Abstract
Backround
Crohn’s disease (CD) is a chronic inflammatory enteropathy characterized by fibrotic strictures. Myofibroblasts (MFB) are stromal cells of the GI tract found in increased numbers in patients with CD and represent the key effector cells involved in pathologic fibrosis. MFB are a known target of TNF-α, a pro-inflammatory cytokine strongly implicated in the pathophysiology of CD. However, the precise mechanisms through which TNF-α contributes to fibrosis remain incompletely understood. Here, we demonstrate for the first time that TNF-α increases MFB migration through the COX-2 and Hsp27 pathways.
Materials and Methods
The human colonic MFB cell line 18Co was grown to confluence on 35×10mm cell culture dishes and used from passages 8–14. An in vitro scratch assay assessed the effect of TNF-α (10 ng/ml) on MFB migration over 24 hours in the presence or absence of several inhibitors (NS398, SB203580, Hsp27 siRNA).
Results
TNF-α significantly increased MFB migration over 24 h. TNF-α also led to the increased expression of COX-2 and stimulated rapid phosphorylation of Hsp27 at Ser-82. TNF-α-induced-COX-2 expression, Hsp27 phosphorylation, and MFB migration were all significantly inhibited by the P38 MAPK inhibitor SB203580 (p<0.05). TNF-α-induced MFB migration was also significantly inhibited by NS398 (p<0.05), a direct inhibitor of COX-2, and by siRNA targeting Hsp27 (p<0.05).
Conclusions
TNF-α stimulates colonic myofibroblast migration through P38 MAPK-mediated activation of COX-2 and Hsp27. Further elucidating these inflammatory signaling pathways may lead to novel therapeutic targets for the treatment of Crohn’s disease related fibrosis and strictures.
Keywords: TNF-α, migration, COX-2, myofibroblast, Hsp27
INTRODUCTION
Crohn’s disease is a chronic, relapsing, immune-mediated condition of the gastrointestinal (GI) tract that is characterized by segmental, transmural inflammation of the bowel wall. The reparative process following recurrent, transmural injury involves reorganization of the stroma and extracellular matrix that leads to fibrosis and the development of intestinal strictures through processes that remain incompletely understood. Myofibroblasts are gastrointestinal stromal cells that regulate the extracellular matrix and are a target of inflammatory mediator signaling [1–4]. Located in the lamina propria, myofibroblasts interact with the overlying epithelium as well as neighboring cell populations to regulate a number of important cellular processes, including mucosal repair, and fibrosis [5], through the secretion of cytokines, growth factors, and inflammatory mediators
TNF-α, a potent 17-kDa pro-inflammatory cytokine, is strongly implicated in the pathogenesis of Crohn’s disease [3, 6] and is known to regulate myofibroblast function [1–3]. TNF-α binds to TNF-α receptor 1 (TNFR1) and TNF-α receptor 2 (TNFR2), leading to a conformational change that creates a multiprotein complex (TRADD, RIP, TRAF-2) resulting in the activation of MAP kinases, including JNK, p38-MAPK, and ERK, as well as the transcription factor NFκB [reviewed in [7]]. Monoclonal antibodies (infliximab, adalimumab, etc) that target TNF-α have been proven to be effective in the treatment of patients with Crohn’s disease, demonstrating the central role that TNF-α plays in the pathophysiology of this disease [1, 8, 9]. However, the exact role of TNF-α in the development of intestinal fibrosis remains unclear. Consequently, the purpose of this study was to determine whether TNF-α regulates the migration of human colonic myofibroblasts, and to determine the underlying cell signaling mechanisms that are involved in this process.
Here, we demonstrate, for the first time, that TNF-α stimulates the migration of human colonic myofibroblasts (18Co) through signaling pathways that involve P38 MAPK, COX-2, and Hsp27. Our results support the notion that TNF-α regulates colonic myofibroblast function in the setting of inflammation and may explain how TNF-α leads to progressive intestinal fibrosis in the setting of recurrent transmural inflammation.
MATERIALS AND METHODS
Cell Culture
CCD-18CO cells were purchased from American Type Culture Collection (Manassas, VA). These cells were originally cultured from a mucosal biopsy of human neonatal colon and are structurally and functionally similar to in situ colonic subepithelial myofibroblasts. This has been validated in numerous previous studies [10–13]. CCD-18CO cells were cultured in DMEM containing 10% FBS, penicillin, streptomycin, fungizone and glutamine at 37 °C in humidified air containing 5% CO2. Cells were passaged in 0.25% trypsin-0.02% EDTA solution. Experiments were performed with cells within passages 8–14.
SDS-PAGE and Immunoblotting
Cells were lysed in Triton buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 5 mM sodium pyrophosphate, 10 mM sodium glycerophosphate, 1% Triton X-100, 50 mM NaF plus 1% Calbiochem Protease Inhibitor Cocktail) and lysates were assayed for protein using the Bradford protein assay and then diluted with 5× Laemmli loading buffer for SDS-PAGE. Equal amounts of protein were then loaded in 4–20% Tris/glycine gels, and electrophoresed for 120 min at 130 Volts constant voltage. Next, the gel was blotted onto a PVDF membrane by electrophoretic transfer at 25 V constant voltage overnight. The membrane was then washed, blocked with 5% milk, and probed with primary antibodies. Appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL) and a chemiluminescent substrate (SuperSignal, Pierce, Rockford, IL) were used to visualize immunoreactive bands. The primary antibodies against pHSP27 and COX2 were purchased from Cell Signaling (Danvers, MA), antibodies against HSP27 and GAPDH were purchased from Santa Cruz (Santa Cruz, CA). Donkey anti-mouse and donkey anti-rabbit secondary antibodies were purchased from Pierce (Rockford, IL).
Scratch Assay
For migration assays, cells were plated in 35 mm dishes until confluent, then the cell monolayer was scratched with a sterile pipette tip to make the scratch gap. Old medium was removed and replaced with serum free medium. Three pictures were randomly taken along the scratch gap and pictured positions were marked with marker (time 0 h). Cells were then cultured for another 18 or 24 h with treatment. Then three more pictures were taken at the same positions as marked previously. Decrease in the scratch area was measured with software Image J and normalized to time 0 control respectively. Cells were treated with SB203580 (5 μM) or NS398 (10 μM) or HSP27 siRNA (100 nM) during the migration test.
SiRNA Transfection
80%–90% confluent cells were transfected with HSP27 siRNA (Cat # 4392420 Ambion, CA) or negative control siRNA (Cat # 4611 Ambion, CA) with Lipofectamine 2000 (Invitrogen, CA) at 100 nM according to the manufacturer’s instructions. Western blotting was then performed to test the silencing effect of siRNA on the expression levels of target proteins.
Materials and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin G potassium, streptomycin, fungizone, and glutamine were purchased from Invitrogen (Carlsbad, CA). TNF-α was purchased from R&D Systems (Minneapolis, MN). HSP27-siRNA was purchased from Ambion (Carlsbad, CA). NS398 was purchased from Calbiochem (San Diego, CA). SB203580 was purchased from Santa Cruz (Dallas, TX).
RESULTS
TNF-α stimulates myofibroblast migration via P38 MAPK
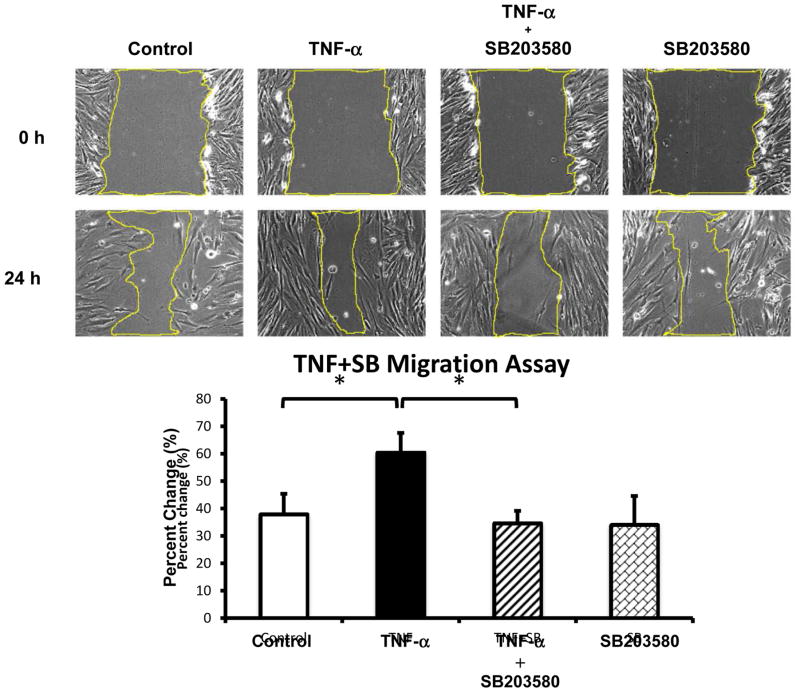
To determine whether TNF-α regulates myofibroblast migration, 18Co cells grown to confluence on a 35mm cell culture plate were scratched with a sterile pipette tip to create a scratch gap, the area of which was measured before and after exposure to TNF-α (10 ng/ml) for 24 h. As demonstrated in Fig 1, after 24 h untreated myofibroblasts migrated to decrease the size of the scratch gap area. However, exposure of 18Co cells to TNF-α enhanced myofibroblast migration, leading to a significant decrease in the scratch gap area compared to untreated cells (p<0.05). This effect was blocked by pre-treatment with the P38 MAPK inhibitor SB203580.
Figure 1. TNF-α stimulates myofibroblast migration via p38 MAPK.
The surface of a confluent monolayer of 18Co cells was scratched with a sterile pipette tip and incubated in serum free medium. Three pictures were randomly taken along the scratch gap and pictured positions were marked (time 0 h). Cells were then treated with 10 ng/ml TNF-α for 24 h, in the presence or absence of SB203580 (5 μM), an inhibitor of p38 MAPK. Three pictures were taken after 24 h at the same positions as marked previously. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as percentage change in scratch gap area. * denotes p < 0.05.
TNF-α leads to enhanced COX-2 expression via P38 MAPK
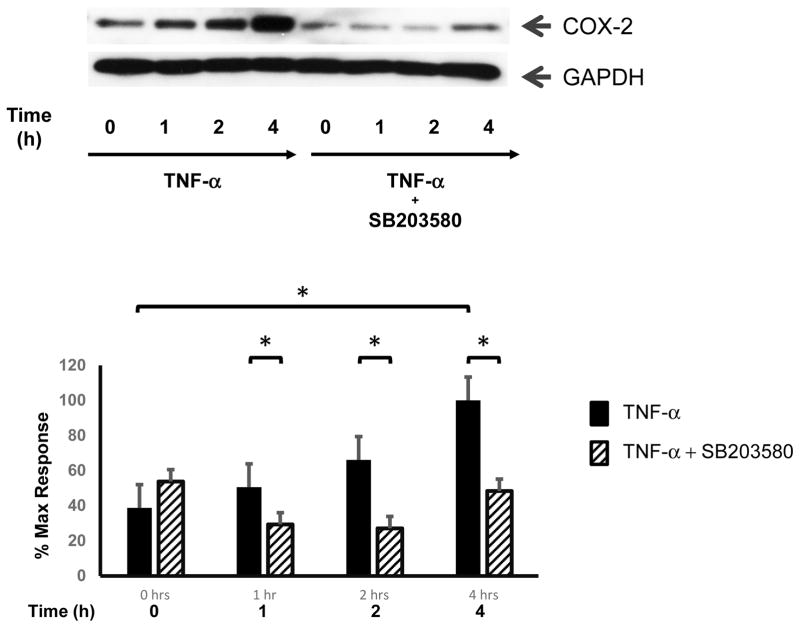
Cyclo-oxygenase-2 (COX-2), also known as prostaglandin G/H synthase 2 (PTGS2), is the rate-limiting enzyme that catalyzes the biosynthesis of prostaglandins G2 and H2, the precursors of prostaglandins (PGs) and thromboxanes [14]. COX-2 and its downstream products have been implicated in promoting cell migration in a variety of cell types [15–17]. To determine whether TNF-α-induced COX-2 expression was involved in myofibroblast migration, 18Co cells were first exposed to TNF-α (10 ng/ml) over 4 h and COX-2 expression was analyzed by Western blot. As shown in Fig 2, exposure of 18Co cells to TNF-α led to a time-dependent increase in COX-2 expression which was statistically significant at 4 h, consistent with previous results [1, 18]. The increased expression of COX-2 induced by TNF-α was inhibited by the P38 MAPK inhibitor SB203580, as was TNF-α-induced myofibroblast migration (Fig 1), suggesting that myofibroblast migration could be mediated by COX-2. Further experimentation was performed to explore this possibility.
Figure 2. TNF-α leads to enhanced COX-2 expression via p38 MAPK.
Confluent 18Co cells were washed and equilibrated in serum-free media for 30 min, then exposed to 10 ng/ml TNF-α for 4 h in the presence or absence of SB203580 (5 μM). Cell lysates were then analyzed by SDS-PAGE and Western blot using an antibody that detects COX-2 protein expression. TNF-α induced a statistically significant increase in COX-2 expression at 4 h, an effect that was inhibited by SB203580. The results shown are the mean ± S.E. n ≥ 3 and are expressed as a percentage of the maximum level of COX-2 expression, displayed in graphical form below. Equal protein loading was verified using an antibody that detects GAPDH. * denotes p < 0.05.
TNF-α leads to rapid and sustained Hsp27 phosphorylation via P38 MAPK
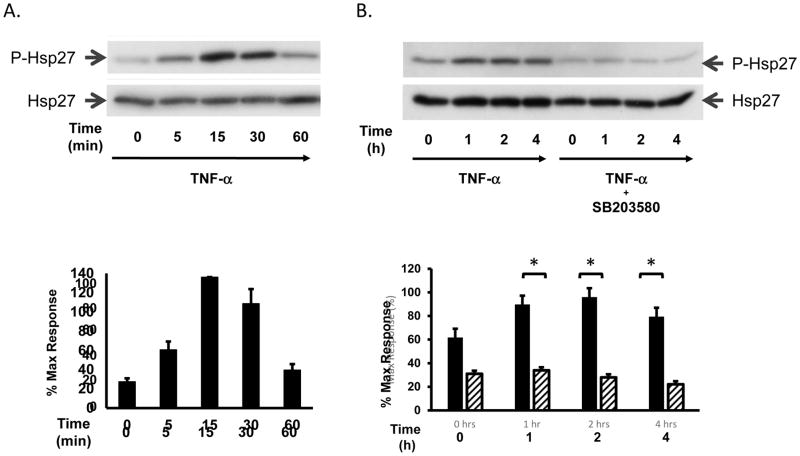
Heat shock protein 27 (Hsp27) is a molecular chaperone regulated by the HSPB1 gene, which is involved in many cellular processes including fibrosis [19, 20], the response to stress [21], and tumorigenesis [21, 22]. Hsp27 is regulated by post-translational phosphorylation at Serine (Ser)-15, -78, -82 and Threonine (Thr-143) residues induced by various kinases [21, 23] and is known to interact with actin and other cytoskeletal elements that may be involved in wound healing and cell migration [19, 24]. As a known downstream target of TNF-α-induced signaling, we sought to determine whether the increase in myofibroblast migration induced by TNF-α was associated with increased signaling activity of Hsp27. 18Co cells were incubated with 10 ng/ml TNF-α and Hsp27 phosphorylation at Serine residue 82 was assessed by Western blot using a site-specific antibody. As shown in Fig 3A, exposure of 18Co cells to TNF-α induced a rapid phosphorylation of Hsp27, evident after 5 min. TNF-α-induced Hsp27 phosphorylation peaked at 15 min followed by a sustained phosphorylation over 4 h (Fig 3B). TNF-α-induced Hsp27 phosphorylation was also completely inhibited by pre-treatment with the P38 MAPK inhibitor SB203580 (Fig 3B).
Figure 3. TNF-α leads to rapid and sustained Hsp27 phosphorylation via p38 MAPK.
Panel A: Confluent 18Co cells were incubated with 10 ng/ml TNF-α for 60 min. The cells were analyzed by Western blot using an antibody that detects Hsp27 phosphorylation at serine residue 82. Equal protein loading was verified using an antibody that detects total Hsp27. The results of three independent experiments is depicted in graphical form below, presented as mean ± S.E. and expressed as a percentage of the maximum level of phosphorylated Hsp27. Panel B: Confluent 18Co cells were incubated with 10 ng/ml TNF-α for 4 h, in the presence or absence of SB203580 (5 μM). The cells were analyzed by Western blot using an antibody that detects Hsp27 phosphorylation at serine residue 82. Equal protein loading was verified using an antibody that detects total Hsp27. The results of three independent experiments are depicted in graphical form below, presented as mean ± S.E. and expressed as a percentage of the maximum level of phosphorylated Hsp27. The solid bars indicate 18Co cells exposed to TNF-α alone. The lined bars depict 18Co cells exposed to TNF-α and SB203580. * denotes p < 0.05.
TNF-α induced Myofibroblast Migration is Inhibited by NS398
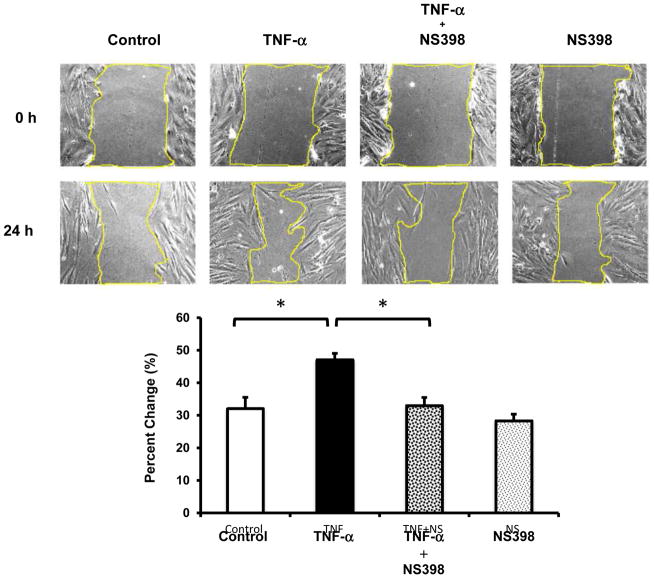
COX-2 is a known downstream target of TNF-α in colonic myofibroblasts [1, 4, 18, 25], is up-regulated in the setting of intestinal inflammation [26, 27], and regulates cell migration in a number of cell types [15–17]. Having demonstrated that TNF-α stimulates COX-2 expression in a P38 MAPK-dependent manner, we sought to determine whether COX-2 was involved in TNF-α-mediated myofibroblast migration. After creation of a scratch gap, confluent 18Co cells were exposed to TNF-α (10 ng/ml) for 24 h in the presence or absence of NS398 (10 μM), a COX-2-specific inhibitor. 18Co cells exposed to TNF-α demonstrated enhanced migration that was statistically significant compared to untreated cells (Fig 4, p<0.05). While exposure to NS398 alone had no effect on myofibroblast migration, NS398 significantly inhibited TNF-α-induced myofibroblast migration (Fig 4).
Figure 4. TNF-α-induced myofibroblast migration is inhibited by NS398.
The surface of a confluent monolayer of 18Co cells was scratched with a sterile pipette tip and incubated in serum free medium. Three pictures were randomly taken along the scratch gap and pictured positions were marked (time 0 h). Cells were then treated with 10 ng/ml TNF-α for 24 h, in the presence or absence of NS398 (10 μM), a COX-2-specific inhibitor. Three pictures were taken after 24 h at the same positions as marked previously. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as percentage change in scratch gap area. * denotes p < 0.05.
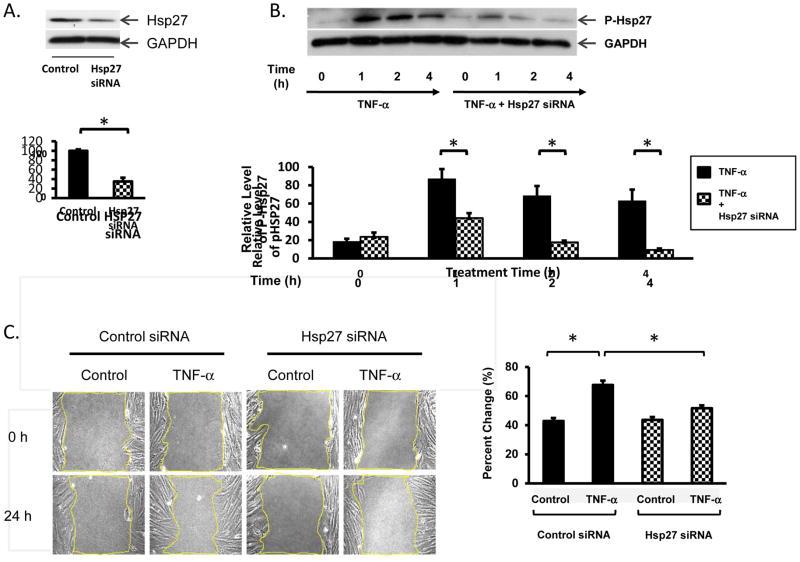
TNF-α-induced Myofibroblast Migration is Inhibited by Hsp27 siRNA
Exposure to TNF-α led to enhanced Hsp27 phosphorylation and a striking increase in myofibroblast migration, effects that were both markedly inhibited by the P38 MAPK inhibitor SB203580. To determine whether Hsp27 was also involved in TNF-α-induced myofibroblast migration, we tested whether siRNA-mediated knockdown of Hsp27 protein in 18Co cells was feasible and whether Hsp27 siRNA had any effect on TNF-α-induced myofibroblast migration. Lysates of 18Co cells treated with targeting or non-targeting Hsp27 siRNAs were analyzed by Western blot to assess the level of Hsp27. As shown in Fig 5A, siRNA targeting Hsp27 produced a significant depletion of Hsp27 protein in 18Co cells. Importantly, this level of Hsp27 depletion correlated with a significant decrease in the level of Hsp27 phosphorylation induced by TNF-α (Fig 5B). Having demonstrated that Hsp27 siRNA is effective in reducing both the expression and phosphorylation of Hsp27 in 18Co cells, we sought to determine what role Hsp27 may have on colonic myofibroblast migration. As shown in Fig 5C, 18Co cells transfected with Hsp27 siRNA demonstrated a significant reduction in myofibroblast migration following exposure to TNF-α compared to 18Co cells transfected with non-targeted siRNA. The results indicate that Hsp27 plays an important role in myofibroblast migration following exposure to TNF-α.
Figure 5. TNF-α-induced myofibroblast migration is inhibited by Hsp27 siRNA.
Panel A: 18Co cells were transfected with either nontargeting (NT) short interfering RNA (siRNA) or with Hsp27 siRNA at 100 nM for 36 h. The level of Hsp27 protein was analyzed by Western blot. The expression of Hsp27 in 18Co cells transfected with Hsp27 siRNA was 35.4 ± 8% compared to 18Co cells transfected with nontargeting siRNA. Antibody against GAPDH was used to verify equal protein loading. Band intensity was analyzed by densitometric scanning and is presented as mean ± SE, n = 3, and expressed as a percentage of the maximum level of Hsp27. * denotes p < 0.05. Panel B: 18Co cells were transfected with 100 nM Hsp27 siRNA or with a non-targeting sequence at the same concentration, as described in Panel A, followed by incubation with TNF-α for 1, 2, and 4 h. Cell lysates were analyzed by Western blot using an antibody specific for phosphorylated Hsp27 at serine residue 82. Antibody against GAPDH was used to verify equal protein loading. Band intensity was analyzed by densitometric scanning and is presented as mean ± SE, n = 3, and expressed as a percentage of the maximum level of phosphorylated Hsp27 at Ser-82. * denotes p < 0.05. Panel C: 18Co cells were transfected with 100 nM Hsp27 siRNA or with a non-targeting sequence as described in Panel A. The surface was then scratched with a sterile pipette tip and cells were treated with 10 ng/ml TNF-α for 24 h. The scratch gap area was measured with software Image J and normalized to time 0 control. The results shown are the mean ± S.E. n ≥ 3 and are expressed as percentage change in scratch gap area. * denotes p < 0.05.
DISCUSSION
Advancements in medical therapies have improved our ability to treat acute Crohn’s disease exacerbations. However, intestinal strictures may still result as a long-term sequelae of episodic transmural injury, often requiring endoscopic or surgical intervention. Myofibroblast migration is an important aspect of wound healing, allowing myofibroblasts to travel towards a wounded area and regulate the extracellular matrix through secretion of matrix metalloproteinases [4] and the deposition of collagen [28, 29].
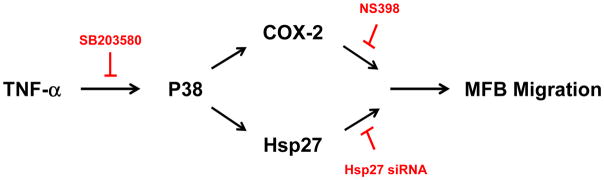
In the present study we demonstrate that TNF-α stimulates myofibroblast migration through signaling pathways that involve P38 MAPK, COX-2, and Hsp27. This is supported by several lines of evidence. First, myofibroblasts exposed to TNF-α demonstrated a significant decrease in scratch gap area compared to untreated cells, a phenomenon that was preceded temporally by TNF-α-induced Hsp27 phosphorylation and COX-2 expression. TNF-α-induced Hsp27 phosphorylation, COX-2 expression, and myofibroblast migration were all inhibited by the P38 MAPK inhibitor SB203580. Furthermore, TNF-α-induced myofibroblast migration was inhibited by both NS398, a COX-2 specific inhibitor, as well as by siRNA targeting Hsp27 (Fig 6). While wound healing may involve both cell migration and proliferation, TNF-α had no effect on myofibroblast proliferation following exposure for 24 h (data not shown), suggesting that the decrease in wound area was not due to an increase in myofibroblast cell number.
Figure 6. Proposed signaling pathways involved in TNF-α-induced myofibroblast migration.
TNF-α stimulated Hsp27 phosphorylation, COX-2 expression, and myofibroblast migration through the P38 MAPK pathway. Myofibroblast migration was inhibited by SB203580, an inhibitor of P38 MAPK, Hsp27 siRNA, and NS398, a COX-2-specific inhibitor. P38: p38 MAPK. MFB: myofibroblast. COX-2: cyclo-oxygenase-2.
Previous studies suggest that COX-2 can regulate myofibroblast migration in an autocrine fashion in both stimulated and unstimulated cells [30–32], though enhanced myofibroblast migration has not been previously demonstrated following exposure to TNF-α. In patients with Crohn’s disease, the stromal compartment has been identified as the major reservoir of COX-2 derived by-products [33–35]. The local production of prostaglandins may have multiple effects on different cell populations, representing a cytoprotective response that not only promotes mucosal healing but also regulates the stromal reaction to transmural injury. In the present study, we have identified a plausible mechanism for myofibroblast migration directly attributable to enhanced stromal COX-2 expression induced by TNF-α.
Hsp27 has been implicated in the regulation of the extracellular matrix [19], the development of fibrosis [20], and cell migration [22], and the P38 MAPK/Hsp27 signaling pathway has been previously implicated in the regulation of cell migration, though in different cell types [36, 37]. Here, we demonstrate that the P38 MAPK/Hsp27 pathway is also involved in the regulation of myofibroblast migration in the setting of inflammation, with siRNA targeting Hsp27 resulting in a near complete inhibition of TNF-α-induced myofibroblast migration. Interestingly, both NS398 and Hsp27 siRNA independently inhibited TNF-α-induced myofibroblast migration, implying that both inputs may be necessary to stimulate myofibroblast migration. Whether these signaling pathways occur in series or in parallel, or whether inhibition of one pathway can be compensated by upregulation of the other remains to be determined, and would depend largely on which specific aspects of migratory function are targeted by COX-2 and Hsp27. Cell migration involves many overlapping and independent processes that regulate cell adhesion, collagen production, modification of the extracellular matrix scaffolding, and the spatial arrangement and polymerization of the actin cytoskeleton.
In conclusion, the present study supports the notion that TNF-α utilizes COX-2 and Hsp27 signaling via P38 MAPK to mediate myofibroblast migration. A growing body of evidence suggests that the myofibroblast plays an important role in the pathophysiology of Crohn’s disease. Future studies will explore how therapies targeting COX-2 and Hsp27 signaling pathways within the myofibroblast could be used in combination with anti-TNF-α therapies for the prevention of Crohn’s-disease related strictures.
Acknowledgments
This work was supported by National Institutes of Health Grant 5KO8DK085136-06 to JY.
Footnotes
Author Contributions: SS - study conception and design, data acquisition, data analysis, article drafting, article revision, final approval; TL – data acquisition, data analysis, article drafting, article revision, final approval; JY – study conception and design, data analysis, article drafting, article revision, final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodriguez Perez CE, et al. TNF-alpha potentiates lysophosphatidic acid-induced COX-2 expression via PKD in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G637–46. doi: 10.1152/ajpgi.00381.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo J, et al. Protein kinase D mediates synergistic expression of COX-2 induced by TNF-{alpha} and bradykinin in human colonic myofibroblasts. Am J Physiol Cell Physiol. 2009;297(6):C1576–87. doi: 10.1152/ajpcell.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armaka M, et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205(2):331–7. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo J, et al. Protein kinase D1 mediates synergistic MMP-3 expression induced by TNF-alpha and bradykinin in human colonic myofibroblasts. Biochem Biophys Res Commun. 2011;413(1):30–5. doi: 10.1016/j.bbrc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell DW, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm SM, et al. A review of infliximab use in ulcerative colitis. Clin Ther. 2008;30(2):223–30. doi: 10.1016/j.clinthera.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 10(1):45. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 8.Khoa ND, et al. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69(4):1311–9. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- 9.Grounds MD, et al. Implications of cross-talk between tumour necrosis factor and insulin-like growth factor-1 signalling in skeletal muscle. Clin Exp Pharmacol Physiol. 2008;35(7):846–51. doi: 10.1111/j.1440-1681.2007.04868.x. [DOI] [PubMed] [Google Scholar]
- 10.Valentich JD, et al. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol. 1997;272(5 Pt 1):C1513–24. doi: 10.1152/ajpcell.1997.272.5.C1513. [DOI] [PubMed] [Google Scholar]
- 11.Shao J, et al. Roles of Myofibroblasts in Prostaglandin E2-Stimulated Intestinal Epithelial Proliferation and Angiogenesis. Cancer Res. 2006;66(2):846–855. doi: 10.1158/0008-5472.CAN-05-2606. [DOI] [PubMed] [Google Scholar]
- 12.Laurens K, et al. Myofibroblast Matrix Metalloproteinases Activate the Neutrophil Chemoattractant CXCL7 From Intestinal Epithelial Cells. Gastroenterology. 2006;130(1):127. doi: 10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Pacheco II, MacLeod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G748–759. doi: 10.1152/ajpgi.00560.2007. [DOI] [PubMed] [Google Scholar]
- 14.Marnett LJ, DuBois RN. COX-2: A Target for Colon Cancer Prevention. Annual Review of Pharmacology and Toxicology. 2002;42(1):55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 15.Saalbach A, et al. Fibroblasts support migration of monocyte-derived dendritic cells by secretion of PGE2 and MMP-1. Exp Dermatol. 2015;24(8):598–604. doi: 10.1111/exd.12722. [DOI] [PubMed] [Google Scholar]
- 16.Lu DY, et al. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J Cell Biochem. 2010;110(1):141–50. doi: 10.1002/jcb.22520. [DOI] [PubMed] [Google Scholar]
- 17.Mayoral R, et al. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis. 2005;26(4):753–61. doi: 10.1093/carcin/bgi022. [DOI] [PubMed] [Google Scholar]
- 18.Yoo J, et al. TNF-alpha and LPA promote synergistic expression of COX-2 in human colonic myofibroblasts: role of LPA-mediated transactivation of upregulated EGFR. BMC Gastroenterol. 2013;13:90. doi: 10.1186/1471-230X-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez E, et al. Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, alpha2beta1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg. 2013;131(2):158e–173e. doi: 10.1097/PRS.0b013e3182789b2b. [DOI] [PubMed] [Google Scholar]
- 20.Wettstein G, et al. Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 2013;27(4):1549–60. doi: 10.1096/fj.12-220053. [DOI] [PubMed] [Google Scholar]
- 21.Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang RC, et al. Proteomic Characterization of Annexin l (ANX1) and Heat Shock Protein 27 (HSP27) as Biomarkers for Invasive Hepatocellular Carcinoma Cells. PLoS One. 2015;10(10):e0139232. doi: 10.1371/journal.pone.0139232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103(2):648–62. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 24.Shin KD, et al. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem. 2005;280(50):41439–48. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 25.Yoo J, et al. TNF-alpha induces upregulation of EGFR expression and signaling in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G805–14. doi: 10.1152/ajpgi.00522.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, DuBois RN. PPARdelta and PGE signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal. 2014;1(6) doi: 10.14800/ics.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–8. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Mia MM, Bank RA. The pro-fibrotic properties of transforming growth factor on human fibroblasts are counteracted by caffeic acid by inhibiting myofibroblast formation and collagen synthesis. Cell Tissue Res. 2015 doi: 10.1007/s00441-015-2285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillibert-Duplantier J, et al. Thrombin inhibits migration of human hepatic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G128–36. doi: 10.1152/ajpgi.00031.2007. [DOI] [PubMed] [Google Scholar]
- 31.Fernando MR, Giembycz MA, McKay DM. Bidirectional crosstalk via IL-6, PGE and PGD between murine myofibroblasts and alternatively activated macrophages enhances anti-inflammatory phenotype in both cells. Br J Pharmacol. 2015 doi: 10.1111/bph.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwanaga K, et al. Prostaglandin E2 promotes wound-induced migration of intestinal subepithelial myofibroblasts via EP2, EP3, and EP4 prostanoid receptor activation. J Pharmacol Exp Ther. 2012;340(3):604–11. doi: 10.1124/jpet.111.189845. [DOI] [PubMed] [Google Scholar]
- 33.Adegboyega PA, et al. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res. 2004;10(17):5870–9. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 34.Benamouzig R, et al. Cyclooxygenase-2 expression and recurrence of colorectal adenomas: effect of aspirin chemoprevention. Gut. 2010;59(5):622–9. doi: 10.1136/gut.2008.175406. [DOI] [PubMed] [Google Scholar]
- 35.Omura N, et al. Cyclooxygenase-deficient pancreatic cancer cells use exogenous sources of prostaglandins. Mol Cancer Res. 2010;8(6):821–32. doi: 10.1158/1541-7786.MCR-09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo Y, et al. Stimulation of Cell Migration by Flagellin Through the p38 MAP Kinase Pathway in Cultured Intestinal Epithelial Cells. J Cell Biochem. 2015 doi: 10.1002/jcb.25272. [DOI] [PubMed] [Google Scholar]
- 37.Usui T, et al. Eukaryotic elongation factor 2 kinase controls proliferation and migration of vascular smooth muscle cells. Acta Physiol (Oxf) 2015;213(2):472–80. doi: 10.1111/apha.12354. [DOI] [PubMed] [Google Scholar]