapse. Patients had a strong aversion to cardiopulmonary and neurologic side effects. Although still statistically significant, there was better toleration of skin-related side effects and flulike symptoms. A risk of relapse that requires hospitalization to manage treatment caused a large decrease in the probability of choosing that DMA. To a lesser extent, oral medications and medications that targeted improvements in mobility and coordination were valued by the respondents. Finally, we found that “no drug” was an important option for the patients in their choice of treatment, and that patients tended to migrate to “no drug” rather than the alternative DMA when the focal drug characteristics changed.
These results can help guide discussions between patients and their clinicians as they consider treatment options. In particular, the findings highlight the importance of talking about any differences in expected severity of relapse, not just expected frequency of relapse, and any known differences in the types of symptoms expected to improve with the treatment alternatives. These results also may guide drug development and regulation, as the benefits and risks of new therapeutic options are considered.
Promise
We make a promise which no other people make, promising to be the serf and slave of our lords, the sick.
— Order of Knights Hospitallers of Saint John of Jerusalem, c 1099-present
Acknowledgments
Financial support for this study was provided entirely by a grant from TEVA Pharmaceuticals. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Nevertheless, representatives from TEVA provided important input over the course of the project. In particular, Cathy Carroll, employed by TEVA at the time of the survey development, provided important insight in the development of the survey attributes and levels.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.OptumRx. Multiple sclerosis insight report [Internet] Eden Prairie, MN: Optum; 2014. Aug, Multiple sclerosis: Background, new developments, key strategies. [cited 2014 Sep 9]. Available from: www.optum.com/content/dam/optum/resources/whitePapers/M53018_G_MS_Insight_Report_ORx_FINAL.pdf. [Google Scholar]
- 2.MS prevalence [Internet] Portland, OR: National Multiple Sclerosis Society; 2015. [cited 2015 Jul 30]. Available from: www.nationalmssociety.org/About-the-Society/MS-Prevalence. [Google Scholar]
- 3.Flachenecker P, Rieckmann P. Early intervention in multiple sclerosis: Better outcomes for patients and society? Drugs. 2003 Aug;63(15):1525–33. doi: 10.2165/00003495-200363150-00001. DOI: https://doi.org/10.2165/00003495-200363150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Devonshire V, Lapierre Y, Macdonell R, et al. GAP Study Group. The Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011 Jan;18(1):69–77. doi: 10.1111/j.1468-1331.2010.03110.x. Doi: https://doi.org/10.1111/j.1468-1331.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients’ benefit-risk preferences: Serious adverse event risks versus treatment efficacy. J Neurol. 2009 Apr;256(4):554–62. doi: 10.1007/s00415-009-0084-2. DOI: https://doi.org/10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 6.Wicks P, Brandes D, Park J, Liakhovitski D, Koudinova T, Sasane R. Preferred features of oral treatments and predictors of non-adherence: Two web-based choice experiments in multiple sclerosis patients. Interact J Med Res. 2015 Mar 5;4(1):e6. doi: 10.2196/ijmr.3776. DOI: https://doi.org/10.2196/ijmr.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson L, Loucks A, Bui C, et al. Patient centered decision making: Use of conjoint analysis to determine risk-benefit trade-offs for preference sensitive treatment choices. J Neurol Sci. 2014 Sep 15;344(1–2):80–7. doi: 10.1016/j.jns.2014.06.030. DOI: https://doi.org/10.1016/j.jns.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Wilson LS, Loucks A, Gipson G, et al. Patient preferences for attributes of multiple sclerosis disease-modifying therapies: Development and results of a ratings-based conjoint analysis. Int J MS Care. 2015 Mar-Apr;17(2):74–82. doi: 10.7224/1537-2073.2013-053. Doi: https://doi.org/10.7224/1537-2073.2013-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: A choice-based conjoint analysis. Ther Adv Neurol Disord. 2014 Nov;7(6):263–75. doi: 10.1177/1756285614555335. DOI: https://doi.org/10.1177/1756285614555335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler B. AlgDesign: Algorithmic experimental design [Internet] AlgDesign; 2014. Oct 15, [cited 2015 Aug 27]. Available from: https://cran.r-project.org/web/packages/AlgDesign/index.html. [Google Scholar]
- 11.Roodman D. Fitting fully observed recursive mixed-process models with cmp. The Stata Journal. 2011;11(2):159–206. [Google Scholar]