Abstract
Objective
Voriconazole, a first line agent for the treatment of invasive fungal infections (IFIs), is metabolized by CYP2C19. A significant portion of patients fail to achieve therapeutic trough concentrations with standard weight-based voriconazole dosing, placing them at increased risk for treatment failure, which can be life threatening. We sought to test the association between CYP2C19 genotype and subtherapeutic voriconazole concentrations in adults with IFIs.
Methods
Adults receiving weight-based voriconazole dosing for the treatment of IFIs were genotyped for the CYP2C19*2, *3, and *17 polymorphisms, and CYP2C19 metabolizer phenotypes were inferred. Steady-state voriconazole trough plasma concentrations and the prevalence of subtherapeutic troughs (<2 mg/L) were compared between patients with the CYP2C19*17/*17 (ultrarapid metabolizers, UMs) or *1/*17 genotypes (rapid metabolizers, RMs) versus those with other genotypes. Logistic regression, adjusting for clinical factors, was performed to estimate the odds of subtherapeutic concentrations.
Results
Of 70 patients included (mean age 51±18 years), 39% were RMs or UMs. Compared to patients with the other phenotypes, RMs/UMs had a lower steady state trough concentration (4.26±2.2 vs. 2.86±2.3, p=0.0093), and a higher prevalence of subtherapeutic troughs (16% vs. 52%, p=0.0028), with an odds ratio of 5.6 (95% confidence interval 1.64–19.24, p=0.0059).
Conclusion
Our findings indicate that adults with the CYP2C19 RM or UM phenotype are more likely to have subtherapeutic concentrations with weight-based voriconazole dosing. These results corroborate previous findings in children and support potential clinical utility of CYP2C19 genotype-guided voriconazole dosing to avoid underexposure in RMs and UMs.
Keywords: invasive fungal infections, voriconazole, subtherapeutic trough plasma concentration, CYP2C19*17, rapid and ultrarapid metabolizer phenotypes
Introduction
Invasive fungal infections (IFIs) are one of the most feared complications of prolonged and profound neutropenia, with mortality rates approaching 90% if left untreated [1,2]. Hence, early diagnosis coupled with timely initiation of optimal doses of effective antifungal agents is critical for favorable patient outcomes [3]. Voriconazole is a broad spectrum, second generation triazole widely used for the treatment of life threatening fungal infections. The Infectious Diseases Society of America (IDSA) recommends it as a first line agent for the treatment of invasive aspergillosis (IA) [4]. This is because of its unequivocal efficacy compared with other agents such as amphotericin B, the former gold standard for IFIs treatment [5,6]. There is a large body of evidence demonstrating that therapeutic success with voriconazole is contingent on achieving a therapeutic trough plasma concentration at steady state [7–9], and response rates approach 100% when trough concentrations of at least 2 mg/L are attained early in the course of therapy [3,10,11]. However, the recommended weight-based voriconazole dosing for the treatment IFIs, which consists of a loading dose of 6 mg/kg every 12 hours for the first 24 hours followed by a maintenance dose of 4 mg/kg every 12 hours, is associated with a wide inter-individual variability in voriconazole exposure [7,12], with reported troughs ranging from 0.2 mg/L to 13.5 mg/L [12,13].
Voriconazole undergoes extensive hepatic metabolism, which is predominantly mediated by the cytochrome P450 (CYP) 2C19 enzyme [14,15]. The gene encoding CYP2C19 is highly polymorphic, with more than 34 variant alleles identified (http://www.cypalleles.ki.se)[16]. The CYP2C19*17 allele is a gain-of-function variant arising from a single nucleotide polymorphism (SNP) in the gene promoter region. The CYP2C19*1/*17 and *17/*17 genotypes confer the rapid metabolizer (RM) and ultrarapid metabolizer (UM) phenotypes, respectively, with increased enzyme activity compared to the normal metabolizer (NM) phenotype (*1/*1 genotype) [17]. Conversely, the *2 and *3 alleles are loss-of-function variants. Intermediate metabolizers (IMs) have a single loss-of-function variant and significant reduction in enzyme activity compared to NMs, while poor metabolizers (PMs), with two loss-of-function variants, have no enzyme activity [18,19].
The CYP2C19 UM phenotype has been associated with subtherapeutic voriconazole trough concentrations in children with IFIs [20]. There are important differences in voriconazole pharmacokinetics between children and adults, with more rapid drug clearance in children,[21,22] limiting the ability to extrapolate findings to adults. Thus, studies in adults are needed. Several single dose pharmacokinetic studies in healthy adults and a small retrospective study of fixed voriconazole dosing in adults with IFIs have shown that voriconazole disposition differed significantly according to CYP2C19 genotype [23–26]. Nonetheless, these findings may not be generalizable to patients with IFIs receiving weight-based voriconazole dosing, the current standard of care for patients with IFIs [4]. Therefore, the primary objective of this study was to test the association between CYP2C19 genotype and voriconazole plasma concentrations in adults receiving standard weight-based dosing for the treatment of IFIs.
Methods
Patient population selection and procedures
This was a prospective cohort study of patients aged 18 years or older with probable or definite IFI based on the European Organization for Research Criteria and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) criteria [27]. All patients were started on standard weight-based voriconazole dosing, consisting of a loading dose of 6 mg/kg every 12 hours for the first 24 hours followed by a maintenance dose of 4 mg/kg every 12 hours [4]. Individuals receiving voriconazole for prophylaxis of fungal infection or with a history of liver transplantation were excluded. Trough plasma concentration was measured on day 5 to 7 after voriconazole initiation (i.e. at steady state). The dose was subsequently adjusted if the trough concentration was outside the therapeutic range (2–6 mg/L) recommended for critically ill patients [10–12,28]. The study protocol was approved by the institutional review board at the University of Florida, and all participants provided written informed consent.
DNA sample collection and isolation
Genomic DNA was collected from each patient for genetic analysis by either mouthwash collection or buccal swabs, and isolated using the Puregene® kit (Qiagen, Valencia, CA, USA) per the manufacturer’s protocol.
CYP2C19 genotyping
The CYP2C19*17 (c.−806C>T; rs12248560), *2 (c.681C>A; rs4244285), and *3 (c.636 G>A; rs4986893) alleles were determined by polymerase chain reaction (PCR) and Pyrosequencing, as previously described [29]. The CYP2C19*1 allele was assigned if the *2, *3, or *17 allele was not detected.
CYP2C19 phenotype assignment
According to nomenclature by the Clinical Pharmacogenetics Implementation Consortium (CPIC) [30], patients with the *1/*17 genotype were classified as RMs, and those with the *17/*17 genotype were classified as UMs. Patients with the one copy of a *2 or *3 allele (e.g. *1/*2,*1/*3, *2/*17) were assigned the IM phenotype, and carriers of two copies (e.g. *2/*2) were assigned the PM phenotype. The NM phenotype was assigned by default to patients without a *2, *3, or *17 allele.
Measurement of voriconazole trough plasma concentration
Plasma samples were stored at −80°C until assayed at the University of Florida Infectious Disease Pharmacokinetics Laboratory, a Clinical Laboratory Improvement Act (CLIA)/College of American Pathologists (CAP) certified laboratory, using a validated high performance liquid chromatography (HPLC) assay. Briefly, concentration was determined using a system consisting of a ThermoFinnegan P4000 HPLC pump (San Jose, CA) with model AS1000 fixed-volume autosampler, a model UV2000 ultraviolet detector, a Gateway Series E computer (Poway, CA), and the Chromquest HPLC data management system. The plasma standard curve for voriconazole ranged from 0.05 to 10.0 mg/L. The absolute recovery of voriconazole from plasma was 94%. The within-sample precision (percent coefficient of variation [CV%]) of validation of a single standard concentration was 1.82%, and the overall validation precision across all standards was 0.55 to 3.71%. No interferences were observed with the measurement of voriconazole with 90 different commonly used medications. Prior to assay, we planned to reanalyze any samples that did not meet quality control criteria (per Standard Operating Procedure).
Data analysis
The Chi square test with one degree of freedom was used to test for genotype deviation from Hardy Weinberg Equilibrium (HWE). The primary endpoint was the prevalence of subtherapeutic trough plasma concentrations at steady state between the CYP2C19 RM/UM phenotype and other phenotypes. A subtherapeutic voriconazole plasma concentration was defined as a trough plasma concentration <2 mg/L on day 5 to 7 of therapy, based on evidence that supports targeting a trough of at least 2 mg/L for treatment success, particularly for critically ill patients [3,10,11]. Secondary endpoints were mean voriconazole trough plasma concentration, prevalence of supratherapeutic trough concentrations (>6 mg/L), and prevalence of trough concentrations <1 mg/L.[31] The prevalence of subtherapeutic trough concentrations was compared between CYP2C19 RM/UMs and other phenotypes using the Chi square test. Simple and multiple logistic regression analyses were performed to estimate the odds of having a subtherapeutic voriconazole trough plasma concentration at steady state with the RM/UM phenotype after adjusting for other covariates such as age, route of voriconazole administration, sex, race, weight, and concomitant medications. Additional comparisons between phenotype groups were done using the Chi square test for categorical data or the Student’s unpaired t-test or Analysis of Variance (ANOVA) for continuous data. Statistical significance was set at a p value <0.05. The inclusion of at least 70 patients, with 14 expected to have the CYP2C19 RM/UM phenotype based on reported phenotype frequencies [16], was estimated to provide 80% power to detect a 30% difference [32] in the prevalence of subtherapeutic trough plasma concentrations between groups with an alpha of 0.05. All statistical analyses were performed with SAS (version 9.3; SAS Institute, Cary, NC).
Results
A total of 81 patients were enrolled. However, voriconazole was discontinued in 11 patients prior to day 5 to 7 (steady state), and thus analysis was limited to 70 patients with trough concentrations drawn at steady state. Their characteristics are summarized in Table 1. The majority were male, Caucasian, and had a recent history of hematopoietic stem cell transplant or induction chemotherapy for hematologic malignancy. Most patients were also receiving pantoprazole, the only proton pump inhibitor (PPI) on formulary, and none was receiving other medications known to induce or inhibit the CYP2C19 enzyme (e.g. rifampicin, carbamazepine, phenytoin, protease inhibitors, or omeprazole).
Table 1.
Patient Characteristics
| Characteristic | n=70 |
|---|---|
| Age (years) | 52.5 ± 18.1 |
| Weight (kg) | 68.6 ± 15 |
| Male sex | 42 (60) |
| Race | |
| Caucasian | 57 (81) |
| African American | 11 (16) |
| Asian | 2 (3) |
| Voriconazole via intravenous route | 44 (63) |
| Voriconazole dose (mg/day) | |
| Loading dose | 880 ± 161 |
| Maintenance dose | 553 ± 120 |
| Comorbidities | |
| Hematopoietic stem cell transplant | 21 (30) |
| Hematologic malignancies | 22 (31) |
| Solid organ transplant | 12 (17) |
| Other* | 15 (21) |
| Concomitant pantoprazole | 50 (71) |
Mean ± SD or No.(%)
Central nervous system fungal infections, fungal endocarditis, inflammatory bowel disease, and connective tissue disorders
CYP2C19 allele frequencies were 0.11 for *2 and 0.22 for *17. The CYP2C19*3 allele was not detected. The distribution of CYP2C19 genotypes and inferred metabolizer phenotypes are shown in Table 2. None of the genotypes deviated from HWE. In all, 39% had the RM or UM phenotype.
Table 2.
Distribution of CYP2C19 genotypes and metabolizer phenotypes
| Genotype | No. (%) | Inferred phenotype | No. (%) |
|---|---|---|---|
| *2/*2 | 1 (1.4) | Poor Metabolizer* | 1 (1.4) |
| *1/*2 | 13 (18.6) | Intermediate Metabolizer* | 14 (20) |
| *2/*17 | 1 (1.4) | ||
| *1/*1 | 28 (40) | Normal Metabolizer | 28 (40) |
| *1/*17 | 24 (34.3) | Rapid Metabolizer | 24 (34.3) |
| *17/*17 | 3 (4.3) | Ultra-rapid Metabolizer | 3 (4.3) |
No patient had the *3 variant
There was a considerable inter-individual variability in voriconazole trough concentrations at steady state, which ranged from 0.26 mg/L to 9.53 mg/L. Trough concentrations <2 mg/L and >6 mg/L (out of therapeutic range) were observed in 30% and 20% of the patients, respectively (Supplementary Figure 1). There was no difference in trough concentration between the NM (4.27±2.4 mg/L) and IM/PM (4.13±1.6 mg/L) groups (p=0.84, Supplementary Table 2), supporting combining these groups for comparison with RMs/UMs. Trough concentration were lower in RMs/UMs compared to patients with other CYP2C19 phenotypes (2.86 ± 2.3 vs. 4.26 ± 2.2, p=0.0093). Mean steady-state trough concentrations were 1.35 ± 0.7 mg/L, 2.97 ± 2.3 mg/L, and 4.26± 2.03 mg/L in patients with the CYP2C19 *17/*17 (UMs), *1/*17 (RMs) and other genotypes, respectively (p=0.02 for both the *17/*17 and *1/*17 genotypes compared to other genotypes, Figure 1).
Figure 1.
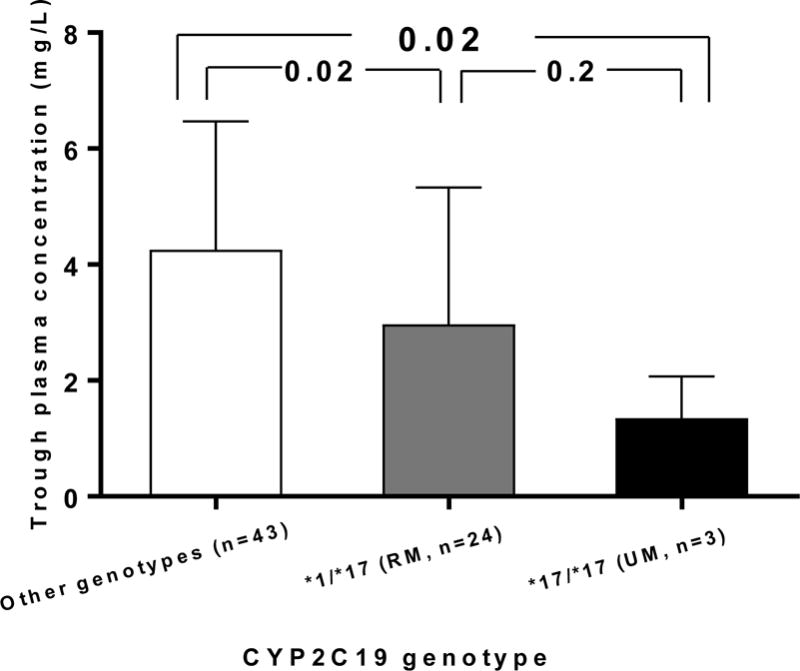
Steady state voriconazole trough plasma concentration in patients with the CYP2C19*17/*17 genotype (ultrarapid metabolizer phenotype, UM), *1/*17 genotype (rapid metabolizer phenotype, RM), and other genotypes (*1/*1, *1/2, *2/2, or *2/*17).
More patients with versus without the RM/UM phenotype had a subtherapeutic trough concentration (52% vs. 16%, p=0.0028). All 3 UMs and 46% of RMs had a subtherapeutic trough (p≤0.01 for each compared to other CYP2C19 phenotypes, Figure 2). On univariate analysis, RM/UM phenotype and weight were the only variables associated with a subtherapeutic trough concentration (Table 3). The association between phenotype and trough concentration remained significant (Table 4) after including other covariates (age, race, sex, body weight, concomitant pantoprazole use, and route of administration) in the model, with an odds ratio of 5.6 (95% CI: 1.64 to 19.24, p=0.0059). The RM/UM phenotype was also more prevalent than other phenotypes among patients with a trough concentration <1 mg/L (27% versus 7%, p=0.031). In contrast, the likelihood of having a supratherapeutic trough concentration (>6 mg/L) at steady state was lower in RMs/UMs compared to NMs (7.4% vs. 35.7%, p=0.03, Figure 3).
Figure 2.
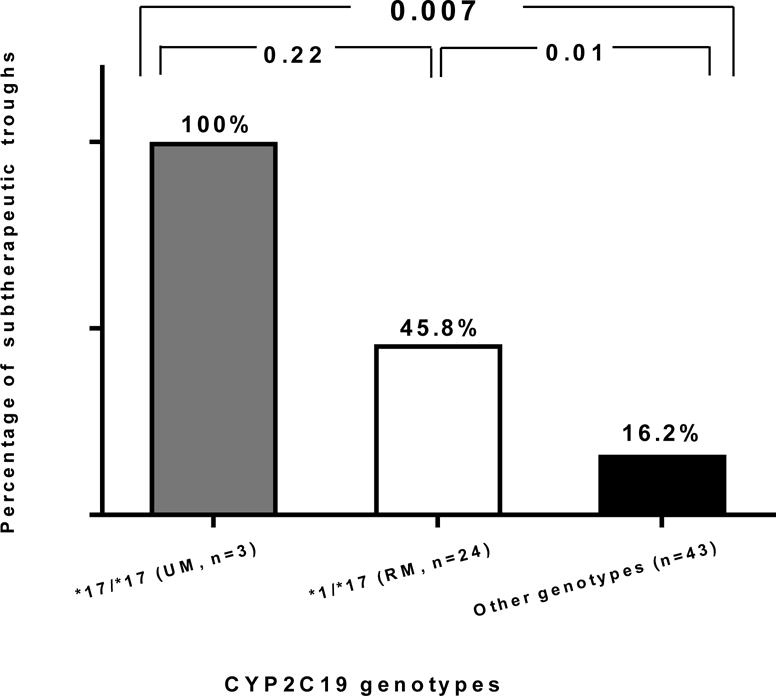
Prevalence of subtherapeutic voriconazole trough plasma concentration at steady state in patients with the CYP2C19*17/*17 genotype (ultrarapid metabolizer phenotype, UM), CYP2C19*1/*17 genotype (rapid metabolizer phenotype, RM) and the other CYP2C19 genotypes (*1/*1, *1/2, *2/2, or 2/*17).
Table 3.
Simple logistic regression analysis for odds of subtherapeutic voriconazole trough plasma concentration at steady state.
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| CYP2C19 RM/UM phenotype | 5.5 | 1.83–16.75 | 0.0024 |
| Weight > 70 kg | 0.3 | 0.1–0.9 | 0.03 |
RM, rapid metabolizer; UM, ultrarapid metabolizer
Table 4.
Multiple logistic regression analysis for odds of subtherapeutic voriconazole trough plasma concentration at steady state
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| CYP2C19 RM/UM phenotype | 5.6 | 1.64–19.24 | 0.0044 |
| Age | 1 | 0.95–1.03 | 0.73 |
| Male sex | 2.2 | 0.47–10.15 | 0.20 |
| African American race | 0.93 | 0.12–7.23 | 0.72 |
| Oral route | 2.8 | 0.67–11.70 | 0.15 |
| Pantoprazole use | 0.37 | 0.10–1.45 | 0.15 |
| Weight >70 kg | 0.18 | 0.04–0.77 | 0.02 |
RM, rapid metabolizer; UM, ultrarapid metabolizer
Figure 3.
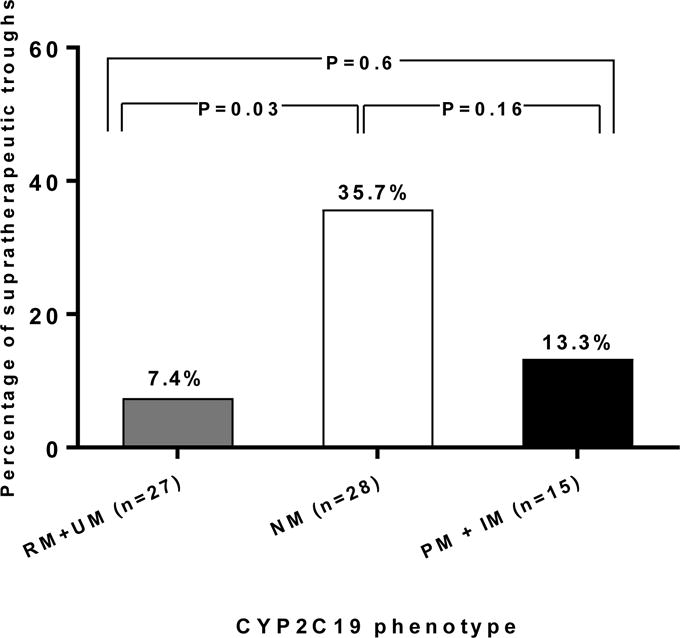
Prevalence of the supratherapeutic voriconazole trough plasma concentration at steady state in CYP2C19 rapid and ultrarapid metabolizers combined (RMs and UMs), normal metabolizers (NMs) and intermediate and poor metabolizers combined (IMs and PMs).
Although not an endpoint of the study, we also examined trough concentrations after dose adjustment in RMs and UMs who initially had a subtherapeutic trough given the paucity of data on appropriate doses with these phenotypes. Voriconazole was either discontinued or switched to an alternative antifungal agent in the majority of patients with a subtherapeutic trough concentration on day 5 to 7. However, in 3 patients who continued on voriconazole (2 UMs and 1 RM), the dose was adjusted to 5 mg/kg. This resulted in a therapeutic trough concentration in two patients - one with the RM phenotype (2.9 mg/L) and one with the UM phenotype (2.4 mg/L) and a trough of 1.85 mg/L in the other UM. No hepatotoxicity or other adverse effects were observed following dose increases.
Discussion
Consistent with previous reports [3,23,33–36], we observed wide inter-individual variability in voriconazole exposure at steady state, with 30% of patients having a subtherapeutic trough concentration on treatment day 5 to 7 with recommended weight-based dosing. Given the severity of illness in patients with invasive fungal infections, it is critical that therapeutic voriconazole concentrations are attained rapidly to prevent adverse outcomes [7–9]. We found that CYP2C19 genotype was a major contributor to risk for voriconazole underexposure, with 100% of those with the *17/*17 genotype (UMs) and nearly 50% of those with the *1/*17 genotype (RMs) failing to achieve a therapeutic trough concentrations (2–6 mg/L) with weight-based dosing. The influence of CYP2C19 genotype on voriconazole underexposure remained significant on logistic regression analysis that included route of administration and use of concomitant pantoprazole, a CYP2C19 inhibitor.
To our knowledge, this is the first prospective study demonstrating an association between CYP2C19 genotype and sub-therapeutic voriconazole trough concentrations in adults receiving weight-based dosing, the currently accepted dosing approach for treatment of IFIs. Our results corroborate previous findings in healthy adults and in adults receiving fixed-dose (versus weight-based) voriconazole [23,32,33,37,38]. Specifically, in a retrospective study of 35 patients receiving voriconazole 200 mg twice daily, Lamoureux et al [23] reported lower voriconazole trough concentrations in CYP2C19*17 allele carriers compared to noncarriers. Despite use of higher doses in our study (mean daily maintenance dose of 553 mg), we still observed a greater likelihood for subtherapeutic troughs among RMs/UMs compared to those with other phenotypes. These data show that use of weight-based dosing versus fixed dosing is not sufficient to overcome the risk for subtherapeutic voriconazole exposure in RMs/UMs.
The CYP2C19 genotype has also been associated with voriconazole exposure in children [20,39]. Compared to children with the *1/*1 genotype, Hicks et al [20] showed significantly lower voriconazole trough concentrations in children with the *17/*17 genotype (UM phenotype), but not *1/*17 genotype (RM phenotype). In contrast, both the *17/*17 and *1/*17 genotypes were associated with lower trough concentrations compared to the *1/*1 genotype in our adult population. Discordant in adults and children may be due to age-related differences in voriconazole pharmacokinetics and differing dose recommendations in pediatrics and adults. Specifically, voriconazole displays linear pharmacokinetics in children, but nonlinear pharmacokinetics in adults [40]. Because of fast metabolic clearance of the drug in pediatric patients, doses ranging from 7 to 10 mg/kg every 12 hours are needed to achieve plasma concentrations comparable to those in adults treated with the 4 mg/kg every 12 hours [22,40,41].
Seventy-one percent of patients in our study were receiving a PPI, specifically pantoprazole. While previous in vivo and in vitro studies demonstrate that PPIs increase voriconazole levels, we observed no association between pantoprazole use and voriconazole disposition [16,42–45]. This finding is consistent with a previous study [20] and may be secondary to the weak inhibitory effect of pantoprazole on CYP2C19 [33,44,45]. Hence, our results should not be extrapolated to other PPIs due to the varying degrees of CYP2C19 enzyme inhibition that exist among the members of this drug class.
The current guidelines from the British Society for Medical Mycology recommend voriconazole trough plasma concentrations of at least 2 mg/L in critically ill patients with a poor prognosis [28]. Alternatively, the 2016 update of the IDSA guidelines for the diagnosis and treatment of aspergillosis recommends a voriconazole trough concentrations of >1 to 1.5 mg/L [4]. Given that most of the patients enrolled in this study were presumed to have impaired host defense mechanisms subsequent to the conditioning chemotherapy regimens and/or receipt of immunosuppressants, we opted to set the therapeutic threshold at 2 mg/L. Nonetheless, we also examined the association between CYP2C19 genotype and trough concentrations <1 mg/L, and found that the likelihood of trough concentrations <1 mg/L was significantly higher with the RM/UM phenotype.
While there are only 3 patients with the RM or UM phenotype in our study with a voriconazole dose adjustment in response to a subtherapeutic concentration, all three had trough levels that were therapeutic or nearly therapeutic level following a 25% dose increase (i.e. from 4 mg/kg to 5 mg/kg). These data suggest that using higher voriconazole doses may be a viable option for patients known to have the CYP2C19 RM or UM phenotype to increase the likelihood of attaining therapeutic concentrations. However, further assessment of dose adjustments based on CYP2C19 genotype is warranted.
In conclusion, our findings indicate that adult patients with IFIs and the CYP2C19 RM or UM phenotype are at increased risk for subtherapeutic trough plasma concentrations of voriconazole with currently recommended weight-based dosing, Hence, the current “one size fits all” approach used in clinical practice for initiating voriconazole treatment is far from optimal. Alternatively, preemptive CYP2C19 genotyping may serve as a valuable tool to help identify patients at risk for voriconazole underexposure with standard weight-based dosing regimens, who may require higher voriconazole doses or alternative therapy to effectively treat IFIs.
Supplementary Material
Acknowledgments
Sources of Funding:
This work supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01007269.
Footnotes
Conflicts of Interests/Disclosures: None
Author Contributions:
I.S.H. wrote the manuscript; I.S.H., L.H.C, J.A.J., J.W.H. and C.A.P. designed the research; All authors reviewed/revised the manuscript; I.S.H, K.P.K., S.J.B., A.M.R., and W.L. performed the research; I.S.H., N.M., and S.S. analyzed data; T.Y.L. and C.A.P. contributed new reagents/analytical tools, and designed the assays.
References
- 1.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32(3):358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18(1):44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16(7):927–933. doi: 10.1111/j.1469-0691.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 5.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 6.Walsh TJ, Lutsar I, Driscoll T, Dupont B, Roden M, Ghahramani P, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J. 2002;21(3):240–248. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 8.Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55(8):1080–1087. doi: 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 9.Bruggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, et al. Therapeutic drug monitoring of voriconazole. Ther Drug Monit. 2008;30(4):403–411. doi: 10.1097/FTD.0b013e31817b1a95. [DOI] [PubMed] [Google Scholar]
- 10.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, et al. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50(4):1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89(5):592–599. doi: 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 12.Trifilio S, Pennick G, Pi J, Zook J, Golf M, Kaniecki K, et al. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer. 2007;109(8):1532–1535. doi: 10.1002/cncr.22568. [DOI] [PubMed] [Google Scholar]
- 13.Cojutti P, Candoni A, Forghieri F, Isola M, Zannier ME, Bigliardi S, et al. Variability of Voriconazole Trough Levels in Haematological Patients: Influence of Co-Medications with CYP Inhibitors and/or with CYP Inhibitors plus CYP Inducers. Basic Clin Pharmacol Toxicol. 2015 doi: 10.1111/bcpt.12530. [DOI] [PubMed] [Google Scholar]
- 14.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31(5):540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 15.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 16.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79(1):103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 18.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269(22):15419–15422. [PubMed] [Google Scholar]
- 19.De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46(4):594–598. [PubMed] [Google Scholar]
- 20.Hicks JK, Crews KR, Flynn P, Haidar CE, Daniels CC, Yang W, et al. Voriconazole plasma concentrations in immunocompromised pediatric patients vary by CYP2C19 diplotypes. Pharmacogenomics. 2014;15(8):1065–1078. doi: 10.2217/pgs.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driscoll TA, Yu LC, Frangoul H, Krance RA, Nemecek E, Blumer J, et al. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob Agents Chemother. 2011;55(12):5770–5779. doi: 10.1128/AAC.00531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael C, Bierbach U, Frenzel K, Lange T, Basara N, Niederwieser D, et al. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob Agents Chemother. 2010;54(8):3225–3232. doi: 10.1128/AAC.01731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamoureux F, Duflot T, Woillard JB, Metsu D, Pereira T, Compagnon P, et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int J Antimicrob Agents. 2016;47(2):124–131. doi: 10.1016/j.ijantimicag.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Lei HP, Li Z, Tan ZR, Guo D, Fan L, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65(3):281–285. doi: 10.1007/s00228-008-0574-7. [DOI] [PubMed] [Google Scholar]
- 25.Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2):196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 26.Scholz I, Oberwittler H, Riedel KD, Burhenne J, Weiss J, Haefeli WE, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68(6):906–915. doi: 10.1111/j.1365-2125.2009.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langaee T, Ronaghi M. Genetic variation analyses by Pyrosequencing. Mutat Res. 2005;573(1–2):96–102. doi: 10.1016/j.mrfmmm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2016 doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luong ML, Al-Dabbagh M, Groll AH, Racil Z, Nannya Y, Mitsani D, et al. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother. 2016;71(7):1786–1799. doi: 10.1093/jac/dkw099. [DOI] [PubMed] [Google Scholar]
- 32.Berge M, Guillemain R, Tregouet DA, Amrein C, Boussaud V, Chevalier P, et al. Effect of cytochrome P450 2C19 genotype on voriconazole exposure in cystic fibrosis lung transplant patients. Eur J Clin Pharmacol. 2011;67(3):253–260. doi: 10.1007/s00228-010-0914-2. [DOI] [PubMed] [Google Scholar]
- 33.Gautier-Veyret E, Fonrose X, Tonini J, Thiebaut-Bertrand A, Bartoli M, Quesada JL, et al. Variability of voriconazole plasma concentrations after allogeneic hematopoietic stem cell transplantation: impact of cytochrome p450 polymorphisms and comedications on initial and subsequent trough levels. Antimicrob Agents Chemother. 2015;59(4):2305–2314. doi: 10.1128/AAC.04838-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chawla PK, Nanday SR, Dherai AJ, Soman R, Lokhande RV, Naik PR, et al. Correlation of CYP2C19 genotype with plasma voriconazole levels: a preliminary retrospective study in Indians. Int J Clin Pharm. 2015;37(5):925–930. doi: 10.1007/s11096-015-0143-y. [DOI] [PubMed] [Google Scholar]
- 35.Miyakis S, van Hal SJ, Solvag CJ, Ray J, Marriott D. Clinician ordering practices for voriconazole therapeutic drug monitoring: experiences of a referral laboratory. Ther Drug Monit. 2010;32(5):661–664. doi: 10.1097/FTD.0b013e3181ea3de6. [DOI] [PubMed] [Google Scholar]
- 36.Trifilio SM, Yarnold PR, Scheetz MH, Pi J, Pennick G, Mehta J. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob Agents Chemother. 2009;53(5):1793–1796. doi: 10.1128/AAC.01316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan A, Burhenne J, Riedel KD, Weiss J, Mikus G, Haefeli WE, et al. Modulators of very low voriconazole concentrations in routine therapeutic drug monitoring. Ther Drug Monit. 2011;33(1):86–93. doi: 10.1097/FTD.0b013e31820530cd. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Zhu H, Sun J, Cheng X, Xie J, Dong H, et al. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents. 2014;44(5):436–442. doi: 10.1016/j.ijantimicag.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Narita A, Muramatsu H, Sakaguchi H, Doisaki S, Tanaka M, Hama A, et al. Correlation of CYP2C19 phenotype with voriconazole plasma concentration in children. J Pediatr Hematol Oncol. 2013;35(5):e219–223. doi: 10.1097/MPH.0b013e3182880eaa. [DOI] [PubMed] [Google Scholar]
- 40.Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez-Llorens X, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48(6):2166–2172. doi: 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob Agents Chemother. 2010;54(10):4116–4123. doi: 10.1128/AAC.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noguerado Asensio A, Rodriguez Barrientos R, Zelaya Castro P, Sanchez Sempere A, Antuna Blanco F, Lutz Garcia E, et al. Use of acid-suppressive medications in hospitalized patients. An Med Interna. 2002;19(11):557–560. [PubMed] [Google Scholar]
- 43.Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7–8):1261–1266. doi: 10.1345/aph.1G703. [DOI] [PubMed] [Google Scholar]
- 44.Zvyaga T, Chang SY, Chen C, Yang Z, Vuppugalla R, Hurley J, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40(9):1698–1711. doi: 10.1124/dmd.112.045575. [DOI] [PubMed] [Google Scholar]
- 45.Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29(9):769–784. doi: 10.2165/00002018-200629090-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


