Abstract
Idiopathic nephrotic syndrome (NS) is the most common glomerular disorder of childhood. Response to initial treatment with corticosteroids is an indicator of prognosis, as resistant patients often present more progressive disease. In this cross-sectional pilot study, we set out to discover a panel of noninvasive biomarkers that could distinguish steroid-resistant nephrotic syndrome (SRNS) from steroid-sensitive nephrotic syndrome (SSNS). Information gleaned from such a panel could yield more individualized treatment plans and prevent unnecessary steroid exposure in patients unlikely to respond. Urine was collected from 50 pediatric patients diagnosed with idiopathic NS at Cincinnati Children’s Hospital Medical Center. Isobaric tags for relative and absolute quantitation (iTRAQ) was used to discover 13 proteins that were differentially expressed in SSNS vs SRNS in a small 5 × 5 discovery cohort. Suitable assays were found for 9 of the 13 markers identified by iTRAQ and were used in a 25 SRNS × 25 SSNS validation cohort. Vitamin D–binding protein (VDBP), alpha-1 acid glycoprotein 1 (AGP1), alpha-1 acid glycoprotein 2 (AGP2), alpha-1-B glycoprotein (A1BG), fetuin-A, prealbumin, thyroxine-binding globulin and hemopexin, and alpha-2 macroglobulin were measured and combined with urine neutrophil gelatinase–associated lipocalin (NGAL), which had been previously shown to distinguish patients with SRNS. Urinary VDBP, prealbumin, NGAL, fetuin-A, and AGP2 were found to be significantly elevated in SRNS using univariate analysis, with area under the receiver operating characteristic curves (AUCs) ranging from 0.65 to 0.81. Multivariate analysis revealed a panel of all 10 markers that yielded an AUC of 0.92 for identification of SRNS. A subset of 5 markers (including VDBP, NGAL, fetuin-A, prealbumin, and AGP2) showed significant associations with SRNS and yielded an AUC of 0.85.
Keywords: Nephrotic syndrome, steroid resistance, focal segmental glomerulosclerosis, minimal change disease, biomarkers
Introduction
Idiopathic nephrotic syndrome (NS) is the leading glomerular disease in pediatric patients, occurring in 16 per 100 000 children.1 Initial presentation of various NS subtypes is similar and includes the presence of proteinuria, edema, hypoalbuminemia, and hypercholesterolemia. Despite initial similarities, NS subtypes have markedly different disease courses and outcomes. Invasive biopsy remains the only method for positive diagnosis, and the 2 most frequent histopathological findings are focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD). Prognosis depends on underlying pathophysiology and response to steroid treatment. Approximately 95% of children with MCD achieve remission following an 8-week course of prednisone (steroid-sensitive nephrotic syndrome [SSNS]) compared with 80% of patients with FSGS who fail to reach remission in response to steroids (steroid-resistant nephrotic syndrome [SRNS]).2 Focal segmental glomerulosclerosis is the most common acquired cause of end-stage renal disease in children and leads to further complications with roughly 30% recurrence posttransplant.3,4
Although kidney biopsies are effective for diagnosis in the adult population, they are not typically performed at presentation in children because therapeutic response better predicts long-term outcomes than histology in the pediatric population, and FSGS is often underdiagnosed due to its focal nature in combination with a smaller core size.2,5 As a result, response to treatment is used as a one-size-fits-most diagnostic tool. The problem with this approach is that a population of children who are unlikely to respond to steroids (FSGS patients) are unnecessarily exposed to steroids and their potential side effects6 and, at the same time, postponing alternative treatments that may have a better chance of success. What are needed are noninvasive diagnostic tests that can predict which patients are more likely to respond to steroids to better inform caregivers to make the appropriate clinical decisions. In this pilot study, we enrolled children diagnosed with idiopathic NS and compared the urine proteome of patients with SSNS to those with SRNS. We employed isobaric tags for relative and absolute quantitation (iTRAQ) labeling techniques for relative quantitation and identification of differentially expressed proteins. These methods, which originated from the isotope-coded affinity tag approach reported by Gygi et al,7 have the added advantages of using labeling chemistry targeted at primary amines (rather than sulfhydryl groups) and the ability to simultaneously measure relative quantities of proteins under multiple conditions.8 Differentially expressed proteins were validated using clinically available tools such as enzyme-linked immunosorbent assay (ELISA) and clinical immunonephelometry.
Methods and Materials
Patients and study design
Under an Institutional Review Board–approved protocol (2013-1715), informed consent was recorded from all participants and/or their legal guardians. Exclusion criteria included history of gross hematuria, active or recurrent urinary tract infection, or NS secondary to systemic disease. The study was performed in accordance with the principles of the Declaration of Helsinki. Urine and clinical data were collected from 50 patients, aged 2 to 19, who were diagnosed with idiopathic NS at Cincinnati Children’s Hospital Medical Center. The samples were collected over a period of 24 months. The study included 20 patients with SRNS (19 of whom had biopsy-proven FSGS) and 30 patients with SSNS. Urine was collected as part of a standard clinical visit, centrifuged at 5000 g for 5 minutes, aliquoted, and stored at −80°C. No more than 2 freeze-thaw cycles were used per sample. For our measurements, each patient is represented by a single sample. Demographic and clinical data, including urinalysis, steroid-response history, most recent serum creatinine, and current remission/relapse status, were recorded at the time of patient enrollment. Estimated glomerular filtration rate was calculated from serum creatinine using the new Schwartz formula9 and classified to chronic kidney disease (CKD) stage.10 Steroid-sensitive nephrotic syndrome was defined as the ability to reach remission within 8 weeks after initial diagnosis in response to steroid treatment, as evidenced by normalization of protein urine reading to a negative reading on a urine dipstick. Steroid-resistant nephrotic syndrome was defined as a failure to respond to standard steroid treatment (2 mg/kg/day) for at least 8 weeks.
Quantitative profiling of urine proteins using isobaric protein labeling and tandem mass spectrometry
Urine samples from 2 subject groups (5 each from SRNS relapse and SSNS relapse) were prepared for quantitative protein profiling using the iTRAQ method8 by following the vendor instructions (Sciex, Toronto, ON, Canada). The patients selected for the 5 × 5 comparison met the following criteria—(1) all patients presented to the clinic with active disease consisting of high-grade proteinuria and (2) had samples collected within 6 months prior to the experiment to ensure the most pristine samples possible. The sample preparation protocol prior to iTRAQ tagging varied from the original vendor protocol; thus, the workflow is summarized here with details of each step provided below. The general sample preparation and analysis workflow included concentration and buffer exchange of each urine sample followed by preparative separation of the proteins on a mini sodium dodecyl sulfate polyacrylamide gel electrophoresis gel, in-gel trypsin digestion and recovery of the peptides, iTRAQ tagging of duplicate SSNS and SRNS samples with the iTRAQ 4-plex reagents (114, 115, 116, 117 reporters), combining the peptide from the 4 samples in equal portions, then subjecting peptides to nanoscale liquid chromatography coupled to tandem mass spectrometry (nanoLC-MS/MS), followed by protein identification and quantitation of the collective data set using the ProteinPilot (PP), ProteinPilot Descriptive Statistics Template (PDST), and Protein Alignment software algorithms (AB Sciex, Toronto, ON, Canada). Additional details of each step in the process are provided below.
Gel electrophoresis and isolation of peptides
Proteins from SRNS and SSNS urine samples were concentrated, and buffer exchanged (2×) with Invitrogen 1× Laemmli buffer using 3 kDa Amicon concentrator cartridge (UFC500396). The protein concentration for each sample was determined using the noninterfering (Ni) protein assay reagents from G-Biosciences (Maryland Heights, MO); 50 µg each from the 5 SRNS and 5 SSNS (10 samples total) were loaded onto separate lanes of a 1-dimensional (1D), 4% to 12% Bis-Tris minigel, then electrophoresed for 15 minutes which was just long enough for the proteins to enter into the gel. The gel region containing the proteins (about 1.5 cm × 2.5 cm) was cut from the gel and subjected to in-gel trypsin digestion and subsequent recovery of peptides as described previously.11
iTRAQ labeling
The isolated peptides from the 10 urine samples (5 SRNS and 5 SSNS) were each divided in half such that technical replicates were available for each sample. The patient samples were paired randomly between groups; 5 pairwise comparative groups (A-E) were tagged using the 4-plex iTRAQ reagents as described previously.8 The 114 and 115 reporter tags were used for the technical replicates of SRNS samples, whereas the 116 and 117 reporter tags were used for the technical replicates of the SSNS samples. After labeling, the samples were mixed together in equal quantities for subsequent separation, identification, and quantitative analysis.
Nanoliquid chromatography coupled to electrospray tandem mass spectrometry
Nanoliquid chromatography coupled to electrospray tandem mass spectrometry analyses were performed on a TripleTOF 5600+ (Sciex) attached to an Eksigent (Dublin, CA) nanoLC-ultra nanoflow system; 2.5 µg of total protein from each 4-plex mixture was loaded (via an Eksigent NanoLC-AS-2 autosampler) onto an IntegraFrit Trap Column (outer diameter of 360 µm, inner diameter of 100 µm, and 25 µm packed bed) from New Objective, Inc. (Woburn, MA) at 2 µL/min in formic acid/H2O 0.1/99.9 (v/v) for 15 minutes to desalt and concentrate the samples. For the chromatographic separation of peptides, the trap column was switched to align with the analytical column, Acclaim PepMap100 (inner diameter of 75 µm, length of 15 cm, C18 particle sizes of 3 µm, and pore sizes of 100 Å) from Dionex-Thermo Fisher Scientific (Sunnyvale, CA). The peptides were eluted using a variable mobile phase (MP) gradient from 95% phase A (formic acid/H2O 0.1/99.9, v/v) to 40% phase B (formic acid/acetonitrile 0.1/99.9, v/v) for 70 minutes, from 40% phase B to 85% phase B for 5 minutes, and then keeping the same MP composition for 5 more minutes at 300 nL/min. The nLC effluent was ionized and sprayed into the mass spectrometer using NANOSpray III Source (AB Sciex). Ion source gas 1 (GS1), ion source gas 2 (GS2), and curtain gas (CUR) were, respectively, kept at 7, 0, and 25 vendor specified arbitrary units. Interface heater temperature and ion spray voltage were kept at 150 C and at 2.3 kV, respectively. Mass spectrometer method was operated in positive ion mode set to go through 4156 cycles for 90 minutes, where each cycle performed 1 time of flight mass spectrometry scan type (0.25 seconds accumulation time, in a 400-1600 m/z window) followed by 20 information dependent acquisition mode MS/MS-scans on the most intense candidate ions having a minimum 150 counts. Each product ion scan was operated under vender specified high-sensitivity mode with an accumulation time of 0.05 seconds and a mass tolerance of 50 mDa. Former MS/MS-analyzed candidate ions were excluded for 10 seconds after its first occurrence, and data were recorded using Analyst-TF (v.1.6) software.
Data analyses of quantitative protein profiling
Individual and merged search from the nano-scale liquid chromatographic tandem mass spectrometry analyses was accomplished using PP software (version 4.5, revision 1656) that uses Paragon algorithm, against a SWISS-PROT database of human protein sequences. A vendor sample type including all biological modifications was selected for the search parameter as variable modification while methylthiocysteine was used as a fixed modification. The output files for the PP database search (*.group file) contain the peptide identification tables, protein identification tables, and relative quantitation data from the iTRAQ reporter ions from each peptide all of which can be exported as Excel spreadsheets for further statistical analysis using the PDST (version 3.005pB). The PDST is a mathematical Excel template that processes the relative quantitation data among the sample sets and provides statistical probabilities related to the confidence of the protein identification in relationship to an inverse (decoy) protein database, and provides P values regarding the relative quantitation of the 4 reporter ions for each protein. For protein identification and quantitative profiling, a minimum of 2 peptides at 99% or greater confidence was required. After confident protein profiles were collected for each of the 5 pairwise comparisons of the SRNS and the SSNS samples, the collective proteins from across all 5 groups were analyzed using the vendor supplied (Sciex) Protein Alignment Template algorithm (version 2.000p). This algorithm allows for the comparison of up to 10 pairwise groups to determine common protein changes across all the groups. The data reported here required that the proteins be detected in a minimum of 3 of the 5 samples and maintained statistical significance of P < .05 based on a t-test versus the null values.
Urine measurements
Urine vitamin D–binding protein (VDBP) was measured using an ELISA kit that is available commercially (R&D Systems, Minneapolis, MN). Test variability (coefficients of variation [CVs]) were 5.9% (intra-assay) and 6.2% (interassay). The urine neutrophil gelatinase–associated lipocalin (NGAL) ELISA was performed using a commercially available assay (NGAL ELISA Kit 036; Bioporto, Grusbakken, Denmark) that specifically detects human NGAL. The intra-assay CV was 2.1% and interassay CV was 9.1%. Alpha-1 acid glycoprotein 2 (AGP2 or orosomucoid 2) was measured using a commercially available ELISA (Abnova—Taipei City, Taiwan) with an intra-assay CV of 4.4% and an interassay CV of 7.2%. Human fetuin-A and alpha-1 acid glycoprotein 1 (AGP1 or orosomucoid) were measured using commercially available ELISAs with CVs (intra/inter) of 5.5%/7.6% and 5.6%/7.2%, respectively. Human thyroxine-binding globulin (TBG) was measured using a commercially available ELISA (Kamiya Biomedical, Seattle, WA). Thyroxine-binding globulin had CVs of 8.2% (intra) and 10.1% (inter). Hemopexin and prealbumin (transthyretin) were measured with commercially available ELISAs (Assaypro, St. Charles, MO) with CVs of (intra/inter) of 4.9%/7.3% and 4.6%/9.0%, respectively. Alpha-2 macroglobulin was measured using immunonephelometry on a Siemens BNII clinical nephelometer (Siemens, Munich, Germany). Alpha-1-B glycoprotein (A1BG) was measured using a lab constructed ELISA as described previously.12
Statistical analysis on selected biomarkers measured at the patient level
Ten biomarkers selected after Quantitative Protein Profiling were further measured at the patient level using a total of 50 patients, 20 with SRNS and 30 with SSNS. Due to small sample numbers, and to add a level of verification to the proteomics methods, the samples from the 5 × 5 comparison were included in this analysis. As all the biomarkers showed right skewness of their empirical distributions, log2 transformations were used to correct the skewness and ensure that parametric statistical models could be used in analyses. Means with original values were presented after taking inverse function of the transformed means (ie, 2transformed mean) estimated from the statistical models. Two steps of statistical analyses were used in the study. In step 1 of analysis of association, each biomarker was compared of its means between SRNS and SSNS groups using 2 sample tests. In step 2 of predictive analysis, multivariate logistical regression models were used to predict SRNS using a panel of biomarkers. Here, we considered 2 candidate panels, one that employed all 10 biomarkers as the panel (or the predictors) in the multivariate logistical model (MLM-10), and the other that chose 5 biomarkers that showed significance in step 1 (MLM-5). The multivariate logistical regression model from each panel would calculate a logit or risk score of SRNS, and the score was evaluated for discriminative or diagnostic accuracy of SRNS using a receiver operating characteristic (ROC) curve. In particular, the overall accuracy could be evaluated using the area under the ROC curve (or AUC), and specific accuracy under a cutoff score could be evaluated using corresponding sensitivity and specificity. The accuracy is considered “outstanding,” “excellent,” “very good,” “fair,” and “poor” if an AUC is “0.9 to 1,” “0.8 to 0.89,” “0.7 to 0.79,” “0.6 to 0.69,” and “<0.6,” respectively, and a sensitivity or specificity is “0.8 to 1,” “0.6 to 0.79,” “0.4 to 0.59,” “0.2 to 0.39,” and “<0.2,” respectively. The comparison between an ROC curve from a multivariate model vs an ROC curve from an individual biomarker was tested using a nonparametric test.13 The same analyses were repeated in a subset of relapsed patients only. Subanalyses on relapsed patients were not performed given too small the sample size, especially those with SRNS (n = 3). All statistical analyses were performed using SAS 9.4 software (SAS, Cary, NC). P values <.05 were considered statistically significant.
Results
Patients
Fifty pediatric patients were enrolled over a 2-year period. Out of the 50 subjects, 20 presented with SRNS and 16 with FSGS on biopsy. Seventeen subjects had active disease and 3 were in remission. Thirty patients demonstrated response to corticosteroid therapy and were labeled SSNS at the time of sample collection, 14 SSNS patients had active proteinuria, and 16 presented without protein in their urine. Seventeen SRNS patients and the active SSNS patients had high-grade proteinuria as diagnosed by urine dipstick at the time of collection. The high-grade test result indicates protein levels greater than 2000 mg/dL. Patient demographics can be seen in Table 1. Steroid-resistant nephrotic syndrome patients differed from SSNS in age (12.3 vs 7.5 years, P < .001), ±hypertension (75% vs 30%, respectively, P = .003), pathologic diagnosis (FSGS vs no biopsy, P < .001), and current steroid therapy (SRNS 45% vs SSNS 87%, P = .001).
Table 1.
Patient Demographics.
| Variable | SRNS (n = 20) | SSNS (n = 30) | P value |
|---|---|---|---|
| Age, years; mean ± SE | 12.3 ± 1.2 | 7.5 ± 0.8 | .001 |
| Sex, %, male | 14 (70) | 20 (68) | NS |
| Pathology, % | |||
| FSGS | 16 (80) | 2 (6.7) | .001 |
| MCD | 1 (5) | 7 (23.3) | |
| Other | 2 (10) | 0 | |
| No biopsy | 1 (5) | 21 (70) | |
| Hypertension | 15 (75) | 9 (30) | .003 |
| Immunosuppressant, % | |||
| Steroid | 9 (45) | 26 (87) | .001 |
| CNI | 4 (20) | 5 (17) | |
| MMF | 3 (15) | 1 (3) | |
| Rituximab | 2 (10) | 4 (13) | |
| CTX | 2 (10) | 3 (10) | |
| ACEI/ARB | 8 (40) | 1 (3) | |
| GFR, mL/min/1.73 m2 | 119 ± 11.4 | 135 ± 6.1 | NS |
| MALB/Cr, mg/mg; ± SE | 2.0 ± 0.6 | 1.5 ± 0.34 | NS |
Abbreviations: FSGS, focal segmental glomerulosclerosis; GFR, glomerular filtration rate; MCD, minimal change disease; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; CTX, cytoxan; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MALB, microalbumin; Cr, creatinine.
iTRAQ profiling for differential proteins in SRNS vs SSNS
Samples from a cohort of 10 patients (5 in each group) were prepared in duplicate (see Table 2) using a 4-plex isotope tagging method (iTRAQ) followed by nanoLC-MS/MS profiling of the sample groups for protein identification and evaluation of quantitative changes as described in the “Materials and Methods” section. Collectively, more than 150 proteins were identified from the sample sets. Of these 150+ proteins identified, 72 proteins were identified and quantified in at least 3 of the 5 pairwise groups (see supplementary Table S1 for complete ratio values for all pairwise comparisons). Importantly, statistical analysis of the protein changes among the patient cohort revealed 13 protein changes with P values <.05. (Table 3). These 13 proteins were selected for further validation in a larger sample group.
Table 2.
iTRAQ pairwise groupings.
| 4-plex group | Sample ID | Tag | Sample group |
|---|---|---|---|
| A | SRNS, sample 007, rep 1 | 114 | Resistant |
| SRNS, sample 007, rep 2 | 115 | ||
| SSNS, sample 002, rep 1 | 116 | Sensitive | |
| SSNS, sample 002, rep 2 | 117 | ||
| B | SRNS, sample 008, rep 1 | 114 | Resistant |
| SRNS, sample 008, rep 2 | 115 | ||
| SSNS, sample 015, rep 1 | 116 | Sensitive | |
| SSNS, sample 015, rep 2 | 117 | ||
| C | SRNS, sample 009, rep 1 | 114 | Resistant |
| SRNS, sample 009, rep 2 | 115 | ||
| SSNS, sample 027, rep 1 | 116 | Sensitive | |
| SSNS, sample 027, rep 2 | 117 | ||
| D | SRNS, sample 012, rep 1 | 114 | Resistant |
| SRNS, sample 012, rep 2 | 115 | ||
| SSNS, sample 021, rep 1 | 116 | Sensitive | |
| SSNS, sample 021, rep 2 | 117 | ||
| E | SRNS, sample 004, rep 1 | 114 | Resistant |
| SRNS, sample 004, rep 2 | 115 | ||
| SSNS, sample 013, rep 1 | 116 | Sensitive | |
| SSNS, sample 013, rep 2 | 117 |
Abbreviations: iTRAQ, isobaric tags for relative and absolute quantitation; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome.
Table 3.
Differential Protein Findings by iTRAQ.
| Accession | Protein name | SSNS/SRNS Group A | SSNS/SRNS Group B | SSNS/SRNS Group C | SSNS/SRNS Group D | SSNS/SRNS Group E | Average log2 | P value |
|---|---|---|---|---|---|---|---|---|
| sp|P02774|VTDB_HUMAN | VDBP | −0.668 | −0.529 | −0.628 | −0.078 | −0.919 | −0.564 | .015 |
| sp|P02765|FETUA_HUMAN | Fetuin-A | −0.530 | −0.466 | −0.365 | −0.278 | −0.410 | .005 | |
| sp|P02790|HEMO_HUMAN | Hemopexin | −0.328 | −0.366 | −0.337 | −0.554 | −0.396 | .005 | |
| sp|P02766|TTHY_HUMAN | Prealbumin | −0.281 | −0.531 | −0.326 | −0.052 | −0.491 | −0.336 | .017 |
| sp|P02647|APOA1_HUMAN | Apolipoprotein A-1 | −0.139 | −0.368 | −0.332 | −0.186 | −0.575 | −0.320 | .014 |
| sp|P01019|ANGT_HUMAN | Angiotensinogen | −0.263 | −0.225 | −0.260 | −0.376 | −0.281 | .003 | |
| sp|P01024|CO3_HUMAN | Complement C3 | −0.323 | −0.098 | −0.208 | −0.059 | −0.211 | −0.180 | .018 |
| sp|P01023|A2MG_HUMAN | Alpha-2 macroglobulin | −0.139 | −0.175 | −0.172 | −0.162 | .005 | ||
| sp|P02763|A1AG1_HUMAN | AGP1 | 0.140 | 0.177 | 0.183 | 0.101 | 0.086 | 0.138 | .002 |
| sp|P05543|THGB_HUMAN | TBG | 0.126 | 0.240 | 0.349 | 0.213 | 0.056 | 0.197 | .017 |
| sp|P19652|A1AG2_HUMAN | AGP2 | 0.238 | 0.066 | 0.317 | 0.459 | 0.247 | 0.265 | .014 |
| sp|P25311|ZA2G_HUMAN | Zinc-alpha-2 glycoprotein | 0.205 | −0.013 | 0.529 | 0.437 | 0.366 | 0.305 | .033 |
| sp|P04217|A1BG_HUMAN | Alpha-1-B glycoprotein | 0.120 | 0.324 | 0.733 | 0.681 | 0.173 | 0.406 | .033 |
Abbreviations: AGPI, alpha-1 acid glycoprotein 1; AGP2, alpha-1 acid glycoprotein 2; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome; TBG, thyroxine-binding globulin; VDBP, vitamin D–binding protein.
Validation
Of the 13 proteins determined to be different between the 2 groups, we were able to find reliable assays for 9 proteins. These proteins were included for validation using ELISA or immunonephelometry in the expanded cohort (N = 50): AGP, AGP2, alpha-1 microglobulin, A1BG, fetuin-A, hemopexin, prealbumin (transthyretin), TBG, and VDBP. In addition, we measured NGAL because we have previously shown to be able to differentiate SSNS from SRNS.14 Table 4 shows that VDBP (P < .001), prealbumin (P < .001), NGAL (P = .001), fetuin-A (P < .001), and AGP2 (P = .03) were all 5.5 to 38 fold higher in SRNS patients than SSNS in the complete cohort.
Table 4.
Summary of biomarkers by SSNS/SRNS.
| VARIABLE | Mean (95% CI) |
Fold (SRNS/SSNS) | P | |
|---|---|---|---|---|
| SRNS | SSNS | |||
| All (N = 50) | ||||
| n = 20 | n = 30 | |||
| VDBP | 2519.41 (669.59–9479.56) | 66.25 (22.46–195.47) | 38.0 | <.001 |
| NGAL | 30.77 (15.01–63.08) | 5.57 (3.10–10.00) | 5.5 | .001 |
| Fetuin-A | 36 723.78 (13 878.94–97 171.38) | 3433.82 (1551.44–7600.15) | 10.7 | <.001 |
| Prealbumin | 20 685.39 (7391.11–57 891.95) | 1649.83 (712.04–3822.76) | 12.5 | <.001 |
| AGP2 | 141.30 (54.38–367.14) | 35.79 (16.41–78.04) | 3.9 | .030 |
| AGP1 | 90.97 (13.43–616.16) | 82.89 (17.38–395.22) | 1.1 | .940 |
| A2MCG | 119.93 (40.33–356.62) | 35.79 (14.70–87.13) | 3.4 | .090 |
| A1BG | 310.97 (146.86–658.43) | 192.57 (104.37–355.31) | 1.6 | .325 |
| TBG | 1136.19 (320.34–4029.90) | 730.91 (259.97–2054.98) | 1.6 | .590 |
| Hemopexin | 4701.67 (1993.48–11 089.00) | 2049.40 (1017.11–4129.39) | 2.3 | .138 |
| Relapse (N = 31) | ||||
| n = 17 | n = 14 | |||
| VDBP | 3708.40 (1010.16–13 613.90) | 353.58 (84.36–1482.06) | 10.5 | .018 |
| NGAL | 33.48 (15.22–73.64) | 7.16 (3.00–17.06) | 4.7 | .011 |
| Fetuin-A | 55 745.38 (23 435.74–132 598.64) | 15 607.72 (6006.81–40 554.13) | 3.6 | .053 |
| Prealbumin | 33 079.70 (12 129.94–90 212.00) | 5000.48 (1655.35–15 105.43) | 6.6 | .014 |
| AGP2 | 171.01 (81.37–359.43) | 266.72 (117.65–604.69) | 0.6 | .422 |
| AGP1 | 141.97 (22.88–881.03) | 1340.72 (179.35–10 022.32) | 0.1 | .103 |
| A2MCG | 137.11 (44.26–424.79) | 110.19 (31.70–383.10) | 1.2 | .795 |
| A1BG | 318.05 (139.00–727.74) | 241.52 (97.01–601.29) | 1.3 | .655 |
| TBG | 1639.78 (419.97–6402.53) | 1237.83 (275.92–5553.08) | 1.3 | .781 |
| Hemopexin | 4019.45 (1583.99–10 199.55) | 3126.86 (1120.64–8724.72) | 1.3 | .717 |
Abbreviations: AGPI, alpha-1 acid glycoprotein 1; AGP2, alpha-1 acid glycoprotein 2; CI, confidence interval; NGAL, neutrophil gelatinase–associated lipocalin; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome; VDBP, vitamin D–binding protein. Italicized font represents biomarker included in MLM-5.
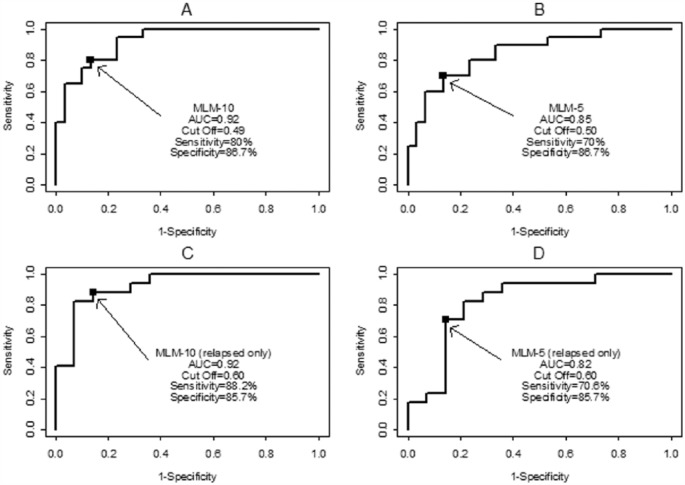
The predictive analyses showed the panel of biomarkers (MLM-5) improved the AUC to 0.85, significantly higher than that of AGP2 or any individual biomarker not selected in the panel. The panel using all 10 biomarkers (MLM-10) yielded an AUC of 0.92, significantly higher than that of any single biomarker (Table 5). Sensitivities and specificities from panels showed excellent outstanding accuracy under suggested cutoff scores (see Table 6 and Figure 1). Table 7 provides the algorithms to calculate the risk scores of SRNS of the panels. For the algorithms, the formula in step 2 is derived from the logistic regression analysis, and the cutoff for step 6 was derived from the ROC curve analysis. To better understand these algorithms, an example is provided to demonstrate the steps to calculate the risk score and draw a conclusion of Steroid Resistant (or Steroid Sensitive), using a predefined cut. The example is based on Model MLM-5; similar calculations apply to other models in the table.
Table 5.
Summary of AUC for detecting SSNS.
| ROC model | AUC (95% CI) | P vs MLM_10 | P vs MLM_5 |
|---|---|---|---|
| All (N = 50) | |||
| MLM-10 | 0.92 (0.85–0.99) | — | .076 |
| MLM-5 | 0.85 (0.74–0.96) | .076 | — |
| VDBP | 0.81 (0.68–0.95) | .052 | .267 |
| NGAL | 0.78 (0.65–0.91) | .020 | .264 |
| Fetuin-A | 0.78 (0.65–0.91) | .016 | .195 |
| Prealbumin | 0.78 (0.65–0.91) | .026 | .286 |
| AGP2 | 0.65 (0.49–0.80) | .001 | .011 |
| AGP1 | 0.55 (0.39–0.71) | .000 | .000 |
| A2MCG | 0.64 (0.48–0.80) | .001 | .027 |
| A1BG | 0.59 (0.42–0.75) | .000 | .008 |
| TBG | 0.56 (0.39–0.73) | .000 | .003 |
| Hemopexin | 0.66 (0.50–0.82) | .002 | .028 |
| Relapse (N = 31) | |||
| MLM-10 | 0.92 (0.83–1.00) | — | .129 |
| MLM-5 | 0.82 (0.66–0.99) | .129 | — |
| VDBP | 0.77 (0.58–0.96) | .105 | .561 |
| NGAL | 0.76 (0.58–0.94) | .037 | .312 |
| Fetuin-A | 0.68 (0.48–0.88) | .016 | .118 |
| Prealbumin | 0.73 (0.55–0.91) | .035 | .215 |
| AGP2 | 0.60 (0.39–0.80) | .003 | .067 |
| AGP1 | 0.57 (0.35–0.79) | .002 | .091 |
| A2MCG | 0.52 (0.30–0.73) | .001 | .023 |
| A1BG | 0.58 (0.36–0.79) | .005 | .051 |
| TBG | 0.57 (0.36–0.78) | .003 | .045 |
| Hemopexin | 0.56 (0.35–0.77) | .003 | 0.080 |
Abbreviations: AGPI, alpha-1 acid glycoprotein 1; AGP2, alpha-1 acid glycoprotein 2; AUC, area under the curve; CI, confidence interval; NGAL, neutrophil gelatinase–associated lipocalin; ROC receiver operating characteristic; SSNS, steroid-sensitive nephrotic syndrome; TBG, thyroxine-binding globulin; VDBP, vitamin D–binding protein. Italicized font indicates a biomarker included in the MLM-5.
Table 6.
Sensitivity and specificity of detecting SSNS using suggested cutoffs from multivariate logistic models.
| Model | AUC (95% CI) | Cutoff probability | Sensitivity | Specificity |
|---|---|---|---|---|
| All (N = 50) | ||||
| MLM-10 | 0.92 (0.85–0.99) | .49 | 80.0% | 86.7% |
| MLM-5 | 0.85 (0.74–0.96) | .50 | 70.0% | 86.7% |
| Relapse (N = 31) | ||||
| MLM-10 | 0.92 (0.83–1.00) | .60 | 88.2% | 85.7% |
| MLM-5 | 0.82 (0.66–0.99) | .60 | 70.6% | 85.7% |
Abbreviations: AUC, area under the receiver operating characteristic curve; SSNS, steroid-sensitive nephrotic syndrome.
Figure 1.
ROC curves using panels of 10 biomarkers (MLM-10) and 5 biomarkers (MLM-5), respectively. A) MLM-10, all patients; B) MLM-5, all patients; C) MLM-10, relapse only; D) MLM-5 relapse only. ROC indicates receiver operating characteristic. Arrows indicate Cut Off values.
Table 7.
Algorithms of computing risk scores of SRNS.
| Step | MLM-10 (any patient) | MLM-5 (any patient) | MLM-10 (relapsed patient only) | MLM-5 (relapsed patient only) |
|---|---|---|---|---|
| 1 | Converting all biomarkers into log2 values | Converting all biomarkers into log2 values | Converting all biomarkers into log2 values | Converting all biomarkers into log2 values |
| 2 | Each biomarker is adjusted by a multiplier in the following: 0.27 × VDBP −0.004 × Prealbumin 0.51 × NGAL 0.37 × fetuin-A 0.50 × AGP2 −0.49 × AGP1 0.22 × A2MCG 0.20 × Hemopexin −0.09 × TBG 0.11 × A1BG |
Each biomarker is adjusted by a multiplier in the following: 0.23 × VDBP −0.03 × Prealbumin 0.32 × NGAL 0.01 × fetuin-A 0.03 × AGP2 |
Each biomarker is adjusted by a multiplier in the following: 0.23 × VDBP 0.07 × prealbumin 0.82 × NGAL 0.41 × fetuin-A 0.42 × AGP2 −0.60 × AGP1 0.24 × A2MCG 0.36 × Hemopexin −0.15 × TBG 0.24 × A1BG |
Each biomarker is adjusted by a multiplier in the following: 0.14 × VDBP 0.16 × prealbumin 0.26 × NGAL −0.05 × fetuin-A −0.47 × AGP2 |
| 3 | Sum of the adjusted biomarkers | Sum of the adjusted biomarkers | Sum of the adjusted biomarkers | Sum of the adjusted biomarkers |
| 4 | Calculate a raw score by subtracting 13.65 from the sum in step 3 | Calculate a raw score by subtracting 3.58 from the sum in step 3 | Calculate a raw score by subtracting 17.27 from the sum in step 3 | Calculate a raw score by subtracting 0.03 from the sum in step 3 |
| 5 | Calculate the risk score by taking 2 to the power of the raw score in step 4 | Calculate the risk score by taking 2 to the power of the raw score in step 4 | Calculate the risk score by taking 2 to the power of the raw score in step 4 | Calculate the risk score by taking 2 to the power of the raw score in step 4 |
| 6 | Compare the risk score with the cutoff point 0.49: SRNS positive if the score > cutoff SRNS negative if the score ⩽ cutoff |
Compare the risk score with the cutoff point 0.50: SRNS positive if the score > cutoff SRNS negative if the score ⩽ cutoff |
Compare the risk score with the cutoff point 0.60: SRNS positive if the score > cutoff SRNS negative if the score ⩽ cutoff |
Compare the risk score with the cutoff point 0.60: SRNS positive if the score > cutoff SRNS negative if the score ⩽ cutoff |
Abbreviations: AGPI, alpha-1 acid glycoprotein 1; AGP2, alpha-1 acid glycoprotein 2; NGAL, neutrophil gelatinase–associated lipocalin; SRNS, steroid-resistant nephrotic syndrome; TBG, thyroxine-binding globulin; VDBP, vitamin D–binding protein.
Assume a patient’s observed values of VDBP, prealbumin, NGAL, fetuin-A, and AGP2 are 250, 900, 24, 1250, and 50, respectively:
Step1: The log2 transferred values are 7.97, 9.81, 4.58, 10.29, and 5.64, respectively.
Step2: The formula is 0.23 × 7.97 − 0.03 × 9.81 + 0.32 × 4.58 + 0.01 × 10.29 + 0.03 × 5.64.
Step3: The sum of step 2 is 3.2765.
Step4: The adjusted sum is 3.2765 − 3.58 = −0.3035.
Step5: The risk score = 2 ^ 0.3035 = 1.23
Step6: As the risk score = 1.23 > 0.5, the patient is considered likely Steroid Resistant based on this model.
Similar conclusions could be reached in the subanalyses on relapsed patients.
Discussion
Patients with SRNS have a more progressive disease course and related poor outcomes when compared patients with SSNS.15-18 The number of cases of FSGS and the resulting SRNS in the pediatric population are continuing to increase.19-21 Currently, the only method of diagnosis is an invasive biopsy, which is not typically performed in children until first-line treatments fail. This results in patients with SRNS getting an unnecessary exposure to high-dose corticosteroids and a delay in initiating a more appropriate treatment. In this study, our objective was to use a robust proteomic technique, iTRAQ, to identify potential biomarkers that could be used to noninvasively distinguish steroid-resistant patients from those whose disease is likely to respond to steroids. Of the 13 differentially expressed proteins identified by iTRAQ, we were able to use ELISA and immunonephelometry to validate a 10-biomarker panel with an excellent discriminatory power to identify SRNS (AUC, 0.92) in both the complete cohort and the subset with active disease. In addition, we demonstrated that by using the 5 markers with significant association to SRNS, we were still able to achieve an AUC of 0.85 in the complete cohort and an AUC of 0.82 in the active disease subjects. This predictive biomarker panel includes VDBP, NGAL, fetuin-A, prealbumin, and AGP2.
Our study is the first to use the iTRAQ profiling to find potential biomarkers to distinguish SRNS from SSNS and validate the findings in a pediatric population. Therefore, it is not surprising that we found markers that differ from earlier studies. For instance, in one such study, Khurana et al22 found that beta-2 microglobulin was associated with SRNS, but a separate validation was never undertaken. Those studies had used techniques, such as surface-enhanced laser desorption ionization time of flight mass spectrometry, that have fallen out of favor due to the fact that they only identify peptide or protein peaks—and one has to separately isolate the protein of the appropriate size and subsequently identify the protein using tandem MS.
The current study adds further validation to the previous findings concerning VDBP and NGAL in SRNS. Vitamin D–binding protein and NGAL have previously been shown to be increased in children with SRNS.14,23 While both had been shown previously to be correlated with proteinuria,24,25 their ability to positively identify patients with SRNS vs SSNS was independent of proteinuria as measured by MALB/Cr.23 It was found that VDBP was independently able to categorize (AUC, 0.87, P < .0002) SRNS and SSNS patients, without relation to proteinuria. This indicates a potential mechanism that leads to increased urine vitamin D binding protein (uVDBP) specifically in patients with SRNS. A plausible mechanism may involve intact megalin and cubulin receptors in the proximal tubule to reabsorb filtered VDBP. As SRNS leads to chronic tubular injury, this could explain increased levels of uVDBP in the urine. Supporting this theory, VDBP was recently shown in a rat model of adriamycin-induced nephrosis to be a biomarker of tubular fibrosis and renal interstitial damage.26
Interestingly, fetuin-A has been shown to work through megalin-mediated endocytosis to counter nephrocalcification in the tubular lumen in rats.27 Therefore, like VDBP, increased excretion of fetuin-A in the urine could be explained by megalin dysfunction and could represent a mechanism for the appearance of these proteins at high levels in the urine of SRNS patients. It has been suggested by some that the normal glomerulus leaks proteins at a nephrotic level, but that those proteins are generally reabsorbed in the proximal tubule by megalin and related proteins.28 However, in the nephrotic kidney, levels of megalin, clathrin, and other important parts of the endocytic pathway are compromised, which leads to albuminuria. Given the number of podocyte mutations discovered in nephrotic diseases such as FSGS,29 it is unlikely that megalin dysfunction accounts for all aspects of the disease, but it remains an intriguing possibility given some of our findings. Research indicates that fetuin-A may well be a biomarker for other forms of renal disease. Inoue et al30 demonstrated that fetuin-A was a risk factor for both microalbuminuria and reduction of glomerular filtration rate in diabetic nephropathy and could therefore be used as a marker to predict progression of the disease. Urinary fetuin-A has also been shown to be a sensitive (94%), yet not especially specific (60%), marker for progression and prediction of renal insufficiency in autosomal dominant polycystic kidney disease. Fetuin-A, similar to NGAL, appears to be a sensitive marker of progression of disease but lacks some specificity to individual disease processes.31,32
Not all of the markers we discovered have a track record as being associated with CKD in the urine. For instance, serum prealbumin levels are often elevated in CKD and are used to evaluate nutritional status in dialysis patients,33 but urinary levels have not appeared to be associated with specific diseases in the literature.
This study has certain limitations that must be presented. Our pilot study was cross-sectional, at a single center, and included a small subject pool, many of which had begun treatment at enrollment. We are therefore limited in the conclusions we can make about the predictive value of the biomarker panel. There exists an inherent age difference between our SRNS patients and our SSNS patients, as well as varying lengths of disease duration. This age difference is unavoidable in a limited population of patients with NS because the different forms tend to occur at different stages of life. For instance, most children with MCD are diagnosed before the age of 5, whereas FSGS occurs mainly after the age of 6.2 The strength of our panel to distinguish SRNS from SSNS (AUC, 0.93; P < .0001) is high enough, however, that it is not likely to represent an artifact of age differences. It is also unfortunate that we did not have access to serum samples for this study, so we could not ascertain whether any of these markers represent high serum levels that are leaking into the urine. Clinically, in the pediatric NS population, our results indicate a level of promise that our biomarker panel could be used to identify patients with the steroid-resistant form of the disease. Of course, our panel is only a tool and, as with any other diagnostic test, must be used in the context of the clinical situation to help inform the decision making of the physician.34
This biomarker panel must now be subjected to a larger, multicenter, prospective study which would allow us to access adequate numbers of patients with primary NS before initial treatments are administered and allow more control over age variation. This type of study would allow us to determine the utility of our panel in the early diagnosis of NS, and to determine whether it could be used to guide treatment, thus limiting exposure to high-dose steroids and their harmful side effects in patients who are not likely to benefit from their use. We could also determine whether any of the biomarkers in our panel change with response to therapy. Biomarkers that can be used in clinical trials as surrogate endpoints can allow for expedited drug development. The development of a noninvasive panel of urinary biomarkers that may be useful in guiding therapy for patients with NS could lead to improved care and better outcomes for patients with this progressive and devastating disease.
Supplementary Material
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1187 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NIH P50 DK096418 to MRB, KDG and PD. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MRB and PD disclose a patent pending for compositions and methods for treating steroid resistant nephrotic syndrome, 14/838,551. Other authors disclose no potential conflicts of interest.
Author Contributions: Study design, data collection, statistical analysis, result interpretation, and manuscript preparation: MRB. Data collection and manuscript preparation: LP, CH, WDH, QM. Statistical Analysis and manuscript preparation: MW, JY. Study design, result interpretation, and manuscript preparation: KDG and PD. All authors reviewed and approved of the final manuscript.
References
- 1. Gipson DS, Massengill SF, Yao L, et al. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. [DOI] [PubMed] [Google Scholar]
- 2. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. [DOI] [PubMed] [Google Scholar]
- 3. Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the transplant registry: the 2006 annual report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant. 2007;11:366–373. [DOI] [PubMed] [Google Scholar]
- 4. Korbet SM. Clinical picture and outcome of primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 1999;14:68–73. [DOI] [PubMed] [Google Scholar]
- 5. Gulati S, Sharma AP, Sharma RK, Gupta A, Gupta RK. Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol. 2002;17:404–408. [DOI] [PubMed] [Google Scholar]
- 6. Neuhaus TJ, Fay J, Dillon MJ, Trompeter RS, Barratt TM. Alternative treatment to corticosteroids in steroid sensitive idiopathic nephrotic syndrome. Arch Dis Child. 1994;71:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. [DOI] [PubMed] [Google Scholar]
- 8. Ross PL, Huang YN, Marchese JN, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 11. Eismann T, Huber N, Shin T, et al. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G266–G274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piyaphanee N, Ma Q, Kremen O, et al. Discovery and initial validation of alpha 1-B glycoprotein fragmentation as a differential urinary biomarker in pediatric steroid-resistant nephrotic syndrome. Proteomics Clin Appl. 2011;5:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 14. Bennett MR, Piyaphanee N, Czech K, Mitsnefes M, Devarajan P. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. [DOI] [PubMed] [Google Scholar]
- 16. Hari P, Bagga A, Jindal N, Srivastava RN. Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol. 2001;16:901–905. [DOI] [PubMed] [Google Scholar]
- 17. Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. [DOI] [PubMed] [Google Scholar]
- 18. Roberti I, Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol. 2010;25:1117–1124. [DOI] [PubMed] [Google Scholar]
- 19. Gulati S, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34:646–650. [DOI] [PubMed] [Google Scholar]
- 20. Bonilla-Felix M, Parra C, Dajani T, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55:1885–1890. [DOI] [PubMed] [Google Scholar]
- 21. Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13:13–18. [DOI] [PubMed] [Google Scholar]
- 22. Khurana M, Traum AZ, Aivado M, et al. Urine proteomic profiling of pediatric nephrotic syndrome. Pediatr Nephrol (Berlin, Germany). 2006;21:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett MR, Pordal A, Haffner C, Pleasant L, Ma Q , Devarajan P. Urinary vitamin D-binding protein as a biomarker of steroid-resistant nephrotic syndrome. Biomarker Insights. 2016;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grymonprez A, Proesmans W, Van Dyck M, Jans I, Goos G, Bouillon R. Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol. 1995;9:278–281. [DOI] [PubMed] [Google Scholar]
- 25. Doorenbos CR, de Cuba MM, Vogt L, et al. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol. 2012;128:56–61. [DOI] [PubMed] [Google Scholar]
- 26. Mirkovic K, Doorenbos CR, Dam WA, et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS ONE. 2013;8:e55887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsui I, Hamano T, Mikami S, et al. Retention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in rats. Am J Physiol Renal Physiol. 2013;304:F751–F760. [DOI] [PubMed] [Google Scholar]
- 28. Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. [DOI] [PubMed] [Google Scholar]
- 29. Lovric S, Ashraf S, Tan W, Hildebrandt F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant. 2016;31:1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inoue K, Wada J, Eguchi J, et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS ONE. 2013;8:e77118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu J, Ding Y, Zhu C, et al. Urinary TNF-alpha and NGAL are correlated with the progression of nephropathy in patients with type 2 diabetes. Exp Ther Med. 2013;6:1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rymarz A, Bartoszewicz Z, Szamotulska K, Niemczyk S. The associations between body cell mass and nutritional and inflammatory markers in patients with chronic kidney disease and in subjects without kidney disease. J Ren Nutr. 2016;26:87–92. [DOI] [PubMed] [Google Scholar]
- 34. Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care. 2015;19:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.