Abstract
Background
In May 2011, a major incident involving phthalates-contaminated foodstuffs occurred in Taiwan. Di-(2-ethylhexyl) phthalate (DEHP) was added to foodstuffs, mainly juice, jelly, tea, sports drink, and dietary supplements. Concerns arose that normal pubertal development, especially reproductive hormone regulation in children, could be disrupted by DEHP exposure.
Objective
To investigate the association between phthalate exposure and reproductive hormone levels among children following potential exposure to phthalate-tainted foodstuffs.
Methods
A total of 239 children aged <12 years old were recruited from 3 hospitals in north, central, and south Taiwan after the episode. Structured questionnaires were used to collect the frequency and quantity of exposures to 5 categories of phthalate-contaminated foodstuffs to assess phthalate exposure in children. Urine samples were collected for the measurement of phthalate metabolites. The estimated daily intake of DEHP exposure at the time of the contamination incident occurred was calculated using both questionnaire data and urinary DEHP metabolite concentrations. Multiple regression analyses were applied to assess associations between phthalate exposure and reproductive hormone levels in children.
Results
After excluding children with missing data regarding exposure levels and hormone concentrations and girls with menstruation, 222 children were included in the statistical analyses. After adjustment for age and birth weight, girls with above median levels of urinary mono-(2-ethyl-5-hydroxyhexyl) phthalate, mono-(2-ethyl-5-oxohexyl) phthalate, and sum of mono-(2-ethylhexyl) phthalate concentrations had higher odds of above median follicle-stimulating hormone concentrations. Girls with above median estimated average daily DEHP exposures following the contamination episode also had higher odds of sex hormone-binding globulin above median levels.
Conclusions
Phthalate exposure was associated with alterations of reproductive hormone levels in girls.
Introduction
Phthalate esters are widely used in chemical synthesis and are added during the manufacture of many items used in daily life [1, 2], including food packaging, cosmetics, pharmaceuticals, insecticides, polyvinyl chloride (PVC) products, construction materials, and medical products. Phthalate esters are not chemically bound to the polymer and therefore easily evaporate, migrate, or release into air, foodstuffs, and related materials. Exposure to phthalate occurs by ingestion, dermal absorption, inhalation, or contact with medical devices [2].
The hypothalamus–anterior pituitary–gonadal (HPG) axis regulates and controls the balance of reproductive hormone levels in the body. In brief, gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus induce the secretion of GnRH that subsequently stimulates the anterior pituitary to synthesize and release luteinizing hormone (LH) and follicle-stimulating hormone (FSH) to the gonads. The gonads, testes in the male and ovaries in the female, then synthesize and release sex hormones (which are steroids) into the somatic circulation, mainly producing testosterone (TT) in males and estradiol (E2) and progesterone (PG) in females. The HPG axis is controlled under negative feedback mechanisms by systemic sex hormone concentrations that inhibit GnRH secretion in the hypothalamus and by pituitary responsiveness to GnRH. Environmental phthalate diesters are endocrine disruptors that may interfere with the normal function of the HPG axis, resulting in distorted levels of reproductive hormones [3, 4] and potentially producing reproductive and developmental toxicity [5–7].
Phthalate diesters are reported to have antiandrogenic and weak estrogenic effects [8–10]. Among phthalate diesters, di-(2-ethylhexyl) phthalate (DEHP) is the most widely used [11]. Animal studies have shown that DEHP exposure is associated with decreased TT concentration, lowered sperm counts, and abnormal testicular development due to its adverse effect on Leydig cells [12, 13]. In human studies, DEHP has been linked with decreased TT levels in children [14, 15]. Moreover, prenatal DEHP exposure is reportedly associated with a shorter anogenital distance in boys [16] and a lower sperm volume in adolescent males [17] that perhaps is associated with subsequent infertility in adulthood [18]. DEHP may also contribute to a decline in semen quality due to the increased production of semen with abnormal heads [19]. Higher E2 levels and elevated E2/TT ratios were also found in male PVC workers who were exposed to higher urinary DEHP metabolite concentrations [20].
In May 2011, a major phthalates-tainted foodstuffs incident occurred in Taiwan. DEHP and diisononyl phthalate were illegally added to foodstuffs to replace palm oil as the clouding agent [21]. Among foodstuffs, the Taiwan Food and Drug Administration (TFDA) included 5 categories that were contaminated, including tea drinks, fruit beverages, sport drinks, fruit juice or jelly, and dietary supplements in capsule or powder form for use by children [22]. Since this incident occurred, members of the general public have expressed concerns about phthalate exposure and health effects. The Ministry of Health and Welfare (MOHW) (past the Taiwan Department of Health) therefore set up special clinics in 128 hospitals across Taiwan to provide consultations and basic health examinations to people who were wary of possible exposure to phthalate-tainted foodstuffs. Those who might expose to DEHP/DiNP contaminated foodstuffs that announced by TFDA via self-report would be considered with suspected high levels of exposure and were then transferred to 1 of 3 expert hospitals located in the north, middle, and south of Taiwan. Accordingly, the National Health Research Institute (NHRI) of Taiwan also began a project, the Risk Assessment of Phthalate Incident in Taiwan (RAPIT), to investigate the association between DEHP exposure and health effects in persons who were potentially exposed to phthalate-tainted foodstuffs.
Children are in a critical stage of development and are more sensitive than adults to environmental pollutant exposure. Children exposed to endocrine disruptors may have long-term and stronger effects on their health compared to adults. It is crucial for reproductive hormones to be well regulated for normal pubertal development in children. In the present study, we investigated the association between phthalate exposure and reproductive hormone levels in children who participated in the RAPIT study.
Materials and methods
Participant recruitment
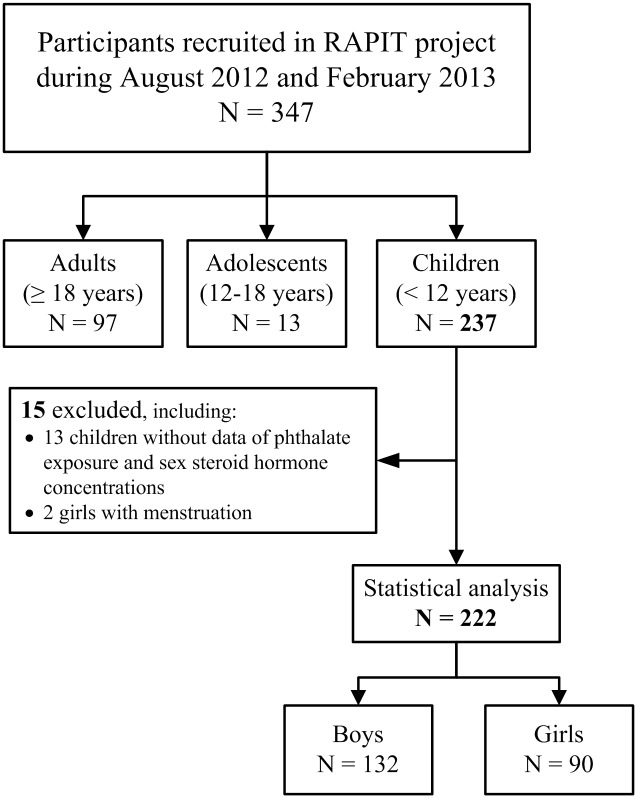
The study subjects were recruited from the RAPIT Project 2011 at 3 expert hospitals, the Ministry of Health and Welfare Hospital in Taipei and Taichung and the Kaohsiung Medical University Hospitals. A total of 347 participants, including 237 children (<12 years old), 13 adolescents (12–18 years old), and 97 adults (≥18 years old) were invited to participate during the period from August 2012 to February 2013 (Fig 1). Subjects or their parents gave informed consent and filled out an exposure assessment questionnaire about phthalate exposure; urine and blood samples were collected for phthalate metabolite and biochemical marker measurement; and participants received physical examinations. Children who provided written consent from both their main caretaker and themselves were our major subjects and were recruited into the present study. The study process was approved by the Research Ethnics Committee of the National Health Research Institutes.
Fig 1. Flow chart of participant recruitment.
Questionnaire
A structured questionnaire was used to collect information about the frequency, duration, and types of daily exposure to 5 major phthalate-contaminated foodstuffs as announced by TFDA. Questionnaires were also used to collect demographic data, diet, histories of atopic disease (asthma, allergic rhinitis, or atopic dermatitis), health status, and indoor environmental conditions.
DEHP metabolites and reproductive hormone measurement
Urine samples of participants were collected and stored in brown glass bottles (National Scientific Supply Company, Claremont CA, USA). We conducted blank tests to ensure that the bottles did not contribute to measure phthalate contaminants. The metabolites of DEHP in spot urine, including mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), were measured by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) as described in a previous study [23, 24, 25]. The water (Sigma-Aldrich, Switzerland) as blank sample was measured simultaneously in every experiment. The measured concentration of blank sample below twofold of the detection limit value for each phthalate metabolite are as standard of quality control. The experiment was performed in the laboratory at National Institute of Environmental Health Sciences, National Health Research Institutes, Taiwan. For external quality assurance of phthalate metabolite measurement, we annually participated in the intercomparison programme in the German External Quality Assessment Scheme for Biological Monitoring (G-EQUAS). Urinary creatinine levels were measured at Union Clinical Laboratory (UCL; Taipei, Taiwan) using an ADVIA 1800 Clinical Chemistry System (Siemens, Erlangen, Germany). Phthalate metabolite measurements were divided by urinary creatinine levels and expressed as “μg/g creatinine” to account for urinary volume correction.
Reproductive hormones in venous blood were also assessed by UCL. LH (mIU/mL), FSH (mIU/mL), E2 (pg/mL), and TT (ng/dL) levels were measured using a chemiluminescence immunoassay (Centaur; Siemens). Free TT (ng/dL) and sex hormone–binding globulin (SHBG, nmol/L) were assessed using an electrochemiluminescence immunoassay (ECLIA) (Elecsys 2010; Roche, Basel, Switzerland). The detection limit values of MEHP, MEHHP, MEOHP, LH, FSH, E2, TT, free TT, and SHBG were 0.7 ng/mL, 0.1 ng/mL, 0.1 ng/mL, 0.07 ng/dL, 0.3 IU/mL, 11.8 pg/mL, 10.0 IU/mL, 0.04 ng/dL, and 0.35 nmole/L, respectively. The participants’ concentrations of DEHP metabolites and reproductive hormones below the detection limit value were replaced by half-of-detection-limit values.
DEHP exposure assessment
Participants’ levels of DEHP exposure were estimated by their urinary concentrations of 3 DEHP metabolites and an exposure assessment questionnaire, as described in a previous study [25]. Briefly, the concentration of urinary DEHP metabolites was considered to be the current background DEHP exposure status and was used to calculate the daily intake of DEHP (AvDIenv) (Eq 1).
| (1) |
UEsum: Sum of three urinary creatinine-adjusted DEHP metabolite concentrations;
CE: Sex-specific body height-based reference values for urinary creatinine excretion in children < 12 years old;
FUE: Molar fraction of excreted metabolite relative to total intake at 24-h post-dosing;
BW: Body weight;
MWDEHP: Molecular weight of DEHP.
Participants’ DEHP exposure to contaminated food products was estimated and reconstructed from the exposure assessment questionnaire, combined with estimated DEHP levels in 2449 foodstuffs reported by the TFDA and the Bureau of Health of Kaohsiung City. We arrived at these estimates by Bayesian models using Markov chain Monte Carlo simulation [25]. Based on the structured questionnaire for 5 major phthalate-contaminated foodstuffs and the self-reported exposure history with more specific detailed information about foodstuffs consumed, we estimated and calculated the AvDI of DEHP exposure levels through contaminated foodstuffs from the questionnaire (AvDIQN) and the self-report (AvDISF) using Eq 2, respectively. Finally, the total daily DEHP exposure (AvDIall) of participants was estimated as the sum of these 3 estimated levels obtained from urine samples and the questionnaire as Eq 3. If exposure to contaminated foodstuffs from self-report was available in that category, the AvDIQN of such food category was counted as zero to avoid double counting and to lower uncertainty [25].
| (2) |
i = 1,…,5; five categories of major phthalate-contaminated foodstuffs;
Yi (mg/L or ppm): DEHP concentration;
Mi (mL or g): amount consumed;
EFi (times*day-1): exposure frequency;
EDi (day): exposure duration
AT (day): average time of exposure
BWb (kg): body weight on May 31, 2011 (the official last day of the incident according to MOHW).
| (3) |
Because there was a time lag of more than one year between the phthalate incident and participant’s recruitment, an additional estimated exposure level, AvDIall_wp (μg/kg bw/day), was used to account for the window period between May 31, 2011, and the date of participant recruitment; this value was calculated by replacing AT (day) with ATwp (day), which included an extra time lag for urinary DEHP metabolite measurement, and we also replaced the body weight value(BWb) (kg) with the body weight at recruitment (BWp) (kg) [25].
Statistical analysis
Data analysis was done with JMP version 10.0 (SAS Institute, Cary, NC, USA) and SPSS version 20.0 (IBM, Armonk, NY, USA). Because boys and girls may have different levels of reproductive hormone concentrations, children were stratified by sex for statistical analysis. Analysis of variance (ANOVA), nonparametric tests, and χ2 tests were applied as appropriate to different groups. Spearman’s rank correlation coefficient was applied to assess the correlation between DEHP exposure parameters and reproductive hormone concentrations in children. Multiple logistic regression was also applied to test the association between DEHP exposure parameters and reproductive hormone concentrations in children. All concentrations of DEHP exposure parameters and reproductive hormones were log-transformed to approximate normality. Because multiple comparisons were done to examine the relationships between DEHP exposure and reproductive hormones, the α value was adjusted, and a p value ≤ 0.0071 (ie, 0.05 divided by 7) was considered statistically significant.
Results
After exclusion of 2 girls with menstruation and 15 children without AvDI or reproductive hormone data, a total of 222 children including 132 boys and 90 girls < 12 years old were recruited into the present study (Fig 1). The characteristics of participants and sex differences are shown in Table 1. No significant differences were found between boys and girls in the cohort in terms of body mass index, age, parental education levels, and birth order. Among boys, the development of pubic hair and external genitalia were all at Tanner Stage I. Among girls, 1.1% were at pubic hair Tanner Stage II. For breast development, 12.2% and 1.1% of girls were in Tanner Stages II and III, respectively (Table 1).
Table 1. Statistical description of participants’ characteristics by sex.
| Variables | All (n = 222) | Boys (n = 132) | Girls (n = 90) | p value¶ |
|---|---|---|---|---|
| Mean (SD) or n (%) | ||||
| Age (years)§ | 5.48 (2.28) | 5.30 (2.22) | 5.75 (2.33) | 0.144 |
| BMI (kg/m2)§ | 16.22 (2.27) | 16.26 (2.13) | 16.17 (2.47) | 0.769 |
| Gestation (weeks)§ | 38.33 (2.16) | 38.23 (2.34) | 38.49 (1.86) | 0.384 |
| Birth weight (kg)§ | 3123.6 (545.3) | 3142.2 (539.3) | 3096.4 (555.7) | 0.541 |
| Birth order | ||||
| 1st | 146 (65.5) | 88 (66.2) | 58 (64.4) | 0.525 |
| ≥ 2nd | 63 (28.4) | 35 (26.5) | 28 (31.1) | |
| ETS exposure during pregnancy | ||||
| Yes | 44 (19.7) | 27 (20.3) | 17 (18.9) | 0.753 |
| No | 177 (79.7) | 104 (78.8) | 73 (91.1) | |
| Mother was active smoker during pregnancy | ||||
| Yes | 5 (2.2) | 3 (2.3) | 2 (2.2) | 0.973 |
| No | 216 (97.3) | 128 (97.0) | 88 (97.8) | |
| Maternal education | ||||
| ≤ 12 years | 0 | 0 | 0 | |
| > 12 years | 209 (94.1) | 123 (93.2) | 86 (95.6) | —& |
| Paternal education | ||||
| ≤ 12 years | 1 (0.4) | 1 (0.7) | 0 | —& |
| > 12 years | 208 (93.7) | 122 (92.4) | 86 (95.6) | |
| Tanner stage | ||||
| Pubic hair | ||||
| Stage I | 132 (100.0) | 89 (98.9) | —& | |
| Stage II | 0 | 1 (1.1) | ||
| Breast development | ||||
| Stage I | —& | 78 (86.7) | —& | |
| Stage II | —& | 11 (12.2) | ||
| Stage III | —& | 1 (1.1) | ||
| Development of external genitalia | ||||
| Stage I | 132 (100.0) | —& | —& | |
¶p value was calculated by χ2 test for categorical variables and Kruskal–Wallis tests for continues variables as compared between boys and girls.
§Mean (SD).
&”—”indicates no analysis.
ETS: environmental tobacco smoke.
Some numbers do not add up to total n because of missing values.
Urinary phthalate metabolite concentration was corrected by urinary creatinine levels. Children without urinary creatinine data due to insufficient urine sample would be therefore excluded in analyses of urinary metabolites. There was no significant different children included and excluded in sex, gestational week, and birthweight. However, the excluded children were younger than those included (mean = 2.81 years v.s. 5.68 years, respectively). The distribution of urinary DEHP metabolite concentrations, estimated DEHP exposure levels (AvDI), and reproductive hormone concentrations are reported in Table 2. The concentrations of urinary MEHP and ΣMEHP were significantly higher in boys than in girls. Boys also had higher levels of background DEHP exposure (AvDIenv) than girls. However, higher FSH, E2, and free TT levels were measured in girls than boys (Table 2). The percentages of children with above the detection limit values of phthalate metabolites and reproductive hormones were also reported in Table 2.
Table 2. Concentration of urinary DEHP metabolites, estimated DEHP exposure, and reproductive hormones in participants by sex (n = 222).
| Boys (n = 132) | Girls (n = 90) | p-value¶ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GM (GSD) | AM (SD) | Median | IQR | % > DL | n | GM (GSD) | AM (SD) | Median | IQR | % > DL | ||
| Urinary DEHP monoesters (μg/g creatinine) | |||||||||||||
| MEHP | 123 | 9.05 (0.48) | 14.59 (15.14) | 10.56 | 5.35–18.83 | 100 | 86 | 5.82 (0.6) | 11.11 (10.95) | 7.93 | 2.66–15.57 | 100 | 0.035 |
| MEHHP | 122 | 47.94 (0.33) | 64.18 (62.74) | 51.51 | 30.80–68.85 | 100 | 86 | 42.89 (0.27) | 51.57 (36.25) | 38.88 | 30.64–61.76 | 100 | 0.095 |
| MEOHP | 121 | 32.88 (0.42) | 44.83 (38.74) | 34.41 | 21.77–49.91 | 100 | 85 | 28.52 (0.39) | 36.58 (27.71) | 28.59 | 22.08–40.24 | 100 | 0.089 |
| ∑MEHP | 121 | 94.96 (0.32) | 124.0 (111.9) | 101.7 | 61.77–135.9 | 100 | 85 | 81.97 (0.27) | 98.5 (69.64) | 78.53 | 58.18–105.2 | 100 | 0.026 |
| Estimated DEHP exposure (μg/kg bw/day) | |||||||||||||
| AvDIenv | 132 | 6.86 (0.32) | 8.99 (8.30) | 7.2 | 4.55–10.2 | —& | 90 | 6.0 (0.26) | 7.09 (4.56) | 5.8 | 4.28–8.30 | —& | 0.039 |
| AvDIall | 132 | 30.0 (0.41) | 47.92 (58.79) | 29.65 | 16.58–51.95 | —& | 90 | 26.96 (0.42) | 44.01 (53.6) | 25.85 | 13.78–47.78 | —& | 0.41 |
| AvDIall_wp | 132 | 16.6 (0.37) | 25.23 (34.53) | 16.25 | 10.0–25.2 | —& | 90 | 15.99 (0.35) | 22.88 (24.89) | 13.2 | 9.28–27.83 | —& | 0.651 |
| Reproductive hormones | |||||||||||||
| LH (mIU/mL) | 132 | 0.053 (0.37) | 0.10 (0.18) | 0.035 | 0.035–0.035 | 23.5 | 90 | 0.051 (0.48) | 0.25 (1.09) | 0.035 | 0.035–0.035 | 14.4 | 0.142 |
| FSH(mIU/mL) | 132 | 0.91 (0.3) | 1.13 (0.75) | 0.9 | 0.6–1.40 | 95.5 | 90 | 2.08 (0.23) | 2.38 (1.33) | 2.0 | 1.6–2.83 | 100 | <0.0001 |
| E2 (pg/mL) | 132 | 8.05 (0.24) | 9.63 (7.02) | 5.9 | 5.9–12.75 | 26.5 | 90 | 9.54 (0.26) | 11.69 (9.94) | 5.9 | 5.9–14.93 | 44.4 | 0.021 |
| TT (ng/dL) | 132 | 5.54 (0.15) | 6.02 (3.63) | 5 | 5.0–5.0 | 9.1 | 90 | 6.18 (0.22) | 7.42 (6.46) | 5.0 | 5.0–5.0 | 16.7 | 0.081 |
| Free TT (ng/dL) | 132 | 0.069 (0.19) | 0.08 (0.058) | 0.06 | 0.05–0.08 | 100 | 90 | 0.087 (0.24) | 0.11 (0.09) | 0.07 | 0.06–0.11 | 100 | 0.0003 |
| SHBG (nmol/L) | 132 | 121.3 (0.19) | 131.2 (46.79) | 137.2 | 95.35–165.8 | 100 | 90 | 104.8 (0.19) | 113.8 (43.01) | 109.6 | 80.9–145.4 | 100 | 0.006 |
¶p-value was calculated by Kruskal-Wallis tests.
Some numbers do not add up to total n because of missing values.
&"—" was indicated not applicable.
Abbreviations: DEHP, di-(2-ethylhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; AvDIall, estimated daily intake of DEHP exposure; AvDIall_wp, AvDIall with window period; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; TT, testosterone; SHBG, sex hormone-binding globulin; GM (GSE), geometric mean (geometrical standard error); AM (SE), arithmetic mean; IQR, interquartile range; DL, detection limit value.
We suggested that birthweight was reflected by the growth and development of the newborn which may be associated with later health status. We analyzed the association between covariables and reproductive hormones. Birthweight tends to correlated with reproductive hormone with borderline significant for TT in girls (n = 90) as shown in S1 Table. We therefore adjusted for birthweight in statistical analyses. Table 3 shows Spearman’s correlation between DEHP exposure and reproductive hormone concentrations stratified by sex. Among children, no significant association was found between urinary DEHP metabolite concentrations and reproductive hormone levels after adjustment for age and birthweight. In terms of estimated DEHP exposure levels in children, when we considered the window period between the exposure incident and participants’ recruitment to our study, AvDIall_wp was positively correlated with SHBG concentrations in girls (β = 0.276, p = 0.009) after adjustment for birth weight and age (Table 3). The result of a sensitivity analysis excluding 4 children suspected to have particular unreliable estimates for AvDI at the episode was shown in S2 Table. The results were similar with or without the exclusions.
Table 3. Spearman's correlation between urinary DEHP monoester and estimated DEHP exposure levels and reproductive hormone concentrations in children by sex (n = 222).
| LH (mIU/mL) | FSH (mIU/mL) | E2 (pg/mL) | TT (ng/dL) | FreeTT (ng/dL) | SHBG (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Un-adj | adj | Un-adj | adj | Un-adj | adj | Un-adj | adj | Un-adj | adj | Un-adj | adj | |
| Boys | ||||||||||||
| Urinary DEHP monoesters (μg/g creatinine) (n = 121) | ||||||||||||
| MEHP | -0.048 | -0.040 | -0.015 | 0.022 | -0.108 | -0.089 | -0.038 | -0.034 | 0.069 | 0.104 | -0.134 | -0.180* |
| MEHHP | -0.181* | -0.112 | -0.066 | 0.059 | -0.035 | -0.018 | -0.138 | -0.044 | -0.082 | 0.035 | 0.040 | -0.103 |
| MEOHP | -0.172# | -0.080 | 0.002 | 0.101 | -0.118 | -0.024 | -0.097 | -0.006 | -0.053 | 0.042 | 0.021 | -0.087 |
| ΣMEHP | -0.183* | -0.103 | -0.042 | 0.088 | -0.087 | -0.049 | -0.127 | -0.024 | -0.028 | 0.060 | -0.014 | -0.125 |
| Estimated DEHP exposure levels (μg/kg bw/day) (n = 131) | ||||||||||||
| AvDIenv | -0.077 | -0.083 | 0.041 | 0.095 | -0.027 | -0.017 | -0.022 | -0.033 | 0.018 | -0.015 | -0.038 | -0.023 |
| AvDIall | -0.086 | -0.056 | -0.152# | -0.098 | -0.073 | -0.059 | -0.165# | -0.008 | -0.228** | -0.100 | 0.242**§ | 0.098 |
| AvDIall_wp | -0.069 | -0.074 | -0.065 | 0.015 | -0.080 | -0.053 | -0.119 | -0.025 | -0.104 | -0.067 | 0.114 | 0.057 |
| Girls | ||||||||||||
| Urinary DEHP monoesters (μg/g creatinine) (n = 86) | ||||||||||||
| MEHP | 0.155 | 0.073 | 0.242* | 0.186# | 0.048 | -0.025 | 0.007 | -0.013 | 0.092 | 0.139 | 0.063 | 0.066 |
| MEHHP | -0.138 | -0.005 | 0.120 | 0.097 | 0.053 | 0.121 | -0.277** | -0.027 | -0.151 | 0.122 | 0.241* | 0.216# |
| MEOHP | -0.136 | -0.149 | 0.191# | 0.014 | -0.007 | -0.013 | -0.262* | -0.110 | -0.122 | 0.022 | 0.214* | 0.147 |
| ΣMEHP | -0.114 | -0.024 | 0.164 | 0.122 | -0.002 | 0.077 | -0.256* | -0.027 | -0.121 | 0.140 | 0.214* | 0.187# |
| Estimated DEHP exposure levels (μg/kg bw/day) (n = 90) | ||||||||||||
| AvDIenv | -0.075 | -0.026 | 0.119 | 0.067 | 0.026 | 0.051 | -0.239* | -0.073 | -0.169 | 0.053 | 0.275** | 0.266* |
| AvDIall | -0.162 | 0.029 | -0.061 | 0.013 | -0.073 | -0.021 | -0.177# | -0.054 | -0.251* | -0.079 | 0.334**§ | 0.233* |
| AvDIall_wp | -0.141 | 0.006 | -0.016 | 0.017 | -0.085 | -0.031 | -0.177# | -0.045 | -0.288**§ | -0.102 | 0.384***§ | 0.276** |
¶Adjusted for age and birth weight.
#p<0.1,
*p<0.05,
**p<0.01,
***p<0.001,
§p<0.0071 indicates a statistical significant correlation.
All concentrations of urinary DEHP monoester, estimated DEHP exposure, and reproductive hormones were log 10 transformed.
Some numbers do not add up to total n because of missing values.
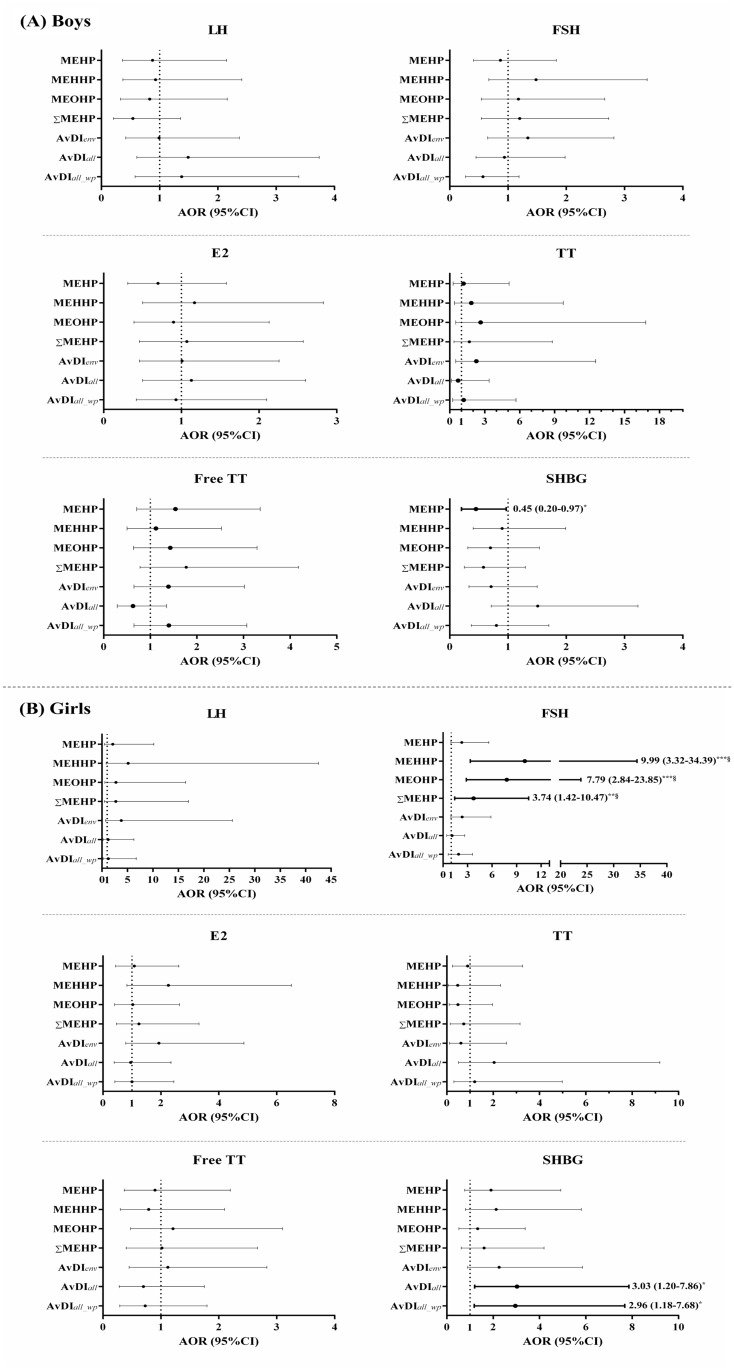
We then grouped FSH, free TT, and SHBG concentrations by median, and divided LH, E2, and TT concentrations into undetected (80.2%, 66.2%, and 87.8% for LH, E2, and TT, respectively) and detected groups. We also grouped DEHP exposure parameters by median to categorize children into high- and low-exposure groups. The crude odds ratios and 95% confidence intervals (OR [95% CI]) for the high and low reproductive hormone concentrations between DEHP exposure parameters grouped by median are shown in S1 Fig. The association between DEHP exposure and reproductive x hormone levels after adjustments for age and birth weight is shown in Fig 2. After adjustment for age and birth weight, urinary MEHP concentrations above the median were marginally negative association with SHBG concentrations in boys (OR [95% CI] = 0.45 [0.20–0.97], p = 0.0446) (Fig 2A). Urinary MEHHP, MEOHP, and ΣMEHP concentrations above the median were positively associated with FSH concentrations in girls (OR [95% CI] = 9.99 [3.32–34.39], p < 0.0001) for MEHHP, 7.79 [2.84–23.85], p < 0.0001) for MEOHP, and 3.74 [1.42–10.47], p = 0.0071) for ΣMEHP) (Fig 2B). Moreover, the estimated AvDI concentration above the median with or without the window period also showed a marginally positive association with SHBG levels in girls (OR [95% CI] = 3.03 [1.20–7.86], p = 0.0188) for AvDIall and 2.96 [1.18–7.68], p = 0.0210) for AvDIall_wp) (Fig 2B). The association between urinary DEHP metabolites and reproductive hormones was not affected by correction for urinary creatinine concentration, as demonstrated by multiple variable regression analysis using urinary creatinine as an independent variable (S3 Table).
Fig 2. Odds ratios (95% confidence intervals) for the high–low reproductive hormone concentrations between DEHP exposure parameters grouped by median as high and low exposures, with adjustments for age and birth weight (n = 222).
The results for (A) boys (n = 132) and (B) girls (n = 90).
Numbers in the figures indicated adjusted OR (95% CI).
Boldface indicates achievement of p < 0.05.
*p < 0.05, **p < 0.01, and ***p < 0.001 (§p<0.0071 indicates a statistical significant).
Abbreviations: DEHP, di-(2-ethylhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; AvDIall, estimated daily intake of DEHP exposure; AvDIall_wp, AvDIall with window period; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; TT, testosterone; SHBG, sex hormone-binding globulin.
Discussion
We found that DEHP exposure was associated with altered reproductive hormone levels in girls who were potentially exposed to phthalate-tainted foodstuffs. Higher FSH and SHBG levels were shown in girls with higher current and past DEHP exposure, respectively.
The estimated background DEHP daily intake level (AvDIenv) in our recruited children was reported slightly higher than those in some other countries but lower than those in previous Taiwan studies (Table 4). From a birth cohort study conducted in central Taiwan, the results showed that the geometric mean (GM) of estimating DEHP daily intake was 8.1 and 10.9 μg/kg bw/day in 2-year-old and 5-year-old children, respectively [26]. In Kaohsiung, Wu et al., measured higher DEHP daily intake levels (median: 11.8 μg/kg bw/day) in children aged 1–10 years and recruited immediately as the phthalate episode occurred [27]. In Australia, daily DEHP intake was reportedly higher in children at the age of 6–15 years old than those in our study [28]. However, lower DEHP exposure levels were reported in Greek children aged 2 years old [29] and in German children 5–6 years old [30]. Lower DEHP exposure levels were also reported in the study by Wittassek et al.’s of German children aged 2–14 years [31]. Our children also had higher DEHP exposure levels than did Danish children [32, 33]. Moreover, the percentage of children whose estimated DEHP daily intake dose exceed European Food Safety Authority (EFSA) guideline (50 μg/kg bw/day) in present study was 26.0% for AvDIall and 9.4% for AVDIall_wp, respectively. However, 6 months after the episode, Wu et al. found that the urinary concentration of four phthalate monoesters in children recruited immediately when the phthalate incident occurred was decreased to normal or background levels [27]. Even so, whether children with higher DEHP exposure during the incident have longer-term effects and related health issues still needs to be further investigated.
Table 4. Median or geometric mean (GM) of estimated DEHP daily intake levels (μg/kg bw/day) in different countries.
| Country | City | Study year | Subjects | Median | Reference |
|---|---|---|---|---|---|
| Taiwan | This study | 2012–3 | ≤ 12 years old (n = 222) | ¶AvDIenv: 6.6 (median) and 6.5 (GM) | |
| Taichung | 2003–4 | 2–3 years (n = 30) | 8.1 | [26] | |
| 2006–7 | 5–6 years (n = 59) | 10.9 | |||
| Kaohsiung | 2011 | 1–10 years (n = 29) | 11.8 | [27] | |
| Australia | —& | 2012 | 6–15 years (n = 387) | 7.8 | [28] |
| Greece | Crete | 2009–11 | 2 years (n = 239) | 4.0 (median) and 3.3 (GM) | [29] |
| Germany | Frankfurt | 2007 | 5–6 years (n = 111) | 4.5 | [30] |
| Berlin | 2001–2 | 2–14 years (n = 239) | 4.3 | [31] | |
| Denmark | —& | 2006–8 | 6–10 years (n = 25 for boys, 24 for girls) | Boys: 5.7 Girls: 5.4 | [32] |
| —& | 2008–9 | 3–6 years (n = 431) | 4.3 | [33] |
¶AvDIenv: a background daily DEHP exposure for comparison to the other studies.
&“—”indicated no information.
In the present study, we found that current DEHP exposures above the median were positively associated with above-median FSH levels in girls who were affected by the phthalate episode. This result was consistent with previous human and animal studies. In a longitudinal follow-up study of 8-year-old children, MEHP correlated significantly with serum FSH levels in girls [10]. Meltzer et al. observed an increase in FSH serum levels in female adult rats following in utero exposure to DEHP [34]. The mechanism underlying the positive association between DEHP exposure and serum FSH levels is still unclear. GnRH that modulates FSH stimulation of the HPG axis was measured at higher levels in rats exposed to higher DEHP concentrations [35, 36]. Moreover, DEHP exposure may regulate the transcription of steroid hormone receptor genes. Ye et al. found that the FSH β subunit gene was upregulated in females exposed to 0.1 mg/L DEHP in marine medaka study [36]. DEHP was also reported to inhibit FSH-stimulated progesterone production [37], and the positive association between DEHP exposure and FSH levels found in the present study may be the positive feedback for the pituitary release of gonadotropic hormones. The unbalanced secretion of FSH may disturb the HPG axis and affect downstream sex hormone secretion and function.
SHBG was also positively associated with DEHP exposure measured by both urinary and estimated levels in girls in the present study. SHBG is a serum glycoprotein mainly produced in the liver, and it binds testosterone and estrogen with high affinity and specificity. SHBG is related to androgen activity because of their ability for binding to TT and therefore regulating the levels of bioavailable free TT. SHBG can be regulated by sex hormones as androgen decreases and estrogen increases SHBG concentration [38, 39]. The positive association of SHBG with DEHP exposure may be due to the anti-androgenic effects of DEHP because testosterone has an inhibitory influence on SHBG [9]. Our results were consistent with anti-androgenic effects of phthalate exposure, and a negative association was found between DEHP and TT concentrations in general, even though the association became statistically non-significant after multiple adjustments due to the limited sample size. Moreover, estrogen is a known SHBG stimulator [39], so elevated SHBG concentrations may also be partially influenced by the estrogenic effects of phthalate exposure.
The strength of this study included recruiting unique participants who possibly were exposed to higher levels of DEHP during the incident in Taiwan. We were able to investigate the effect of high-does DEHP exposure on children’s health in general population. Second, we applied Bayesian models to reconstruct exposure status before the incident occurred using a well-designed exposure questionnaire and urinary measurements.
There are limitations in this study. The cross-sectional study design makes it hard to interpret any causal relationships between DEHP exposure and reproductive hormone levels. However, the results were consistent with previous studies that showed that DEHP had antiandrogenic and estrogenic effect in children [15, 40]. These children should be followed to investigate the effects of exposure to phthalate-tainted foodstuffs on children’s health [41]. Second, the range of the children’s age was wide (mean = 5.48 years; range = 1 to approximately 11.6 years) in the present study. The concentrations of reproductive hormone vary throughout life before puberty [42, 43]. A more narrowly defined age group and a larger sample size should be studied in further investigations. Third, because of the small sample size, we could not consider more potential covariates, such as socioeconomic status, diet, and stress. However, the educational levels of the parents of participating children were almost all at the college level. Factors that closely correlate with parental education levels, such as socioeconomic status and lifestyle [44] may be similar in our cohort of children. Thus, potential confounding by these factors may not be severe in the present study. Fourth, only one measurement of urine and blood samples was done in our study and may not represent the long-term pattern of phthalate exposure and reproductive hormone concentrations. Thus, we assumed that the usage of phthalate-related products was fairly constant in the children’s daily life and that the urinary concentration of phthalate monoesters was therefore representative of their steady-state concentrations. Fifth, we did not investigate the presence of other known environmental endocrine disruptors such as bisphenol A, alkylphenols, or other phthalate esters that may be associated with reproductive hormone concentrations [45, 46]. The effects of multiple environmental exposures on reproductive hormone levels should be investigated in further studies. Sixth, the study was begun one year after the phthalate incident. The participants’ recall bias about using phthalate-tainted products is a concern. Finally, we only had children who possibly were exposed to higher levels of DEHP but without an appropriate control group, and therefore we did not fully determine if such effects were similar (or more adverse) in the general population.
In conclusion, one year after the episode, the exposure to DEHP resulted in altered concentrations of reproductive hormones. In particularly, past DEHP exposure was associated with SHBG in girls. These children should be followed to investigate the long-term effect of exposure to phthalate-tainted foodstuffs on pubertal development.
Supporting information
(PDF)
(PDF)
(PDF)
The results of (A) Boys (n = 132) and (B) Girls (n = 90).
Bold line indicated achievement of p < 0.05.
*p < 0.05, **p < 0.01, and ***p < 0.001 (§p<0.0071 indicates a statistical significant).
Abbreviations: DEHP, di-(2-ethylhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; AvDIall, estimated daily intake of DEHP exposure; AvDIall_wp, AvDIall with window period; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; TT, testosterone; SHBG, sex hormone-binding globulin.
(TIF)
Acknowledgments
The authors thank all participants who joined the RAPIT study. The authors thank the collaborating hospitals for clinical examinations and participant recruitment, UCL for biomedical measurement, and NHRI for urinary phthalate metabolite measurement. The authors thank Ms. Fang-Zu Lin, Yi-Chun Chang, Yin-HanWang, and Yuh-An Chen for helping to collect the interview data, conduct the field work and analyze urinary DEHP metabolite concentrations. The authors also thank all experts and the study group for the RAPIT study. RAPIT study members include Chao A. Hsiung (coordinating principal investigator, hsiung@nhri.org.tw, Institute of Population Health Sciences, National Health Research Institutes, Miaoli, Taiwan), Shu-Li Wang (National Environmental Health Research Center, National Institute of Environmental Health Sciences, National Health Research Institutes, Miaoli, Taiwan), Chu-Chih Chen (Institute of Population Health Sciences, National Health Research Institutes, Miaoli, Taiwan), Ming-Tsang Wu (Department of Public Health, College of Health Sciences, Kaohsiung Medical University, Kaohsiung, Taiwan), Mei-Lien Chen (Institute of Environmental and Occupational Health Sciences, College of Medicine, National Yang Ming University, Taipei, Taiwan), Bai-Hsiun Chen (Department of Laboratory Medicine and Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan), Wen-Harn Pan (Institute of BioMedical Science, Academia Sinica, Taipei, Taiwan), Ching-Chang Lee (Department of Environmental and Occupational Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan), and Po-Chin Huang (National Environmental Health Research Center, National Institute of Environmental Health Sciences, National Health Research Institutes, Miaoli, Taiwan) (all principal investigators).
Data Availability
All data in the study are available upon request to the National Health Research Institute. Due to legal restrictions imposed by the government of Taiwan in relation to the "Personal Information Protection Act", data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw/index1.php).
Funding Statement
Financial support received from the Department of National Health Research Institutes, Taiwan, is gratefully acknowledged (EH-102-SP-01, EH-102-PP-05, EH-103-SP-01, EH-103-PP05, EH-104-SP-01, and EH-104-PP05) and Ministry of Science and Technology (NSC100-3114-Y-043-005). The funding agents had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates—the human biomonitoring approach. Molecular nutrition & food research. 2011;55(1):7–31. [DOI] [PubMed] [Google Scholar]
- 2.Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk analysis: an official publication of the Society for Risk Analysis. 2006;26(3):803–24. [DOI] [PubMed] [Google Scholar]
- 3.Ljungvall K, Spjuth L, Hulten F, Einarsson S, Rodriguez-Martinez H, Andersson K, et al. Early post-natal exposure to low dose oral di(2ethylhexyl) phthalate affects the peripheral LH-concentration in plasma, but does not affect mating behavior in the post-pubertal boar. Reproductive toxicology. 2006;21(2):160–6. 10.1016/j.reprotox.2005.07.012 [DOI] [PubMed] [Google Scholar]
- 4.Yeung BH, Wan HT, Law AY, Wong CK. Endocrine disrupting chemicals: Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis. 2011;1(3):231–9. 10.4161/spmg.1.3.18019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. Journal of Endocrinology. 2007;194(3):603–9. 10.1677/JOE-07-0238 [DOI] [PubMed] [Google Scholar]
- 6.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauser R, Calafat AM. Phthalates and human health. Occupational and environmental medicine. 2005;62(11):806–18. 10.1136/oem.2004.017590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environmental health perspectives. 2007;115(3):390–6. 10.1289/ehp.9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lottrup G, Andersson AM, Leffers H, Mortensen GK, Toppari J, Skakkebaek NE, et al. Possible impact of phthalates on infant reproductive health. International journal of andrology. 2006;29(1):172–80; discussion 81–5. 10.1111/j.1365-2605.2005.00642.x [DOI] [PubMed] [Google Scholar]
- 10.Su PH, Chen JY, Lin CY, Chen HY, Liao PC, Ying TH, et al. Sex steroid hormone levels and reproductive development of eight-year-old children following in utero and environmental exposure to phthalates. PloS one. 2014;9(9):e102788 10.1371/journal.pone.0102788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heindel JJ, Gulati DK, Mounce RC, Russell SR, Lamb JCt. Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundamental and applied toxicology: official journal of the Society of Toxicology. 1989;12(3):508–18. [DOI] [PubMed] [Google Scholar]
- 12.Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223(1–2):144–55. 10.1016/j.tox.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 13.Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115(3):390–6. 10.1289/ehp.9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM, et al. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reproductive toxicology. 2014;47:70–6. 10.1016/j.reprotox.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouritsen A, Frederiksen H, Sorensen K, Aksglaede L, Hagen C, Skakkebaek NE, et al. Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: associations with adrenal androgen levels and puberty. The Journal of clinical endocrinology and metabolism. 2013;98(9):3755–64. 10.1210/jc.2013-1284 [DOI] [PubMed] [Google Scholar]
- 16.Bornehag CG, Carlstedt F, Jonsson BA, Lindh CH, Jensen TK, Bodin A, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2015;123(1):101–7. 10.1289/ehp.1408163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelsson J, Rylander L, Rignell-Hydbom A, Lindh CH, Jonsson BA, Giwercman A. Prenatal phthalate exposure and reproductive function in young men. Environmental research. 2015;138:264–70. 10.1016/j.envres.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 18.Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. The Journal of clinical endocrinology and metabolism. 2013;98(6):2230–8. 10.1210/jc.2012-4057 [DOI] [PubMed] [Google Scholar]
- 19.Wang YX, You L, Zeng Q, Sun Y, Huang YH, Wang C, et al. Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environmental research. 2015;142:1–9. 10.1016/j.envres.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 20.Fong JP, Lee FJ, Lu IS, Uang SN, Lee CC. Relationship between urinary concentrations of di(2-ethylhexyl) phthalate (DEHP) metabolites and reproductive hormones in polyvinyl chloride production workers. Occupational and environmental medicine. 2015;72(5):346–53. 10.1136/oemed-2014-102532 [DOI] [PubMed] [Google Scholar]
- 21.Li JH, Ko YC. Plasticizer incident and its health effects in Taiwan. The Kaohsiung journal of medical sciences. 2012;28(7 Suppl):S17–21. 10.1016/j.kjms.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Wu MT, Wu CF, Wu JR, Chen BH, Chen EK, Chao MC, et al. The public health threat of phthalate-tainted foodstuffs in Taiwan: the policies the government implemented and the lessons we learned. Environ Int. 2012;44:75–9. 10.1016/j.envint.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Lin LC, Wang SL, Chang YC, Huang PC, Cheng JT, Su PH, et al. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere. 2011;83(8):1192–9. Epub 2011/01/29. 10.1016/j.chemosphere.2010.12.079 [DOI] [PubMed] [Google Scholar]
- 24.Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a taiwanese birth cohort. PloS one. 2015;10(4):e0123309 10.1371/journal.pone.0123309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CC, Wang SL, Wu MT, Wang YH, Huang PC, Chen BH, et al. Exposure Estimation for Risk Assessment of the Phthalate Incident in Taiwan. PloS one. 2016;11(3):e0151070 10.1371/journal.pone.0151070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S, Ku HY, Su PH, Chen JW, Huang PC, Angerer J, et al. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere. 2011;82(7):947–55. Epub 2010/11/16. 10.1016/j.chemosphere.2010.10.073 [DOI] [PubMed] [Google Scholar]
- 27.Wu CF, Chen BH, Shiea J, Chen EK, Liu CK, Chao MC, et al. Temporal changes of urinary oxidative metabolites of di(2-ethylhexyl)phthalate after the 2011 phthalate incident in Taiwanese children: findings of a six month follow-up. Environmental science & technology. 2013;47(23):13754–62. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann C, Uhl M, Weiss S, Koch HM, Scharf S, Konig J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. International journal of hygiene and environmental health. 2015;218(5):489–99. 10.1016/j.ijheh.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 29.Myridakis A, Fthenou E, Balaska E, Vakinti M, Kogevinas M, Stephanou EG. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort). Environ Int. 2015;83:1–10. 10.1016/j.envint.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 30.Koch HM, Wittassek M, Bruning T, Angerer J, Heudorf U. Exposure to phthalates in 5–6 years old primary school starters in Germany—a human biomonitoring study and a cumulative risk assessment. International journal of hygiene and environmental health. 2011;214(3):188–95. 10.1016/j.ijheh.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 31.Wittassek M, Heger W, Koch HM, Becker K, Angerer J, Kolossa-Gehring M. Daily intake of di(2-ethylhexyl)phthalate (DEHP) by German children—A comparison of two estimation models based on urinary DEHP metabolite levels. International journal of hygiene and environmental health. 2007;210(1):35–42. 10.1016/j.ijheh.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Frederiksen H, Aksglaede L, Sorensen K, Skakkebaek NE, Juul A, Andersson AM. Urinary excretion of phthalate metabolites in 129 healthy Danish children and adolescents: estimation of daily phthalate intake. Environmental research. 2011;111(5):656–63. 10.1016/j.envres.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 33.Beko G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G. Children's phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PloS one. 2013;8(4):e62442 10.1371/journal.pone.0062442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reproductive toxicology. 2015;51:47–56. 10.1016/j.reprotox.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Li N, Zhu J, Yu G, Guo K, Zhou L, et al. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reproductive toxicology. 2014;46:141–7. 10.1016/j.reprotox.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 36.Ye T, Kang M, Huang Q, Fang C, Chen Y, Shen H, et al. Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma). Aquatic toxicology. 2014;146:115–26. 10.1016/j.aquatox.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 37.Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. The Journal of endocrinology. 2007;194(3):603–9. 10.1677/JOE-07-0238 [DOI] [PubMed] [Google Scholar]
- 38.Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin—from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29(5):749–60. 10.1016/j.beem.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Pasquali R, Vicennati V, Bertazzo D, Casimirri F, Pascal G, Tortelli O, et al. Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-Menopause-Health Group. Metabolism: clinical and experimental. 1997;46(1):5–9. [DOI] [PubMed] [Google Scholar]
- 40.Frederiksen H, Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, et al. High urinary phthalate concentration associated with delayed pubarche in girls. International journal of andrology. 2012;35(3):216–26. 10.1111/j.1365-2605.2012.01260.x [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Arguelles DB, Papadopoulos V. Prenatal phthalate exposure: epigenetic changes leading to lifelong impact on steroid formation. Andrology. 2016;4(4):573–84. 10.1111/andr.12175 [DOI] [PubMed] [Google Scholar]
- 42.Kuiri-Hanninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Hormone research in paediatrics. 2014;82(2):73–80. 10.1159/000362414 [DOI] [PubMed] [Google Scholar]
- 43.Hoyt LT, Falconi AM. Puberty and perimenopause: reproductive transitions and their implications for women's health. Soc Sci Med. 2015;132:103–12. 10.1016/j.socscimed.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76. [DOI] [PubMed] [Google Scholar]
- 45.Costa EM, Spritzer PM, Hohl A, Bachega TA. Effects of endocrine disruptors in the development of the female reproductive tract. Arquivos brasileiros de endocrinologia e metabologia. 2014;58(2):153–61. [DOI] [PubMed] [Google Scholar]
- 46.Knez J. Endocrine-disrupting chemicals and male reproductive health. Reproductive biomedicine online. 2013;26(5):440–8. 10.1016/j.rbmo.2013.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
The results of (A) Boys (n = 132) and (B) Girls (n = 90).
Bold line indicated achievement of p < 0.05.
*p < 0.05, **p < 0.01, and ***p < 0.001 (§p<0.0071 indicates a statistical significant).
Abbreviations: DEHP, di-(2-ethylhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; AvDIall, estimated daily intake of DEHP exposure; AvDIall_wp, AvDIall with window period; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; TT, testosterone; SHBG, sex hormone-binding globulin.
(TIF)
Data Availability Statement
All data in the study are available upon request to the National Health Research Institute. Due to legal restrictions imposed by the government of Taiwan in relation to the "Personal Information Protection Act", data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw/index1.php).