Abstract
Background
The definition of a vertebral fracture is usually based on the presence of a deformation of the vertebral body and this can be misleading in the presence of a fracture without radiologic collapse with the definition of occult osteoporotic vertebral fractures (OOVFs). STIR sequence of MRI images showing hyperintensity signal was the most confirmative screening examination used to determine the presence of painful OOVFs. To date, clinical management of OOVFs has been rarely discussed.
Material/Methods
Between 2011 and 2013, 89 patients suffering from painful OOVFs underwent 142 percutaneous balloon kyphoplasty (PKP) procedures. Outcome data (mean variation of anterior and middle vertebral body height, visual analog scale [VAS] scores, Oswestry Disability Index [ODI] scores, and SF-36 scores) were recorded preoperatively, postoperatively, and at 1 month, 6 months, and 2 year after treatment, to evaluate the results.
Results
We successfully treated 89 patients (142 vertebral bodies) with PKP. Cement leakages were observed in 12 (8.45%) treated vertebral bodies and there were 5 new adjacent vertebral fractures during the follow-up period. The mean variation of anterior and middle vertebral body height changed from 96.5±3.4% preoperatively to 97.2±2.5% postoperatively (p>0.05) and from 96.3±2.8% preoperatively to 97.9±3.1% postoperatively (p>0.05), respectively. The mean VAS scores were reduced significantly from pre-surgery to post-surgery (8.3±1.2 to 2.9±0.7; p<0.05), as was the ODI score (76.4±12.5 to 26.7±5.6; p<0.05). The SF-36 scores, including Bodily Pain (BF), Vitality (VT), Physical Function (PF), and Social Functioning (SF), all showed notable improvement (P<0.05). These variations were maintained during the 2-year follow-up period.
Conclusions
PKP is a safe and effective method in the treatment of painful OOVFs.
MeSH Keywords: Kyphoplasty, Osteoporotic Fractures, Spinal Fractures, Vertebroplasty
Background
Osteoporosis, which is a systemic disorder, is characterized by low bone density that leads to fragile bones and higher fractures risks [1,2]. Osteoporotic vertebral compression fracture (OVCF) is a common cause of pain and disability in the elderly population, affecting nearly one-quarter of individuals over the age of 50 during their lifetime. Worldwide, there are approximately 1.4 million new OVCFs reported each year, with approximately 750 000 annually in the United States alone [1–3]. OVCFs often result in significant pain that often leads to decreased mobility, loss of independence, and subsequent loss of bone density, and have negative effects on the respiratory and digestive systems. The direct medical costs associated with OVCFs have been estimated at $13.8 billion annually in the US alone, and indirect costs in lost productivity, pain, and suffering are even greater [4,5]. Conservative management, including bed rest, painkillers, and bracing, may help to reduce pain over weeks or months; however, in frail elderly patients, long periods of inactivity are associated with higher rates of pneumonia, decubitus ulcers, venous thromboembolism, and even death. Invasive surgery is not the optimal treatment due to their usually old age and associated comorbidities [6,7].
Today, vertebral augmentation procedures, including percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP), are commonly used in the treatment of OVCFs [8–10]. Both interventions involve injecting cement into the collapsed vertebral body. PKP utilizes inflatable bone tamps to reduce fracture, restore vertebral anatomy, and control cement injection. Since the first report of PVP in 1987, the use of PVP and PKP for the treatment of OVCFs has been dramatically increased during the last 20 years. Potency and effectiveness of these procedures have repeatedly been proven in numerous articles [9,11,12]. Recently, a US large claims database analysis showed that PKP was associated with a significant reduction in mortality compared to conservative treatment and PVP. PKP is now considered to be as minimally invasive and effective as conventional PVP for rapid pain relief in patients with painful OVCFs resistant to conservative treatment [11,13,14].
In established osteoporosis, the definition of a vertebral fracture is usually based on the presence of a deformation of the vertebral body on lateral radiographs [15]. Early diagnosis of OVCFs is crucial and the detection of vertebral fractures is based primarily on the identification of vertebral collapse. However, this can be misleading in the presence of a fracture without radiologic collapse, and the definition of occult osteoporotic vertebral fractures (OOVFs) has been established [16,17]. Patients highly suspected to have a fresh occult vertebral fracture, who have persistent pain and normal X-ray and CT images, should undergo additional radiologic tests, especially MRI. MRI is the most confirmative screening examination used to determine the presence of painful OOVFs; T2 and STIR sequences of MRI images show hyperintensity signal because of bone edema in the affected vertebra [18]. OOVFs are disabling causes of severe low back pain, requiring bed rest and increasing the risk of comorbidities [15]. Moreover, these patients usually require high doses of painkillers, especially for multiple OOVFs, which can cause serious adverse effects.
Pham et al. [17] and Mao et al. [18] reported that 79% of patients with OOVF developed a “classic” vertebral collapse within 3 months after the first radiograph. Thus, it is essential and necessary to enhance the vertebral body with occult fracture to prevent further vertebral collapse. Because the number OOVFs patients was small and usually mixed with other OVCFs cases, clinical treatment of OOVFs has been rarely discussed. Few reports exist documenting the outcomes of vertebral augmentation procedure as an alternative to traditional conservative therapy for painful OOVFs, most of which are limited to case reports and small series of patients [17–19]. In this report, we present a series of 89 patients with painful OOVFs treated with PKP, and we assess the radiological and clinical outcomes. The aim of the research was to assess the effectiveness and safety of PKP in the management of painful OOVFs.
Material and Methods
Patients
The research was approved by Ethics Committee of the First Affiliated Hospital of Soochow University (Suzhou, China).
From January 2011 to December 2013, 923 kyphoplasties were performed in 688 patients at our institution. Among them, 89 patients (55 females and 34 males), aged 56–79 years, had 1 or 2 OOVFs, and underwent 142 PKP procedures. The mean time since onset of pain to the intervention was 1.25 months in all patients. The levels treated by PKP were distributed as shown in Figure 1. Bone mineral density was preoperatively measured as an indicator of osteoporosis with dual-energy X-ray absorptiometry in all cases. We only included the osteoporotic patients (according to the World Health Organization; these patients have a T-score −2.5 or lower, meaning a bone density that is 2.5 standard deviations below the mean of a 30-year-old man/woman [7]). The mean T-score was −3.1.
Figure 1.
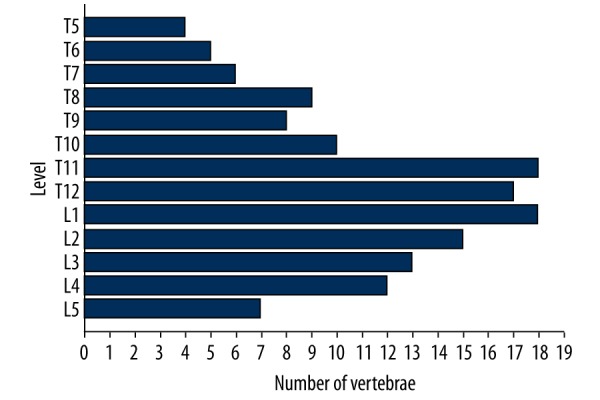
Distribution of vertebral bodies treated by PKP (n=142).
Occult osteoporotic vertebral fractures (OOVFs) were defined as vertebral fractures without radiographic measurable compression [17]. Diagnosis of painful OOVFs was established by clinical symptoms and radiographic evaluation. The indications for PKP were OOVFs with acute pain and not responding to nonoperative treatment, which included all types of medications, nerve blocks, exercises, and osteoporotic medications to strengthen the bone. Patients included in our research underwent a plain X-ray, magnetic resonance imaging (MRI), and multislice computed tomography (MSCT) to evaluate the main cause of pain and to plan the treatment. MSCT was also performed to study the vertebral structures before the PKP procedure. MRI was the most confirmative screening examination used to determine the presence of painful OOVFs; STIR sequences of MRI images show hyperintensity signal because of bone edema in the affected vertebra [18]. Patients with local infections, non-correctable coagulation disorders, and other systemic diseases were excluded from the treatment.
Operative technique
All the PKP procedures were supervised by senior spinal surgeons in our department. Patients received general anesthesia and were positioned prone on a radiolucent operation table. Using image guidance X-rays, a small incision was made and a probe was placed into the vertebral space at the fracture site. The bone was drilled and a balloon (Kyphon Inc., Sunnyvale, California) was inserted on each side. The balloon was then inflated slowly for injecting bone cement. During the cement injection, fluoroscopic monitoring was conducted in both planes. After completion of the PKP procedures, patients were monitored for 8 hours postoperatively.
Radiographic and clinical assessment
Radiographic assessment and clinical examinations were performed preoperatively, and at 1 month, 6 months, and 2 years after treatment. The anterior and middle vertebral heights were obtained from standing lateral radiographs for compromised and adjacent uncompromised control vertebrae. For each vertebra, the normal height of the compromised vertebrae was estimated from the mean of the measurements from the closest uncompromised vertebral cephalad and caudalad to the treated level. Vertebral body height variations were calculated by the following method: (fractured vertebral body height/normal vertebral body height) ×100% [20,21].
Back pain was measured using a visual analog scale (VAS scores) with values from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain imaginable.
Oswestry Disability Index (ODI) scores, which are used to assess functional capacity, were also documented, in which a lower percentage indicates a better health status. The ODI has been shown to have high test-retest reliability and is the most commonly recommended condition-specific outcome measure in patients with acute and chronic low back pain.
The SF-36 questionnaire is an in-depth and broad-ranging health-related quality of life evaluation consisting of 36 questions, where a higher score indicates a better health status. The results are categorized into 8 domains; Bodily Pain (BP), Vitality (VT), Physical Function (PF), and Social Function (SF) have been shown to have high reliability and responsiveness in spinal injury patients and were selected for evaluation in our study [22–24].
Complications such as bleeding, infection, stroke, pulmonary embolism, and cardiac arrest were recorded. The rates of cement leakage outside the vertebral body were measured postoperatively using radiographs and CT scans.
Statistical analysis
Mean changes, including mean values and standard deviations, in anterior vertebral body height variation and middle vertebral body height variation, SF-36 score, VAS and ODI score, were evaluated and analyzed with SPSS software (SPSS 19.0, Inc., Chicago). Comparisons between different time points were performed using a paired Student’s t test and tested by the “repeated-measures analysis of variance” (ANOVA). Differences were considered as statistically significant with a P value of less than 0.05.
Results
We performed a 2-year follow-up for the 89 patients. Significant improvements in SF-36 score, VAS, and ODI score were observed postoperatively and at 1 month, 6 months, and 2 years after treatment. Five new adjacent vertebral fractures in 89 patients were detected during the 2-year follow-up period.
Safety and efficacy
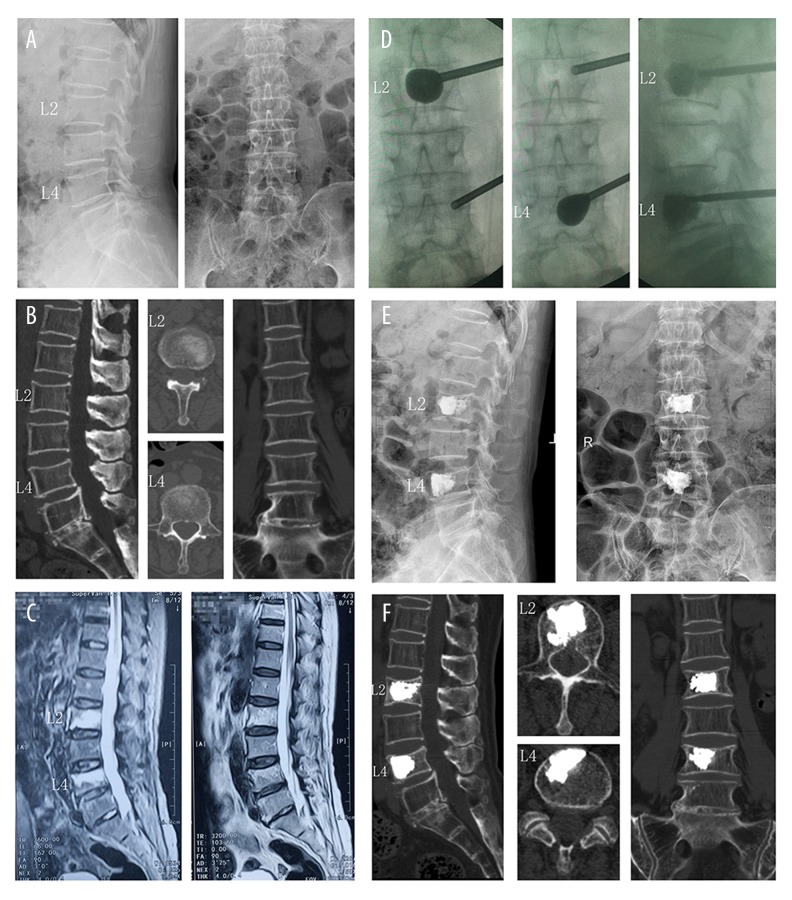
Involved vertebrae were located from levels T5 to L5 (n=142). The levels were T5 (n=4), T6 (n=5), T7 (n=6), T8 (n=9), T9 (n=8), T10 (n=10), T11 (n=18), T12 (n=17), L1 (n=18), L2 (n=15), L3 (n=13), L4 (n=12), and L5 (n=7) (Figure 1). The operation time was 28.5±8.6 minutes per treated level. The injected cement volume was 5.2±1.3 ml and the time of fluoroscopy was 10.5±1.4 minutes for each level. There were 50 cases (81 vertebral bodies) done with bilateral access and 39 cases (61 vertebral bodies) with unilateral access. A representative case of a 77-year-old female patient with L2 and L4 OOVFs who received PKP treatment using unilateral access is shown in Figure 2. Osteoporosis was confirmed by pathology examination. There were no treatment-related complications such as bleeding, infection, stroke, pulmonary embolism, and cardiac arrest. Furthermore, our patients showed no reactions with the bone cement. General properties of the patients are summarized in Table 1.
Figure 2.
A 77-year-old female patient with acute back pain in L2 and L4 region. (A, B) Initial plain radiograph and CT reconstruction showed insignificant fracture lines in L2 and L4 vertebral body with no radiologic collapse. (C) L2 and L4 had MRI signal changes in STIR and T2WI sequence, demonstrating an extensive bone marrow edema in the vertebral body. (D) Intraoperative images showed L2 and L4 were treated by PKP with unilateral access. (E, F) Postoperative plain radiograph and CT reconstruction images showed cement distribution was diffuse and homogeneous and cement leakage was not noticed
Table 1.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Patient | |
| Number | 89 |
| Total number of OOVFs | 142 |
| Number of OOVFs treated per patient | 1.6 (1–2) |
| Age (years) | 67.5±11.5 |
| Gender (F/M) | 55/34 |
| BMD T score | −3.1±0.6 |
| Fracture age (months) | 1.25±1.15 |
| Follow-up (months) | 24 |
| Fracture region (number/percentage) | |
| T5 through T9 vertebrae | 32/(22.5%) |
| T10 through L2 vertebrae | 78/(55%) |
| L3 through L5 vertebrae | 32/(22.5%) |
| Kyphoplasty procedure | |
| Operation time per vertebrae (minutes) | 28.5±8.6 |
| Fluoroscopy time per vertebrae (minutes) | 10.5±1.4 |
| Bipedicular access (vertebraes) | 81 |
| Unilateral access (vertebraes) | 61 |
| Injected cement volume (mL) | 5.2±1.3 |
| Cement leakage | |
| Number of vertebraes | 12/(8.45%) |
| Location | |
| Venous plexus | 2 (1.41%) |
| Paravertebral soft tissues | 6 (4.23%) |
| Adjacent disks | 4 (2.82%) |
| New fractures | |
| Recollapse of treated vertebrae | 0 |
| Adjacent vertebral fractures | 5 (3.52%) |
Data are mean ± standard deviation; median, with the range in parentheses; or number of findings, with the percentage in parentheses.
Radiographic measurement
On the basis of comparison of pre-operative and every postoperative data, anterior vertebral body height and middle vertebral body height were maintained in all cases. The mean anterior vertebral body height variation changed from 96.5±3.4% preoperatively to 97.2±2.5% postoperatively (p>0.05) and maintained at 95.7±3.4% at 2 years postoperatively. The mean middle vertebral body height variation changed from 96.3±2.8% preoperatively to 97.9±3.1% postoperatively and maintained at 96.7±4.4% at 2 years postoperatively. Statistically, no significant difference was detected at the immediate postoperative time and every follow-up assessment compared with the preoperative value.
Clinical evaluation
The mean VAS score of these patients decreased from 8.3±1.2 preoperatively to 2.9±0.7 postoperatively and maintained at 3.0±1.2 at 2 years postoperatively, and the ODI score decreased from 76.4±12.5 preoperatively to 26.7±5.6 postoperatively and maintained at 27.8±8.3 at 2-year postoperatively (Table 2). The SF-36 scores for Vitality (VT), Bodily Pain (BF), Social Functioning (SF), and Physical Function (PF) all showed notable improvement (P<0.05) (Table 3). Statistically significant differences were observed at the immediate postoperative time and each follow-up assessment compared with the pre-operative value.
Table 2.
Mean improvement in VAS and ODI scores.
| Preoperative | Postoperative | 1-Month follow up | 6-Month follow up | 2-Year follow up | |
|---|---|---|---|---|---|
| VAS | 8.3±1.2 | 2.9±0.7* | 2.3±1.1* | 2.8±1.6* | 3.0±1.2* |
| ODI | 76.4±12.5 | 26.7±5.6* | 19.5±6.8* | 22.9±11.3* | 27.8±8.3* |
P<0.001 compared to preoperative value.
Table 3.
Mean improvement of SF-36 scores.
| Preoperative | Postoperative | 1-Month follow up | 6-Month follow up | 2-Year follow up | |
|---|---|---|---|---|---|
| BP | 15.3±4.6 | 45.1±9.3* | 54.2±6.9* | 58.4±13.0* | 55.8±17.1* |
| PF | 29.8±9.2 | 56.5±11.1* | 58.7±12.4* | 59.6±10.2* | 54.3±12.8* |
| VT | 32.1±11.7 | 51.2±10.1* | 53.6±8.8* | 58.6±12.9* | 55.2±7.6* |
| SF | 19.5±8.3 | 37.0±12.3* | 41.2±8.9* | 44.5±9.4* | 43.0±8.6* |
P<0.05 compared to preoperative value.
Cement extravasation
The postoperative CT images of the PKP-treated vertebrae indicated a cement extravasation in 12 vertebras, representing 8.45% of all treated levels. Cement extravasation was located in the paravertebral soft tissues (6 leaks), vertebral venous plexus (2 leaks), and adjacent disk area (4 leaks). However, there were no major complications such as pulmonary embolism or epidural leakage.
Discussion
Osteoporosis is a systemic bone disorder characterized by reduced bone mass and degradation of skeletal microarchitecture. It is a growing global public health problem in the elderly. Approximately 750 000 new cases of osteoporosis are observed in the United States annually. Osteoporotic vertebral compression fractures (OVCFs) are one of the most common fractures associated with osteoporosis. In addition to acute or chronic pain, OVCFs are also associated with progressive spinal deformity, impaired physical function, decreased quality of life, and increased mortality in this population. The direct medical costs associated with OVCFs have been estimated at $13.8 billion annually in the US alone, and indirect costs in lost productivity, pain, and suffering are even greater [25–27]. Conservative management includes analgesics, bed rest, external bracing, and a combination of these treatments. However, there are still limitations of these methods. Long-term bed rest often leads to further demineralization and may predispose the patients to future OVCFs [28,29]. As for medication of anti-inflammatory drugs and certain types of analgesics, it may be difficult for patients to tolerate the adverse effects. In frail elderly patients, long periods of inactivity are associated with higher rates of pneumonia, decubitus ulcers, venous thromboembolism, and even death. In case of failure of these non-surgical methods, until the beginning of the 1980s the only options left were surgical stabilization and spine straightening via dorsal instrumentation. However, there were limits to the application of this type of surgery, especially with patients in relatively poor general condition [3,30].
PVP and PKP are 2 minimally invasive surgical procedures in which bone cement is injected into the fractured vertebral body. Clinical results indicate both are effective in relieving back pain caused by the OVCFs, especially those that are refractory to conservative treatment. The main difference between the 2 procedures is that PKP involves the use of an inflatable bone tamp, in an attempt to create a cavity in the vertebral body prior to cement injection, while PVP does not [31–33]. Recently, a US large claims database analysis showed that PKP was associated with a significant reduction in mortality compared to conservative treatment and PVP. PKP is now considered to be as minimally invasive and effective as conventional PVP for rapid pain relief in patients with painful OVCFs resistant to conservative treatment [20,25,31].
Early diagnosis of OVCFs is important, and the detection of vertebral fractures is based primarily on the presence of a deformation of the vertebral body on lateral radiographs [15]. Occult osteoporotic vertebral fractures (OOVFs), which imply symptomatic vertebral fracture without measurable radiographic compression, can be misleading [17]. MRI is a very important diagnostic tool for early detection of OOVFs. The typical MRI finding in acute compression fracture is hypointensity in T1-weighted image, heterogeneous intensity or hyperintensity on T2-weighted image, and hyperintensity on fat-suppressed T2-weighted image or on short-inversion time-inversion recovery image [18]. In our study, numerous fractures would have been missed on standard radiographs, only being confirmed by MRI. Therefore, patients highly suspect of acute osteoporotic vertebral fracture, with acute symptomatic pain and normal X-ray and CT images, should undergo an additional imaging test, especially MRI.
The pathophysiological process of OOVFs is obscure. Histological study demonstrated trivial microtrabeculae fracture and end-plate fracture in the involved vertebral bodies [16,17]. The etiology of back pain due to vertebral fractures is unclear. Teyssedou et al. [25] showed that back pain was equally likely to occur with each type of vertebral fracture. Lee et al. [34] reported that there was little correlation between the degree of collapse of the vertebral body and the level of pain. OOVFs are important causes of severe back pain, leading to chronic disability similar to that in patients with severe vertebral collapses [17,18]. Most vertebral fractures without significant initial deformation developed a “classic” vertebral collapse at follow-up. The radiologic worsening was observed approximately 2 to 3 months after the initial radiograph [15,16]. Pham et al. [17] revealed that most OOVFs developed a “classical” vertebral fracture with apparent vertebral body collapse in patients with conservative treatment. Therefore, it seems essential and necessary to enhance vertebral bodies with OOVFs to prevent possible vertebral collapse.
Clinicians may miss OOVFs, which leads to delayed diagnosis or misdiagnosis [15]. Current treatment recommendations vary from analgesia to surgery. Despite the benefits of vertebral augmentation procedures, the application of PVP or PKP for the treatment of OOVFs is controversial [15,17,34]. Reports discussing the vertebral augmentation procedure as an alternative to traditional medical therapy for the treatment of OOVFs are scarce. There are several reports on the results of PVP and PKP in treating OOVFs; favorable results were reported, but, unfortunately, the literature data is mostly limited to case reports and small series of patients, and high-quality assessment methods are lacking. Pham et al. [17] reported that 21 cases of OOVFs in 16 patients presented with a typical history of acute back pain with no substantial vertebral deformation on the initial plain radiographs. Kanchiku et al. [35] reported that 10 out of 95 OVCFs showing signal intensity changes on MRI were difficult to identify on plain radiographs due to almost no collapse of the vertebral body. Mao et al. [18] reported results in 45 patients with OOVFs who underwent PVP and PKP. They found notable pain alleviation and improved functional activity after PKP intervention and did not detect delayed collapse of the treated vertebral bodies. Pereira et al. [36] evaluated the clinical outcome and technical feasibility of percutaneous sacroplasty in treating insufficiency fractures of the sacrum. They performed 67 sacroplasty in 58 consecutive patients with intractable pain from osteoporotic fractures or from sacral tumors. They found the VAS score decreased significantly after treatment, with only minor complications.
The purpose of our study was to describe our experience and to assess the safety and effectiveness of PKP in patients with painful OOVFs. In a consecutive series of 89 patients with painful OOVFs, who had 142 levels of treatment in a 2-years follow-up period, we found PKP leading to a remarkable remission of pain and improvement of function. Significant improvement in VAS score, ODI score, and SF-36 score was observed postoperatively and at each follow-up time. The results revealed that PKP is effective in the management of painful OOVFs. The exact mechanism by which these procedures achieve pain relief remains controversial. It is likely that pain relief is obtained through stabilization of the fracture by the cement. Another explanation proposed is that the injected bone cement causes thermal necrosis and chemotoxicity of the intraosseous pain receptors [37,38].
There have been numerous studies evaluating the anatomical distribution of OVCFs, which were consistently showing 2 prevalent peaks of vertebral fractures: the first in the mid-thoracic spine region (T7/T8) and another in the thoraco-lumbar junction [35–37]. Vertebral fractures in the lower lumbar area, including L3, L4, and L5 level, are quite uncommon [39–41]. Painful OOVFs are uncommon in osteoporotic patients and its anatomical distributions are rarely discussed. Our study revealed the peak of OOVFs is the thoracolumbar junction area, representing 55% of overall spine fractures, which was consistent with OVCFs. It also shows that the lower lumbar vertebrae are frequently affected, representing 22.5% of overall spine fractures. It is likely that the lower lumbar area suffers less biomechanical stress, which could explain the temporary preservation of vertebral bodies weakened by a fracture [15,36]. In these patients, OOVFs are disabling causes of severe low back pain, and conservative treatments often fail. Minimally invasive procedures, such as PKP and PVP, are effective treatment options that can result in rapid pain relief and rehabilitation, and prevent delayed vertebral collapse.
Cement leakage remains a significant clinical problem associated with PVP and PKP procedures. The less frequent cement leakage with PKP is probably because this technique creates a cavity that allows for a more viscous cement to be injected under lower pressure. Moreover, the expansion of the balloon compacts cancellous bone in the intravertebral cavity, which may also reduce the rate of cement leakage [42,43]. In our collective, asymptomatic cement leak was seen in 8.45% of treated cases, notably lower than the documented average for OVCFs. This may be attributed to the relatively intact vertebral cortex and end-plate of the occult fractured vertebral body [17]. In our experience, some leakage via the deficiency in the vertebral wall can be avoided by good operative technique. The injection of cement must be done slowly and carefully. Once cement leakage is detected, the operator may adjust the needle direction or stop the injection immediately. The injection process was stopped when the cement reached the margins of the vertebral body. Based on these individual skills, no symptomatic cement extravasation occurred in our cases; however, the significance of an asymptomatic leak remains unclear. It had been suggested that cement leaks into the adjacent intervertebral disc space affect the mechanical loading of either the disc or adjacent vertebrae, which might predispose the patient to an adjacent-level fracture [44–46]. In our study, there were 5 new adjacent vertebral fractures during the follow-up period: 3 fractures were associated with cement leakage nearby and 2 fractures were related to sandwich vertebra. A sandwich vertebra is an intact vertebral body located between 2 previously cemented vertebras. Because of double load shifts, the sandwich vertebra was prone to an increased risk of adjacent vertebral fracture [47]. However, adjacent-level fracture can also reflect systematic weakening of bone in the osteoporotic spine. We therefore believe this phenomenon may also represent the natural progression of osteoporosis in this series. Hence, the long-term impacts of these asymptomatic cement leaks and sandwich vertebral fractures need further research.
This study has some limitations. First, this study was performed retrospectively, which produces weaker evidence than a prospective study. Second, only patients who underwent PKP were included in our study; there was no control or alternative treatment, such as PVP. Further additional prospective studies are needed to assess the effectiveness and safety of PKP in treatment of painful OOVFs.
Conclusions
To achieve earlier detection and diagnosis of painful OOVFs, suspected patients with osteoporosis who have symptomatic pain and normal X-ray and CT images should undergo further MRI testing. Patients with incapacitating vertebral pain can be treated with, and benefit from, PKP. PKP seems to be a safe and effective treatment for painful OOVFs.
Footnotes
Disclosure
None of the authors has a financial interest or other interest in any of the products, devices, or methods mentioned in this article.
Source of support: This project is supported by the National Natural Science Foundation of China (No.81301646 to Sun Zhi-Yong and 81401768 to Lin Jun), the Natural Science Foundation of Jiangsu Province (No. BK20140289 to Lin Jun)
References
- 1.Nas OF, Inecikli MF, Hacikurt K, et al. Effectiveness of percutaneous vertebroplasty in patients with multiple myeloma having vertebral pain. Diagn Interv Radiol. 2016;22(3):263–68. doi: 10.5152/dir.2016.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee S, Yeh J, Ellamushi H. Pain and functional outcomes following vertebroplasty for vertebral compression fractures – A tertiary centre experience. Br J Neurosurg. 2016;30(1):57–63. doi: 10.3109/02688697.2015.1096901. [DOI] [PubMed] [Google Scholar]
- 3.El-Fiki M. Vertebroplasty, kyphoplasty, lordoplasty, expandable devices, and current treatment of painful osteoporotic vertebral fractures. World Neurosurg. 2016;91:628–32. doi: 10.1016/j.wneu.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Liang L, Chen X, Jiang W, et al. Balloon kyphoplasty or percutaneous vertebroplasty for osteoporotic vertebral compression fracture? An updated systematic review and meta-analysis. Ann Saudi Med. 2016;36(3):165–74. doi: 10.5144/0256-4947.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch JA, Chandra RV. Resurrection of evidence for vertebroplasty? Lancet. 2016;388(10052):1356–57. doi: 10.1016/S0140-6736(16)31356-3. [DOI] [PubMed] [Google Scholar]
- 6.Clark WA. Vertebroplasty is not a do-not-do treatment. Med J Aust. 2016;204(2):71–e1. doi: 10.5694/mja15.01201. [DOI] [PubMed] [Google Scholar]
- 7.Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408–16. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 8.Bornemann R, Jansen TR, Otten LA, et al. Comparison of radiofrequency kyphoplasty and balloon kyphoplasty in combination with posterior fixation for the treatment of vertebral fractures. J Back Musculoskelet Rehabil. 2016 doi: 10.3233/BMR-140224. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Barr JD. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg. 2016;8(7):765–66. doi: 10.1136/neurintsurg-2016-012279. [DOI] [PubMed] [Google Scholar]
- 10.Awwad A, Le Jeune I, Kumaran M, Sosin MD. A rock in a hard place: Cement pulmonary emboli after percutaneous vertebroplasty. Int J Cardiol. 2016;208:162–63. doi: 10.1016/j.ijcard.2016.01.176. [DOI] [PubMed] [Google Scholar]
- 11.Baz AB, Akalin S, Kilicaslan OF, et al. Efficiency of balloon kyphoplasty in the treatment of osteoporotic vertebral compression fractures. Kobe J Med Sci. 2016;62(3):E49–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Aparisi F. Vertebroplasty and kyphoplasty in vertebral osteoporotic fractures. Semin Musculoskelet Radiol. 2016;20(4):382–91. doi: 10.1055/s-0036-1592431. [DOI] [PubMed] [Google Scholar]
- 13.Zhang GQ, Gao YZ, Chen SL, et al. Comparison of percutaneous vertebroplasty and percutaneous kyphoplasty for the management of Kummell’s disease: A retrospective study. Indian J Orthop. 2015;49(6):577–82. doi: 10.4103/0019-5413.168752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zapalowicz K, Radek M. Percutaneous balloon kyphoplasty in the treatment of painful vertebral compression fractures: Effect on local kyphosis and one-year outcomes in pain and disability. Neurol Neurochir Pol. 2015;49(1):11–15. doi: 10.1016/j.pjnns.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.De Kong L, Meng LC, Shen Y, et al. Effect of shape and severity of vertebral fractures on the outcomes of kyphoplasty. Acta Orthop Belg. 2013;79(5):565–71. [PubMed] [Google Scholar]
- 16.Wu CT, Lee SC, Lee ST, Chen JF. Classification of symptomatic osteoporotic compression fractures of the thoracic and lumbar spine. J Clin Neurosci. 2006;13(1):31–38. doi: 10.1016/j.jocn.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Pham T, Azulay-Parrado J, Champsaur P, et al. “Occult” osteoporotic vertebral fractures: Vertebral body fractures without radiologic collapse. Spine (Phila Pa 1976) 2005;30(21):2430–35. doi: 10.1097/01.brs.0000184303.86932.77. [DOI] [PubMed] [Google Scholar]
- 18.Mao H, Zou J, Geng D, et al. Osteoporotic vertebral fractures without compression: key factors of diagnosis and initial outcome of treatment with cement augmentation. Neuroradiology. 2012;54(10):1137–43. doi: 10.1007/s00234-012-1018-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Kuh SU, Chin DK, et al. Kyphoplasty versus vertebroplasty: Restoration of vertebral body height and correction of kyphotic deformity with special attention to the shape of the fractured vertebrae. J Spinal Disord Tech. 2012;25(6):338–44. doi: 10.1097/BSD.0b013e318224a6e6. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Liu H, Wang S, et al. Review of percutaneous kyphoplasty in China. Spine (Phila Pa 1976) 2016;41(Suppl 19):B52–58. doi: 10.1097/BRS.0000000000001804. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Sribastav SS, Ye F, et al. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: A meta-analysis of the literature. Pain Physician. 2015;18(3):209–22. [PubMed] [Google Scholar]
- 22.Ahn DK, Lee S, Kim DG, Shin WS. Percutaneous vertebroplasty using fresh frozen allogeneic bone chips as filler. Clin Orthop Surg. 2014;6(1):49–55. doi: 10.4055/cios.2014.6.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannavale A, Salvatori FM, Wlderk A, et al. Percutaneous vertebroplasty with the rotational fluoroscopy imaging technique. Skeletal Radiol. 2014;43(11):1529–36. doi: 10.1007/s00256-014-1925-3. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Fan S, Zhao F. Percutaneous balloon kyphoplasty of osteoporotic vertebral compression fractures with intravertebral cleft. Indian J Orthop. 2014;48(1):53–59. doi: 10.4103/0019-5413.125498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teyssedou S, Saget M, Pries P. Kyphopasty and vertebroplasty. Orthop Traumatol Surg Res. 2014;100(1 Suppl):S169–79. doi: 10.1016/j.otsr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Yu CW, Hsieh MK, Chen LH, et al. Percutaneous balloon kyphoplasty for the treatment of vertebral compression fractures. BMC Surg. 2014;14:3. doi: 10.1186/1471-2482-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L, Chen Z, Sun M, et al. A preliminary study of the safety and efficacy of radiofrequency ablation with percutaneous kyphoplasty for thoracolumbar vertebral metastatic tumor treatment. Med Sci Monit. 2014;20:556–63. doi: 10.12659/MSM.889742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamae T, Fujimoto Y, Yamada K, et al. Efficacy of percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures with intravertebral cleft. Open Orthop J. 2015;9:107–13. doi: 10.2174/1874325001509010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie P, Zhao Y, Li G. Efficacy of percutaneous vertebroplasty in patients with painful vertebral metastases: A retrospective study in 47 cases. Clin Neurol Neurosurg. 2015;138:157–61. doi: 10.1016/j.clineuro.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Elnoamany HA. Influence of vertebral bone marrow edema on outcome in non-acute osteoporotic patients treated with percutaneous vertebroplasty. Asian Spine J. 2016;10(3):436–42. doi: 10.4184/asj.2016.10.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm K. [Vertebroplasty– state of the art]. Radiologe. 2015;55(10):847–52. doi: 10.1007/s00117-015-0013-6. [in German] [DOI] [PubMed] [Google Scholar]
- 32.Xing Y, Zhang G, Tian W. [Cause analysis of back pain after vertebroplasty or kyphoplasty]. Zhonghua Yi Xue Za Zhi. 2015;95(29):2342–45. [in Chinese] [PubMed] [Google Scholar]
- 33.Abbad N, Lemeunier L, Cotten A, et al. [Efficacy and tolerance of vertebroplasty and kyphoplasty for vertebral osteoporotic fractures at Lille University Hospital]. Presse Med. 2016;45(5):552–55. doi: 10.1016/j.lpm.2016.01.031. [in French] [DOI] [PubMed] [Google Scholar]
- 34.Lee YL, Yip KM. The osteoporotic spine. Clin Orthop Relat Res. 1996;(323):91–97. doi: 10.1097/00003086-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Kanchiku T, Taguchi T, Kawai S. Magnetic resonance imaging diagnosis and new classification of the osteoporotic vertebral fracture. J Orthop Sci. 2003;8(4):463–66. doi: 10.1007/s00776-003-0665-3. [DOI] [PubMed] [Google Scholar]
- 36.Pereira LP, Clarencon F, Cormier E, et al. Safety and effectiveness of percutaneous sacroplasty: A single-centre experience in 58 consecutive patients with tumours or osteoporotic insufficient fractures treated under fluoroscopic guidance. Eur Radiol. 2013;23(10):2764–72. doi: 10.1007/s00330-013-2881-3. [DOI] [PubMed] [Google Scholar]
- 37.Elnoamany H. Percutaneous vertebroplasty: A first line treatment in traumatic non-osteoporotic vertebral compression fractures. Asian Spine J. 2015;9(2):178–84. doi: 10.4184/asj.2015.9.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elnoamany H. Percutaneous vertebroplasty: A new serial injection technique to minimize cement leak. Asian Spine J. 2015;9(6):855–62. doi: 10.4184/asj.2015.9.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu JJ, Shen MJ, Meng B, et al. Percutaneous kyphoplasty for the treatment of osteoporotic thoracolumbar fractures with neurological deficit: Radicular pain can mimic disc herniation. Int J Clin Exp Med. 2014;7(8):2360–64. [PMC free article] [PubMed] [Google Scholar]
- 40.Gan M, Zou J, Zhu X, et al. Balloon kyphoplasty for osteoporotic spinal fractures with middle column compromise. Injury. 2014;45(10):1539–44. doi: 10.1016/j.injury.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Civelek E, Cansever T, Yilmaz C, et al. The retrospective analysis of the effect of balloon kyphoplasty to the adjacent-segment fracture in 171 patients. J Spinal Disord Tech. 2014;27(2):98–104. doi: 10.1097/bsd.0b013e31824e9b98. [DOI] [PubMed] [Google Scholar]
- 42.Wichlas F, Trzenschik H, Tsitsilonis S, et al. Biomechanical behavior of MRI-signal-inducing bone cements after vertebroplasty in osteoporotic vertebral bodies: An experimental cadaver study. Clin Biomech (Bristol, Avon) 2014;29(5):571–76. doi: 10.1016/j.clinbiomech.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Toru U, Coskun T, Acat M, et al. Pulmonary cement embolism following percutaneous vertebroplasty. Case Rep Pulmonol. 2014;2014:851573. doi: 10.1155/2014/851573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omidi-Kashani F, Hasankhani EG, Ebrahimzadeh MH, et al. Percutaneous vertebroplasty in Iranian patients with osteoporotic vertebral fractures. Arch Bone Jt Surg. 2013;1(1):9–13. [PMC free article] [PubMed] [Google Scholar]
- 45.Jay B, Ahn SH. Vertebroplasty. Semin Intervent Radiol. 2013;30(3):297–306. doi: 10.1055/s-0033-1353483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson J, Wang MY. Balloon Kyphoplasty. Neurosurgery. 2009;65(1):N12–13. [Google Scholar]
- 47.Wang L, Yang H, Shi Y, et al. Sandwich vertebral fracture in the study of adjacent-level fracture after vertebral cement augmentation. Orthopedics. 2012;35(8):e1225–30. doi: 10.3928/01477447-20120725-24. [DOI] [PubMed] [Google Scholar]