Abstract
Background
Adult leg length is influenced by nutrition in the first few years of life. Adult head circumference is an indicator of brain growth. There is a limited literature linking short legs and small skulls to an increased risk for cognitive impairment and dementia in late life.
Methods
One phase cross-sectional surveys of all over 65 year old residents (n=14,960) in 11 catchment areas in China, India, Cuba, Dominican Republic, Venezuela, Mexico and Peru. The cross-culturally validated 10/66 dementia diagnosis, and a sociodemographic and risk factor questionnaire were administered to all participants, and anthropometric measures taken. Poisson regression was used to calculate prevalence ratios for the effect of leg length and skull circumference upon 10/66 Dementia, controlling for age, gender, education and family history of dementia.
Results
The pooled meta-analysed fixed effect for leg length (highest vs. lowest quarter) was 0.82 (95% CI, 0.68-0.98) and for skull circumference 0.75 (95% CI, 0.63-0.89). While point estimates varied between sites, the proportion of the variability attributable to heterogeneity between studies as opposed to sampling error (I2) was 0% for leg length and 22% for skull circumference. The effects were independent and not mediated by family history of dementia. The effect of skull circumference was not modified by educational level or gender, and the effect of leg length was not modified by gender.
Conclusions
Since leg length and skull circumference are said to remain stable throughout adulthood into old age, reverse causality is an unlikely explanation for the findings. Early life nutritional programming, as well as neurodevelopment may protect against neurodegeneration.
Keywords: Anthropometry, Growth and Development, Aged, Developing Countries, Etiology
Background
For dementia, as for other late-life chronic diseases, there is growing awareness that risk can accumulate across the life course, and that there may be critical periods for the operation of particular risk factors including early in life (Whalley et al., 2006). Much of the interest has focussed upon the possible relevance to dementia of the foetal and developmental origins of adult disease hypotheses, with programming by undernutrition in very early life exacerbated by overnutrition in later life leading to increased risk, mediated through cardiovascular and metabolic disease (Whalley et al., 2006;Miller and O'Callaghan, 2008). Factors influencing neurodevelopment may also be implicated. Limited evidence, from just two long-term historical cohort studies, supports a protective effect of intellectual development in the early years of life against the later onset of both dementia (Whalley et al., 2000;Riley et al., 2005) and neurodegeneration (Riley et al., 2005). Such data, spanning the life course, are hard to come by, but leg length and skull circumference measured in late-life may provide useful information about the nutritional environment in early life, and brain development, respectively.
Skull circumference and dementia
Brains and skull vaults grow rapidly, with 95% of growth achieved by age six (Lenroot and Giedd, 2006). Up to this age there is a near perfect correlation between skull circumference and brain size (Bartholomeusz et al., 2002). Maximal brain proportions are reached at age 11.5 years in girls and 14.5 years in boys (Lenroot and Giedd, 2006). Thereafter, overall brain size decreases while skull dimensions remain constant. Thus, skull dimensions can be considered to be a long-term and stable marker of early-life brain size. In the USA, skull circumference was inversely associated with progression of Alzheimer’s Disease (AD) and with the extent of cognitive impairment in an older community sample (Reynolds et al., 1999). Five cross-sectional studies have reported an inverse association between skull circumference and prevalent AD: three community-based studies in USA (Schofield et al., 1997), Brazil (Scazufca et al., 2008), and Korea (Kim et al., 2008), and two on communities of Catholic nuns in the US (Mortimer et al., 2003) and Germany (Bickel et al., 2006). There are two published negative reports (Jenkins et al., 2000;Edland et al., 2002), both of which were small case-control studies with cases recruited from clinical services, and for which the exposure was intracranial volume measured using MRI. Only one cohort study has been carried out, in which an inverse association was reported with incident Alzheimer’s Disease among Japanese Americans (Borenstein et al., 2001). Taken together, these findings suggest a robust and independent association. In the US, the association was concentrated in the lowest fifth of skull circumference, was independent of age, height, gender, education and APOE status, and seemed not to have been accounted for either by pre-morbid intelligence or weight loss secondary to dementia (Schofield et al., 1997). In Korea the effect was independent of age, education and rural residence (Kim et al., 2008). In the US Nun’s study there was evidence to support an a priori hypothesis of an interaction between head circumference and education (Mortimer et al., 2003). Other reports of effect modification; that the effect was limited to or more pronounced in women (Kim et al., 2008), or to those with one or more APOE e4 alleles (Bickel et al., 2006;Borenstein et al., 2001) were post hoc and not supported by formal tests for interaction.
Leg length and dementia
In childhood, leg length is more strongly associated with economic conditions than trunk length (Gunnell et al., 1998). Recent findings from a British cohort study, suggest that adult leg length is particularly sensitive to diet in infancy (under 5 years), specifically breast feeding and energy intake at age four (independent of birth weight) with trunk length more associated with factors operating over longer periods between infancy and puberty, such as childhood serious illness and parental separation (Wadsworth et al., 2002). Increasing population height (assumed to be secondary to improved nutrition in childhood) is also principally accounted for by increasing leg length rather than symmetrical increases in leg and trunk length (Tanner et al., 1982). Leg length was inversely cross-sectionally related to dementia prevalence in Brazil (Scazufca et al., 2008), among women in a population-based study in Korea (Kim et al., 2003), and with cognitive impairment among African Caribbean migrants in the UK (Mak et al., 2006). Knee height was inversely associated with incident dementia among women in the USA (Huang et al., 2008); this was a post hoc finding, and the interaction between gender and knee length was not statistically significant. In a later wave of the Korean study, the interaction between gender and leg length was tested as an a priori hypothesis and found to be statistically significant; the effect of leg length was also found to be independent of skull circumference (Kim et al., 2008).
The 10/66 Dementia Research Group programme of population-based surveys in 17 sites in 12 low and middle income countries (LAMIC) in Latin America, Africa, India and China will provide a unique resource for comparative studies of the aetiology, as well as the prevalence, impact and cost of dementia (Prince et al., 2007). We have already published findings from one 10/66 DRG centre, Cuba, where skull circumference, but not leg length was independently inversely associated with dementia prevalence (Llibre et al., 2008). The aims of the current analyses are
to test the hypotheses that longer leg lengths and larger skull circumferences are associated with a lower prevalence of dementia in large, representative population-based surveys, checking for consistency of findings across a wide variety of cultural settings in Latin America, India and China
- to assess the specificity of the effects of leg length and skull circumference and to explore potential mechanisms, specifically
- whether the effect of leg length is accounted for by skull circumference (suggesting a specific neurodevelopmental rather than a general nutritional programming effect)
- whether the effect of skull circumference is accounted for by leg length or total height (nutritional programming or overall skeletal frame, rather than a neurodevelopmental effect)
- whether the effects of leg length and skull circumference are accounted for by family history of dementia (suggesting a shared genetic predisposition)
- to test for effect modification, with a priori hypotheses based upon previously observed associations
- is the effect of leg length modified by gender (Kim et al., 2003;Kim et al., 2008;Huang et al., 2008) (stronger in women)?
- is the effect of skull circumference modified by education (Mortimer et al., 2003) (stronger among the least educated), or gender (Kim et al., 2008) (stronger in women)?
Methods
The full 10/66 population-based study protocols have already been published in an open access journal (Prince et al., 2007). Details of specific relevance to this analysis are provided here.
Settings and study design
Cross-sectional comprehensive one phase surveys were conducted of all residents aged 65 and over in 11 geographically defined catchment area sites in seven LAMIC (India, China, Cuba, Dominican Republic, Venezuela, Mexico and Peru). After door-knocking to establish eligibility (age and residence), those consenting to participate received the full 2-3 hour assessment, comprising participant and informant interviews, physical examination, and phlebotomy. Interviews were carried out in participants’ homes. The target sample size for each country was 2000 to 3000 (see Table 1). China, India, Peru and Mexico split recruitment between urban and rural sites; other countries included urban sites only. A sample of 2,000 would allow estimation of a typical dementia prevalence of 4.5% with a precision of +/-0.9%. Rural and urban samples of 1,000 each would provide precision of +/-1.2%. All studies were approved by local ethical committees and the King’s College London Research Ethics Committee. Participation was on the basis of signed informed consent.
Table 1.
Sample characteristics, by site
| Demographic variables | Cuba N (%) |
DR N (%) |
Peru (Urban) N (%) |
Peru (Rural) N (%) |
Venezuela N (%) |
Mexico (Urban) N (%) |
Mexico (Rural) N (%) |
China (Urban) N (%) |
China (Rural) N (%) |
India (Urban) N (%) |
India (Rural) N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 65-69 years | 760 (25.9) | 533 (26.5) | 375 (27.2) | 179 (32.4) | 813 (42.7) | 245 (24.4) | 299 (29.9) | 316 (27.2) | 383 (38.2) | 415 (41.5) | 331 (33.1) |
| 70-74 years | 789 (26.9) | 520 (25.9) | 25.5 (52.7) | 141 (25.5) | 461 (24.2) | 329 (32.8) | 252 (25.2) | 362 (31.2) | 296 (29.5) | 318 (31.8) | 350 (35.0) |
| 75-79 years | 639 (21.8) | 397 (19.7) | 298 (21.6) | 101 (18.3) | 340 (17.9) | 205 (20.5) | 221 (22.1) | 254 (21.9) | 202 (20.2) | 144 (14.4) | 177 (17.7) |
| 80+ years | 749 (25.5) | 561 (27.9) | 355 (25.7) | 131 (23.7) | 290 (15.2) | 223 (22.3) | 228 (22.8) | 228 (19.7) | 121 (12.1) | 124 (12.4) | 141 (14.1) |
| Missing values | 7 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 |
| Female gender | 1913 (65.0) | 1325 (66.0) | 888 (64.3) | 295 (53.4) | 1215 (63.8) | 666 (66.4) | 602 (60.2) | 661 (57.0) | 556 (55.5) | 571 (57.7) | 545 (54.5) |
| Missing values | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 |
| Education | |||||||||||
| None | 75 (2.5) | 392 (19.7) | 37 (2.7) | 84 (15.4) | 154 (8.1) | 227 (22.6) | 327 (32.7) | 232 (20.0) | 579 (57.8) | 428 (42.7) | 660 (66.1) |
| Minimal | 655 (22.3) | 1022 (51.3) | 90 (6.5) | 141 (25.9) | 438 (23.1) | 354 (35.3) | 510 (51.0) | 153 (13.2) | 114 (11.4) | 234 (23.3) | 195 (19.5) |
| Completed primary | 979 (33.3) | 370 (18.6) | 460 (33.5) | 267 (49.1) | 950 (50.1) | 229 (22.8) | 122 (12.2) | 303 (26.1) | 259 (25.8) | 212 (21.1) | 116 (11.6) |
| Completed secondary | 728 (24.8) | 135 (6.8) | 481 (35.0) | 36 (6.6) | 263 (13.9) | 99 (9.9) | 25 (2.5) | 335 (28.9) | 45 (4.5) | 87 (8.7) | 26 (2.6) |
| Tertiary | 499 (17.0) | 73 (3.7) | 305 (22.2) | 16 (2.9) | 92 (4.8) | 94 (9.4) | 16 (1.6) | 137 (11.8) | 5 (0.5) | 42 (4.2) | 2 (0.2) |
| Missing values | 8 | 19 | 8 | 8 | 7 | 0 | 0 | 0 | 0 | 2 | 0 |
| Dementia |
Cuba N (%) |
DR N (%) |
Peru (Urban) N (%) |
Peru (Rural) N (%) |
Venezuela N (%) |
Mexico (Urban) N (%) |
Mexico (Rural) N (%) |
China (Urban) N (%) |
China (Rural) N (%) |
India (Urban) N (%) |
India (Rural) N (%) |
| Family history of dementia | 525 (17.8) | 187 (9.3) | 132 (9.6) | 19 (3.4) | 157 (8.4) | 40 (4.0) | 41 (4.1) | 5 (0.4) | 1 (0.1) | 3 (0.3) | 11 (1.1) |
| Missing values | 10 | 4 | 8 | 1 | 52 | 0 | 0 | 0 | 0 | 2 | 0 |
| 10/66 Dementia | 315 (10.8) | 235 (11.7) | 128 (9.3) | 36 (6.5) | 109 (5.7) | 85 (8.5) | 85 (8.5) | 81 (7.0) | 56 (5.6) | 75 (7.5) | 106 (10.6) |
| Missing values | 20 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 |
| Anthropometric indices |
Cuba Mean (SD) |
DR Mean (SD) |
Peru (Urban) Mean (SD) |
Peru (Rural) Mean(SD) |
Venezuela Mean (SD) |
Mexico (Urban) Mean(SD) |
Mexico (Rural) Mean(SD) |
China (Urban) Mean (SD) |
China (Rural) Mean(SD) |
India (Urban) Mean(SD) |
India (Rural) Mean (SD) |
| Leg length | 85.3 (7.4) | 87.1 (6.9) | 87.2 (7.7) | 84.2 (7.1) | 86.7 (8.8) | 85.6 (6.5) | 82.7 (6.1) | 87.9 (7.0) | 86.9(11.4) | 90.8 (6.6) | 90.9 (6.7) |
| Missing values | 131 | 85 | 84 | 19 | 421 | 20 | 19 | 29 | 6 | 51 | 63 |
| Trunk length | 73.5 (9.5) | 72.0 (8.1) | 68.3 (8.4) | 70.2 (9.4) | 71.2 (7.3) | 68.8 (7.7) | 70.0 (7.5) | 72.0 (5.4) | 75.1 (9.3) | 62.2 (7.9) | 63.7 (7.0) |
| Missing values | 159 | 120 | 103 | 32 | 452 | 40 | 53 | 32 | 6 | 83 | 77 |
| Skull | |||||||||||
| circumference | 55.8 (1.9) | 56.3 (2.3) | 55.3 (2.2) | 55.7 (2.0) | 55.2 (2.3) | 55.0 (2.1) | 54.4 (1.9) | 55.2 (2.0) | 55.0 (3.6) | 53.0 (2.3) | 53.6 (1.8) |
| Missing values | 57 | 46 | 31 | 12 | 400 | 20 | 9 | 16 | 16 | 24 | 23 |
| Total N | 2944 | 2011 | 1381 | 552 | 1904 | 1002 | 1000 | 1160 | 1002 | 1005 | 999 |
Protocols and procedures
Each site had a project coordinator and 4-10 interviewers; generally lay graduates, although Cuba and China used medical doctors. Assessments were translated into Ibero-American Spanish (with country-specific adaptations, where necessary), Tamil (India) and Mandarin (China). All centres had already been extensively trained in the main diagnostic assessments (see below) for the dementia diagnostic pilot study (Prince et al., 2003). Further one week project planning meetings were held for all PIs before starting field work. Group meetings for investigators from all sites were held approximately six monthly during the life of the project. All researchers were retrained in study protocol and procedures and structured interviewing techniques, supported by a standardized operating procedure. Field interviews were regularly checked and supervised. The London-based coordinators, CF and MP visited each centre at least twice during the field work phase. In Cuba data was collected directly onto laptop computers using computerized questionnaires driven by EpiData 2.0 software, incorporating conditional skips and range checks. In other sites data was first collected onto paper. Data was extracted into SPSS, and cleaning, processing of derived variables and diagnostic algorithms was carried out using SPSS syntax files. Data consistency was checked regularly in London throughout the data gathering period.
Measures
The 10/66 population-based study interview generates information regarding dementia diagnosis, mental disorders, physical health, anthropometry, demographics, an extensive dementia and chronic diseases risk factor questionnaire, disability, health service utilisation, care arrangements and caregiver strain (Prince et al., 2007). Only the assessments relevant to the current analysis of associations between leg length, skull circumference and dementia prevalence will be described in detail here.
Anthropometric measures
Skull circumference was measured using a cloth tape measure encircling the nuchal tuberosity and the brow. Leg length was measured, standing, from the highest point of the iliac crest to the lateral malleolus. Standing height was also measured, and trunk length was calculated as the difference between standing height and leg length. All dimensions were measured to the nearest centimetre.
Sociodemographic variables
Age was formally determined during interview from stated age, official documentation and informant report, and, if discrepant, age according to an event calendar. We also recorded sex, and level of education (none/ did not complete primary/ completed primary/ secondary/ tertiary).
Dementia
10/66 dementia diagnosis was allocated to those scoring above a cutpoint of predicted probability for dementia, calculated using coefficients derived from a logistic regression equation developed, calibrated and validated cross-culturally in the 25 centre 10/66 pilot study (Prince et al., 2003). This diagnosis, the outcome for this study, is hereafter referred to as ‘10/66 dementia’. The coefficients are linked to the outputs from a) a structured clinical mental state interview, the Geriatric Mental State (Copeland et al., 1986), b) two cognitive tests; the Community Screening Instrument for Dementia (CSI’D’) COGSCORE (Hall et al., 1993) and the modified CERAD 10 word list learning task with delayed recall (Ganguli M. et al., 1996), and c) informant reports of cognitive and functional decline from the CSI’D’ RELSCORE (Hall et al., 1993). In the pilot study, this algorithm developed on one half of the sample and tested on the other, identified dementia with a sensitivity of 94% and specificity of 97% in high education and 94% in low education controls (Prince et al., 2003). The cross-culturally developed and validated 10/66 dementia diagnosis algorithm proved to be more sensitive to mild and moderate cases than the DSM-IV criterion in Cuba (Prince et al., 2008), and our study of prospective validity in India confirmed a relative lack of sensitivity of the DSM-IV criterion in India (Joteeswaran et al., 2010). The prevalence of 10/66 dementia in all sites has already been published (Llibre Rodriguez et al., 2008). The severity of dementia (questionable/ mild/ moderate/ severe) was assessed in all participants according to the Clinical Dementia Rating (CDR).
Analyses
We used release 1.7 of the 10/66 data archive (February 2008) and STATA version 9.2 for all analyses.
For each site, we have
described the participants’ characteristics; age, sex, educational level, 10/66 dementia diagnosis, and mean leg length, trunk length and skull circumference
assessed the independent associations of age, sex and education with leg length and skull circumference using general linear modeling, reporting beta coefficients and R2 (the % proportion of variance in the outcome explained by the covariate), and the bivariate association between leg length and skull circumference using Spearman correlations.
modeled the effects of leg length (quarters) and skull circumference (quarters) on 10/66 dementia prevalence, controlling for age, gender, education and family history of dementia, providing mutually adjusted prevalence ratios derived from a Poisson working model with robust 95% confidence intervals adjusted for household clustering. We fitted the model separately for each site and then used a fixed effects meta-analysis to combine them, estimating the degree of heterogeneity using Higgins’ I2 (Higgins and Thompson, 2002) with approximate 95% confidence intervals.
tested for effect modification of the effects of leg length on 10/66 dementia by gender and of skull circumference upon 10/66 dementia by gender and education, by extending the models described in 3. above by the appropriate interaction terms.
tested for the specificity of the associations between a) leg length and 10/66 dementia, by controlling additionally for skull circumference and trunk length, and b) skull circumference and 10/66 dementia, by controlling additionally for leg length and overall height.
Results
Sample characteristics
In all, 14,960 interviews were completed in the 11 sites in seven countries. Response proportions varied between 72% and 98%, and were 80% or higher in all but two sites (urban China and urban India) (Llibre Rodriguez et al., 2008). Demographic ageing was more advanced in the Latin American centres and in urban China, compared with rural China and India (Table 1). Educational levels were highest in the urban sites in Cuba, Peru and Venezuela. In the Dominican Republic, rural Peru and Mexico a fifth to a third lack any education, whilst in rural China and India having no education was the norm. Women participants predominated over men in all sites. The prevalence of 10/66 dementia varied between 5.6% and 11.7% by site. There was significant between centre variation (see Table 1) in skull circumference (smaller skulls in Indian centres; F=226.6, p<0.001), leg length (shorter legs in rural Latin American sites, and longer legs in Indian sites; F=106.3, p<0.001) and trunk length (shorter trunks in India; F=256.0, p<0.001). Study site accounted for 13.7% of the variance in skull circumference, 7.0% of the variance in leg length and 15.6% of the variance in trunk lengths.
Correlates of skull circumference and leg length
Both skull circumference and leg length were consistently and significantly associated with gender (Table 2) with men having larger skulls and longer legs in all sites. In most sites, older people had smaller skulls and shorter legs, consistent with a birth cohort effect. This effect was not apparent, or less apparent, in the least developed sites. The effect of education was less consistent, and smaller throughout. However, there was a general tendency for better education to be associated with larger skulls and longer legs. Leg length and skull circumference were significantly correlated in all sites other than rural China, with a median Spearman’s correlation of 0.21 (interquartile range 0.11 to 0.28). A family history of dementia was not associated with either leg length or skull circumference in any site.
Table 2.
Linear regression coefficients and R2(% of the variance explained) for the independent effects of age, gender and education upon skull circumference and leg length, by site
| Site | Parameter | Skull circumference | Leg length | ||||
|---|---|---|---|---|---|---|---|
| Age (per year) | Gender (male vs. female) | Education (per level of education) | Age (per year) | Gender (male vs. female) | Education (per level of education) | ||
| Cuba | Beta coeff. R2 | -0.04 (-0.05 to -0.03) 2.0% |
1.5 (1.3 to 1.6) 13.5% |
-0.01 (-0.08 to 0.05) 0.0% |
-0.09 (-0.13 to -0.05) 0.8% |
2.3 (1.7 to 2.8) 2.1% |
0.86 (0.60 to 1.12) 1.5% |
| DR | Beta coeff. R2 | -0.05 (-0.06 to -0.03) 2.3% |
1.2 (1.0 to1.4) 6.2% |
0.14 (0.03 to 0.24) 0.3% |
-0.11 (-0.15 to -0.07) 1.7% |
5.1 (4.5 to 5.7) 12.4% |
0.23 (-0.07 to 0.53) 0.1% |
| Peru (urban) | Beta coeff. R2 | -0.02 (-0.04 to -0.01) 0.6% |
1.6 (1.4 to 1.9) 12.6% |
0.08 (-0.04 to 0.19) 0.1% |
-0.12 (-0.18 to-0.07) 1.3% |
3.4 (2.5 to 4.2) 4.3% |
0.67 (0.24 to 1.11) 0.7% |
| Peru (rural) | Beta coeff. R2 | -0.05 (-0.07 to-0.02) 2.8% |
1.0 (0.6 to 1.3) 5.9% |
0.19 (0.01 to 0.38) 0.8% |
-0.04 (-0.12 to 0.05) 0.1% |
2.9 (1.6 to 4.2) 3.8% |
0.03 (-0.64 to 0.70) 0.0% |
| Venezuela | Beta coeff. R2 | -0.03 (-0.05 to -0.01) 0.9% |
2.1 (1.8 to 2.3) 18.6% |
0.10 (-0.02 to 0.22) 0.2% |
-0.10 (-0.17 to -0.03) 0.5% |
4.5 (3.6 to 5.4) 5.9% |
0.73 (0.22 to 1.23) 0.5% |
| Mexico (urban) | Beta coeff. R2 | -0.05 (-0.06 to -0.03) 2.3% |
1.9 (1.6 to 2.1) 18.8% |
0.02 (-0.08 to 0.12) 0.0% |
-0.10 (-0.16 to -0.04) 1.2% |
5.3 (4.6 to 6.1) 15.7% |
0.85 (0.54 to 1.16) 2.8% |
| Mexico (rural) | Beta coeff. R2 | -0.02 (-0.04 to 0.00) 0.5% |
0.9 (0.6 to1.1) 5.0% |
0.00 (-0.14 to 0.14) 0.0% |
-0.07 (-0.12 to -0.02) 0.6% |
4.5 (3.8 to 5.3) 13.0% |
0.39 (-0.05 to 0.83) 0.3% |
| China (urban) | Beta coeff. R2 | -0.01 (-0.03 to 0.01) 0.1% |
1.4 (1.2 to1.7) 11.7% |
0.09 (0.00 to 0.18) 0.4% |
-0.17 (-0.23 to -0.11) 3.0% |
7.0 (6.2 to 7.7) 23.2% |
0.51 (0.23 to 0.79) 1.1% |
| China (rural) | Beta coeff. R2 | -0.02 (-0.05 to 0.02) 0.1% |
1.1 (0.6 to 1.6) 1.8% |
0.24 (-0.02 to 0.49) 0.3% |
-0.12 (-0.23 to 0.0) 0.4% |
7.3 (5.7 to 8.7) 8.3% |
0.01 (-0.76 to 0.78) 0.0% |
| India (urban) | Beta coeff. R2 | 0.01 (-0.02 to 0.03) 0.1% |
0.9 (0.6 to 1.2) 3.8% |
0.24 (0.12 to 0.37) 1.5% |
0.01 (-0.06 to 0.08) 0.0% |
3.6 (2.7 to 4.5) 6.3% |
-0.08 (-0.46 to 0.30) 0.0% |
| India (rural) | Beta coeff. R2 | -0.03 (-0.05 to 0.00) 0.8% |
1.0 (0.7 to 1.2) 5.8% |
0.06 (-0.09 to 0.21) 0.1% |
-0.05 (-0.12 to 0.01) 0.3% |
6.4 (5.5 to 7.2) 17.7% |
0.49 (-0.05 to 1.02) 0.3% |
Associations of skull circumference and leg length with dementia
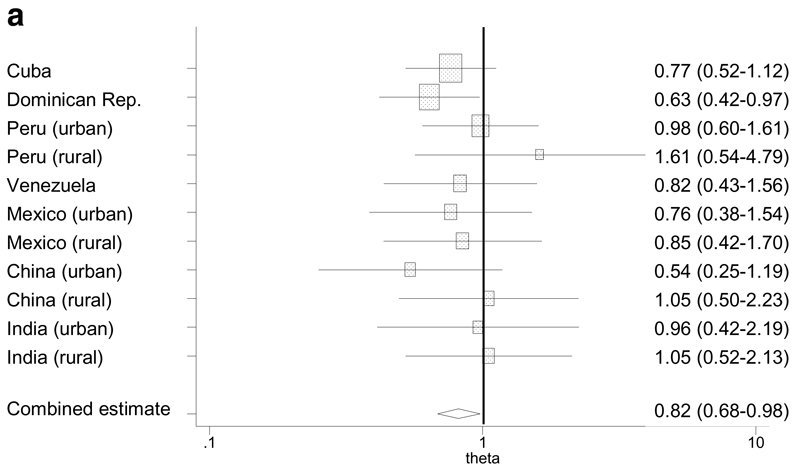
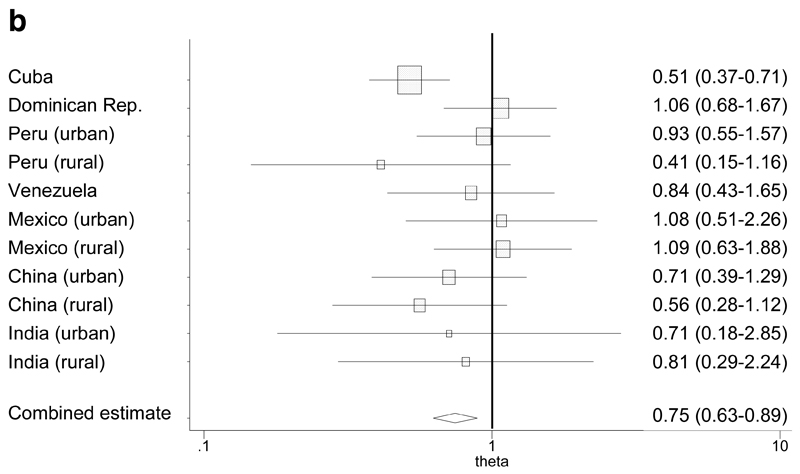
We modeled the effect of a) leg length (quarters) and b) skull circumference (quarters) upon prevalence of 10/66 dementia, controlling for the potential confounding effects of age, gender, education and family history of dementia. The pattern of association varied between sites, and, in most sites, was neither strictly linear nor indeed monotonic in form (Table 3). Statistically significant inverse linear trends were only apparent for the effect of leg length in the Dominican Republic, and for skull circumference in Cuba and rural Peru. For ease of interpretation, and to synthesise findings across sites in a meaningful way allowing for the variability in forms of association across sites, we present the findings from the multivariate Poisson regressions as meta-analytical forest plots of prevalence ratios comparing a) the quarter with the longest legs with the quarter with the shortest legs (Figure 1a), and b) the quarter with the largest skulls with the quarter with the smallest skulls (Figure 1b). Although the effect of leg length was statistically significant only in the Dominican Republic, and the effect of skull circumference was statistically significant only in Cuba, the formal test for heterogeneity of estimates was not statistically significant for either leg length (Q=5.8, 10 df, p=0.83; Higgins I2 = 0%, 95% CI, 0-60%) or skull circumference (Q=12.9, 10 df, p=0.23; Higgins I2 = 22%, 95% CI, 0-61%). The pooled fixed effect comparing the quarter with the longest legs with the quarter with the shortest legs was 0.82 (95% CI, 0.68-0.98). This estimate changed very little when controlling also for skull circumference, but the protective effect strengthened after adjusting for trunk length (Table 4). The pooled fixed effect comparing the quarter with the largest skulls with the quarter with the smallest skulls was 0.75 (95% CI, 0.63-0.89). This attenuated somewhat when controlling also for leg length, but changed very little when controlling also for height (Table 4). None of the interaction terms tested; leg length by gender, skull circumference by gender and skull circumference by education; was statistically significant, either in any site, or when summarized as pooled fixed effect estimates (Table 4). As a sensitivity analysis, we also meta-analysed the effect of leg length in (centimetres) and skull circumference (in centimetres) on dementia prevalence when deployed as linear variables, using the adjusted prevalence ratios in Table 3. Dementia prevalence declined with each centimetre increase in leg length (PR 0.988, 95% CI, 0.981-0.995) and with each centimetre increase in skull circumference (PR 0.961, 95% CI 0.940-0.986). The formal test for heterogeneity of estimates was not statistically significant for either exposure (leg length Q=13.3, 10 df, p=0.83; Higgins I2 =25%, and skull circumference Q=17.2, 10 df, p=0.07; Higgins I2 = 41.9%).
Table 3.
The effects of leg length and skull circumference on prevalence of dementia expressed as prevalence ratios (with 95% confidence intervals) controlling for age, gender, education and family history of dementia
| Cuba | DR | Peru (urban) | Peru (rural) | Venezuela | Mexico (urban) | Mexico (rural) | China (urban) | China (rural) | India (urban) | India (rural) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leg length quarter | |||||||||||
| 1 (shortest) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 | 0.91 (0.70-1.19) | 0.74 (0.54-1.02) | 0.64 (0.39-1.07) | 0.78 (0.32-1.90) | 0.63 (0.33-1.20) | 1.08 (0.69-1.69) | 1.17 (0.69-1.98) | 1.05 (0.62-1.77) | 0.95 (0.42-2.17) | 1.33 (0.56-3.17) | 0.97 (0.49-1.90) |
| 3 | 1.11 (0.85-1.46) | 0.60 (0.42-0.87) | 0.73 (0.44-1.19) | 1.36 (0.52-3.56) | 0.95 (0.56-1.63) | 0.97 (0.54-1.74) | 0.89 (0.49-1.61) | 0.76 (0.41-1.42) | 1.66 (0.85-3.25) | 1.18 (0.53-2.65) | 0.79 (0.40-1.56) |
| 4 (longest) | 0.77 (0.52-1.12) | 0.63 (0.42-0.97) | 0.98 (0.60-1.61) | 1.62 (0.54-4.79) | 0.82 (0.43-1.56) | 0.76 (0.38-1.54) | 0.85 (0.42-1.70) | 0.54 (0.25-1.19) | 1.05 (0.50-2.23) | 0.96 (0.42-2.19) | 1.05 (0.52-2.13) |
| Per 1 cm increase in leg length | 0.99 (0.98-1.01) | 0.97 (0.96-0.99) | 0.99 (0.97-1.01) | 1.02 (0.97-1.08) | 1.00 (0.98-1.02) | 0.99 (0.96-1.02) | 0.98 (0.95-1.02) | 0.97 (0.94-1.01) | 1.01 (0.99-1.04) | 1.00 (0.97-1.03) | 0.99 (0.95-1.02) |
| Skull circumference quarter | |||||||||||
| 1 (smallest) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 2 | 0.63 (0.48-0.83) | 1.37 (0.89-2.12) | 0.95 (0.60-1.51) | 0.58 (0.26-1.28) | 1.24 (0.62-2.46) | 0.93 (0.47-1.80) | 0.97 (0.52-1.81) | 0.64 (0.38-1.06) | 0.67 (0.33-1.37) | 0.94 (0.58-1.55) | 0.82 (0.56-1.21) |
| 3 | 0.56 (0.41-0.78) | 0.99 (0.61-1.63) | 1.35 (0.81-2.26) | 0.31 (0.09-1.05) | 1.34 (0.76-2.36) | 1.28 (0.61-2.65) | 1.30 (0.73-2.30) | 0.62 (0.31-1.23) | 1.05 (0.55-2.03) | 1.69 (0.81-3.53) | 0.39 (0.12-1.24) |
| 4 (largest) | 0.51 (0.37-0.71) | 1.07 (0.68-1.67) | 0.93 (0.55-1.58) | 0.41 (0.15-1.17) | 0.84 (0.43-1.65) | 1.08 (0.51-2.26) | 1.09 (0.63-1.89) | 0.71 (0.39-1.29) | 0.56 (0.28-1.13) | 0.71 (0.18-2.85) | 0.81 (0.29-2.24) |
| Per 1 cm increase in skull circumference | 0.89 (0.84-0.95) | 0.98 (0.93-1.04) | 1.01 (0.93-1.10) | 0.83 (0.71-0.97) | 1.03 (0.95-1.13) | 0.96 (0.86-1.08) | 1.00 (0.91-1.10) | 0.93 (0.84-1.03) | 0.98 (0.93-1.03) | 1.00 (0.90-1.11) | 0.90 (0.81-1.01) |
Figure 1a. Forest plot for the meta-analysis of independent associations (PRs*) between leg length (longest vs. shortest quarters) and dementia.
* Prevalence ratios, adjusting for age, gender, educational level and family history of dementia
Figure 1b. Forest plot for the meta-analysis of independent associations (PRs*) between skull circumference (longest vs. shortest quarters) and dementia.
* Prevalence ratios, adjusting for age, gender, educational level and family history of dementia
Table 4.
Pooled fixed effects meta-analysed across sites (prevalence ratios with 95% confidence intervals) for the main effects of leg length and skull circumference on 10/66 dementia, with tests for hypothesized interactions by gender and education
| Leg length – main effect (quarter with longest vs. quarter with shortest legs) | Skull circumference – main effect (quarter with largest vs. quarter with smallest skulls) | ||
|---|---|---|---|
| Model 1 Controlling for age, gender, education and family history of dementia |
0.82 (0.68-0.98) | Model 1 Controlling for age, gender, education and family history of dementia |
0.75 (0.63-0.89). |
| Model 2 As for model 1, plus skull circumference |
0.84 (0.70-1.01) | Model 2 As for model 1, plus leg length |
0.82 (0.69-0.99) |
| Model 3 As for model 1, plus trunk length |
0.72 (0.59-0.87) | Model 3 As for model 1, plus height |
0.74 (0.62-0.89) |
| Leg length – interaction terms | Skull circumference – interaction terms | ||
| Interaction of leg length and gender (M vs. F), controlling for covariates as per model 1 | 0.99 (0.88-1.12) | Interaction of skull circumference and gender (M vs. F), controlling for covariates as per model 1 | 1.02 (0.90-1.16) |
| Interaction of skull circumference and education, controlling for covariates as per model 1 | 0.96 (0.91-1.01) | ||
Finally, given concerns that reverse causality or mortality bias might have accounted for these associations, we tested, among people with dementia, in each site, the hypotheses that dementia severity (Clinical Dementia Rating - CDR) would be inversely associated with leg length and skull circumference, that is that those with more severe dementia would have shorter legs and smaller skulls. These hypotheses were supported only in Cuba, with respect to skull circumference (oneway ANOVA weighted linear term, F=2.32, p=0.03). Pooling data across sites, there was no association between CDR and either leg length (for which there was a non-significant trend towards longer legs in those with more severe dementia - F=2.86, p=0.09), or skull circumference (F=1.94, p=0.16)
Discussion
This study provides the strongest and most consistent evidence yet for the salience of early life development to the subsequent risk of dementia. Both larger skull circumferences and longer legs were independently associated with a lower prevalence of dementia. The effect of skull circumference was not explained by overall height, and the effect of leg length was accentuated after controlling for trunk height. Although men, younger people and the better educated had larger skulls and longer legs, the effects of leg length and skull circumference on dementia were largely independent of these potential confounders. There was no association between either leg length or skull circumference and a family history of dementia, and the effect of these exposures on dementia risk was not explained by a family history of the disease. Shared genetic predisposition seems therefore unlikely to explain the findings. The independent effects of skull circumference after controlling for leg length or overall height, and of leg length after controlling for skull circumference suggest the possibility that different mechanisms may be in play. Although adult leg length and height is mainly influenced by early growth and its determinants (Gigante et al., 2009), legs continue to grow for up to a decade after skull growth is complete. Skull circumference and leg length may therefore be markers of different phases, and, possibly, different aspects of the developmental process. Neurodevelopment seems a credible mediator of the association between skull circumference and dementia. Shorter limb length in Western populations is associated with cardiovascular risk factors in later life, particularly those linked to the insulin resistance syndrome (Smith et al., 2001). This may be a mediating pathway, but, given the complex bidirectional relationships between cardiovascular risk factors and dementia this possibility will be better explored in the incidence phase of our project. We could not confirm previously reported interactions between educational level and skull circumference (Mortimer et al., 2003), gender and leg length (Kim et al., 2003;Kim et al., 2008;Huang et al., 2008), or gender and skull circumference (Kim et al., 2008).
We have demonstrated these associations in large population-based studies, encompassing rural and urban catchment area sites in the Caribbean, Latin America, India and China. We have been able to use meta-analytical techniques to increase the precision of our estimates of the protective effects of long legs and large skulls across these culturally and racially diverse settings. With no clear evidence of heterogeneity, the use of fixed effect meta-analysis to pool these estimates is appropriate, the underlying assumption being that the true effect size is the same in all sites, and that any observed variation is accounted for by sampling error. While our studies are cross-sectional, reverse causality seems an unlikely explanation of these associations; longitudinal CT studies of people with dementia have not suggested that their cranial dimensions decrease with progression of the disease (Jenkins et al., 2000), and leg length, in contrast to trunk height, is thought to be relatively stable across the adult life course. In our Chennai centre, reassessment of skull circumferences and leg lengths three years after baseline indicated that leg lengths did appear to shrink, but at a similar rate in those with and without dementia, while skull circumferences remained stable; the moderate agreement for the repeated measures, particularly for skull circumference suggested an important degree of random measurement error, which would have led to an underestimation of the true effect of these exposures upon dementia risk (Jotheeswaran et al., 2010). While differential mortality remains a possible explanation, the finding from a previous study that small skull circumference is also associated with the mildest stage of AD runs counter to this theory (Schofield et al., 1997). In our Chennai centre, neither leg length nor skull circumference was associated with subsequent mortality, and these associations were not significantly modified by dementia status at baseline; if anything, the data suggested that the effect of mortality bias would have been to underestimate the true association (Jotheeswaran et al., 2010). Finally, of relevance both to reverse causality and mortality bias, we found no evidence among people with dementia, for an association between dementia severity and either leg length or skull circumference.
Apart from the cross-sectional design, there are two main weaknesses to our study. First, we have only studied dementia syndrome as the outcome. Previous studies have indicated that skull circumference and leg length are associated with Alzheimer’s Disease, but not vascular dementia. Again, this is likely to have led to an underestimation of the effect in our study. Second, we have not yet completed APOE genotyping for most centres. We have not therefore been able either to adjust for APOE genotype (an important risk factor for dementia), or to test for effect modification by APOE genotype (two studies have suggested that the effect of skull circumference may be stronger in those with one or more APOE e4 alleles (Bickel et al., 2006;Borenstein et al., 2001)). However, those studies that have controlled for APOE genotype have not found it to be a confounding factor in the estimation of these associations (Kim et al., 2008;Borenstein et al., 2001).
An incidence phase of the 10/66 Dementia Research Group studies (Prince et al., 2007), now under way, focuses on aetiology, examining the roles of racial admixture, micronutrient deficiency, cardiovascular disease and its risk factors on incident dementia and stroke. In the course of this we will also assess the effect of skull circumference and leg length on incidence risk of dementia. Uniquely we will have repeated anthropometric measures on all participants in the incidence phase, enabling us to test directly for reverse causality, and, in the event of no secular trend in leg length and skull circumference we will be able to use the mean of the repeated measures to reduce random measurement error in the ascertainment of the leg length and skull circumference exposures, hence reducing regression dilution bias.
Acknowledgements
The 10/66 Dementia Research Group’s research has been funded by the Wellcome Trust Health Consequences of Population Change Programme (GR066133 – Prevalence phase in Cuba and Brazil; GR08002- Incidence phase in Peru, Mexico, Argentina, Cuba, Dominican Republic, Venezuela and China), the World Health Organisation (India, Dominican Republic and China), the US Alzheimer’s Association (IIRG – 04 – 1286 - Peru, Mexico and Argentina), and FONDACIT (Venezuela). The Rockefeller Foundation supported our recent dissemination meeting at their Bellagio Centre. Alzheimer’s Disease International has provided support for networking and infrastructure.
Footnotes
Conflict of interest
None
Description of authors’ roles
All of the authors worked collectively to develop the protocols and methods described in this paper. Martin Prince led the research group and Cleusa Ferri acted as research coordinator. Juan Llibre Rodriguez (Cuba), Daisy Acosta (Dominican Republic), Mariella Guerra (Peru), Aquiles Salas (Venezuela), Ana Luisa Sosa (Mexico), KS Jacob (Vellore, India), Joseph D Williams (Chennai, India) and Yueqin Huang (China) were principal investigators responsible for the field work in their respective countries. Martin Prince wrote the first draft, with Alan Dangour, Ricardo Uauy and Emiliano Albanese. He also conducted the analyses with statistical support from Michael Dewey. Other authors reviewed the manuscript, provided further contributions and suggestions. All authors read and approved the final manuscript.
Reference List
- Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Bickel H, Riemenschneider M, Kurz A. [Associations between dementia and head circumference as a measure of brain reserve--results from the Bavarian School sisters study] Psychiatrische Praxis. 2006;33:138–144. doi: 10.1055/s-2005-915464. [DOI] [PubMed] [Google Scholar]
- Borenstein GA, et al. Head circumference and incident Alzheimer's disease: modification by apolipoprotein E. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- Copeland JRM, Dewey ME, Griffith-Jones HM. A computerised psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychological Medicine. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- Edland SD, et al. Total intracranial volume: normative values and lack of association with Alzheimer's disease. Neurology. 2002;59:272–274. doi: 10.1212/wnl.59.2.272. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Chandra V, Gilbey J. Cognitive test performance in a community-based non demented elderly sample in rural India: the Indo-US cross national dementia epidemiology study. International Psychogeriatrics. 1996;8:507–524. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- Gigante DP, Nazmi A, Lima RC, Barros FC, Victora CG. Epidemiology of early and late growth in height, leg and trunk length: findings from a birth cohort of Brazilian males. European Journal of Clinical Nutrition. 2009;63:375–381. doi: 10.1038/sj.ejcn.1602949. [DOI] [PubMed] [Google Scholar]
- Gunnell DJ, et al. Childhood leg length and adult mortality: follow up of the Carnegie (Boyd Orr) Survey of Diet and Health in Pre-war Britain. Journal of Epidemiology and Community Health. 1998;52:142–152. doi: 10.1136/jech.52.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KS, et al. The development of a dementia screeing interview in two distinct languages. International Journal of Methods in Psychiatric Research. 1993;3:1–28. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Huang TL, Carlson MC, Fitzpatrick AL, Kuller LH, Fried LP, Zandi PP. Knee height and arm span: a reflection of early life environment and risk of dementia. Neurology. 2008;70:1818–1826. doi: 10.1212/01.wnl.0000311444.20490.98. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Archives of Neurology. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Joteeswaran AT, Williams J, Prince MJ. The predictive validity of the 10/66 Dementia diagnosis in Chennai, India - a three year follow-up study of cases identified at baseline. Alzheimer's Disease and Associated Disorders. 2010 doi: 10.1097/WAD.0b013e3181d5e540. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotheeswaran AT, Williams JD, Stewart RJ, Prince MJ. Could Reverse Causality or Selective Mortality Explain Associations Between Leg Length, Skull Circumference and Dementia? A south Indian Cohort Study. International Psychogeriatrics. 2010 doi: 10.1017/S1041610210001171. In Press. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Kim SW, Yang SJ, Yoon JS. Associations between head circumference, leg length and dementia in a Korean population. International Journal of Geriatric Psychiatry. 2008;23:41–48. doi: 10.1002/gps.1833. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Yoon JS. Limb length and dementia in an older Korean population. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:427–432. doi: 10.1136/jnnp.74.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Llibre Rodriguez JJ, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llibre RJ, et al. The Prevalence, Correlates and Impact of Dementia in Cuba. A 10/66 Group Population-Based Survey. Neuroepidemiology. 2008;31:243–251. doi: 10.1159/000165362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak Z, Kim JM, Stewart R. Leg length, cognitive impairment and cognitive decline in an African-Caribbean population. International Journal of Geriatric Psychiatry. 2006;21:266–272. doi: 10.1002/gps.1458. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism. 2008;57(Suppl 2):S44–S49. doi: 10.1016/j.metabol.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. Journal of Clinical and Experimental Neuropsychology. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- Prince M, et al. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ, et al. The 10/66 Dementia Research Group's fully operationalised DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: a population validation study. BMC Public Health. 2008;8:219. doi: 10.1186/1471-2458-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MD, Johnston JM, Dodge HH, DeKosky ST, Ganguli M. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurology. 1999;53:228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiology of Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Scazufca M, et al. Risk factors across the life course and dementia in a Brazilian population: results from the Sao Paulo Ageing & Health Study (SPAH) International Journal of Epidemiology. 2008;37:879–890. doi: 10.1093/ije/dyn125. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer's disease in a population-based study of aging and dementia. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- Smith GD, Greenwood R, Gunnell D, Sweetnam P, Yarnell J, Elwood P. Leg length, insulin resistance, and coronary heart disease risk: the Caerphilly Study. Journal of Epidemiology and Community Health. 2001;55:867–872. doi: 10.1136/jech.55.12.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Hayashi T, Preece MA, Cameron N. Increase in length of leg relative to trunk in Japanese children and adults from 1957 to 1977: comparison with British and with Japanese Americans. Annals of Human Biology. 1982;9:411–423. doi: 10.1080/03014468200005951. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. International Journal of Epidemiology. 2002;31:383–390. [PubMed] [Google Scholar]
- Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurology. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]