Abstract
Most patients with advanced non-small cell lung cancer (NSCLC) have a poor prognosis and receive limited benefit from conventional treatments, especially in later lines of therapy. In recent years, several novel therapies have been approved for second- and third-line treatment of advanced NSCLC. In light of these approvals, it is valuable to understand the uptake of these new treatments in routine clinical practice and their impact on patient care. A systematic literature search was conducted in multiple scientific databases to identify observational cohort studies published between January 2010 and March 2017 that described second- or third-line treatment patterns and clinical outcomes in patients with advanced NSCLC. A qualitative data synthesis was performed because a meta-analysis was not possible due to the heterogeneity of the study populations. A total of 12 different study cohorts in 15 articles were identified. In these cohorts, single-agent chemotherapy was the most commonly administered treatment in both the second- and third-line settings. In the 5 studies that described survival from the time of second-line treatment initiation, median overall survival ranged from 4.6 months (95% CI, 3.8–5.7) to 12.8 months (95% CI, 10.7–14.5). There was limited information on the use of biomarker-directed therapy in these patient populations. This systematic literature review offers insights into the adoption of novel therapies into routine clinical practice for second- and third-line treatment of patients with advanced NSCLC. This information provides a valuable real-world context for the impact of recently approved treatments for advanced NSCLC.
Introduction
Brief background on NSCLC
Globally, lung cancer is the second most common newly diagnosed cancer and the leading cause of cancer death. In 2012, there were an estimated 1.8 million new lung cancer cases and almost 1.6 million deaths [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 83% of all newly diagnosed lung cancers, and most patients (70%) are diagnosed with advanced disease [2]. Despite treatment advancements, overall survival remains poor with 5-year survival estimates globally ranging from 10% to 20% [3–5].
Lung cancer imposes a substantial economic and societal burden [6]. In Europe, lung cancer–related premature mortality cost an estimated €17 billion in 2008 [7]. In the United States, annual lung cancer treatment expenditures were estimated at $13.1 billion USD in 2014 and lost productivity due to premature lung cancer deaths was an additional estimated $36.1 billion USD [2, 8, 9].
Rationale
Systemic therapy can provide a meaningful clinical benefit for patients with advanced NSCLC, and several new therapies have been approved since 2010 [10]. However, there is a paucity of published information describing how these therapies are used in real-world clinical practice, especially for second and later lines of therapy. Increased understanding of how these treatments are used in routine clinical practice and the associated clinical outcomes may provide insights into how these therapies benefit patients, inform strategies that support development of new therapies, and ultimately decrease the global economic and societal burden of NSCLC.
Objectives
The primary objective of this study was to describe treatment patterns and survival outcomes among patients who received second-line treatment for advanced NSCLC in routine clinical practice. The study also describes treatment patterns and available information on survival for the subset of patients who received third-line treatment.
Approach
To achieve these objectives, we conducted a systematic literature review and qualitative evidence synthesis of observational studies.
Methods
Literature search strategy
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. A review protocol does not exist. A tiered search string that included a combination of keywords and medical subject headings (MeSH; S1 Table) was used to search the following databases: BIOSIS Previews, Current Contents Search, Embase, Gale Group PROMT, International Pharmaceutical Abstracts, Medline, and SciSearch.
Study selection criteria
Observational cohort studies that included detailed information on second- and third-line treatment patterns in patients with advanced NSCLC were eligible for inclusion. Only English-language studies published in peer-review journals between January 1, 2010, and March 1, 2017, were considered. This date range was intended to capture current real-world data on advanced NSCLC treatment patterns in order to account for changes in treatment guidelines related to newly approved therapies and biomarker-guided treatment decisions. Studies that described only one type of therapy, case reports, clinical trials, conference abstracts, and Delphi panels were excluded. Additionally, studies that introduced selection bias with patient selection criteria like requiring platinum-based chemotherapy or a specific mutation were excluded since the intent of the qualitative review was to describe the treatment patterns in a broad, unselected patient population.
Study selection process
Article titles and abstracts were initially screened by one reviewer (JD) to identify articles that potentially fulfilled the study selection criteria. Full-text articles were independently evaluated for inclusion by 2 reviewers (JD and MM). Additional publications were identified through examination of references cited by the included publications and were included if they also fulfilled the selection criteria.
Data extraction
Two independent reviewers extracted key information from each publication, including study location, time period, methods, patient and tumor characteristics, treatment regimens by line of therapy, survival outcomes, response rates, and biomarker testing information. If multiple publications described a single study, the extracted data were combined, and each publication was referenced to reflect this circumstance. Discrepancies during study data extraction were resolved by consensus between the reviewers with reference to the articles. If data differed between 2 publications describing the same study, data from the more recent publication were used.
Main study measures
The main study measures included the proportion of treatment regimens used in second- and third-line advanced NSCLC treatment and overall treatment rates in first-, second-, and third-line therapy. Treatment lines were defined based on the reporting authors’ definitions. Single-agent chemotherapies included systemic anti-cancer therapies other than targeted therapies that were prescribed as monotherapy. Combination chemotherapies comprised 2 or more systemic anti-cancer therapies, including both chemotherapies and targeted therapies. Single-agent targeted therapies included any targeted therapy prescribed as monotherapy. Investigational drugs included any drugs administered as part of a clinical trial. In studies that did not explicitly report treatment proportions among patients receiving second- or third-line treatment, the proportion was estimated based on the number of patients receiving a specific treatment regimen and the total number of patients receiving second- or third-line treatment. Survival outcomes were reported based on the original definitions described in each study.
Assessment of bias
Risk of bias and methodological quality of the studies were assessed using the 2013 RTI framework created for the Agency for Healthcare Research and Quality for observational studies [12]. Two authors independently performed this assessment (S2 Table).
Results
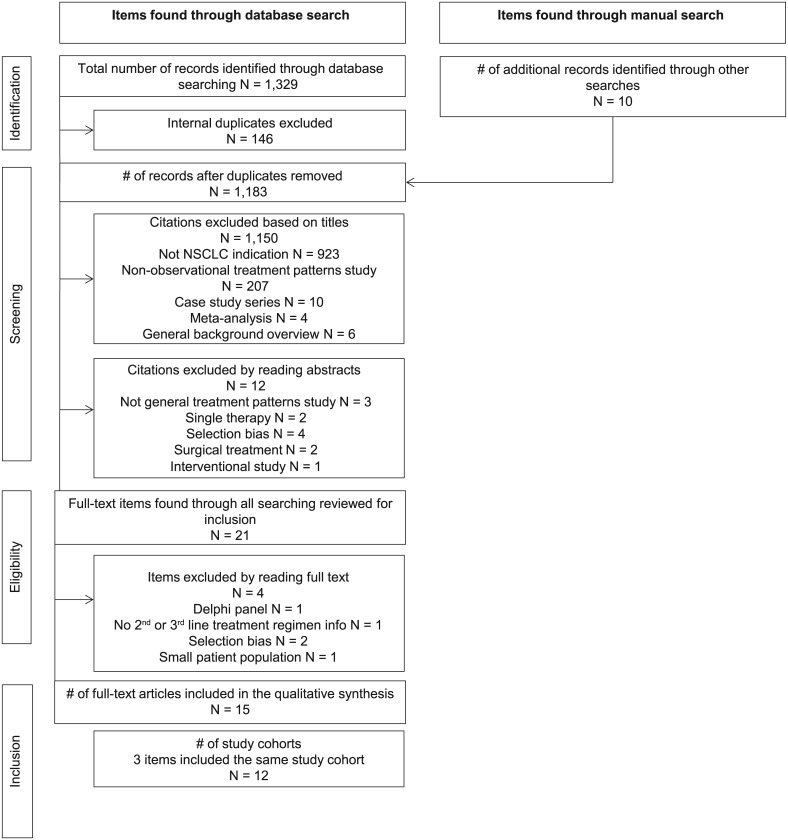
The literature search yielded 1,329 citations, and 10 additional citations were identified through examination of references cited by the included publications (Fig 1). After applying the selection criteria, 15 articles describing 12 different study cohorts were included in the qualitative data synthesis. Of the 12 study cohorts, 7 were retrospective medical record reviews or database analyses and 5 were prospective single-center or multicenter cohorts (Table 1). The study cohorts included patients from Europe (7), North America (3), Asia (1) and South America (1). In general, there was minimal risk of bias within the studies, although several studies had issues that could lead to confounding of the reported outcomes, either due to the length of time during which the study was conducted or missing information on key study variables such as histology or use of oral therapies. Overall, the study results were judged as believable after accounting for limitations.
Fig 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of studies identified from the systematic literature search [11].
Table 1. Studies evaluating treatment patterns in patients with advanced non-small cell lung cancer by country.
| Country | Setting (enrollment period) | Study Population (n) | Selection Criteria | Advanced NSCLC Stage Distribution | Median Patient Age, years (range) | Male (%) | Never Smoker | NSCLC Histology | Definition of Second- and Third-Line Treatment | Study Aims |
|---|---|---|---|---|---|---|---|---|---|---|
| Brazil [23] | 2 cancer research and treatment centers in Sao Paolo (1990–2008) | Adults with cytological or histological diagnosis of stage IV NSCLC (2,673) | 1. Cytological or histological diagnosis of stage IV NSCLC 2. Admitted for cancer management according to clinical practice guidelines of each institution |
IV: 100% | 63 (24–89) | 69% | NR | Adenocarcinoma 33% Squamous 31% Large cell 27% NSCLC NOS 10% |
Systemic treatment administered after discontinuing first-line chemotherapy, either for intolerance or for progressive or recurrent disease | 1. Evaluate patient characteristics of patients with NSCLC 2. Evaluate outcomes of treatment for metastatic disease, with emphasis on second- and third-line chemotherapy |
| Canada [20] | Linked healthcare databases in Ontario Province (2005–2009) | All patients diagnosed with stage IV NSCLC (8,113) | 1. Stage IV NSCLC 2. Diagnosed pre-mortem |
IV: 100% | 68 (28–96) | 54% | NR | Adenocarcinoma 39% Squamous 14% Other 4% NSCLC NOS 43% |
Cancer Care Ontario Activity Level Reporting and New Drug Funding Program databases included treatment records with line of therapy documented | Examine practice patterns with respect to systemic treatment, survival outcomes, and changes in chemotherapy costs over time |
| Europe: Finland, Netherlands, Germany, Portugal, UK [14] | Routine clinical practice in 5 countries (April 2003-September 2004) | Chemonaive adults aged ≥18 years with confirmed stage IIIB or IV NSCLC not participating in a clinical trial (975) | 1. Chemo-naive patients aged ≥18 years 2. Not participating in a clinical trial 3. Provided written informed consent to participate |
IIIB: 35% IV: 65% |
65 (32–90) | 71% | NR | NR | Treatment lines were documented by study physicians | 1. Investigate potential associations between first-line chemotherapy, general patient and disease characteristics, and outcomes in Europe 2. Evaluate the effect of non–treatment-related factors on outcome in the setting of routine medical care in Europe |
| Europe and South America: France, Germany, Portugal, Finland, Denmark, UK, Sweden, Netherlands, Israel, Romania, Peru [18] | 129 physician practices routinely involved in NSCLC treatment in 11 countries (October 2006-January 2008) | Adults 18+ years with advanced NSCLC who had progressed after first-line chemotherapy and who were about to start second-line treatment (1,013) | 1. Initiation of second-line treatment on, after, or not more than 30 days before date of informed consent 2. Not participating in a clinical trial 3. Provided written informed consent to participate |
IIIB: 17% IV: 78% Unknown: 5% |
63 (32–86) | 69% | 15% | Adenocarcinoma 52% Squamous 24% Large cell 8% Other 16% |
Patients were enrolled around the time of second-line chemotherapy initiation; data were provided by study physicians | 1. Assess choice of and time from initiation of second-line treatment to treatment discontinuation in patients with NSCLC 2. Assess reasons for treatment discontinuation 3. Evaluate impact of treatment discontinuation on patient outcomes, including OS and PFS |
| France [21] | Bas-Rhin population-based cancer registry (1998–2005) | Patients newly diagnosed with stage IIIB (wet) or IV NSCLC and treated in the Bas-Rhin medical network (1,047) |
|
IIIB: 9% IV: 92% |
65 | 75% | 13% | Adenocarcinoma 49% Squamous 35% Large cell 13% Other 3% |
Treatment data were collected during the course of clinical care |
|
| Germany [24] | Speciality hospital near Munich (January 2003-July 2007) | Treatment-naive patients with histologically confirmed stage IIIB and IV NSCLC (406) | 1. Histologically confirmed primary NSCLC 2. Admitted to hospital for diagnostics and/or therapy |
IIIB: 16% IV: 84% |
65 (57–72) | 63% | 25% | Adenocarcinoma 59% Squamous 27% Large cell <1% Other 14% |
Treatment data were collected during the course of clinical care | 1. Present the “real-life conditions” of patients with advanced NSCLC, with a focus on the sequence of different lines of therapy 2. Give information on the complete course of radiotherapy, surgery, and systemic therapy 3. Identify possible prognostic factors for disease control, PFS, and OS for first 3 lines of systemic therapy |
| Germany [19] | Single academic institution in Heidelberg (January 2004-December 2006) | Patients with stage IV NSCLC (493) | 1. No second primary cancers 2. Patients consented in writing for analyses of their data |
IIIB: 4% IV: 96% |
62 (34–86) | 67% | 7.50% | Adenocarcinoma 59% Squamous 19% Large cell 22% |
Treatment data were collected from medical records of patients treated at the institution | 1. Identify patients most likely to benefit from subsequent lines of systemic therapy 2. Understand how clinical study results translate into clinical practice |
| Italy [15] | 60 oncology and pneumology centers in Italy (July 2011-January 2012) | Adults aged 18+ years with stage IIIB or IV NSCLC with disease progression after first-line treatment within 6 months prior to study enrollment (603) | 1. ≥18 years of age 2. Histological or cytological stage IIIB-IV NSCLC diagnosis 3. Confirmed disease progression after first-line treatment within 6 months before study enrollment 4. Provided written informed consent to participate |
IIIB: 25% IV: 75% |
65 (28–84) | 70% | 25% | Adenocarcinoma 72% Squamous 17% Large cell 2% NSCLC NOS 4% Other 5% |
Second-line treatment defined by the clinician as any chemotherapy and/or targeted therapy administered according to routine clinical practice or within a clinical trial subsequent to first-line progression | 1. Describe second-line treatment in the clinical setting 2. Describe clinical practice related to biomarker identification in terms of execution, results, and patient features 3. Describe outcome for patients after second-line NSCLC treatment in terms of the proportion of patients who received third-line treatment according to routine clinical practice |
| Italy [17] | 74 oncology and pneumology centers in Italy (January 2007-March 2008) | Adults age 18+ years with newly diagnosed inoperable stage IIIB or IV NSCLC (987) | 1. ≥18 years of age 2. Newly diagnosed inoperable NSCLC stage IIIB (T4 with pleural effusion and/or N3 metastatic supraclavicular lymph nodes) or IV 3. No concomitant secondary tumors 4. Provided written informed consent to participate |
IIIB: 22% IV: 78% |
66 (35–86) | 75% | 18% | Adenocarcinoma 44% Squamous 28% Large cell 4% NSCLC NOS 24% |
Second- and third-line medical treatments defined as any chemotherapy or targeted therapy previously not administered | 1. Describe the evolving approaches of the Italian oncologists in the treatment of advanced NSCLC |
| Japan [13] | Single academic institution in Tokyo (July 2002-June 2006) | Patients with histologically or cytologically proven, unresectable stage IIIB or IV or recurrent NSCLC who had received first-line chemotherapy (599) | 1. Histologically or cytologically proven unresectable stage IIIB to IV or recurrent NSCLC 2. Received first-line chemotherapy at the institute |
IIIB: 22% IV: 55% Recurrent: 23% |
62 (31–81) for patients who received first or second line; 59 (26–76) for patients who received ≥third line | 65% | 34% | Adenocarcinoma 77% Squamous 12% Large cell 4% NSCLC NOS 7% Other <1% |
Treatment data were collected from medical records of patients treated at the institution | Describe use of and outcomes for third- and fourth-line chemotherapy for NSCLC after approval of gefitinib in Japan in 2002 |
| United States [27] | Network of 1200 community-based oncology practices (January 2007-June 2011) | Adults aged 18+ years with stage IIIB or IV non-squamous NSCLC who initiated second-line chemotherapy (1,168) | 1. Receipt of care at a site utilizing the full iKM EHR capabilities at time of treatment 2. ≥2 visits within the study period 3. ≥18 years at date of initiating second-line therapy 4. ECOG performance status <3 at date of initiating second-line therapy 5. Not enrolled in a clinical trial 6. No other major cancers |
IIIB: 17% IV: 83% |
NR (59% ≥65) | 54% | 11% | Non-squamous 100% | Treatment data were collected from the EHR for patients treated by clinicians in the US Oncology network of community-based oncologists | Evaluate treatment patterns, NSCLC biomarker testing rates, clinical outcome, and hospitalization among patients receiving second-line treatment for advanced NSCLC in a US community-based setting |
| United States [26] | SEER-Medicare linked healthcare databases (2001–2009) | Patients diagnosed with stage IIIB or IV squamous NSCLC (17,133) | 1. Primary stage IIIB with pleural effusion (2004–2009) or stage IV squamous NSCLC (2001–2009) or initial diagnosis at an earlier disease stage with later development of metastases 2. No other primary cancers 3. Aged >65 years 4. Continuous enrollment in Medicare Parts A and B for 6 months before to end of study or death |
IIIB: 17% IV: 83% |
75.3 (mean) | 62% | NR | Squamous 100% | Systemic therapy regimens and cycles were defined using previously published methods that define algorithms based on procedure and diagnostic codes | Assess demographic and clinical characteristics, OS by treatment status, treatment patterns, common systemic treatment regimens used in different therapy lines, and healthcare resource use and costs among patients with metastatic squamous NSCLC enrolled in the US Medicare system |
Patient characteristics
Median age at advanced NSCLC diagnosis ranged from 59 to 68 years in 10 studies that reported this information (Table 1) [13–25]. Males comprised a larger proportion of the study population in all cohorts. There were 2 studies that included only patients with a single NSCLC histology—one with only squamous patients, and one with only non-squamous patients [26, 27]. Among the other studies, adenocarcinoma was the most common NSCLC histology, with the proportion of adenocarcinoma patients ranging from 33% in Brazil to 77% in Japan [13, 23]. Two studies enrolled only stage IV patients [20, 23]. The proportion of patients who never smoked was highest in the Japanese cohort [13,]. Five studies reported performance status after first-line therapy or at initiation of second-line therapy, with most patients having a Karnofsky Performance Status of 70 or better or an Eastern Cooperative Oncology Group score of 0 or 1 [15, 16, 18, 21, 24, 27].
Treatment patterns
Second-line treatment
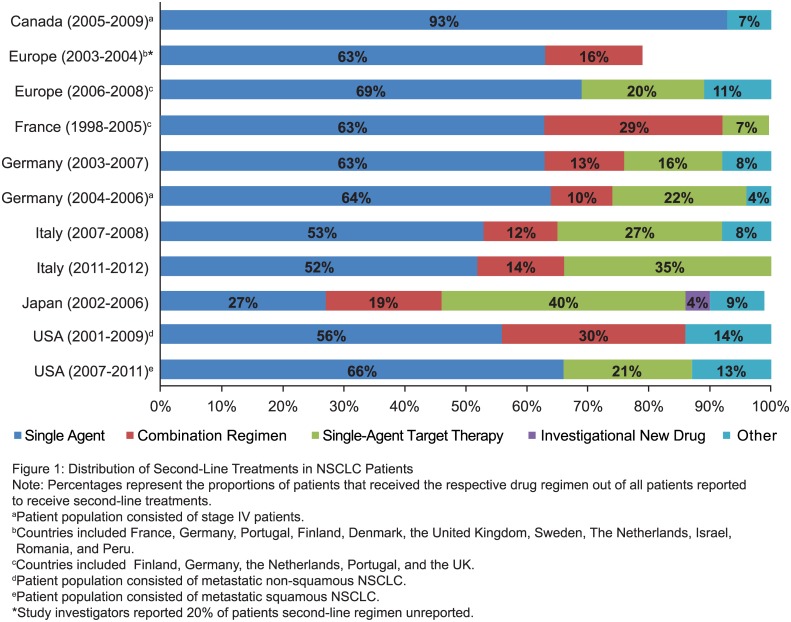
Fig 2 and Table 2 describe the distribution of second-line treatment regimens in each of the studies. The proportion of patients receiving second-line therapy among the studies varied depending on how the study cohort was selected and what treatments were included. In studies that followed patients from initial NSCLC diagnosis, the proportion of patients who received second-line treatment ranged from 8% in a population-based Canadian study that did not include oral therapies (epidermal growth factor receptor [EGFR] tyrosine kinase inhibitors [TKIs]) to 53% in a German study at a single institution [20, 24, 25].
Fig 2. Second-line systemic regimen composition by country and time period.
Note: Percentages represent the proportions of patients who received the respective drug regimen out of all patients reported to receive second-line treatments.
a Per study investigators, 20% of patients’ second-line regimens were unreported.
Germany (2004–2006) and Canada (2005–2009): Patient population consisted of stage IV patients.
Europe (2003–2004): Countries included France, Germany, Portugal, Finland, Denmark, the United Kingdom, Sweden, the Netherlands, Israel, Romania, and Peru.
Europe (2006–2008): Countries included Finland, Germany, the Netherlands, Portugal, and the United Kingdom.
South Korea (2003–2008): All patients had platinum-based first-line therapy.
United States (2007–2011): Patient population consisted of metastatic non-squamous NSCLC.
United States (2001–2009): Patient population consisted of metastatic squamous NSCLC.
NSCLC, non-small cell lung cancer.
Table 2. Summary of second-line treatment patterns.
| Country | Reference | Number of Patients Enrolled | Patients With First-Line Treatment, n (%)a | Patients With Second-Line Treatment, n (%)a | Overall Second-Line Treatment Regimen Distribution, n (%)b | Distribution of Single-Agent Treatments, n (%)b | Distribution of Combination Regimens, n (%)b | Distribution of Targeted Therapy, n (%)b |
|---|---|---|---|---|---|---|---|---|
| Brazilc | Younes et al, 2011 | 2673 | 1548 (58%) | 625 (40.4%) | Non-platinum based 95% Platinum based 5% |
NR | NR | NR |
| Canadac | Sacher et al, 2015 | 8113 | 1944 (24%) | 609 (31.3%) | Single agent 93% Other 7% |
Docetaxel 52% Pemetrexed 41% |
NR | NR |
| Europed | Bischoff et al, 2010 | 975 | 975 (100%) | 285 (29.2%) | Single agent 63% Combination regimen 16% |
Taxane 120 (42%) Vinorelbine 31 (11%) Gemcitabine 29 (10%) |
NR | NR |
| Europee | Moro-Sibilot et al, 2010; Vergnenegre et al, 2012 (SELECTTIONN) |
1013 | 1013 (100%) | 1013 (100%) | Single agent 700 (69%) Targeted therapy 206 (20%) Other regimens 106 (11%) |
Pemetrexed 468 (46%) Docetaxel 232 (23%) |
NR | Erlotinib 207 (20%) |
| Francef | Carpentier, 2016 | 1,047 | 863 (82%) | 431 (41.2%) | Single agent 273 (64%) Combination regimen 126 (29%) Targeted therapy 32 (7%) |
Docetaxel 100 (23%) Pemetrexed 17 (4%) Other 156 (36%) |
Cisplatin based: 48 (11%) Carboplatin based: 78 (18%) |
Erlotinib or gefitinib 32 (7%) |
| Germany | Zietemann, 2011; Zietemann, 2010 | 406 | 406 (100%) | 213 (52.5%) | Single agent 134 (63%) Combination regimen 28 (13%) Targeted therapy 35 (16%) Other regimen 16 (8%) |
Docetaxel 66 (31%) Gemcitabine 31 (15%) Vinorelbine 24 (11%) Pemetrexed 13 (6%) |
Gemcitabine + mitomycin 10 (5%) Carboplatin + paclitaxel 7 (3%) Carboplatin + vinorelbine 5 (2%) Carboplatin + gemcitabine 3 (1%) Carboplatin + docetaxel 3 (1%) |
Erlotinib 20 (9%) Gefitinib 15 (7%) |
| Germanyc | Reinmuth et al, 2013 | 493 | 352 (71%) | 183 (52%) | Single agent 117 (64%) Combination regimen 18 (10%) Targeted therapy 41 (22%) Other 7 (4%) |
NR | Platinum based 18 (10%) | EGFR-TKI 41 (22%) |
| Italy | De Marinis et al, 2014; Gridelli et al, 2014 (LIFE) |
541 | 541 (100%) | 464 (85.8%) | Single agent 241 (52%) Combination regimen 63 (14%) Targeted therapy 163 (35%) |
Docetaxel 118 (25%) Pemetrexed 68 (15%) Gemcitabine 26 (6%) Vinorelbine 23 (5%) Carboplatin 2 (<1%) Cisplatin 2 (<1%) Paclitaxel 2 (<1%) |
Carboplatin + gemcitabine 15 (3%) Cisplatin + pemetrexed 14 (3%) Docetaxel + gemcitabine 9 (2%) Carboplatin + paclitaxel 8 (2%) Carboplatin + pemetrexed 7 (2%) Cisplatin + gemcitabine 5 (1%) Other 5 (1%) |
Erlotinib 149 (32%) Gefitinib 9 (2%) Bevacizumab combination 3 (1%) Crizotinib 2 (<1%) |
| Italy | Gridelli et al, 2011 | 987 | 790 (80%) | 275 (35%) | Single agent 146 (53%) Combination regimen 34 (12%) Targeted therapy 73 (27%) Clinical trial 22 (8%) |
Pemetrexed 56 (20%) Docetaxel 46 (17%) Gemcitabine 19 (7%) Vinorelbine 19 (7%) Cisplatin or carboplatin 3 (1%) Other 3 (1%) |
Carboplatin + vinorelbine 6 (2%) Carboplatin + pemetrexed 6 (2%) Carboplatin + gemcitabine 4 (1%) Docetaxel + vinorelbine 2 (1%) Carboplatin + paclitaxel 2 (1%) Cisplatin + docetaxel 2 (1%) Cisplatin + gemcitabine 1 (<1%) Other 6 (2%) |
Erlotinib 73 (27%) |
| Japan | Asahina et al, 2012 | 599 | 599 (100%) | 415 (69%) | Single agent 114 (27%) Combination regimen 80 (19%) Targeted therapy 167 (40%) Investigational new drug 15 (4%) Other 39 (9%) |
Docetaxel 114 (27%) | Carboplatin + paclitaxel 64 (15%) Carboplatin + gemcitabine 16 (4%) |
Gefitinib 167 (40%) |
| United Statesg | Pan et al, 2013 | 1168 | 1168 (100%) | 1168 (100%) | Single agent 781 (66%) Targeted therapy 241 (21%) Other 146 (13%) |
Pemetrexed 635 (54%) Docetaxel 117 (10%) Gemcitabine 29 (2%) |
NR | Erlotinib 205 (18%) Bevacizumab combination 36 (3%) |
| United Statesh | Davis et al, 2015 | 17,133 | 7029 (41%) | 3405 (20%) | Single agent 1916 (56%) Combination regimen 1012 (30%) Other 477 (14%) |
Gemcitabine 550 (16%) Docetaxel 459 (14%) Pemetrexed 376 (11%) Vinorelbine 207 (6%) Paclitaxel 206 (6%) Carboplatin 118 (3%) |
Carboplatin + paclitaxel 458 (14%) Carboplatin + gemcitabine 299 (9%) Carboplatin + docetaxel 181 (5%) Gemcitabine + vinorelbine 74 (2%) |
NR |
Abbreviations: EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; NR, not reported; NSCLC, non-small cell lung cancer.
aPercentage of patients with first-line treatment out of all patients
bPercentage of patients with second-line treatment.
cOnly consisted of patients with stage IV NSCLC.
dCountries included France, Germany, Portugal, Finland, Denmark, the United Kingdom, Sweden, the Netherlands, Israel, Romania, and Peru.
eCountries included Finland, Germany, the Netherlands, Portugal, and the United Kingdom.
fOther includes vinorelbine, gemcitabine, paclitaxel, adriamycin, epirubicin, mitomycin, ifosfamide, VP16, cyclophosphamide, 5-fluroruracil, and topotecan
gOnly included patients who received planned second-line treatment and had non-squamous histology.
hOnly included patients who had squamous histology.
In 10 studies that provided detailed information on prescribed treatment regimens, single-agent chemotherapy was the most commonly used second-line treatment regimen except in one study from Japan. In the Japanese study, single-agent targeted therapy was the most commonly prescribed second-line treatment [13]. Docetaxel, pemetrexed, and gemcitabine were among the top 3 most frequently used single-agent chemotherapeutics across the studies [15–22, 24–27]. In Japan gefitinib was the most frequently used second-line single-agent targeted-therapy [13]. Outside of Asia, the most frequently used single-agent targeted therapy was erlotinib [15–19, 22, 24, 25, 27].
Pemetrexed was one of the most commonly prescribed second-line treatments in the studies that included patients treated after its initial approval in 2004 [15–18, 22, 24, 25, 27]. The study conducted in Canada reported that pemetrexed use increased to 70% among treated second-line patients in 2008, when government funding was approved for pemetrexed [20].
Second-line treatment with EGFR-TKIs increased in the European studies after initial approval of erlotinib in 2005 [15–19, 21, 22, 24, 25]. This uptake occurred prior to the widespread introduction of EGFR mutation testing, which was not required at the time of initial erlotinib approval.
Platinum-based doublet chemotherapy was administered in all 7 studies that described the use of second-line combination chemotherapy regimens [13, 15–17, 19, 21, 24–26]. The most frequent use of combination chemotherapy was reported in a study that included only patients with squamous histology [26].
Third-line treatment
Nine of the 12 studies reported detailed information on patients who received third-line therapy [15–19, 21–25]. In these studies, approximately 30% of patients received third-line treatment (S1 Table). Single-agent chemotherapy accounted for about 50% of third-line treatments in general, and targeted therapy accounted for almost 40% [15–18, 21, 22, 24, 25]. Docetaxel, gemcitabine, vinorelbine, and pemetrexed were the most frequently administered third-line chemotherapies. Erlotinib was the most common single-agent targeted therapy in the third-line setting in all countries.
Other lines of therapy
All 12 studies reported details about the distribution of first-line treatment regimens. The most frequently administered first-line treatment across all countries was platinum-doublet chemotherapy (S3 Table). Three European studies reported use of targeted therapy in the first-line setting. In an Italian cohort, gefitinib, bevacizumab, and erlotinib were administered to 6%, 4%, and 1% of first-line treated patients, respectively [15, 16]. Erlotinib was administered to 3% of first-line patients in a German cohort [24, 25]. Only 4 patients (0.4%) in a French cohort received an EGFR-TKI in first-line therapy [21].
In Japan, fourth-line chemotherapy was reported in 17.7% of the patients who received first-line chemotherapy. The top 3 anti-cancer treatment regimens in the fourth-line setting were single-agent S-1 (22%), docetaxel (21%), and gefitinib (21%) [13]. In Brazil, 2.5% of all patients with stage IV NSCLC (4.3% of the patients who received first-line chemotherapy) received fourth-line chemotherapy [23].
Survival outcomes
Eleven of 12 studies included in this review presented overall survival (OS) data (Table 3) [13, 15–27]. Five studies reported OS from time of metastatic NSCLC (mNSCLC) diagnosis [19, 20, 21, 23, 26]. Three of these studies reported the median OS among patients with advanced NSCLC who received second-line therapy. Median OS from mNSCLC diagnosis date ranged from 8.7 months among patients who received only first- and second-line (ie, no third-line) treatments in a single-center German cohort to 17 months among patients who received at least second-line treatment in a single-center Brazilian cohort [19, 20, 23].
Table 3. Summary of survival outcomes by definition of survival time.
| Region/Country | Source | Number of Patients | Study Setting | Study Period | Median OS, months (first line) | Median OS, months (second line) | Median OS, months (third line) | Median OS (BSC only) | OS Definition |
|---|---|---|---|---|---|---|---|---|---|
| Time from mNSCLC diagnosis to death/study end | |||||||||
| Brazil | Younes et al, 2011 | 2673 | Single institution | 1990–2008 | 11.0 | 17.0 | 4.0 | Time from initial mNSCLC diagnosis to date of last consultation or death | |
| Canada | Sacher et al, 2015 | 8113 | Population based | 2005–2009 | 8.2 (7.7–8.6) for patients who received first line only | 16.2 (15.1–17.0) for patients who received first and second line | 3.3 (3.2–3.4) | Time from date of mNSCLC diagnosis (K-M) | |
| France | Carpentier et al, 2016 | 1047 | Population based | 1998–2005 | 8.3 (7.6–9.1) | 1.3 (1.1–1.7) | Time from date of mNSCLC diagnosis to date of death, visit to the medical center, or end of study period (K-M) | ||
| Germany | Reinmuth et al, 2013 | 493 | Single institution | 2004–2006 | 2.0 | 5.3 | Time from date of mNSCLC diagnosis (K-M) | ||
| United States | Davis et al, 2015 | 17,133 | Population based | 2001–2010 | 8 (for patients who received any cancer-directed treatment) | 2.0 | Time from date of mNSCLC diagnosis to death or end of study period (K-M) | ||
| Time from start of first-line chemotherapy until death/study end | |||||||||
| Europe (multiple) | Bischoff et al, 2010 (ACTION study) | 975 | Provider based | 2003–2006 | 9.3 (8.6–10.3) | Time from start of chemotherapy until death or time of last follow-up | |||
| Europe (Italy) | Gridelli et al, 2011 (SUN study) | 790 | Multiple institutions | 2007–2008 | 9.1 (8.1–10.0) | Time from start of first-line chemotherapy until last day patient was known to be alive | |||
| Time from start of each chemotherapy line until death/study end | |||||||||
| Japan | Asahina et al, 2012 | 599 | Single institution | 2002–2006 | 15.3 (13.8–16.5) | 12.8 (10.7–14.5) | 12.0 (9.3–14.2) | Time from first day of each chemotherapy line until death or last day of follow-up period | |
| Germany | Reinmuth et al, 2013 | 493 | Single institution | 2004–2006 | 7.6 (6.8–8.5) | 6.2 (5.0–7.4) | 5.2 (3.5–7.0) | Time from the beginning of the respective line of systemic therapy | |
| Europe (Germany) | Zietemann, 2010 and 2011 | 405 | Single institution | 2003–2008 | 8.9 (8.2–10.1) | 4.6 (3.8–5.7) | 3.8 (2.6–5.4) | Time from first day of each chemotherapy line until death or last day of follow-up period; OS given in days in study and converted to months (days/30) | |
| Time from start of second-line chemotherapy until death/study end | |||||||||
| Europe (multiple) | SELECTTION study—2010, 2012 | 1013 | Provider based | 2006–2008 | ACA: 8.1 (6.9–9.0) other NSCLC: 6.2 (5.5–6.8) | Time from start of second-line chemotherapy until death or date of last contact | |||
| United States | Pan et al, 2013 | 1168 | Provider based | 2007–2011 | 7.5 (6.6–8.4) | Time from start of second-line chemotherapy until death or date of last follow-up visit (K-M) | |||
| Time from the start of third-line chemotherapy until death/study end | |||||||||
| France | Carpentier et al, 2016 | 226 | Population based | 1998–2005 | TKI: 5.9 (4.2–10.1) | Time from initiation of third line treatment to date of death, vital status, or end of study period (K-M) | |||
| No outcome data provided | |||||||||
| Europe (Italy) | LIFE study—2014, 2014 | 541 | Multiple institutions | 2011–2012 | No survival reported | ||||
Abbreviations: ACA, adenocarcinoma; BSC, best supportive care; K-M, Kaplan-Meier; mNSCLC, metastatic non-small cell lung cancer; NSCLC, non-small cell lung cancer; OS, overall survival; TKI, tyrosine kinase inhibitor.
In 5 studies that described median OS from time of second-line treatment initiation in routine clinical practice, median OS ranged from 4.6 months (95% CI, 3.8–5.7) to 12.8 months (95% CI, 10.7–14.5) [13, 19, 22, 24, 25, 27]. Median OS estimates were close to 8 months in 2 studies that reported this information specifically for patients with adenocarcinoma in the second-line setting [18, 22, 27]. Median OS estimates were reported from the time of third-line treatment initiation in 4 studies and median OS ranged from 3.8 (95% CI, 2.6–5.4) to 12.0 months (95% CI, 9.3–14.2) [13, 24, 25]
Biomarkers
Two studies reported biomarker testing and treatments prescribed based on biomarker status, one in Italy (LIFE) and one in the US that included only patients with adenocarcinoma (Table 4) [15, 16, 27]. In the US study, EGFR testing frequency increased significantly from 2.3% between 2007 and 2009 to 32% in the first 6 months of 2011 [27]. The LIFE study, which was conducted in 2011–2012, reported that 60% of Italian patients received biomarker testing [15, 16]. In this study, biomarker testing was more frequent among patients who were younger, female, never-smokers, and those with adenocarcinomas.
Table 4. Summary of biomarker testing frequency and mutation prevalence by country.
| United States [27] | Italy [15, 16] | |
|---|---|---|
| Study population | Advanced non-squamous NSCLC patients who initiated second-line treatment | Advanced NSCLC patients with disease progression after first-line treatment |
| Study enrollment period | 2007–2011 | 2011–2012 |
| Timing of biomarker testing | Before or at initiation of second-line treatment | Between advanced NSCLC diagnosis and baseline study visit |
| Biomarker testing frequency | N (%) | N (%) |
| Any biomarker | NR | 314 (58%) |
| EGFR | 128 (11%) | 311 (57%) |
| KRAS | 40 (3%) | 77 (14%) |
| ALK | 28 (2%) | 74 (14%) |
| Biomarker mutation prevalence (among tested patients) | ||
| EGFR | 24 (19%) | 65 (21%) |
| KRAS | 8 (20%) | 17 (22%) |
| ALK | 1 (4%) | 17 (23%) |
Abbreviations: EGFR, epidermal growth factor receptor; NR, not reported; NSCLC, non-small cell lung cancer.
Both studies provided evidence of biomarker-driven therapy choices within their cohorts. The LIFE study reported that 26 of 37 (70%) patients who received EGFR-TKIs as first-line therapy were known to have EGFR-activating mutations prior to first-line treatment [15, 16]. In the US cohort, 50% of 24 patients with known EGFR mutations received erlotinib as second-line treatment compared with 17% of the 89 EGFR wild-type patients. The use of erlotinib was significantly more common in patients with EGFR mutations compared with EGFR-wild type patients (P < .001) [27].
Discussion
The objective of this study was to describe real-world treatment patterns and survival outcomes for patients with advanced NSCLC who received second-line or later treatments, through a review of recently published observational studies. To our knowledge, there are no systematic reviews that summarize this information. By qualitatively reviewing this information from multiple countries, we provide a broad overview of how patients are managed in this setting around the world.
Overall, the retrieved studies showed how newly approved therapies are rapidly integrated into clinical practice, and how clinical practice evolves in response to therapeutic advances. In addition, most studies demonstrated a high level of clinician adherence to international treatment guidelines, such as those from European Society of Medical Oncology and the US National Comprehensive Cancer Network [28, 29]. Among the studies that focused solely on patients with non-squamous NSCLC, single-agent pemetrexed, docetaxel, or EGFR-TKIs were the most common treatment choices, which aligns with current guidelines. However, there were examples of non-adherence to guidelines, namely use of platinum-doublet chemotherapies as second-line treatment. This was highest in the all-squamous cohort, which is consistent with the limited number of approved second-line treatments for this particular subgroup of patients with advanced NSCLC that were available at the time these studies were conducted [26]. Moreover, while specific patient-level information was not available from these studies, it is possible that use of platinum-doublet chemotherapy in the second-line setting could be motivated by oncologists’ perceptions of the superiority of these particular treatment regimens.
Survival after initiation of second-line treatment was reported in most studies, and was generally consistent with outcomes reported from pemetrexed, docetaxel, and erlotinib clinical trials [30–33]. The consistency of survival outcomes suggests that clinical trial results may be generalizable to real-world patient populations. Additionally, the data confirm the limited survival benefit of treatments that were available at the time these studies were conducted. An exception to this was the lower survival among second-line patients in Germany reported by Zietemann and Duell compared with outcomes reported from other European studies with similar outcome definitions [19, 22]. No explicit reason for the lower survival reported in this study was identified based on patient and tumor characteristics, or the study setting. The range of survival outcomes reported by the studies included in this review exemplifies the variation that can occur across multiple studies and may be due to individual-level patient differences. In addition, these survival differences highlight the importance of examining population-level data and outcomes from multiple observational studies to understand the range of outcomes experienced in routine clinical practice and the limited ability to draw conclusions from studies that do not report detailed patient-level data.
In most studies that reported the proportion of patients with advanced NSCLC treated across lines of therapy, between one-third to one-half of those who received first-line treatment also received second-line therapy [14, 16, 20, 21, 23–26]. None of these studies provided information to explain why patients did not receive later lines of therapy. However, Gridelli et al reported that poor performance status and older age were the most common reasons patients did not receive first-line treatment [17]. These reasons, in addition to a poor response to first-line treatment, may be why patients with advanced NSCLC do not receive later lines of therapy. Newer treatments such as immunotherapies may offer these patients an opportunity to receive treatment because of their more favorable toxicity profiles compared with existing treatments [34, 35]. Additional studies that examine why patients are not treated could help identify opportunities to increase the proportion of patients with advanced NSCLC who derive benefit from treatment.
There was limited information reported on biomarker-guided therapy decisions in the studies included in this review, likely due to the time periods covered by the studies. In the studies covering more recent time periods, the rapid uptake seen in the use of targeted therapies and biomarker testing is encouraging. It reveals that biomarker-driven treatment decisions are being integrated into routine clinical practice. This information provides a positive outlook for the uptake of newer targeted therapies in the second- and third-line settings. The rapid uptake of anaplastic lymphoma kinase (ALK) inhibitors such as crizotinib and ceritinib in patients with advanced NSCLC harboring ALK mutations provides further evidence that biomarker-directed therapy is an important treatment option for these patients [36].
Biomarker testing may impact uptake of newly approved targeted therapies. More information on how and when biomarker testing is performed in routine clinical practice is needed, since it is unclear in the included studies whether biomarker testing was more commonly performed prior to first-line versus second-line treatment. Across the European studies, erlotinib use increased despite approval of EGFR mutation testing in Europe after erlotinib approval. This suggests a trend among clinicians of using new therapies when clinically appropriate, despite limitations in access to biomarker testing. These trends imply positive uptake of new immunotherapies like programmed death-ligand 1/programmed death 1 (PD-L1/PD-1) checkpoint inhibitors despite evolving biomarker testing guidelines.
A few limitations should be considered when interpreting the information provided in this review. A meta-analysis could not be conducted due to the heterogeneity of the study designs and data presentation in the included studies. There were substantial differences in patient selection procedures, data collection methods, and outcome measure definitions. Thus, a qualitative data synthesis was the most appropriate way to summarize these studies [37]. Moreover, the review was robust and systematic, which renders the search and presentation of results transparent and reproducible. Additionally, publication bias could influence the evidence presented in this review; however, studies were found from multiple countries. Finally, conclusions from the results presented herein are limited by the observational design of the included studies.
Conclusions
Real-world studies of second- and third-line treatment patterns in advanced NSCLC provide insights into how evidence from clinical trials impacts clinical practice. The studies included in this qualitative systematic review demonstrate the limited clinical benefit of the second- and third-line treatments for advanced NSCLC that were available at the time these studies were conducted. Within recent years, several novel therapies have been approved for use in these settings, and it will be important to determine the benefit that these and other new treatments may provide to patients with advanced NSCLC.
Supporting information
This table contains the search string that was used to perform the systematic review.
(DOCX)
This table contains the assessment of bias for each study.
(DOCX)
This table contains the summary of third-line treatments by country.
(DOCX)
This document contains a completed PRISMA checklist.
(DOC)
Acknowledgments
Thanks to scientific communications leaders Cindy Yun and Alicia Chung of Genentech, Inc., for medical writing support.
This study was sponsored by F. Hoffmann-La Roche, Ltd. Medical writing assistance for this manuscript was provided by Health Interactions, Inc, paid for by F. Hoffmann-La Roche, Ltd.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was sponsored by F. Hoffmann-La Roche, Ltd. Ms. Davies, Dr. Waterkamp and Dr. McCusker are employed by F. Hoffmann-La Roche. F. Hoffmann-La Roche provided support in the form of salaries for authors [JD, MM, DW] and also played a role in the decision to publish, and preparation of the manuscript, but did not play a role in the study design, data collection and analysis. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5):e359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; 2015. http://seer.cancer.gov/csr/1975_2012/. [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. ; CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015; 385(9972):977–1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta SK. Role of Crizotinib in previously treated non-small-cell lung cancer. South Asian J Cancer. 2014; 3(2):138–40. 10.4103/2278-330X.130468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. ; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002; 346(2):92–8. 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 6.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012; 380:1840–50. 10.1016/S0140-6736(12)60919-2 [DOI] [PubMed] [Google Scholar]
- 7.Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer‐related mortality in Europe. Int J Cancer. 2015; 136:e136–45. 10.1002/ijc.29105 [DOI] [PubMed] [Google Scholar]
- 8.Bradley CJ, Dahman B, Given CW. Treatment and survival differences in older Medicare patients with lung cancer as compared with those who are dually eligible for Medicare and Medicaid. J Clin Oncol. 2008; 26(31):5067–73. 10.1200/JCO.2008.16.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011; 103(2):117–28. 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DH, Schiller JH, Bunn PA Jr. Recent clinical advances in lung cancer management. J Clin Oncol. 2014; 32(10):973–82. 10.1200/JCO.2013.53.1228 [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012; 65(2):163–78. 10.1016/j.jclinepi.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 13.Asahina H, Sekine I, Horinouchi H, Nokihara H, Yamamoto N, Kubota K, et al. Retrospective analysis of third-line and fourth-line chemotherapy for advanced non–small-cell lung cancer. Clin Lung Cancer. 2013; 13(1):39–43. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff HG, van den Borne B, Pimentel FL, Arellano J, Langer F, Leschinger MI, et al. Observation of the treatment and outcomes of patients receiving chemotherapy for advanced NSCLC in Europe (ACTION study). Curr Med Res Opin. 2010; 26(6):1461–70. 10.1185/03007991003799180 [DOI] [PubMed] [Google Scholar]
- 15.de Marinis F, Ardizzoni A, Fontanini G, Grossi F, Cappuzzo F, Novello S, et al. ; LIFE Study Team. Management of Italian patients with advanced non–small-cell lung cancer after second-line treatment: results of the longitudinal phase of the LIFE observational study. Clin Lung Cancer. 2014; 15(5):338–45. 10.1016/j.cllc.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Gridelli C, de Marinis F, Ardizzoni A, Novello S, Fontanini G, Cappuzzo F, et al. ; LIFE study team. Advanced non-small cell lung cancer management in patients progressing after first-line treatment: results of the cross-sectional phase of the Italian LIFE observational study. J Cancer Res Clin Oncol. 2014; 140(10):1783–93. 10.1007/s00432-014-1715-2 [DOI] [PubMed] [Google Scholar]
- 17.Gridelli C, Ardizzoni A, Barni S, Crinò L, Caprioli A, Piazza E, et al. Medical treatment choices for patients affected by advanced NSCLC in routine clinical practice: results from the Italian observational “SUN” (Survey on the lUng cancer maNagement) study. Lung Cancer. 2011;74(3):462–8. 10.1016/j.lungcan.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Moro-Sibilot D, Vergnenegre A, Smit EF, Toy E, Parente B, Schmitz S, et al. Second-line therapy for NSCLC in clinical practice: baseline results of the European SELECTTION observational study. Curr Med Res Opin. 2010; 26(11):2661–72. 10.1185/03007995.2010.525489 [DOI] [PubMed] [Google Scholar]
- 19.Reinmuth N, Payer N, Muley T, Hoffmann H, Herth FJ, Villalobos M, et al. Treatment and outcome of patients with metastatic NSCLC: a retrospective institution analysis of 493 patients. Respir Res. 2013; 14:139 10.1186/1465-9921-14-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacher AG, Le LW, Lau A, Earle CC, Leighl NB. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: Are patients undertreated? Cancer. 2015; 121(15):2562–9. 10.1002/cncr.29386 [DOI] [PubMed] [Google Scholar]
- 21.Carpentier O, Selvaggi L, Jégu J, Purohit A, Prim N, Velten M, Quoix E. Modern Treatments in Advanced Non–Small-Cell Lung Cancer: Temporal Trends and Effect on Survival. A French Population-Based Study. Clinical lung cancer. 2015. November 30;16(6):496–506. 10.1016/j.cllc.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 22.Vergnenegre A, Smit EF, Toy E, Parente B, Schmitz S, Kraaij K, et al. Second-line therapy for non-small cell lung cancer in clinical practice: final results and treatment pathways from the SELECTTION observational study. Curr Med Res Opin. 2012; 28(8):1253–62. 10.1185/03007995.2012.703133 [DOI] [PubMed] [Google Scholar]
- 23.Younes RN, Pereira JR, Fares AL, Gross JL. Chemotherapy beyond first-line in stage IV metastatic non-small cell lung cancer. Rev Assoc Med Bras. 2011; 57(6):686–91. [DOI] [PubMed] [Google Scholar]
- 24.Zietemann V, Duell T. Every-day clinical practice in patients with advanced non-small-cell lung cancer. Lung Cancer. 2010; 68(2):273–7. 10.1016/j.lungcan.2009.06.023 [DOI] [PubMed] [Google Scholar]
- 25.Zietemann V, Duell T. Prevalence and effectiveness of first-, second-, and third-line systemic therapy in a cohort of unselected patients with advanced non-small cell lung cancer. Lung Cancer. 2011; 73(1):70–7. 10.1016/j.lungcan.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 26.Davis KL, Goyal RK, Able SL, Brown J, Li L, Kaye JA. Real-world treatment patterns and costs in a US Medicare population with metastatic squamous non-small cell lung cancer. Lung Cancer. 2015; 87(2):176–85. 10.1016/j.lungcan.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 27.Pan IW, Mallick R, Dhanda R, Nadler E. Treatment patterns and outcomes in patients with non-squamous advanced non-small cell lung cancer receiving second-line treatment in a community-based oncology network. Lung Cancer. 2013; 82(3):469–76. [DOI] [PubMed] [Google Scholar]
- 28.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. ; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27(suppl 5):v1–v27. 10.1093/annonc/mdw326 [DOI] [PubMed] [Google Scholar]
- 29.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 2.2017. www.nccn.org.
- 30.Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist. 2005; 10(7):461–6. 10.1634/theoncologist.10-7-461 [DOI] [PubMed] [Google Scholar]
- 31.Hanna NH. Second-line chemotherapy for non-small-cell lung cancer: recent data with pemetrexed. Clin Lung Cancer. 2004; 5(Suppl 2):S75–9. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18(10):2095–103. 10.1200/JCO.2000.18.10.2095 [DOI] [PubMed] [Google Scholar]
- 33.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. ; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353(2):123–32. 10.1056/NEJMoa050753 [DOI] [PubMed] [Google Scholar]
- 34.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373(17):1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373(2):123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frampton JE. Crizotinib: a review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs. 2013; 73(18):2031–51. 10.1007/s40265-013-0142-z [DOI] [PubMed] [Google Scholar]
- 37.Deeks JJ, Higgins J, Altman DG. Analysing data and undertaking meta-analyses In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd; 2008. p. 243–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table contains the search string that was used to perform the systematic review.
(DOCX)
This table contains the assessment of bias for each study.
(DOCX)
This table contains the summary of third-line treatments by country.
(DOCX)
This document contains a completed PRISMA checklist.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.