Abstract
Background:
Growth hormone-releasing peptides (GHRPs) constitute a group of small synthetic peptides that stimulate the growth hormone secretion and the downstream axis activity. Mounting evidences since the early 1980s delineated unexpected pharmacological cardioprotective and cytoprotective properties for the GHRPs. However, despite intense basic pharmacological research, alternatives to prevent cell and tissue demise before lethal insults have remained as an empty niche in the clinical armamentarium. Here, we have rigorously reviewed the investigational development of GHRPs and their clinical niching perspectives.
Methodology:
PubMed/MEDLINE databases, including original research and review articles, were explored. The search design was date escalated from 1980 and included articles in English only.
Results and Conclusions:
GHRPs bind to two different receptors (GHS-R1a and CD36), which redundantly or independently exert relevant biological effects. GHRPs’ binding to CD36 activates prosurvival pathways such as PI-3K/AKT1, thus reducing cellular death. Furthermore, GHRPs decrease reactive oxygen species (ROS) spillover, enhance the antioxidant defenses, and reduce inflammation. These cytoprotective abilities have been revealed in cardiac, neuronal, gastrointestinal, and hepatic cells, representing a comprehensive spectrum of protection of parenchymal organs. Antifibrotic effects have been attributed to some of the GHRPs by counteracting fibrogenic cytokines. In addition, GHRP family members have shown a potent myotropic effect by promoting anabolia and inhibiting catabolia. Finally, GHRPs exhibit a broad safety profile in preclinical and clinical settings. Despite these fragmented lines incite to envision multiple pharmacological uses for GHRPs, especially as a myocardial reperfusion damage-attenuating candidate, this family of “drugable” peptides awaits for a definitive clinical niche.
Keywords: GHRP, GHS, GH, cardioprotection, cytoprotection
Introduction
The family of peptidyl growth hormone (GH) secretagogues with broad cytoprotective properties came to light by the American endocrinologist Cyril Bowers, who observed that chemical analogs of enkephalin amide showed GH-releasing activity upon their incorporation to pituitary cultures. GHRP-6 (His-DTrp-Ala-Trp-DPhe-Lys-NH2) appeared as the first in-line synthetic peptide that specifically elicited GH dosage-related release in vitro and in vivo.1 Afterward, a heptapeptide, GHRP-1, and two other hexapeptides, GHRP-2 and hexarelin, were synthesized and addressed by basic and only sporadic clinical studies.
GH-releasing peptide (GHRP) family members began to be distinguished by their ability to prevent cardiac cell demise and to induce the restoration of critical cardiac functions upon ischemia/reperfusion episodes.2,3 A novel generation of promising cardioprotective agents had started to rise and set a bridge between endocrinology and cardiology.
Although the history of some of the foremost biomedical discoveries is permeated by serendipity,4 we deem that the well-established pivotal role of the GH/insulin-like growth factor-1 (IGF-1) axis for cardiomyocyte physiology, and the subtle alterations of this axis within the pathogenicity of dilated cardiomyopathy (DCM) and left ventricular (LV) dysfunction, ignited the idea of assessing the potentiality of GHRP to alleviate cardiac pathologies.5 It was far to be anticipated on those early days, however, that the GHRP-mediated cardiotropic and cytoprotective effects are superior to those shown by the exogenous administration of GH and are not shared by GH-releasing hormone (GHRH) and that, importantly, GHRPs exert their pharmacological actions via GH-independent pathways that obviously represented another turning point in this history.3
The progresses obtained and the experiences accrued along the years of work with these pioneer synthetic, nonendogenous GHRPs gradually led to the discovery of ghrelin, the 28-aminoacid hormone with GH-releasing action secreted by gastric cells6,7 with orexigenic, cardioprotective, and cytoprotective abilities for a myriad of cell populations.8–12
GHRP-6 is a small molecular weight peptide, effective when orally administered, stable, and economically low priced than others.13 Our observation that GHRP-6 intravenous administration proved to be safe in a dose scale-up clinical trial in healthy human volunteers is significantly important.14 Our demonstration that there is no in vivo pharmacological interaction between the peptide and a well-validated cardiovascular drug such as the beta blocker agent metoprolol is also relevant for GHRP-6 pharmacological “positioning”.15 Since for years, GHRP-6 has been the platform of our experimental work; we address particular attention to its investigational development as for hexarelin and GHRP-2.
The goal of this review is to offer a summary of the most relevant achievements of the pharmacological knowledge with synthetic GHRP (GHRP-6, GHRP-2, and hexarelin) in a historical perspective line. General cyto- and cardioprotection fields are specially focused, since all these agents have contributed to the discovery of novel functions and mechanisms involved in cellular survival, senescence, and death. We deem that cardiologists, clinicians, and basic and clinical pharmacologists would receive some benefit from this text, in correspondence to the futuristic pharmacological opportunities offered by these agents. To date, cytoprotection remains as an orphan niche in contemporary medical armamentarium.
Search methodology
The search strategy was based on the PubMed/MEDLINE electronic databases including original research and review articles. The search was progressively date escalated from 1980 and included articles in English only. The search terms were as follows: growth hormone secretagogues (GHS), GHRP, growth hormone secretagogue receptor (GHS-R), CD36, cardiac ischemia/reperfusion, cardiac stunning, heart failure, cytoprotection, and cardioprotection.
Pharmacological repositioning of GHRP
Despite their potent and reproducible GH-releasing activity, the clinical use of GHRPs as orally active growth-promoting agents and anabolic antiaging drugs remains to be confirmed.13 Accordingly, the early years’ enthusiasm as an alternative for GH replacement therapy faded away soon after their discovery.16 Nevertheless, it is likely that the myocardial, vascular, and multiorgan expression of the GHRP receptors may have contributed to reinforce the cardiovascular application stream of these peptides.
A remarkable specific (125)I-Tyr-Ala-hexarelin binding was observed in the human cardiovascular system where the highest binding levels were detected in ventricles, followed by atria, aorta, coronaries, carotid, endocardium, and vena cava. In other experiments on H9c2, cardiomyocyte-specific GHRP binding was found along with a potent antiapoptotic activity.3 The primarily investigated receptor was the growth hormone secretagogue receptor type 1a (GHS-R1a), which was detected in isolated human cardiomyocytes, myocardium, and aorta samples.17 It has been recently shown that GHS-R1a is a sort of “promiscuous receptor” involved in many systems and behavioral patterns such as reward, feeding, and memory, which makes it an attractive pharmacological target.18 Years later, the synthetic GHRP hexarelin was acknowledged as a ligand of another protein identified as CD36, a scavenger receptor that is expressed in various tissues, including monocytes/macrophages and the endothelial microvasculature. Activation of CD36 in perfused hearts by hexarelin was shown to increase coronary perfusion pressure in a dose-dependent manner. Contrariwise, this effect was lacking in hearts from CD36-null mice and hearts from spontaneous hypertensive rats genetically deficient in CD36.19,20 Thus, it is currently accepted that two cardiac receptor subtypes mediate the pharmacological actions of GHRP-6, GHRP-2, and hexarelin.21,22
Cardioprotective evidences
The importance of limiting myocardial ischemia/reperfusion injury has been appreciated since Braunwald23,24 proposed that the extent and severity of tissue damage were not predetermined at the onset of ischemia, but could be modified by therapeutic manipulations applied during ischemia. Few years ago, a National Institutes of Health (NIH) expert’s panel concluded that cardioprotection is at a crossroads since approaches to identify cardioprotective therapies have been disappointing during the past 30 years.25 This may be related to the fact that the multiple candidates assayed so far target one single pathogenic event of the multiple damage cascade involved in myocardial damage and failure.25
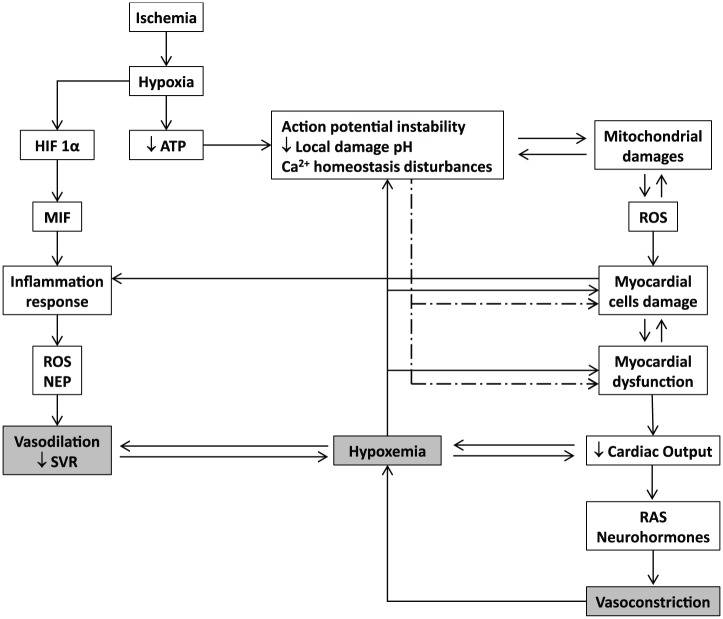
Myocardial ischemia/reperfusion damage entails multiple molecular and biochemical mechanisms that each alone is sufficiently injurious to disturb an organ whose mechanical performance is dependent upon the stability of ionic/electrical pumps. Oxidative stress, intracellular calcium overload, pH changes, mitochondrial dysfunction, inflammation, and excessive neurohormones are part of an interactive and self-perpetuating continuum of the myocardial injury cascade (Figure 1). The evidences obtained along the years of experimental screening of the synthetic GHRP suggest that each single member of this family of peptides is able to simultaneously counteract different injurious operators in the myocardial ischemic event.
Figure 1.
Basic molecular pathophysiological cascade of acute myocardial infarction. Hypoxia triggers an acute failure in mitochondrial respiratory function when the diffusible oxygen stores become exhausted. Adenosine triphosphate reserves are rapidly depleted, and there is a respiratory shift toward an anaerobic profile. Lactate, H+ ions, CO2, and potassium accumulate may lead to arrhythmias, microendothelial damage, myocardiocytes stunning, and cell death. Adenosine triphosphate (ATP) depletion is irrevocably ligated to the inability of maintaining the normal negative resting membrane potential, to an alteration of calcium homeostasis (intracellular Ca2+ ([Ca2+]i) overload), which may eventually lead to different patterns of abnormal cardiac contraction. Mitochondrial functionality becomes abnormal, establishing the so-called “open pore” (mitochondrial permeability transition pore [mPTP]), leading to local cell death. In this scenario, mitochondria turn into an active ROS manufacturing plant that increases and perpetuates mitochondrial damages and dysfunction. The failure of myocardial contractility (contractility depression) is a precocious and multifactorial consequence of ischemia, which may eventually lead to reduced cardiac output and heart failure. This situation may translate into a self-perpetuated vicious circle, thus amplifying the ischemic episode and the myocardial wall stress. The local inflammatory reaction is a useful but critical operator within the myocardial ischemia/reperfusion damage process. Hypoxia itself activates the HIF-α/MIF axis and the consequent downstream inflammatory cascade. The locally secreted pro-inflammatory cytokines are involved in a self-perpetuating process in the ROS chain reaction, inflammation, and cellular damage.
Experimental studies in 1997 proved that hexarelin could reverse the cardiac dysfunction in GH-deficient animals immunized by the administration of an anti-GHRH serum. Ex vivo and in vivo systems converged to document that hexarelin progressively and globally improved LV function even under postischemic scenarios. These experiments showed that the synthetic secretagogue protective activity was independent from any further stimulation derived from the somatotropic function.26 In 1998, this group demonstrated that hexarelin protected against postischemic ventricular dysfunction in senescent hearts of aged male rats. Both ex vivo and in vivo, GHRPs offered a striking heart protection against reperfusion stunning, improved ventricular pressures and volumes, and reduced CK concentration in perfusate. Again, they sustained the concept that the protection afforded by the peptide is likely due to a direct cardiotropic action that appeared far greater than that induced by GH administration in a concurrent control group.27 A more defining protocol was assumed in 1999 as the study included hypophysectomized rats, to ascertain whether hexarelin had non-GH-mediated protective effects on the heart. The authors showed that hexarelin attenuated the ischemia/reperfusion damage and prevented elevation of LV end-diastolic pressure, coronary perfusion pressure, reactivity of the coronary vasculature to angiotensin II, and the release of creatine kinase in hypophysectomized animals.28 These three experiments were pivotal to define GHRP intrinsic cardioprotective ability.
In 1999, seven adult patients with GH deficiency and LV failure received hexarelin administrations. The GH response to hexarelin was negligible in these patients. Moreover, hexarelin administration increased their left ventricular ejection fraction (LVEF) without changing catecholamine levels, mean blood pressure (MBP), or cardiac output. For the first time, the acute administration of hexarelin proved to induce a positive inotropic effect in humans, which is GH independent and mediated by specific myocardial receptors for a GH secretagogue peptide.29 A subsequent study involving hexarelin administration to normal adults, severe GH-deficient patients (N = 7), and patients with severe ischemic DCM (N = 12) confirmed that the acute administration of hexarelin exerts a GH-independent positive inotropic effect likely mediated by specific GHRP myocardial receptors.30 This pioneering group subsequently evaluated the cardiac performances of the acute hexarelin administration (2.0 µg/kg, i.v.) in patients undergoing bypass surgery in comparison to patients given GH-releasing hormone, recombinant human GH, or placebo. The study concluded that the acute administration of hexarelin improved cardiac performance without any relevant variation in systemic vascular resistance and induced a reduction of wedge pressure and, significantly, that these cardiotropic effects were not shown by the other concurrent interventions.31
These studies on human subjects were paralleled by contemporary experimental progresses in basic science, which demonstrated that hexarelin enhanced H9c2 cardiomyocyte proliferation in a dose-dependent manner. Since these were in vitro experiments, they completely excluded a potential intervention of the GH axis and clearly indicated a direct GHRP binding to cardiac cells membranes.32 Weekers et al33 demonstrated that 14 days of pretreatment with GHRP-2, but not GH, selectively protected against the postischemic diastolic dysfunction and myocardial stunning of excised hearts submitted to ischemia/reperfusion in isolated, perfused rabbit hearts.
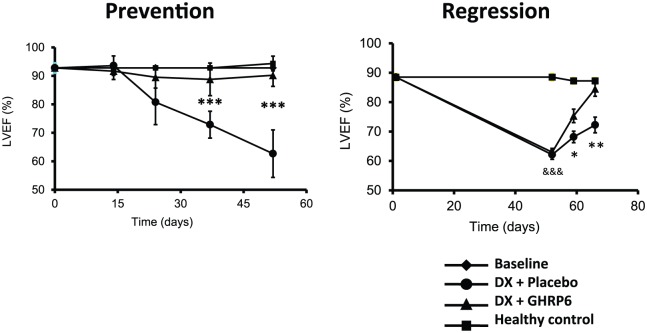
TO-2 hamster model of DCM was characterized by progressive LV dilation, LV wall thinning, LV systolic dysfunction, and reduced life span; both GHRP-2 and GHRP-6 ameliorated all the dysfunctional ventricular parameters and reduced the progression of the DCM.34 We also examined the potential impact of GHRP-6 in a rat model of DCM/heart failure induced by doxorubicin (DX). The concurrent administration of GHRP-6 was undertaken with the purpose to study the potential prophylactic impact before the cardiac function demise. As part of the prolonged treatment with DX, the concurrent administration of GHRP-6 completely prevented failure of cardiac function, which was evaluated as the percentage of ejection fraction by echocardiography (Figure 2, prevention). This effect significantly increased the survival of animals. Similar results were obtained in the therapeutic administration schedule, with functional recovery of cardiac muscle to physiological levels (Figure 2, regression), also attenuating systemic damages and, consequently, decreasing the mortality rates of rats. In the experimental model of DX-induced cardiac and systemic damage, GHRP-6 additionally attenuated various extracardiac damages observed in the renal tubular and bronchoalveolar epithelial structures as in the hepatic parenchyma.35
Figure 2.
Effect of GHRP-6 on the left ventricular ejection fraction in an experimental model of dilated cardiomyopathy (DCM). Echocardiography data were derived from our DCM model including the prevention and the regression study protocols in rats.35 The prevention protocol conceived the concurrent administration of GHRP-6 as part of a prolonged treatment with doxorubicin. The concomitant GHRP-6 completely prevented cardiac function failure evaluated as the percentage of ejection fraction by echocardiography. The regression approach examined the GHRP-6 intervention once LVEF was already deteriorated. As shown, the therapeutic administration schedule introduced a full functional recovery of cardiac muscle. Data corresponding to percentage of ejection fraction (%EF) are represented as a mean value ± standard error of the mean for each experimental group. (*), (**), and (***) represent the statistically significant differences between groups treated either with placebo or GHRP-6, according to Student’s t-test.
In more recent years, these data were further substantiated using again the TO-2 hamster DCM biomodel in which GHRP-2 reduced the progression of LV remodeling, dysfunction, and the ensued myocardial fibrosis by an antioxidant mechanism.36 The abovementioned myocardial fibrotic process amelioration reveals an additional potential use for GHRP in an unmet medical need. Chronic treatment with hexarelin in spontaneously hypertensive rats, in addition to decreasing ventricular hypertrophy, diastolic dysfunction, and high blood pressure, significantly reduced cardiac fibrosis by decreasing interstitial and perivascular myocardial collagen deposition and myocardial hydroxyproline content. Mechanistically, hexarelin treatment increased matrix metalloproteinase (MMP)-2 and MMP-9 activities and decreased myocardial mRNA expression of tissue inhibitor of metalloproteinase (TIMP)-1.37
Our group has contributed to validate the potential antifibrotic abilities of GHRP-6 in animal models of liver cirrhosis38 and hypertrophic scars,39 in which via a peroxisomal proliferator-activated receptor gamma (PPARγ)-driven cascade, GHRP-6 intervention reduced TGF-β1 and connective tissue growth factor (CTGF) expression, which translated in a dramatic reduction in the accumulation of collagen and other extracellular matrix (ECM) proteins.
A seminal report by a Merck Research Laboratories group dated 2003 demonstrated for the first time that chronic treatment with GHRP-6 (21 days) prevented sudden death in a canine model of DCM and subsequently subjected to acute myocardial infarction (AMI). In the meantime, the mortality rates for the vehicle and GH-treated groups were about 50%. Although the authors do not precise the mechanism underlying the 100% survival in the GHRP-6 group, an enhanced regional myocardial compensatory function of the nonischemic zone was assumed.40 This notion could be validated at least in part by the fact that the cardiotropic effects shown by GHRP-1, GHRP-2, GHRP-6, and hexarelin in cardiomyocytes and isolated, denervated, perfused hearts are mediated by an elevation of Ca2+ influx through the voltage-gated calcium channel, triggering Ca2+ release from thapsigargin-sensitive intracellular stores, which translated in a positive inotropic response without a chronotropic effect.41 More recent data confirm the ability of hexarelin and other secretagogue peptides that bind and activate the GHS-R1a, to control the cardiac action potential and reduce apoptosis of cardiomyocytes, derived from isolated hearts subjected to ischemia/reperfusion episodes.42
Consistent with these data, our group observed a transient inotropic effect of about 15 minutes in both healthy and infarcted rabbits following a single GHRP-6 intravenous bolus (400 µg/kg). Echocardiography recordings indicated a 15%–20% elevation of the ejection fraction as an increase in shortening fraction (Juan Valiente Mustelier and Jorge Berlanga Acosta, unpublished observations, 2007). More recent studies based on isolated murine hearts that underwent periods of ischemia and reperfusion (I/R) confirm that pre- or posttreatments with hexarelin for instance prevented the intracellular disturbances in Ca+2 transients through recovery of p-PLB after the I/R insult.43 Other studies involving adult Wistar rat ventricular myocytes have confirmed the positive inotropic response induced by hexarelin and other secretagogue peptides that bind the GHS-R1a, which activates protein kinase C signaling cascade.44
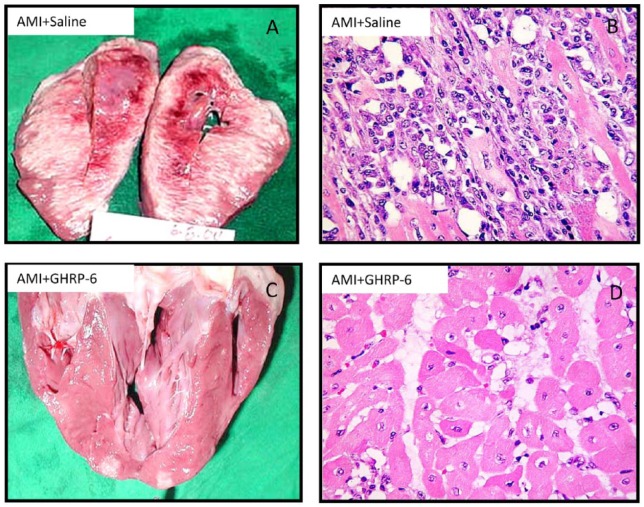
In 2005, we undertook a porcine model of AMI via left circumflex artery occlusion for 1 hour followed by a 72-hour reperfusion period. GHRP-6 rescued ischemic myocardium from death for over 70% of the area at risk (Figure 3), and that in addition to enhance survival signaling pathways/gene expression of the PI-3K, AKT1, and BCL2 pathways, GHRP-6 decreased reactive oxygen species (ROS) spillover, the inflammatory marker CRP, and preserved the antioxidant defenses.45 These antioxidant and anti-inflammatory properties have also been attributed to GHRP-2 when its antiatherogenic potential was examined in ApoE(−/−) mice so that 12/15-lipoxygenase, interferon gamma, and macrophage migration inhibitory factor (MMF) gene expression were accounted. Furthermore, in cultured aortic smooth muscle cells, GHRP-2 prevented the generation of peroxides, the downregulation of IGF-1 receptor, and the commitment of apoptosis.46
Figure 3.
Morphological evidences representative of the GHRP-6 effect in a porcine model of myocardial infarction. Effect of the GHRP-6 on AMI size and severity. (A, B) Macroscopic and histological images of AMI damage in animals treated with placebo. (C, D) Macroscopic and histological images representative of the GHRP-6 cardioprotective effect. Histological fragments were in every case collected from apparently normal zones, adjacent to the AMI necrotic core. Rats treated with GHRP-6 exhibited mostly preserved or marginally damaged (sarcoplasmic edema) myofibrils. No myofibrolysis was observed, although a number of ghost nuclei appeared. (H/E, ×20 magnification).
Hexarelin via CD36 occupation increases the expression of multiple genes involved in fatty acid mobilization in adipocytes toward the mitochondrial oxidative phosphorylation, and many of these upregulated genes are known targets of PPARγ. Consistent with this, electron microscopy of hexarelin-treated adipocytes reflects highly organized cristae formation that spans the entire width of mitochondria, with a concomitant cytochrome c oxidase activity enhancement. Although this signaling and activation cascade has not been described for myocardial cells so far, the potential existence of these phosphorylative and mitochondriogenic mechanisms in the heart, and its potential amplification by GHRP ligands, may eventually contribute to myocardial salvage during critical ischemia periods.47 In a more recent study based on a myocardial infarction model, and addressed to examine whether hexarelin treatment can compensate for ghrelin deficiency in ghrelin-knockout mice, the mortality within two weeks was significantly lower in the hexarelin (6.7%) and ghrelin groups (14.3%) than in the vehicle group (50%). Furthermore, hexarelin was more effective than ghrelin as judged by the ejection fraction and other LV-dependent physiological constants as dP/dt max and dP/dt min, which is a measure of LV global contractility.48
In extracardiac models of striated muscles atrophy, GHRP-2 exerted a potent myoprotective effect, presumably via the direct agonistic stimulation of the GHS-R1a since no elevation of IGF-1 transcript was observed.49 Thus, it is likely that GHRP cardioprotective effects in scenarios of DCM may be somehow mediated by a trophic or anabolic mechanism. Based on the benefits of GHRP-6 on muscle functions, a newly synthesized GHRP-6-biotin conjugate tested on cultured myoblasts showed that it induced the expression of myogenic proteins and IGF-1 levels similar to the concentrations of energy metabolites and the corresponding enzymes. Practical applications of the GHRP-6-biotin conjugate could include amelioration of sarcopenia and/or cardiac cachexia.50
Extracardiac cytoprotective actions of GHRP
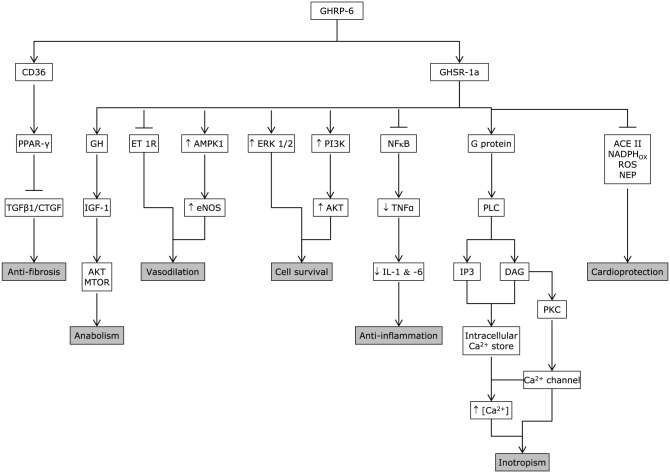
As judged by the PubMed outcomes, the cytoprotective effects of synthetic peptidyl GHRP appear far less studied in noncardiac, parenchymal epithelial organs or multiple organ systems than in the cardiovascular system. However, the results of the reviewed studies are consistent with a broad cytoprotective influence for various organs by reducing inflammation and preventing necrosis and/or apoptosis. The mechanism of GHRP-mediated pharmacological actions is shown in Figure 4.
Figure 4.
GHRP mechanism of action. GHRPs are endowed with the ability to bind two different receptors that seem to mediate its cytoprotective and other pharmacological properties (GHS-R1a and CD36). The main biological properties/pharmacological actions of GHRP-6 as cyto- and cardioprotective candidates are summarized as follows: Inotropic: mediated by an elevation of Ca2+ influx via PLC/DAG/PKC, through the voltage-gated calcium channel, triggering Ca2+ release from thapsigargin-sensitive intracellular stores, which translated in a positive inotropic response without a chronotropic effect. Anti-fibrotic: via upregulation of PPARγ, which is followed by a transforming growth factor-beta (TGF-β), CTGF, and platelet-derived growth factor (PDGF) downregulation. Anti-inflammatory: blunts NFκB expression and activation. Cell survival: it involves the phosphatidylinositol 3-kinase/RAC-alpha serine/threonine-protein kinase (PI-3K/AKT1) pathway, as the induction of the hypoxia-inducible factor-1 alpha (HIF-1α). Cardioprotective: as shown, it involves different biological actions that converge to enhance cardiomyocytes survival. Vasodilatory: it seems to involve e-NOS upregulation and endothelin activity reduction. Anabolic: it is mediated by the IGF-1/AKT1 and mTOR pathway activity.
Years ago, our group examined the cytoprotective effects exerted by the GHRP-6 preventive administration in the hepatic tissue subjected to I/R, as in other distal organs from the ischemic site (ie, lungs, kidneys, and small intestine). Histological and biochemical results allowed us to conclude that the pharmacological preconditioning induced by the GHRP-6 treatment attenuated I/R liver damage. Besides respiratory distress syndrome like pulmonary changes, intestinal transmural infarct and acute tubular necrosis in kidneys were significantly reduced. These results indicated for the first time a systemic cytoprotective effect for the GHRP-6, suggesting its potential efficacy to control the inflammatory response associated with acute I/R and shock, which eventually originated multiple organs damage (MOD). Cytoprotection induced by GHRP-6 treatment was also related to the attenuation in the generation of ROS and preservation of the antioxidant defense reserves. Histological analysis as the assessment of myeloperoxidase activity evidenced a clear anti-inflammatory GHRP-6-induced effect in the liver and remote organs. Moreover, the molecular mechanism mediating the action of GHRP-6 peptide was shown to involve the phosphatidylinositol 3-kinase/RAC-alpha serine/threonine-protein kinase (PI-3K/AKT1) pathway, as the induction of the hypoxia-inducible factor-1 alpha (HIF-1α) all committed in cellular survival.51 Subsequently, Granado et al52 examined the potential anti-inflammatory impact of GHRP-2 in lipopolysaccharide (LPS)-challenged rats. GHRP-2 administration attenuated the effects of LPS on the elevation of circulating levels of transaminases, nitrites/nitrates, and tumor necrosis factor-alpha (TNF-α), via direct interaction with liver nonparenchymal cells. Globally, the exogenous administration of these two synthetic GHRPs appeared to exert a potent hepatoprotective role by attenuating the inflammatory response orchestrated by liver-resident macrophages. Another line of evidences document the benefits of 15-daily injections of GHRP-2 (100 μg/kg) in arthritic rats, so that the treatment ameliorated the external symptoms of arthritis and decreased the circulating levels of interleukin 6 (IL-6) as the nitrite/nitrate release from peritoneal macrophages in vitro. This experiment extrapolated the counter-inflammatory properties of GHRP-2 to a nonepithelial organ and suggested again a direct interaction with ghrelin receptor of immune cells.53 Similarly, effects have been attributed to ghrelin by inhibiting the inflammatory response via AKT1-activated pathway with a concomitant reduction of myeloperoxidase activity, the rate of apoptosis, and oxidative stress.54 All these data suggest that GHRPs exert a mutually inclusive beneficial effect by directly protecting parenchymal organs epithelial cells, and simultaneously by modulating the magnitude of the inflammatory response by direct interaction with the effector immune cells. Supporting the protective effect of GHRP-6 on epithelial organs, a recent study has excellently described and dissected the mechanistic bases on how GHRP-6 prevented gastric mucosal damage induced by water immersion restraint (WRS) and other forms of stress. The data indicated that the protective effect of GHRP-6 on WRS-induced gastric mucosal injury is somehow mediated by peripherally suppressing the vagal efferent effect on the stomach, including gastric acid secretion. Although more studies are clearly demanded, the present findings open the possibility to use GHRP-6 in preventing Curling ulcers.55
Additionally and not less relevant, GHRP-6 appears as an excellent partner to combine with other molecules (ie, epidermal growth factor [EGF]) because their exclusive actions seem to achieve a kind of synergism, useful to target the multiples nodes of complex pathophysiological processes, and thus to enhance tissue repair processes.56 Garcia del Barco and coworkers in our group have opened unprecedented avenues, by combining GHRP-6 and EGF as a therapeutic approach to ameliorate the damages of multiple sclerosis,57 peripheral axonal pathology,58 and brain ischemia in animal models.59,60 They have demonstrated that in all these experimental substrates the combined action of GHRP-6 and EGF is associated with a better outcome in both clinical and pathological fields.
Finally, an exciting medical opportunity could be opened for synthetic GHRP to treat the threatening cancer-associated anorexia–cachexia syndrome in advanced-stage cancer patients. Although the mechanistic bases of this syndrome are not fully understood, it represents a major impediment for the course of chemotherapy. In a rodent model of cancer-bearing chemotherapy, GHRP-2 administration increased appetite/food intake and prolonged median survival time, which certainly suggests that GHRP-2 may improve the quality of life of cancer patients by correcting its nutritional and metabolic states.61 These data may also incite to further studies in the search for a potential niche for GHRP to counteract the catabolic states of prolonged critical illness, invasive surgeries, severe burn traumas, etc.
Concluding remarks
Drug discovery is an uncertain ground in which disappointments and rewards are encountered. Most of those who have been involved in GHRP research have enjoyed clear-cut data, which in most of the cases are all in with very few outs. Exceptionally, a pharmacologically active agent appears to be endowed with such a variety of useful properties as to make it highly drugable. The fact that synthetic GHRPs bind at least two different and biologically significant receptors that seem not to be redundant in nature and are largely represented in most organs and tissues broadens their biological activities and increases their pharmacological potentialities. This suggests that GHRPs may stimulate multiple cells and simultaneously trigger different signaling pathways. The information gathered so far in terms of the molecular cytoprotective mechanism of GHRPs is inconclusive and fragmentary, which has become difficult to disclose the hidden facts behind their biological effects. Nevertheless, it is reasonable that these molecules share the ability to knock life-sensitive pathways and restore critical organelle physiology at very proximal levels. Beyond their ability to enhance the survival of a diversity of cells and tissues before adverse episodes, GHRP members exert an agonistic effect of the GH/IGF-1 axis, promoting anabolia and deterring catabolism and sarcopenia.
Despite all these pharmacological advantages and that GHRPs exhibit a broad safety profile, their clinical development has been erratic and irregular. This has been a deterrence factor for their definitive positioning within cardiology and intensive care medicine for years. In the meantime, novel drugs and therapeutic strategies are demanded to protect organs and tissues exposed to ischemia and other lethal insults in the clinical practice.
Footnotes
Peer Review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 328 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations: AKT1, RAC-alpha serine/threonine-protein kinase; AMI, acute myocardial infarction; CTGF, connective tissue growth factor; DCM, dilated cardiomyopathy; dP/dt, the rate of left ventricle maximal pressure rise in early systole; DX, doxorubicin; ECM, extracellular matrix; EGF, epidermal growth factor; ERK1/2, extracellular signal-regulated kinase 1/2; GH, growth hormone; GHRH, growth hormone-releasing hormone; GHRPs, growth hormone-releasing peptides; GHS, growth hormone secretagogues; GHS-R, growth hormone secretagogue receptor; GHS-R1a, growth hormone secretagogue receptor type 1a; HIF-1α, hypoxia-inducible factor-1 alpha; I/R, ischemia and reperfusion; IGF-1, insulin-like growth factor-1; IL-1β, interleukin-1 beta; IL-6, interleukin 6; LPS, lipopolysaccharide; LV, left ventricle; LVEF, left ventricular ejection fraction; MBP, mean blood pressure; MIF, macrophage migration inhibitory factor; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; MOD, Multiple Organs Damage; NEP, nitrosylation end products; NIH, National Institute of Health; PDGF, platelet-derived growth factor; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1 alpha; PI-3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PPARγ, peroxisome proliferator-activated receptor gamma; RAS, rennin–angiotensin system; rhGH, recombinant human growth hormone; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; TIMP, tissue inhibitor of metalloproteinase; TNF-α, tumor necrosis factor alpha.
Author Contributions: Conceived and designed the experiments: JBA, AAC, DGBH, YMM, ARU, AGO, VFC, FHB, GGN. Analyzed the data: JBA, AAC, DGBH, YMM, ARU, AGO, VFC, FHB. Wrote the first draft of the manuscript: JBA. Contributed to the writing of the manuscript: JBA, AGO, YMM. Agree with manuscript results and conclusions: JBA, AAC, DGBH, YMM, ARU, AGO, VFC, FHB, QB, GGN. Jointly developed the structure and arguments for the paper: JBA, AGO, GGN. Made critical revisions and approved final version: QB, GGN. All authors reviewed and approved of the final manuscript.
References
- 1. Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114(5):1537–1545. [DOI] [PubMed] [Google Scholar]
- 2. Corneli G, Gasco V, Prodam F, Grottoli S, Aimaretti G, Ghigo E. Growth hormone levels in the diagnosis of growth hormone deficiency in adulthood. Pituitary. 2007;10(2):141–149. [DOI] [PubMed] [Google Scholar]
- 3. Muccioli G, Broglio F, Valetto MR, et al. Growth hormone-releasing peptides and the cardiovascular system. Ann Endocrinol (Paris). 2000;61(1):27–31. [PubMed] [Google Scholar]
- 4. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broglio F, Fubini A, Morello M, et al. Activity of GH/IGF-I axis in patients with dilated cardiomyopathy. Clin Endocrinol (Oxf). 1999;50(4):417–430. [DOI] [PubMed] [Google Scholar]
- 6. Bowers CY. Growth hormone-releasing peptide (GHRP). Cell Mol Life Sci. 1998;54(12):1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharya SK, Andrews K, Beveridge R, et al. Discovery of PF-5190457, a potent, selective, and orally bioavailable ghrelin receptor inverse agonist clinical candidate. ACS Med Chem Lett. 2014;5(5):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khatib MN, Simkhada P, Gode D. Cardioprotective effects of ghrelin in heart failure: from gut to heart. Heart Views. 2014;15(3):74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kishimoto I, Tokudome T, Hosoda H, Miyazato M, Kangawa K. Ghrelin and cardiovascular diseases. J Cardiol. 2012;59(1):8–13. [DOI] [PubMed] [Google Scholar]
- 11. Pietra C, Takeda Y, Tazawa-Ogata N, et al. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle. 2014;5(4):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Root AW, Root MJ. Clinical pharmacology of human growth hormone and its secretagogues. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2(1):27–52. [PubMed] [Google Scholar]
- 13. Broglio F, Arvat E, Gottero C, et al. Natural and synthetic growth hormone secretagogues: do they have therapeutic potential? Treat Endocrinol. 2003;2(3):153–163. [DOI] [PubMed] [Google Scholar]
- 14. Selman-Housein-Bernal KH, Hernández-Bernal F, Abreu-Cruz AA, Valenzuela-Silva C, Berlanga-Acosta J, López-Saura PA. Seguridad clínica del péptido liberador de la hormona de crecimiento-6 (GHRP-6) en voluntarios sanos. Investigaciones Médico Quirúrgicas. 2014;6(1):81–91. [Google Scholar]
- 15. Valiente-Mustelier J, Garcia del Barco D, Guillen-Nieto G, et al. Cardiotropic effect of GHRP-6: in vivo characterization by echocardiography. Biotecnología Aplicada. 2013;30:285–289. [Google Scholar]
- 16. Marleau S, Mulumba M, Lamontagne D, Ong H. Cardiac and peripheral actions of growth hormone and its releasing peptides: relevance for the treatment of cardiomyopathies. Cardiovasc Res. 2006;69(1):26–35. [DOI] [PubMed] [Google Scholar]
- 17. Zhang G, Yin X, Qi Y, et al. Ghrelin and cardiovascular diseases. Curr Cardiol Rev. 2010;6(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wellman M, Abizaid A. Growth hormone secretagogue receptor dimers: a new pharmacological target (1,2,3). eNeuro. 2015;2(2):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodart V, Febbraio M, Demers A, et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res. 2002;90(8):844–849. [DOI] [PubMed] [Google Scholar]
- 20. Demers A, McNicoll N, Febbraio M, et al. Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J. 2004;382(pt 2):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao Y, Tokudome T, Kishimoto I. The cardiovascular action of hexarelin. J Geriatr Cardiol. 2014;11(3):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosa RM, Zhang Z, Shao R, Deng C, Chen J, Chen C. Implications of ghrelin and hexarelin in diabetes and diabetes-associated heart diseases. Endocrine. 2015;49(2):307–323. [DOI] [PubMed] [Google Scholar]
- 23. Braunwald E. Clinical efforts to reduce myocardial infarct size – the next step. J Cardiovasc Pharmacol Ther. 2011;16(3–4):349–353. [DOI] [PubMed] [Google Scholar]
- 24. Braunwald E. Cardiovascular science: opportunities for translating research into improved care. J Clin Invest. 2013;123(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolli R, Becker L, Gross G, et al. ; NHLBI Working Group on the Translation of Therapies for Protecting the Heart from Ischemia. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95(2):125–134. [DOI] [PubMed] [Google Scholar]
- 26. De Gennaro Colonna V, Rossoni G, Bernareggi M, Muller EE, Berti F. Cardiac ischemia and impairment of vascular endothelium function in hearts from growth hormone-deficient rats: protection by hexarelin. Eur J Pharmacol. 1997;334(2–3):201–207. [DOI] [PubMed] [Google Scholar]
- 27. Rossoni G, De Gennaro Colonna V, Bernareggi M, Polvani GL, Muller EE, Berti F. Protectant activity of hexarelin or growth hormone against postischemic ventricular dysfunction in hearts from aged rats. J Cardiovasc Pharmacol. 1998;32(2):260–265. [DOI] [PubMed] [Google Scholar]
- 28. Locatelli V, Rossoni G, Schweiger F, et al. Growth hormone-independent cardioprotective effects of hexarelin in the rat. Endocrinology. 1999;140(9):4024–4031. [DOI] [PubMed] [Google Scholar]
- 29. Bisi G, Podio V, Valetto MR, et al. Cardiac effects of hexarelin in hypopituitary adults. Eur J Pharmacol. 1999;381(1):31–38. [DOI] [PubMed] [Google Scholar]
- 30. Broglio F, Benso A, Valetto MR, et al. Growth hormone-independent cardiotropic activities of growth hormone-releasing peptides in normal subjects, in patients with growth hormone deficiency, and in patients with idiopathic or ischemic dilated cardiomyopathy. Endocrine. 2001;14(1):105–108. [DOI] [PubMed] [Google Scholar]
- 31. Broglio F, Guarracino F, Benso A, et al. Effects of acute hexarelin administration on cardiac performance in patients with coronary artery disease during by-pass surgery. Eur J Pharmacol. 2002;448(2–3):193–200. [DOI] [PubMed] [Google Scholar]
- 32. Pettersson I, Muccioli G, Granata R, et al. Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J Endocrinol. 2002;175(1):201–209. [DOI] [PubMed] [Google Scholar]
- 33. Weekers F, Van HE, Isgaard J, Van den Berghe G. Pretreatment with growth hormone-releasing peptide-2 directly protects against the diastolic dysfunction of myocardial stunning in an isolated, blood-perfused rabbit heart model. Endocrinology. 2000;141(11):3993–3999. [DOI] [PubMed] [Google Scholar]
- 34. Iwase M, Kanazawa H, Kato Y, et al. Growth hormone-releasing peptide can improve left ventricular dysfunction and attenuate dilation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2004;61(1):30–38. [DOI] [PubMed] [Google Scholar]
- 35. Cibrian-Vera D, Berlanga-Acosta J, Guevara L, et al. Efecto citoprotector cardíaco y extracardíaco del péptido GHRP6. Biotecnología Aplicada. 2008;25(3):276–278. [Google Scholar]
- 36. Kato Y, Iwase M, Ichihara S, et al. Beneficial effects of growth hormone-releasing peptide on myocardial oxidative stress and left ventricular dysfunction in dilated cardiomyopathic hamsters. Circ J. 2010;74(1):163–170. [DOI] [PubMed] [Google Scholar]
- 37. Xu X, Ding F, Pang J, et al. Chronic administration of hexarelin attenuates cardiac fibrosis in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2012;303(6):H703-H711. [DOI] [PubMed] [Google Scholar]
- 38. Berlanga-Acosta J, Vázquez-Blomquist D, Cibrian-Vera D, et al. Growth Hormone Releasing Peptide 6 (GHRP6) reduces liver fibrosis in CCl4 chronically intoxicated rats. Biotecnología Aplicada. 2012;29:60–72. [Google Scholar]
- 39. Mendoza-Marí Y, Fernández-Mayola M, Aguilera-Barreto A, et al. Growth hormone-releasing peptide 6 enhances the healing process and improves the esthetic outcome of the wounds. Plast Surg Int. 2016;2016:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen YT, Lynch JJ, Hargreaves RJ, Gould RJ. A growth hormone secretagogue prevents ischemic-induced mortality independently of the growth hormone pathway in dogs with chronic dilated cardiomyopathy. J Pharmacol Exp Ther. 2003;306(2):815–820. [DOI] [PubMed] [Google Scholar]
- 41. Xu XB, Cao JM, Pang JJ, et al. The positive inotropic and calcium-mobilizing effects of growth hormone-releasing peptides on rat heart. Endocrinology. 2003;144(11):5050–5057. [DOI] [PubMed] [Google Scholar]
- 42. Ma Y, Zhang L, Launikonis BS, Chen C. Growth hormone secretagogues preserve the electrophysiological properties of mouse cardiomyocytes isolated from in vitro ischemia/reperfusion heart. Endocrinology. 2012;153(11):5480–5490. [DOI] [PubMed] [Google Scholar]
- 43. Ma Y, Zhang L, Edwards JN, Launikonis BS, Chen C. Growth hormone secretagogues protect mouse cardiomyocytes from in vitro ischemia/reperfusion injury through regulation of intracellular calcium. PLoS One. 2012;7(4):e35265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun Q, Ma Y, Zhang L, Zhao YF, Zang WJ, Chen C. Effects of GH secretagogues on contractility and Ca2+ homeostasis of isolated adult rat ventricular myocytes. Endocrinology. 2010;151(9):4446–4454. [DOI] [PubMed] [Google Scholar]
- 45. Berlanga J, Cibrian D, Guevara L, et al. Growth-hormone-releasing peptide 6 (GHRP6) prevents oxidant cytotoxicity and reduces myocardial necrosis in a model of acute myocardial infarction. Clin Sci (Lond). 2007;112(4):241–250. [DOI] [PubMed] [Google Scholar]
- 46. Titterington JS, Sukhanov S, Higashi Y, Vaughn C, Bowers C, Delafontaine P. Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in ApoE-/- mice but does not reduce atherosclerosis. Endocrinology. 2009;150(12):5478–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodrigue-Way A, Demers A, Ong H, Tremblay A. A growth hormone-releasing peptide promotes mitochondrial biogenesis and a fat burning-like phenotype through scavenger receptor CD36 in white adipocytes. Endocrinology. 2007;148(3):1009–1018. [DOI] [PubMed] [Google Scholar]
- 48. Mao Y, Tokudome T, Kishimoto I, et al. Hexarelin treatment in male ghrelin knockout mice after myocardial infarction. Endocrinology. 2013;154(10):3847–3854. [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto D, Ikeshita N, Matsubara T, et al. GHRP-2, a GHS-R agonist, directly acts on myocytes to attenuate the dexamethasone-induced expressions of muscle-specific ubiquitin ligases, Atrogin-1 and MuRF1. Life Sci. 2008;82(9–10):460–466. [DOI] [PubMed] [Google Scholar]
- 50. Lim CJ, Jeon JE, Jeong SK, et al. Growth hormone-releasing peptide-biotin conjugate stimulates myocytes differentiation through insulin-like growth factor-1 and collagen type I. BMB Rep. 2015;48(9):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cibrian D, Ajamieh H, Berlanga J, et al. Use of growth-hormone-releasing peptide-6 (GHRP-6) for the prevention of multiple organ failure. Clin Sci (Lond). 2006;110(5):563–573. [DOI] [PubMed] [Google Scholar]
- 52. Granado M, Martin AI, Lopez-Menduina M, Lopez-Calderon A, Villanua MA. GH-releasing peptide-2 administration prevents liver inflammatory response in endotoxemia. Am J Physiol Endocrinol Metab. 2008;294(1):E131-E141. [DOI] [PubMed] [Google Scholar]
- 53. Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288(3):E486-E492. [DOI] [PubMed] [Google Scholar]
- 54. Cao Y, Tang J, Yang T, et al. Cardioprotective effect of ghrelin in cardiopulmonary bypass involves a reduction in inflammatory response. PLoS One. 2013;8(1):e55021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guo S, Gao Q, Jiao Q, Hao W, Gao X, Cao JM. Gastric mucosal damage in water immersion stress: mechanism and prevention with GHRP-6. World J Gastroenterol. 2012;18(24):3145–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rodriguez SS, Gonzalez NL, Garcia Del Barco HD, et al. Role of epidermal growth factor and growth hormone-releasing peptide-6 in acceleration of renal tissue repair after kanamycin overdosing in rats. Iran J Kidney Dis. 2014;8(5):382–388. [PubMed] [Google Scholar]
- 57. García Del Barco D, Montero E, Coro-Antich RM, et al. Coadministration of epidermal growth factor and growth hormone releasing peptide-6 improves clinical recovery in experimental autoimmune encephalitis. Restor Neurol Neurosci. 2011;29(4):243–252. [DOI] [PubMed] [Google Scholar]
- 58. García Del Barco D, Perez-Saad H, Rodriguez V, et al. Therapeutic effect of the combined use of growth hormone releasing peptide-6 and epidermal growth factor in an axonopathy model. Neurotox Res. 2011;19(1):195–209. [DOI] [PubMed] [Google Scholar]
- 59. Garcia Del Barco-Herrera D, Martinez NS, Coro-Antich RM, et al. Epidermal growth factor and growth hormone-releasing peptide-6: combined therapeutic approach in experimental stroke. Restor Neurol Neurosci. 2013;31(2):213–223. [DOI] [PubMed] [Google Scholar]
- 60. Subiros N, Perez-Saad HM, Berlanga JA, et al. Assessment of dose-effect and therapeutic time window in preclinical studies of rhEGF and GHRP-6 coadministration for stroke therapy. Neurol Res. 2015;38(3):187–195. [DOI] [PubMed] [Google Scholar]
- 61. Perboni S, Bowers C, Kojima S, Asakawa A, Inui A. Growth hormone releasing peptide 2 reverses anorexia associated with chemotherapy with 5-fluoruracil in colon cancer cell-bearing mice. World J Gastroenterol. 2008;14(41):6303–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]