Abstract
Altered regulation of insulin secretion by glucose is characteristic of individuals with type 2 diabetes mellitus, although the mechanisms that underlie this change remain unclear. We have now generated mice that lack the λ isoform of PKC in pancreatic β cells (βPKCλ–/– mice) and show that these animals manifest impaired glucose tolerance and hypoinsulinemia. Furthermore, insulin secretion in response to high concentrations of glucose was impaired, whereas the basal rate of insulin release was increased, in islets isolated from βPKCλ–/– mice. Neither the β cell mass nor the islet insulin content of βPKCλ–/– mice differed from that of control mice, however. The abundance of mRNAs for Glut2 and HNF3β was reduced in islets of βPKCλ–/– mice, and the expression of genes regulated by HNF3β was also affected (that of Sur1 and Kir6.2 genes was reduced, whereas that of hexokinase 1 and hexokinase 2 genes was increased). Normalization of HNF3β expression by infection of islets from βPKCλ–/– mice with an adenoviral vector significantly reversed the defect in glucose-stimulated insulin secretion. These results indicate that PKCλ plays a prominent role in regulation of glucose-induced insulin secretion by modulating the expression of genes important for β cell function.
Introduction
Type 2 diabetes mellitus is characterized by insulin resistance in peripheral tissues and functional failure of pancreatic β cells. The importance of the β cells of islets of Langerhans in the pathogenesis of type 2 diabetes is reflected in the development of potential new treatments such as islet transplantation and regenerative islet cell therapy. The roles of molecules important in β cell function in vivo have recently begun to be examined by gene targeting. Signaling by receptor tyrosine kinases has thus been implicated in the regulation both of β cell mass (1, 2) and of insulin secretion (3–5). For example, deletion of the insulin receptor specifically in mouse β cells resulted in impairment of glucose-stimulated insulin secretion and a reduction in β cell mass with age, effects that led to the development of glucose intolerance (6). Ablation of the IGF-1 receptor in mouse β cells also resulted in impaired insulin secretion but did not affect β cell morphology (7, 8). Silencing of the insulin receptor or IGF-1 receptor by RNA interference in MIN6 cells blocked activation of PI3K and consequently inhibited glucose-induced insulin secretion (9). 3-Phosphoinositide–dependent kinase–1 is thought to be a key mediator of PI3K signaling and contributes to the activation of AGC protein kinases, including Akt, p70 S6 kinase, and atypical isoforms of PKC (10). Mice that express a constitutively active form of Akt1 exhibit an increased β cell mass (11, 12), whereas ablation of p70 S6 kinase 1 was associated with a reduced β cell size (13). Atypical PKC isoforms also might therefore be expected to participate in regulation of β cell growth and insulin secretion. In fact, the atypical isoform PKCλ is expressed in pancreatic β cells (14). There have been so far several reports suggesting the role of atypical PKC in insulin secretion (15, 16), insulin synthesis (15, 16), and proliferation (17) in pancreatic β cells. However, direct evidence for the function of PKCλ in β cells in vivo has not been available.
The purpose of this study was to establish a mouse line in which the PKCλ gene is deleted specifically in pancreatic β cells in order to determine the role of this isozyme in these cells in vivo. Our results show that glucose-stimulated insulin secretion is altered in such mice in a manner similar to that apparent in type 2 diabetes, and they suggest that this change is attributable to altered regulation of gene expression important for β cell function.
Results
Generation ofβ cell–specific PKC λ knockout mice.
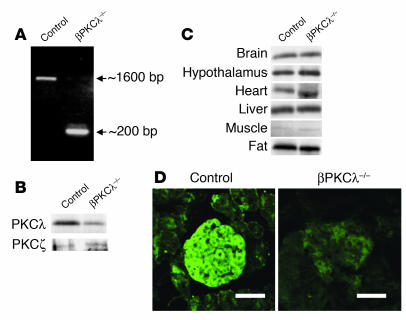
Pancreatic β cell–specific PKCλ knockout (βPKCλ–/–) mice were generated by breeding of mice (PKCλflox/flox) that harbor a modified endogenous PKCλ gene, in which exon 5 is flanked by loxP sites (K. Akimoto et al., unpublished observations), with mice that express Cre recombinase under the control of the promoter of the rat insulin 2 gene (RIP-Cre mice) (18). To evaluate the efficiency of Cre recombinase expression in pancreatic β cells, we performed PCR analysis with primers targeted to regions external to the loxP sites of PKCλflox/flox mice and with genomic DNA extracted from the islets of βPKCλ–/– mice and PKCλflox/flox mice (used as control animals thereafter). Whereas the control animals yielded a PCR product of about 1,600 bp, the βPKCλ–/– mice yielded a major product of about 200 bp and a minor product of about 1,600 bp (Figure 1A), which indicates that the loxP-flanked region containing exon 5 of the PKCλ gene was efficiently removed by Cre recombinase in the β cells of βPKCλ–/– mice.
Figure 1.
Generation of β cell–specific PKCλ knockout mice. (A) PCR analysis of genomic DNA isolated from islets of control (PKCλflox/flox) and βPKCλ–/– mice. The primers were targeted to regions external to the loxP sites of PKCλflox/flox mice. (B and C) Immunoblot analysis of PKCλ in the islets (B) or the brain, hypothalamus, heart, liver, skeletal muscle, and fat (C) of control and βPKCλ–/– mice. Tissue homogenates were subjected to immunoprecipitation and subsequent immunoblot analysis with antibodies against PKCλ. The expression of PKCλ in islets was similarly analyzed. (D) Immunostaining of islets in pancreatic sections of control and βPKCλ–/– mice with antibodies against PKCλ and FITC-conjugated secondary antibodies. Scale bars: 50 μm.
We next examined the expression of PKCλ in various tissues by immunoblot analysis. The amount of PKCλ in islets of βPKCλ–/– mice was reduced by about 80% compared with that in islets of control mice (Figure 1B), indicating the virtually complete loss of PKCλ expression in β cells, given that these cells account for about 80% of the islet cell mass. In contrast, the expression of PKCζ, another atypical isoform of PKC, was not affected in the islets of βPKCλ–/– mice (Figure 1B). The abundance of PKCλ in the brain, hypothalamus, heart, liver, skeletal muscle, and fat was also similar for βPKCλ–/– and control mice (Figure 1C). Furthermore, hypothalamus from βPKCλ–/– and control mice was hybridized in situ to probe the complementarity to loxP-flanked exon 5 of the PKCλ gene. The expression of PKCλ mRNA was marginally reduced by about 20% in βPKCλ–/– mice (data not shown). Immunostaining of pancreatic sections confirmed the efficient ablation of PKCλ in β cells of βPKCλ–/– mice (Figure 1D). Together, these results indicate that loss of PKCλ expression occurred mainly in pancreatic β cells of βPKCλ–/– mice.
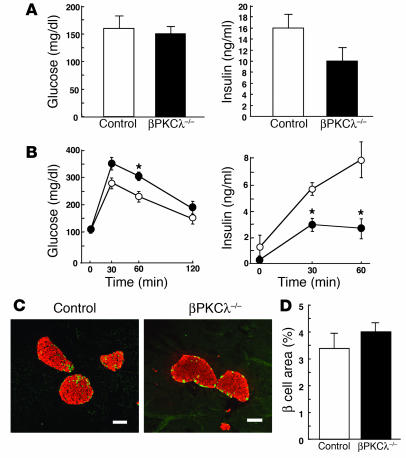
Metabolic characteristics ofβPKCλ –/– mice.
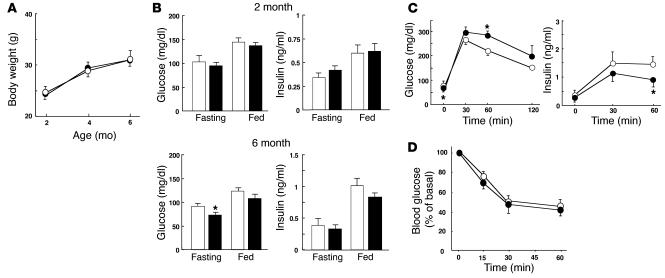
The rate of increase in body weight did not differ between βPKCλ–/– and control mice (Figure 2A). To assess the possible effects of PKCλ ablation in β cells on glucose metabolism, we measured blood glucose and plasma insulin concentrations in 2- and 6-month-old βPKCλ–/– and control mice. There were no significant differences in blood glucose and plasma insulin levels between βPKCλ–/– and control mice at 2 months. At 6 months, the blood glucose concentration of βPKCλ–/– mice was significantly lower than that of control mice in the fasting state (Figure 2B). The blood glucose level in the fed state as well as plasma insulin concentrations in the fasting or fed state did not differ between the 2 groups of animals, however. Intraperitoneal glucose tolerance tests in animals that had fasted overnight revealed that βPKCλ–/– mice had abnormal glucose tolerance, with the blood glucose concentration 60 minutes after the glucose load being significantly higher in these mice than in the control animals (Figure 2C). The insulin response to glucose was also impaired in βPKCλ–/– mice compared with that in control mice, with the plasma insulin level 60 minutes after the glucose load being significantly lower in the former than in the latter. Insulin tolerance tests did not reveal a significant difference in the insulin sensitivity of peripheral tissues between control and βPKCλ–/– mice (Figure 2D), which excludes the possibility that βPKCλ–/– mice are insulin resistant.
Figure 2.
Effect of β cell–specific ablation of PKCλ on glucose metabolism. (A) Growth curves of control (open circles) and βPKCλ–/– (filled circles) mice. Mice were weighed at 2, 4, and 6 months. (B) Blood glucose and plasma insulin concentrations of 6-month-old control (white bars) and βPKCλ–/– (black bars) mice in the fasting or fed state. (C) Intraperitoneal glucose tolerance tests performed in control and βPKCλ–/– mice that had fasted overnight. (D) Blood glucose concentrations during insulin tolerance testing in control and βPKCλ–/– mice. Data are means ± SE of values from 25 (A), 28 (B), 10 (C), or 6 (D) animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice.
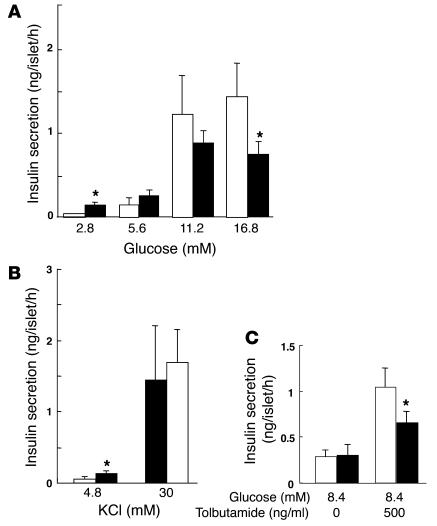
To investigate further the effect of PKCλ ablation on insulin secretion, we examined glucose- or KCl-stimulated insulin release with islets isolated from βPKCλ–/– and control mice. Glucose induced a concentration-dependent increase in insulin secretion from control islets in static incubations, with the extent of insulin release at 16.8 mM glucose being about 20 times that at 2.8 mM glucose (Figure 3A). In contrast, the insulin response of islets from βPKCλ–/– mice was significantly greater (3-fold at 2.8 mM glucose) than that of control islets at low glucose concentrations but significantly smaller (about 50% at 16.8 mM glucose) than that of control islets at high glucose concentrations. The islets of βPKCλ–/– mice exhibited a normal secretory response to a high concentration of KCl (Figure 3B), which elicits insulin release by inducing membrane depolarization. These results thus indicate that depletion of PKCλ in β cells results in a specific impairment in glucose-stimulated insulin secretion and consequent glucose intolerance.
Figure 3.
Impairment of glucose-stimulated insulin secretion in isolated islets of βPKCλ–/– mice. Insulin release in response to the indicated concentrations of glucose (A), KCl (B), or tolbutamide (C) was measured with islets isolated from control (white bars) or βPKCλ–/– (black bars) mice. Data are means ± SE of values from 6 animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice.
Morphology and insulin content of islets ofβPKCλ –/– mice.
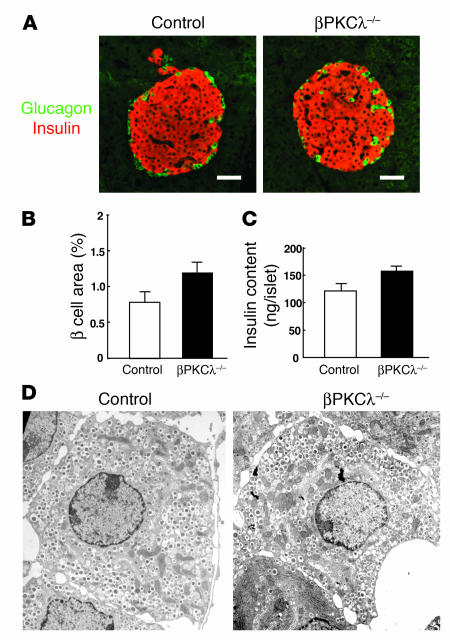
We next determined the possible effects of β cell–specific deficiency of PKCλ on pancreatic morphology. Immunostaining of pancreatic sections from 6-month-old animals with antibodies against insulin and against glucagon revealed a normal islet architecture in βPKCλ–/– mice (Figure 4A). Quantitative analysis also revealed no significant difference in the β cell area per pancreas between βPKCλ–/– and control mice (Figure 4B). The α cell area per pancreas was also normal in βPKCλ–/– mice (data not shown). Furthermore, the total insulin content of islets of βPKCλ–/– mice was similar to that of control islets (Figure 4C). These results indicate that islet growth and insulin biosynthesis are not impaired in βPKCλ–/– mice. Electron microscopy also revealed that the ultrastructure of β cells, including the number and distribution of insulin-containing dense-core granules, is similar in βPKCλ–/– and control mice (Figure 4D), which suggests that the impairment of glucose-stimulated insulin secretion in βPKCλ–/– mice is not due to an obvious disruption of cellular architecture or a failure to form insulin secretory granules.
Figure 4.
Lack of effect of β cell–specific ablation of PKCλ on islet morphology and insulin content. (A) Immunostaining of pancreatic sections from 6-month-old control and βPKCλ–/– mice with antibodies against insulin (red) and glucagon (green). Scale bars: 50 μm. (B) Quantitation of β cell area as a percentage of total pancreatic area in control and βPKCλ–/– mice. Data are means ± SE of values from 4 mice of each genotype. (C) Insulin content of isolated islets. Data are means ± SE of values from 4 mice of each genotype. (D) Electron microscopy of β cells of control and βPKCλ–/– mice. Magnification, ×10,000.
Effect of a high-fat diet on insulin secretion inβPKCλ –/– mice.
We investigated the effect of PKCλ deficiency on β cell function in the insulin-resistant state by feeding control and βPKCλ–/– mice a high-fat diet for up to 21 weeks from weaning. The increases in body weight were similar for the 2 types of mice on this diet (data not shown). Random measurement of blood glucose concentration in the fed state also did not reveal any significant difference between βPKCλ–/– and control mice (Figure 5A). The plasma insulin concentration in the fed state tended to be lower in βPKCλ–/– mice than in control animals, although this difference was not statistically significant. The blood glucose concentration apparent 60 minutes after a glucose challenge was significantly higher in βPKCλ–/– mice than in controls (Figure 5B). The plasma insulin concentrations apparent after a glucose load were higher in control mice fed the high-fat diet than in those fed a normal diet, reflecting the insulin-resistant state of the former. The insulin levels after a glucose challenge were markedly lower in βPKCλ–/– mice fed the high-fat diet than in control mice on this diet.
Figure 5.
Effects of a high-fat diet on β cell phenotype in βPKCλ–/– mice. (A) Blood glucose and plasma insulin concentrations in the fed state of control and βPKCλ–/– mice on a high-fat diet. Data are means ± SE of values from 12 animals of each genotype. (B) Intraperitoneal glucose tolerance tests in control (open circles) and βPKCλ–/– (filled circles) mice after 15 weeks on the high-fat diet. Data are means ± SE of values from 6 animals of each genotype. *P < 0.05 (ANOVA) versus the corresponding value for control mice. (C) Immunostaining of pancreatic sections from control and βPKCλ–/– mice after 15 weeks on the high-fat diet with antibodies against insulin (red) and glucagon (green). Scale bars: 100 μm. (D) Quantitation of β cell area as a percentage of total pancreatic area in control and βPKCλ–/– mice after 15 weeks on the high-fat diet. Data are means ± SE of values from 3 mice of each genotype.
We also examined islet morphology in mice fed the high-fat diet. Compared with those fed normal chow, control mice fed the high-fat diet exhibited an increase in islet mass to compensate for their insulin resistance. Islet mass in βPKCλ–/– mice fed the high-fat diet was similar to that in the control animals on this diet (Figure 5, C and D). These results indicate that the β cells of βPKCλ–/– mice grow like those of control mice during the development of insulin resistance, whereas the insulin secretory response to glucose is blunted in the knockout mice.
Expression of genes important forβ cell function inβPKC λ–/– mice.
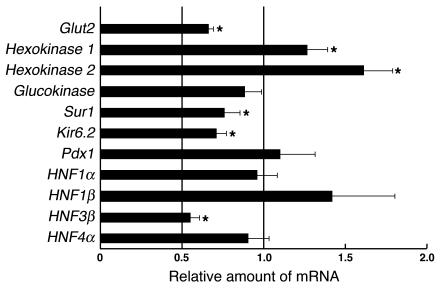
We analyzed the expression of several genes important for β cell function by real-time RT-PCR analysis of total RNA isolated from islets (Figure 6). The amounts of mRNAs for the glucose transporter Glut2 and the Sur1 and Kir6.2 subunits of the ATP-sensitive K+ channel were significantly reduced by 25–35% in βPKCλ–/– mice compared with those in control mice, which suggests that the impairment of glucose-stimulated insulin secretion in the former animals might result, at least in part, from a deficiency of glucose-sensing proteins. In accordance, the islets of βPKCλ–/– mice exhibited reduced secretory response to tolbutamide, a sulfonylurea reagent, which elicits insulin release by the closure of the ATP-sensitive K+ channel (Figure 3C). The abundance of the mRNA for the transcription factor HNF3β was also reduced by 45% in the islets of βPKCλ–/– mice. In contrast, the amounts of mRNAs for the high-affinity hexokinase isoforms 1 and 2 were increased in βPKCλ–/– mice. The abundance of mRNAs for glucokinase, Pdx1, HNF1α, HNF1β, and HNF4α did not differ between βPKCλ–/– and control mice. Gene expression profiling with an oligonucleotide microarray confirmed the results obtained by real-time RT-PCR analysis (data not shown).
Figure 6.
Altered gene expression in the islets of βPKCλ–/– mice. The abundance of mRNAs for the indicated proteins was determined by real-time RT-PCR analysis of total RNA isolated from islets of control and βPKCλ–/– mice. The amounts of the mRNAs in βPKCλ–/– mice are expressed relative to those in control animals. Data are means ± SE of triplicates for pooled total RNA samples from 6 mice of each genotype and are representative of a total of 3 similar experiments. *P < 0.05 (ANOVA) versus the corresponding value (1.0) for control mice.
Restoration of PKCλ expression in islets ofβPKCλ –/– mice.
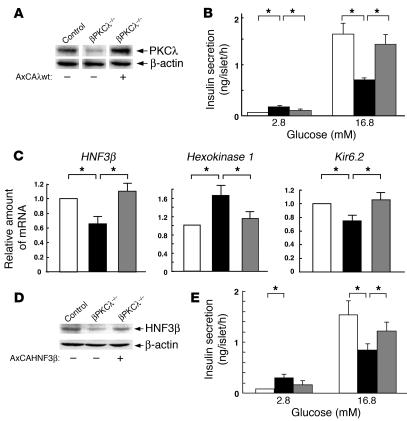
We next examined the effect of restoration of PKCλ expression with an adenoviral vector in islets from βPKCλ–/– mice on glucose-stimulated insulin secretion. Transfection of islets from control or βPKCλ–/– mice with a control vector encoding β-galactosidase (AxCALacZ) had no effect on glucose-induced insulin release (data not shown). The abundance of PKCλ in βPKCλ–/– mouse islets transfected with the vector for wild-type PKCλ (AxCAλwt) was approximately twice that of endogenous PKCλ in islets from control mice (Figure 7A). Restoration of PKCλ expression in islets from the knockout mice normalized the insulin secretory response to glucose (Figure 7B); the hypersecretion of insulin at low glucose concentrations and the impaired insulin secretion at high glucose concentrations apparent with βPKCλ–/– mouse islets were thus both significantly reversed by expression of recombinant PKCλ.
Figure 7.
Effects of adenovirus-mediated restoration of PKCλ or HNF3β expression on insulin secretion in islets of βPKCλ–/– mice. (A–C) Islets isolated from control mice or βPKCλ–/– mice were infected with an adenovirus encoding either β-galactosidase (AxCALacZ) or wild-type PKCλ (AxCAλwt). The islets were then either subjected to immunoblot analysis with antibodies against PKCλ or β actin (A); assayed for insulin secretion in the presence of 2.8 or 16.8 mM glucose (white bars, control islets plus AxCALacZ; black bars, βPKCλ–/– islets plus AxCALacZ; gray bars, βPKCλ–/– islets plus AxCAλwt) (B); or subjected to real-time RT-PCR analysis of mRNAs for HNF3β, hexokinase 1, or Kir6.2 (C). (D and E) Islets isolated from control or βPKCλ–/– mice were infected with either AxCALacZ or an adenovirus encoding wild-type HNF3β (AxCAHNF3β). The islets were then either subjected to immunoblot analysis with antibodies against HNF3β or β actin (D) or assayed for insulin secretion in the presence of 2.8 or 16.8 mM glucose (E). Data are means ± SE of values from 6 mice (B and E) or of triplicates for pooled total RNA samples from 5 mice (C). *P < 0.05 (ANOVA) for the indicated comparisons.
The expression of HNF3β and Kir6.2 genes was also increased in βPKCλ–/– mouse islets transfected with AxCAλwt compared with that in those transfected with AxCALacZ (Figure 7C). Similarly, the increased abundance of hexokinase 1 mRNA apparent in βPKCλ–/– mouse islets was reduced by restoration of PKCλ expression. These results thus indicate that the altered patterns of both insulin secretion and islet gene expression apparent in βPKCλ–/– mice are directly attributable to the lack of PKCλ.
Effect of HNF3β on glucose-stimulated insulin secretion inβPKCλ –/– mouse islets.
Our results suggested that HNF3β might be a key mediator of PKCλ action in the regulation of insulin secretion in β cells. We therefore examined the effect of expression of recombinant wild-type HNF3β in islets isolated from βPKCλ–/– mice on glucose-stimulated insulin secretion. Transfection of βPKCλ–/– mouse islets with an adenoviral vector for HNF3β (AxCAHNF3β) restored the abundance of this transcription factor to the level apparent in islets from control mice (Figure 7D). The insulin secretory response of βPKCλ–/– mouse islets to a high glucose concentration was also significantly increased by expression of recombinant HNF3β (Figure 7E). The increase in the basal secretion of insulin by βPKCλ–/– mouse islets also appeared to be partially reversed by transfection with AxCAHNF3β, although this effect was not statistically significant. These results indicate that HNF3β might function downstream of PKCλ in the regulation of glucose-induced insulin secretion.
Discussion
We have shown that mice deficient in PKCλ in pancreatic β cells exhibit an increased basal rate of insulin secretion and impairment of glucose-stimulated insulin secretion without any obvious abnormality in β cell morphology. Both glucose-induced transcriptional activation of the insulin gene promoter and glucose-stimulated insulin secretion were previously shown to be inhibited by the PKC-specific inhibitor calphostin C (Ro31-8220) but not by Go6976, an inhibitor of classical and novel PKC isoforms; this implicated atypical PKC in these effects of glucose (15, 16). With the use of isoform-specific pseudosubstrates to inhibit either classical (α, β, and γ) isoforms of PKC or the atypical isoform PKCζ, Buteau et al. (17) showed that the latter enzyme mediates glucagon-like peptide–1–induced proliferation of pancreatic β cells. However, most of the previous studies of the possible roles of atypical isoforms of PKC in β cells have been performed with inhibitors, and the antibody used to detect atypical PKC did not discriminate between the λ and ζ isoforms. To obtain direct evidence for the function of PKCλ in β cells, we therefore generated mice that lack this isozyme specifically in these cells with the use of the Cre-loxP system. No compensatory increase in the abundance of PKCζ was apparent in the islets of the βPKCλ–/– mice. RIP-Cre mice have been demonstrated to express Cre recombinase in pancreatic β cells, and also in a subset of neurons in hypothalamus (19). In situ hybridization of brain slices for PKCλ revealed a marginal decrease (approximately 20%) in βPKCλ–/– mice. It is possible that the reduced expression of PKCλ in hypothalamus might affect insulin secretory response via the neuronal pathway. However, we believe that the phenotype of βPKCλ–/– mice is mainly attributable to the decrease in PKCλ levels in pancreatic β cells, because (a) we observed the impaired glucose-induced insulin secretion and the altered gene expression in islets isolated from βPKCλ–/– mice ex vivo, and (b) ex vivo normalization of PKCλ expression in islets isolated from βPKCλ–/– mice resulted in a significant restoration of the impairment of insulin secretion and an alteration of gene expression (Figure 7, A–C).
Complexes of atypical PKC isoforms, PAR-3, and PAR-6 have been shown to be indispensable for cell polarization in Caenorhabditis elegans embryos (20), Drosophila epithelial cells (21) and neuroblasts (22), and mammalian epithelial cells (23). Expression of a dominant negative mutant of PKCλ thus disrupted the formation of apical-basal polarity in cultured Madin-Darby canine kidney cells (23). We therefore examined whether ablation of PKCλ resulted in disruption of β cell polarity and dysregulation of insulin secretion in βPKCλ–/– mice. Electron microscopy did not reveal any obvious structural abnormality related to β cell polarity in βPKCλ–/– mice, however. In addition, βPKCλ–/– mice manifested impairment of insulin secretion stimulated by glucose but not of that stimulated by KCl, suggesting that the exocytosis of insulin granules remained intact. Together, these results indicate that PKCλ does not contribute substantially to the polarity or structure of β cells, at least in our mice, in which the PKCλ gene is deleted in mature β cells after the onset of Cre expression driven by the rat insulin 2 gene promoter. Several previous studies have also suggested that PKCλ mediates growth factor signaling that leads to cell proliferation, differentiation, and survival (24–26). However, islet size and insulin content did not differ substantially between βPKCλ–/– and control mice. Moreover, the extent of β cell apoptosis appeared unaffected in βPKCλ–/– mice (data not shown).
PKCλ mediates induction of the expression of the gene for SREBP-1c by either insulin or active PI3K in hepatocytes (27). Indeed, PKCλ contains a functional nuclear localization signal in its NH2-terminal region (28) and undergoes rapid nucleocytoplasmic shuttling in response to stimuli such as PDGF and nerve growth factor (29). Atypical PKC has also been implicated in the phosphorylation and activation of the transcription factor Pdx1 in response to glucose stimulation in β cells (15). IGF-1 increased the transcriptional activity of the gene for the cytochrome P450 side-chain cleavage enzyme by promoting an interaction between PKCλ and polypyrimidine tract–binding protein–associated splicing factor in the nucleus, and it increased the amount of PKCλ in the nucleus of a granulosa cell line (30). These various observations suggest that PKCλ participates in nuclear events such as the regulation of gene expression.
With the use of quantitative gene expression analysis, we have now shown that ablation of PKCλ affected the expression of genes that encode components of the glucose-sensing mechanism of β cells. The lack of PKCλ thus resulted in increased expression of the genes for the high-affinity hexokinase isoforms 1 and 2, which mediate glucose sensing in the physiological range of blood glucose concentration. Overexpression of hexokinase 1 or 2 has previously been shown to result in an increase in basal insulin release from β cells (31, 32), similar to that observed in the present study with islets derived from βPKCλ–/– mice. The loss of PKCλ from β cells also resulted in downregulation of the expression of the genes for Sur1 and Kir6.2, confirmed by the reduced secretory response to tolbutamide (Figure 3C). These subunits of the ATP-sensitive K+ channel respond to changes in the intracellular ATP/ADP concentration ratio and thereby couple cellular metabolism to membrane electrical activity and insulin secretion. Studies of knockout mice have shown that the loss of ATP-sensitive K+ channels results in impairment of glucose-stimulated insulin secretion (33, 34). In addition, Sur1 knockout mice exhibited increased basal plasma insulin levels, because the rate of return to the basal rate of insulin secretion after a fall in blood glucose concentration was reduced, resulting in hypoglycemia during fasting that was similar to that observed in βPKCλ–/– mice. The ATP-sensitive K+ channel and hexokinases 1 and 2 have been shown to be targets of HNF3β regulation in β cells (32, 35). Together, these various observations suggest that the downregulation of HNF3β induced by PKCλ ablation is responsible for the changes in the expression of the genes for hexokinases 1 and 2, Sur1, and Kir6.2, and that these changes then result in the altered insulin secretion apparent in the islets of βPKCλ–/– mice. Expression of the Glut2 gene was also reduced in islets of βPKCλ–/– mice. The Glut2 transporter mediates glucose uptake by pancreatic β cells, the first step in the signaling cascade responsible for glucose-stimulated insulin secretion. Ablation of Glut2 from mouse β cells resulted in loss of the initial phase of glucose-induced insulin release; the second phase was preserved but diminished (36), consistent with the phenotype of βPKCλ–/– mice.
To verify the role of PKCλ in insulin secretion suggested by our results, we examined the effects of restoration of expression of this enzyme with the use of an adenoviral vector in islets isolated from βPKCλ–/– mice. Restoration of PKCλ expression indeed normalized glucose-stimulated insulin secretion as well as the abundance of mRNAs for HNF3β, hexokinase 1, and Kir6.2 in the βPKCλ–/– islets, consistent with our conclusion that PKCλ regulates the expression of genes whose products mediate the induction of insulin release by glucose. We also examined the effects of normalization of HNF3β expression in islets derived from βPKCλ–/– mice. HNF3β (Foxa-2) is a member of the hepatocyte nuclear factor 3 and forkhead family of transcription factors that also includes HNF3α (Foxa-1) and HNF3γ (Foxa-3). HNF3 proteins play important roles in maintaining glucose homeostasis through regulation of gene expression in the liver. HNF3β is thought to act upstream of Pdx1, HNF1α, and HNF4α during pancreatic development. In mature β cells, HNF3β regulates the expression of genes important for β cell function, including those for Pdx1, Nkx6.2, Sur1, and Kir6.2 (35, 37). A dominant negative form of HNF3β was also shown to reduce the expression of the genes for Sur1 and Kir6.2 by 70% in INS-1 cells (32). The cells expressing this HNF3β mutant also exhibited upregulation of hexokinase 1 and hexokinase 2 as well as failure to regulate Pdx1, consistent with our present results. In contrast to our results, however, expression of the HNF3β mutant resulted in downregulation of HNF1α and HNF4α; it is possible that the abundance of HNF1α and HNF4α mRNAs was unaffected in the islets of βPKCλ–/– mice because the amount of HNF3β mRNA was reduced by only 40–50% and thus might still be sufficient to allow normal transcription of the HNF1α and HNF4α genes. Although mutations of the HNF3β gene are not a common cause of maturity-onset diabetes of the young, a loss-of-function mutation (A86T) of this gene has been associated with type 2 diabetes (38), suggesting that HNF3β gene mutations might confer predisposition to this disease. Indeed, normalization of HNF3β expression in islets isolated from βPKCλ–/– mice resulted in a significant reversal of the impairment of glucose-induced insulin secretion. Taken together, we conclude that PKCλ plays a prominent role in the regulation of glucose-induced insulin secretion by modulating the gene expression of HNF3β, Glut2, hexokinase, Sur1, and Kir6.2, and most likely also other effector molecules important for β cell function.
Ablation of the IGF-1 receptor in mouse β cells has been shown to impair insulin secretion, possibly as a result of a reduction in the levels of expression of Glut2, glucokinase, and HNF3β (7). The β cell–specific IGF-1 receptor knockout mouse and our βPKCλ–/– mice thus both show similar changes in insulin secretion and in gene expression. Silencing of the IGF-1 receptor by RNA interference also inhibited PI3K activity and glucose-stimulated insulin secretion in MIN6 cells and increased the extent of basal insulin release (9). Given that PI3K and 3-phosphoinositide–dependent kinase–1 contribute to the signaling pathway that links receptor tyrosine kinases to PKCλ (24, 29, 39), PKCλ might mediate signaling from the IGF-1 receptor to gene transcription in pancreatic β cells.
Hypersecretion of insulin at basal blood glucose levels and impairment of glucose-stimulated insulin secretion are characteristics of type 2 diabetes (40), and lipotoxicity manifests a similar phenotype of insulin secretion (41). Further elucidation of the intracellular signaling mediated by PKCλ in pancreatic β cells might thus provide a basis for the development of new therapeutic strategies for diabetes.
Methods
Animals and genotyping.
Mice (PKCλflox/flox) harboring a “floxed” PKCλ gene were generated by homologous recombination (K. Akimoto et al., unpublished observations). Mice expressing the Cre recombinase under the control of the rat insulin 2 gene promoter were generated as described previously (18). The genetic background of mice was derived from a hybrid of C57BL/6, 129sv, and DBA-2 strains. Animals were maintained under a 12-hour-light, 12-hour-dark cycle and fed either normal chow or a high-fat diet from the time of weaning (3 weeks old) for a 21-week period. The high-fat diet contained 30% (wt/wt) fat (14% bovine fat, 14% porcine fat, 2% soybean oil) (42). Mice were genotyped by PCR analysis with primers (5′ and 3′, respectively) specific for the Cre transgene (5′-ATGTCCAATTTACTGACCG-3′, 5′-CGCCGCATAACCAGTGAAAC-3′) or for the floxed PKCλ allele (5′-CATGCAGTGTACTGGCATAGCCACC-3′, 5′-AGAGGCAGCCAAAGCCCTGCTCTCC-3′). PCR was performed for 39 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 45 seconds or for 35 cycles of 94°C for 30 seconds, 66°C for 30 seconds, and 72°C for 30 seconds, respectively. This study was performed according to the guidelines of the Animal Ethics Committee of Kobe University Graduate School of Medicine.
Immunoprecipitation and immunoblot analysis.
For determination of the abundance of PKCλ and PKCζ, total tissue homogenates were subjected to immunoprecipitation with rabbit polyclonal antibodies against PKCλ (αλ190) or against PKCζ (αζ170) (14), and the resulting precipitates were subjected to immunoblot analysis with mouse mAbs against PKCλ/ι (Transduction Laboratories) or rabbit polyclonal antibodies against PKCζ (Upstate Biotechnology Inc.), respectively. Goat polyclonal antibodies against HNF3β obtained from Santa Cruz Biotechnology Inc. were used to determine the abundance of HNF3β by immunoblot analysis.
Analysis of metabolic parameters.
Blood samples were collected from the tail vein and plasma samples were separated by centrifugation of blood in a microcentrifuge for 5 minutes at 4°C. Blood glucose level was determined with a glucometer (Glutest Pro; Sanwa Kagaku Kenkyusho Co.). Plasma insulin concentration was measured with an ELISA kit with a mouse insulin standard (Shibayagi Co.) or by RIA with a rat insulin standard (Eiken Chemical Co.). All assays were performed in duplicate (43).
Intraperitoneal glucose tolerance and insulin tolerance test.
Mice were deprived of food for 16 hours and then anesthetized with pentobarbital (30 mg per kilogram of body weight). Blood was collected immediately before as well as 30, 60, and 120 minutes after the intraperitoneal injection of glucose (1.5 mg/kg) (44). For insulin tolerance testing, mice in the randomly fed state were injected with 0.75 U/kg body weight of human regular insulin. Blood glucose and plasma insulin levels were measured as described above.
Assay of insulin secretion from isolated islets.
Islets were isolated from 6-month-old mice by collagenase digestion and subsequent centrifugation over a Histopaque (Sigma-Aldrich) gradient as described previously (44). For assay of insulin release, 5 islets were manually selected, incubated in Krebs-Ringer solution, and stimulated at 37°C with various concentrations of either glucose for 1 hour, KCl for 30 minutes, or tolbutamide for 1 hour. The islets were then collected by centrifugation, and the supernatant was assayed for insulin content by RIA as described above. For measurement of islet insulin content, islets were solubilized in acid-ethanol solution (74% ethanol, 1.4% HCl) overnight at 4°C before insulin RIA.
Immunostaining and morphometric analysis of islets.
The pancreas was removed from 6-month-old mice, weighed, fixed overnight in Bouin’s solution, and embedded in paraffin. Consecutive 4-μm-thick sections were cut from the tissue and mounted on glass slides. After rehydration, the sections were stained with H&E. Immunostaining for insulin and glucagon was performed with guinea pig antibodies against insulin and rabbit antibodies against glucagon (DAKO Japan); immune complexes were detected with secondary antibodies conjugated with Cy3 or FITC, respectively (Jackson ImmunoResearch Laboratories Inc.). Immunostaining for PKCλ was performed with antibodies against PKCλ (αλ190). For quantitation of β cell area, 4 animals of each genotype were analyzed at 6 months of age. Sections of paraffin-embedded pancreas were immunostained at 200-μm intervals to avoid measurement of the same islets twice. Images of β cells and of the entire pancreas were obtained with a digital camera (EOS D30; Canon Inc.) and analyzed with the use of NIH Image 1.60 software, as described previously (45). The β cell area was expressed as a percentage of the total pancreatic area surveyed.
Electron microscopy.
Two PKCλflox/flox and 2 βPKCλ–/– mice were anesthetized with ether and subjected to intracardial perfusion with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4). The pancreas was excised from each mouse, cut into small pieces, and immersed overnight in the same fixative. The tissue was then exposed to 2% OsO4, treated with 2% uranyl acetate, dehydrated with ethanol, and embedded in Epon 812 (Nacalai Tesque). For light microscopy, 1-μm-thick sections were cut and stained with toluidine blue. For electron microscopy, thin sections were stained with uranyl acetate and lead citrate before examination with a Hitachi 7100 electron microscope (Hitachi Ltd.).
Quantitation of mRNA by real-time RT-PCR.
Total cellular RNA was isolated from islets of PKCλflox/flox and βPKCλ–/– mice with the use of an RNeasy kit (QIAGEN Sciences). Real-time RT-PCR analysis of the total RNA pooled from 6 animals of each genotype was performed with an ABI PRISM 7900 Sequence Detection System (Applied Biosystems); 36B4 mRNA was used as an internal standard as previously described (27). Each reaction was performed in triplicate.
Infection of isolated islets with adenoviral vectors.
Adenoviral vectors encoding β-galactosidase or wild-type mouse PKCλ have been described previously (46). Complementary DNA encoding human HNF3β was kindly provided by M. Stoffel (Rockefeller University, New York, New York, USA), and an adenoviral vector encoding HNF3β was generated with an adenovirus expression kit (Takara Shuzo Co.) as described previously (47). Isolated islets were maintained in RPMI 1640 medium that contained 11.1 mM glucose and was supplemented with 10% FBS. About 200 islets were infected with the adenoviral vectors at an MOI of 10 PFUs per cell. Experiments were performed 24 hours after infection.
Statistical analysis.
Data are expressed as means ± SE and were analyzed with ANOVA. A P value of less than 0.05 was considered statistically significant.
Acknowledgments
We thank M.A. Magnuson for Ins-Cre mice, T. Kitamura and T. Aizawa for helpful discussions, and K. Satomura and M. Kawasaki for technical assistance. This work was supported by grants from the Intellectual Cluster Formation Project, and from the 21st Century COE Program “Center of Excellence for Signal Transduction Disease: Diabetes Mellitus as Model,” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M. Kasuga).
Footnotes
See the related Commentary beginning on page 16.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J. Mol. Endocrinol. 2000;24:303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 2.Withers DJ, et al. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 3.Leibiger IB, Leibiger B, Moede T, Berggren PO. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 S6 kinase and CaM kinase pathways. Mol. Cell. 1998;1:933–938. doi: 10.1016/s1097-2765(00)80093-3. [DOI] [PubMed] [Google Scholar]
- 4.Leibiger B, et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol. Cell. 2001;7:559–570. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 5.Persaud SJ, Harris TE, Burns CJ, Jones PM. Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J. Mol. Endocrinol. 1999;22:19–28. doi: 10.1677/jme.0.0220019. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni RN, et al. Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat. Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- 8.Xuan S, et al. Defective insulin secretion in pancreatic β cells lacking type 1 IGF receptor. J. Clin. Invest. 2002;110:1011–1019. doi:10.1172/JCI200215276. doi: 10.1172/JCI15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva Xavier G, Qian Q, Cullen PJ, Rutter GA. Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic β-cell glucose sensing revealed by RNA silencing. Biochem. J. 2004;377:149–158. doi: 10.1042/BJ20031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MR, et al. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle RL, et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 12.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 2001;108:1631–1638. doi:10.1172/JCI200113785. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pende M, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 14.Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J. Biol. Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 15.Furukawa N, et al. Possible involvement of atypical protein kinase C (PKC) in glucose-sensitive expression of the human insulin gene: DNA-binding activity and transcriptional activity of pancreatic and calphostin C-sensitive but phorbol 12-myristate 13-acetate (PMA) and Go 6976-insensitive pathway. Endocr. J. 1999;46:43–58. doi: 10.1507/endocrj.46.43. [DOI] [PubMed] [Google Scholar]
- 16.Harris TE, Persaud SJ, Jones PM. Atypical isoforms of PKC and insulin secretion from pancreatic β-cells: evidence using Go 6976 and Ro 31-8220 as PKC inhibitors. Biochem. Biophys. Res. Commun. 1996;227:672–676. doi: 10.1006/bbrc.1996.1567. [DOI] [PubMed] [Google Scholar]
- 17.Buteau J, et al. Protein kinase Cζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 18.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol. Cell. Biol. 2004;24:258–269. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabuse Y, et al. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- 21.Muller HA. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev. Dyn. 2000;218:52–67. doi: 10.1002/(SICI)1097-0177(200005)218:1<52::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Doe CQ, Bowerman B. Asymmetric cell division: fly neuroblast meets worm zygote. Curr. Opin. Cell Biol. 2001;13:68–75. doi: 10.1016/s0955-0674(00)00176-9. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, et al. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkoy G, Perander M, Overvatn A, Johansen T. Reversion of ras- and phos-phatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase C lambda is accompanied by the loss of constitutive nuclear mitogen-activated protein kinase/extracellular signal-regulated kinase activity. J. Biol. Chem. 1997;272:11557–11565. doi: 10.1074/jbc.272.17.11557. [DOI] [PubMed] [Google Scholar]
- 25.Akimoto K, et al. Atypical protein kinase C lambda binds and regulates p70 S6 kinase. Biochem. J. 1998;335:417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki A, Akimoto K, Ohno S. Protein kinase Cλ/ι (PKCλ/ι): a PKC isotype essential for the development of multicellular organisms. J. Biochem. 2003;133:9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, et al. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J. Clin. Invest. 2003;112:935–944. doi:10.1172/JCI200318816. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perander M, Bjorkoy G, Johansen T. Nuclear import and export signals enable rapid nucleocytoplasmic shuttling of the atypical protein kinase C λ. J. Biol. Chem. 2001;276:13015–13024. doi: 10.1074/jbc.M010356200. [DOI] [PubMed] [Google Scholar]
- 29.Akimoto K, et al. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 30.Urban RJ, Boedenburg YH, Jiang J, Denner L, Chedrese J. Protein kinase C iota enhances the transcriptional activity of the porcine P450 side chain cleavage insulin-like response element. Am. J. Physiol. Endocrinol. Metab. 2004;286:E975–E979. doi: 10.1152/ajpendo.00520.2003. [DOI] [PubMed] [Google Scholar]
- 31.Becker TC, BeltrandelRio H, Noel RJ, Johnson JH, Newgard CB. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 1994;269:21234–21238. [PubMed] [Google Scholar]
- 32.Wang H, Gauthier BR, Hagenfeldt-Johansson KA, Iezzi M, Wollheim CB. Foxa2 (HNF3β) controls multiple genes implicated in metabolism-secretion coupling of glucose-induced insulin release. J. Biol. Chem. 2002;277:17564–17570. doi: 10.1074/jbc.M111037200. [DOI] [PubMed] [Google Scholar]
- 33.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. J. Biol. Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- 34.Miki T, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sund NJ, et al. Tissue-specific deletion of Foxa2 in pancreatic β cells results in hyperinsulinemic hypoglycemia. Genes Dev. 2001;15:1706–1715. doi: 10.1101/gad.901601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillam MT, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat. Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 37.Lee CS, et al. Foxa2 controls Pdx1 gene expression in pancreatic β-cells in vivo. Diabetes. 2002;51:2546–2551. doi: 10.2337/diabetes.51.8.2546. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Q, et al. Identification of missense mutations in the hepatocyte nuclear factor-3β gene in Japanese subjects with late-onset Type II diabetes mellitus. Diabetologia. 2000;43:1197–1200. doi: 10.1007/s001250051512. [DOI] [PubMed] [Google Scholar]
- 39.Kotani K, et al. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porte D. Banting lecture 1990: β-cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 41.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 42.Inoue H, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 2004;10:168–174. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 43.Kido Y, et al. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitamura T, et al. Preserved pancreatic β-cell development and function in mice lacking the insulin receptor-related receptor. Mol. Cell. Biol. 2001;21:5624–5630. doi: 10.1128/MCB.21.16.5624-5630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kido Y, et al. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and β-cell compensation to insulin resistance. J. Biol. Chem. 2002;277:36740–36747. doi: 10.1074/jbc.M206314200. [DOI] [PubMed] [Google Scholar]
- 46.Sakaue H, et al. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol. Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura T, et al. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol. Cell. Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]