Abstract
Vitamin D controls calcium homeostasis and the development and maintenance of bones through vitamin D receptor activation. Prolonged therapy with rifampicin or phenobarbital has been shown to cause vitamin D deficiency or osteomalacia, particularly in patients with marginal vitamin D stores. However, the molecular mechanism of this process is unknown. Here we show that these drugs lead to the upregulation of 25-hydroxyvitamin D3-24-hydroxylase (CYP24) gene expression through the activation of the nuclear receptor pregnane X receptor (PXR; NR1I2). CYP24 is a mitochondrial enzyme responsible for inactivating vitamin D metabolites. CYP24 mRNA is upregulated in vivo in mice by pregnenolone 16α-carbonitrile and dexamethasone, 2 murine PXR agonists, and in vitro in human hepatocytes by rifampicin and hyperforin, 2 human PXR agonists. Moreover, rifampicin increased 24-hydroxylase activity in these cells, while, in vivo in mice, pregnenolone 16α-carbonitrile increased the plasma concentration of 24,25-dihydroxyvitamin D3. Transfection of PXR in human embryonic kidney cells resulted in rifampicin-mediated induction of CYP24 mRNA. Analysis of the human CYP24 promoter showed that PXR transactivates the sequence between –326 and –142. We demonstrated that PXR binds to and transactivates the 2 proximal vitamin D–responsive elements of the human CYP24 promoter. These data suggest that xenobiotics and drugs can modulate CYP24 gene expression and alter vitamin D3 hormonal activity and calcium homeostasis through the activation of PXR.
Introduction
Vitamin D is essential for the maintenance of calcium homeostasis and for the development and maintenance of bones. Before acquiring its biological potential, vitamin D is transformed into 25-hydroxyvitamin D3 [25(OH)D3], the major form of vitamin D in the circulation, by two 25-hydroxylases (the mitochondrial CYP27A, and the microsomal CYP2R1) mainly expressed in the liver (1, 2). The 25(OH)D3 is further hydroxylated in the 1α position to form 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], by the CYP27B1 mainly expressed in the kidney (3). The active form of vitamin D3, 1α,25(OH)2D3, elicits most of its biological effects by binding to a high-affinity receptor, the vitamin D receptor (VDR; NR1I1) (4). After binding 1α,25(OH)2D3, VDR forms heterodimers with the retinoid X receptor (RXR; NR2B1) and then binds to and transactivates the vitamin D–responsive elements (VDREs) present in the regulatory region of target genes (5). The classical VDREs consist of a direct repeat of nuclear receptor half-sites separated by 3 nucleotides (DR3) (6). In the classical vitamin D–responsive organs, including the intestine, bone, kidney, and parathyroid gland, vitamin D3–activated VDR plays a central role in the regulation of calcium and phosphate homeostasis, bone mineralization, inhibition of cell growth, and parathyroid hormone synthesis (3, 7). VDR is also expressed in many other nonclassical vitamin D–responsive organs, including the liver (8, 9).
The potent role of 1α,25(OH)2D3 in the regulation of serum calcium and phosphate levels requires a tight control of its blood concentration. This is reached through the balance between the rates of synthesis and degradation. CYP24 is a multifunctional 24-hydroxylase and the major catabolic enzyme of vitamin D (10). It directs the side-chain oxidation and cleavage of 1,25(OH)2D and 25(OH)D to catabolic carboxylic acid end products. Notably, this enzyme converts 1α,25(OH)2D3 into 1α,24,25(OH)3D3, a product with decreased hormonal activity (3), and further into calcitroic acid, known as the final metabolite of 1α,25(OH)2D3 excreted in bile. Hypocalcemia increases the serum levels of 1α,25(OH)2D3 through stimulation of 1α-hydroxy-lase (2) and suppresses the CYP24 activity through increased levels of parathyroid hormone (10). Inversely, hypercalcemia depresses 1α-hydroxylase and increases the 24-hydroxylase activity, and high levels of 1α,25(OH)2D3 induce the 24-hydroxylase activity through VDR (11). The important role of the CYP24 gene has been further demonstrated in CYP24 knockout mice, which exhibit high ambient 1α,25(OH)2D3 levels and abnormal bone mineralization (12).
Osteomalacia is a metabolic bone disease, characterized by a defect of bone mineralization that can be caused by vitamin D deficiency or genetic alterations of vitamin D metabolism, vitamin D action, or other factors involved in the phosphocalcic metabolism. This pathology has also been suspected to result from the adverse effects of drugs. Some of the drugs most frequently associated with osteomalacia are phenobarbital, phenytoin, rifampicin, and carbamazepine (13–17). Indeed, long-term antiepileptic drug therapy, such as phenobarbital or carbamazepine, is a known risk factor for bone loss and fractures (13). In addition, prolonged therapy with rifampicin can cause vitamin D deficiency or osteomalacia (18), particularly in patients with marginal vitamin D stores (19). Notably, 2 weeks of rifampicin treatment reduces the levels of circulating 25-hydroxyvitamin D and 1α,25-dihydroxyvitamin D in healthy subjects (20). The molecular mechanism of this drug-induced osteomalacia or vitamin deficiency is unknown. However, one interesting feature is that rifampicin (21), phenobarbital (22), phenytoin (23), and carbamazepine (24) are inducers of the hepatic cytochrome P450s (CYPs) involved in drug metabolism, i.e., CYP2B and CYP3A. These observations suggested to us that the orphan nuclear receptor pregnane X receptor (PXR; NR1I2 [ref. 25]) might be involved in this phenomenon. PXR has been shown to mediate CYP2 and CYP3 gene induction (25, 26) and is activated by a wide variety of commonly used drugs, notably rifampicin (25) and phenobarbital (25) but also carbamazepine (27) and phenytoin (27).
In close similarity to VDR and the enzymes involved in vitamin D biosynthesis and metabolism, PXR is expressed in intestine, kidney, and liver (25, 28), and both receptors share around 60% similarity of amino acids in their DNA-binding domains. Moreover, even though their ligand-binding domains share only 37% identity, lithocholic acid and derivatives act as ligands for both PXR and VDR (29). PXR interacts with its cognate response elements in the 5′-flanking regions of target genes by forming heterodimers with RXRα. Typically, these elements contain 2 copies of the AG(G/T)TCA hexanucleotide organized as a direct repeat with a 3- to 4-bp spacer (DR3, DR4), or an everted repeat separated by 6 bp (ER6) (30). Some of these elements are also recognized by VDR after heterodimerization with RXR, as shown with iNOS (31), CYP3A4 (32, 33), CYP2B6, and CYP2C9 (8). These observations suggest to us that these receptors are capable of regulating common genes through the same cis-acting elements. This could represent an example of functional nuclear receptor cross-talk, suggesting that PXR activators might interfere with VDR-controlled physiological processes. Here, we test this hypothesis and provide data suggesting that PXR is involved in drug-induced osteomalacia by upregulating CYP24, the major enzyme responsible for the biological catabolism of vitamin D hormone.
Results
Induction of CYP24 gene expression and CYP24 activity by PXR activators in human hepatocytes.
We first investigated the effects of PXR activators on the expression of key proteins involved in vitamin D3 homeostasis, i.e., the mitochondrial 25-hydroxylase CYP27A, the microsomal 25-hydroxylase CYP2R1, the 1α-hydroxylase CYP27B, the 24-hydroxylase CYP24, and VDR. Forty-eight hours after plating, human hepatocytes were treated with 1α,25(OH)2D3 (50 nM) or PXR activators such as rifampicin (10 μM), hyperforin (2 μM), carbamazepine (20 μM), and phenobarbital (500 μM) for 48 hours. CYP24, CYP2R1, VDR, CYP27B, and CYP27A mRNAs were then analyzed by real-time quantitative RT-PCR using the LightCycler apparatus (Roche Diagnostics Corp.), in parallel with CYP3A4 mRNA as a control for PXR activation. We evaluated the induction ratios (mRNA levels in treated cells compared with control cells after normalization with respect to GAPDH signal) obtained from the analysis of 5 different cultures from 5 different liver donors. GAPDH and β-actin mRNA levels (data not shown) were used as quality controls of RNA preparations and RT reactions. As expected, vehicle-treated cells expressed very low levels of CYP24 (cycle threshold [Ct] = 37 ± 2 cycles), VDR (Ct = 32 ± 4), and CYP27B (Ct = 32 ± 2) mRNAs compared with CYP27A (Ct = 24 ± 1), CYP3A4 (Ct = 25 ± 5), CYP2R1 (Ct = 26 ± 2), and GAPDH (Ct = 17 ± 2).
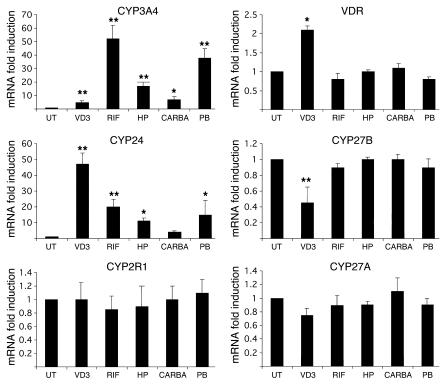
As shown in Figure 1, expression of CYP3A4 mRNA was significantly induced by all of the compounds tested, although more modestly by 1α,25(OH)2D3 (5-fold induction). In addition, expression of CYP24 mRNA was strongly induced by 1α,25(OH)2D3 (47-fold induction). Interestingly, expression of CYP24 mRNA was significantly (P < 0.05) increased by rifampicin (20-fold induction), hyperforin (11-fold induction), and phenobarbital (12-fold induction). Carbamazepine was a weak, nonsignificant (P > 0.07) inducer of CYP24. The relative induction potency of rifampicin, hyperforin, carbamazepine, and phenobarbital was identical for CYP3A4 and CYP24 genes: i.e., rifampicin > phenobarbital > hyperforin > carbamazepine. In contrast, 1α,25(OH)2D3 was a weak inducer of CYP3A4 compared with rifampicin or the other PXR activators but appeared as the most potent inducer of the CYP24 gene. As previously observed in other tissues (34), 1α,25(OH)2D3 produced a significant decrease (50% inhibition, P < 0.01) of the CYP27B1 mRNA level (Figure 1). In contrast, rifampicin, hyperforin, or phenobarbital did not affect the expression of this mRNA. The level of VDR mRNA in human hepatocytes was not affected by the drugs used, except by 1α,25(OH)2D3 (2.2-fold increase). The levels of mRNA encoding 2 enzymes involved in the 25-hydroxylation of vitamin D3 (i.e., the mitochondrial CYP27A and the recently “de-orphanized” microsomal CYP2R1) were not affected by any of the compounds tested, even by 1α,25(OH)2D3.
Figure 1.
Effect of 1α,25(OH)2D3, rifampicin, hyperforin, carbamazepine, and phenobarbital on CYP24, CYP2R1, CYP27B, CYP27A, VDR, and CYP3A4 mRNAs in human hepatocytes. Human hepatocytes were cultured for 48 hours in the absence (UT) or presence of the indicated compounds: 50 nM 1α,25(OH)2D3 (VD3), 20 μM rifampicin (RIF), 2 μM hyperforin (HP), 20 μM carbamazepine (CARBA), or 500 μM phenobarbital (PB). Total RNA was isolated using TRIZOL reagent. One microgram of total RNA was reverse-transcribed, and CYP3A4, CYP24, CYP2R1, CYP27B, CYP27A, VDR, and GAPDH mRNAs were quantified by real-time RT-PCR analysis using the LightCycler apparatus (Roche Diagnostics Corp.). Data presented are means ± SE (from 5 different cultures from 5 different liver donors) of the ratio of mRNA levels in treated cells to corresponding levels in untreated cells, normalized with respect to GAPDH mRNA levels, which themselves exhibited no significant variation. Statistically significant inductions compared with those in untreated cells are marked with asterisks: *P < 0.05 and **P < 0.01.
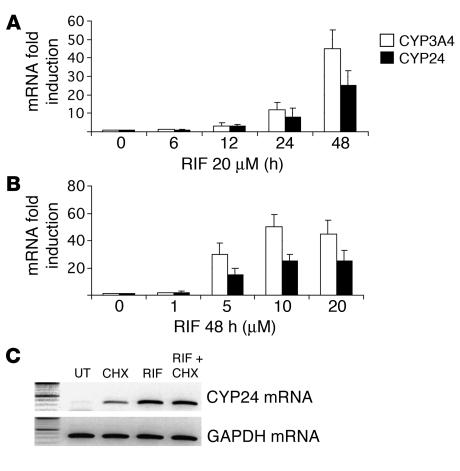
Next, we compared the extent of mRNA induction of the main PXR target gene, CYP3A4, to the extent of mRNA induction of CYP24 at various time points (from 6 to 48 hours with 20 μM rifampicin; Figure 2A), and with increasing concentrations of rifampicin (from 1 to 20 μM after 48 hours of treatment; Figure 2B). As shown, both the time-course and the dose-response profiles of induction were similar for CYP3A4 and CYP24 mRNAs. To determine whether xenobiotics induce CYP24 mRNA directly or indirectly, human hepatocytes were treated with rifampicin in the absence or presence of the protein synthesis inhibitor cycloheximide (CHX) for 24 hours, and RNA was analyzed by semiquantitative RT-PCR using primers for CYP24 and GAPDH as control. As shown in Figure 2C, the rifampicin-mediated induction of CYP24 mRNA was not affected by CHX, although the latter compound alone increased the expression of CYP24 mRNA. It is likely that this results from an mRNA stabilization effect of this compound. In contrast, the GAPDH mRNA level was not affected by these treatments.
Figure 2.
Effects of rifampicin on CYP24 gene transcription in human hepatocytes. (A) Time course of induction of CYP24 and CYP3A4 by rifampicin (RIF). Hepatocytes were cultured for the indicated times with or without 20 μM rifampicin. Total RNA was reverse-transcribed and analyzed by quantitative RT-PCR using primers for CYP3A4 (white bars), CYP24 (black bars), and GAPDH. Data presented are means ± SE (from 3 different cultures from 3 different liver donors) of the ratio of mRNA levels in treated cells to corresponding levels in untreated cells, normalized with respect to GAPDH mRNA levels. (B) Dose-dependent induction of CYP24 and CYP3A4 by rifampicin. Hepatocytes were cultured for 48 hours in the absence or presence of increasing concentrations of rifampicin, from 1 μM to 20 μM. Total RNA was extracted and analyzed by quantitative RT-PCR for CYP24 (black bars), CYP3A4 (white bars), and GAPDH mRNA content. Data presented are means ± SE (from 3 different cultures from 3 different liver donors) of the ratio of mRNA levels in treated cells to corresponding levels in untreated cells, normalized with respect to GAPDH mRNA levels. (C) Rifampicin has direct transcriptional effects on CYP24. Hepatocytes from liver donor number 220 were untreated (UT) or pretreated with 10 μg/ml CHX for 1 hour before addition of 20 μM rifampicin. Total RNA was harvested 24 hours later, reverse-transcribed, and analyzed by semiquantitative RT-PCR for CYP24 (45 cycles) and GAPDH (25 cycles) mRNA content.
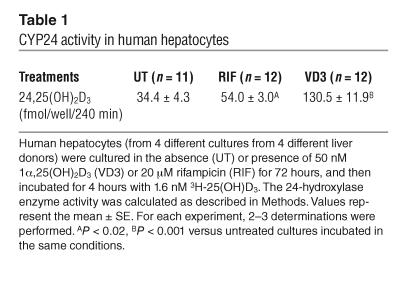
To investigate whether CYP24 mRNA accumulation coincided with an increase in the rate of 24-hydroxylation of vitamin D3, the metabolism of 3H-25(OH)D3 was evaluated. For this purpose, human hepatocytes were treated with 1α,25(OH)2D3 (50 nM) or 20 μM rifampicin for 72 hours, and then incubated for 4 hours with 3H-25(OH)D3. A significant increase of 24,25(OH)2D3 production was observed both after 1α,25(OH)2D3 treatment (3.79-fold induction, P < 0.001) and after rifampicin treatment (1.58-fold induction, P < 0.02) (Table 1). The potency of these compounds to increase the rate of 24-hydroxylation of 25(OH)D3 parallels their ability to induce CYP24 mRNA accumulation [i.e., 1α,25(OH)2D3 > rifampicin].
Table 1.
CYP24 activity in human hepatocytes
In vivo induction of mouse CYP24 mRNA and 24,25(OH)2D3 plasma concentration by PXR agonists.
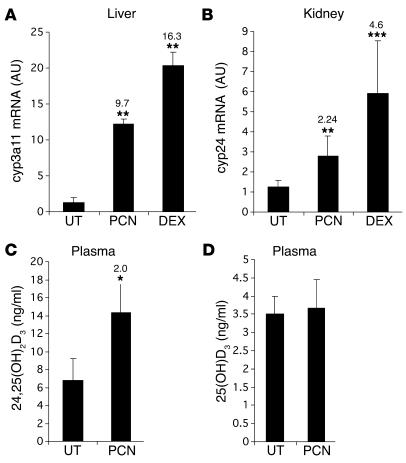
To determine whether activators of PXR induce CYP24 in vivo, mice (n = 5) were treated for 6 days with dexamethasone or pregnenolone 16α-carbonitrile, 2 well-known murine PXR agonists (28), or corn oil plus 5% DMSO (vehicle). Total RNA was prepared from liver and kidney and analyzed for cyp3a11, cyp24, and cyclophilin (internal control) gene expression by real-time PCR. As expected, the cyp3a11 mRNA level was significantly (P < 0.01) and strongly increased by dexamethasone (16.3-fold) and pregnenolone 16α-carbonitrile (9.7-fold) in the liver of treated mice in comparison to the level in vehicle-treated mice (Figure 3A). More interestingly, dexamethasone and pregnenolone 16α-carbonitrile provoked a significant increase of cyp24 mRNA expression in kidney, 4.6-fold (P = 0.0005) and 2.2-fold (P = 0.0019) induction, respectively (Figure 3B). In addition, we measured changes in the plasma concentration of vitamin D metabolites [24,25(OH)2D3 and 25(OH)D3] after 1 week of pregnenolone 16α-carbonitrile treatment. As shown in Figure 3C, pregnenolone 16α-carbonitrile provoked a significant increase of 24,25(OH)2D3 plasma level (2.01-fold induction, P < 0.03), while no significant effect was observed on 25(OH)D3 circulating level (Figure 3D).
Figure 3.
In vivo modulation of CYP24 by PXR agonists. (A and B) Effect of pregnenolone 16α-carbonitrile and dexamethasone on cyp24 mRNA abundance. Mice (n = 5) were injected i.p. for 6 consecutive days with dexamethasone (DEX; 10 mg/kg/d), pregnenolone 16α-carbonitrile (PCN; 100 mg/kg), or corn oil (UT). Total RNA from liver or kidney was prepared, and 1 μg was reverse-transcribed. The relative levels of cyp3a11 and cyp24 mRNAs were determined in duplicate for each mouse by real-time PCR using cyp3a11-specific (A) and cyp24-specific (B) primers. Cyclophilin mRNA levels were used as a reference standard. Data are means ± SE of the ratio of mRNA levels in treated mice to corresponding levels in untreated mice, normalized with respect to cyclophilin mRNA levels. (C and D) Effect of pregnenolone 16α-carbonitrile on vitamin D3 metabolites in mouse plasma. Mice (n = 5) were injected i.p. for 6 consecutive days with pregnenolone 16α-carbonitrile (100 mg/kg) or corn oil and plasma samples. Pooled mouse plasma (1–2 mice, 300 μl, n = 3) or 50-μl plasma samples (n = 5) were analyzed for 24,25(OH)2D3 (C) or 25(OH)D3 metabolites (D), respectively, as described in Methods. Statistically significant expressions compared with untreated mice are marked with asterisks: *P < 0.05, **P < 0.01, and ***P < 0.005. Fold change relative to control mice is indicated.
PXR transactivates human CYP24 gene promoter in Hek293 cells.
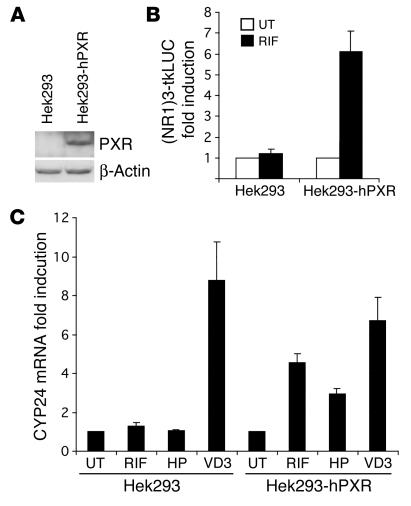
The results presented above suggest that PXR is involved in the regulation of the CYP24 gene. To further document this point, we first established a stable human embryonic kidney cell line (Hek293) expressing the human PXR protein. For this purpose, Hek293 cells were transfected with pIRES-hPXR/neo expression plasmid and cultured 3 weeks in a selection medium containing 600 μg/μl G418. Several colonies were amplified and tested for their ability to express PXR protein, and 1 of them was used for subsequent studies. As shown in Figure 4A, this cell line (Hek293-hPXR) expresses human PXR protein, as assessed by Western blotting performed with an anti–human PXR serum, while the parental Hek293 cells do not. Moreover, in contrast to the parental cells, these cells respond to rifampicin, as observed in transient transfection assays using a CYP2B6 (NR1)3-tkLUC reporter construct, which contains 3 repeats of a functional PXR-responsive element of the CYP2B6 gene (26) (Figure 4B). We then analyzed the effect of 1α,25(OH)2D3 (50 nM), rifampicin (10 μM), or hyperforin (2 μM) on the endogenous CYP24 mRNA level in both parental and PXR-expressing Hek293 cell lines. As shown in Figure 4C, treatment with 1α,25(OH)2D3 for 48 hours produced 9- and 7-fold inductions of CYP24 mRNA in the parental cells (reflecting the endogenous expression of VDR) and in Hek293-hPXR cells, respectively. In contrast, while rifampicin and hyperforin had no effect on CYP24 mRNA expression in control cells, these compounds produced 4.5- and 3-fold increases of CYP24 mRNA in the Hek293-hPXR cells, respectively.
Figure 4.
Rifampicin induces the CYP24 gene in a Hek293 cell line stably transfected with PXR. (A) Expression of human PXR in parental and pIRES-hPXR/neo–transfected Hek293 cells. Whole-cell extracts were prepared from parental Hek293 cells or pIRES-hPXR/neo–transfected cells (Hek293-hPXR). Proteins were separated on 10% SDS-PAGE, and PXR and β-actin expression was monitored by Western blotting (Santa Cruz Biotechnology Inc.). (B) Rifampicin activates an NR1-LUC reporter gene in Hek293-hPXR cells. Parental Hek293 or Hek293-hPXR cell lines were transiently transfected with CYP2B6 NR1-LUC reporter gene together with pRSV-β-gal transfection control plasmid. After transfection, cells were treated with 0.1% DMSO (UT; white bars) or 10 μM rifampicin (RIF; black bars). Values represent β-gal–corrected luciferase activities normalized to the corresponding level in untreated Hek293 cells and are the average of duplicates ± SE. These were replicated in independent experiments. (C) Rifampicin and hyperforin induce the CYP24 gene in Hek293-hPXR cells. Parental Hek293 or Hek293-hPXR cell lines were untreated (UT) or treated with 50 nM 1α,25(OH)2D3 (VD3), 10 μM rifampicin (RIF), or 2 μM hyperforin (HP) in triplicate. Forty-eight hours later, total RNA was extracted and analyzed by quantitative RT-PCR for CYP24 and GAPDH mRNA content. Values represent the average ± SE.
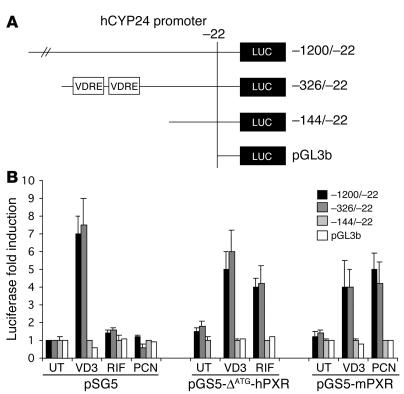
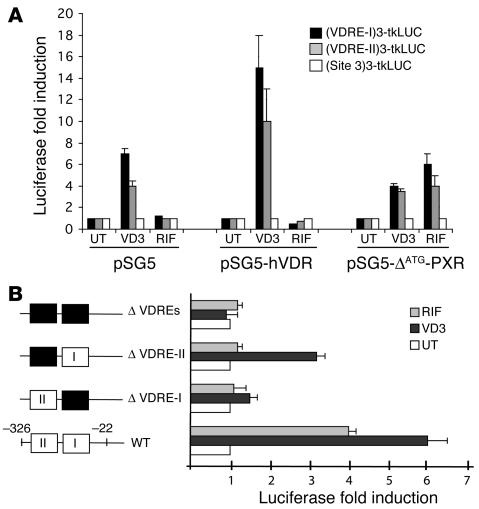
The cloning of the human CYP24 promoter has been reported (35). To determine the nature of the cis-acting elements involved in PXR-mediated CYP24 gene expression, analysis of promoter transcriptional activity was undertaken. Various deletions of the human CYP24 promoter were constructed into a promoterless luciferase reporter vector (pGL3basic; Figure 5A). These constructs were tested for their ability to respond to PXR by transient cotransfection assays in Hek293 cells. In response to rifampicin, the luciferase activity of the 1.2-kb promoter fragment (–1,200 to –22) exhibited a 4-fold increase under cotransfection with human PXR cDNA expression vector (Figure 5B). The magnitude of the response was identical to that observed with the –326 to –22 construct. However, the –144 to –22 construct did not respond to rifampicin. Similar results were obtained with pregnenolone 16α-carbonitrile and a mouse PXR expression vector. Therefore, the –326 to –144 region of the CYP24 promoter confers response to both human and murine PXR. Interestingly, this region is responsive to 1α,25(OH)2D3 even in the absence of cotransfection of the receptor expression vector (Figure 5B), because Hek293 cells express endogenous VDR (36). Indeed, 2 VDREs have been defined within the –300 to –150 region of the human and mouse CYP24 promoter that confer response of the promoter to both 1α,25(OH)2D3 (35) and RXR ligands (37).
Figure 5.
PXR transactivates the proximal human CYP24 promoter. (A) VDREs present in the human CYP24 (hCYP24) gene. Schematic representation of the human CYP24 constructs used in this work. (B) Ligand-activated PXR transactivates the –326 to –143 promoter region of human CYP24. Hek293 cells were transiently transfected with human PXR (pSG5-ØATG-hPXR), mouse PXR (pSG5-mPXR), or control expression vector (pSG5) in the presence of reporter vectors driven by the indicated human CYP24 promoter sequences, together with pRSV-β-gal transfection control plasmid. Cells were treated with vehicle (0.1% DMSO; UT), 50 nM 1α,25(OH)2D3 (VD3), 10 μM rifampicin (RIF), or 10 μM pregnenolone 16α-carbonitrile (PCN) for 24 hours. Cells were then harvested and analyzed for both luciferase and β-gal activities. Values represent β-gal–corrected luciferase activities normalized to the corresponding level in untreated Hek293 cells and are the average of duplicates ± SE. These were replicated in independent experiments.
PXR binds to and transactivates the VDRE-I and VDRE-II motifs of human CYP24 promoter.
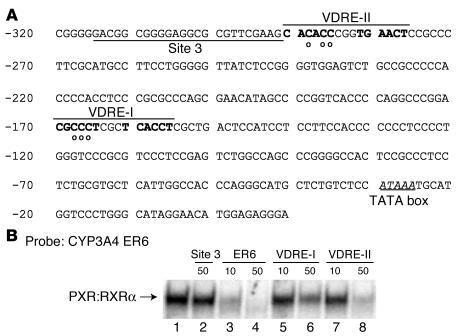
We have previously reported that VDR binds to and transactivates PXR-responsive elements present on the promoter of several CYP genes, including CYP2C9, CYP2B6, and CYP3A4. This suggested that these receptors could share DNA-responsive elements and target genes (8). The –316 to –144 region of the human CYP24 promoter contains 3 potential binding sites for PXR: 2 VDREs previously identified, –294 to –274 (VDRE-II) and –174 to –151 (VDRE-I), and the –316 to –291 region (CYP24 site 3), which contains sequences that are homologous with various repeat motifs (Figure 6A). The ability of these sequences to bind to the PXR:RXRα heterodimer was tested by competition in electrophoretic mobility shift assays using the proximal PXR-responsive element of CYP3A4 (ER6 motif [ref. 25]). PXR and RXRα were prepared by in vitro translation of their cDNAs. As expected, PXR:RXRα heterodimer produced a retarded band with the CYP3A4 ER6 probe (Figure 6B, lane 1), which was decreased by an excess of ER6 unlabeled probe (lanes 3 and 4). Interestingly, the 2 CYP24 VDREs produced a dose-dependent decrease of PXR:RXRα binding to the CYP3A4 ER6 probe (Figure 6B, lanes 5–8), which suggests direct competition between the CYP3A4 ER6 and VDRE motifs in the interaction with PXR:RXRα heterodimers. In contrast, the CYP24 –316 to –291 region (site 3) failed to compete with the CYP3A4 ER6 probe (Figure 6B, lane 2), which suggests that this element is not required for PXR-mediated CYP24 gene induction.
Figure 6.
CYP2A4 VDREs compete with the CYP3A4 ER6 probe for binding to the PXR:RXRα heterodimer in electrophoretic mobility shift assay. (A) PXR-responsive region of CYP24 promoter sequence. VDRE sequences (VDRE-II, –294 to –274, and VDRE-I, –174 to –151) are overlined, and half-sites are indicated in boldface letters. The region between –316 and –291 (site 3), which contains sequences that are homologous to various direct repeats, is underlined. Circles under nucleotides denote those nucleotides that were changed in mutation constructs. The TATA box is indicated. (B) CYP24 VDRE-II and VDRE-I motifs compete with the CYP3A4 ER6 probe for binding to the PXR:RXRα heterodimer. Radiolabeled CYP3A4 ER6 oligonucleotide (50,000 cpm) was incubated in the presence of PXR and RXRα proteins prepared by in vitro translation using a transcription-translation coupled system (lane 1). In parallel experiments, incubation was performed in the presence of a 50-fold molar excess of the CYP24 site 3 region (lane 2), or a 10- to 50-fold molar excess of unlabeled CYP3A4 ER6 (lanes 3 and 4), CYP24 VDRE-I (lanes 5 and 6), and VDRE-II (lanes 7 and 8).
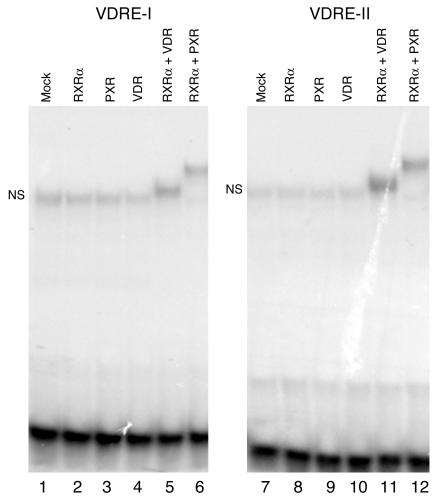
To test the direct interaction between PXR and the 2 VDREs of the CYP24 promoter, oligonucleotides VDRE-I (Figure 7, lanes 1–6) and VDRE-II (lanes 7–12) were end-labeled and subjected to electrophoretic mobility shift assay. Note that the reticulocyte lysate produced a nonspecific retarded band (Figure 7, NS). Nevertheless, in vitro–synthesized PXR:RXRα and VDR:RXRα heterodimers efficiently bound to the 2 CYP24 VDRE motifs (Figure 7, lanes 5, 6, 11, and 12). This binding was observed only in the presence of the heterodimerization partner RXRα, while neither PXR, RXRα, nor VDR alone bound to these sequences. These results strongly suggest that the PXR:RXRα heterodimer binds to the 2 VDREs of the human CYP24 promoter.
Figure 7.
PXR and VDR bind as heterodimers with RXRα to the VDRE-I and VDRE-II motifs of the human CYP24 promoter in electrophoretic mobility shift assay. Radiolabeled CYP24 VDRE-I (lanes 1–6) and VDRE-II (lanes 7–12) oligonucleotides (50,000 cpm) were incubated in the absence or presence of PXR, RXRα, or VDR protein, alone or in association as indicated, before being loaded onto the gel. Mock: control TNT lysate. NS, nonspecific band.
To demonstrate further the role of CYP24 VDRE-I and VDRE-II motifs in PXR-mediated CYP24 gene induction, we used heterologous promoter constructs containing 3 repeats of CYP24 site 3 (–316 to –291), VDRE-I, or VDRE-II elements upstream of the minimal thymidine kinase promoter (tkLUC). Transient cotransfection assays were performed on Hek293 cells with these reporter plasmids and VDR or PXR cDNA expression vectors (Figure 8A). As expected, both VDRE-I and VDRE-II reporter constructs exhibited increased luciferase activity in response to 1α,25(OH)2D3, even in the absence of the cotransfected receptor (7- and 4-fold induction, respectively). This induction was increased in the presence of VDR. Interestingly, in both VDRE-I and VDRE-II reporter constructs, rifampicin produced an increase in reporter activity (approximately 4- to 6-fold induction) only in the presence of PXR cotransfection. In contrast, the luciferase activity of the site 3 reporter construct was affected neither by 1α,25(OH)2D3 treatment nor by rifampicin-activated PXR. We then introduced specific mutations into VDRE-I and VDRE-II motifs in the native CYP24 promoter (–326 to –22) by means of PCR-based site-directed mutagenesis. The effect of PXR on these mutated CYP24 gene promoters was investigated by transient transfection experiments. As shown in Figure 8B, the mutation in VDRE-I caused a strong decrease in both the VDR-dependent and the PXR-dependent luciferase induction. On the other hand, introduction of the mutation into VDRE-II retained half of the VDR response, while it completely abolished PXR-dependent luciferase induction. Double mutations in both VDRE-I and VDRE-II completely abolished both VDR and PXR response. These results demonstrate that PXR binds to and transactivates the 2 VDRE motifs present in the human CYP24 promoter.
Figure 8.
Functional and mutational analysis of human CYP24 VDREs. (A) Thymidine kinase promoter luciferase reporter vectors (pGL3tkLUC) driven by 3 repeats of oligonucleotides from the human CYP24 promoter (site 3, VDRE-I, and VDRE-II) were cotransfected with control expression plasmid (pSG5), human VDR (pSG5-hVDR), or human PXR (pSG5-ØATG-hPXR) into Hek293 cells, together with pRSV-β-gal transfection control plasmid. Cells were treated with vehicle (0.1% DMSO; UT), 50 nM 1α,25(OH)2D3 (VD3), or 10 μM rifampicin (RIF) for 24 hours. Cells were then harvested and analyzed for both luciferase and β-gal activities. Values represent β-gal–corrected luciferase activities normalized to the corresponding level in untreated Hek293 cells and are the average of duplicates ± SE. They were replicated in independent experiments. (B) Hek293 cells were transiently cotransfected with pSG5-ØATG-hPXR expression vector along with various human CYP24 promoter sequence–containing reporter constructs, together with pRSV-β-gal transfection control plasmid. Cells were treated with vehicle, 50 nM 1α,25(OH)2D3, or 10 μM rifampicin for 24 hours. Reporter constructs were as follows: –316 to –22 wild-type sequence in pGL3b (WT) or point mutants of VDRE-I (–168CCC mutated to GTT; ØVDRE-I), VDRE-II (–289CACC to AAAA; ØVDRE-II), or both VDREs (ØVDREs). Values represent β-gal–corrected luciferase activities normalized to the corresponding level in untreated Hek293 cells and are the average of duplicates ± SE. They were replicated in independent experiments.
Discussion
Recent studies have shown that the orphan nuclear receptor PXR plays a central role in the transcriptional regulation of enzymes and transporters involved in detoxification, such as CYP3A4 and MDR1 (38). PXR is activated by a number of pharmaceutical agents, including rifampicin, SR12813, taxol, clotrimazole, phenobarbital, hyperforin, the herbal antidepressant St. John’s wort (39), and HIV protease inhibitors such as ritonavir and saquivanir (40). PXR functions as a sensor to regulate xenobiotic clearance in the liver and intestine. Indeed, gene knockout studies have confirmed the role of PXR in the regulation of the metabolism of endogenous steroids and dietary and xenobiotic compounds (41). Interestingly, recent studies have demonstrated that PXR and VDR might signal through the same DNA-responsive elements, notably in iNOS (31), CYP3A4 (32, 33), CYP2B6 (8), and CYP2C9 (8) genes. In addition, VDR (29) and PXR (42) share ligands, such as the secondary bile acid lithocholic acid. These observations suggest the possibility of functional cross-talk between PXR and VDR.
The results described here show that PXR activators increase the expression of CYP24, a VDR target gene, not only in cultured cells (primary culture of human hepatocytes and embryonic kidney cell line) but also in vivo in mice. In fact, we observed that CYP24 and CYP3A4, a typical PXR target gene, displayed similar dose-dependent and kinetic profiles of induction by PXR agonists, suggesting a common mechanism of regulation of these genes. CYP24 is a multifunctional enzyme that directs the side-chain oxidation and cleavage of 25(OH)D3 and 1,25(OH)2D3 to catabolic carboxylic acid end products (43). It is well established that induction of the rat CYP24 gene by 1,25(OH)2D3 is mediated by 2 VDREs located about 100 bp apart within the 300-bp proximal promoter (35). In human hepatocytes, PXR-mediated CYP24 mRNA accumulation was not inhibited by CHX pretreatment, which suggests a direct effect of PXR on CYP24 gene expression. In addition, transfection studies in human embryonic kidney cells demonstrated that both human PXR and mouse PXR directly transactivate the CYP24 promoter. Moreover, we found that PXR binds to and transactivates the 2 proximal VDREs present in the CYP24 promoter. Point mutations within the 5′ half-site of either the upstream VDRE (ØVDRE-II) or the downstream VDRE (ØVDRE-I) led to a total inhibition of PXR-mediated induction of luciferase activity, which suggests that both sites are required for an efficient PXR-mediated CYP24 gene expression. Such cooperation between several PXREs is frequently observed within PXR target genes. In summary, our results demonstrate that CYP24 is a VDR target gene as well as a PXR target gene. Moreover, we observed that, in vivo in mice, activation of PXR by pregnenolone 16α-carbonitrile significantly increased the circulating level of 24,25(OH)2D3 (2-fold induction). These results fully prove our hypothesis and are in agreement with those previously obtained by others. Indeed, pharmacological doses of dexamethasone, another mouse PXR agonist and cyp3a inducer (28), increase intestinal and renal cyp24 mRNA accumulation and enzyme activity (44).
Since 24-hydroxylation is the initial enzymatic step in the catabolism of 1,25(OH)2D3, increased cyp24 gene expression and 24(OH)-ase activity might be expected to result in lower cellular concentrations of 1,25(OH)2D3 and, therefore, lower expression of VDR target genes (Figure 9). Indeed, many reports show that drugs now identified as PXR activators produce vitamin D deficiency and increase the risk of bone fracture. It is well-known that long term treatment with phenytoin, phenobarbital, rifampicin, or carbamazepine impairs bioavailability of vitamin D and bone mineralization, leading to osteomalacia and osteoporosis (13, 15–18, 20, 45–47). Consistent with this, it has been observed that the circulating level of 25(OH)D3 or 1,25(OH)2D3 is decreased by these drugs (18, 20, 48, 49). In addition, osteopenia, osteoporosis, and osteonecrosis are the most significant bone disorders affecting patients suffering from HIV infection (50), and HIV-infected patients receiving antiretroviral therapy have a high prevalence of reduced bone mineral density (51). The mechanisms of these phenomena are complex and unclear. However, a part of these effects might be the consequence of CYP24 gene upregulation induced by the activation of PXR. Indeed, protease inhibitors have been suspected to play some role in this process (52, 53), and 2 of them, ritonavir and saquinavir (27, 40), are PXR agonists.
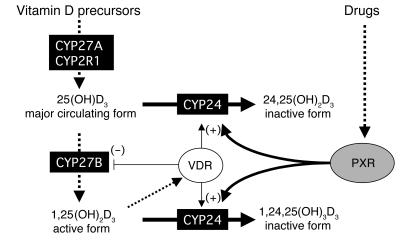
Figure 9.
Proposed model for PXR-mediated drug-induced osteopenia or osteomalacia. While 1α,25(OH)2D3 maintains vitamin D homeostasis via downregulation of vitamin D3 biosynthesis enzymes (CYP27B) and upregulation of the vitamin D–inactivating enzyme (CYP24), drugs that activate PXR may be responsible for the acceleration of vitamin D catabolism through the upregulation of CYP24, leading to vitamin D deficiency and eventually to osteopenia or osteomalacia.
In the most classical view of molecular toxicology, undesirable effects of xenobiotics are primarily ascribed to the chemical reactivity of the parent compounds and/or of some of their metabolites that are produced during oxidative metabolism mediated notably by CYPs (e.g., covalent binding to proteins and DNA, effects on membrane lipids, etc.). A second aspect of the undesirable effects of xenobiotics results from the fact that these compounds may affect the level and activity of xenobiotic detoxification systems (interactions, induction, and inhibition). In this respect, although PXR evolved to protect the body from xenobiotic-mediated toxic effects, its activation by some drugs represents the basis for a common class of potentially life-threatening drug interactions in which one drug accelerates the metabolism of another (54). The cross-talk described here between PXR and VDR represents another aspect of the undesirable effects of xenobiotics, in which a xenobiotic-mediated increase in endobiotic-compound metabolism/catabolism can lead to endocrine system perturbation.
Methods
Chemicals.
DMEM, DMSO, dexamethasone, rifampicin, 1α,25(OH)2D3, carbamazepine, hyperforin, pregnenolone 16α-carbonitrile, and culture medium additives were from Sigma-Aldrich. The radioisotope γ-[32P]d-ATP was from Amersham International. Gelatin was purchased from Touzart-Matignon, neutral charcoal from NORIT, and dextran 20 from OSI Pharmaceuticals. Unlabeled 25(OH)D3 was a gift from Laboratoires Roussel-Uclaf, 24,25(OH)2D3 was a gift from M.L. Uskokovic (F. Hoffmann-La Roche Ltd.), and 26,27-3H-dihydroxyvitamin D3 (specific activity, 23 Ci/mmol) was purchased from Amersham Biosciences Europe GmbH. Chromatography solvents (n-hexane and isopropanol), methanol, and chloroform were purchased from Merck SA.
Plasmids.
The following plasmids have been described previously: pSG5-ØATG-hPXR (28), pSG5-mPXR (28), pSG5-mRXRα (8), and pSG5-hVDR (8). pIRES-hPXR/neo plasmid was generated by PCR amplification (High-Fidelity PCR system; F. Hoffmann-La Roche Ltd.) of cDNA encoding amino acids 1–434 of human PXR using oligonucleotides 5′-GGGTGTGGGGAATTCACCACCATGGAGGTGAGACCCAAAGAAAGC and 5′-GGGTGTGGGGGATCCTCAGCTACCTGTGATGCCG and insertion into pIRESneo (BD Biosciences — Clontech) digested with EcoRI/BamHI.
Human CYP24 promoter deletions were obtained by PCR (Pfu polymerase; Promega Corp.) using CYP24 forward 18-mer oligonucleotide primers (–1,200 to –1,182, –326 to –308, or –144 to –126) with an overhanging SalI site immediately upstream of the 5′-most base of the deletion construct; a common 3′ primer, TGCATTTATGGAGACAGA (CYP24 –39 to –22); and the human CYP24 (–1,250 to +99) pBLCAT3 plasmid as a template (a gift from H.F. DeLuca, University of Wisconsin — Madison, Madison, Wisconsin, USA). PCR products were then digested by SacI and subcloned in SacI/SmaI–linearized pGL3basic vector (Promega Corp.) to produce –1,200-LUC, –326-LUC, and –144-LUC constructs.
pGL3tkLUC plasmid was obtained by ligation of the HindIII/BglII tk-SEAP vector fragment (BD Biosciences — Clontech), corresponding to the minimal 147nt long tk promoter, into the HindIII/BglII–linearized pGL3basic vector. Oligonucleotides corresponding to the individual CYP2B6 NR1 (55), CYP24 site 3 (–316 to –291), and VDREs (VDRE-II, –294 to –274, and VDRE-I, –174 to –151) (37) were synthesized with BamHI and BglII ends, annealed, and ligated, and 3 copies were subcloned into the BamHI/BglII–digested pGL3tk. The resulting (NR1)3-tkLUC, (site 3)3-tkLUC, (VDRE-II)3-tkLUC, and (VDRE-I)3-tkLUC were verified by sequencing.
The specific mutations of the VDREs in the native CYP24 promoter (–326 to –22) were introduced by PCR-based site-directed mutagenesis (QuikChange PCR-mediated site-directed mutagenesis kit; Stratagene) using –326-LUC vector as a template to produce ØVDRE-I (–168CCC mutated to GTT) and ØVDRE-II (–289CACC mutated to AAAA). The ØVDRE-II vector was then used as a template to produce the ØVDRE-I + ΔVDRE-II (ΔVDREs) construct.
Primary culture of human hepatocytes.
Hepatocytes were prepared from lobectomy segments resected from adult patients for medically required purposes unrelated to our research program. The use of these human hepatic specimens for scientific purposes has been approved by the French National Ethics Committee (Paris, France). Hepatocytes were prepared and cultured according to a previously published procedure (56). The cells were plated into collagen-coated dishes at 0.17 × 106 cells per square centimeter in a hormonally and chemically defined medium consisting of a mixture of Williams’s E medium and Ham’s F12 medium (1:1 in volume). Forty-eight hours after plating, cells were cultured in the presence of the indicated concentrations of compounds.
Quantitative PCR.
Total RNA was extracted from cells or frozen mouse tissues using TRIZOL reagent (Invitrogen Corp.), according to the manufacturer’s instructions. Purity was confirmed by spectrophotometry. cDNAs were synthesized from 1–2 μg of total RNA using the SuperScript II first-strand synthesis system for PCR (Invitrogen Corp.) at 42°C for 60 minutes, in the presence of random hexamers (Amersham Pharmacia Biotech). Quantification of mRNAs was performed using the LightCycler apparatus (Roche Diagnostics Corp.). Two microliters of diluted RT reaction (1:10) was used for real-time PCR amplification. The following program was used: a denaturation step at 95°C for 8 minutes, and 50 cycles of PCR (denaturation, 95°C, 15 seconds; annealing, 67°C, 10 seconds; extension, 72°C, 20 seconds). In all cases, the quality of the PCR product was assessed by monitoring of a fusion step at the end of the run. Mouse sense and reverse primers, respectively, were as follows: cyp24a1, 5′-CTCCCTATGGATGCAGTATGTATAGTG and 5′-TTTAAAAACGTTGTCAGTAGGTCATAACT; cyp3a11, 5′-GAAGGAAAGCCGCCTGGATT and 5′-GGGTGAGTGGCCAAGGAATG; cyclophilin, 5′-TGGAGAGCACCAAGACAGACA and 5′-TGCCGGAGTCGACAATGAT. Human sense and reverse primers, respectively, were as follows: CYP3A4, 5′-CACAAACCGGAGGCCTTTTG and 5′-ATCCATGCTGTAGGCCCCAA; GAPDH, 5′-GGTCGGAGTCAACGGATTTGGTCG and 5′-CAAAGTTGTCATGGATGACC; CYP24 (NM_000782), 5′-GTGGCTCCAGCCAGACCCTA and 5′-GGCGAGGTTGGTACGAGGTG; CYP27B (NM_000785), 5′-CGAGAAGGACCTGGAGTCTG and 5′-TCTGGGACACGAGAATTTCC; CYP27A (NM_000784), 5′-GCTTCCTCTTCCCCAAGAAC and 5′-ACTGCAGGCCCACTTTCTTA; VDR (NM_000376), 5′-AGCAGCGCATCATTGCCATA and 5′-CAGCATGGAGAGCTGGGACA. Human and mouse CYP24, CYP2R1, CYP27B, CYP27A, and VDR mRNA amplified fragments were purified using the NucleoSpin extract kit (Macherey-Nagel Co.) and verified by sequencing.
25(OH)D3 24-hydroxylase assay.
Human hepatocytes plated in collagen-coated dishes were treated for 72 hours with 50 nM 1,25(OH)2D3, 20 μM rifampicin, or 500 μM phenobarbital. After rinsing with medium, 1.6 nM 3H-25(OH)D3 (0.05 μCi) were added, and the cells were incubated for an additional 4 hours in 1 ml medium. The medium was removed and the 3H-vitamin D3 derivatives present in the medium were extracted by addition of 2 ml methanol plus 2 ml chloroform. The chloroform extract was cochromatographed with 100 ng unlabeled 24,25(OH)2D3 using a straight-phase HPLC system (Beckman Coulter Inc.) equipped with a 4.6 × 250 mm Ultrasphere Si column (Altex; Beckman Coulter Inc.), and equilibrated with n-hexane and isopropanol (95:5) (flow rate 1.5 ml/min). Absorbance at 254 nm was continually monitored, and effluent fractions were collected every minute. After evaporation to dryness, fractions were dissolved in Ultima Gold scintillation fluid (Packard Instrument Co.), and the radioactivity was measured. The 24-hydroxylase enzyme activity (femtomoles per culture well per 240 minutes) was calculated based on the percentage of the injected radioactivity recovered in the 24,25(OH)2D3 regions after HPLC. The data were subjected to an overall statistical evaluation using ANOVA. Test groups were compared with respective untreated control groups using the Mann-Whitney nonparametric U test.
Animals and treatments.
Male mice (C57BL/6; Charles River Laboratories France) were housed in a pathogen-free animal facility under a standard cycle of 12 hours light, 12 hours dark. After 1 week of acclimatization, pregnenolone 16α-carbonitrile (100 mg/kg/d) or dexamethasone (10 mg/kg/d) was injected in corn oil plus 5% DMSO through 6 successive daily administrations (n = 5 for each group). The mice were killed 4 hours after the last pregnenolone 16α-carbonitrile administration, and plasma and tissues (liver and kidney) were snap-frozen in liquid nitrogen.
Measurements of vitamin D metabolites in mouse plasma.
The 25(OH)D3 metabolite was extracted from 50-μl plasma samples (n = 5) with methanol-chloroform, and chromatographed on Amprep C18 Mini-Columns (RPN1900; Amersham Biosciences Europe GmbH) to separate 25(OH)D3 from vitamin D (57). The 24,25(OH)2D3 metabolite was extracted from 300-μl plasma samples (pool of 1–2 mice, n = 3) with methanol-chloroform, and chromatographed on a straight-phase HPLC system (Beckman Coulter Inc.) using 92% n-hexane and 8% isopropanol as solvents. The 25(OH)D3 region from Amprep C18 chromatography and the 24,25(OH)2D3 region from HPLC were dried down and dissolved in ethanol. They were then assayed by the competitive protein-binding assay of Preece et al. (58), with 2 modifications: serum vitamin D–binding protein was obtained from vitamin D–fed and not from vitamin D–deficient rats, and gelatin (3 g/l) was used instead of albumin. The quality of the results was controlled by simultaneous assessment of external standards (Vitamin D External Quality Assessment Scheme [DEQAS]).
Cell culture and transfections.
Hek293 cells (human embryonic kidney cells) were obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10% FCS, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Corp.). Transfection of plasmid DNA was performed in single batches with FuGENE 6 (Roche Diagnostics Corp.) according to the manufacturer’s instructions. For reporter analysis, transfections were performed using 80,000 cells, 150 ng of luciferase-based reporter plasmids, and expression plasmids for mouse PXR (50 ng of pSG5-mPXR.1), human PXR (50 ng of pSG5-ØATG-hPXR), or human VDR (25 ng of pSG5-hVDR). As internal control, 50 ng of pRSV-β-gal (Promega Corp.) was added. After 12–16 hours, the medium was changed, and fresh medium containing 5% delipidated charcoal-stripped serum (Sigma-Aldrich) and 0.1% DMSO (vehicle) or inducers was added. Twenty-four hours after the medium was changed, the cells were harvested in reporter lysis buffer (Promega Corp.), and cell extracts were analyzed for luciferase and β-gal activities as described previously (8). Luciferase values were normalized with β-gal values calculated by division of the maximal response by the response elicited with vehicle.
Stable Hek293 clones expressing human PXR (Hek293-hPXR) were obtained by transfection of Hek293 cells with pIRES-hPXR/neo and, after 3 weeks of selection, with 600 μg/ml G418. Individual clones were then amplified and analyzed for PXR mRNA and protein expression levels.
Western blot analysis.
Whole-cell lysates (106 cells) were prepared from Hek293 or stable transfectant Hek293-hPXR cell lines, with 300 μl of 100 mM Tris (pH 8.5), 250 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM DTT, and antiprotease tablet from Roche Diagnostics Corp. Following centrifugation (12,000 g for 10 minutes at 4°C), the supernatant was collected and stored at –80°C. Protein content was determined by bicinchoninic acid (BCA) protein assay (Interchim). Fifty-microgram extracts were subjected to 10% SDS-PAGE and transferred to PROTRAN nitrocellulose membrane (Schleicher & Schuell BioScience Inc.). Immunoblotting was carried out with actin antibody (I-19) and PXR antibody (N-16), both purchased from Santa Cruz Biotechnology Inc. Chemiluminescence detection was performed using HRP-conjugated secondary antibodies and the Amersham Biosciences Europe GmbH ECL kit.
Electrophoretic mobility shift assays.
Double-stranded oligonucleotides corresponding to the 2 VDREs and site 3 of human CYP24 were synthesized (Invitrogen Corp.): VDRE-I (–174 to –151), CCGGACGCCCTCGCTCACCTCGCTGA; VDRE-II (–294 to –274), CGAAGCACACCCGGTGAACTCCGG; site 3 (–316 to –291), GGAGGGCGGGGAGGCGCGTTCGAAG. The CYP3A4 ER6 probe and binding procedure have previously been described (8). Probes were end-labeled with 32P-ATP using T4 polynucleotide kinase (Promega Corp.) and then incubated for 20 minutes at room temperature with 1 μl of in vitro–synthesized PXR, VDR, or RXRα (transcription and translation–coupled [TNT-coupled] transcriptional translation; Promega Corp.). DNA-protein complexes were subjected to electrophoresis on a 4% polyacrylamide gel (30:1 acrylamide/bis-acrylamide) in 0.5× TBE (1× TBE = 89 mM Tris, 89 mM boric acid, 2 mM EDTA). Gels were dried and subjected to autoradiography with XAR film (Eastman Kodak Co. Scientific Imaging Systems).
Acknowledgments
We are grateful to Sanofi-Synthélabo France SA for financial support. We thank L. Pichard-Garcia for the preparation of human hepatocytes, and F. Lasserre and C. Bétouliéres (Institut National de la Recherche Agronomique) for assistance in execution of animal studies.
Footnotes
See the related Commentary beginning on page 32.
Nonstandard abbreviations used: CHX, cycloheximide; Ct, cycle threshold; PXR, pregnane X receptor; RXR, retinoid X receptor; VDR, vitamin D receptor; VDRE, vitamin D–responsive element.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1. J. Biol. Chem. 2003;278:38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wikvall K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form. Int. J. Mol. Med. 2001;7:201–209. doi: 10.3892/ijmm.7.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am. J. Physiol. 1999;277:F157–F175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 4.Carlberg C, Polly P. Gene regulation by vitamin D3. Crit. Rev. Eukaryot. Gene Expr. 1998;8:19–42. doi: 10.1615/critreveukargeneexpr.v8.i1.20. [DOI] [PubMed] [Google Scholar]
- 5.Kato S. The function of vitamin D receptor in vitamin D action. J. Biochem. 2000;127:717–722. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- 6.Carlberg C. The concept of multiple vitamin D signaling pathways. J. Investig. Dermatol. Symp. Proc. 1996;1:10–14. [PubMed] [Google Scholar]
- 7.Dusso AS, Brown AJ. Mechanism of vitamin D action and its regulation. Am. J. Kidney Dis. 1998;32(Suppl. 2):S13–S24. doi: 10.1053/ajkd.1998.v32.pm9808140. [DOI] [PubMed] [Google Scholar]
- 8.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J. Biol. Chem. 2002;277:25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 9.Segura C, et al. Vitamin D receptor ontogenesis in rat liver. Histochem. Cell Biol. 1999;112:163–167. doi: 10.1007/s004180050403. [DOI] [PubMed] [Google Scholar]
- 10.Suda T, Shinki T, Kurokawa K. The mechanisms of regulation of vitamin D metabolism in the kidney. Curr. Opin. Nephrol. Hypertens. 1994;3:59–64. doi: 10.1097/00041552-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ohyama Y, et al. Identification of a vitamin D-responsive element in the 5′-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J. Biol. Chem. 1994;269:10545–10550. [PubMed] [Google Scholar]
- 12.St-Arnaud R, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 13.Andress DL, et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch. Neurol. 2002;59:781–786. doi: 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 14.Burt R, Freston JW, Tolman KG. The influence of phenobarbital on biotransformation of 25-hydroxycholecalciferol. J. Clin. Pharmacol. 1976;16:393–398. doi: 10.1002/j.1552-4604.1976.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 15.Karaaslan Y, Haznedaroglu S, Ozturk M. Osteomalacia associated with carbamazepine/valproate. Ann. Pharmacother. 2000;34:264–265. doi: 10.1345/aph.19099. [DOI] [PubMed] [Google Scholar]
- 16.O’Hare JA, Duggan B, O’Driscoll D, Callaghan N. Biochemical evidence for osteomalacia with carbamazepine therapy. Acta Neurol. Scand. 1980;62:282–286. doi: 10.1111/j.1600-0404.1980.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 17.Shah SC, Sharma RK, Hemangini, Chitle AR. Rifampicin induced osteomalacia. Tubercle. 1981;62:207–209. doi: 10.1016/0041-3879(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 18.Brodie MJ, et al. Rifampicin and vitamin D metabolism. Clin. Pharmacol. Ther. 1980;27:810–814. doi: 10.1038/clpt.1980.115. [DOI] [PubMed] [Google Scholar]
- 19.Chan TY. Osteomalacia during rifampicin and isoniazid therapy is rare in Hong Kong. Int. J. Clin. Pharmacol. Ther. 1996;34:533–534. [PubMed] [Google Scholar]
- 20.Brodie MJ, et al. Effect of rifampicin and isoniazid on vitamin D metabolism. Clin. Pharmacol. Ther. 1982;32:525–530. doi: 10.1038/clpt.1982.197. [DOI] [PubMed] [Google Scholar]
- 21.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J. Clin. Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichard L, et al. Identification of the rabbit and human cytochromes P-450IIIA as the major enzymes involved in the N-demethylation of diltiazem. Drug Metab. Dispos. 1990;18:711–719. [PubMed] [Google Scholar]
- 23.Raucy JL. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab. Dispos. 2003;31:533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 24.Bertilsson L, Tybring G, Widen J, Chang M, Tomson T. Carbamazepine treatment induces the CYP3A4 catalysed sulphoxidation of omeprazole, but has no or less effect on hydroxylation via CYP2C19. Br. J. Clin. Pharmacol. 1997;44:186–189. doi: 10.1046/j.1365-2125.1997.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann JM, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA. Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol. Pharmacol. 2001;60:427–431. [PubMed] [Google Scholar]
- 27.Luo G, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab. Dispos. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- 28.Kliewer SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 29.Makishima M, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 30.Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. . Biochim. Biophys. Acta. 2003; 1619:243–253. doi: 10.1016/s0304-4165(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 31.Toell A, Kroncke KD, Kleinert H, Carlberg C. Orphan nuclear receptor binding site in the human inducible nitric oxide synthase promoter mediates responsiveness to steroid and xenobiotic ligands. J. Cell. Biochem. 2002;85:72–82. [PubMed] [Google Scholar]
- 32.Schmiedlin-Ren P, Thummel PE, Fisher JM, Paine MF, Watkins PB. Induction of CYP3A4 by 1 alpha,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab. Dispos. 2001;29:1446–1453. [PubMed] [Google Scholar]
- 33.Thummel KE, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol. Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 34.Theodoropoulos C, Demers C, Delvin E, Menard D, Gascon-Barre M. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clin. Endocrinol. (Oxf). 2003;58:489–499. doi: 10.1046/j.1365-2265.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. . Biochim. Biophys. Acta. 1995; 1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 36.Wagner KD, Wagner N, Sukhatme VP, Scholz H. Activation of vitamin D receptor by the Wilms’ tumor gene product mediates apoptosis of renal cells. J. Am. Soc. Nephrol. 2001;12:1188–1196. doi: 10.1681/ASN.V1261188. [DOI] [PubMed] [Google Scholar]
- 37.Zou A, Elgort MG, Allegretto EA. Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D-responsive elements and elicit additive effects with 1,25-dihydroxyvitamin D3. J. Biol. Chem. 1997;272:19027–19034. doi: 10.1074/jbc.272.30.19027. [DOI] [PubMed] [Google Scholar]
- 38.Roy-Chowdhury J, Locker J, Roy-Chowdhury N. Nuclear receptors orchestrate detoxification pathways. Dev. Cell. 2003;4:607–608. doi: 10.1016/s1534-5807(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 39.Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J. Nutr. 2003;133(Suppl. 7):2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- 40.Dussault I, et al. Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J. Biol. Chem. 2001;276:33309–33312. doi: 10.1074/jbc.C100375200. [DOI] [PubMed] [Google Scholar]
- 41.Xie W, Evans RM. Pharmaceutical use of mouse models humanized for the xenobiotic receptor. Drug Discov. Today. 2002;7:509–515. doi: 10.1016/s1359-6446(02)02251-1. [DOI] [PubMed] [Google Scholar]
- 42.Staudinger JL, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omdahl JL, Bobrovnikova EA, Choe S, Dwivedi PP, May BK. Overview of regulatory cytochrome P450 enzymes of the vitamin D pathway. Steroids. 2001;66:381–389. doi: 10.1016/s0039-128x(00)00157-4. [DOI] [PubMed] [Google Scholar]
- 44.Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J. Endocrinol. 2000;164:339–348. doi: 10.1677/joe.0.1640339. [DOI] [PubMed] [Google Scholar]
- 45.Chung S, Ahn C. Effects of anti-epileptic drug therapy on bone mineral density in ambulatory epileptic children. Brain Dev. 1994;16:382–385. doi: 10.1016/0387-7604(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 46.D’Erasmo E, Ragno A, Raejntroph N, Pisani D. Drug-induced osteomalacia. Recenti Prog. Med. 1998;89:529–533. [PubMed] [Google Scholar]
- 47.Munch UH, Lupi G, Kistler HJ. Osteomalacia and antiepileptic drugs: study on the pathogenesis. Schweiz. Med. Wochenschr. 1975;105:608–615. [PubMed] [Google Scholar]
- 48.Tomita S, Ohnishi J, Nakano M, Ichikawa Y. The effects of anticonvulsant drugs on vitamin D3-activating cytochrome P-450-linked monooxygenase systems. J. Steroid Biochem. Mol. Biol. 1991;39:479–485. doi: 10.1016/0960-0760(91)90241-v. [DOI] [PubMed] [Google Scholar]
- 49.Jubiz W, Haussler MR, McCain TA, Tolman KG. Plasma 1,25-dihydroxyvitamin D levels in patients receiving anticonvulsant drugs. J. Clin. Endocrinol. Metab. 1977;44:617–621. doi: 10.1210/jcem-44-4-617. [DOI] [PubMed] [Google Scholar]
- 50.Glesby MJ. Bone disorders in human immunodeficiency virus infection. Clin. Infect. Dis. 2003;37(Suppl. 2):S91–S95. doi: 10.1086/375884. [DOI] [PubMed] [Google Scholar]
- 51.Mondy K, Tebas P. Emerging bone problems in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 2003;36(Suppl. 2):S101–S105. doi: 10.1086/367566. [DOI] [PubMed] [Google Scholar]
- 52.Tebas P, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Rivera J, et al. Relationship between low bone mineral density and highly active antiretroviral therapy including protease inhibitors in HIV-infected patients. HIV Clin. Trials. 2003;4:337–346. doi: 10.1310/4X0H-UVMJ-BHYW-CPFB. [DOI] [PubMed] [Google Scholar]
- 54.Moore JT, Kliewer SA. Use of the nuclear receptor PXR to predict drug interactions. Toxicology. 2000;153:1–10. doi: 10.1016/s0300-483x(00)00300-0. [DOI] [PubMed] [Google Scholar]
- 55.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 56.Pichard L, et al. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol. Pharmacol. 1992;41:1047–1055. [PubMed] [Google Scholar]
- 57.Zeghoud, F., Jardel, A., Guillozo, H., Nguyen, T.M., and Garabedian, M. 1991. Micromethod for the determination of 25-hydroxyvitamin D. In Vitamin D: gene regulation, structure-function analysis, and clinical application. Proceedings of the Eighth Workshop on Vitamin D, Paris, France, July 5–10, 1991. A.W. Norman, R. Bouillon, and M. Thomasset, editors. Walter de Gruyter. Berlin, Germany/New York, New York, USA. 662–663.
- 58.Preece MA, O’Riordan JH, Lawson DEM, Kodicek E. A competitive protein binding assay for 25-hydroxycholecalciferol and 25-hydroxyergocalciferol in serum. Clin. Chim. Acta. 1974;54:235–242. doi: 10.1016/0009-8981(74)90241-1. [DOI] [PubMed] [Google Scholar]