Abstract
BACKGROUND & AIMS
The gut microbiota affects intestinal permeability and mucosal mast cells (MMCs) responses. Activation of MMCs has been associated with absorption of dietary fat. We investigated whether the gut microbiota contributes to the fat-induced activation of MMCs in rats, and how antibiotics might affect this process.
METHODS
Adult male Sprague-Dawley rats were given streptomycin and penicillin for 4 days (n = 6–8) to reduce the abundance of their gut flora, or normal drinking water (controls, n = 6–8). They underwent lymph fistula surgery and after an overnight recovery were given an intraduodenal bolus of intralipid. We collected intestinal tissues and lymph fluid and assessed activation of MMCs, intestinal permeability, and fat transport parameters.
RESULTS
Compared with controls, intestinal lymph from rats given antibiotics had reduced levels of mucosal mast cell protease II (produced by MMCs) and decreased activity of diamine oxidase (produced by enterocytes) (P < .05). Rats given antibiotics had reduced intestinal permeability in response to dietary lipid compared with controls (P < .01). Unexpectedly, antibiotics also reduced lymphatic transport of triacylglycerol and phospholipid (P < .01), concomitant with decreased levels of mucosal apolipoproteins B, A-I, and A-IV (P < .01). No differences were found in intestinal motility or luminal pancreatic lipase activity between rats given antibiotics and controls. These effects were not seen with an acute dose of antibiotics or 4 weeks after the antibiotic regimen ended.
CONCLUSIONS
The intestinal microbiota appears to activate MMCs after the ingestion of fat in rats; this contributes to fat-induced intestinal permeability. We found that the gut microbiome promotes absorption of lipid, probably by intestinal production of apolipoproteins and secretion of chylomicrons.
Keywords: Microbe, Digestion, APOA-I, APOA-IV
The gastrointestinal tract harbors a rich and diverse community of bacteria consisting of approximately 500–1000 species of >1013 cells, which is collectively known as the gut microbiota.1 The gut epithelium is one of the largest and most important barriers between the outside environment and host, forming a selective barrier that permits absorption of macronutrients while maintaining an effective defense against bacteria and other toxins. Intestinal mucosal mast cells (MMCs), which reside in the lamina propria underneath the epithelium, are important sentinel cells that sense entry of foreign material into the mucosa and orchestrate an inflammatory response to prevent the material from entering the systemic circulation. Upon sensing, MMCs are activated and degranulated, releasing cytokines, histamine, proteoglycans, and proteases. While important in host defense, MMC activation also contributes to epithelial barrier dysfunction and an array of gastrointestinal disorders, such as food allergies,2 irritable bowel syndrome,3 and inflammatory bowel disease.4
While the gut microbiota is shown to play a major role in inflammation,5,6 this influence is modulated by daily physiological events, such as the digestion and absorption of dietary fat. For example, the presence of gut bacteria was found to increase intestinal permeability and inflammation associated with high-fat feeding7,8 and the effects were abolished with antibiotic treatment.7 Studies in our laboratory revealed that ingestion of dietary fat activates intestinal MMCs by a mechanism partially linked to the formation and secretion of chylomicrons.9 As a result, a significant amount of histamine is released into intestinal lymph, which in excess causes allergies or inflammation.10 What is not known is the mechanism by which dietary fat activates intestinal MMCs. Fat absorption facilitates the absorption of bacterial lipopolysaccharide (LPS) from the gut,7,11 which associates with chylomicron remnants.12 Because intestinal MMCs are activated by bacterial products, such as LPS13 or Escherichia coli hemolysin,14 we hypothesized that the gut microbiota is responsible for the lipid-induced activation of intestinal MMCs.
In this study, we aimed to modify the gut microbiota by an antibiotic regimen designed to reduce the load of gut bacteria in rats maintained on a standard chow diet. Using a well-established conscious lymph fistula model, we examined markers for MMC activation and assessed intestinal permeability in response to a dietary fat load in antibiotic-treated vs untreated control rats. Additionally, we examined whether antibiotic-related changes of the gut microbiota affected the process of fat absorption.
Methods
Animals
Only adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) that weighed 300–350 g were used. The animals were acclimated to our animal facility under a 12:12-hour light–dark cycle for at least 2 weeks before experiments and provided with standard rodent chow ad libitum. Room temperature and humidity were maintained at 70°F–74°F and 40%–60%, respectively. All procedures performed were in accordance with the University of Cincinnati Internal Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Antibiotics Treatment Regimen
The rats in the experimental group were administered streptomycin (4 mg/mL) and penicillin (2 mg/mL) in drinking water ad libitum for 4 days before surgery. This antibiotic regimen reduces total aerobic gut bacterial population levels from 106 to <102 colony-forming units of bacteria per gram of cecum.15 The control group was given normal drinking water ad libitum for 4 days.
Lymph Fistula Surgery With Duodenal Cannulation
The lymph fistula surgical procedure and postoperative care were performed as described previously.16 Briefly, animals were fasted overnight before surgery. Under isoflurane anesthesia, the superior mesenteric lymph duct was cannulated according to Bollman et al,17 with slight modification. A cyanoacrylate adhesive (tissue glue) was used to fix the cannula. A second cannula was inserted approximately 1.5 cm down into the duodenum via a fundus incision of the stomach. Postoperatively, the animals were kept in Bollman restraining cages, which prevented the animals from chewing and damaging the cannula. Despite restraints, the animals had considerable freedom to move forward, backward, and laterally.
Fat Absorption Study and Lymph Collection
After surgery, the animals were given an intraduodenal infusion of a 5% glucose-saline solution with or without antibiotics at a rate of 3 mL/h for 8 hours. The infusion was then switched to saline alone (with or without antibiotics) for overnight infusion until the next morning. Fasting lymph was collected for 1 hour before administering lipid. Liposyn III (Hospira, Lake Forest, IL) was given as a single bolus through the duodenal cannula. After the bolus, saline was infused continuously into the duodenum until the end of the study. Lymph was collected every hour for 6 hours.
Measurement of Rat Mucosal Mast Cell Protease II
Due to termination of the rat mucosal mast cell protease II (RMCPII) enzyme-linked immunosorbent assay (ELISA) kit by Moredun Scientific (Edinburgh, Scotland), we were only able to measure RMCPII levels by Western blotting. As a result, measuring levels in the lymph is the only way for studying RMCPII secretion by MMCs because serum levels are too low for detection by Western blot. Briefly, lymph samples were loaded onto 4%–15% polyacrylamide gradient gels and electrotransferred to polyvinylidene difluoride membranes. After transfer, the membranes were treated with a blocking reagent (5% nonfat milk and 0.1% Tween 20 in Tris-buffered saline). After blocking, membranes were incubated with RMCPII antibody (Moredun Scientific) diluted 1:1000 at 4°C overnight. After rinsing with Tris-buffered saline with 0.1% Tween 20, the membranes were further incubated with peroxidase-conjugated anti-goat secondary antibody at 1:5000 for 1 hour and developed with Immobilon Western Chemiluminescent Horseradish Peroxidase Substrate (Millipore Corporation, Billerica, MA) according to the manufacturer’s protocol. Band densities were quantified with Total Lab Quant Analysis Software (TL100; FOTODYNE Hartland, WI).
Diamine Oxidase Activity Measurement
Diamine oxidase (DAO) activity was measured by a radiometric assay described by Forget et al18 with slight modifications. The reaction mixture consisted of 50 μL enzyme and 50 μL substrate. The substrate consisted of cold and labeled putrescine (10 μL [3H]-putrescine; Amersham Biosciences, Piscataway, NJ; per 1000 μL 0.9 mM unlabeled putrescine) prepared in 0.1 M sodium phosphate buffer at pH 7.2. The reaction mixture was incubated at 37°C for 30 minutes and stopped by adding 10 μL of 0.1 mM aminoguanidine, an inhibitor of DAO, followed by 50 μL sodium carbonate (pH 12.2). The reaction product Δ1-pyrroline was extracted twice with 500 μL toluene. Radioactivity was determined by liquid scintillation counting. Activity was dependent on time and quantity of putrescine and enzyme. DAO activity was calculated in milliunits per milliliter of lymph, where 1 U oxidized 1 mmol putrescine per hour at 37°C.
To test for direct effects of antibiotics on DAO activity, the assay was performed in duplicate under standard conditions but with penicillin-G added at 4 mg/mL and streptomycin at 2 mg/mL, which would be the dose used in our rat experiments. The control assays had an equivalent volume of water added. Samples were taken during a 1-hour time course and processed according to our standard protocol as described.
Evaluation of Intestinal Permeability
One hundred and fifty milligrams of 4 kDa fluorescein isothiocyanate (FITC)-dextran (Sigma Aldrich, St Louis, MO) was given intraduodenally as a bolus 2 hours before lipid infusion according to a previous study,19 with slight modifications. FITC-dextran is used as a fluorescent marker to measure paracellular transport and mucosal barrier dysfunction.20 Fasting lymph was collected for 1 hour, and lymph was collected continuously after lipid administration for 4 hours. Lymphatic FITC concentration was measured by spectrophotofluorometry with an excitation wavelength of 485 nm and emission wavelength of 528 nm.
Measurement of Lymphatic Outputs of Triacylglycerol, Phospholipid, and Apolipoproteins
Lymph triacylglycerol was measured by chemical assay using a total triacylglycerol kit (Randox Laboratories, Kearneysville, WV). Lymph phospholipids were measured by chemical assay using a phospholipid kit (Wako Diagnostics, Mountain View, CA). Both assays were performed according to manufacturer’s protocols. Measurements of lymphatic apolipoprotein (Apo) outputs were determined by Western blotting, as described previously by Hayashi et al.21
Measurement of Lipid Hydrolysis and Intestinal Motility Using Radiolabeled Lipid
To determine whether the antibiotics impacted motility, we used another cohort of antibiotic-treated and untreated lymph fistula animals. Following our standard 4-day treatment with antibiotics, surgery, and an overnight recovery, the animals were given an intraduodenal bolus of Liposyn III labeled with 3H triolein and followed by a continuous infusion of saline for 6 hours. The animals were sacrificed and their small intestines were excised and cut into 4 equal-length segments. Luminal contents of each segment were collected by washing 3 times with 2 mL 1% sodium taurocholate saline. An aliquot of the pooled washes from each segment was taken for radioactivity determination.
Determination of Acute Effects of Antibiotics
To determine whether antibiotics had a direct effect on fat absorption and MMC activation, we studied another 2 groups of lymph fistula rats. The animals were given strictly 5% glucose-saline after surgery (with no antibiotics) at a rate of 3 mL/h until 16 hours before the fat absorption study. The 5% glucose-saline was replaced with saline (no antibiotics) and infused overnight until the next morning. Before administering the lipid bolus, antibiotics were administered continuously into the duodenum (4 mg/mL streptomycin and 2 mg/mL penicillin in saline) for only 1 hour. Liposyn III was administered intraduodenally and followed with saline, as described. Lymphatic RMCPII and triacylglycerol outputs were determined as described.
Recovery of the Gut Microbiota From Antibiotics
In a different cohort of animals, we administered our 4-day antibiotic treatment regimen and saline as a control and waited 4 weeks to allow restoration of the gut microbiota before performing the fat absorption experiments and RMCPII measurements as described.
Fecal Collection, DNA Extraction, and Polymerase Chain Reaction Amplification of 16S Ribosomal RNA
After the 4-day regimen, fecal pellets were collected and stored at −80°C until analysis. DNA was extracted using a standard phenol:chloroform extraction method, as described previously.22 Real-time quantitative polymerase chain reaction amplification of 16S ribosomal RNA (rRNA) was performed with a Roche 480 II Light Cycler (Roche, Indianapolis, IN). 16S rRNA gene-specific primers were used to target specific bacterial genera; 16S rRNA (forward: 5′-ATG GYT GTC GTC AGC TCG TG-3′ (reverse: 5′-GGG TTG CGC TCG TTG C-3′). Genes were quantified by determining a standard curve for gene copy number by cloning primer sequences into pCR4-TOPO plasmids and gene copy number was determined from stool as described previously.23,24
Sequencing of 16S Ribosomal RNA Gene Using Illumina Mi-Seq Platform
To assess bacterial community structure, primers specific for 16S rRNA V4-V5 region (forward: 338F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and reverse: 806R: 5′-GGAC-TACHVGGGTWTCTAAT-3′) that contained Illumina 3′ adapter sequences, as well as a 12-bp barcode were used. Sequences were generated by an Illumina MiSeq DNA platform at Argonne National Laboratory and analyzed by the program Quantitative Insights Into Microbial Ecology (QIIME).25 Operational taxonomic units (OTUs) were picked at 97% sequence identity using open reference OTU picking against the Greengenes database (May 2013 release).26 OTUs were quality-filtered based on default parameters set in the open-reference OTU command in QIIME and sequences were rarified to an equal sampling depth of 30,000 reads per sample for all saline control samples. We were unable to assess 16S taxonomic structure in antibiotic samples due to low abundance 16S rRNA. Representative sequences were aligned via PyNAST,25 taxonomy assigned using the RDP Classifier,27 and a phylogenetic tree was built using FastTree.28
Statistical Analysis
Data shown are mean values ± SEM. To compare groups during the lipid infusion, 2-way repeated-measures analyses of variance were used. A t test was used for other analyses comparing only 2 groups. Differences were considered significant at P < .05.
Results
Antibiotic Treatment Suppressed Lipid-Induced Activation of Intestinal Mucosal Mast Cells
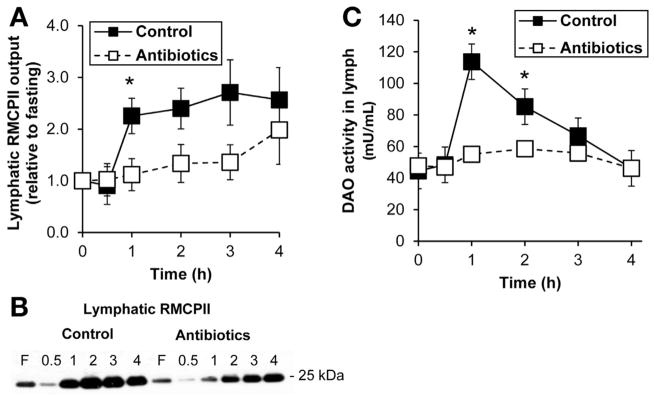
We previously showed that dietary fat stimulated the release of RMCPII into lymph,9 which is a specific marker for rat intestinal MMC degranulation.29 Lymphatic RMCPII secretion, normally increased in response to lipid, was decreased significantly (P < .05) in the antibiotic-treated group compared with controls (Figure 1A and B). There was no difference in lymph flow rates between the 2 groups of animals (2.33 ± .40 mL/h control vs 2.26 ± .22 mL/h antibiotics). This decrease is apparently not due to direct effects of antibiotics because treating the intestine for 1 hour with antibiotics did not affect RMCPII output (data not shown). Taken together, the data suggest that our antibiotic treatment regimen, which dramatically reduces the gut microbiota, suppressed the activation of intestinal MMCs by dietary fat absorption.
Figure 1.
Antibiotic treatment suppressed mucosal mast cell activation. (A) Lymphatic RMCPII output in saline-treated control (n = 8) and antibiotic-treated (n = 8) rats given an intraduodenal infusion of LiposynIII. (B) A representative Western blot showing RMCPII output in lymph. (C) Lymph DAO activity between the 2 groups (n = 8 each). Data are expressed as mean ± SEM; *P < .05.
We also previously showed that a dietary fat load stimulated the activity of DAO in lymph.30 Intestinal DAO is stored in plasma membrane-associated vesicles of enterocytes31 and is the major enzyme that catabolizes histamine,32 which is secreted by activated MMCs. Consistent with our previous report,30 an intraduodenal bolus of Liposyn III dramatically increased the activity of DAO in lymph after 1 hour, which returned to baseline levels after 4 hours, as seen in the control group (Figure 1C). In contrast, the antibiotic-treated group did not show an increase in DAO activity in lymph and was significantly lower than the control group (P < .05) (Figure 1C). These data suggest that antibiotic-mediated effects of the gut microbiota reduced the release of DAO into lymph. Although antibiotics might possibly have direct effects on DAO activity, adding penicillin-G at 4 mg/mL and streptomycin at 2 mg/mL, the dose used in our rat experiments, to the enzymatic assay for DAO showed no such effect (data not shown).
Antibiotic Treatment Reduced Intestinal Permeability in Response to Fat Absorption
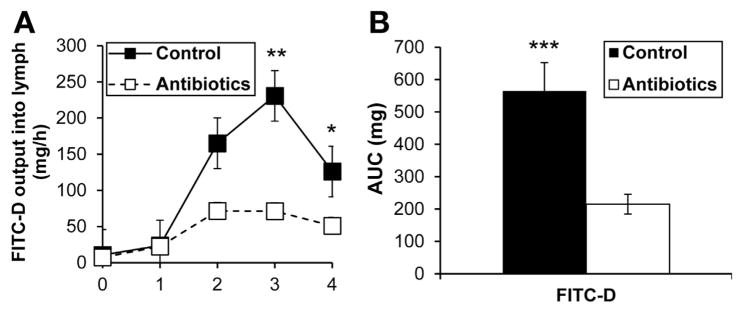
Because MMC activation is linked to increased intestinal permeability,33,34 we studied the effect of antibiotic treatment on the transport of duodenally administered FITC-conjugated dextran (molecular weight: 4000 daltons) into lymph in response to lipid. As expected, lymphatic FITC levels increased dramatically after 2 hours in the control group, but did not increase as dramatically in the antibiotic-treated group in response to lipid (Figure 2A). Area under the curve was dramatically lower (P < .001) in the antibiotic-treated group (Figure 2B), indicating clearly that antibiotic treatment decreased intestinal permeability during fat absorption, which is probably linked to reduced MMC activation.
Figure 2.
Antibiotic treatment reduced intestinal permeability in response to dietary lipid. (A) Lymphatic output of FITC-labeled dextran to measure intestinal permeability in control (n = 6) and antibiotic-treated (n = 10) rats given Liposyn III. (B) Total (area under the curve [AUC]) lymph FITC levels over 4 hours. Data are expressed as mean ± SEM; *P < .05; **P < .01; ***P < .001.
Antibiotic Treatment Reduced Fat Absorption
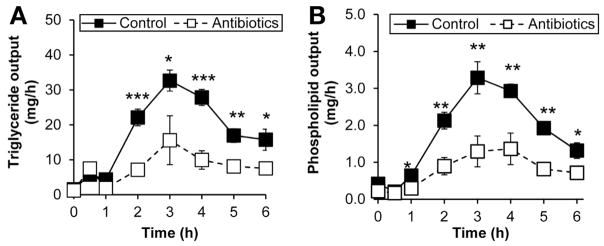
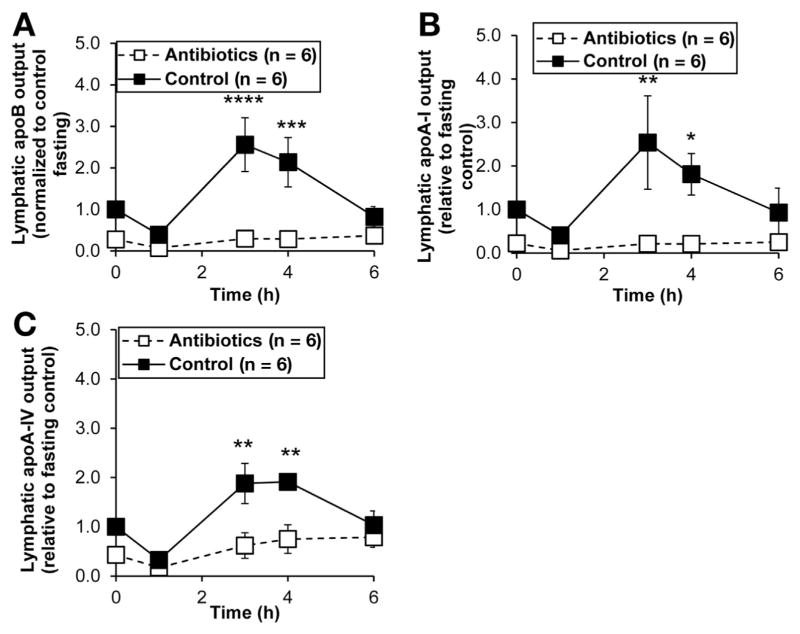
Because we previously showed a dose-dependent effect of dietary fat on the activation of intestinal MMCs,9 we wondered whether antibiotic treatment altered the absorption of dietary fat. Intriguingly, the lymphatic outputs of both triacylglycerol and phospholipid were suppressed in the antibiotic-treated group (Figure 3A and B), suggesting that antibiotic treatment limited the absorption of dietary lipids and subsequent production of chylomicrons. Thus, we would expect the lymphatic transport of chylomicron-associated apolipoproteins to be significantly reduced. As expected, antibiotic treatment dramatically reduced outputs of ApoB, ApoA-I, and ApoA-IV into lymph (Figure 4A, B, and C), suggesting that antibiotic-associated bacterial changes altered the intracellular machinery involved in the formation and packaging of chylomicrons. Because only one ApoB associates with a chylomicron particle,35 the decrease in ApoB output indicates that fewer chylomicron particles are secreted with antibiotic treatment.
Figure 3.
Antibiotics treatment reduced the lymphatic outputs of (A) triglycerides and (B) phospholipids. Saline-treated control (n = 8) and antibiotic-treated (n = 8) rats were given an intraduodenal infusion of LiposynIII lipid. Data are expressed as mean ± SEM; *P < .05; ** P < .01; ***P < .001.
Figure 4.
Antibiotic treatment suppressed the intestinal outputs of (A) ApoB, (B) ApoA-I, and (C) ApoA-IV into the mesenteric lymph. Saline-treated control (n = 6) and antibiotic-treated (n = 6) rats were given an intraduodenal infusion of LiposynIII. Data are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .005; ****P < .001.
To rule out acute and direct effects of antibiotics, we administered antibiotics continuously into the duodenum 1 hour before administering dietary lipid. Short-term antibiotic treatment did not affect the lymphatic outputs of triacylglycerol or RMPCII (data not shown). Taken together, our data suggest that the presence of a normal gut flora is required for efficient lipid absorption and the production of chylomicrons, and antibiotic treatment reduces these effects. These findings have profound implications in the interaction between microbiome and lipid metabolism and the mechanisms involved will be explored in future studies.
Antibiotic-Treatment Did Not Affect Intestinal Motility or Digestion
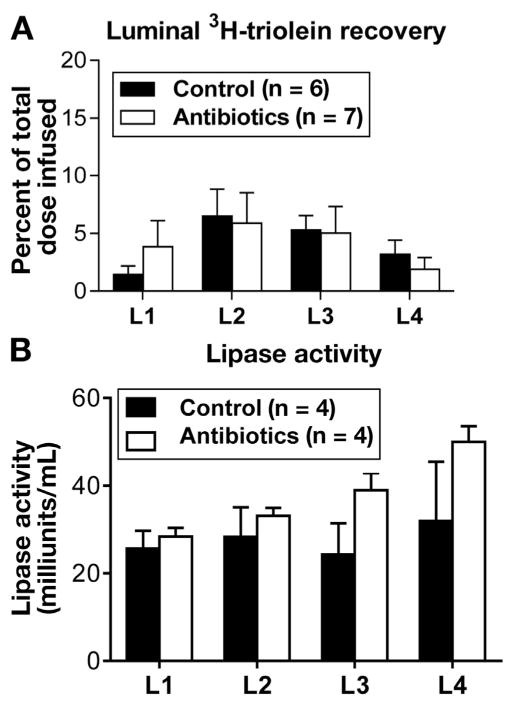
Because the gut microbiota plays an important role in intestinal motility,36,37 and antibiotic treatment is recently reported to have an independent effect on motility by affecting enteric neurons,38 we studied the effect of our antibiotic regimen on intestinal motility. Figure 5A shows the distribution of radioactive triolein (expressed as percent total radioactive triolein infused) in each of the 4 small intestinal segments. There was no difference in any of the segments between the 2 groups of animals. To rule out the effects of antibiotics on lipid digestion, such as the secretion of pancreatic enzymes that hydrolyze fats,39 we compared luminal pancreatic lipase activity between antibiotic-treated and control groups. Antibiotic treatment did not affect pancreatic function, as determined by lipase enzyme activity in the 4 segments of the small intestine between the 2 groups of animals (Figure 5B). Thus, we conclude that the decrease in the lymphatic transport of lipids in response to our antibiotic treatment was not related to motility or fat digestion.
Figure 5.
Antibiotic treatment did not affect (A) intestinal motility and (B) luminal fat digestion. To look at effects of antibiotics on intestinal motility, lymph fistula animals given either antibiotics (n = 7) or saline (n = 6) for 4 days were given an intraduodenal bolus of intralipid labeled with 3H triolein. Radioactivities from luminal contents of the intestinal segments (L1, duodenum; L2 and L3, jejunum; L4, ileum) were measured by liquid scintillation. To measure luminal digestion, luminal pancreatic lipase activity was assayed. Lymph-intact animals equipped with duodenal tubes were treated for 4 days with (n = 4) or without antibiotics (n = 4). On the experimental day, a lipid bolus was administered intraduodenally and, after 4 hours, the luminal contents of small intestinal segments were assayed for lipase activity.
Recovery From Antibiotic Treatment Restored Mucosal Mast Cell Activation and Fat Absorption
In a different cohort of animals, we administered our 4-day antibiotic treatment regimen and saline as a control, and waited 4 weeks to allow for restoration of the gut microbiota before performing experiments. Upon restoration of the gut microbiota, there were no differences in lymphatic lipid transport (Figure 6A) and MMC activation (Figure 6B) between the 2 groups of animals, strengthening the observations that changes in MMC activation and lipid transport are directly related to alterations in the gut microbiota by antibiotic treatment.
Figure 6.
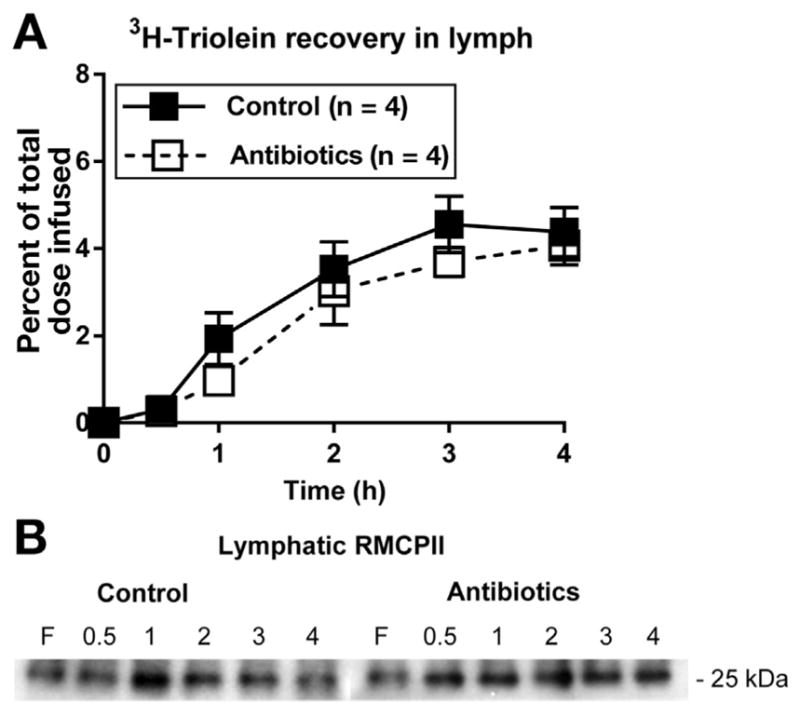
Restoring the microbiota after antibiotic treatment restored (A) fat absorption and (B) MMC activation as measured by RMCPII output into lymph. After the 4-day antibiotic treatment regimen (n = 4) and saline as a control (n = 4), the animals were allowed to recover for 4 weeks before performing experiments.
Antibiotic-Associated Changes on Microbiota Composition
To determine whether our results were dependent on changes in microbial composition or microbial load, Illumina Mi-Seq of the 16S rRNA gene and targeted real-time quantitative polymerase chain reaction for 16S gene copy number was performed using fecal DNA. Based on the targeted real-time quantitative polymerase chain reaction, there was no amplification of the 16S rRNA gene from the antibiotic samples, whereas there was a mean of 3,429,459 gene copies/ng DNA from the saline control animals. To be absolutely certain of our data, we performed the DNA extraction twice and submitted each set of samples on 2 different sequencing runs. There were too few sequencing reads from the antibiotic-treated animals to assess taxonomic structure. In contrast, we had no problem characterizing microbiota composition in the saline controls animals. At the phylum-level (Figure 7A), our control animals have a ratio that is about equal to that of firmicutes to bacteroidetes and, at the class level (Figure 7B), the dominate class is clostridia. The results show that our antibiotic treatment significantly reduced the microbial load to the extent that the 16S rRNA cannot be amplified. We conclude that our observations throughout this study are not due to changes in microbial composition, but more likely to a dramatic reduction of a microbiota.
Figure 7.
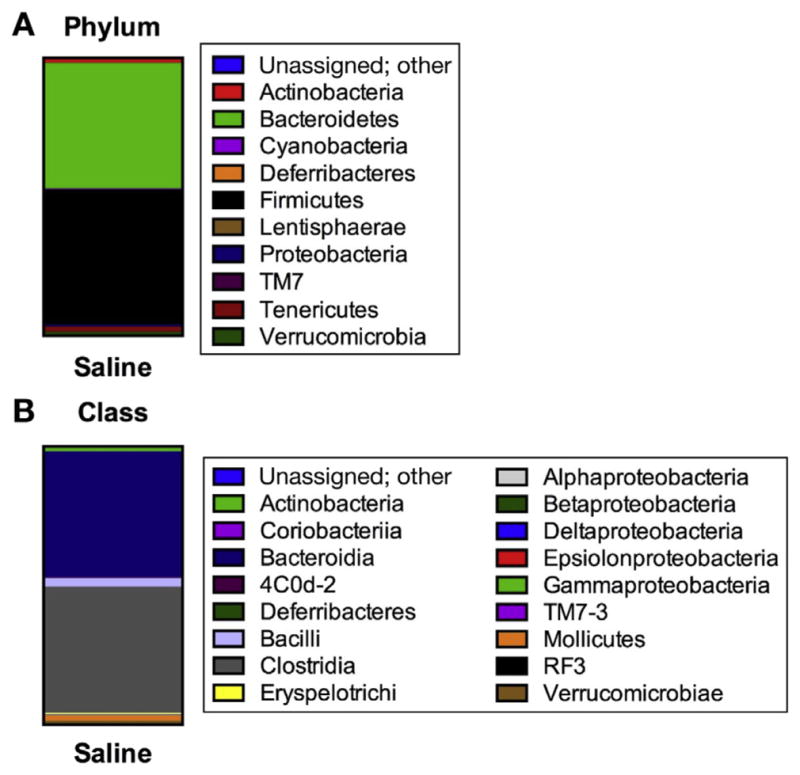
Gut microbial composition depicting phyla (A) and class (B) of saline-treated control animals. Antibiotic treatment resulted in no amplification of the 16S rRNA gene and thus we were unable to assess fecal microbiota composition. Taxonomic structure of 16S rRNA gene was assessed using the Illumina Mi-Seq platform and results analyzed using Quantitative Insights Into Microbial Ecology software.
Discussion
Using conscious lymph fistula rats, we report that gut bacteria are involved in the fat-induced activation of intestinal MMCs through a mechanism that involves the absorption of dietary fat. Our antibiotic treatment regimen, designed to dramatically lower bacteria load in the gut, has resulted in such low bacterial counts that we were not able to get a taxonomic characterization of the remaining microbiome (Figure 7). The antibiotic treatment significantly decreased the activation of intestinal MMCs to release mediators such as protease RMCPII in response to dietary fat. Additionally, the intestinal release of DAO into lymph, as well as gut permeability, were significantly reduced with antibiotic treatment, which are normally both increased in response to dietary fat. Interestingly, these changes were also associated with an unexpected decrease in lipid absorption. The decrease in lymphatic lipid transport was also associated with a marked reduction in intestinal ApoB secretion. Because ApoB is critical for the formation and secretion of intestinal chylomicron particles, it is tempting to speculate that the reduction in ApoB secretion may be responsible for the decreased lymphatic lipid transport. However, it may not be straightforward to determine the causal relationship between ApoB and lipid transport in the in vivo setting. It should also be noted that the intestinal secretions of ApoA-I and ApoA-IV were also reduced significantly. We have revealed for the first time a link between the gut microbiota and the activation of intestinal MMCs in the physiological process of fat absorption.
The conscious lymph fistula model is demonstrated to be a sensitive model for measuring substances secreted directly by the intestine, such as gastrointestinal hormones.16,40 This model offers several advantages for our study at hand. First, the model allows collection of mesenteric lymph directly after a stimuli—in this case a dietary lipid challenge—and therefore allows measurements of substances before they enter the blood and become metabolized. Second, because the flow of lymph is much slower than the flow of portal blood (2–5 mL/h lymph vs 14–20 mL/min blood flow for an adult rat),41 substances secreted are less dilute if measured from the lymph. Third, many degrading enzymes are present at lower concentrations in lymph than in portal or systemic blood, such as dipeptidyl peptidase IV, which degrades the incretins.42 With lower levels of degrading enzymes, RMCPII or incretin concentrations are higher in lymph than in blood. We previously reported that RMCPII concentration was 10-fold higher in lymph than serum during fasting and 100-fold higher during the postprandial period,9 as measured by ELISA assay. Unfortunately, the ELISA kit is no longer commercially available and the only way to measure RMCPII is by Western blot analysis, which is not sensitive enough to detect its low levels in serum (data not shown). With its lymphatic levels being significantly higher, measuring RMCPII in lymph is the only sensitive way to detect changes in RMCPII section by MMCs. Fourth, the lymph fistula model allows us to measure the absorption of high-molecular-weight dextran into lymph as an indicator of epithelial barrier permeability. Fifth, dietary lipids can be administered directly into the duodenum, which bypasses the stomach and reduces variability related by stomach emptying. Finally, because the animals are conscious during the study, our experiments lack complications from anesthesia, which was previously shown to affect lymph flow and lipid transport.43
Previously, we demonstrated that dietary fat activates intestinal MMCs to release RMCPII.9 We confirm the data in this report and further show that this mechanism is linked to the presence of the gut microbiota (Figures 1A and B). These effects were not a result of direct acute effects of antibiotics (data not shown), and the effects were abolished when antibiotic-treated animals were allowed regrowth of their microbiota (Figure 6). Although the activation of intestinal MMCs partially depends on the production of chylomicrons,9 what remained unclear is how lipid absorption interacts with MMCs in order to induce their activation. Because MMCs are distributed in the lamina propria, it seemed unlikely that MMCs directly sense the fatty acids in the intestinal lumen. However, intestinal MMCs are well documented to contribute to colitis, secretory diarrhea, and food allergies, which reflects on the ability of MMCs to detect allergens or harmful bacterial products44,45 through receptor interactions, such as complement receptor 3,46,47 CD48,48 soluble CD14,13 and Toll-like receptors.49–51 Activated MMCs in these pathological states impair the intestinal barrier52–56 and increase permeability, which enhances the flux of bacterial products or food antigens into the intestinal tissue. It is not entirely clear whether MMCs are direct sensors of the antigens, are involved in crosstalk with other immune cells,57 or are activated by other signals derived from the gut.58 Nonetheless, in this study, we show that MMCs are less activated when the burden of gut bacteria is reduced significantly, suggesting a link between bacteria and the lipid-induced activation of mast cells.
Along with a reduction in MMC activation, we show that antibiotic treatment significantly decreased intestinal permeability in response to dietary lipid (Figure 2). Dietary lipid has been linked to increased intestinal permeability in a number of studies,33,59–61 which likely contributes to the pathogenesis of inflammatory bowel disease in humans.62 The activation of MMCs is also shown to affect intestinal permeability. RMPCII secreted by activated MMCs suppresses the expression of tight junction–associated proteins zonula occludens-1 and occludin.34 MMCs also release histamine, which increases lymph flow and vascular permeability30 and enables transport of chylomicrons across the lamina propria. This passage involves possible perforation of the basement membrane, which is coupled with loosened epithelial junctions. While we did not measure histamine release in this study, we did find that antibiotic treatment significantly lowered DAO activity in lymph (Figure 1C). DAO is a major enzyme responsible for catabolizing histamine, and is coupled with the release of histamine from MMCs by dietary fat.30 Therefore, the reduced permeability seen with antibiotic treatment may be due to reduced activation of MMCs and the secretion of their mediators.
Based on the data, we speculate that dietary fat absorption and chylomicron production damage the intestinal epithelium, leading to a flux of bacterial products into intestinal tissue, which consequently activates MMCs. Bacterial LPS has been shown to absorb concomitantly with dietary fat.7,11,12 In humans, fat intake (but not protein or carbohydrate intake) has been linked to endotoxemia in men on controlled diets.63 This result was mirrored in mice, where a 4-week, high-fat diet correlated with an increase in plasma LPS, although the effect was not seen under conditions of a high-carbohydrate diet.63 While plasma LPS was not measured in our study, the data combined with others support the idea that gut microbial products can be carried on chylomicrons and thus activate MMCs.
Unexpectedly, antibiotic treatment reduced the absorption of dietary lipid and production of chylomicrons, as measured by the intestinal output of triacylglycerol and phospholipid into lymph (Figure 3). Additionally, these effects were concomitant with reduced lymphatic outputs ApoB, ApoA-I, and ApoA-IV (Figure 4). ApoB is the main structural protein of a chylomicron and one ApoB protein exists on every chylomicron particle.64 ApoA-IV is presumed to facilitate the assembly of chylomicrons.65 These data suggest that chylomicron formation and secretion is dramatically affected under conditions of low microbiota. One variable that may affect lipid transport is intestinal motility, which has recently been shown to be altered by antibiotic treatment.38 However, we did not find an effect on motility by antibiotic treatment, as measured by the distribution of radiolabeled-lipid uptake along the length of the intestine (Figure 5A). Additionally, we did not see differences in pancreatic lipase activity (Figure 5B). It is not clear how antibiotic treatment affects apolipoprotein output in enterocytes because our dose of penicillin and streptomycin is not known to affect mammalian cells. The question of antibiotics treatment on the production and secretion of apolipoproteins is probably best studied in vitro, as there are too many variables in vivo.
The observations that short-term antibiotic treatment did not affect lymphatic lipid output, or markers of MMC activation ruled out the possibility of an independent effect of antibiotics on these parameters. A study that examined germ-free and conventionally raised mice concluded that the gut microbiota did not influence lipid absorption66; however, this conclusion was drawn from postprandial plasma triacylglycerol measurements after an intragastric lipid gavage. While the lymph fistula model allowed us to observe a profound influence of the microbiota on lipid transport into lymph, it remains unclear how the gut bacteria might affect the physiological process of fat absorption. Furthermore, animals with reduced gut microbiota resulting from antibiotic treatment are very different from animals raised in a germ-free environment. Thus, comparisons of findings from these studies should be interpreted with caution.
In conclusion, our studies suggest that the gut microbiota is a key player in the activation of intestinal MMCs by dietary lipid, which may lead to increased gut permeability. We also reveal a novel role of gut bacteria in facilitating the absorption of dietary lipids. Whether this effect is specific to fat or extends to carbohydrate or protein absorption remains to be investigated. A better understanding of this interaction may provide insight into the etiology and treatment of obesity and its associated complications.
Acknowledgments
Funding
This study was supported by grants DK092138, DK059630, and DK097268.
Abbreviations used in this paper
- Apo
apolipoprotein
- DAO
diamine oxidase
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- LPS
lipopolysaccharide
- MMC
mucosal mast cell
- OUT
operational taxonomic unit
- RMCPII
rat mucosal mast cell protease II
- rRNA
ribosomal RNA
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Forbes EE, Groschwitz K, Abonia JP, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 4.Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol. 2007;13:3027–3030. doi: 10.3748/wjg.v13.i22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 8.Lam YY, Ha CW, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Y, Sakata Y, Yang Q, et al. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1292–G1300. doi: 10.1152/ajpgi.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 11.Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal S, Witta J, Zhong J, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Brenner SA, Zacheja S, Schaffer M, et al. Soluble CD14 is essential for lipopolysaccharide-dependent activation of human intestinal mast cells from macroscopically normal as well as Crohn’s disease tissue. Immunology. 2014;143:174–183. doi: 10.1111/imm.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer S, Sellge G, Lorentz A, et al. Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J Immunol. 2008;181:1438–1445. doi: 10.4049/jimmunol.181.2.1438. [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Magnotti LJ, Ding J, et al. Influence of gut microflora on mesenteric lymph cytokine production in rats with hemorrhagic shock. J Trauma. 2002;52:1178–1185. doi: 10.1097/00005373-200206000-00026. disciussion 1185. [DOI] [PubMed] [Google Scholar]
- 16.Lu WJ, Yang Q, Sun W, et al. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1130–G118. doi: 10.1152/ajpgi.00400.2007. [DOI] [PubMed] [Google Scholar]
- 17.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med. 1948;33:1349–1352. [PubMed] [Google Scholar]
- 18.Forget P, Grandfils C, van Cutsem JL, et al. Diamine oxidase and disaccharidase activities in small intestinal biopsies of children. Pediatr Res. 1984;18:647–649. doi: 10.1203/00006450-198407000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 20.Joly Condette C, Khorsi-Cauet H, Morliere P, et al. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS One. 2014;9:e102217. doi: 10.1371/journal.pone.0102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi H, Nutting DF, Fujimoto K, et al. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res. 1990;31:1613–1625. [PubMed] [Google Scholar]
- 22.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vital M, Penton CR, Wang Q, et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1:8. doi: 10.1186/2049-2618-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis P, Young P, Holtrop G, et al. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 25.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson S, Mackeller A, Newlands GF, et al. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology. 1987;62:621–627. [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Y, Sakata Y, Li X, et al. Lymphatic diamine oxidase secretion stimulated by fat absorption is linked with histamine release. Am J Physiol Gastrointest Liver Physiol. 2013;304:G732–G740. doi: 10.1152/ajpgi.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwelberger HG, Hittmair A, Kohlwein SD. Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamm Res. 1998;47(Suppl 1):S60–S61. doi: 10.1007/s000110050273. [DOI] [PubMed] [Google Scholar]
- 32.Schayer RW, Kennedy J, Smiley RL. Studies on histamine-metabolizing enzymes in intact animals. II J Biol Chem. 1953;205:739–748. [PubMed] [Google Scholar]
- 33.Scudamore CL, Thornton EM, McMillan L, et al. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995;182:1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scudamore CL, Jepson MA, Hirst BH, et al. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur J Cell Biol. 1998;75:321–330. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- 35.Karpe F, Hamsten A, Uffelman K, et al. Apolipoprotein B-48. Methods Enzymol. 1996;263:95–104. doi: 10.1016/s0076-6879(96)63007-9. [DOI] [PubMed] [Google Scholar]
- 36.Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson BE, Midtvedt T, Strandberg K. Effects of microbial contamination on the cecum enlargement of germfree rats. Scand J Gastroenterol. 1970;5:309–314. [PubMed] [Google Scholar]
- 38.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazer AC, Sammons HG. The formation of mono- and di-glycerides during the hydrolysis of triglyceride by pancreatic lipase. Biochem J. 1945;39:122–128. doi: 10.1042/bj0390122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Yang Q, Huesman S, et al. The role of apolipoprotein A-IV in regulating glucagon-like peptide-1 secretion. Am J Physiol Gastrointest Liver Physiol. 2015;309:G680–G687. doi: 10.1152/ajpgi.00075.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Protoc Mouse Biol. 2012;2:219–230. doi: 10.1002/9780470942390.mo120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Alessio D, Lu W, Sun W, et al. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2163–R2169. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 43.Redgrave TG. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970;49:465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009;31:185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 45.Kraneveld AD, Sagar S, Garssen J, et al. The two faces of mast cells in food allergy and allergic asthma: the possible concept of Yin Yang. Biochim Biophys Acta. 2012;1822:93–99. doi: 10.1016/j.bbadis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Sher A, McIntyre SL. Receptors for C3 on rat peritoneal mast cells. J Immunol. 1977;119:722–725. [PubMed] [Google Scholar]
- 47.Sher A, Hein A, Moser G, et al. Complement receptors promote the phagocytosis of bacteria by rat peritoneal mast cells. Lab Invest. 1979;41:490–499. [PubMed] [Google Scholar]
- 48.Malaviya R, Gao Z, Thankavel K, et al. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci U S A. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Supajatura V, Ushio H, Nakao A, et al. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 50.McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol. 2001;70:977–984. [PubMed] [Google Scholar]
- 51.Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demaude J, Salvador-Cartier C, Fioramonti J, et al. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrier L, Berard F, Debrauwer L, et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 55.McDermott JR, Bartram RE, Knight PA, et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderholm JD, Yang PC, Ceponis P, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 57.Bischoff SC, Kramer S. Human mast cells, bacteria, and intestinal immunity. Immunol Rev. 2007;217:329–337. doi: 10.1111/j.1600-065X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 58.van Diest SA, Stanisor OI, Boeckxstaens GE, et al. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012;1822:74–84. doi: 10.1016/j.bbadis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreira AP, Texeira TF, Ferreira AB, et al. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 61.Kirpich IA, Feng W, Wang Y, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 63.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 64.Albers JJ, Kennedy H, Marcovina SM. Evidence that Lp [a] contains one molecule of apo[a] and one molecule of apoB: evaluation of amino acid analysis data. J Lipid Res. 1996;37:192–196. [PubMed] [Google Scholar]
- 65.Weinberg RB, Gallagher JW, Fabritius MA, et al. ApoA-IV modulates the secretory trafficking of apoB and the size of triglyceride-rich lipoproteins. J Lipid Res. 2012;53:736–743. doi: 10.1194/jlr.M019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velagapudi VR, Hezaveh R, Reigstad CS, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]