Abstract
IN BRIEF Many patients with type 2 diabetes require high basal insulin doses, necessitating multiple injections, increasing patient burden, and resulting in reduced treatment adherence. This randomized, controlled, crossover trial compared the efficacy, safety, and patient-reported outcomes for a concentrated formulation of insulin degludec (200 units/mL) to those of insulin glargine in patients requiring high doses of basal insulin. By offering equivalent glycemic control while reducing the rate of confirmed hypoglycemia and the number of injections required for administration, insulin degludec 200 units/mL may be preferred by patients with type 2 diabetes who require high basal insulin doses.
Many people with type 2 diabetes are also overweight or obese; this comorbidity increases insulin resistance, leading to a requirement for high doses of basal insulin to achieve glycemic targets (1). Data from the phase 3a insulin degludec (IDeg) clinical program indicate that ∼20% of patients with type 2 diabetes require high doses of basal insulin (>80 units/day). This increases injection frequency because the dose exceeds the maximum that can be delivered in a single administration, which also increases regimen complexity. Indeed, injection frequency is a well-recognized barrier that can reduce treatment adherence, thus compromising glycemic control (2,3). Hypothetically, therefore, a more concentrated insulin formulation, if accompanied by the ability to deliver higher doses of basal insulin in a single injection, would improve patient adherence and health-related quality of life (HRQoL).
IDeg, a basal insulin analog with an ultra-long duration of action, has been shown to be as effective as insulin glargine 100 units/mL (IGlar U100) with fewer hypoglycemic episodes, particularly nocturnal episodes (4–9). IDeg is available in two formulation strengths: 100 units/mL and 200 units/mL (IDeg U200), which share identical pharmacokinetic and pharmacodynamic properties (10). However, IDeg U200 facilitates the delivery of up to 160 units with a single injection, compared with a maximum of 80 units for the 100 units/mL formulation. A treat-to-target clinical trial (in accordance with U.S. Food and Drug Administration guidance for treating to similar glycemic control to enable interpretable comparison of hypoglycemia [11]) compared the efficacy and safety of IDeg U200 with IGlar U100. Both agents were combined with metformin in insulin-naive participants with type 2 diabetes. The primary objective of noninferiority of IDeg U200 to IGlar U100, as assessed by A1C change from baseline, was met (12). Additionally, IDeg U200 was associated with significantly greater fasting plasma glucose (FPG) reductions and significantly lower rates of confirmed and nocturnal confirmed hypoglycemia (12).
The primary objective of this study (BEGIN: HIGH DOSE) was to confirm these findings of noninferiority. The study also aimed to investigate patient-reported outcomes (PROs) for IDeg U200 compared with IGlar U100, both administered once daily with metformin to insulin-experienced patients with type 2 diabetes who required ≥81 units/day of IGlar U100.
Methods
Trial Design
This 32-week, open-label, crossover, treat-to-target trial was conducted at 28 U.S. sites. Blinding was not considered appropriate because of the difference in strength between the respective formulations. The study was completed in compliance with the Declaration of Helsinki (13) and the International Conference on Harmonization Good Clinical Practice Guidelines (14). Institutional review boards reviewed and approved the protocol for each site, and all participants provided written, informed consent before participating. This trial is registered with ClinicalTrials.gov as NCT01570751.
Participants
Adults with type 2 diabetes for ≥6 months who had been receiving 65–100 units/day IGlar U100 once daily in combination with metformin and one other oral antidiabetic drug (OAD) for at least 3 months and who had A1C levels ≥7.5% were eligible for inclusion. Patients presenting with any of the following criteria were excluded: clinically significant cardiovascular, hepatic, renal, or oncological disease; recurrent severe hypoglycemia; hypoglycemia unawareness; pregnancy; or proliferative retinopathy.
Treatments
Eligible patients received IGlar vials for a 16-week run-in period during which treatment was optimized using a treat-to-target approach to ensure stable and improved A1C before randomization to have similar baseline conditions at the start of each of the two treatment periods (11,15). All OADs except for metformin were discontinued. Participants’ daily metformin doses remained unaltered throughout the trial. Basal insulin was injected at the same time of day as preferred by the patient throughout the trial.
Patients requiring ≥81 units/dayof IGlar U100 at the end of the run-in period were randomized 1:1 to one of two 16-week treatment sequences: IDeg U200 once daily (3 mL FlexTouch; Novo Nordisk, Bagsværd, Denmark) (16) followed by once-daily IGlar U100 (Lantus 100 units/mL, 3 mL SoloSTAR; Sanofi U.S., St. Louis, Mo.) or vice versa. At 16 weeks, participants were switched directly to the other formulation without a washout period. Basal insulin doses were titrated based on the average of three consecutive prebreakfast, self-monitored plasma glucose (SMPG) measurements. IDeg U200 or IGlar U100 were injected subcutaneously in the thigh, abdomen, or upper arm, with rotation of injection sites within the same region from one injection to the next. In instances in which the dose of insulin exceeded the maximum that could be delivered in a single injection (160 units for IDeg U200 or 80 units for IGlar U100), the dose was split across as many injections as required, which were administered at the same time point and in the same bodily region as chosen by the patient.
Endpoints
The primary endpoint was change in A1C from baseline at 16 weeks of treatment. Secondary efficacy and safety endpoints included A1C responders; change in FPG; number of severe, confirmed, and nocturnal confirmed hypoglycemic episodes; PROs; adverse events (AEs); insulin dose; body weight; and standard physiological and laboratory tests. Confirmed hypoglycemic events were defined as episodes of SMPG of <3.1 mmol/L (<56 mg/dL) or severe episodes requiring assistance. Hypoglycemic episodes occurring between 12:01 a.m. and 05:59 a.m. (inclusive) were classified as nocturnal. Laboratory analyses were performed by Quintiles Central Laboratories (Marietta, Ga.).
Statistical Analysis
Only endpoints derived after 16 weeks of randomized treatment (i.e., at the end of each treatment period) were analyzed statistically. Unless otherwise specified, missing values (including intermittent missing values) were imputed using the last-observation-carried-forward method. Only values obtained after the first 8 weeks in each treatment period were carried forward to avoid carryover effects. Endpoints derived in the first treatment period were not carried over to the second treatment period. All endpoints were summarized descriptively at each visit using observed data.
Mean estimated treatment differences (ETDs, or ratios) were calculated together with two-sided 95% confidence intervals (CIs) for all endpoints analyzed statistically. Noninferiority was considered confirmed if the upper bound of the two-sided 95% CI for the mean A1C ETD was ≤0.4%. A linear mixed model with period and treatment as fixed effects and subject as a random effect was used for statistical modeling of A1C, FPG, and PRO measures. The number of hypoglycemic events was analyzed using a negative binomial regression model with a log-link and logarithm of the exposure time (100 patient-years) as offset, including the same fixed effects and random effect as for the primary endpoint. Sample size was determined using a t statistic assuming a one-sided test of size 2.5% and a mean ETD of D = 0%. Based on prior experience, an estimate for the standard deviation (SD) of 1.0% for A1C was used in the sample size calculation, which was performed using SAS 9.1.3 software (SAS Institute, Cary, N.C.). The minimum sample size required to meet the primary objective with at least 85% power was 144 patients in the per-protocol set. All endpoint analyses were based on the full analysis set (all randomized subjects). The primary analysis was repeated using the per-protocol analysis set. Safety endpoints were summarized using the safety analysis set (all participants receiving at least one dose of IDeg U200 or IGlar U100).
Results
Patient Characteristics
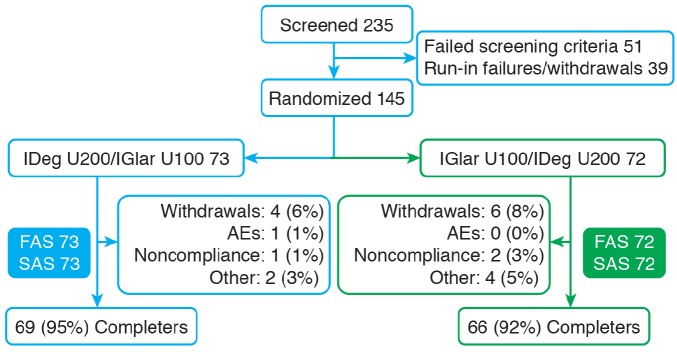
The patient disposition is illustrated in Figure 1. Following randomization, the patient demographics and characteristics for each treatment sequence were highly comparable (Table 1).
FIGURE 1.
Patient disposition throughout the trial. FAS, full analysis set; SAS, safety analysis set.
TABLE 1.
Patient Baseline Characteristics
| Characteristic | IDeg U200/IGlar U100 Full Analysis Set(n = 73) | IGlar U100/IDeg U200Full Analysis Set(n = 72) |
|---|---|---|
| Female/male (%) | 42.5/57.5 | 33.3/66.7 |
| Race: white/black/Asian/other (%) | 91.8/6.8/1.4/0.0 | 86.1/12.5/1.4/0.0 |
| Ethnicity: Hispanic or Latin American (%) | 39.7 | 45.8 |
| Age (years) | 54.7 ± 10.2 | 55.8 ± 9.0 |
| Weight (lb) | 234.8 ± 52.6 | 229.1 ± 52.2 |
| BMI (kg/m2) | 36.9 ± 6.7 | 35.4 ± 6.6 |
| Duration of diabetes (years) | 12.1 ± 6.7 | 12.1 ± 7.9 |
| A1C (%) | 8.0 ± 1.1 | 8.3 ± 1.4 |
| A1C (mmol/mol)* | 63.9 | 67.2 |
| FPG (mg/dL) | 135.6 ± 58.6 | 153.3 ± 74.6 |
Values are mean ± SD unless otherwise stated. *Calculated, not measured.
Efficacy Endpoints
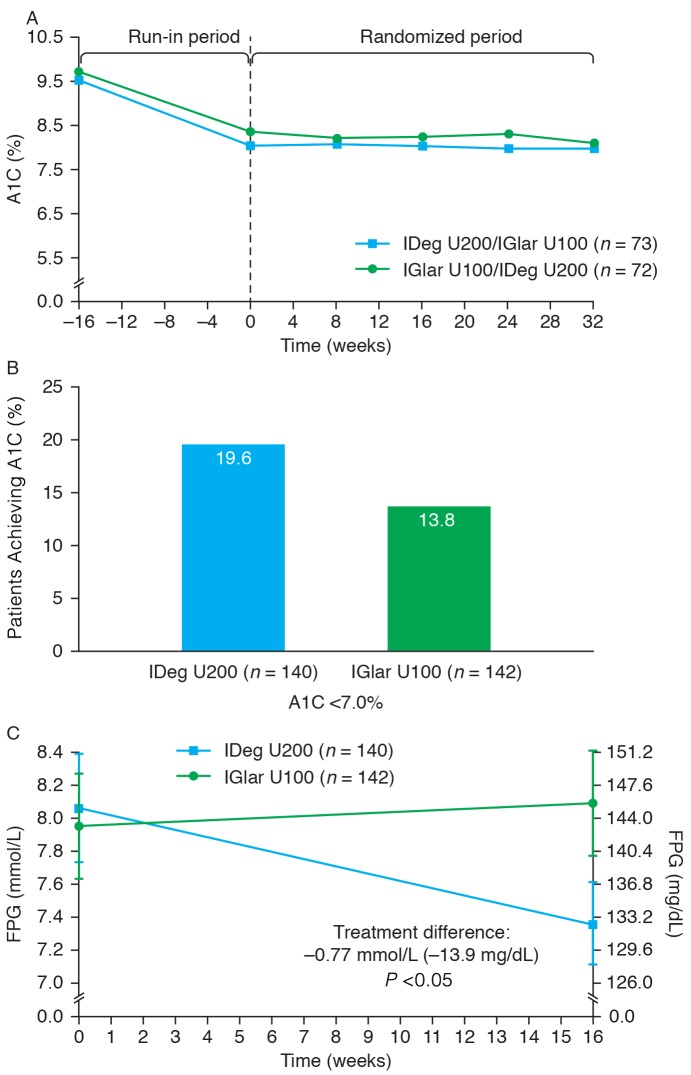
For both treatment sequences, the largest reduction in A1C occurred during the run-in period, as would be predicted from the treat-to-target protocol. After 16 weeks of treatment, the primary endpoint was met, and noninferiority was confirmed (mean ETD [IDeg U200–IGlar U100] −0.06%, 95% CI −0.21 to 0.09) (Figure 2A). Sensitivity analyses supported the primary analysis (data not shown). The mean reduction in A1C was −0.12% with IDeg U200 and −0.06% with IGlar U100. At 16 weeks, 19.6 and 13.8% of patients on IDeg U200 and IGlar U100, respectively, achieved an A1C <7.0% (Figure 2B). The mean change in FPG from baseline to 16 weeks was significantly greater for patients treated with IDeg U200 (−0.82 mmol/L) than with IGlar U100 (−0.05 mmol/L), with an ETD (IDeg U200–IGlar U100) of −0.77 mmol/L (95% CI −1.39 to −0.15, P <0.05) (Figure 2C).
FIGURE 2.
Change in A1C over time (A), proportion of patients achieving target A1C <7.0% (B), and change in FPG over time (C).
Patient-Reported Outcomes
Patient HRQoL was evaluated using the Short Form 36v2 (SF-36v2) questionnaire (17), and insulin device satisfaction was measured using the Treatment-Related Impact Measure–Diabetes Device (TRIM-DD), which comprises eight items covering device functioning (including ease of learning to use the device) and device bother (including physical discomfort when using the device, size, and use in public) (18). Preferred treatment was assessed using categorical answers.
After 16 weeks, there were no statistically significant differences between IDeg U200 and IGlar U100 in any of the SF-36v2 questionnaire domains or summary scores (data not shown). At baseline, TRIM-DD scores for both devices were high (mean range 76.1–78.0 on a 0–100 scale, with higher scores indicating a higher device satisfaction). However, significantly more participants reported less device bother with the IDeg U200 FlexTouch than with the IGlar SoloSTAR pen (ETD 6.01 [95% CI 2.23–9.78], P <0.05) (Table 2). Similarly, the IDeg U200 device scored significantly higher than the IGlar U100 device with regard to device function (ETD 8.40 [95% CI 5.15–11.65], P <0.05) (Table 2), resulting in a significantly higher overall score in favor of the IDeg U200 device compared with the IGlar U100 device (ETD 7.50 [95% CI 4.79–10.21], P <0.05) (Table 2). At the end of the trial, based on their experience of both treatments, more patients (54.5%) preferred IDeg U200 compared with IGlar U100 (20.0%); 15.9% had no preference, and 2.8% did not know which device they preferred.
TABLE 2.
Patient-Reported Outcomes: TRIM-DD
| IDeg U200 (n = 140) | IGlar U100 (n = 142) | ETD (95% CI), P | |
|---|---|---|---|
| Device function (score) | |||
| Baseline | 76.1 | 76.8 | 8.40 (5.15–11.65), <0.05 |
| Week 16 | 86.1 | 77.9 | |
| Device bother (score) | |||
| Baseline | 78.0 | 77.7 | 6.01 (2.23–9.78), <0.05 |
| Week 16 | 90.9 | 84.9 | |
| Total (score) | |||
| Baseline | 76.8 | 77.2 | 7.50 (4.79–10.21), <0.05 |
| Week 16 | 87.9 | 80.5 | |
Device function comprises five items: using the device, functioning of the device, ease in learning how to use the device, delivery of the correct full dose of medication, and adjustment of medication for small dose changes. Device bother comprises three items: physical discomfort related to use of the device, using the device in public, and size of the device (18).
Safety Endpoints
The incidence and relative rates of hypoglycemia for this trial are summarized in Table 3. Four patients experienced severe hypoglycemia with IDeg U200 and one with IGlar U100. Overall, the rates of severe, confirmed, and nocturnal confirmed hypoglycemia were low for both IDeg U200 and IGlar U100. The estimated mean rate of confirmed hypoglycemia was statistically significantly lower with IDeg U200 than with IGlar U100 (estimated rate ratio 0.594 [95% CI 0.391–0.901], P <0.05). Nocturnal confirmed hypoglycemia rates were numerically lower but not statistically different for IDeg U200 compared with IGlar U100 (Table 3).
TABLE 3.
Summary of Hypoglycemia Data
| IDeg U200 (n = 140) |
IGlar U100 (n = 142) |
IDeg U200 Versus IGlar U100 |
||||
|---|---|---|---|---|---|---|
| Incidence(% [n]) | Episodes/PYE (n) | Incidence(% [n]) | Episodes/PYE (n) | Rate Ratio | 95% CI | |
| Severe | 2.9 (4) | 0.12 | 0.7 (1) | 0.02 | 5.12 | 0.492–53.14 |
| Confirmed | 26.4 (37) | 1.92 | 36.6 (52) | 2.88 | 0.59* | 0.391–0.901 |
| Nocturnal confirmed | 9.3 (13) | 0.38 | 11.3 (16) | 0.63 | 0.66 | 0.290–1.480 |
P <0.05, safety analysis set. None of the severe hypoglycemic episodes led to withdrawal or were linked to other AEs. Only one severe hypoglycemic episode was reported as serious; it occurred during the follow-up period (3 days after last drug date [IDeg]). According to the patient’s wife, the patient had not eaten that day. The patient was treated orally with orange juice by his wife and recovered on the same day. PYE, patient-year of exposure.
The observed mean change in body weight from baseline was numerically smaller with IDeg U200 than with IGlar U100 (0.4 vs. 1.0 kg) but did not reach statistical significance (−0.62 kg [95% CI −1.25 to 0.01]). Mean insulin doses were comparable between IDeg U200 and IGlar U100 after 16 weeks (157.3 vs. 152.2 units), with 50.4 and 94.1% of patients, respectively, needing to split their dose into multiple injections. Of those who needed to split their dose, more patients administered four or more injections with IGlar U100 than with IDeg U200 (10.3 vs. 2.2%). The average number of injections was lower for IDeg U200 than for IGlar U100 at both week 4 (IDeg U200 1.38 vs. IGlar U100 2.27) and week 16 (IDeg U200 1.62 vs. IGlar U100 2.40).
AE rates were comparable for IDeg U200 and IGlar U100 (event rate per 100 patient-years of exposure 246 vs. 260, respectively). The most common AE was nasopharyn-gitis, and the majority of treatment-emergent AEs were mild or moderate in severity. There were no deaths in this trial, and the event rate for severe AEs was similar between treatments.
Discussion
The IDeg U200 formulation and device provides the same number of units of insulin as IGlar 100 units/mL in a smaller volume, postulated to offer particular benefits to patients requiring high doses of insulin by reducing the burden imposed by multiple injections. This trial aimed to confirm the noninferiority of IDeg U200 compared with IGlar U100 in providing glycemic control and to investigate patient satisfaction and HRQoL for people with type 2 diabetes requiring high insulin doses.
After 16 weeks of treatment, noninferiority was confirmed with respect to change in A1C from baseline. A significantly greater reduction in FPG was seen with IDeg U200 than with IGlar U100, a trend previously observed in phase 3a trials with both IDeg formulations (100 and 200 units/mL) compared with IGlar U100 (4,6,8,12,19). Moreover, although HRQoL did not differ according to treatment as measured by the SF-36v2, patients reported greater treatment satisfaction with IDeg U200 than with IGlar U100. This was true both in terms of significantly higher ratings for device function and less device bother for the IDeg U200 pen compared with the IGlar U100 pen and in terms of a higher proportion of patients preferring treatment with IDeg U200 than with IGlar U100.
Injection frequency is a known barrier to adherence that has a negative effect on patient HRQoL (2,3); therefore, by reducing the number of injections required, IDeg U200 may result in a lower burden of disease. In terms of safety parameters, the rate of confirmed hypoglycemia was significantly lower, and the rate of nocturnal confirmed hypoglycemia was numerically lower with IDeg U200 than with IGlar U100. A previous study demonstrated a similar trend toward a reduction in nocturnal hypoglycemia with IDeg U200 compared with IGlar U100 (12). Patients also experienced less weight gain with IDeg U200 than with IGlar U100, although the difference failed to reach statistical significance.
Mean insulin doses were similar between treatments after 16 weeks; however, although patients were able to reduce their A1C levels without significant hypoglycemia, not all were able to reach the American Diabetes Association target of 7%. This reveals the limits of basal insulin alone and demonstrates that, for many patients with longstanding disease, further treatment intensification may be necessary. However, this trial reflects the real-world setting in which many patients are on high basal insulin doses without intensification and re-affirms that more proactive intensification is required to achieve glycemic goals. No safety issues were identified with IDeg U200, and there were no differences between IDeg U200 and IGlar U100 with respect to AEs, withdrawal rates, or standard laboratory safety parameters.
The main strength of this trial lies in its randomized, crossover design. However, the necessity of an open-label trial could have introduced bias. This was addressed by minimizing participants’ familiarity with insulin pen devices through exclusive en-rollment of patients who were only familiar with IGlar vials.
Conclusion
This trial confirms the noninferiority of IDeg U200 to IGlar U100 in terms of reduction in A1C from baseline. In addition, IDeg U200 significantly reduced confirmed hypoglycemia compared with IGlar U100, and PRO measures indicate that IDeg U200 may be preferred to IGlar U100 by patients with type 2 diabetes requiring high-dose insulin.
Acknowledgments
The authors thank the trial investigators, staff, and participants. They also thank Watermeadow Medical, funded by Novo Nordisk, for providing medical writing and editorial assistance. This study (ClinicalTrials.gov number NCT01513473) was funded by Novo Nordisk A/S. Data from this study were presented as a poster (Abstract 1040-P) at the American Diabetes Association’s 75th Scientific Sessions, 5–9 June 2015, in Boston, Mass.
Duality of Interest
Dr. Warren has participated on advisory boards for Eli Lilly and Novo Nordisk and in speakers’ bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Vivus. He has also received research support from Boehringer Ingelheim, Eli Lilly, Forest Laboratories, Janssen, Merck Sharp & Dohme, Mylan, Novo Nordisk, NPS Pharmaceuticals, Pfizer, Sanofi, Takeda, and VPI. Dr. Chaykin has participated in a speaker’s bureau for Novo Nordisk. Dr. Jabbour has served on advisory panels and as a consultant for AstraZeneca, Eli Lilly, and Janssen. Dr. Sheikh-Ali has received research support from Eli Lilly, Hanmi, and Novo Nordisk. Dr. Hansen and Mr. Nielsen are employed by and own stocks/shares in Novo Nordisk A/S. Dr. Norwood has received research support from Eli Lilly, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 2.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005;20:479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care 2010;33:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinman B, Philis-Tsimikas A, Cariou B, et al. ; NN1250-3579 (BEGIN Once Long) Trial Investigators. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care 2012;35:2464–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garber AJ, King AB, Del Prato S, et al. ; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-long acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes. Lancet 2012;379:1498–1507 [DOI] [PubMed] [Google Scholar]
- 6.Meneghini L, Atkin SL, Gough SC, et al. ; NN1250-3668 (BEGIN FLEX) Trial Investigators. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomised, open-label, parallel-group, treat to target trial in individuals with type 2 diabetes. Diabetes Care 2013;36:858−864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onishi Y, Iwamoto Y, Yoo SJ, Clauson P, Tamer SC, Park S. Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, Pan-Asian, treat-to-target trial. J Diabetes Invest 2013;4:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodbard HW, Cariou B, Zinman B, et al. ; BEGIN Once Long trial investigators. Comparison of insulin degludec with insulin glargine in insulin-naïve subjects with type 2 diabetes: a 2-year randomized, treat-to-target trial. Diabet Med 2013;30:1298–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratner RE, Gough SC, Mathieu C, et al. . Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab 2013;15:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsatko S, Deller S, Zahiragic S, et al. . Ultra-long-acting insulin degludec: two different formulations (U100 and 200 U/mL) are bioequivalent and show similar pharmacodynamics (Abstract 2349-PO). Diabetes 2011;60(Suppl. 1):A624 [Google Scholar]
- 11.U.S. Department of Health and Human Services , U.S. Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. Draft Guidance. February 2008. Available from http://www.fda.gov/downloads/Drugs/Guidance ComplianceRegulatoryInformation/Guidances/ucm071624.pdf. Accessed 10 July 2015 [Google Scholar]
- 12.Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Diabetes Care 2013;36:2536−2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Last amended by the 59th WMA General Assembly, Seoul, Korea, 2008. Available from: http://www.wma.net/en/30publications/10policies/b3/. Accessed 10 July 2015 [Google Scholar]
- 14.International Conference on Harmonisation ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1), Step 4. 10–6-1996. Available from https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 10 July 2015
- 15.Spilker B. Guide to Clinical Studies and Developing Protocols. New York, N.Y, Raven Press, 1984 [Google Scholar]
- 16.Datapharm. eMC : Tresiba 200 units/mL Pre-filled Pen (FlexTouch) summary of product characteristics. Available from http://www.medicines.org.uk/emc/medicine/27363/SPC/Tresiba+200+units+mL+Pre-filled+Pen+(FlexTouch). Accessed 10 July 2015
- 17.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s Manual for the SF-36v2 Health Survey. 2nd ed. Lincoln, R.I., QualityMetric Incorporated, 2007 [Google Scholar]
- 18.Brod M, Hammer M, Christensen T, Lessard S, Bushnell DM. Understanding and assessing the impact of treatment in diabetes: the Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device). Health Qual Life Outcomes 2009;7:83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieu C, Hollander P, Miranda-Palma B, et al. ; NN1250-3770 (BEGIN: Flex T1) Trial Investigators. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab 2013;98:1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]