Abstract
Cytokinesis in fission yeast requires the function of an actomyosin-based contractile ring whose constriction is dependent on a signaling module termed the septation initiation network (SIN). In response to minor perturbation of the ring, the duration of SIN signaling is extended concurrently with a delay in nuclear cycle progression. These mechanisms require the conserved phosphatase Clp1p/Flp1p and facilitate the successful completion of cytokinesis, thereby increasing cellular viability. To isolate novel components of this cytokinesis monitoring system, we screened a genome-wide bank of protein kinase deletion mutants and identified Lsk1p, a nuclear-localized protein kinase. Similar to clp1Δ mutants, and in contrast to wild type, lsk1Δ cells are unable to maintain the integrity of the actomyosin ring upon treatment with low doses (0.3 μM) of latrunculin A. However, unlike clp1Δ mutants, lsk1Δ cells are competent to delay nuclear cycle progression after cytokinetic failure. In addition, lsk1Δ mutants suppress the lethal, multiseptate phenotype conferred by hyperactivation of the SIN, demonstrating that Lsk1p is a positive regulator of this module. In this report, we demonstrate that Lsk1p acts in parallel to Clp1p to promote actomyosin ring stability upon checkpoint activation. Our studies also establish that actomyosin ring maintenance and nuclear cycle delay in response to cytokinetic perturbation can be genetically resolved into independent pathways.
INTRODUCTION
In Schizosaccharomyces pombe, as in more complex, multicellular eukaryotes, cytokinesis occurs through the use of a contractile actomyosin ring. The primary actin ring forms from F-actin cables as cells enter mitosis and matures during anaphase, forming a progressively more tightly packed and thickened structure. The actomyosin ring then constricts upon completion of anaphase B concurrently with the deposition of a division septum (Arai and Mabuchi, 2002). Essential for the constriction of the actomyosin ring is a complex regulatory module referred to as the septation initiation network (SIN). This network localizes to the spindle pole body (SPB) and functions to ensure that cytokinesis occurs once per cell cycle subsequent to the completion of mitosis (reviewed in Simanis, 2003). The SIN includes several kinases (Cdc7p, Sid1p, and Sid2p) that act in a linear pathway and are essential for the correct temporal formation of the division septum (McCollum and Gould, 2001).
Although the SIN is essential for ring constriction and septum formation, it is not required for ring assembly (the ring persists until after anaphase, but eventually disassembles in SIN mutant backgrounds; Wu et al., 2003; Mishra et al., 2004). In contrast to the SIN, the so-called rng genes are essential for the proper assembly of the actomyosin ring. Although some rng mutations seem to affect the interphase distribution of F-actin (cdc3, cdc8), other rng mutants (cdc12, cdc15) have a lesser effect and seem to perturb ring formation and/or stability more specifically (reviewed in Le Goff et al., 1999). In addition to the essential components of the SIN, many other genes affecting aspects of SIN signaling and division septum assembly have been isolated, including scw1, dma1, zfs1, par1, and clp1 (Murone and Simanis, 1996; Beltraminelli et al., 1999; Cueille et al., 2001; Jiang and Hallberg, 2001; Le Goff et al., 2001; Trautmann et al., 2001; Karagiannis et al., 2002).
Recently, we have shown that minor perturbation of the actomyosin ring triggers a checkpoint mechanism that ensures completion of cytokinesis in a manner dependent on the protein phosphatase Clp1p/Flp1p and the SIN (Mishra et al., 2004). The cytokinesis checkpoint is activated in mutants affecting several components of the actomyosin ring as well as by treatment with drugs such as latrunculin A (LatA), which affect the rate of actin polymerization (Ayscough et al., 1997). Two cellular responses contribute to enhanced viability upon perturbation of the actomyosin ring. These include a G2 delay, as well as a mechanism that promotes the reassembly and maintenance of the actomyosin ring (Mishra et al., 2004). Recent studies have shown that the G2 delay likely results from Clp1p-dependent activation of Wee1p (Cueille et al., 2001; Trautmann et al., 2001) and instability of Cdc25p (Esteban et al., 2004; Wolfe and Gould, 2004). The molecular mechanism leading to actomyosin ring maintenance is relatively poorly understood. To identify additional components that regulate this checkpoint mechanism, we screened a bank of protein kinase deletion mutants for hypersensitivity to doses of the actin polymerization inhibitor latrunculin A that do not affect the viability of wild-type cells.
In this report, we describe the characterization of a previously uncharacterized kinase, latrunculin sensitive kinase knockout (lsk1), derived from this screen. Similar to clp1Δ cells, lsk1Δ mutants are inviable in media containing 0.3 μM LatA. However, in contrast to clp1Δ, lsk1Δ mutants are competent to delay nuclear cycle progression and fail only in maintaining the integrity of the actomyosin ring. Furthermore, failure to maintain the ring stems from a role for Lsk1p in positively regulating the SIN. Significantly, as predicted by a branched cytokinesis checkpoint model, we demonstrate the ability to resolve two independent pathways required for cytokinesis checkpoint function.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
All S. pombe strains used in this study (Table 1) were cultured in YES media (Alfa et al., 1993) with constant shaking. All genetic crosses and general yeast techniques were performed using standard methods (Moreno et al., 1991). Elutriation was performed using a Beckman elutriation chamber (JE 5.0) according to the manufacturer's instructions. Latrunculin A was purchased from Molecular Probes (Eugene, OR).
Table 1.
Strain list
| Strain | Genotype | Source |
|---|---|---|
| MBY192 | ura4-D18 leu1-32 h− | Laboratory collection |
| MBY1768 | lsk1::ura4 ura4-D18 leu1-32 h− | This study |
| MBY2295 | act1-LR::ura4 ura4-D18 leu1-32h+ | This study |
| MBY2405 | lsk1::ura4 act1-LR::ura4 ura4-D18 leu1-32 h+ | This study |
| MBY664 | rlc1GFP::leu1 leu1-32 ura4-D18 ade6-210 h+ | Laboratory collection |
| MBY2406 | lsk1::ura4 rlc1GFP::leu1 leu1-32 ura4-D18 h+ | This study |
| MBY2407 | lsk1::ura4 clp1::ura4 ura4-D18 leu1-32 h+ | This study |
| MBY2408 | lsk1::ura4 rng3-65 ura4-D18 ade6-21xleu1-32 h− | This study |
| MBY2409 | clp1::ura4 rng3-65 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2410 | lsk1::ura4 clp1::ura4 rng3-65 ura4-D18 ade6-21xleu-32h+ | This study |
| MBY2411 | lsk1::ura4 cdc15-140 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2119 | clp1::ura4 cdc15-140 ura4-D18 ade6-21x | Laboratory collection |
| MBY2412 | lsk1::ura4 clp1::ura4 cdc15-140 ura4-D18 ade6-21x h− | This study |
| MBY2413 | lsk1::ura4 myo2-E1 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2117 | clp1::ura4 myo2-E1 ura4-D18 leu1-32 ade6-21xh− | Laboratory collection |
| MBY2414 | lsk1::ura4 clp1::ura4 myo2-E1 ura4-D18 ade6-21xleu1-32 h− | This study |
| MBY978 | clp1GFP::kan ura4-D18 leu1-32 ade6-21xh+ | D. McCollum |
| MBY2415 | cdc7GFP::ura4 ade6-21x leu1-32 ura4-D18 his3-D1 h+ | C. Albright |
| MBY2416 | lsk1::ura4 clp1GFP::kan ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2417 | lsk1::ura4 cdc7GFP::ura4 ura4-D18 leu1-32 h+ | This study |
| MBY2418 | lsk1::ura4 cdc14-118 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2419 | lsk1::ura4 sid1-239 ura4-D18 leu1-32 h+ | This study |
| MBY2420 | lsk1::ura4 cdc11-123 ura4-D18 h+ | This study |
| MBY2421 | lsk1::ura4 sid4-A1 ura4-D18 leu1-32 h+ | This study |
| MBY2422 | lsk1::ura4 sid3-106 ura4-D18 leu1-32 h+ | This study |
| MBY2423 | lsk1::ura4 cdc7-24 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2424 | lsk1::ura4 mob1-R4 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2425 | lsk1::ura4 sid2-250 ura4-D18 leu1-32 h+ | This study |
| MBY2426 | lsk1::ura4 plo1-1 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2427 | lsk1::ura4 cdc16-116 ura4-D18 leu1-32 h+ | This study |
| MBY2428 | lsk1GFP::ura4 ura4-D18 leu1-32 h− | This study |
| MBY2429 | lsk1GFP::ura4 cdc14-118 ura4-D18 ade6-21xleu1-32 h+ | This study |
| MBY2430 | lsk1GFP::ura4 cdc16-116 ura4-D18 ade6-21xleu1-32h+ | This study |
| MBY2124 | clp1::ura4 cdc16-116 ura4-D18 leu1-32 ade6-21x h− | Laboratory collection |
| MBY286 | cdc16-116 leu1-32 ura4-D18 ade6-210 h+ | Laboratory collection |
| MBY1463 | plo1-1 leu1-32 ura4-D18 ade6-21xh− | D. McCollum |
| MBY95 | cdc14-118 ade6-210 ura4D18 leu1-32 h+ | Lab collection |
| MBY2309 | nmt41-CHD-GFP::leu1 ura4-D18 leu1-32 ade6-216 h− | D. McCollum |
| MBY2529 | lsk1::ura4 nmt41-CHD-GFP::leu1 ura4-D18 leu1-32 ade6-216 | This study |
| MBY2530 | sid4GFP::kanR rlc1GFP::leu1 leu1-32 ura4-D18 ade6-21x | This study |
| MBY2531 | lsk1::ura4 sid4GFP-kanR rlc1GFP::leu1 leu1-32 ura4-D18 ade6-21x | This study |
Deletion of the lsk1 Gene
The entire open reading frame of the lsk1 gene was deleted as part of a genome-wide study of fission yeast protein kinase knockouts (Bimbó, Balasubramanian, and Liu, unpublished data). Deletions were performed using polymerase chain reaction (PCR)-based homologous recombination (forward: 5′-TCA CAG ATT GCG TGT AAT TCT CTT CAT TGT TTA GGA ATA TTC CTT TTT TTA TTT TTT ATT TTT TTT AAC CCT GTT AAA TGC AAC AGC TAT GAC CGA GCT AGG TCG TGA AGA GGG ACC CTC ACT AAA GGG AAC-3′; reverse: 5′-ATG AAA TAT AAA GAT TAT TTT TTT TAA ACA TTT GTC CAG CTT GCA TAG CTT CGC CTT GAC AAA ATT TTC TGG CTC ATT TAT TGT AAA ACG ACG GCC ACT GGG TTG AGC CGA AGA GGC ACT ATA GGG CGA ATT GG-3′). After amplification the ura4-based disruption cassette was transformed into ura4-D18 leu1-32 strain by using the lithium acetate method to obtain integrants. The desired integration was confirmed by colony PCR.
Lsk1p-GFP Fusion
A C-terminal fragment of the lsk1 gene was PCR amplified (forward: 5′-GGG GGG AAT TCT GAG AAC AGC GGA AAA GAA CAT TT-3′; reverse: 5′-GGG GGC CCG GGTC TTT TAG ATT TTC GTT TTT TAC-3′) using the Expand high-fidelity PCR system (Roche Diagnostics, Indianapolis, IN) and cloned into the unique EcoRI and SmaI sites of the pJK210 vector. The desired integration was confirmed by PCR. Integrants displayed wild-type resistance to 0.3 μM LatA and were unable to suppress the lethal multi-septate phenotype conferred by the cdc16-116 mutation.
Construction of the act1-LR Allele
An N-terminal fragment of the act1 gene was PCR amplified with a reverse primer incorporating the R183A and D184A mutations (forward primer #1: 5′-GTC GTC CTG CAG ATG GAA TAA GAA GAA ATC GC-3′; reverse primer #1: 5′-TAG TCA GTC AAG GCA GCA CCG GCG AGA TCA AGA CG-3). Similarly, a C-terminal fragment of the act1 gene was PCR amplified with a forward primer incorporating the R183A and D184A mutations (forward primer #2: 5′-CGT CTT GAT CTC GCC GGT GCT GCC TTG ACT GAC-3′; reverse primer #2: 5′-GTC GTC GCG GCC GCT TAG AAG CAC TTA CGG-3′). A full-length product containing the desired mutations was then PCR amplified using forward primer #1, reverse primer #2, and the products of the first two reactions as template. The full-length product was then cloned into the PstI and NotI sites of the pJK210 vector. The vector was linearized with NcoI and transformed into a ura4-D18 leu1-32 strain to obtain integrants. Resistance to LatA was tested by plating on EMM media. In contrast to wild-type the act1-LR mutant was capable of colony formation on plates containing up to 5 μM latrunculin A.
Fluorescence Microscopy
Images were obtained using a Leica DMIRE2 microscope equipped with Uniblitz shutter and CoolSnap HQ charge-coupled device camera (Photometrics, Tucson, AZ) driven by MetaMorph 6.1 software (Universal Imaging, Downingtown, PA). Cell staining with 4′6,-diamidino-2-phenylindole (DAPI), aniline blue, and Alexafluor-488-conjugated phalloidin were performed as described previously (Balasubramanian et al., 1998). Time-lapse videos were obtained using logarithmically growing cells placed on top of a 0.5% agarose pad (in YES). The agarose filled the cavity of a depression slide (catalog no. 7103; Sail brand) that was covered with a Matsunami coverslip (Matsunami Trading, Tokyo, Japan); thus, cells were gently trapped between the agarose and coverslip. Three-dimensional (3D) reconstructions were performed by taking Z-series (200-nm sections) of wide-field fluorescent images and deconvolving using the AutoDeblur/AutoVisualize 9.2 package (Auto-quant Imaging, Watervliet, NY). In experiments involving quantitation of Alexafluor-488 phalloidin upon LatA treatment, maximum projections of Z-stacks were obtained using the Metamorph Multi-dimensional Data Set utility. Total integrated intensity of a given cell, minus an equal area of background total integrated intensity, was calculated using the MetaMorph Region Statistics utility. The velocity of calponin-homology domain fused to green fluorescent protein (CHD-GFP) patches was determined using the “Track Objects” application included in MetaMorph Software. Immunofluorescence was performed as described previously (Balasubramanian et al., 1998). Rabbit anti-GFP (A-6455; Molecular Probes, Eugene, OR) and mouse anti-TAT1 primary antibodies were used at 1:800 and 1:200 dilutions, respectively. Goat anti-rabbit IgG (A-11008; Molecular Probes), and goat anti-mouse IgG (A-11020 secondary antibodies; Molecular Probes) were used at a dilution of 1:1000.
RESULTS
Reverse Genetics Identifies a Serine/Threonine Protein Kinase Essential for Viability upon Treatment with 0.5 μM Latrunculin A
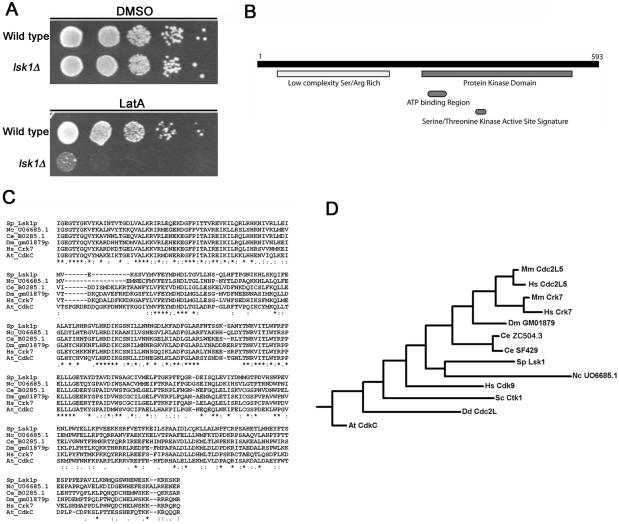
Kinase signaling plays a critical role in all checkpoint responses examined thus far. Hence, in an attempt to identify kinases involved in the regulation of the cytokinesis checkpoint, we screened a bank of fission yeast protein kinase knockouts for sensitivity to low concentrations of the actin depolymerizing drug LatA. We have shown previously that cells defective for the checkpoint display a marked hyper-sensitivity to LatA in the range of 0.2 to 0.5 μM and are inviable in growth medium containing the drug (Mishra et al., 2004). One kinase mutant derived from this screen, lsk1, became of particular interest because it was unable to proliferate upon treatment with 0.5 μM LatA. In contrast, wild-type cells were viable and capable of forming colonies even at low cell densities (Figure 1A). lsk1 defines an open reading frame (SPAC2F3.15) encoding a putative serine/threonine protein kinase of 593 amino acids (Figure 1B). Lsk1p is most closely related to members of the cyclin-dependent kinase family (Liu and Kipreos, 2000) and has homologues in budding yeast, Arabidopsis, Dictyostelium, Caenorhabditis elegans, Drosophila, and mammals (Figures 1, C and D). The N-terminal half of the protein contains low-complexity serine- and arginine-rich sequences that show little homology to proteins in current databases (Figure 1B).
Figure 1.
Loss of Lsk1p confers a hypersensitivity to 0.5 μM latrunculin A. (A) Ten-fold serial dilutions of logarithmically growing wild-type and lsk1Δ cells were plated onto YES plates containing 0.5 μM LatA or DMSO (solvent control) and incubated at 32°C for 3 d. (B) Schematic representation of Lsk1p showing the C-terminal Ser/Thr kinase domain, and N-terminal low complexity Ser/Arg-rich region. (C) ClustalW alignments of Lsk1p and related proteins. Sp, S. pombe; Nc, Neurospora crassa; Ce, C. elegans; Dm, Drosophila melanogaster; Hs, Homo sapiens; At, Arabidopsis thaliana. (D) Phylogenetic relationship of Lsk1p to related proteins inferred using the Fitch-Margoliash algorithm available in the PHYLIP 3.5 software package (http://evolution.genetics.washington.edu/phylip.html). Mm, Mus musculus; Sc, S. cerevisiae; Dd, Dictyostelium discoideum.
lsk1Δ Mutants Cannot Complete Cytokinesis When Treated with 0.3 μM LatA
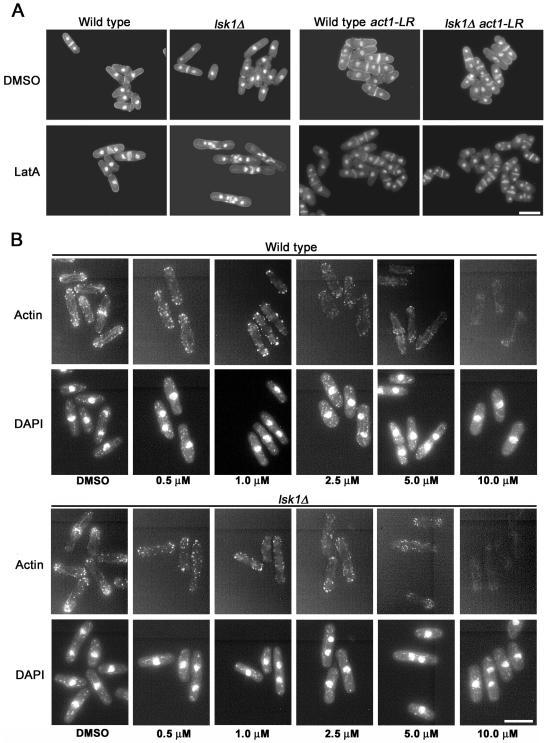
To more closely examine why lsk1Δ mutants were inviable upon LatA treatment, cells grown in liquid culture were examined microscopically after 5-h exposure to the drug. Interestingly, the lsk1Δ mutant displayed severe cytokinesis defects. In contrast to wild-type cells, which were able to form functional (although misshapen) septa, lsk1Δ mutants were unable to successfully complete division septum formation (Figure 2A). Importantly, lsk1Δ mutants did not display any obvious phenotypes in the absence of LatA and were able to form fully functional septa that were indistinguishable from wild-type (Figure 2A).
Figure 2.
Hypersensitivity of lsk1Δ mutants to LatA stems from an inability to complete septum formation. (A) Cells of the indicated genotype were grown to mid-log phase at 32°C and then treated with 0.3 μM LatA for 5 h before being fixed and stained with DAPI (nuclei) and aniline blue (cell wall/septa). Bar, 10 μm. (B) Wild-type and lsk1Δ mutants were grown to mid-log phase at 32°C and treated for 20 min with the indicated concentration of LatA. Cells were subsequently fixed and stained with Alexafluor-488 phalloidin (actin) and DAPI (nuclei). Bar, 10 μm.
To ensure that the observed phenotypes were not due to nonspecific effects of LatA on molecules other than actin, we examined the effects of the drug in cells where critical residues (R183, D184) in the actin molecule were altered to alanine by site-directed mutagenesis (see Materials and Methods). These residues were chosen because the alteration of the homologous residues in Saccharomyces cerevisiae result in a complete desensitization to LatA (Belmont et al., 1999). Interestingly, lsk1+ and lsk1Δ cells carrying the LatA-resistant allele of actin (referred to as act1-LR for LatA resistant) were indistinguishable from one another both in the presence and absence of LatA (Figure 2A). Thus, the cytokinesis defects observed in lsk1Δ mutants are due specifically to the perturbation of actin.
lsk1Δ Cells Do Not Have a Generalized Defect in the Architecture of the Actin Cytoskeleton
Because the mutant displayed a hypersensitivity to LatA, we proceeded to test for a generalized defect in the architecture of the actin cytoskeleton by using Alexafluor-488 phalloidin. As part of this analysis, we also examined whether F-actin in lsk1Δ cells was more rapidly depolymerized in the presence of LatA relative to wild type. Because LatA affects the rate of actin polymerization in a concentration-dependent manner (Rupes et al., 2001), this was achieved by incubating cells with differing concentrations of the drug for the same duration. Interestingly, untreated, logarithmically growing lsk1Δ cells were indistinguishable from wild type in terms of F-actin distribution (Figure 2B). Furthermore, no gross differences in the depolymerization of F-actin were observed because both wild-type and lsk1Δ cells required 20-min treatment with 10 μM LatA to achieve complete depolymerization of interphase F-actin (Figure 2B). To provide a more quantitative analysis, Z-stacks of wild-type and lsk1Δ mutants treated as in Figure 2B also were obtained and the total integrated Alexafluor-488 phalloidin fluorescent intensity of maximum projections determined (see Materials and Methods). No significant differences in fluorescent intensity were observed when comparing wild-type and lsk1Δ cells upon treatment with increasing concentrations of LatA (Table 2).
Table 2.
Total integrated fluorescent intensity (± SD) of wild-type and lsk1Δ mutants stained with Alexafluor-488 phalloidin after 20-min treatment with the indicated concentration of LatA
| Treatment | Wild type | lsk1Δ | P value |
|---|---|---|---|
| DMSO | 4366 ± 899 | 3997 ± 1131 | 0.39 |
| 0.5 μM LatA | 1759 ± 450 | 1507 ± 596 | 0.26 |
| 1.0 μM LatA | 806 ± 274 | 1004 ± 304 | 0.16 |
| 2.0 μM LatA | 685 ± 246 | 786 ± 260 | 0.34 |
| 5.0 μM LatA | 393 ± 118 | 449 ± 151 | 0.32 |
| 10.0 μM LatA | 330 ± 158 | 404 ± 96 | 0.17 |
P values obtained from t tests comparing wild-type and lsk1Δ cells are shown in the right-most column.
As an alternate approach to determine whether the lsk1Δ phenotype could be due to alterations in actin dynamics, we examined actin patch movement by using cells expressing an integrated copy of the Rng2p CHD-GFP (Wachtler et al., 2003). In a manner analogous to Coronin-GFP fusion (Pelham and Chang, 2001), the CHD-GFP fusion protein, which colocalizes with actin, can be used to monitor the movement of actin patches by using high-speed (2 frames/s) time-lapse fluorescence microscopy (see Materials and Methods). The velocity of actin patch movement calculated in our study (Table 3) is similar to that described using coronin-GFP as a marker of actin patches (Pelham and Chang, 2001). No significant differences in actin patch velocity were observed when comparing wild-type and lsk1Δ cells treated with dimethyl sulfoxide (DMSO) or with 0.3 μM LatA (see Supplementary Time-Lapse Videos 1-4 and Table 3). Together, these results indicated that lsk1Δ cells did not have a generalized defect in the architecture or dynamics of the actin cytoskeleton and thus implied that Lsk1p played a more specific role in regulating the formation and/or stability of cytokinetic structures in the presence of 0.3 μM LatA.
Table 3.
CHD-GFP patch velocity in micrometers per second (± SD) of wild-type and lsk1Δ mutants in the presence or absence of 0.3 μ M LatA (Time-Lapse Videos 1-4)
| Treatment | Wild type | lsk1Δ | P value |
|---|---|---|---|
| DMSO | 0.24 ± 0.08 | 0.26 ± 0.10 | 0.61 |
| 0.3 μM LatA | 0.22 ± 0.07 | 0.23 ± 0.08 | 0.45 |
P values obtained from t tests comparing wild-type and lsk1Δ cells are shown in the right-most column. Images were captured at 0.5-s intervals.
lsk1Δ Mutants Are Able to Form, but Not Maintain, Actomyosin Rings in the Presence 0.3 μM LatA
To further investigate the role of Lsk1p in the regulation of cytokinesis, we next examined the kinetics of actin ring assembly upon LatA treatment. Wild-type and lsk1Δ mutants were synchronized by centrifugal elutriation, treated with LatA or DMSO (solvent control), and examined using Alexafluor-488 phalloidin. The profiles of DMSO treated wild-type and lsk1Δ cells were similar with a peak in actin ring formation occurring at 60 min (Figure 3, A-C). In contrast, the response of lsk1Δ cells differed dramatically upon LatA treatment compared with wild-type. Wild-type cells were able to form rings (with similar kinetics to DMSO controls) and in addition were able to maintain these rings for the duration of the experiment. lsk1Δ mutants, on the other hand, were also able to form rings (peak in ring formation at 60 min was slightly decreased compared with DMSO controls), but they were unable to maintain the ring in the presence of the drug.
Figure 3.
lsk1Δ mutants are able to form but not maintain actomyosin rings upon treatment with 0.3 μM LatA. Wild-type (A) and lsk1Δ mutants (B) were synchronized in early G2 phase by centrifugal elutriation, and released into YES media containing 0.3 μM LatA or DMSO. Cells were subsequently fixed at 20-min intervals and stained with Alexafluor-488 phalloidin, and DAPI. Representative micrographs are shown in C. A single representative trial out of three is shown. Bar, 10 μm.
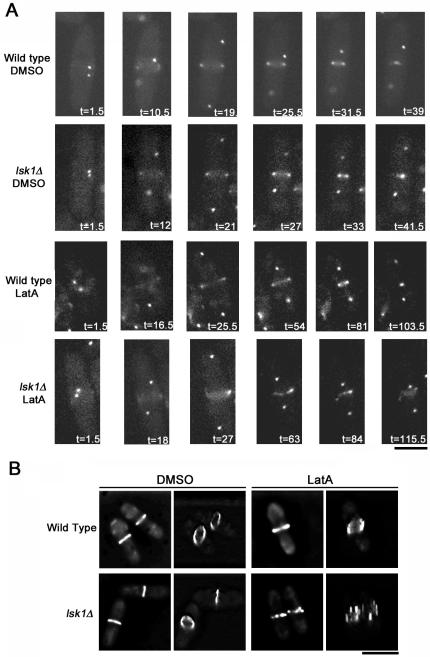
To determine what was becoming of actin rings in the presence of 0.3 μM LatA, we examined ring dynamics by using time-lapse microscopy. We thus constructed strains carrying a GFP-tagged version of the myosin regulatory light chain Rlc1p, which localizes to the actomyosin ring (Naqvi et al., 2000). In addition, these strains also expressed Sid4-GFP (Chang and Gould, 2000), which acted as a marker for the SPB and allowed us to measure the timing of ring assembly relative to a common temporal reference (SPB separation). On treatment with DMSO, wild-type and lsk1Δ cells displayed similar kinetics both in terms of the timing of ring assembly relative to SPB separation (13.7 ± 1.9 vs. 14.0 ± 1.6 min, respectively) as well as the completion of ring constriction (42 ± 3 vs. 43 ± 2.6 min, respectively) (Supplementary Time-Lapse Videos 5 and 6). LatA-treated wild-type cells (Supplementary Time-Lapse Video 7) formed either malformed Rlc1p-GFP rings (1 of 8 cells) that still attempted to constrict, normal rings that completed constriction with durations ranging from 72 to 114 min post-SPB separation (5 of 8 cells), or abnormal rings that displayed a fragmentation similar to lsk1Δ cells (2 of 8). In contrast, 16 of 19 lsk1Δ cells displayed a fragmentation of the Rlc1p-GFP ring (Supplementary Time-Lapse Video 8), whereas 3 of 19 formed what seemed to be normal rings that completed constriction with times ranging from 70.5 to 90 min post-SPB separation.
To more closely examine the physical structure of actomyosin rings, DMSO and LatA-treated wild-type and lsk1Δ strains expressing Rlc1p-GFP were examined by generating 3D reconstructions of deconvolved Z-stacks. These experiments clearly showed a fragmentation of the actomyosin ring in lsk1Δ mutants upon challenge with LatA in later stages of mitosis (Figure 4B).
Figure 4.
Actomyosin rings fragment in lsk1Δ mutant backgrounds in the presence of 0.3 μM LatA. (A) Time-lapse microscopy of wild-type (Supplementary Time-Lapse Videos 5 and 7) and lsk1Δ cells (Supplementary Time-Lapse Videos 6 and 8) carrying an integrated copy of both Rlc1-GFP and Sid4-GFP. Cells were treated with DMSO (Supplementary Time-Lapse Videos 5 and 6) or 0.3 μM LatA (Supplementary Time-Lapse Videos 7 and 8) before being imaged at 90-s intervals. Bar, 5 μm. (B) Maximum projections (left-hand panels) and tilted 3D reconstructions (right-hand panels) of deconvolved Z-stacks of wild-type and lsk1Δ cells expressing rlc1-GFP treated with 0.3 μM LatA, or DMSO, for 30 min. Bar, 10 μm.
Lsk1p Acts in Parallel to Clp1p to Promote Stability of Actomyosin Rings
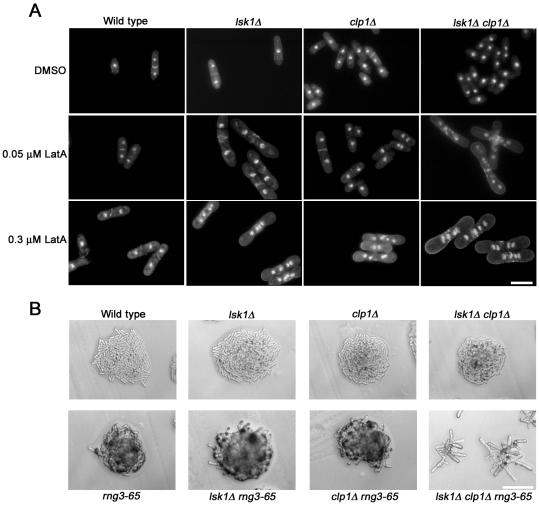
Up to this point, the behavior of lsk1Δ mutants seemed extremely similar to that displayed by clp1Δ cells (Mishra et al., 2004). Both mutants showed little or no cytokinesis phenotype under normal growth conditions, but they displayed a hypersensitivity to LatA that stemmed from a failure to maintain the integrity of the actomyosin ring (Mishra et al., 2004; Figures 3 and 4). We were thus interested to determine whether Lsk1p and Clp1p were acting in a simple linear pathway that controlled cytokinesis checkpoint function. To answer this question, lsk1Δ clp1Δ double mutants were examined at differing concentrations of LatA (0-0.3 μM) for 5 h. If Lsk1p and Clp1p were acting in a linear pathway, one would expect to observe similar phenotypes when comparing lsk1Δ, clp1Δ, and lsk1Δ clp1Δ double mutants. On the other hand, if Lsk1p and Clp1p were acting in parallel one would expect to observe an additive effect in the double mutant. Remarkably, even at 0.05 μM LatA, and in contrast to the respective single mutants, double mutants could not successfully complete division septum assembly, and accumulated multiple nuclei (Figure 5A). Quantitation of actin rings in these experiments also confirmed that lsk1Δ and clp1Δ cells were acting in parallel in terms of actomyosin ring stability (Table 4).
Figure 5.
Lsk1p and Clp1p act in parallel pathways. (A) Cells of the indicated genotype were grown to mid-log phase at 32°C and then treated with varying concentrations of LatA for 5 h. Cells were subsequently stained with both aniline blue (cell wall/septa) and DAPI (nuclei). Bar, 10 μm. (B) Cells of the indicated genotype were freshly streaked to YES plates and incubated for 18 h at 29°C. Bar, 50 μm.
Table 4.
Percentage of cells (± SD) with actin rings after 5-h treatment with the indicated concentration of LatA.
| Treatment | Wild type | lsk1Δ | clp1Δ | lsk1Δ clp1Δ |
|---|---|---|---|---|
| DMSO | 11.7 ± 2.1 | 10.9 ± 2.0 | 12.4 ± 1.3 | 11.3 ± 1.8 |
| 0.05 μM LatA | 16.0 ± 2.9 | 8.4 ± 1.9 | 11.2 ± 1.9 | 0.3 ± 0.1 |
| 0.3 μM LatA | 21.9 ± 5.9 | 1.9 ± 0.6 | 0 | 0 |
In addition to drug treatment, we also examined the effects of ring damage by using a temperature-sensitive rng mutation, rng3-65. rng3 encodes a member of the UCS domain family of proteins that are thought to play a role in ensuring the proper folding of myosin heads (Wong et al., 2000). Interestingly, lsk1Δ rng3-65 and clp1Δ rng3-65 double mutants displayed a more severe rng phenotype compared with rng3-65 single mutants (our unpublished data), but were viable at 29°C. In contrast, lsk1Δ clp1Δ rng3-65 triple mutants were inviable under these same conditions (Figure 5B). Similar additive effects were observed when using the myo2-E1 (myosin II heavy chain; Wong et al., 2000) and cdc15-140 (PCH domain protein; Fankhauser et al., 1995) mutations (our unpublished data). Together, these results strongly suggested that Lsk1p and Clp1p were acting in parallel. Thus, Lsk1p could not be a simple upstream activator or downstream effector of the Clp1p-dependent cytokinesis checkpoint.
lsk1Δ Mutants Are Competent to Delay Nuclear Cycle Progression upon Cytokinesis Checkpoint Activation
Although Lsk1p was clearly required for ring integrity, we were interested to determine whether it, like Clp1p, was also important for G2 delay in response to perturbations of the actomyosin ring. We thus examined the kinetics of nuclear cycle progression in LatA-treated cells by using populations synchronized by centrifugal elutriation. Interestingly, although the kinetics of nuclear accumulation in the checkpoint negative clp1Δ mutant were similar in LatA and DMSO controls, nuclear accumulation in LatA-treated lsk1Δ mutants was delayed for ∼2 h (LatA treated lsk1Δ cells initiated a second round of nuclear division at t = 270 min, whereas DMSO-treated lsk1Δ mutants, as well as LatA-treated clp1Δ mutants, initiated a second round of mitosis at t = 150 min; Figure 6.). This suggested that lsk1Δ mutants were competent to delay cell cycle progression upon treatment with LatA. To determine whether this delay was a consequence of checkpoint activation or some other aspect of the lsk1Δ phenotype, lsk1Δ clp1Δ cells also were examined in the same way. Importantly, the 2-h delay in nuclear cycle progression observed in lsk1Δ cells was eliminated in lsk1Δ clp1Δ mutant backgrounds, indicating that the cell cycle block in lsk1Δ cells was dependent on Clp1p activity.
Figure 6.
lsk1Δ mutants are competent to delay cell cycle progression upon activation of the cytokinesis checkpoint. Cells of the indicated genotype were synchronized in early G2 phase by centrifugal elutriation and released into YES media at 32°C in the presence of 0.3 μM LatA or DMSO. Cells were subsequently fixed at 30-min intervals, stained with aniline blue (cell wall/septa) and DAPI (nuclei), and scored for nuclear number and fragmented septa. A single representative trial of three is shown.
Loss of Lsk1p Does Not Prevent Activation of the Cytokinesis Checkpoint
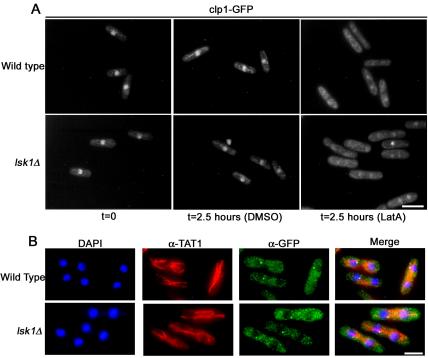
Because lsk1Δ cells were competent to delay cell cycle progression, we reasoned that the cytokinesis checkpoint was being activated normally in lsk1Δ backgrounds and that Lsk1p was simply affecting the arm of the checkpoint that enhanced actomyosin ring stability (perhaps by modulating SIN function). To test these assumptions, we began by examining two known markers of cytokinesis checkpoint activation, Clp1p-GFP and Cdc7p-GFP. Clp1p, a nucleolar protein in interphase, is present in the cytoplasm during cytokinesis. Cdc7p, on the other hand, is present at both SPBs in early mitosis, but only at a single SPB during cytokinesis. Furthermore, these proteins are maintained at these subcellular locations for prolonged durations upon activation of the cytokinesis checkpoint. Thus, the presence of these two cell cycle-regulated proteins in the cytoplasm, and at a single SPB, respectively, are thought to signify active SIN signaling (Trautmann et al., 2001; Mishra et al., 2004).
Clp1p-GFP signal was examined in elutriated cells at 2.5 h post-LatA treatment (a time point where DMSO controls had just completed their first division and LatA-treated cells were still delayed in cytokinesis). Intriguingly, Clp1p-GFP was localized outside the nucleolus in both wild-type and lsk1Δ mutants treated with LatA (Figure 7A). As a further test, we also examined Cdc7p-GFP localization in asynchronous cultures after 3-h treatment with LatA. As expected, Cdc7p-GFP was found at a single SPB in 88 and 83% of wild-type and lsk1Δ cells respectively (Figure 7B). Together with our earlier results, this analysis indicated that Lsk1p was not required for checkpoint initiation but instead was important for maintaining integrity of the actomyosin ring subsequent to the establishment of a checkpoint active state.
Figure 7.
Loss of Lsk1p does not prevent activation of the cytokinesis checkpoint. (A) Wild-type and lsk1Δ cells carrying an integrated GFP-tagged version of Clp1p were synchronized in early G2 phase by centrifugal elutriation and then released into YES media containing 0.3 μM LatA or DMSO at 32°C. Cells were subsequently fixed and examined using GFP autofluorescence. Bar, 10 μm. (B) Wild-type and lsk1Δ cells carrying an integrated GFP-tagged version of Cdc7p were grown to mid-log phase and treated with 0.3 μM LatA or DMSO for 3 h. Cells were subsequently, fixed and stained with DAPI (nuclei) and antibodies specific for microtubules and GFP (see Materials and Methods). Bar, 5 μm.
Lsk1p Is a Positive Regulator of the SIN
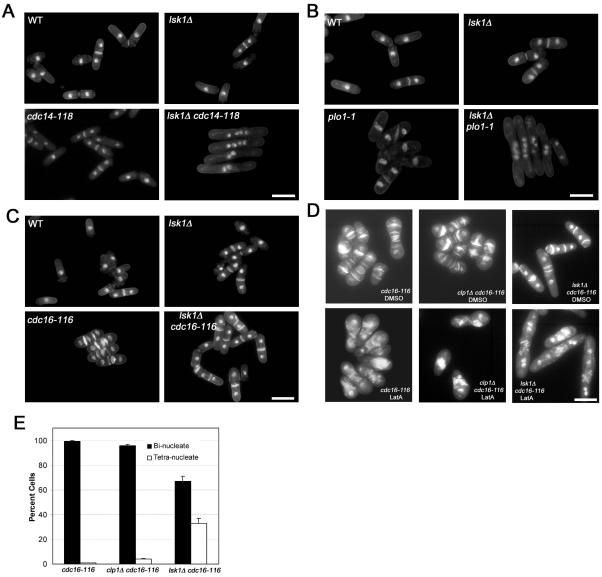
Because our data suggested that Lsk1p was solely affecting the actomyosin ring stability branch of the cytokinesis checkpoint, and because the SIN is essential for maintaining integrity of the actomyosin ring, we examined whether Lsk1p could be influencing activity of the SIN. To this end, we crossed lsk1Δ strains to mutants affecting various components of the septation initiation network (SIN). Interestingly, deletion of lsk1 lowered the restrictive temperature of the cdc14-118 and sid1-239 alleles (these mutations abrogate SIN activation and confer a phenotype in which division septum formation, but not nuclear cycle progression, is blocked; Fankhauser and Simanis, 1993b; Guertin et al., 2000) (Figure 8A; our unpublished data). Double mutants between lsk1Δ and other SIN components (cdc11, sid4, spg1, cdc7, sid2, and mob1) did not display any overt negative interactions (our unpublished data).
Figure 8.
Lsk1p is a positive regulator of the SIN. (A) Cells of the indicated genotype were grown to mid-log phase at 24°C and then shifted to 30°C for 4 h. Cells were subsequently fixed and stained with aniline blue (cell wall/septa) and DAPI (nuclei). Bar, 10 μm. (B) Cells of the indicated genotype were grown to mid-log phase at 24°C and then shifted to 36°C for 4 h. Cells were subsequently fixed and stained with aniline blue (cell wall/septa) and DAPI (nuclei). Bar, 10 μm. (C) Cells of the indicated genotype were grown to mid-log phase at 24°C and then shifted to 36°C for 4 h. Cells were subsequently fixed and stained with aniline blue (cell wall/septa) and DAPI (nuclei). Bar, 10 μm. (D) Cells of the indicated genotype were grown to mid-log phase at 24°C and then shifted to 36°C for 6 h in the presence of 0.3 μM LatA or DMSO. Cells were subsequently fixed and stained with aniline blue (cell wall/septa) and DAPI (nuclei). Bar, 10 μm. (E) Quantitation of bi- and tetranucleate cells for strains treated as in D.
We next tested for genetic interactions between lsk1Δ and mutants of Plo1p. The Plo1p kinase plays multiple, complex roles in both cytokinesis and mitosis and is thought to act as an upstream activator of the SIN (Tanaka et al., 2001). The temperature-sensitive plo1-1 allele used in this work is viable at 36°C, but it displays defects in the positioning of the actomyosin ring and septum (Bahler et al., 1998). Interestingly, lsk1Δ plo1-1 double mutants were synthetically lethal at 36°C and displayed a typical SIN mutant phenotype (Figure 8B).
These data suggested that Lsk1p was acting as a positive regulator of the SIN. As a further test of this model, we examined the effects of the lsk1Δ mutation in a temperature-sensitive cdc16-116 mutant background (where the SIN is in a constitutively hyperactivated state; Minet et al., 1979; Fankhauser et al., 1993a). If Lsk1p was indeed a positive regulator of the SIN, then one would predict that the loss of Lsk1p might suppress the defects associated with the cdc16-116 allele. Remarkably, deletion of lsk1 (in the absence of LatA) could rescue the lethal, multiseptate phenotype conferred by the cdc16-116 mutation at 36°C (Figure 8C). Although the double mutants displayed a much higher proportion of septated cells, including those that were multiseptate, they were viable and capable of colony formation. Together, genetic analysis indicated that Lsk1p was acting as a positive regulator of the SIN. Thus, the failure of lsk1Δ mutants to maintain integrity of the ring upon LatA treatment is most simply explained by a reduced capacity for SIN signaling.
The Loss of Lsk1p Is Partially Epistatic to cdc16-116 upon Treatment with 0.3 μM LatA
Because lsk1Δ mutations could rescue cdc16-116 defects, we were interested to determine whether cdc16-116 could in turn rescue the LatA sensitivity of lsk1Δ mutants. If this were the case, then this would indicate that Lsk1p and Cdc16p were acting in opposition to regulate actomyosin ring stability. To this end lsk1Δ cdc16-116 double mutants were treated with LatA upon temperature shift-up to 36°C. Interestingly, it became clear that lsk1Δ was partially epistatic to cdc16-116 upon treatment with 0.3 μM LatA. After 6 h, and in contrast to cdc16-116, and clp1Δ cdc16-116 mutants (which arrested as binucleate cells with multiple misshapen septa), lsk1Δ cdc16-116 double mutants accumulated a significant proportion of tetra-nucleate cells with spot like deposits of septum material (Figure 8, D and E). The ability of the lsk1 deletion to abrogate the cdc16-116 phenotype suggests that Lsk1p acts downstream of Cdc16p to promote stability and constriction of the actomyosin ring.
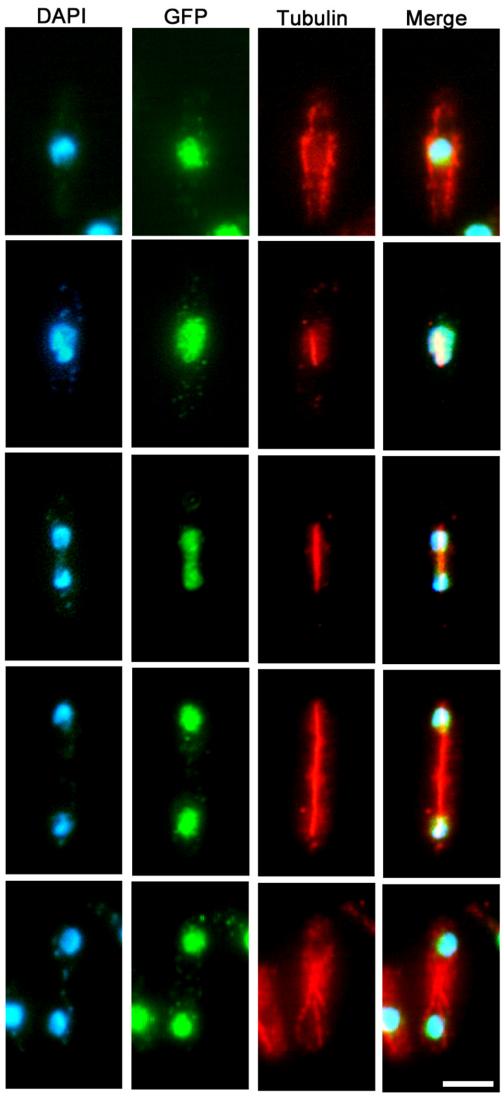
Lsk1p Localizes to the Nucleus
Because members of the SIN localize to the SPB and/or medial ring, we constructed a C-terminal GFP fusion to determine the intracellular localization of Lsk1p (see Materials and Methods). Unlike members of the SIN, Lsk1p was localized to the nucleus during all cell cycle phases (Figure 9), and it was not detected at the spindle pole bodies or the actomyosin ring. GFP signal was not altered by treatment with LatA, or in cdc14-118 or cdc16-116 mutant backgrounds (our unpublished data).
Figure 9.
Lsk1p localizes to the nucleus during all phases of the cell cycle. Cells carrying an integrated copy of a C-terminally tagged copy of Lsk1p-GFP were grown to mid-log phase, fixed, and then stained with DAPI (nuclei) and antibodies specific for microtubules and GFP (see Materials and Methods). Bar, 5 μm.
DISCUSSION
Because the formation and constriction of the actomyosin ring is essential for maintaining the viability of actively growing fission yeast cells it is not surprising that S. pombe has developed a checkpoint system monitoring its structure (Mishra et al., 2004). Because protein kinases (for example Chk1p, Cds1p, Bub1p; Rhind and Russell, 2000; Yamaguchi et al., 2003) play essential roles in all checkpoint responses examined thus far, we reasoned that it might be possible to identify novel components of the cytokinesis monitoring system by screening an available genome-wide bank of fission yeast protein kinase deletion mutants (Bimbó, Balasubramanian, and Liu, unpublished data). The screening method was based on the identification of mutants displaying a hyper-sensitivity to 0.5 μM of the actin depolymerizing drug LatA, a known checkpoint activator.
This approach was validated because the protein kinase mutant described in this report, lsk1Δ, exhibited no obvious defects in the interphase architecture of the actin cytoskeleton, but it did display conspicuous genetic interactions with known SIN components, as well as the Plo1p kinase, an upstream activator of the SIN. Finding a previously uncharacterized SIN regulator in this way strongly supports the notion that low-dose LatA treatment is indeed a useful tool for perturbation of the actomyosin ring and the discovery of new cytokinesis and/or checkpoint mutants. This smaller scale screen also suggests that a full-scale genome-wide screen of fission yeast deletion mutants (as has been performed in budding yeast) would be highly informative.
Genetic analysis implicates Lsk1p as a positive regulator of the SIN because the lsk1Δ mutant rescues the lethal, multiseptate phenotype conferred by the cdc16-116 allele (a mutation that results in a constitutive hyperactivation of the SIN; Figure 8). However, in contrast to core SIN components, Lsk1p is not essential for ring constriction under normal growth conditions. In fact, cytokinesis defects could not be observed in logarithmically growing populations of lsk1Δ mutants even when >3000 cells were scored (our unpublished data). Its nonessential nature thus suggested that Lsk1p might be specifically triggered upon checkpoint activation to positively regulate the SIN and thereby promote actomyosin ring stability. However, the observation that lsk1Δ cdc16-116 double mutants are viable in the absence of LatA implies that Lsk1p functions as a positive SIN regulator even in the absence of damage to the actomyosin ring. This is to say it seems more likely that a decrease in SIN signal in lsk1Δ mutants simply becomes limiting for constriction only under conditions in which actomyosin ring function is already compromised.
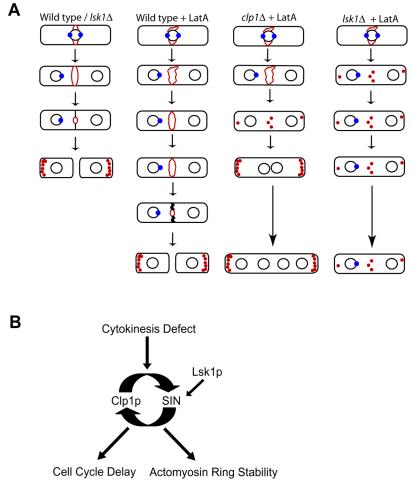
With respect to actomyosin ring stability, lsk1Δ mutants behave in a similar manner to clp1Δ cells (which also show a ring fragmentation phenotype upon treatment with 0.3 μM LatA). This result thus raised the possibility that Lsk1p and Clp1p were acting in a simple linear pathway in the regulation of SIN signaling. However, despite similar phenotypes (Figures 3 and 4; Mishra et al., 2004), further analysis clearly demonstrated that Lsk1p and Clp1p were acting in parallel (Figure 5) and in addition highlighted two crucial differences between these mutants. First, clp1Δ cdc16-116 mutants, in contrast to lsk1Δ cdc16-116 strains, are inviable at the restrictive conditions and display a similar phenotype to cdc16-116 single mutants (Figure 8). Second, lsk1Δ mutants, unlike clp1Δ cells, are competent to delay cell cycle progression, despite being unable to maintain the integrity of the actomyosin ring (Figures 6 and 10A).
Figure 10.
Summary and model. (A) Summary of the behavior of indicated mutant genotypes upon challenge with 0.3 μM LatA. Actin patches and rings are shown in red. Nuclei are shown as black circles. Cdc7p localization to the spindle pole body (a marker of active SIN) is shown schematically as a blue circle. Wild-type or lsk1Δ cells under normal growth conditions (column 1); wild-type, clp1Δ, and lsk1Δ cells upon treatment with 0.3 μM Lat A (columns 2, 3, and 4 respectively). (B) Model. Lsk1p acts as a positive regulator of the SIN promoting integrity of the actomyosin ring upon activation of the cytokinesis checkpoint.
The ability of lsk1Δ cells to delay cell cycle progression is of crucial importance for two reasons. First, it demonstrates the ability to genetically separate the two independent pathways of checkpoint function through mutation. Second, it strongly suggests that Lsk1p is not required for the establishment of a checkpoint active state, but instead implies that Lsk1p is acting as an essential component of ring stability subsequent to checkpoint initiation. Such a role is consistent with our findings that Lsk1p is nonessential for the maintenance of either Clp1p in the cytoplasm, or Cdc7p at the SPB (Figures 7 and 10A). This is also consistent with the observation that the lsk1Δ phenotype is partially epistatic to cdc16-116 upon treatment with 0.3 μM LatA (Figure 8, D and E). To date, the only mutations identified capable of abrogating the cdc16-116 phenotype have been in SIN components downstream of Cdc16p. Together, the simplest model would have Lsk1p modulating SIN activity at a point downstream of Cdc16p (promoting the integrity and constriction of the actomyosin ring upon perturbation) (Figure 10B). The mechanism by which Lsk1p acts to modulate SIN activity remains unknown and may be direct or indirect.
Interestingly, the cell cycle delay in lsk1Δ mutants is abrogated not only in lsk1Δ clp1Δ backgrounds (Figure 6) but also in lsk1Δ wee1-50 double mutants, which behave similarly to clp1Δ cells (our unpublished data). Furthermore, the ability of the lsk1Δ mutation, but not wee1-50, to suppress cdc16-116 (our unpublished observations) also strongly suggests that Lsk1p is not affecting ring stability merely by modulating Cdc2p kinase in a simple linear pathway. Conversely, mutations in cdr1/nim1, which result in a down-regulation of Cdc2p kinase activity through modulation of Wee1p, are also unable to rescue cdc16-116 mutants (our unpublished data). Furthermore, unlike clp1Δ cells, lsk1Δ mutants do not display a semiwee or wee phenotype (our unpublished data).
Given that hyperactivation of the SIN suppresses both the cell cycle delay, and actomyosin ring stability defects of clp1Δ cells in response to low-dose LatA treatment (Mishra et al., 2004), it is intriguing to note that the lsk1Δ mutation causes deficiencies in only the branch that controls actomyosin ring maintenance. If Lsk1p is acting as a positive regulator of the SIN, and the SIN is part of a positive feedback loop affecting cell cycle progression, then why does the loss of Lsk1p have little or no effect on checkpoint-induced cell cycle delay? This question can be answered by hypothesizing that the amount of signal generated by the positive feedback loop does not affect each branch of the checkpoint equally. In other words, less output may be required to maintain the cell cycle block than is needed to maintain stability of the ring. Thus, in an lsk1Δ background the level of output from the SIN may be decreased to a range where the function of one branch (cell cycle delay) is relatively unperturbed, whereas the other (ring stability) is more strongly affected. Interestingly, this differential effect also would ensure, as the signal from the positive feedback loop increases during a normal, wild-type cell cycle, that the pathway controlling cell cycle delay would be activated before the pathway controlling stability and constriction of the ring. Thus, the linking of the SIN and Clp1p in a positive feedback loop provides the cell with a simple way to temporally coordinate two entirely distinct physical processes.
Supplementary Material
Acknowledgments
We thank D. McCollum and C. Albright for strains used in this study as well as K. Gull for α-TAT1 antibodies. We also thank all members Temasek Life Sciences Laboratory, in particular members of the yeast and fungal biology laboratories, for support and useful discussions during the course of this work. This work was supported by research funds from Temasek Life Sciences Laboratory. J. K. is a recipient of a Singapore Millennium Foundation postdoctoral fellowship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-06-0502. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0502.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alfa, C., Fantes, P., Hyams, J., McLeod, M., and Warbrick, E. (1993). Experiments with Fission Yeast: A Laboratory Course Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Arai, R., and Mabuchi, I. (2002). F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 115, 887-898. [DOI] [PubMed] [Google Scholar]
- Ayscough, K. R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D. G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., Steever, A. B., Wheatley, S., Wang, Y., Pringle, J. R., Gould, K. L., and McCollum, D. (1998). Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 143, 1603-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M. K., McCollum, D., Chang, L., Wong, K. C., Naqui, N. I., He, X., Sazer, S., and Gould, K. L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont, L. D., Patterson, G. M., and Drubin, D. G. (1999). New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell Sci. 112, 1325-1336. [DOI] [PubMed] [Google Scholar]
- Beltraminelli, N., Murone, M., and Simanis, V. (1999). The S. pombe zfs1 gene is required to prevent septation if mitotic progression is inhibited. J. Cell Sci. 112, 3103-3114. [DOI] [PubMed] [Google Scholar]
- Chang, L., and Gould, K. L. (2000). Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast. Proc. Natl. Acad. Sci. USA 97, 5249-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueille, N., Salimova, E., Esteban, V., Blanco, M., Moreno, S., Bueno, A., and Simanis, V. (2001). Flp1, a fission yeast orthologue of the S. cerevisiae cdc14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J. Cell Sci. 114, 2649-2664. [DOI] [PubMed] [Google Scholar]
- Esteban, V., Blanco, M., Cueille, N., Simanis, V., Moreno, S., and Bueno, A. (2004). Role for the Cdc14-family phosphatase Flp1p at the end of the cell cycle in controlling the rapid degradation of the mitotic inducer Cdc25p in fission yeast. J. Cell Sci. 117, 2461-2468. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., Marks, J., Reymond, A., and Simanis, V. (1993a). The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 12, 2697-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., and Simanis, V. (1993b). The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol. Biol. Cell 4, 531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., Reymond, A., Cerutti, L., Utzig, S., Hofmann, K., and Simanis, V. (1995). The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell 82, 435-444. [DOI] [PubMed] [Google Scholar]
- Guertin, D. A., Chang, L., Irshad, F., Gould, K. L., and McCollum, D. (2000). The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 19, 1803-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., and Hallberg, R. L. (2001). Correct regulation of the septation initiation network in Schizosaccharomyces pombe requires the activities of par1 and par2. Genetics 158, 1413-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis, J., Oulton, R., and Young, P. G. (2002). The Scw1 RNA-binding domain protein regulates septation and cell-wall structure in fission yeast. Genetics 162, 45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff, X., Utzig, S., and Simanis, V. (1999). Controlling septation in fission yeast: finding the middle, and timing it right. Curr. Genet. 35, 571-584. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., Buvelot, S., Salimova, E., Guerry, F., Schmidt, S., Cueille, N., Cano, E., and Simanis, V. (2001). The protein phosphatase 2A B'-regulatory subunit par1p is implicated in regulation of the S. pombe septation initiation network. FEBS Lett. 508, 136-142. [DOI] [PubMed] [Google Scholar]
- Liu, J., and Kipreos, E. T. (2000). Evolution of cyclin-dependent kinases (CDKs) and CDK-activating kinases (CAKs): differential conservation of CAKs in yeast and metazoa. Mol. Biol. Evol. 17, 1061-1074. [DOI] [PubMed] [Google Scholar]
- McCollum, D., and Gould, K. L. (2001). Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11, 89-95. [DOI] [PubMed] [Google Scholar]
- Minet, M., Nurse, P., Thuriaux, P., and Mitchison, J. M. (1979). Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 137, 440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, M., Karagiannis, J., Trautmann, S., Wang, H., McCollum, D., and Balasubramanian, M. K. (2004). The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 117, 3897-3910. [DOI] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795-823. [DOI] [PubMed] [Google Scholar]
- Murone, M., and Simanis, V. (1996). The fission yeast dma1 gene is a component of the spindle assembly checkpoint, required to prevent septum formation and premature exit from mitosis if spindle function is compromised. EMBO J. 15, 6605-6616. [PMC free article] [PubMed] [Google Scholar]
- Naqvi, N. I., Wong, K. C., Tang, X., and Balasubramanian, M. K. (2000). Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat. Cell Biol. 2, 855-858. [DOI] [PubMed] [Google Scholar]
- Pelham, R. J., and Chang, F. (2001). Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat. Cell Biol. 3, 235-244. [DOI] [PubMed] [Google Scholar]
- Rhind, N., and Russell, P. (2000). Chk1 and Cds 1, linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113, 3889-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes, I., Webb, B. A., Mak, A., and Young, P. G. (2001). G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol. Biol. Cell 12, 3892-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis, V. (2003). Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 116, 4263-4275. [DOI] [PubMed] [Google Scholar]
- Tanaka, K., Petersen, J., MacIver, F., Mulvihill, D. P., Glover, D. M., and Hagan, I. M. (2001). The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe. EMBO J. 20, 1259-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann, S., Wolfe, B., Jorgensen, P., Tyers, M., Gould, K. L., and McCollum, D. (2001). Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr. Biol. 11, 931-940. [DOI] [PubMed] [Google Scholar]
- Wachtler, V., Rajagopalan, S., and Balasubramanian, M. K. (2003). Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 116, 867-874. [DOI] [PubMed] [Google Scholar]
- Wolfe, B. A., and Gould, K. L. (2004). Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 23, 919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, K. C., Naqvi, N. I., Iino, Y., Yamamoto, M., and Balasubramanian, M. K. (2000). Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 113, 2421-2432. [DOI] [PubMed] [Google Scholar]
- Wu, J., Kuhn, J. R., Kovar, D. R., and Pollard, T. D. (2003). Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723-734. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, S., Decottignies, A., and Nurse, P. (2003). Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 22, 1075-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.