Summary
Oral squamous cell carcinoma (OSCC) is the most common cancer of the oral cavity and constitutes 95% of all cancers of this area. Men are affected twice as commonly as women, primarily if they are over 50 years of age. Forty percent of the lesions are localized in the tongue and 30% in the floor of the oral cavity. OSCC often affects upper and lower gingiva, buccal mucous membrane, the retromolar triangle and the palate. The prognosis is poor and the five-year survival rate ranges from 20% (OSCC in the floor of the mouth) to 60% (OSCC in the alveolar part of the mandible). Treatment is difficult, because of the localization and the invasiveness of the available methods. The diagnosis is made based on a histopathological examination of a biopsy sample.
The low detection rate of early oral SCC is a considerable clinical issue. Although the oral cavity can be easily examined, in the majority of cases oral SCC is diagnosed in its late stages. It is difficult to diagnose metastases in local lymph nodes and distant organs, which is important for planning the scope of resection and further treatment, graft implantation, and differentiation between reactive and metastatic lymph nodes as well as between disease recurrence and scars or adverse reactions after surgery or radiation therapy.
Imaging studies are performed as part of the routine work-up in oral SCC. However, it is difficult to interpret the results at the early stages of the disease. The following imaging methods are used – dental radiographs, panoramic radiographs, magnetic resonance imaging with diffusion-weighted and dynamic sequences, perfusion computed tomography, cone beam computed tomography, single-photon emission computed tomography, hybrid methods (PET/CT, PET/MRI, SPECT/CT) and ultrasound. Some important clinical problems can be resolved with the use of novel modalities such as MRI with ADC sequences and PET.
The aim of this article is to describe oral squamous cell carcinoma as it appears in different imaging methods considering both their advantages and limitations.
MeSH Keywords: Carcinoma, Squamous Cell; Cone-Beam Computed Tomography; Diagnostic Imaging; Diffusion Magnetic Resonance Imaging; Perfusion Imaging
Background
Oral squamous cell carcinoma (OSCC) is the most frequent cancer of the oral cavity, constituting 95% of neoplasms localized in this area. OSCC can be localized in the floor of the mouth, tongue, upper and lower gingiva, buccal mucous membrane, the retromolar triangle and the palate. Annually, there are over half a million of new cases of oral SCC diagnosed around the world. In Poland, 2000 cases resulting in 1100 deaths have been noted since 2013. Five percent of all cancer-related deaths are due to cancers of the oral cavity. In recent years, the male-to-female ratio has changed from 5: 1 to 2: 1. This is related to the fact that more and more women smoke tobacco. Oral cancer is most commonly diagnosed in the sixth decade of life; however, a rapid increase in morbidity in people below 50 years of age has been seen recently. At the time of diagnosis, one in ten patients has a secondary cancerous lesion or metastases in the local lymph nodes [1–4].
The major risk factors of oral cancer include tobacco smoking, alcohol abuse, human papilloma virus infection (HPV, primarily HPV-16), Ebstein-Barr virus infection (EBV), candidiasis, precancerous lesions (leukoplakia, erythroplakia, hyperkeratosis, papillomas, pachydermia, dysplasia), industrial pollution, occupational hazards (asbestos, chrome, formaldehyde etc.), inadequate oral hygiene, improper dental prostheses, diet low in vitamins, fruits and vegetables, UV-radiation exposure, pale skin complexion, immunological defects and Plummer-Vinson syndrome [4,5].
The most common initial symptoms of oral cancer are ulcerations of the mucous membrane in the oral cavity, pain, redness of mucous membrane, displacement of teeth, Vincent’s sign symptom, buccal and the floor of the mouth muscle contracture, trismus in cases when the medial pterygoid muscles are affected, halitosis, dysphagia and odynophagia. However, these symptoms can also result from inflammatory conditions. The most common mistakes in the differential diagnosis is to qualify neoplastic lesion as an odontogenic inflammation. It is crucial to early detect cancer lesions and evaluate them with appropriate imaging techniques in order to properly stage the disease [4,5].
Despite the constant advancement of surgery and radiation therapy, the treatment of oral cancers is difficult due to the localization and an invasive nature of the available treatment methods. Over the recent years, the five-year survival rates for oral cancer have not improved. They are 20–30% for cancers of the floor of the mouth, 40% for cancers of the tongue and 60% for cancers of the alveolar processes [1,5–7]. Patients often present when the disease shows high clinical stage [1]. Patients who are diagnosed early have a better long-term survival ranging from 60% to 90%. In contrast, in cases of late diagnosis the long-term survival ranges from 20% to 50%, and is less than 5% after palliative treatment. Because of the poor outcomes and high mortality rates, new methods of treatment aimed at molecular targets are being developed [8].
For staging of oral cancers, the TNM classification is used (Table 1) [9].
Table 1.
TNM classification according to WHO.
| T – Primary tumor size | Tx primary tumor cannot be assessed |
| T0 no evidence of primary tumor | |
| Tis carcinoma in situ | |
| T1 tumor 2 cm or less in the largest dimension | |
| T2 tumor larger than 2 cm but smaller than 4 cm in the largest dimension | |
| T3 tumor larger than 4 cm in the largest dimension | |
| T4a (lip) tumor invades the cortical bone, inferior alveolar nerve, floor of the mouth or skin (chin or nose) | |
| T4a (oral cavity) tumor invades the cortical bone, into deep/extrinsic muscles of the tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), maxillary sinus or skin of the face | |
| T4b (lip and oral cavity) tumor invades the masticator space, pterygoid plates, or skull base; or encases internal carotid artery Note: Superficial erosions of the bone/tooth socket alone; or a primary tumor of the gums, are not sufficient to classify a tumor as T4 | |
| N –Regional lymph nodes | Nx regional lymph nodes cannot be assessed |
| N0 no regional lymph node metastasis | |
| N1 metastasis in a single ipsilateral lymph node, 3 cm or smaller in the largest dimension | |
| N2 N2a metastasis in a single ipsilateral lymph node, larger than 3 cm but smaller than 6 cm in the largest dimension N2b metastasis in multiple ipsilateral lymph nodes, none larger than 6 cm in the largest dimension N2c metastasis in bilateral or contralateral lymph nodes, none larger than 6 cm in the largest dimension | |
| N3 metastasis in a lymph node larger than 6 cm in the largest dimension Note: Mid-line nodes are considered as ipsilateral nodes | |
| M – Distant metastasis | Mx distant metastasis cannot be assessed |
| M0 no distant metastasis | |
| M1 distant metastasis |
Negative predictive factors in oral cancers include tumor size, scope of infiltration of the surrounding tissues and lymph node metastases. In case of tumors localized in the floor of the mouth or in the tongue, metastases form rapidly thorough the lymphatic vessels with cervical lymph nodes affected most frequently. Due to a quick disease progression and the resultant cachexia, distant metastases are seen rarely. If they occur, the lung is the most commonly affected site [10,11].
The aim of this article is to describe oral squamous cell carcinoma as it appears in different diagnostic methods considering their advantages and limitations.
Imaging Methods in Oral Cancer
There are many imaging techniques that can be used for the diagnosis of cancers in the oral cavity. The most commonly used modalities used for both diagnosis and the planning of treatment include magnetic resonance imaging (MRI), computed tomography (CT) and positron emission tomography (PET). Moreover, oftentimes a biopsy sample is also taken. Other modalities include plain radiography, orthopantomography (OPG), cone beam computed tomography (CBCT), multidetector computed tomography (MDCT), computed tomography perfusion (CTP), diffusion-weighted MRI (DW-MRI), dynamic contrast-enhanced MRI (DCE-MRI), whole body MRI (WB-MRI), ultrasonography (USG), single-photon emission computed tomography (SPECT) as well as hybrid techniques such as ECT/CT, CT/MRI, PET/CT, PET/MRI with radiopharmaceuticals – 2-deoxy-2-[18F]fluoro-D-glucose, (18F-FDG), 18F-3-fluoro-alpha-methyltyrosine, (18F-FAMT) and L-1-[11C]-tyrosine, (C-TYR) [12,13].
An appropriate use of the above-mentioned imaging modalities assists in staging of the tumor, assessment of its vascularity, determination of metastases in both local lymph nodes and distant organs. Moreover, imaging studies help in planning the scope of resection and further treatment, graft implantation, and differentiation between reactive and metastatic lymph nodes as well as between disease recurrence and scars or adverse reactions after surgery or radiation therapy. Table 2 presents proposed use of imaging in the diagnosis of oral cancer.
Table 2.
Diagnostic algorithm for detection, staging and follow-up of patients with oral squamous cell carcinoma.
| Diagnostic algorithm – oral squamous cell carcinoma | ||
|---|---|---|
| 1) Detection | 2) Staging – TNM classification | 3) Follow-up after 3, 6, 12, 24 and 36 months |
| a) Physical examination, tumor sample for microscopic study, ultrasound-guided lymph node biopsy | a) Primary tumor size:
|
a) Evaluation of the scope of surgery:
|
b) Incidental finding in studies performed for other diseases of the facial skeleton:
|
b) Metastases in regional lymph nodes
|
b) Response to radiation therapy and chemotherapy:
|
c) Distant metastases:
|
c) Detection of recurrence:
|
|
d) Disease dissemination:
| ||
Plain radiography
Dentists routinely order plain radiographs of the teeth, occlusal radiographs or panoramic radiographs. These modalities can suggest malignant lesions. Oral SCC can initially develop in the bones as the primary intraosseous squamous cell carcinoma (PIOSCC), which is a very rare type of cancer originating from the remnants of the dental epithelium (epithelial cell rests of Malassez and enamel epithelium). The diagnosis of PIOSCC is based of the following three criteria – 1) no contact with mucous membrane or skin, 2) exclusion of distant metastasis in a 6-month follow-up, 3) histopathological confirmation of squamous cell carcinoma [14,15].
The characteristic features of malignant lesions in plain radiographs include – atrophy of cortical lamina, osteolytic defects – both single and multilocular with an initial osteosclerotic capsule. In later stages, the ridges of bone defects become sharp and the teeth lose their bony support at the site of infiltration (Figures 1, 2).
Figure 1.
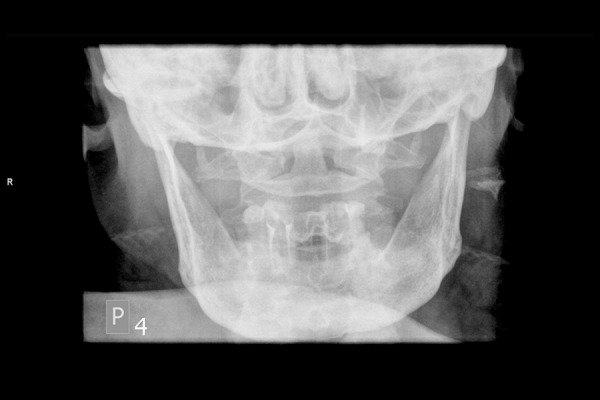
Radiography of the mandible. Discrete radiolucency in bone tissue on the right side.
Figure 2.
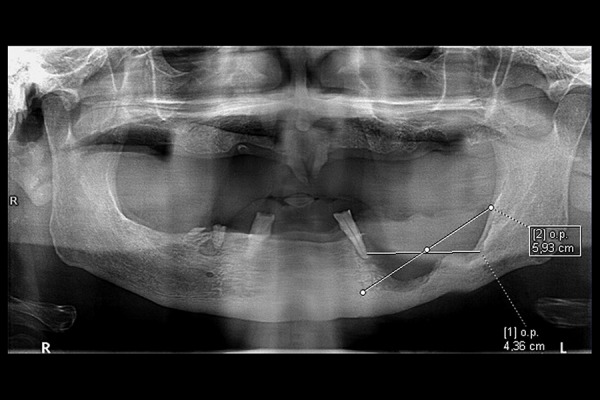
Panoramic radiograph. Bowl-shaped bone defect in the left part of the mandibular body, approximately 5.9 cm in length. The osteolytic lesion reaches the alveolus of the lower left canine that had lost distal bony support.
On panoramic radiographs, the involvement of the facial bones cannot be assessed, because when the osseous tissue loss is greater than 30%, the radiolucency is significantly increased. Plain radiography is not helpful in assessing soft tissues. It has been shown that the assessment of bone involvement on panoramic radiographs has a sensitivity of 75% and specificity of 100%, respectively [16].
Chest radiographs are not helpful in assessing early pulmonary dissemination of oral cancers and low-dose CT (LDCT) is used routinely. It is a very accurate modality that can either confirm or exclude pulmonary metastases.
Computed tomography
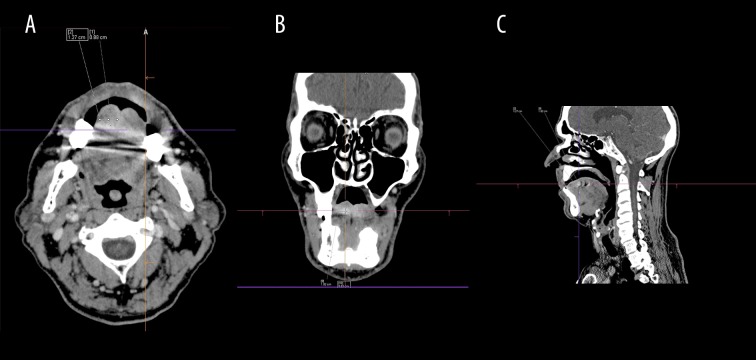
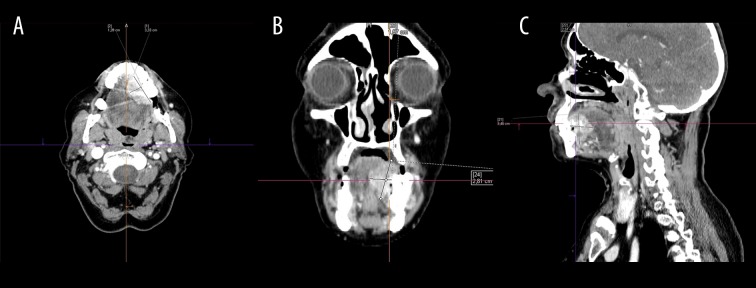
Computed tomography is a standard tool for detecting the primary tumors as well as their local bone infiltration. Special attention should be given to small lesions with a high degree of differentiation (low malignancy), since contrast enhancement can be scarce (Figure 3A–3C). However, lesions with a low degree of differentiation can be easily distinguished from the surrounding tissues (Figure 4A–4C) [13]. Contrast-enhanced CT can accurately determine lymph node metastases, which can initially look normal despite the presence of micrometastases detected in microscopic studies [17].
Figure 3.
Contrast-enhanced computed tomography. There is a tumor in the tongue (1 cm in diameter) on the right (T1 stadium). On the histopathological examination, it was diagnosed as a highly differentiated oral squamous cell carcinoma (G1) (A) axial view (B) coronal view (C) sagittal view.
Figure 4.
Contrast-enhanced computed tomography. There is a tumor in the tongue (3.5×2.8×1.6 cm) on the right (T2 stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with moderate/low degree of differentiation (G2/3) (A) axial view (B) coronal view (C) sagittal view.
The sensitivity of CT in detecting tumors is 41–82% (specificity 82–100%) and in determining bone infiltration 63–80% (specificity 81–100%), respectively. Exclusion of lymph node metastasis is performed with 74% sensitivity and 85% specificity. CT cannot unambiguously differentiate between recurrence, surgical scars and adverse reactions after radiation therapy. However, the changes seen in serial CT studies can give information regarding the underlying process. The sensitivity and specificity of detecting recurrence and assessing treatment response are 50% and 88% respectively [13,16–18].
Multi-detector CT (MDCT) can precisely determine the boundaries of the tumor. In cancers localized in the retromolar triangle, Arya et al. showed that with the use of puffed-cheek MDCT (axial slice thickness – 2.5 mm, slice thickness for axial, coronal and sagittal reconstruction – 0.625 mm) the sensitivity and specificity of determining mandible and bone marrow involvement can be increased to 83–94% and 90–92%, respectively. MDCT can very precisely determine the involvement of the inferior alveolar nerve (up to 100%). Similar observations were made by Vidiri et al. [19,20].
Perfusion computed tomography
By assessing perfusion of the tumor site, it is possible to better evaluate the involvement of the surrounding tissues. The tumor is characterized by an increased blood volume (BV) and blood flow (BF) in comparison to healthy tissues. This results from neoangiogenesis in the tumor. It is suggested that perfusion CT is superior to CT as regards the assessment of muscle involvement. The sensitivity of CTP in detecting local lymph node metastases is 67% (specificity 53%). Importantly, CTP can distinguish between nonspecific changes after radiation therapy or chemotherapy and local recurrence, which is not possible with CT. Higher values of BF and BV correspond to better responses to chemotherapy and therefore CTP can potentially assess the efficacy of this mode of treatment [13,21,22].
Cone beam computed tomography (CBCT)
Isovolumetric tomography is an intensely developed diagnostic tool. The usefulness of CBCT in detecting osteolysis has been confirmed (sensitivity 89–93%, specificity 60–96.5%). CBCT is more accurate than panoramic radiography and comparable to MRI, CT and bone scintigraphy [18]. Moreover, CBCT is increasingly used by dentists in everyday practice which can result in an improved detection of oral cancers. However, CBCT is limited by a poor assessment of soft tissues.
CT fluoroscopy-guided biopsy
CT fluoroscopy is a minimally invasive imaging technique that enables a real-time assessment. It can be used for taking biopsies of oral cancers. However, it has not been described so far in a clinical setting in the available literature.
Magnetic resonance imaging (MRI)
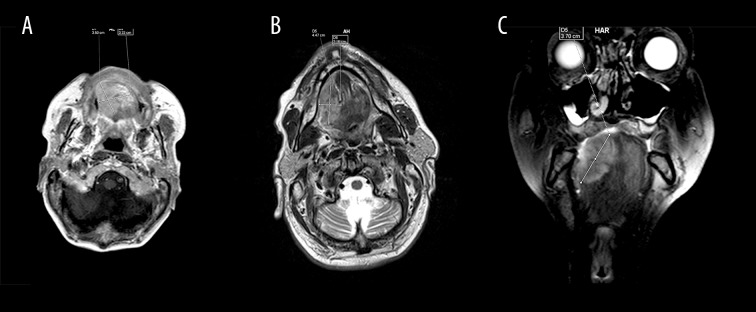
The protocol of an MRI study includes the following sequences – T1, T2, STIR, TIRM, DWI, perfusion with and without a contrast agent. MRI enables the detection of very small lesions, assessment of local spread of the tumor, planning surgery, evaluation of complications that can occur during and after surgery. MRI can determine the involvement of local soft tissues, bone marrow and bones (sensitivity 82%, specificity 66.7%) as well as vessels and nerves. Thus, it assists in choosing the method and scope of surgical resection. It is estimated that oral cancer can infiltrate the bone tissue of the maxilla or mandible in 12–56% of cases. MRI is a better modality in comparison to plain radiography and comparable to CBCT and CT – with similar sensitivity and specificity. However, MRI has a higher sensitivity with respect to the assessment of soft tissues (Figure 5A–5C) [12,13,18,23].
Figure 5.
Magnetic resonance imaging after contrast administration (A) T1 sequence, axial view, tumor in the tongue (3.5×3.3 cm) on the right (T2 stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with moderate/low degree of differentiation (G2/3). (B) T2, axial view, tumor in the tongue (4.5×2.2 cm) on the right (T2 stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with moderate/low degree of differentiation (G2/3). (C) T2, coronal view. There is a tumor in the tongue (3.5×3.3 cm) on the right (T2 stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with moderate/low degree of differentiation (G2/3).
MRI can effectively detect both local lymph node metastases and distant metastases of oral cancers (features N and M). MRI can differentiate between metastases and can assess the size of lymph node clusters (sensitivity 51–74%, specificity 95–100%). After diagnosing oral cancer, MRI should be used in order to assess a possible involvement of the neck and chest. Unfortunately, MRI can give falsely negative results. In cases of micrometastases in the lymph nodes (approximately 20% of patients), DWI-based MRI and hybrid methods can be helpful [24].
MRI can assist in planning the scope of resection and further reconstruction, graft implantation, and differentiation between disease recurrence and scars after surgery.
In patients with ferromagnetic prostheses in the facial skeleton, such as amalgamate fillings, dental crowns, tooth bridges, steel screws after osteosynthesis, MRI can be disturbed by numerous artifacts.
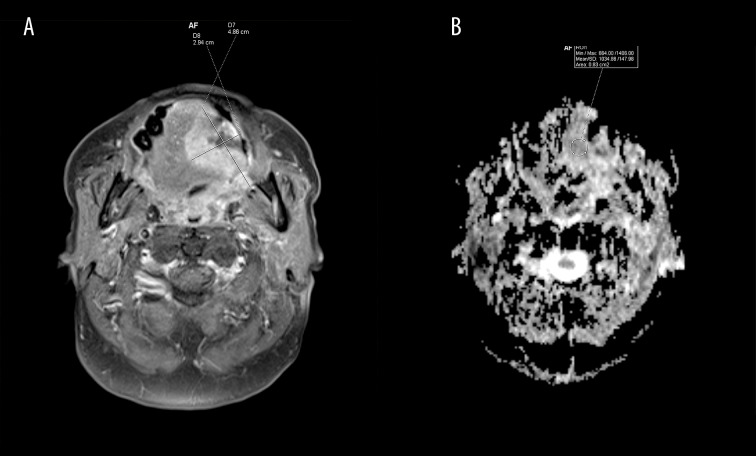
Diffusion-weighted imaging MRI (DWI-MRI)
In MRI, areas with decreased values of diffusion appear hyperintese (Figure 6A). DWI detects local lymph node metastases with 98% sensitivity and 88% specificity. DWI-MRI of lymph nodes smaller than 1 cm is superior to MRI (7% increase in sensitivity). However, this difference is not seen in lymph nodes larger than 1 cm. Maps of apparent diffusion coefficients (ADC) allow for a quantitative analysis of diffusion. In ADC maps, reduction of diffusion is seen as hypointense areas in comparison to DWI (Figure 6B). The sensitivity and specificity of ADC maps in detecting lesions is 94% and 91%, respectively [25–28].
Figure 6.
Magnetic resonance imaging after contrast administration (A) FAT SAT, T1 sequence, axial view. Tumor (4.9×2.9 cm, T3 stadium) with a decreased diffusion coefficient. (B) Apparent-diffusion-coefficient map (ADC map), axial view (mean value of 1034.86×106 mm2/s).
DWI can precisely detect structures involved by cancer and it can also differentiate between reactive and metastatic lymph nodes. Therefore, it should be used for lymph node assessment before surgery. Moreover, DWI can be helpful in assessing early response of the tumor to chemotherapy (as early as after 1–2 cycles of chemotherapy).
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)
Dynamic MRI after administration of a contrast agent enables the assessment of tumor perfusion and extralymphatic dissemination before surgery (sensitivity 96%, specificity 100%). Some studies suggest that, based on DCE-MRI, the microvasculature and perfusion of the tumor can be evaluated, which can help predict the outcome. However, recently this has been called into question. Nevertheless, DCE-MRI is a valuable tool in the diagnosis of oral cancer [13,29–32].
There are also possibilities to assess tumor perfusion without administering a contrast agent.
Single-photon emission computed tomography (SPECT)
SPECT allows for mapping metabolic activity of the tumor with the use of gamma radiation. The sources of radiation are isotopes such as 3-D 99mTcDPD (technetium-dicarboxy propan SPECT) or 99mTechnetium methoxy isobutyl isonitrile (MIBI) [33].
In the early stages of oral cancer, sentinel lymph node biopsy (SLNB) is of high importance. If the sentinel lymph node is not involved by the disease process (based on a microscopic study), lymphatic metastases can be excluded with a high probability. The most commonly used techniques for the detection of sentinel nodes includes 1) lymphoscintigraphy before surgery, 2) SPECT/CT, and 3) injection of the patent blue dye. The procedure can be difficult when lymph nodes are densely clustered or when the sentinel node is close to the site of injection and isotope administration (shine-through phenomenon). Freehand SPECT (fhSPECT) is a more accurate method which is used for planning biopsy. It is based on an intraoperative 3D imaging with the use of 3 gamma cameras – two cameras are placed above the patient and the third one is held by the surgeon who can movie it freely around the patient. The whole process is supported by a system that registers the positions of the cameras. Freehand SPECT can determine the location of the sentinel node, its distance from the skin and relation to the surrounding structures. It also evaluates the flow of lymph into the sentinel node so that the physician can change the scope of resection by selectively removing metastatic lymph nodes [34–37].
The assessment of the involvement of the mandible with 99mTc SPECT has almost 100% sensitivity and 14.3% specificity [16,38]. Leitha et al. reported a combined use of 99mTc-DPD SPECT and 99mTc-hekso-2-methyloxyisobutyl isonitrile (MIBI) SPECT that had 100% sensitivity and 17% specificity [39].
Positron emission tomography (PET)
PET with 18F-fluorodeoxyglucose evaluates tissue metabolic activity (Figure 7). It is used when planning adjuvant treatment and predicting survival without recurrence. It can be used for the detection of metastatic lymph nodes (sensitivity 83%, specificity 88%). It allows for an estimation of the risk of recurrence. Other methods that can be used to evaluate local infiltration include (Figure 8A, 8B) PET/MRI, SPECT/CT, CT/MRI with radiopharmaceuticals – 2-deoxy-2[18F]fluoro-D-glucose (18F-FDG), 3-deoxy-3-18F-fluorothymidine (FLT), L-3-18F-fluoro-alfa-methylothyrosine (18F-FAMT) and L-1-[11C]-thyrosine (C-TYR). 18F-FAMT PET/CT should not be used to assess bone infiltration and tumor boundaries; however, it can determine initial proliferation activity of cancer cells. PET/MRI has a higher sensitivity in assessing soft tissue infiltration in comparison to MRI. 18F-FAMT can be used to assess bone marrow involvement [13,40–42].
Figure 7.
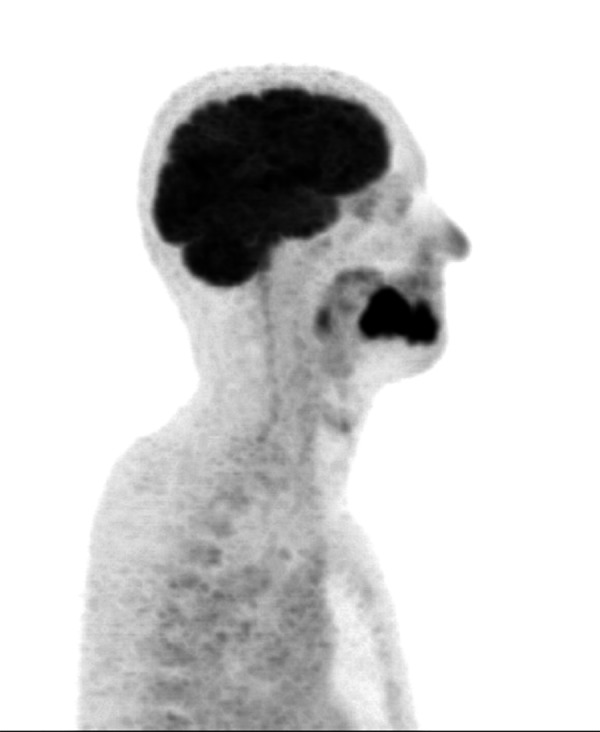
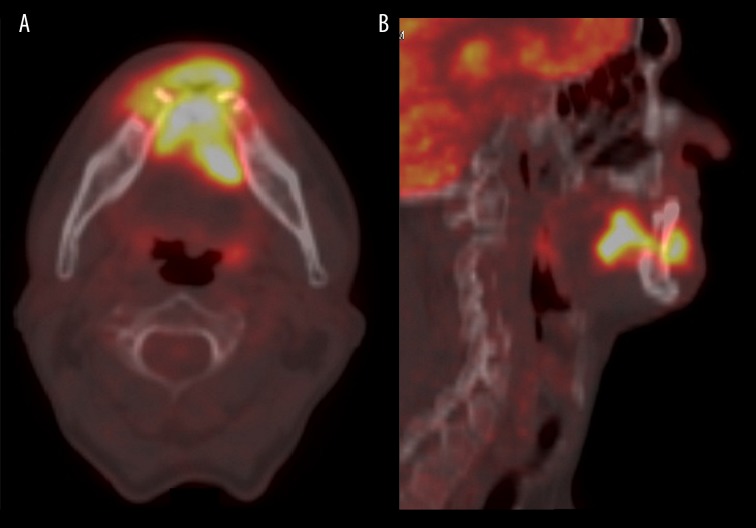
18F-FDG PET, sagittal view. Tumor of the tongue infiltrating the mandible (T4a stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with low degree of differentiation (G3).
Figure 8.
(A) 18F-FDG PET. Tumor of the tongue infiltrating the mandible (T4a stadium). On the histopathological examination, it was diagnosed as an oral squamous cell carcinoma with low degree of differentiation (G3). (A) axial view (B) sagittal view.
Moreover, PET is used to look for the primary tumor site when metastases are found earlier (carcinoma of unknown primary, CUP). PET is also routinely used for detecting distant metastases of known primary tumors [38]. FDG PET/CT can be important in assessing subclinical recurrence of oral cancer.
FDG PET/MR is not superior to MRI and FDG/PET in detecting metastatic lymph nodes. In this respect, FDG PET/CT is more efficacious than CT/MRI. PET/CT is an efficacious method of detecting disease recurrence and distant metastases.
As regards differentiation between surgical scars and disease recurrence, FDG/PET and PET/CT are superior to CT and MRI. PET is recommended in stage IV and should not be used as the method of choice in earlier stages. Whole body imaging with FDG/PET improves analisis of the TNM classification [13,40–43].
Ultrasonography
Ultrasonography is used to evaluate superficial lesions, lymph nodes and to guide needle aspiration biopsies (NAB). NAB is used in order to confirm metastatic lymph nodes. Chaukar et al. reported that ultrasonography is insufficient as a stand-alone method for evaluating cervical lymph nodes (sensitivity 79%, specificity 69%) [17].
In oral cancer, intraoral USG with color-Doppler can also be used in order to assess the involvement of lymph nodes. It can show an increased vascularity within the tumor (blood flow), microvascular changes, size of the lesion and its thickness and the distance between mucous membrane and the front of the tumor [44]. The formation of new vessels can have a prognostic value [45,46]. Intrapapillary capillary loops of the mucous membrane have been recently studied with narrow-band imaging. This helps localize lesions of the mucous membrane and therefore early stages of cancer. However, it cannot detect lesions beyond mucous membrane [47].
Ultrasound imaging can be helpful is assessing lymph nodes following a radical surgical resection with or without adjuvant radiation therapy. According to Wu-Chia et al., ultrasound-guided fine needle aspiration (UsFNA) results in an improved assessment of disease recurrence in lymph nodes. Moreover, they showed that secondary lesions not exposed to radiation therapy had a lower frequency of calcification than lymph nodes treated with radiation. Radiotherapy has an effect on image of the classical ultrasound, however, it does not limit UsFNA [48].
Conclusions
It is crucial to be aware of the basic clinical data of patients and their tumors when interpreting the results of imaging studies. In order to determine the site and size of the tumor, CT and MRI should be performed as initial studies. They can help determine the involvement of soft tissues and bones (CT is more helpful).
For the evaluation of the locoregional lymph nodes, ultrasonography, MRI, CT and PET can be employed. In turn, hybrid techniques coupled with PET should be used in order to look for distant metastases.
In conclusion, all imaging modalities have their advantages and disadvantages. Therefore, it is important to properly combine them in order to attain the highest possible efficacy and sensitivity, as this can significantly improve patient outcomes.
References
- 1.Kordek R, Jassem J, Jeziorski A, et al. Oncology Handbook for students and practitioners. 3rd ed. Gdansk: Via Medica; 2007. pp. 147–58. [Google Scholar]
- 2.Gajecka M, Rydzanic M, Jaskula-Sztul R, et al. Reduced DNA repair capacity in laryngeal cancer subjects. A comparison of phenotypic and genotypic results. Adv Otorhinolaryngol. 2005;62:25–37. doi: 10.1159/000082460. [DOI] [PubMed] [Google Scholar]
- 3.Kawecki A. Epithelial neoplasms of the head and neck organs. In: Krzakowski M, Warzocha K, Kawecki A, et al., editors. Guidelines for diagnostic and therapy of malignant tumors in 2013. 1st ed. I. Gdansk: VM Media; 2013. pp. 1–32. [Google Scholar]
- 4.Didkowska J, Wojciechowska U. Malignant tumors in Poland in 2013. Warsaw: National Cancer Registry; 2015. pp. 52–54.pp. 82–84. [Google Scholar]
- 5.Stachura J, Domagała W. Pathology: A word about the disease. 2nd ed. I. Krakow: Polish Academy of Knowledge; 2008. pp. 156–217. [Google Scholar]
- 6.Cohen Y, Goldenberg-Cohen N, Shalmon B, et al. Mutational analysis of PTEN/PIK3CA/AKT pathway in oral squamous cell carcinoma. Oral Oncol. 2011;47(10):946–50. doi: 10.1016/j.oraloncology.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y-S, Hsu H-T, Ko Y-C, et al. Combined mutational analysis of RAS, BRAF, PIK3CA, and TP53 genes in Taiwanese patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(1):110–16. doi: 10.1016/j.oooo.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Szyfter K, Golusiński W. Molecular cancer biology of the head and neck in the age genomics and proteomics. Contemp Oncol. 2006;10(8):385–90. [Google Scholar]
- 9.Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 165–211. [Google Scholar]
- 10.Lewandowski L, Piorunek T, Kasprzyk M, Skowronek J. Procedure multidisciplinary metastases cancer oral cavity head and neck into the lungs. Czas Stomat. 2006;59(1):37–41. [Google Scholar]
- 11.Morandi L, Tarsitano A, Gissi D, et al. Clonality analysis in primary oral squamous cell carcinoma and related lymph-node metastasis revealed by TP53 and mitochondrial DNA next generation sequencing analysis. J Craniomaxillofac Surg. 2015;43:208–13. doi: 10.1016/j.jcms.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Sarrión Pérez MG, Bagán JV, Jiménez Y, et al. Utility of imaging techniques in the diagnosis of oral cancer. J Craniomaxillofac Surg. 2015;43(9):1880–94. doi: 10.1016/j.jcms.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Blatt S, Ziebart T, Krüger M, Pabst AM. Diagnosing oral squamous cell carcinoma: How much imaging dowe really need? A review of the current literature. J Craniomaxillofac Surg. 2016;44(5):538–49. doi: 10.1016/j.jcms.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Liu SL, Ye WM, Zheng J. STAT3 is a key pathway in primary intraosseous squamous cell carcinoma arising from an odontogenic keratocyst. Am J Transl Res. 2015;7(10):1860–69. [PMC free article] [PubMed] [Google Scholar]
- 15.Kacmarzyk T, Stypułkowka J, Tomaszewska R, Czopek J. Handbook for students and doctors. 1st ed. Warsaw: Library quintessence; 2009. Odontogenic tumors and tumor-like cancers of the maxillary and mandibule bones; pp. 214–16. [Google Scholar]
- 16.Kushraj T, Chatra L, Shenai P, Rao PK. Bone invasion in oral cancer patients: A comparison between Orthopantamograph, conventional computed tomography, and single positron emission computed tomography. J Cancer Res Ther. 2011;7(4):438–41. doi: 10.4103/0973-1482.92012. [DOI] [PubMed] [Google Scholar]
- 17.Chaukar D, Dandekar M, Kane S, et al. Relative value of ultrasound, computed tomography and positron emission tomography imaging in the clinically node-negative neck in oral cancer. Asia Pac J Clin Oncol. 2016;12(2):332–38. doi: 10.1111/ajco.12255. [DOI] [PubMed] [Google Scholar]
- 18.Linz C, Müller-Richter UD, Buck AK, et al. Performance of cone beam computed tomography in comparison to conventional imaging techniques for the detection of bone invasion in oral cancer. Int J Oral Maxillofac Surg. 2015;44:8–15. doi: 10.1016/j.ijom.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Arya S, Rane P, Sable N, et al. Retromolar trigone squamous cell cancers: A reappraisal of 16 section MDCT for assessing mandibular invasion. Clin Radiol. 2013;68(12):680–88. doi: 10.1016/j.crad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Vidiri A, Guerrisi A, Pellini R, et al. Multi-detector row computed tomography (MDCT) and magnetic resonance imaging (MRI) in the evaluation of the mandibular invasion by squamous cell carcinomas (SCC) of the oral cavity. Correlation with pathological data. J Exp Clin Cancer Res. 2010;29:73. doi: 10.1186/1756-9966-29-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trojanowska A. Squamous cell carcinoma of the head and neck – The role of diffusion and perfusion imaging in tumor recurrence and follow-up. Rep Pract Oncol Radiother. 2011;16(6):207–12. doi: 10.1016/j.rpor.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trojanowska A, Grzycka-Kowalczyk L, Trojanowski P, et al. Computed tomography perfusion examination is helpful in evaluating the extent od oropharyngeal and oral cancer. Pol J Radiol. 2011;76(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Higuchi T, Arisaka Y, et al. Clinical significance of 18F-a-methyl tyrosine PET/CT for the detection of bone marrow invasion in patients with oral squamous cell carcinoma: comparison with 18F-FDG PET/CT and MRI. Ann Nucl Med. 2013;27:423–30. doi: 10.1007/s12149-013-0701-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Roh JL, Kim JS, et al. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing central compartment neck dissection for squamous cell carcinoma of the larynx, hypopharynx, and esophagus. Oral Oncol. 2013;49(5):449–53. doi: 10.1016/j.oraloncology.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberger R, Wojtek P, Konopka M, et al. Clinical application of perfusion imaging computed tomography and diffusion and perfusion magnetic resonance imaging in detecting early changes in ischemic stroke. Via Medica Udar Mozgu. 2004;6(2):71–78. [Google Scholar]
- 26.Hejduk B, Bobek-Billewicz B, Jedrzejewska M. Application of MRI imaging DWI-evaluation of lymph nodes in patients with head and neck cancer – a preliminary report. Pol J Radiol. 2007;72(1):207–8. [Google Scholar]
- 27.Bobek-Billewicz B. Diffusion-weighted MRI (DWI-MR) – oncological applications. Pol J Radiol. 2010;75(1):53. [Google Scholar]
- 28.Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR imaging in the head and neck. Radiology. 2012;263(1):19–32. doi: 10.1148/radiol.11101821. [DOI] [PubMed] [Google Scholar]
- 29.Shukla-Dave A, Lee NY, Jansen JFA, et al. Dynamic contast-enhanced magnetic resonance imaging as a predictorof outcome in head and neck suamous cell carcinoma patients with nodal metastases. Int J Radi Oncol Biol Phys. 2011;82(5):1837–44. doi: 10.1016/j.ijrobp.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen JFA, Schoder H, Lee NY, et al. Tumor metabolism and perfusion in head and neck squamous cell carcinoma: pretreatment multimodality imaging with 1H magnetic resonance spectroscopy, dynamic contrast-enhanced MRI, and [18F]FDG-PET. Int J Radi Oncol Biol Phys. 2012;82(1):299–307. doi: 10.1016/j.ijrobp.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chikui T, Kitamoto E, Kami Y, et al. Dynamic contrast-enhanced MRI of oral squamous cell carcinoma: a preliminary study of the correlations between quantitative parameters and the clinical stage. Br J Radiol. 2015;88(1050):20140814. doi: 10.1259/bjr.20140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein JM, Homer JJ, West CM. Dynamic contrast-enhanced magnetic resonance imaging biomarkers in head and neck cancer: Potential to guide treatment? A systematic review. Oral Oncol. 2014;50(10):963–70. doi: 10.1016/j.oraloncology.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Uribe S, Rojas LA, Rosas CF. Accuracy of imaging methods for detection of bone tissue invasion in patients with oral squamous cell carcinoma. Dentomaxillofac Radiol. 2013;42(6):20120346. doi: 10.1259/dmfr.20120346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heuveling DA, van Weert S, Karagozoglu KH, de Bree R. Evaluation of the use of freehand SPECT for sentinel node biopsy in early stage oral carcinoma. Oral Oncol. 2015;51(3):287–90. doi: 10.1016/j.oraloncology.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Alkureishi LW, Burak Z, Alvarez JA, et al. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Ann Surg Oncol. 2009;16:3190–210. doi: 10.1245/s10434-009-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bluemel C, Herrmann K, Müller-Richter U. Freehand SPECT-guided sentinel lymph node biopsy in early oral squamous cell carcinoma. Head Neck. 2014;36(11):E112–16. doi: 10.1002/hed.23596. [DOI] [PubMed] [Google Scholar]
- 37.Mihaljevic AL, Rieger A, Belloni B, et al. Transferring innovative freehand SPECT to the operating room: first experiences with sentinel lymph node biopsy in malignant melanoma. Eur J Surg Oncol. 2014;40(1):42–48. doi: 10.1016/j.ejso.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Schimming R, Juengling FD, Lauer G, et al. Computer-aided 3-D 99mTc-DPD-SPECT reconstruction to assess mandibular invasion by intraoral squamous cell carcinoma: Diagnostic improvement or not? J Craniomaxillofac Surg. 2000;28(6):325–30. doi: 10.1054/jcms.2000.0171. [DOI] [PubMed] [Google Scholar]
- 39.Leitha T, Glaser C, Pruckmayer M. Technetium-99-MIBI in primary and recurrent head and ne6ck tumours: contribution of bone SPECT image fusion. J Nucl Med. 1998;39:1166–71. [PubMed] [Google Scholar]
- 40.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100(10):712–20. doi: 10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 41.Krabbe CA, Balink H, Roodenburg JL, et al. Performance of 18F-FDG PET/contrast-enhanced CT in the staging of squamous cell carcinoma of the oral cavity and oropharynx. Int J Oral Maxillofac Surg. 2011;40(11):1263–70. doi: 10.1016/j.ijom.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Krabbe CA, van der Werff-Regelink G, Pruim J, et al. Detection of cervical metastases with (11)C-tyrosine PET in patients with squamous cell carcinoma of the oral cavity or oropharynx: A comparison with (18)F-FDG PET. Head Neck. 2010;32(3):368–74. doi: 10.1002/hed.21192. [DOI] [PubMed] [Google Scholar]
- 43.Haerle SK, Fischer DR, Schmid DT, et al. 18F-FET PET/CT in advanced head and neck squamous cell carcinoma: an intra-individual comparison with 18F-FDG PET/CT. Mol Imaging Biol. 2011;13(5):1036–42. doi: 10.1007/s11307-010-0419-5. [DOI] [PubMed] [Google Scholar]
- 44.Ariji Y, Goto M, Fukano H. Role of intraoral color Doppler sonography in predicting delayed cervical lymph node metastasis in patients with early-stage tongue cancer: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):246–53. doi: 10.1016/j.oooo.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Eshghyar N, Mohammadi N, Rahrotaban S, et al. Endoglin (CD105) positive microvessel density and its relationship with lymph node metastasis in squamous cell carcinoma of the tongue. Arch Iran Med. 2011;14:276–80. [PubMed] [Google Scholar]
- 46.Okada Y. Relationships of cervical lymph node metastasis to histopathological malignancy grade, tumor angiogenesis, and lymphatic invasion in tongue cancer. Odontology. 2010;98:153–59. doi: 10.1007/s10266-010-0131-6. [DOI] [PubMed] [Google Scholar]
- 47.Takano JH, Yakushiji T, Kamiyama I, et al. Detecting early oral cancer: Narrowband imaging system observation of the oral mucosa microvasculature. Int J Oral Maxillofac Surg. 2010;39:208–13. doi: 10.1016/j.ijom.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Lo WC, Cheng PW, Wang CT. The effect of radiotherapy on ultrasoundguided fine needle aspiration biopsy and the ultrasound characteristics of neck lymph nodes in oral cancer patients after primary treatment. PLoS One. 2016;11(3):e0149346. doi: 10.1371/journal.pone.0149346. [DOI] [PMC free article] [PubMed] [Google Scholar]