Abstract
Background
Recent evidence reveals that the inflammatory microenvironment is associated with tumor migration, invasion, and metastasis. Tumor necrosis factor-α (TNF-α) play a vital role in regulation of the inflammatory process in tumor development. Nuclear factor-kappa B (NF-κB) is one of the key transcription factors which regulate processes in tumor promotion. The aim of this study was to explore the role of NF-κB on the invasion and migration of oral squamous cell carcinoma (OSCC).
Material/Methods
The IKKβ and p65 mRNA and protein levels were determined by quantitative RT-PCR and western blot. Wound scratch healing assays and transwell migration assays were used to evaluate the effect of TNF-α and BAY11-7082 on the migration of the OSCC cell lines (HN4, HN6, and CAL27).
Results
We observed a significant increase of the expression level of IKKβ and p65 in OSCC cells from the experimental group at 24 h, 48 h, and 72 h after TNF-α stimulation. Invasion and metastasis of OSCC cells was obviously improved after the TNF-α stimulation. Invasion and metastasis ability of OSCC cells was inhibited in the suppression group, and no significant changes were observed in expression level of IKKβ and p65 after the use of BAY11-7082.
Conclusions
Our results suggest that TNF-α enhances the invasion and metastasis ability of OSCC cells via the NF-κB signaling pathway.
MeSH Keywords: Carcinoma, Squamous Cell; eIF-2 Kinase; I-kappa B Kinase; NF-kappa B; TNF-Related Apoptosis-Inducing Ligand
Background
Epidemiological studies report about 700 000 cases (including 300 000 new-onset cases) of oral cancer worldwide in the past 5 years, with 145 000 deaths [1,2]. Although there has been improvement in diagnosis and treatment of oral cancer, prognosis of oral squamous cell carcinoma (OSCC) is still poor, the 5-year survival rate is low, and the average 5-year survival rate is about 50% [3]. The high mortality of OSCC is mainly due to invasion and metastasis in tumor cells. A growing number of studies have confirmed that the inflammatory tumor microenvironment was associated with the development, invasion, and metastasis of tumor cells [4,5].
Tumor necrosis factor alpha (TNF-α) is secreted by macrophages and plays an vital role in the process of infection and immune response [6]. According to research on renal cell carcinoma, small doses of TNF-α in the tumor microenvironment can enhance tumor cell proliferation, invasion, and metastasis, increase white blood cells, form blood vessels, and induce upregulation of other cytokines (e.g., angiogenesis factor and matrix metalloproteinases), and trigger epithelial-mesenchymal transition (EMT) of tumor cells [7]. In addition, an animal experiment also found that silencing the TNF-α gene can inhibit the proliferation and migration of gastric cancer cells [8]. Nevertheless, how TNF-α promotes invasion and metastasis of tumor cells is unclear. Extensive research on the TNF-α/NF-κB/Snail pathway confirmed that TNF-α can regulate the expression of transcription factor Snail to induce EMT and promote the invasive and migratory activities of tumor cells [9].
The NF-κB pathway is activated by TNF-α to promote tumor cell proliferation and inhibition of apoptosis, and enhances the tumor angiogenesis ability and the potential of invasion and metastasis [10]. The NF-κB signaling pathway includes receptors and the proximal signal link protein, IκB kinase complexes, and IκB and NF-κB dimers. Normally, NF-κB is hidden by combining with IκB when cells are stimulated by various inflammatory stimuli such as growth factors and infectious microbes, activating IKK, which leads to IκB degradation, and release of NF-κB dimers [11,12]. Then, NF-κB is transferred to the nucleus with further activation, and combines with the gene to promote transcription of the target gene. NF-κB is a transcription factor for different biological processes: immune response; cell growth, proliferation, survival and apoptosis; stress response; embryogenesis; and development [13]. NF-κB is also essential to human health, but the abnormal activation of the NF-κB can cause a variety of autoimmunity, inflammation, and malignant diseases, including rheumatoid arthritis, atherosclerosis, inflammatory bowel disease, multiple sclerosis, and malignant tumors [14,15]. Recent data showed that IKK is involved in the main pathway of proinflammatory genes, and expression of IKK plays a central role in TNF-α-mediated NF-κB activation and expression [16].Therefore, inhibition of the NF-κB signaling pathway could be of use in treating cancer and inflammatory diseases.
Based on the above studies, the present study explored the role of NF-κB in OSCC progression. We observed invasive and migratory activities of OSCC after activation of the NF-κB pathway by TNF-α and inhibition of NF-κB pathway signaling with the use of an inhibitor (BAY11-7082).
Material and Methods
Cell culture
Three human OSCC cell lines (HN4, HN6, and CAL27) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). OSCC cells were cultured in DMEM/F12 (Gibco, France) supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 U/ml penicillin, and streptomycin (100 μg/ml) at 37°C in a 5% CO2 humidified incubator. The cells were seeded in 6-well plates and maintained until they reached 80–90% confluence. Next, the cells were starved overnight in serum-free DMEM/F12. The experimental group cells were stimulated with 10 ng/ml TNF-α for 3 h, 6 h, 12 h, 24 h, 48 h, and 72 h. HN6 cells were stimulated with 5 uM BAY11-7082, HN4, and CAL27 cells, as the suppression group, were stimulated with 10 uM BAY11-7082 for 24 h, 48 h, and 72 h, respectively. No TNF-α or BAY11-7082 was added to the control group.
Real-Time polymerase chain reaction (RT-PCR)
Total RNA of the experimental group, suppression group, and control group OSCC cells was extracted using the TRIzol reagent (Ambion, USA). RNA was reverse-transcribed into cDNA with a one-step RT-PCR kit (TIANGEN Biotech Co., Ltd. Beijing China) at 37°C for 60 min. Real-time quantitative PCR, using RealMaster Mix (SYBR Green) (Tiangen) with a 7800 ABI RT-PCR System (Applied Biosystems, Foster City, CA, USA). PCR proceeded under the conditions of 95°C for 30 s, 95°C for 15 s, 60°C for 30 s, and 68°C for 30 s (40 cycles). The relative gene expression was calculated using the 2(−ΔΔCT) method in at least 3 independent experiments. The resultant mRNA was normalized to its own B-actin. The primers used for the RT-PCR were as followed:
IKKβ (5′-ACCTCGAGACCAGCGAACTG-3′,
5′-TGCTATCCGGGCTTCCACTG-3′),
P65 (5′-TCCTGTGCGTGTCTCCATGC-3′,
-TGGCTGATCTGCCCAGAAGG-3′),
B-action (5′-GCCGGGACCTGACTGACTAC-3,
5′-CGGAGTACTTGCGCTCAGGA-3′).
SDS-PAGE and western blotting
HN4, HN6, and CAL27 OSCC cells were seeded in 6-well plates (105 cells/well), and, after various treatments, they were lysed in pre-warmed Laemmli buffer (Sigma, USA). Each sample was electrophoresed through 10% SDS polyacrylamide gels. These membranes were subsequently blocked for 1 h with 5% non-fat milk in TBST at room temperature and then incubated overnight at 4°C with primary antibodies. A 1: 500 dilution of IKKβ, p65, and a 1: 1000 dilution of B-actin was used. Blots were quantified by Amersham Imager 600 (GE USA). Blots were analyzed using ImageJ software.
Wound healing assay
HN4, HN6, and CAL27 OSCC cells were seeded in 6-well plates and cultured to reach 90% confluency in complete medium. Next, a sterile 10-ul pipet tip was used to scrape 3 wounds through the cell monolayer, and the cells were gently rinsed with PBS. The experimental group cells were treated with 10 ng/ml TNF-α in serum-free medium. The suppression group cells were treated with BAY11-7082 in serum-free serum. Pictures were taken immediately before treatment as the control and after treatment for 6 h, 12 h, and 24 h on an inverted microscope (Olympus).
Transwell migration assay
HN4, HN6, and CAL27 OSCC cells suspended in 200 ul of serum-free medium were loaded onto an upper 8-μm pore size chamber inserted in a 24-well cell culture plate. The lower chamber was filled with DMEM/F12 supplemented with 10% FBS as a chemoattractant. The cells were allowed to adhere before being treated with 10 ng/ml TNF-α and then incubated for 24 h at 37°C in 5% CO2. After this incubation, the inserts were removed and the remaining non-migrating cells on the upper surface of the membrane were removed with a cotton swab. The cells that migrated to the lower surface of the membrane were fixed with 10% paraformaldehyde (Beyotime) for 30 min at room temperature, washed with PBS, and then stained with 0.1% crystal violet (Beyotime). Then, the cells were examined using an inverted microscope at 20× magnification. Pictures of 5 randomly chosen fields were taken, and migrating cells were counted using Image J software.
Statistical analysis
The assays were repeated in 3 or more independent experiments and results are expressed as the mean ± S.D. Statistical significance was assessed using the independent-samples t test. A P-value of less than 0.05 was considered to be statistically significant.
Results
TNF-α activates NF-κB signaling pathway
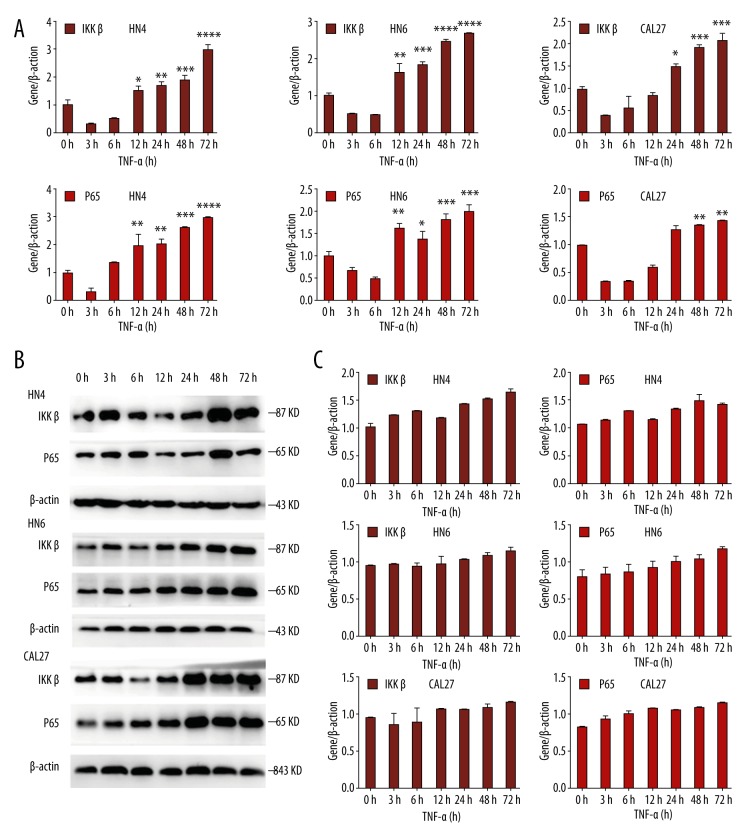
We observed that IKKβ and p65 in HN4, HN6, and CAL27 cells increased significantly after TNF-α stimulation. After being treating with 10 ng/ml TNF-α for 0 h, 3 h, 6 h, 12 h, 24 h, 48 h, and 72 h, RT-PCR and western blot analysis revealed that the level of IKKβ and p65 were significantly increased after 24 h, 48 h, and 72 h (Figure 1A, 1B). Western blot analysis showed statistically significant differences (Figure 1C). The above results indicate that TNF-α activates the NF-κB pathway in oral cancer cells.
Figure 1.
OSCC cells were treated with TNF-α (10 ng/ml) for the indicated times. (A) The mRNA level of IKKβ and p65 was analyzed by real-time RT-PCR. β-actin was used as a control. (B) Western blot analysis was performed to assess the expression of IKKβ and p65 at the protein level. β-actin was used as a loading control. (C) Statistical analysis of western blot analysis. Each bar represents the mean ±S.D. * P<0.05, ** P<0.05, *** P<0.05, **** P<0.05.
BAY11-7082 blocks NF-κB signaling pathway
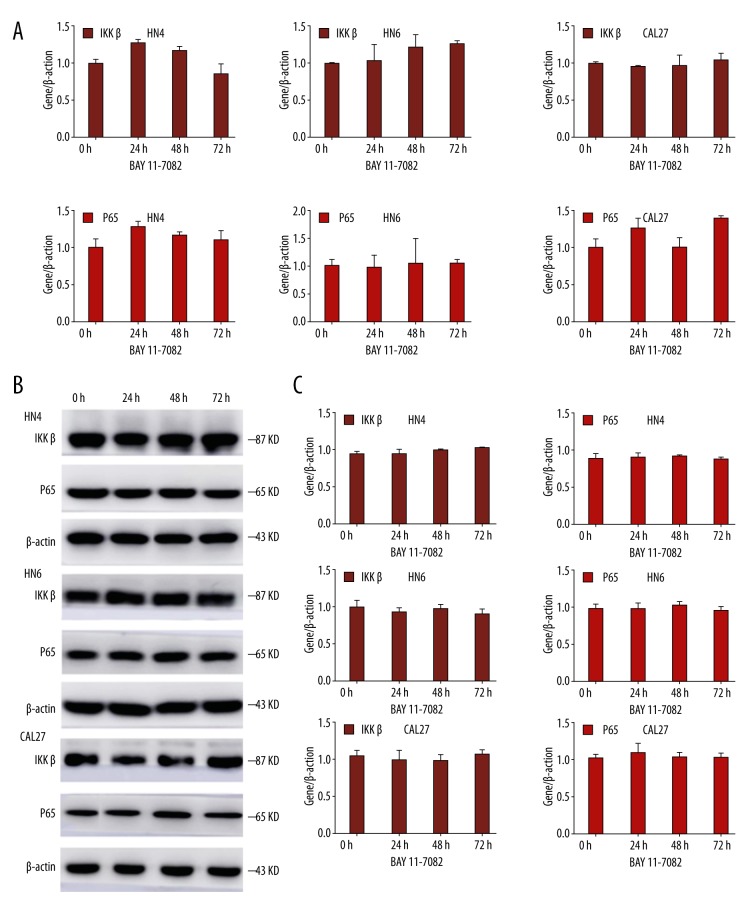
Firstly, HN4, HN6, and CAL27 cells were treated with BAY11-7082 before 1-h TNF-α stimulation. After being treated with BAY11-7082 for 0 h, 24 h, 48 h, and 72 h, real-time RT-PCR and western blot analysis reveal that the level of IKKβ and p65 were not significantly changed after 24 h,48 h, and 72 h (Figure 2A, 2B). The results of western blot analysis indicated no statistically significant difference (Figure 2C), showing that the NF-κB signaling pathway was not activated by TNF-α after blocking by BAY11-7082 in oral cancer cells.
Figure 2.
OSCC cells were treated with BAY11-7082 1 h before TNF-α stimulation for the indicated times. (A) The mRNA level of IKKβ and p65 was analyzed by real-time RT-PCR. β-actin was used as a control. (B) Western blot analysis was performed to assess the expression of IKKβ and p65 on the protein level. β-actin was employed as a loading control. (C) The statistics analysis of western blot analysis. Each bar represents the mean ±S.D. The difference was not significant, P>0.05.
BAY11-7082 inhibits migration and invasion of OSCC cells promoted by TNF-α
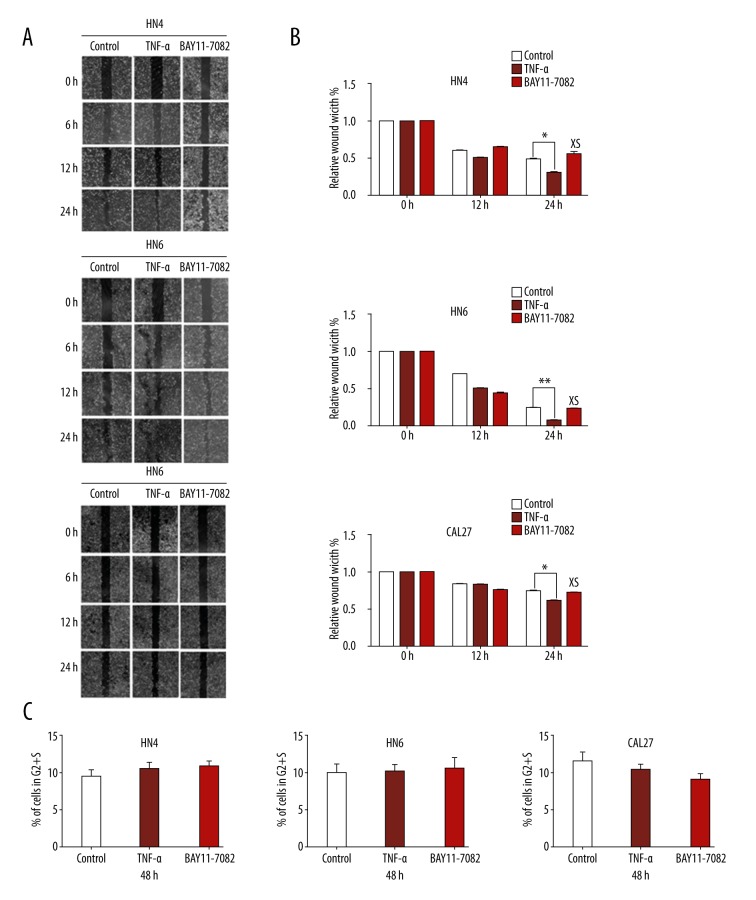
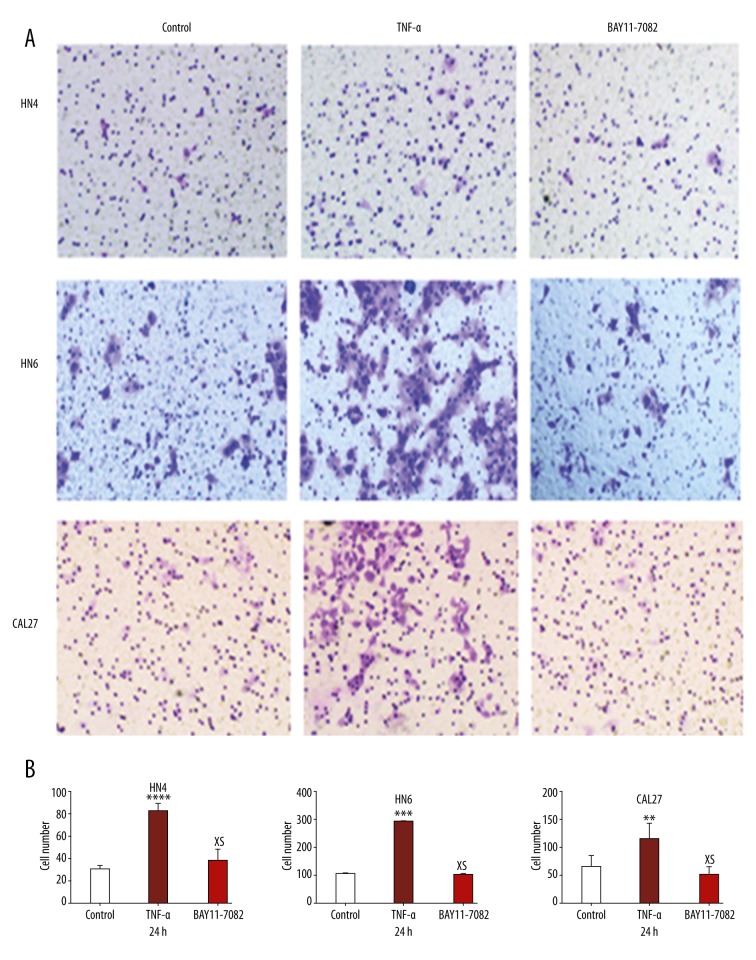
OSCC cells in the experimental group treated with TNF-α almost closed the scratch wound at 24 h, but the suppression group and control group could not, especially in HN4 and HN6 cells (Figure 3A). TNF-α promotes the migration ability of OSCC cells. There was no significant difference in migration ability between the BAY11-7082 treatment group and the control group (Figure 3B). The photographs of transwell invasion assay demonstrate that the experimental group cells treated with TNF-α were more invasive than in the suppression group and control group (Figure 4A). The quantitative analysis confirmed that the invasive abilities were significantly increased in HN4 (2.785-fold), HN6 (2.512-fold), and CAL27 (2.058-fold) after TNF-α treatment (Figure 4B). There was no significant difference in invasive ability between the BAY11-7082 treatment group and the control group (Figure 4C).
Figure 3.
TNF-α enhanced migration behavior of OSCC cells. (A) Photographs were taken at the same position of the wound at the indicated time points (×40 magnification). (B) Quantitative analysis of cells migration in 12 h and 24 h. The data were calculated as the mean ±S.D.* P<0.05. ** P<0.05; NS – not significant. (C) The flow cytometric cell-cycle analysis showed that there was no significant difference of proliferation ability between the TNF-α treatment group, the BAY11-7082 treatment group, and the nontreatment group, P>0.05.
Figure 4.
(A) Pictures presenting the cells penetrating the Matrigel basement membrane matrix after 24 h. (B) Quantitative analysis of cell invasion in 5 different random fields. The data were calculated as the mean ±S.D. * P<0.05; NS – not significant.
Thus, we conclude that BAY11-7082 inhibits the migratory and invasive ability of OSCC cells promoted by TNF-α.
Discussion
Our results showed that the mRNA and protein expression of IKKβ and P65 in OSCC cells increase after TNF-α stimulation, and the invasion and metastasis ability of OSCC cells are enhanced. After adding BAY11-7082 to OSCC cells, the stimulating effect of TNF-α decreased. The expression of IKKβ and P65 was unchanged compared with the control group. The invasion and metastasis ability of OSCC cells was reduced. The results show that TNF-α promotes oral cancer cell invasion and metastasis by activating the NF-κB signaling pathway. The stimulating effect of TNF-α in OSCC cells decreased significantly but did not disappear completely after inhibiting the NF-κB signaling pathway. There may be other signaling pathways involved. TNF-α can promote oral cancer cell invasion and metastasis, and this effect may be associated with activation of the NF-κB signaling pathway.
Cancer recurrence and death in cancer patients are mostly due to invasion and metastasis of tumor cells. Tumor metastasis involves tumor cells spreading from the primary site to regional lymph nodes or spreading to a distant site through a variety of ways. Tumor cells grow and proliferate after arriving in other tissues and organs, forming secondary tumors with the same nature as the primary tumor. Tumor cells mainly metastasize through lymphatic and blood vessels. Tumor cells are surrounded by the tumor microenvironment, which contains a variety of cell types (e.g., epithelial cells, fibroblasts, and inflammatory cells), as well as extracellular matrix and extracellular molecules (e.g., TNF-alpha and TGF-beta) [17,18]. The tumor microenvironment also plays an important role in the occurrence, development, and metastasis of oral cancer [19].
TNF-α in the tumor microenvironment acts as an inflammatory mediator that triggers EMT of tumor cells and promotes tumor metastasis [20]. Signaling pathways and transcription factors activated by TNF-α are considered key to invasion and metastasis in cancer cells. Cancer cells are more dependent on transcription factors than are normal cells. The growth of cancer cells is inhibited and the apoptosis of cancer cells is promoted through targeted inhibition of transcription factors and signaling pathways [21]. TNF-α promotes oral cancer cell invasion and metastasis, which relies on NF-κB signaling pathway activation [22,23].
Infinite proliferation and anti-apoptosis are the basic characteristics of tumor cells. NF-κB interacts with CyclinD1, Cyclin E, and c-myc to promote tumor cell proliferation [24], and promotes survival and inhibits apoptosis through Bcl-2 and Bcl-xL,which regulate transcription of the cellular inhibitor of apoptosis [25]. NF-κB has also been found to regulate the expression of matrix metalloproteinases (MMPs), especially the expression of MMP9 [26], which degrades and reshapes the extracellular matrix to enhance invasion of tumor cells. Several studies showed that the expression of Snail, Slug [27], ZEB1/2 [28], and Twist1 [29] is altered via NF-κB activation and triggers EMT. A breast cancer model experiment found that ENT was reversed and metastasis was reduced through inhibiting NF-κB [30]. Research on pneumonia demonstrated that TNF-α and TGF-β upregulated Snail Twist1, Slug, and ZEB2, dependent on NF-κB activation, and triggered EMT [31].
In addition to the NF-κB signaling pathway, there are some other pathways related to tumor cell invasion and biological behavior. Akca et al. [32] found that PI3K/Akt/NF-κB pathway activation can enhance lung cancer cells invasive ability. The PI3K/Akt signaling pathway upregulated Snail and Slug to inhibit of the expression of E-cadherin; and promoted the degradation by elevating MMPS, which directly induced EMT and strengthened the invasion ability [33].Watson confirmed that liver cancer cells secreted VEGF, activating the p38MAPK signaling pathway, and promoting cell adhesion and angiogenesis [34]. A study on colon cancer and prostate cancer cells found that TNF-α induced Snail stabilization through the AKT/GSK signaling pathway to induce EMT [35]. In colorectal tumor transplantation, model tumor growth was inhibited by suppressing the Notch and Wnt pathways [36]. mRNA might play a tumor-suppressive role in OSCC [37,38].
NF-κB occupies an important position in the study of oral cancer. Cultivation of oral cancer cells in vitro found that TNF-α and NF-κB had high expression levels [39]. Our previous experiments showed that after the application of TNF-a stimulation in OSCC cultivated in vitro in a simulated tumor microenvironment, Snail of OSCC cells was upregulated, and invasion of OSCC cells also increased, probably via activation of the NF-κB pathway [40].
Conclusions
Our results suggest that TNF-α enhances the invasion and metastasis ability of oral cancer cells via the NF-κB signaling pathway.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Provincial Natural Science Research Project of Anhui College (No. KJ2014A269)
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 3.Global oral cancer incidence. Br Dent J. 2016;221:288. doi: 10.1038/sj.bdj.2016.672. [DOI] [PubMed] [Google Scholar]
- 4.Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–49. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL, Ruvolo PP. Introduction to tumor microenvironment regulation of cancer cell survival, metastasis, inflammation, and immune surveillance. Biochim Biophys Acta. 2016;1863:379–81. doi: 10.1016/j.bbamcr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Mosaffa F, Kalalinia F, Lage H, et al. Pro-inflammatory cytokines interleukin-1 beta, interleukin 6, and tumor necrosis factor-alpha alter the expression and function of ABCG2 in cervix and gastric cancer cells. Mol Cell Biochem. 2012;363:385–93. doi: 10.1007/s11010-011-1191-9. [DOI] [PubMed] [Google Scholar]
- 7.Ho MY, Tang SJ, Chuang MJ, et al. TNF-alpha induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3beta-dependent mechanism. Mol Cancer Res. 2012;10:1109–19. doi: 10.1158/1541-7786.MCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 8.Sun Z, Meng Y, Liu G, et al. Effect of interleukin-1beta and tumor necrosis factor alpha gene silencing on mouse gastric cancer cell proliferation and migration. Oncol Lett. 2016;11:2559–65. doi: 10.3892/ol.2016.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Liu Y, Qian Y, et al. Antimetastatic effects of Celastrus orbiculatus on human gastric adenocarcinoma by inhibiting epithelial-mesenchymal transition and NF-kappaB/snail signaling pathway. Integr Cancer Ther. 2015;14:271–81. doi: 10.1177/1534735415572880. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birbach A, Gold P, Binder BR, et al. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem. 2002;277:10842–51. doi: 10.1074/jbc.M112475200. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Hayden MS. Celebrating 25 years of NF-kappaB research. Immunol Rev. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima S, Kitamura M. Bidirectional regulation of NF-kappaB by reactive oxygen species: A role of unfolded protein response. Free Radic Biol Med. 2013;65:162–74. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Westbrook AM, Szakmary A, Schiestl RH. Mechanisms of intestinal inflammation and development of associated cancers: Lessons learned from mouse models. Mutat Res. 2010;705:40–59. doi: 10.1016/j.mrrev.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in aging and disease. Aging Dis. 2011;2:440–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 17.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: A critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–48. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, Jiang YC, Sun CK, Chen QM. Role of the tumor microenvironment in tumor progression and the clinical applications (Review) Oncol Rep. 2016;35:2499–515. doi: 10.3892/or.2016.4660. [DOI] [PubMed] [Google Scholar]
- 19.Patil S, Rao R, Raj T. Potential role of tumor microenvironment in the progression of oral cancer. J Contemp Dent Pract. 2015;16:i–ii. [PubMed] [Google Scholar]
- 20.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. J Pathol. 2013;230:241–48. doi: 10.1002/path.4188. [DOI] [PubMed] [Google Scholar]
- 21.Yedida GR, Nagini S, Mishra R. The importance of oncogenic transcription factors for oral cancer pathogenesis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:179–88. doi: 10.1016/j.oooo.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JR, Jo K, Lee Y, et al. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-alpha gene expression and constitutive NF-kappaB activation. Carcinogenesis. 2012;33:77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–44. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-kappaB: Critical mechanisms in immune function and cancer. Immunol Rev. 2012;246:327–45. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 25.Chu ZL, McKinsey TA, Liu L, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–62. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricca A, Biroccio A, Del Bufalo D, et al. bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–96. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Barbera MJ, Puig I, Dominguez D, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–54. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 28.Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–24. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 29.Pham CG, Bubici C, Zazzeroni F, et al. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–35. doi: 10.1128/MCB.01219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-kappaB-Snail signaling in breast cancer cells. Oncol Rep. 2013;29:117–24. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 31.Kumar M, Allison DF, Baranova NN, et al. NF-kappaB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS One. 2013;8:e68597. doi: 10.1371/journal.pone.0068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akca H, Demiray A, Tokgun O, Yokota J. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer. 2011;73:302–9. doi: 10.1016/j.lungcan.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Kang MH, Oh SC, Lee HJ, et al. Metastatic function of BMP-2 in gastric cancer cells: The role of PI3K/AKT, MAPK, the NF-kappaB pathway, and MMP-9 expression. Exp Cell Res. 2011;317:1746–62. doi: 10.1016/j.yexcr.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Watson JL, Greenshields A, Hill R, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol Carcinog. 2010;49:13–24. doi: 10.1002/mc.20571. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Wang HS, Zhou BH, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer PLoS One 20138e56664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Yang X, Miao Y, et al. Inhibition of cell proliferation and tumor growth of colorectal cancer by inhibitors of Wnt and Notch signaling pathways. Oncol Lett. 2016;12:3695–700. doi: 10.3892/ol.2016.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Liu L, Fu H, et al. Association of decreased expression of serum miR-9 with poor prognosis of oral squamous cell carcinoma patients. Med Sci Monit. 2016;22:289–94. doi: 10.12659/MSM.895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bu J, Bu X, Liu B, et al. Increased expression of tissue/salivary transgelin mRNA predicts poor prognosis in patients with Oral Squamous Cell Carcinoma (OSCC) Med Sci Monit. 2015;21:2275–81. doi: 10.12659/MSM.893925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuta H, Osawa K, Shin M, et al. Selective inhibition of NF-kappaB suppresses bone invasion by oral squamous cell carcinoma in vivo. Int J Cancer. 2012;131:E625–35. doi: 10.1002/ijc.27435. [DOI] [PubMed] [Google Scholar]
- 40.Zhou JP, Gao ZL, Zhou ML, et al. Snail interacts with Id2 in the regulation of TNF-alpha-induced cancer cell invasion and migration in OSCC. Am J Cancer Res. 2015;5:1680–91. [PMC free article] [PubMed] [Google Scholar]