Abstract
Introduction
To better understand the associations between history of tobacco use and survival outcomes, cigarette use was prospectively surveyed in 687 previously untreated patients with cancer of the oral cavity (271), oropharynx (257), larynx (135) or hypopharynx (24).
Methods
Kaplan-Meier and Cox models explored associations of tobacco use intensity (packs/day), duration (years of use) and timing prior to diagnosis with overall and disease specific survival and recurrence free time.
Results
Cigarette use duration, timing, and intensity were significant predictors for all outcomes in univariate analysis. Never smoking and pack years were not significantly associated with outcomes after adjustment for prognostic factors such as stage, comorbidities, and HPV status which were strongly associated with clinical outcomes.
Conclusions
The findings confirm the association between smoking history and survival and the importance of clinical variables in evaluating smoking as a prognostic factor. Timing, intensity, and duration of cigarette use should be considered with other prognostic factors when considering risk stratification for treatment planning.
Keywords: tobacco, epidemiology, head and neck cancer, survival, recurrence, comorbidities, intensity, duration
Introduction
Approximately 35- 55% of patients with head and neck squamous cell carcinoma (HNSCC) experience locoregional recurrence or distant metastasis within two years of initial diagnosis (1, 2) and are at high risk for developing a second primary (3). There are many established factors associated with higher rates of recurrence, including tumor site, stage, Human Papillomavirus status, and diet (4). Patients with Human Papillomavirus positive (HPV(+)) oropharyngeal cancer on average have better prognoses, which led to recommendations to de-escalate aggressive treatment and limit toxicity for this group, particularly if they have a favorable smoking history (5). Tobacco is a strong risk factor for the development of HNSCC via documented cellular, molecular, and epigenetic effects (6-8). Previous studies have shown that all-cause mortality is worse among survivors who continue to smoke compared to never smokers, but this same benefit is not seen when comparing patients who recently quit smoking to patients who continue to smoke after diagnosis (9). However, analyzing smoking history is complex (10-12), and studies in lung squamous cell carcinoma have indicated that duration of smoking as well as time since quitting is associated with incidence (13) and survival (14-16).
It is essential to better define the association between tobacco use and HNSCC survival and oncologic outcomes. This is best derived from prospective, structured smoking assessments to properly understand the potential role of smoking in risk stratification treatment models that include other prognostic factors. Smoking is associated with lifestyle and health variables, such as alcohol use, BMI and comorbidities. All of these may also be associated with clinical outcomes of interest in HNSCC patients. Thus, there is strong interest in examining the risk of tobacco use on long term survival and recurrence rates in HNSCC, particularly in combination with other established prognostic factors. These data will be instrumental in selecting the appropriate cohorts for potential treatment intensification or de-escalation.
From an epidemiologic perspective, smoking status is frequently stratified into three groups of never, current, and former smokers. However, such a classification does not fully account for the intensity and duration of tobacco exposure. Intensity is usually defined in terms of cigarettes per day and duration in years of use. Since a long history of high intensity tobacco use is associated with many adverse outcomes, and may contribute to carcinogenesis and treatment sensitivity, we hypothesize that separate consideration of recent and remote tobacco exposure groups may aid in better understanding the impact of tobacco use on oncologic and survival outcomes.
In this paper we assessed the effects of lifetime cigarette exposure in a prospectively collected, unselected population of previously untreated, incident HNSCC patients. This cohort of 687 is actively followed as part of an epidemiologic study. The study protocol includes annual questionnaires and extensive quality control and completeness checks, and thus constitutes very high quality data on which to study questions regarding the potential importance of tobacco and other risk factors on survival and recurrence outcomes.
Patients and Methods
Full details are provided in the online supplementary materials, below is a brief description of the study design, the patients and the methods.
Recruitment
From November 2008 through July 2013, every previously untreated, incident adult HNSCC patient with primary disease evaluated in the Head and Neck Oncology Program of the University of Michigan (UM; Ann Arbor, MI) Comprehensive Cancer Center was prospectively screened for eligibility and 92% signed a written informed consent and were enrolled. This unselected study population represented 28% of incident HNSCC cases in the State of Michigan. The study was approved by a University of Michigan Institutional Review Board.
Variable Definitions
The date of diagnosis was the date the patient was diagnosed with a biopsy confirmed squamous cell carcinoma at UM. Comorbidity was assessed at diagnosis through medical chart review using the Adult Comorbidity Evaluation 27 (ACE-27) (17), a validated instrument to grade the severity of comorbidities in patients with cancer. Prior HNSCC was defined as a previous primary tumor in the head and neck more than five years earlier. Self-reported smoking history was collected at the time of enrollment and included age of initiation, cessation, and smoking status, categorized into never, current (including patients who quit within 12 months of diagnosis), or former (quit over 12 months prior to diagnosis). Cigar, pipe, and smokeless tobaccos were frequently missing age of initiation and cessation. There were very few non-cigarette smokers in our cohort who only used pipe or cigar tobacco. Because it is known that the risk of HNSCC is not elevated among ever cigarette smokers who also us cigar and pipe tobaccos (18), only cigarette data were further analyzed. The cumulative quantity of cigarettes smoked in the recent past (less than 10 years before diagnosis) and at remote times (earlier than 10 years before diagnosis) were considered. These cumulative amounts were calculated by multiplying the intensity of use (pack years) by the duration of use during each period, as shown in Supplementary Figure 1. Duration of use was determined by the cigarette initiation and cessation dates.
Follow-up
Patients were followed at NCCN guideline intervals for routine cancer care and surveillance. Tumor status (recurrence, persistent disease, second primary) was updated annually during a medical record review and annual surveys. Deaths were confirmed through the Social Security Death Master File, yearly surveys, family notification, and medical record reviews. Survival time was censored to 2/1/14 or the last known contact date for subjects lost to follow-up. Tumor status was censored to the last date of each subject's annual medical record review (19); the last data observation occurred in September 2014. Deaths due to other causes were censored at date of death for disease specific survival time (DST).
Study Population
The study population of 687 subjects was comprised of patients mainly with oral cavity and oropharynx primary sites (39% and 37%, respectively) and Stage IV (59%) disease (Table 1). The mean age at diagnosis was 61 years (SD: 12 years). Among all smokers, the mean age of initiation was 24 years (SD: 10 years); among former smokers, the mean age of cessation was 46 years (SD: 15 years). Treatment modalities included surgery alone (25%) or surgery with adjuvant radiation or chemoradiation (20%), chemoradiation alone (40%), radiation alone (7%), or palliative/unknown (8%) treatment. Ten percent of patients were never rendered disease free after treatment. Recurrence patterns were local only (25%), regional ± locoregional (36%), and distant ± locoregional (39%). Median follow-up for overall survival was 30 months and the estimated two year overall survival rate was 78%. Median follow-up for recurrence-free time was 24 months and the estimated two year recurrence- free rate was 75%.
Table 1. Univariate Cox proportional hazards model for survival outcome.
| OST | RFT | DST | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. of Patients | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at Dx | 10 years | 687 | 1.44 (1.26, 1.64) | <0.0001 | 1.26 (1.10, 1.43) | 0.0007 | 1.21 (1.02, 1.43) | 0.03 |
| Genderb | Male (ref) | 504 | (ref) | (ref) | (ref) | |||
| Female | 183 | 1.31 (0.94, 1.83) | 0.11 | 1.13 (0.80, 1.60) | 0.48 | 1.21 (0.78, 1.86) | 0.39 | |
| Married | Married | 436 | 0.55 (0.40, 0.74) | 0.0001 | 0.65 (0.48, 0.89) | 0.007 | 0.66 (0.44, 0.98) | 0.04 |
| Not Marrieda | 251 | (ref) | (ref) | (ref) | ||||
| Prior Cab | 108 | 1.04 (0.69, 1.57) | 0.86 | 0.86 (0.55, 1.34) | 0.51 | 0.85 (0.48, 1.50) | 0.58 | |
| Prior HNSCCc | 31 | 2.83 (1.60, 5.02) | 0.0004 | 3.10 (1.81, 5.30) | <0.0001 | 3.25 (1.63, 6.49) | 0.0008 | |
| Ca Stage | I/Cis (ref) | 108 | (ref) | (ref) | (ref) | |||
| II | 79 | 3.24 (1.32, 7.95) | 0.01 | 1.47 (0.58, 3.70) | 0.41 | 2.50 (0.60, 10.47) | 0.21 | |
| III | 97 | 4.14 (1.78, 9.61) | 0.0009 | 3.04 (1.41, 6.57) | 0.005 | 5.66 (1.63, 19.69) | 0.006 | |
| IV | 403 | 4.90 (2.28, 10.50) | <0.0001 | 3.98 (2.02, 7.84) | <0.0001 | 7.64 (2.41, 24.21) | 0.0005 | |
| Disease Site | oral cavity (ref) | 271 | (ref) | (ref) | (ref) | |||
| hypopharynx | 24 | 1.60 (0.80, 3.18) | 0.19 | 1.97 (1.01, 3.82) | 0.05 | 1.91 (0.81, 4.49) | 0.14 | |
| Larynx | 135 | 0.69 (0.45, 1.05) | 0.08 | 0.80 (0.52, 1.22) | 0.30 | 0.90 (0.54, 1.51) | 0.69 | |
| Oropharynx | 257 | 0.58 (0.40, 0.83) | 0.003 | 0.74 (0.51, 1.06) | 0.10 | 0.64 (0.40, 1.03) | 0.07 | |
| ACE comorbidity | none (ref) | 183 | (ref) | (ref) | (ref) | |||
| Mild | 322 | 2.29 (1.44, 3.63) | 0.0005 | 1.64 (1.07, 2.52) | 0.02 | 1.71 (1.00, 2.95) | 0.05 | |
| Moderate | 125 | 2.66 (1.58, 4.47) | 0.0002 | 1.96 (1.20, 3.21) | 0.007 | 1.93 (1.03, 3.63) | 0.04 | |
| Severe | 57 | 3.99 (2.20, 7.22) | <0.0001 | 2.61 (1.46, 4.65) | 0.001 | 3.13 (1.53, 6.40) | 0.002 | |
| BMI | 1 unit increase | 683 | 0.95 (0.92, 0.98) | 0.001 | 0.97 (0.94, 0.99) | 0.02 | 0.95 (0.92, 0.99) | 0.01 |
| HPV status | HPV- (ref) | 222 | (ref) | (ref) | (ref) | |||
| HPV+ | 140 | 0.39 (0.24, 0.64) | 0.0002 | 0.48 (0.30, 0.78) | 0.003 | 0.42 (0.23, 0.77) | 0.005 | |
| Unknown | 325 | 0.91 (0.65, 1.26) | 0.56 | 0.89 (0.64, 1.25) | 0.51 | 0.79 (0.52, 1.22) | 0.29 | |
| Alcohol Use | never (ref) | 67 | (ref) | (ref) | (ref) | |||
| Current | 450 | 0.75 (0.45, 1.26) | 0.27 | 0.66 (0.40, 1.07) | 0.09 | 0.72 (0.38, 1.38) | 0.33 | |
| Former | 170 | 1.11 (0.64, 1.92) | 0.72 | 0.88 (0.52, 1.51) | 0.65 | 1.01 (0.51, 2.02) | 0.97 | |
| Cigarette Use | never (ref) | 184 | (ref) | (ref) | (ref) | |||
| current | 282 | 2.07 (1.32, 3.25) | 0.002 | 1.31 (0.87, 1.98) | 0.20 | 1.87 (1.05, 3.31) | 0.03 | |
| former | 221 | 2.11 (1.33, 3.36) | 0.002 | 1.51 (0.99, 2.30) | 0.06 | 2.18 (1.22, 3.90) | 0.008 | |
| Pack Years | 10 unit increase | 687 | 1.10 (1.05, 1.15) | <0.0001 | 1.07 (1.02, 1.12) | 0.007 | 1.09 (1.03, 1.16) | 0.005 |
| Years Quit | never smoker | 184 | 0.48 (0.31, 0.76) | 0.002 | 0.77 (0.51, 1.16) | 0.21 | 0.53 (0.30, 0.94) | 0.03 |
| quit 10+ years | 158 | 0.93 (0.64, 1.37) | 0.73 | 1.09 (0.74, 1.62) | 0.67 | 1.13 (0.70, 1.82) | 0.61 | |
| quit <10 years | 59 | 1.26 (0.77, 2.06) | 0.36 | 1.33 (0.79, 2.23) | 0.29 | 1.20 (0.62, 2.32) | 0.60 | |
| current smoker | 279 | (ref) | (ref) | (ref) | ||||
| Remote Pack Years | 10 unit increase | 680 | 1.13 (1.07, 1.19) | <0.0001 | 1.09 (1.03, 1.16) | 0.004 | 1.12 (1.04, 1.20) | 0.002 |
| Recent Pack Years | 10 unit increase | 680 | 1.20 (0.98, 1.64) | 0.07 | 1.10 (0.89, 1.36) | 0.40 | 1.12 (0.86, 1.46) | 0.38 |
Abbreviations: OST=Overall Survival Time, RFT=Recurrence-Free Time, DST=Disease Specific Survival Time, No.=Number, HR=Hazard Ratio, CI=Confidence Interval, HNSCC=Head and Neck Squamous Cell Cancer, Cis=insitu, ACE=Adult Comorbidity Evaluation-27 summary score, BMI=Body Mass Index, HPV= Human papillomavirus
HPV Status
HPV status was determined from biopsy or surgical resection formalin-fixed, paraffin-embedded blocks for 362 subjects using previously reported and validated PCR methods (20). HPV status was unable to be determined for 325 subjects. Subjects with equivocal or missing HPV status were included in study and given an HPV status of “unknown”. Among subjects with adequate DNA or tissue specimens, 84% of oropharynx cancers were HPV(+), 12% of oral cavity, 13% of larynx, and 25% of hypopharynx.
Statistical Analysis
Chi-square testing and analysis of variance assessed differences in clinical and epidemiological characteristics with tobacco use. The Kaplan-Meier method was used to estimate rates and graphically visualize overall survival (OST), recurrence-free (RFT), and disease specific survival (DST) time. Time-to-event outcomes were defined from diagnosis to death any cause (OST), time to disease recurrence (RFT), or time to death from HNSCC malignancy. forFor patients with persistent disease, time to recurrence was defined as one day.
Single variable and multivariable Cox proportional hazard models were used to test associations between clinical and epidemiological variables with OST, RFT, and DST. Covariates included were age, gender, marital status, history of prior HNSCC or other cancer, stage, tumor site, comorbidity score, BMI, HPV status, alcohol, and tobacco use. Differences in outcome by planned treatment were explored, though ultimately eliminated from the final multivariable models because of the high collinearity observed between disease site and treatment plan. The multivariable analysis excluded 4 subjects missing BMI information, resulting in a total of 683 subjects for analysis. HPV status was defined in three groups; positive, negative and unknown. To address potential bias introduced by creating a category for missing HPV status, sensitivity analyses were performed using multiple imputation and inverse probability weighting. The results from this are shown in the online supplementary materials section. A post-hoc subset analysis was also performed analyzing HPV(+) and HPV(-) cancers separately.
Results
Univariate Clinical and Demographic Effects on Outcome
Clinical variables associated with worse OST, RFT, and DST included increasing patient age, single marital status, history of prior HNSCC (> 5 years previously), tumor stage, disease site, comorbidity score, lower BMI, and HPV negative status (Table 1). We noted a 3-5% decrease in relative risk per year for every one unit increase in BMI. One of the strongest predictors of all measures of relapse and survival was comorbidity score, with a clear trend by extent of comorbidities. The effect of age and comorbidities were strongest for OST, whereas the effects of stage and prior HNSCC were strongest for DST.
Univariate Smoking Effects on Outcome
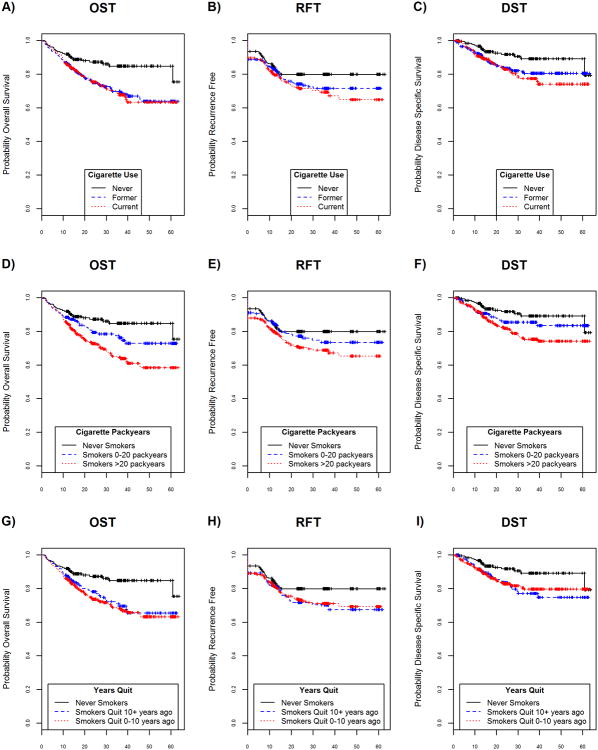
Cigarette use (never, current, former) was significantly associated with an increased risk of death from all causes and disease-specific death. Never smoking was associated with improved overall (Figure 1A), relapse-free (Figure 1B), and disease-specific survival (Figure 1C). Significantly worse OST, RFT, DST were associated with every 10 year increase in pack years of use (HR (95% CI): 1.10 (1.05, 1.15), 1.07 (1.02, 1.12), 1.09 (1.03, 1.16), respectively, Table 1). As expected, smokers of over 20 pack years had significantly worse outcomes compared to never smokers (Figures 1D – 1F). When former smokers were categorized by year since quitting, there was little separation between quitting within 10 years or quitting more than 10 years before diagnosis in OST, RFT, or DST (Figures 1G – 1I), although all outcomes were consistently worse in smokers compared to nonsmokers.
Figure 1.
Survival benefits according to smoking status (A-C), pack years (D-F), and years since quit (G-I). Panels A, B, C: unadjusted OST, RFT, and DST, respectively, by cigarette use (never, former, current). Never smokers showed significantly better survival outcomes than smokers in univariable Cox models. Interestingly, there were no significant survival differences between former and current smokers. Panels D, E, F: unadjusted OST, RFT, and DST, respectively, by pack years (never smoker, smokers 0-20 pack years, and smokers > 20 pack years). Never smokers had significantly better survival outcomes than smokers (OST p-value=0.01 in univariable Cox model). There were apparent differences in each category of survival events with a 7-10% increase risk of outcome for every 10 pack year increase in cigarette use (Cox Proportional models for OST, RFT, DST; p=0.01, 0.007, 0.005, respectively). Panels G, H, I: unadjusted OST, RFT, and DST, respectively, by years since quitting (never smoker, smoker quit 10+ years ago, smoker quit 0-10 years ago). When grouped by year since quitting, there were no significant differences comparing remote and quitters.
Recent Tobacco Use Compared to Remote Use
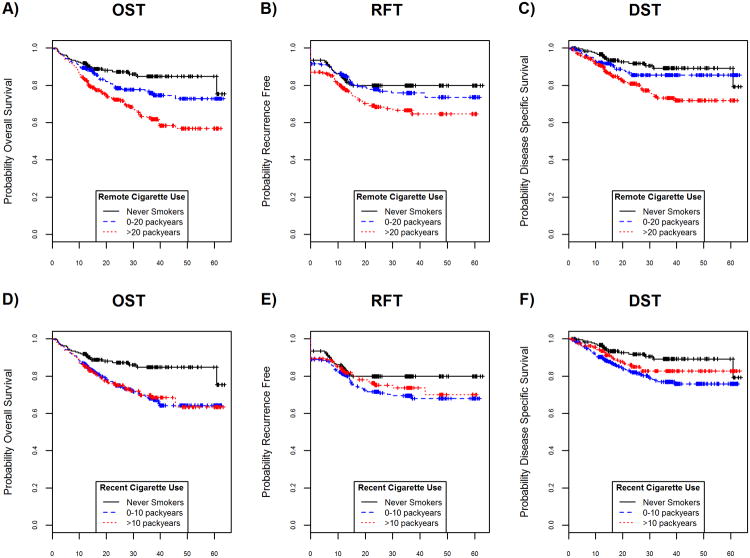
To better understand the influence of cigarette smoking on outcome measures, smoking history was separated into recent and remote use, derived from the patient's age at initiation, years of duration, and intensity. Graphically, we observed that the amount of remote cigarette use among smokers (accumulated up to 10 years prior to diagnosis) was associated with worse survival and recurrence free times (Figure 2A-2C). Although current cigarette use appeared associated with worse survival and recurrence free times when compared to never smokers, the amount of recent cigarette use (accumulated within 10 years of diagnosis) was not (Figure 2D-2F). Among smokers, differences in outcome (HR (95% CI) per 10 pack years) by intensity of remote use were significant for worse OST, RFT and for DST survival (1.11 (1.04, 1.18), 1.09 (1.02, 1.17), and 1.11 (1.02, 1.20), respectively). Among recent users, no evidence of meaningful differences in outcome by intensity of recent use were found as evidenced in graphical representation and the fact that the hazard ratios for each outcome were wide and contained 1.00 (HR (95% CI) per 10 pack years for OST, RFT, DST: 0.94 (0.68, 1.30), 1.05 (0.75, 1.46), 0.98 (0.64, 1.49), respectively).
Figure 2.
Association of remote or recent pack years with survival. A, B, C, unadjusted OST, RFT, DST, respectively, for pack years comparing never smokers to 0-20 pack years and >20 pack years for remote use smokers. For remote use, significant differences in OST favored light pack years compared to heavy pack years while RFT and DST were more similar among light and never smokers. D, E, F, unadjusted OST, RFT, DST, respectively, for pack years comparing never smokers to 0-20 pack years and >20 pack years for recent use smokers. For recent use, moderate
Multivariable Clinical, Demographic and Smoking Effects on Outcome
In multivariable analysis, history of prior HNSCC, tumor stage, comorbidity score and HPV status remained significant prognostic factors for all outcome measures while smoking history was no longer statistically significant in the risk-adjusted models (Table 2). Age and BMI remained statistically significant for overall survival. Interestingly, pack years of use among cigarette smokers was not significantly associated with the outcome measures after adjustment for confounding clinical factors. Sensitivity analyses did not suggest any changes to the conclusions reported after applying alternative strategies for missing HPV data (Supplementary materials, Table 1).
Table 2. Multivariable Cox proportional hazards model for survival outcomes.
| OST | RFT | DST | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. of Patients | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | 10 years unit | 683 | 1.30 (1.12, 1.51) | 0.0005 | 1.16 (1.00, 1.35) | 0.05 | 1.12 (0.93, 1.36) | 0.24 |
| Gender | Male | 500 | (ref) | (ref) | (ref) | |||
| Female | 183 | 1.07 (0.74, 1.54) | 0.73 | 0.95 (0.65, 1.39) | 0.79 | 1.08 (0.68, 1.73) | 0.74 | |
| Married | Married | 434 | 0.77 (0.55, 1.09) | 0.14 | 0.78 (0.55, 1.10) | 0.15 | 0.90 (0.58, 1.39) | 0.63 |
| Not Married | 249 | (ref) | (ref) | (ref) | ||||
| Prior Cancer | 108 | 0.83 (0.53, 1.30) | 0.41 | 0.73 (0.46, 1.16) | 0.18 | 0.67 (0.37, 1.23) | 0.20 | |
| Prior HNSCC | 31 | 2.31 (1.25, 4.26) | 0.008 | 2.73 (1.54, 4.84) | 0.0006 | 2.77 (1.31, 5.87) | 0.008 | |
| Ca Stage | I/Cis | 107 | (ref) | (ref) | (ref) | |||
| II | 78 | 3.24 (1.31, 8.02) | 0.01 | 1.40 (0.55, 3.54) | 0.48 | 2.37 (0.56, 9.97) | 0.24 | |
| III | 97 | 5.02 (2.14, 11.80) | 0.0002 | 3.34 (1.53, 7.31) | 0.003 | 6.58 (1.87, 23.19) | 0.003 | |
| IV | 401 | 7.20 (3.29, 15.73) | <0.0001 | 5.03 (2.50, 10.11) | <0.0001 | 10.54 (3.26, 34.05) | <0.0001 | |
| Disease Site | Oral Cavity | 269 | (ref) | (ref) | (ref) | |||
| Hypopharynx | 23 | 0.76 (0.35, 1.66) | 0.50 | 1.11 (0.53, 2.33) | 0.79 | 0.92 (0.37, 2.31) | 0.86 | |
| Larynx | 135 | 0.52 (0.33, 0.82) | 0.005 | 0.66 (0.42, 1.04) | 0.08 | 0.62 (0.35, 1.08) | 0.09 | |
| Oropharynx | 256 | 0.72 (0.47, 1.11) | 0.14 | 0.81 (0.52, 1.25) | 0.33 | 0.72 (0.41, 1.26) | 0.25 | |
| ACE comorbidity | none | 182 | (ref) | (ref) | (ref) | |||
| mild | 320 | 1.63 (1.00, 2.67) | 0.05 | 1.27 (0.81, 2.01) | 0.30 | 1.35 (0.75, 2.41) | 0.31 | |
| moderate | 125 | 2.66 (1.58, 4.47) | 0.003 | 1.69 (1.00, 2.87) | 0.05 | 1.81 (0.92, 3.54) | 0.08 | |
| severe | 56 | 2.69 (1.38, 5.25) | 0.004 | 1.95 (1.01, 3.78) | 0.05 | 2.72 (1.21, 6.12) | 0.02 | |
| BMI | 1 unit increase | 683 | 0.96 (0.93, 0.99) | 0.01 | 0.98 (0.95, 1.00) | 0.09 | 0.97 (0.93, 1.00) | 0.06 |
| HPV status | HPV- | 221 | (ref) | (ref) | (ref) | |||
| HPV+ | 140 | 0.41 (0.22, 0.73) | 0.003 | 0.48 (0.27, 0.84) | 0.01 | 0.37 (0.17, 0.77) | 0.008 | |
| Unknown | 322 | 0.83 (0.58, 1.20) | 0.32 | 0.79 (0.55, 1.15) | 0.22 | 0.73 (0.46, 1.17) | 0.20 | |
| Alcohol Use | never | 66 | (ref) | (ref) | (ref) | |||
| current | 448 | 1.21 (0.68, 2.15) | 0.51 | 0.86 (0.50, 1.49) | 0.60 | 0.96 (0.48, 1.92) | 0.90 | |
| former | 169 | 1.16 (0.64, 2.12) | 0.62 | 0.90 (0.51, 1.61) | 0.72 | 0.99 (0.47, 2.07) | 0.98 | |
| Cigarette Use | never | 183 | 0.76 (0.45, 1.28) | 0.30 | 0.95 (0.59, 1.53) | 0.83 | 0.81 (0.42, 1.54) | 0.81 |
| Pack Years | 10 unit increase | 683 | 1.05 (0.99, 1.11) | 0.13 | 1.05 (0.98, 1.12) | 0.14 | 1.04 (0.96, 1.13) | 0.33 |
Abbreviations: No.=Number, OST=Overall Survival Time, RFT=Recurrence-Free Time, DST=Disease Specific Survival Time, HR=Hazard Ratio, CI=Confidence Interval, HNSCC=Head and Neck Squamous Cell Cancer, Cis=insitu, ACE=Adult Comorbidity Evaluation-27 summary score, BMI=Body Mass Index, HPV= Human papillomavirus
In multivariable analysis, remote (> than 10 years prior to diagnosis) cigarette use was not a significant prognostic variable after adjustment for other prognostic factors (HR (95% CI) per 10 pack years: 1.03 (0.95, 1.12), 1.05 (0.97, 1.15), 1.08 (0.97,1.20), respectively for OST, RFT, DST).
Tobacco Use, HPV status and Outcome
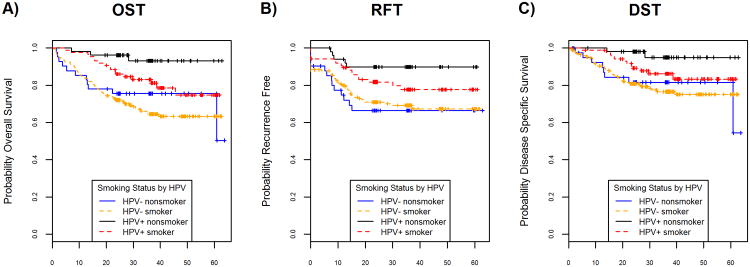
In HPV(+) patients, for overall (Figure 3A), recurrence-free (Figure 3B), and disease-specific survival (Figure 3C), nonsmokers showed marginal improvements in survival compared to smokers. Outcomes were better for HPV(+) patients compared to all HPV(-) patients, regardless of smoking history. In univariate analysis, HPV(+) nonsmokers had better outcomes compared to HPV(+) smokers though only OST reached statistical significance (HR (95% CI): 0.22 (0.05, 0.94), 0.36 (0.12, 1.08), 0.16 (0.02, 1.20) for OST, RFT, DST respectively). In multivariable analysis, HPV(+) nonsmokers compared to HPV(+) smokers had improvements in outcome that did not achieve statistical significance (HR (95% CI): 0.22 (0.05, 1.04), 0.34 (0.11, 1.06), 0.15 (0.02, 1.31) for OST, RFT, DST respectively), after adjustment for confounding factors.
Figure 3.
Survival benefits according to HPV status by smoking history. A, B, C: unadjusted OST, RFT, DST, respectively, for HPV status by smoking history (HPV negative nonsmoker (n=44), HPV negative smoker (n=178), HPV positive nonsmoker (n=52), HPV positive smoker (n=88)). Differences in outcomes for HPV positive patients were evident in univariate analysis according to smoking history; however, outcomes for HPV negative patients were similar comparing nonsmokers and ever smokers.
In HPV(-) patients, there was little survival difference between nonsmokers and smokers for overall (Figure 3A), recurrence-free (Figure 3B), and disease-specific survival (Figure 3C). In univariate analysis, HPV(-) nonsmokers showed marginal improvements in overall survival compared to HPV(-) smokers (HR (95% CI): 0.88 (0.48, 1.59)). For recurrence-free and disease-specific survival in HPV(-) patients, never smoking status showed no significant improvements in survivorship compared to patients with a history of ever smoking (HR (95% CI): 1.21 (0.68, 2.14), 0.97 (0.47, 2.00)) for RFT and DST respectively. In multivariable analysis, among patients with HPV(-) disease, OST, RFT, and DST were not significantly associated with never or ever smokers (HR (95% CI): 1.45 (0.72, 2.94), 1.62 (0.82, 3.19), 1.52 (0.65, 3.55)) respectively, after adjustment for confounding factors.
Association of Tobacco Use and Clinical Variables
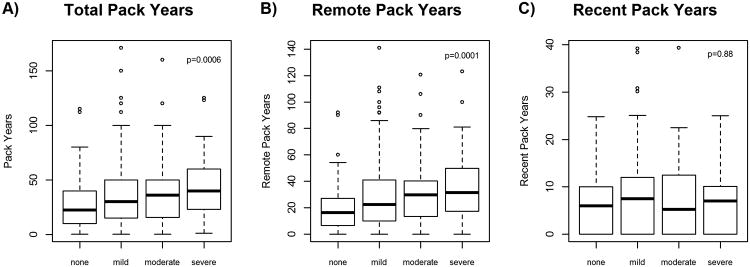
Smoking status (never, current, former) was strongly associated with comorbidity score, age, tumor site, BMI, and HPV status, all of which were significantly associated with outcomes (Table 3). Among subjects with a smoking history, cigarette pack years, both recent and remote were significantly associated with age and disease site (Table 4). Increased comorbidity score was significantly associated with pack years among smokers (Table 4; p<.001) with a clear monotonic trend (Figure 4A). When taking into account duration and intensity of smoking, remote cigarette use (Figure 4B; p<.0001) was more strongly associated with severity of comorbidity score than recent cigarette use (Figure 4C; p=0.88).
Table 3. Association of Smoking Status with clinical and epidemiologic characteristics.
| Variable | Subgroup | No. of Patients | % of Patients Never Smokers | % of Patients Current Smokers | % of Patients Former Smokers | Chi-square p-value |
|---|---|---|---|---|---|---|
| Age Category | <60 | 345 | 30 | 50 | 21 | <0.001 |
| 60-75 | 246 | 20 | 39 | 41 | ||
| >75 | 96 | 35 | 14 | 51 | ||
| Gender | Male | 504 | 26 | 41 | 33 | 0.55 |
| Female | 183 | 28 | 43 | 29 | ||
| Married | Married | 251 | 18 | 56 | 26 | <0.001 |
| Not Married | 436 | 32 | 33 | 36 | ||
| Prior Cancer | No | 579 | 27 | 42 | 31 | 0.48 |
| Yes | 108 | 26 | 37 | 37 | ||
| Prior HNSCC | No | 656 | 27 | 42 | 31 | 0.07 |
| Yes | 31 | 29 | 23 | 48 | ||
| Stage | I/Cis | 108 | 28 | 42 | 31 | 0.37 |
| II | 79 | 28 | 41 | 32 | ||
| III | 97 | 16 | 48 | 35 | ||
| IV | 403 | 29 | 39 | 32 | ||
| Disease Site | Larynx | 135 | 4 | 66 | 30 | <0.001 |
| Oral Cavity | 271 | 29 | 41 | 30 | ||
| Oropharynx | 257 | 37 | 27 | 35 | ||
| Hypopharynx | 24 | 17 | 46 | 38 | ||
| ACE comorbidity | none | 183 | 40 | 36 | 24 | <0.001 |
| mild | 322 | 23 | 43 | 34 | ||
| moderate | 125 | 19 | 44 | 37 | ||
| severe | 57 | 19 | 42 | 39 | ||
| BMI category | underweight (<18.5) | 33 | 3 | 76 | 21 | <0.001 |
| normal weight (18.5-24.9) | 233 | 19 | 54 | 27 | ||
| overweight (25-29.9) | 238 | 30 | 30 | 40 | ||
| obese (30+) | 179 | 37 | 33 | 30 | ||
| HPV | HPV- | 222 | 20 | 50 | 30 | 0.001 |
| HPV+ | 140 | 37 | 31 | 31 | ||
| Unknown | 325 | 27 | 39 | 34 | ||
| Alcohol Use | never | 67 | 48 | 31 | 21 | <0.001 |
| current | 450 | 29 | 42 | 29 | ||
| former (quit >12 months) | 170 | 13 | 43 | 44 |
Abbreviations: No.=Number. %=proportion of patients within subgroup (row). HNSCC=Head and Neck Squamous Cell Cancer, Cis=insitu, ACE=Adult Comorbidity Evaluation-27 summary score, BMI=Body Mass Index, HPV= Human papillomavirus
Table 4.
Associations of Clinical and Epidemiologic Characteristics with Cumulative Pack Years Remote and Recent among Current and Former Smokers.
| Variable | Subgroup | No. of patients | Mean (std) Total Pack years | p-value | Mean (std) Remote Pack Years | p-value | Mean (std) Recent Pack Years | p-value |
|---|---|---|---|---|---|---|---|---|
| Age Category | <60 | 243 | 30.2 (23.8) | <0.001 | 21.5 (18.5) | <0.001 | 9.1 (7.4) | <0.001 |
| 60-75 | 198 | 40.0 (27.6) | 33.4 (23.4) | 6.6 (6.8) | ||||
| >75 | 62 | 36.6 (34.4) | 34.0 (31.1) | 3.2 (5.5) | ||||
| Gender | Male | 372 | 36.1 (26.5) | 0.09 | 28.8 (22.6) | 0.10 | 7.6 (7.4) | 0.23 |
| Female | 131 | 31.4 (28.8) | 24.9 (24.2) | 6.7 (6.8) | ||||
| Married | Married | 206 | 38.1 (27.1) | 0.03 | 29.6 (23.4) | 0.14 | 8.9 (7.1) | <0.001 |
| Not Married | 297 | 32.6 (27.0) | 26.5 (22.9) | 6.3 (7.1) | ||||
| Prior Ca | No | 423 | 34.1 (27.2) | 0.18 | 26.8 (22.7) | 0.03 | 7.6 (7.4) | 0.09 |
| Yes | 80 | 38.6 (27.1) | 32.9 (24.8) | 6.1 (6.3) | ||||
| Prior HNCa | No | 481 | 34.7 (27.3) | 0.48 | 27.4 (23.0) | 0.12 | 7.5 (7.3) | 0.01 |
| Yes | 22 | 38.9 (25.2) | 35.3 (24.6) | 3.6 (4.7) | ||||
| Stage | I/Cis | 78 | 35.9 (29.2) | 0.47 | 28.0 (23.9) | 0.47 | 7.9 (7.5) | 0.55 |
| II | 57 | 37.6 (25.6) | 31.2 (21.1) | 7.7 (6.5) | ||||
| III | 81 | 37.7 (26.9) | 29.6 (23.0) | 8.1 (7.9) | ||||
| IV | 287 | 33.3 (27.0) | 26.5 (23.3) | 7.0 (7.1) | ||||
| Disease Site | Larynx | 129 | 42.3 (25.0) | <0.001 | 32.9 (20.9) | 0.002 | 9.7 (7.1) | <0.001 |
| Oral Cavity | 193 | 36.1 (30.1) | 28.6 (25.4) | 7.7 (7.2) | ||||
| Oropharynx | 161 | 27.5 (23.1) | 22.6 (20.6) | 5.1 (6.8) | ||||
| Hypopharynx | 20 | 34.9 (27.4) | 27.6 (24.7) | 6.8 (6.8) | ||||
| Comorbidities | none | 109 | 26.3 (22.0) | <0.001 | 19.9 (17.4) | <0.001 | 7.0 (6.7) | 0.88 |
| mild | 247 | 35.9 (28.1) | 28.5 (23.8) | 7.6 (7.4) | ||||
| moderate | 101 | 37.3 (27.4) | 30.0 (22.9) | 7.3 (7.5) | ||||
| severe | 46 | 43.9 (28.3) | 37.0 (26.7) | 7.0 (6.8) | ||||
| BMI category | underweight (<18.5) | 32 | 40.5 (31.1) | 0.16 | 31.8 (25.7) | 0.34 | 8.7 (6.9) | 0.01 |
| normal weight (18.5-24.9) | 188 | 34.0 (25.0) | 26.5 (20.7) | 7.9 (7.0) | ||||
| overweight (25-29.9) | 167 | 32.2 (25.3) | 26.6 (22.0) | 5.9 (6.5) | ||||
| obese (30+) | 113 | 38.5 (31.8) | 30.4 (27.5) | 8.2 (8.3) | ||||
| HPV | HPV- | 175 | 38.2 (25.4) | 0.03 | 30.2 (21.8) | 0.03 | 8.4 (6.8) | 0.07 |
| HPV+ | 86 | 28.7 (28.5) | 22.1 (23.9) | 6.7 (7.9) | ||||
| Unknown | 235 | 34.6 (27.7) | 28.0 (23.5) | 6.8 (7.2) | ||||
| Alcohol Use | never | 35 | 37.6 (33.0) | 0.22 | 29.3 (27.6) | 0.07 | 8.3 (9.8) | 0.44 |
| current | 320 | 33.3 (25.6) | 26.0 (21.2) | 7.5 (7.1) | ||||
| former (quit >12 months) | 148 | 37.7 (28.8) | 31.3 (25.6) | 6.8 (6.8) | ||||
| Cigarette Use | never | |||||||
| current | 282 | 40.4 (26.9) | <0.001 | 29.0 (22.1) | 0.19 | 11.6 (6.3) | <0.001 | |
| former (quit >12 months) | 221 | 27.7 (25.9) | 26.2 (24.3) | 1.9 (4.0) |
Abbreviations: No.=Number, std=standard deviation, HNSCC=Head and Neck Squamous Cell Cancer, Cis=insitu, ACE=Adult Comorbidity Evaluation-27 summary score, BMI=Body Mass Index, HPV= Human papillomavirus
Figure 4.
Pack years and comorbidity score boxplots among ever smokers. A, Overall pack years differ by comorbidity score (none, mild, moderate, severe) with an ANOVA test p-value=0.0006. B, Remote use pack years also differ by comorbidity (none, mild, moderate, severe) with an ANOVA test p-alue=0.0001. C, Recent use pack years did not differ significantly by comorbidity score (none, mild, moderate, severe) with an ANOVA test p-value=0.88. Comorbidities were associated with pack years in a monotonic trend when accounting for the intensity and duration of smoking habit.
Discussion
In this large, prospective cohort of HNSCC patients, we have confirmed the simple association of cigarette use with overall survival (21, 22). When considering only smoking status, never smokers were consistently identified as a prognostically favorable patient group for OST, RFT, and DST survival. Similarly, pack years showed a modest increased hazard of death for every 10 pack years of use in univariate analysis. However, in multivariable analysis, we found that both smoking status and pack years were not significant after adjustment for other clinical factors including medical comorbidities, history of prior HNSCC, and BMI. When former smokers were grouped by year since quitting, there was not significant separation in OST, RFT, or DST curves. This study demonstrates that a more refined consideration of cigarette use based upon timing and intensity may be useful when considering HNSCC outcomes. In particular, cigarette use at earlier times may be more clinically relevant than is more recent cigarette use.
Our data also demonstrate that comorbidities are highly related to pack years, particularly with remote use, in bivariate analysis. The univariate effect of remote cigarette use diminishes in multivariate analyses, and comorbidities have a strong effect on outcome, particularly on overall survival. Since tobacco use is a likely contributing factor to these comorbidities, it is possible that some of the negative impact of smoking on outcomes in HNSCC patients is mediated through comorbidities. These findings could have potential implications on the use of smoking history in risk stratification for patients with HNSCC (5, 23-25). It is likely that a more refined approach considering temporal smoking habits along with comorbidities would afford a more predictive model.
Remote cigarette use was significantly associated with outcomes, history of prior HNSCC, and comorbidities whereas recent use was not. Greater pack years were associated in an increasing monotonic trend with more severe comorbidity. We hypothesize that patients who initiated intense smoking for a long duration spanning their lifetime may accumulate more somatic mutations (7, 26-28) than patients who smoked less or more recently, and the implications from a carcinogenesis standpoint merit further study. Lifetime smoking had a significant impact on OST, and this could be phenotypically associated with increased smoking related comorbidities such as cardiovascular and pulmonary disease, contributing to a poorer survival chance after cancer treatment. Comorbidities at diagnosis have been correlated with survival (29) and have been externally validated as an independent predictor of survival in a prognostic HNSCC model (30). In another study, a model of comorbidities, clinical, and pathological information predicted survival better than pathological TNM staging (31). Management of comorbidities at diagnosis may be a key factor for improving survival rates in HNSCC patients. Survivors who continue smoking compared to patients who never smoked are at higher risk of a recurrence or second primary (32). Perhaps continued tobacco exposure exacerbates medical comorbidities in a manner that replicates the biology and medical condition of remote smokers. Further understanding of the biological and clinical impact of smoking during treatment is required (33).
Our data did not demonstrate alcohol use as a prognostic factor, even in univariate analyses, in contrast to other studies which found alcohol use as a strong prognostic factor for HNSCC patients (34, 35). In multivariable and bivariate analyses, increased BMI was associated with both longer survival and less tobacco use. Low BMI, as a measure of nutritional status, was prospectively associated with increased risk of HNSCC mortality among smokers (36), increased risk of death during chemoradiation (37), and a negative prognostic factor post-treatment (38). Other studies found obesity to be adversely associated with disease-specific survival in patients with tongue cancer (39). Smoking and BMI are likely related through the various metabolic effects of smoking on cell physiology, modification of dietary habits related to smoking (40), hormonal effects mediated by nicotine (41), and via other confounders (42).
Subjects with HPV(+) disease, had better OST than HPV(-) subjects, regardless of smoking history. In HPV(+) patients, smoking history was only marginally significant and was no longer significantly associated with survival outcomes after adjusting for other prognostic factors. In multivariable analysis, HPV(-) nonsmokers and ever smokers did not have significantly different OST, RFT, DST. It is an important and unique finding that HPV(-) nonsmokers did not fare noticeably better. For tumor recurrence, other factors including stage, comorbidity score, and history of prior HNSCC had stronger and more consistent associations. Although previous studies that reported a strong association between survival, extent of tobacco use and HPV status (5, 23, 43) these studies relied on highly selected clinical trial data, which have strong selection bias for patients with low comorbidity and may have incomplete smoking histories. In contrast, our detailed smoking history was obtained through self-reported surveys in an unselected prospectively collected cohort. Our contrasting results could also be partly due to the contemporary epidemiologic shift of HPV related oropharyngeal cancer with increasing numbers of non-smokers over the last two decades (44-46).
This study analyzed the associations of cigarette use with oncologic and survival outcomes in head and neck cancer in a large, carefully studied prospective cohort. Smoking status was consistently correlated with worse oncologic and survival outcomes across our univariate analyses. In multivariable analyses we identified high comorbidity score, history of prior HNSCC, negative HPV status, increasing cancer stage and low BMI as highly significant characteristics that negatively affect OST, RFT and DST. However, in our patient population, smoking history was not an independent prognostic factor after adjusting for these other significant covariates. Our findings suggest that continued efforts at identifying and understanding the interactions of smoking and other health behaviors with other patient characteristics will be important in developing new patient risk profiles for use in personalized treatment strategies.
Supplementary Material
Supplemental Table 1. Hazard Ratio (95% Confidence Interval) for selected covariates from multivariable Cox model analyses with different strategies for missing HPV status.
Acknowledgments
Funding: This work was supported by the University of Michigan Head and Neck Specialized Program of Research Excellence through funding from the National Cancer Institute at the National Institutes of Health (grant number NIH/NCI P50CA097248).
Footnotes
Financial disclosures: None.
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, Colevas AD, et al. Head and neck cancers. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9(6):596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 3.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120(10):1507–13. doi: 10.1002/cncr.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur AE, Peterson KE, Rozek LS, Taylor JM, Light E, Chepeha DB, et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. The American journal of clinical nutrition. 2013;97(2):360–8. doi: 10.3945/ajcn.112.044859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(17):2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General's report: “The health consequences of smoking--50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–6. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobus SL, Warren GW. The biologic effects of cigarette smoke on cancer cells. Cancer. 2014;120(23):3617–26. doi: 10.1002/cncr.28904. [DOI] [PubMed] [Google Scholar]
- 8.Humans IWGotEoCRt. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2012;100(Pt E):1–538. [PMC free article] [PubMed] [Google Scholar]
- 9.Sitas F, Weber MF, Egger S, Yap S, Chiew M, O'Connell D. Smoking cessation after cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(32):3593–5. doi: 10.1200/JCO.2014.55.9666. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DC. Invited commentary: is it time to retire the “pack-years” variable? Maybe not! American journal of epidemiology. 2014;179(3):299–302. doi: 10.1093/aje/kwt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. American journal of epidemiology. 2002;156(9):813–23. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 12.Vlaanderen J, Portengen L, Schuz J, Olsson A, Pesch B, Kendzia B, et al. Effect modification of the association of cumulative exposure and cancer risk by intensity of exposure and time since exposure cessation: a flexible method applied to cigarette smoking and lung cancer in the SYNERGY Study. American journal of epidemiology. 2014;179(3):290–8. doi: 10.1093/aje/kwt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos A, Guida F, Cenee S, Cyr D, Schmaus A, Radoi L, et al. Cigarette smoking and lung cancer in women: results of the French ICARE case-control study. Lung cancer. 2011;74(3):369–77. doi: 10.1016/j.lungcan.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. Journal of epidemiology and community health. 1978;32(4):303–13. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer research. 2003;63(19):6556–62. [PubMed] [Google Scholar]
- 16.Knoke JD, Shanks TG, Vaughn JW, Thun MJ, Burns DM. Lung cancer mortality is related to age in addition to duration and intensity of cigarette smoking: an analysis of CPS-I data. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13(6):949–57. [PubMed] [Google Scholar]
- 17.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. Jama. 2004;291(20):2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 18.Wyss A, Hashibe M, Chuang SC, Lee YC, Zhang ZF, Yu GP, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. American journal of epidemiology. 2013;178(5):679–90. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: A report of University of Michigan's nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) J Biomed Inform. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walline HM, Komarck C, McHugh JB, Byrd SA, Spector ME, Hauff SJ, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: comparison of multiple methods. JAMA otolaryngology-- head & neck surgery. 2013;139(12):1320–7. doi: 10.1001/jamaoto.2013.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp L, McDevitt J, Carsin AE, Brown C, Comber H. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(11):2579–90. doi: 10.1158/1055-9965.EPI-14-0311. [DOI] [PubMed] [Google Scholar]
- 22.Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(12):1969–75. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillison ML, H J, Westra W, Chung C, Jordan R, Rosenthal D, Nguyen-Tan P, Spanos WJ, Redmond KP, Ang K, Radiation Therapy Oncology Group, editors. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129. Journal of Clinical Oncology.2009 ASCO Annual Meeting Proceedings (Post-Meeting); 2009 May 20; [Google Scholar]
- 25.Huang SH, Xu W, Waldron J, Siu L, Shen X, Tong L, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM Stage and Prognostic Groups for Human Papillomavirus-Related Oropharyngeal Carcinomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(8):836–45. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 26.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumanski JP, Rasi C, Lonn M, Davies H, Ingelsson M, Giedraitis V, et al. Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science. 2015;347(6217):81–3. doi: 10.1126/science.1262092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenker NS, Polidoro S, van Veldhoven K, Sacerdote C, Ricceri F, Birrell MA, et al. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Human molecular genetics. 2013;22(5):843–51. doi: 10.1093/hmg/dds488. [DOI] [PubMed] [Google Scholar]
- 29.Yung KC, Piccirillo JF. The incidence and impact of comorbidity diagnosed after the onset of head and neck cancer. Archives of otolaryngology--head & neck surgery. 2008;134(10):1045–9. doi: 10.1001/archotol.134.10.1045. [DOI] [PubMed] [Google Scholar]
- 30.Datema FR, Ferrier MB, Vergouwe Y, Moya A, Molenaar J, Piccirillo JF, et al. Update and external validation of a head and neck cancer prognostic model. Head & neck. 2013;35(9):1232–7. doi: 10.1002/hed.23117. [DOI] [PubMed] [Google Scholar]
- 31.Okuyemi OT, Piccirillo JF, Spitznagel E. TNM staging compared with a new clinicopathological model in predicting oral tongue squamous cell carcinoma survival. Head & neck. 2014;36(10):1481–9. doi: 10.1002/hed.23486. [DOI] [PubMed] [Google Scholar]
- 32.Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Beane Freeman L, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(35):3989–95. doi: 10.1200/JCO.2014.56.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassig AA, Yueh B, Joseph AM. The effect of smoking on perioperative complications in head and neck oncologic surgery. The Laryngoscope. 2012;122(8):1800–8. doi: 10.1002/lary.23308. [DOI] [PubMed] [Google Scholar]
- 34.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(2):541–50. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do KA, Johnson MM, Doherty DA, Lee JJ, Wu XF, Dong Q, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer causes & control : CCC. 2003;14(2):131–8. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 36.Gaudet MM, Patel AV, Sun J, Hildebrand JS, McCullough ML, Chen AY, et al. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(3):497–503. doi: 10.1158/1055-9965.EPI-11-0935. [DOI] [PubMed] [Google Scholar]
- 37.Chang PH, Yeh KY, Huang JS, Lai CH, Wu TH, Lan YJ, et al. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2013;270(6):1909–15. doi: 10.1007/s00405-012-2290-2. [DOI] [PubMed] [Google Scholar]
- 38.Chang PH, Wang CH, Huang JS, Lai CH, Wu TH, Lan YJ, et al. Low body mass index at 3 months following adjuvant chemoradiation affects survival of postoperative locally advanced oral cavity cancer patients. The Laryngoscope. 2012;122(10):2193–8. doi: 10.1002/lary.23450. [DOI] [PubMed] [Google Scholar]
- 39.Iyengar NM, Kochhar A, Morris PG, Morris LG, Zhou XK, Ghossein RA, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120(7):983–91. doi: 10.1002/cncr.28532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obesity research. 2005;13(8):1466–75. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 41.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. International journal of obesity. 2005;29(2):236–43. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 42.El-Zein M, Parent ME, Nicolau B, Koushik A, Siemiatycki J, Rousseau MC. Body mass index, lifetime smoking intensity and lung cancer risk. International journal of cancer Journal international du cancer. 2013;133(7):1721–31. doi: 10.1002/ijc.28185. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(4):1226–35. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(36):4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and - unrelated oral squamous cell carcinomas in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(4):612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 46.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Hazard Ratio (95% Confidence Interval) for selected covariates from multivariable Cox model analyses with different strategies for missing HPV status.