Engineered nanomaterials (ENs) comprise a diverse suite of structures manufactured from a range of distinct components that are having an ever-increasing number of applications in industry, commerce and medicine (Figure 1). ENs reported to be produced in the largest quantities include TiO2, SiO2, ZnO, AlOx, FeOx, silver, carbon nanotubes, fullerenes and quantum dots, at levels ranging from 0.5 – 5,500 tonnes/year worldwide [1]. The negative impact of ENs on the environment and human health due to off-target effects is of major concern and requires ongoing investigation. A recent database (NanoE-Tox), compiled from 224 articles published over the preceding decade, was created in order to obtain an overview of the environmental effects of eight chemically distinct ENs where toxicity toward crustaceans (Daphnia magna), fish, algae or bacteria was the primary readout [2]. The purposes of such databases are to: 1) gain an understanding of the relative toxicities of various ENs and how these relate to their physicochemical properties, 2) decipher the mechanism(s) by which their toxicities occur and, 3) provide an alternative to ethically questionable, time consuming and costly animal studies, through the development of predictive models that derive from these databases. NanoE-Tox reported the most ecotoxic ENs to be made from silver, followed by ZnO and CuO, with the primary mechanism of toxicity of these ENs being dissolution of ions, however, direct cell membrane damage, oxidative stress and genotoxicity were also potentially contributing factors for Ag and ZnO ENs. A lack of sufficient information regarding the various physicochemical parameters (primary size, shape, surface charge, surface area) meant that their contributions to cytotoxicity could not be conclusively defined.
Figure 1. Ring charts depicting the proportion of published articles reporting current or potential applications of indicated ENs in industry, commerce or medicine.
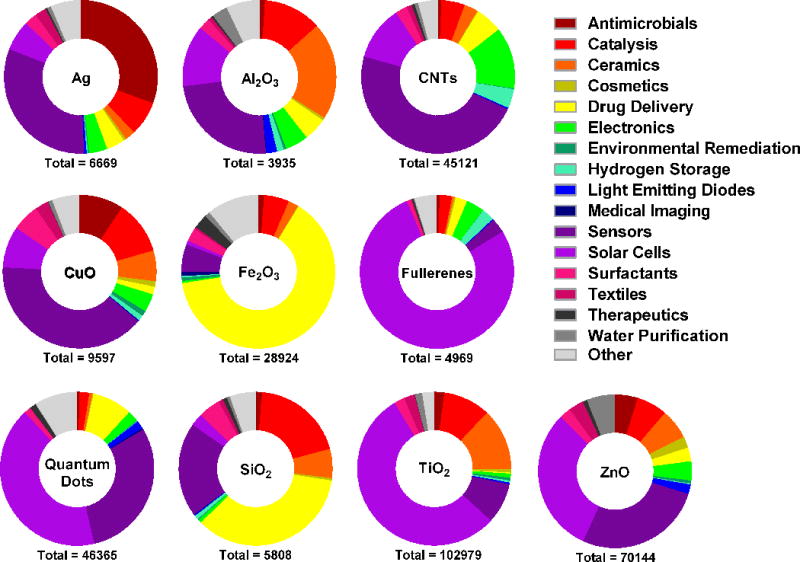
The data was generated using Thomson Reuters Web of Science citation indexing service and was based on a search for articles in all databases using the indicated search terms (below). The initial search for each EN (below) was followed by refined searches using one of the following terms: antimicrobials, catalysis, ceramics, cosmetics, “drug delivery”, electronics, “environmental remediation”, “hydrogen storage”, “medical imaging”, sensors, surfactants, textiles, therapeutics, “water purification”, “solar cells”, “reinforced composites”, optics, “coatings and pigments”, antioxidants, magnetics, “quantum computing”, photonics, “light emitting diodes”. Initial search for silver ENs: Nano* AND silver. For carbon nanotubes: “carbon nanotub*”. For copper oxide ENs: Nano* AND “copper oxide”. For iron oxide ENs: Nano* AND “iron oxide”. For fullerenes: Nano* AND fullerenes. For titanium dioxide ENs: Nano* AND “titanium dioxide” OR titania. For zinc oxide EN: Nano* AND “zinc oxide”. For silica ENs: Nano* AND silica. For quantum dots: Nano* AND “quantum dots”. For aluminum oxide ENs: Nano* AND “aluminum oxide”. N.B. the total number of applications shown for each EN are a likely to be an overestimate due to the limitations of the search algorithm which also includes articles where search terms have appeared in “KeyWords Plus” category only.
There is currently a dearth of empirical data published concerning the impact of ENs, and the influence of their various physicochemical parameters, on human health. Nevertheless, since the lungs are one of the primary routes of inadvertent entry of ENs there has been an increasing number of studies using human lung cells in vitro to determine the potential of ENs to cause inflammation, genotoxicity, oxidative stress or cytotoxicity. Although in vitro data often cannot substitute for results obtained in vivo, it has been shown to be feasible in specific circumstances. For example, an in vitro model assessing the pro-inflammatory potential of ultrafine particles on the bronchoalveolar adenocarcinoma-derived lung cell line, A549, generated findings strongly correlative with in vivo results [3]. Additionally, in vitro studies assessing the cytotoxicity of CuO ENs (and ZnO ENs) on A549 cells were shown to correlate well with in vivo effects on lungs [4]. At this stage the paucity of in vitro data assessing the impact of ENs on lung cells places limitations on the understanding of how EN mediate their cytotoxic effects. Whilst a picture is developing of these mechanisms, it is predominantly derived from studies of ENs using one cell line, A549 (Figure 2), and therefore more studies using other lung cell lines, preferably in parallel, are required (Figure 3). In lieu of waiting for exhaustive data on their effects on human health, certain ENs are surface modifiable such that their impact on human health could be significantly reduced. For instance, we have recently demonstrated that surface modifying amorphous silica nanoparticles (∼50 nm diameter) with amine groups reduced their ability to promote inflammatory responses in mice lungs [5]. This modification made no significant change in the size of these ENs and is believed to reduce their oxidative potential by decreasing the amount of exposed surface silanol groups.
Figure 2. Schematic illustrating potential mechanisms by which indicated ENs induce toxicity or inflammatory potential in lung cells.
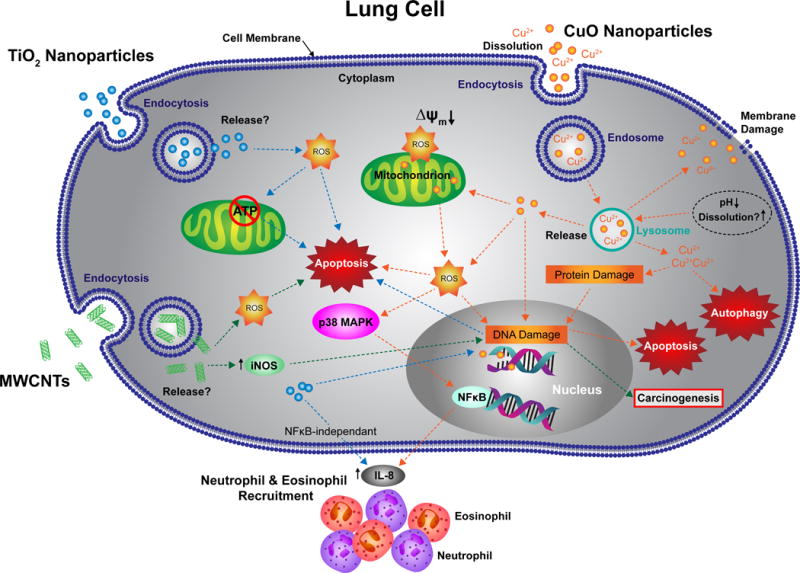
Proposed mechanisms by which copper oxide (CuO) ENs (orange arrows), titanium dioxide (TiO2) ENs (blue arrows) or multiwalled carbon nanotubes (MWCNTs) (green arrows) mediate toxic or pro-inflammatory effects in A549 cells. These proposed pathways were derived from published articles, all involving the A549 cell line, retrieved from a search, using Thomson Reuters Web of Science citation indexing service (search details are the same as those outlined for Figure 3 (A) (see Figure 3 legend)). ROS = reactive oxygen species, iNOS = inducible nitric oxide synthase, MAPK = mitogen-activated protein kinase.
Figure 3. Histograms highlighting the paucity of in vitro studies that assess the impact of ENs on lung cell health and inflammatory potential. (A).
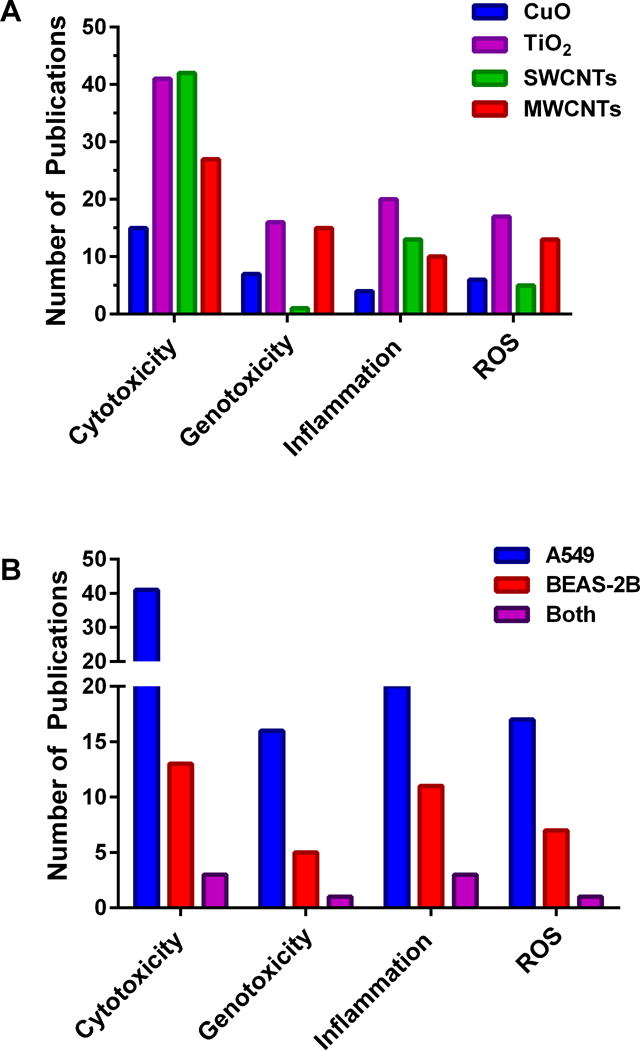
Number of published articles (2006 – 2016) documenting the potentially detrimental effects (in vitro) of copper oxide ENs, titanium oxide ENs, single walled carbon nanotubes (SWCNTs), or multi-walled carbon nanotubes (MWCNTs) on the human adenocarcinoma cell line, A549 (representative of alveolar basal epithelia). (B) Comparison of the number of published articles documenting the potentially detrimental effects of titanium oxide ENs on A549 or BEAS-2B (a cell line representative of normal human bronchial epithelia) or both cell lines studied in parallel (indicated as both). The findings shown here for titanium oxide ENs represent a general trend for ENs where a greater number of articles have been published involving A549 compared to BEAS-2B. Data was generated using Thomson Reuters Web of Science citation indexing service, and based on a search for articles in all databases using the following search terms.: (A) For copper oxide ENs: Nano* AND “copper oxide” or CuO. For titanium dioxide ENs: Nano* AND “titanium dioxide” OR TiO2. For SWCNTs: “carbon nanotubes” NOT multi-walled. For MWCNTs: “carbon nanotubes” AND multi-walled OR multiwalled. The search for each EN was then followed by a refined search using A549, followed by another refined search for cytotox*, genotox*, inflamm* or “ROS”. (B) Following a search for titanium dioxide ENs (described above) a refined search was performed using A549 or BEAS-2B or A549 followed by BEAS-2B (both). The results from each search were modified further by scrutinizing each citation and eliminating any irrelevant publications, thereby increasing the accuracy of the resulting data.
It is becoming increasingly apparent that each EN formulation requires testing for potential harm to health as opposed to invoking generalized assumptions since the alteration of one physicochemical parameter within the formulation can have a significant impact on downstream biological processes. As an example, we have recently shown that CuO ENs (nanoparticles), physically and chemically similar in all respects apart from size (4 nm versus 24 nm) had significantly different impacts on viability of, and ROS production in, lung cells [6]. The finding that 4 nm CuO NPs were less toxic than 24 nm CuO NP coincided with lower intracellular levels of Cu for the former suggesting that the smaller NPs (on a per weight basis) were less readily taken up by the lung cells in vitro. These results are in contrast with expectations based on findings with ENs made from other metals such as TiO2 (size range 14 – 196 nm), Au (1.4 nm versus 15 nm; 10 – 50 nm versus 100 – 200 nm) and Ag (15 – 55 nm) where smaller ENs correlated with higher cytotoxicity and highlights that each EN requires intensive analysis in regards to its potentially deleterious biological effects [7]. Understanding the impact of EN on lung tissue function and health would also pave the way for potentially useful nanomedicines, particularly those aimed at local delivery of drugs to lung cancer patients. Currently, however, there is understandable reluctance in the medical community to use inhalation delivery of EN-based nanomedicines particularly for less life-threatening lung pathologies, such as asthma, and this is primarily due to the knowledge gap with respect to the harmful effects of these potential nanomedicines on patients as well as occupational health concerns. Recently, a promising EN-based vaccine was developed in our laboratory to prevent dust mite-induced asthma [8]. The vaccine comprising a house dust mite antigen, Der p2, and the Toll-like receptor-9 agonist, CpG oligodeoxynucleotide, co-loaded into nanoparticles (300 nm) made from the biodegradable, non-toxic and FDA-approved polymer poly(lactic-co-glycolic acid), was administered subcutaneously rather than via the lungs and was shown to be effective in a mouse model of dust mite-induced asthma, demonstrating protection against Der p2-induced airway hyperresponsiveness and allergic airway inflammation. It is, however, evident that certain lung pathologies, such as lung cancer, will benefit from direct delivery of ENs to the lungs via, for example inhalation local delivery [9]. In summation, with the inevitable role that nanomedicines are to play, combined with the increased production of ENs for consumer and industrial applications, there is an onus on the scientific community to vigilantly interrogate, in a considered and multidisciplinary fashion, the potential hazards of ENs to both human health and the environment.
References
- 1.Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res. 2012;14(9) [Google Scholar]
- 2.Juganson K, Ivask A, Blinova I, Mortimer M, Kahru A. NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J Nanotechnol. 2015;6:1788–1804. doi: 10.3762/bjnano.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho WS, Duffin R, Bradley M, Megson IL, MacNee W, Lee JK, Jeong J, Donaldson K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part Fibre Toxicol. 2013;10(1):55. doi: 10.1186/1743-8977-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris AS, Adamcakova-Dodd A, Lehman SE, Wongrakpanich A, Thorne PS, Larsen SC, Salem AK. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol Lett. 2016;241:207–215. doi: 10.1016/j.toxlet.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongrapanich A, Mudunkotuwa IA, Geary SM, Morris AS, Mapuskar KA, Spitz DR, Grassian VH, Salem AK. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environmental Science: Nano. 2016 doi: 10.1039/c5en00271k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 8.Joshi VB, Adamcakova-Dodd A, Jing X, Wongrakpanich A, Gibson-Corley KN, Thorne PS, Salem AK. Development of a poly (lactic-co-glycolic acid) particle vaccine to protect against house dust mite induced allergy. Aaps J. 2014;16(5):975–985. doi: 10.1208/s12248-014-9624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]


