Abstract
Introduction
Diffusion weighted MRI (dMRI) is a method sensitive to pathological changes affecting tissue microstructure. Most dMRI studies in schizophrenia, however, have focused solely on white matter. There is a possibility, however, that subtle changes in diffusivity exist in gray matter (GM). Accordingly, we investigated diffusivity in GM in patients with recent onset schizophrenia.
Methods
We enrolled 45 patients and 21 age and sex-matched healthy controls. All subjects were evaluated using the short form of the Wechsler Adult Intelligence Scale, the Positive and Negative Syndrome Scale (PANSS), and the video based social cognition scale. DMRI and T1W images were acquired on a 3 Tesla magnet, and mean Fractional Anisotropy (FA), Trace (TR) and volume were calculated for each of the 68 cortical GM Regions of Interest parcellated using FreeSurfer.
Results
There was no significant difference of FA and GM volume between groups after Bonferroni correction. For the dMRI measures, however, patients evinced increased TR in the left bank of the superior temporal sulcus, the right inferior parietal, the right inferior temporal, and the right middle temporal gyri. In addition, higher TR in the right middle temporal gyrus and the right inferior temporal gyrus, respectively, was associated with decreased social function and higher PANSS score in patients with schizophrenia.
Conclusion
This study demonstrates high sensitivity of dMRI to subtle pathology in GM in recent onset schizophrenia, as well as an association between increased diffusivity in temporal GM regions and abnormalities in social cognition and exacerbation of psychiatric symptoms.
Keywords: schizophrenia, gray matter, diffusivity, MRI
1. Introduction
In the last 30 years, various analytical methods that utilize brain magnetic resonance imaging (MRI) have been developed and used in studies of schizophrenia, as well as in other disorders. Nonetheless, the core pathologic changes within the brain have not been clearly delineated and the majority of findings from MRI studies have shown a great deal of variability with the exception of decreased brain volume and increased ventricular volume (Shenton et al., 2001). Finding core pathologic changes has proven to be extremely difficult in part because of the heterogeneity of demographic and clinical characteristics of subjects and in part because of the limitations of the analytic methods used in neuroimaging.
Various MRI modalities and advanced analytic methods have been developed over the past several years in order to improve precision for detecting and characterizing structural pathology in schizophrenia. There is, for example, an increase in interest in exploring white matter connectivity in schizophrenia using diffusion weighted imaging (dMRI). DMRI is a very sensitive method sensitive to microstructural abnormalities (Beaulieu, 2002; Kanaan et al., 2005) including demyelination, axonal loss, edema, and inflammation (Assaf and Pasternak, 2007). Positive dMRI findings that are frequently reported in schizophrenia include: 1) decreased fractional anisotropy (FA) within fibers connecting prefrontal and temporal lobes, such as cingulum bundle, uncinate fasciculus, corpus callosum, and arcuate fasciculus (Abdul-Rahman et al., 2012; Foong, 2000; Kubicki et al., 2007; 2002; 2003; Price et al., 2005); 2) correlations between dMRI and clinical characteristics, for example, the correlations between prefrontal WM anisotropy and negative symptoms, cingulum bundle and executive functions, and uncinate fasciculus FA and declarative episode memory (Kubicki et al., 2007). Moreover, abnormalities in WM are detectable in schizophrenia (Guo et al., 2012; Kasai et al., 2003; Quan et al., 2013), in high risk patients (Hohenberg et al., 2014; Hoptman et al., 2008; Muñoz Maniega et al., 2008), and in patients’ non psychotic relatives (Camchong et al., 2009; Knöchel et al., 2012).
To date, most dMRI studies in schizophrenia are focusing on white matter. However, subtle structural GM changes at or before schizophrenia onset have been associated with schizophrenia, and multiple biological processes suggested to explain those changes. For example, it is well known that many intrinsic connections exist in GM (Barbas and Pandya, 1989; Tardif and Clarke, 2001). In addition, some studies report that decreases in membrane, axon terminals, dendrites, and dendritic spines are among the causes of decreased GM volume in schizophrenia (Bennett, 2011; Costa et al., 2001; Glantz and Lewis, 2000). T1W imaging has been traditionally used for structural, volumetric analysis. Such measures, however, focus only on the gross, anatomical differences, and thus do not capture micro-structural abnormalities. Microstructural changes (which frequently occur before gross volume changes observed) are related to myelin, cell membranes and intracellular organelles, restricted movement of water molecules, all of which result in a measureable difference in the diffusion of water molecules (Uluğ et al., 1999). Moreover, several studies have also taken advantage of the use of dMRI to investigate microscopic changes in GM, which likely predate any gross, structural changes, in other neurodegenerative diseases such as Alzheimer’s disease, Creutzfeldt-Jakob disease and multiple sclerosis (Kincses et al., 2014; Pirko et al., 2007; Weston et al., 2015; Zerr et al., 2009).
The most common measures used in dMRI studies are the magnitude and the anisotropy of the diffusion tensor (Alexander et al., 2007). There are several measures derived from combinations of the eigenvalues (λs) of the diffusion tensor that describe the magnitude of the diffusion including radial diffusivity {(λ2 + λ3)/2}, axial diffusivity (λ1), and trace (λ1 + λ2 + λ3). According to these definitions, axial and radial diffusivity are apparent diffusivities in the directions parallel and perpendicular to the diffusion tensor, respectively, and trace is the sum of diffusivities in all three directions (Beaulieu, 2002). In white matter, where myelinated axons are organized parallel in bundles, axial diffusivity (aligned with predominant diffusion direction in a given voxel) is more specific to axonal degeneration, radial diffusivity is modulated by myelin, and trace is nonspecific, but a sensitive measure of any ongoing pathology (Alexander et al., 2007; Assaf and Pasternak, 2007; Mori et al., 1999; Song et al., 2005). In gray matter, however, where cell bodies and their processes are predominant components of the tissue, axial and radial diffusivity measures lose their biological meaning. Trace, being sensitive to cellularity, cell necrosis, and edema (Alexander et al., 2011), is thus considered a more appropriate, more robust, and more sensitive measure of diffusivity in gray matter. Accordingly, we chose trace as the main measure of diffusivity in gray matter.
We hypothesized that the amplitude of diffusion would be increased and anisotropy of diffusion would be decreased in gray matter in patients with recent onset schizophrenia compared with healthy controls.
2. Methods
2.1. Subjects
Subjects were enrolled from Asan Medical Center which is a university–affiliated hospital. Patients who were right-handed and those patients who were between the ages of 20–40 years old were eligible for the study. Any patients with diseases that affect the functioning of the brain were excluded. Also, patients were excluded if they were unable to complete neuropsychological testing or the MRI scanning session. Subjects within the patient group had a diagnosis of schizophrenia made by a psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR) criteria, and they also evinced psychotic symptoms such as delusions or hallucinations for less than 5 years. In addition, subjects in the control group did not have any Axis I psychiatric diagnosis in themselves or in their first-degree relatives based on DSM-IV-TR.
We enrolled 91 subjects, but excluded 15 cases due to poor image quality or incidental brain lesions. We then excluded 10 additional patients because their diagnoses changed to other psychotic disorders such as bipolar disorder when we re-evaluated them 1–6 months after the enrollment. The final dataset consisting of sixty-six subjects (patients: N=45; controls: N=21) was used for the analysis.
Written informed consent was obtained from all subjects. Ethical approval for the study was obtained from the local Institutional Review Board.
2.2. Assessment of symptoms, neurocognition and social cognition
Assessment of symptoms, neurocognition, and social cognition was completed within one week from the date of the MRI examination. All subjects were evaluated using an age and sex adjusted short form of the Wechsler Adult Intelligence Scale (WAIS), which consisted of 6 subtests including digit span, vocabulary, arithmetic, picture arrangement, block design, and digit symbol. Patients’ psychiatric symptoms were evaluated by a psychiatrist using the Positive and Negative Syndrome Scale (PANSS).
Social cognition for all subjects was measured by the video based social cognition scale (VISC) (Goh et al., 2008; Jang, 2007). VISC consists of 20 video scripts that present a socially inappropriate situation. Subjects watch the video and record answers to questions about these situations. Each question has a 0–2 scoring scale, with a maximum total score of 40.
2.3. MRI protocol
MR scans were performed with an 8 channel SENSE head coil on a 3 Tesla scanner (Philips Achieva). dMRI images were acquired with an echo planar imaging (EPI) dMRI sequence. One baseline(b=0) image and 32 diffusion gradient directions with b=1000 s/mm2 were also acquired. Scan parameters were as follow: field of view (FOV): 224*224*135 mm, voxel size: 2*2*3 mm3, echo time (TE): 70 ms, flip angle: 90°, repetition time (TR): 5422 ms. Structural T1 MRI images with turbo field echo were acquired and scan parameters were as follow: FOV: 240*240*170, voxel size 1*1*1 mm3, TE: 4.6 ms, TR: 9 ms, flip angle: 8°.
2.4. Image processing
dMRI images were upsampled to 1*1*1 mm3 voxel size using Slicer V. 4.4 (Fedorov et al., 2012; “Slicer,” n.d.). Subsequently, motion and eddy current-induced distortions were corrected using affine registration of all gradient volumes with the first b=0 volume (FLIRT: FMRIB software, oxford, UK)(Jenkinson et al., 2002; Jenkinson and Smith, 2001). To exclude the meninges or CSF, we eroded the boundary voxels of each dMRI image. Diffusion tensors were estimated using in-house software based on weighted-least-squares with an added procedure to correct tensors with negative eigenvalues. We then calculated scalar diffusion measures (FA and Trace [TR]) per each subject.
T1 images were parcellated into discrete anatomical regions using the Desikan-Killiany atlas of FreeSurfer V. 5.3 (Fischl et al., 2002) and all parcellated ROIs (N=68) were used in subsequent analysis. We registered a T1 image into a b=0 baseline image of dMRI using a non-linear registration method, part of the Advanced Normalization Tools (ANTs). We then transformed FreeSurfer parcellated labels into dMRI using the same registration transformation. We calculated the mean for the diffusion measures (FA and TR) for each transformed ROI of each subject.
2.5. Statistical analysis
We performed group comparisons using Student t test (for equal variance) or Welch’s t test (for unequal variance) for each of the measures (FA, TR and volume) for each ROI. Significance threshold (p<0.000735) was adjusted using the Bonferroni correction because of the multiple comparisons of ROIs (N=68)
To evaluate the correlations between clinical symptoms, general IQ, or social cognition and diffusion measures in each ROI, we performed Pearson correlation analysis only within the patient group and only for the ROIs that showed significant group difference. All statistical analyses were performed using R packages (ver. 3.2)(www.R-project.org) and STATA (Stata corp., ver 13, Tx, USA).
3. Results
3.1. Demographic data
The final data set included 66 subjects (21 controls and 45 patients). There was no significant difference in age between the control and patient groups (29.3 ± 5.0 vs. 28.6 ± 6.2 respectively; df=64, t=0.478, p=0.634). The male to female ratio of the patient group was 40.0% and was higher than that of controls (33.3%) but statistically not significant (X2=0.270, p=0.603). Patients showed significantly lower IQ than healthy controls (97.5 ± 16.1 vs. 122.0 ± 7.2, t=8.523, p<0.0001, analyzed by Welch t test). Also, patients showed significantly decreased VISC (26.7 ± 10.2 vs. 34.5 ± 5.9, t=3.836, p=0.0003, analyzed by Welch t test). Mean duration of illness was 1.2 ± 1.7 years and PANSS total score was 61.5 ± 15.7 (positive score: 16.2 ± 6.7; negative score: 16.9 ± 7.3) (Table 1).
Table 1.
Demographic and clinical information
| Healthy control | Schizophrenia | Healthy vs. Schizophrenia | |
|---|---|---|---|
| Number of subjects | 21 | 45 | |
| Male (%) | 7 (33.3%) | 18(40.0%) | X2= 0.2704, p=0.603 |
| Age (years) | 29.3±5.0 | 28.6±6.2 | t=0.4782, p=0.6342 |
| IQ | 122.0±7.2 | 97.5±16.1 | t=8.523, p<0.0001* |
| VISC | 34.5±5.9 | 26.7±10.2 | t=3.836, p=0.0003* |
| Duration of illness (years) | 1.2±1.7 | ||
| PANSS (N=41) | |||
| Total score | 61.5±15.7 | ||
| Positive score | 16.2±6.7 | ||
| Negative score | 16.9±7.3 | ||
| General score | 28.3±6.9 | ||
| Total duration of antipsychotics use (months, N=38) | |||
| Atypical antipsychotics (N=38) | 19.7±21.3 | ||
| Typical antipsychotics (N=2) | 2.3±1.8 | ||
| Lithium (N=4) | 9.1±9.0 | ||
| Olanzapine equivalent dose of antipsychotics at MRI scan (mg/day, N=45) | |||
| Atypical antipsychotics (N=45) | 15.5±8.6 | ||
| Typical antipsychotics (N=3) | 19.3±26.6 | ||
Unequal variance, analyzed by Welch t test
Abbreviations: IQ (Intelligence Quotient), VISC (video based social cognition scale), PANSS (Positive And Negative Syndrome Scale
We added information about the duration of medication and olanzapine equivalent (Gardner et al., 2010) dose at the time of the MRI scan into the Results section and Table 1. Please note that the information about duration of medication for 7 patients was not available.
3.2. Comparisons of volume and diffusivity in Gray Matter
After correction for estimated intracranial volume, we compared the volume of each ROIs in GM using Student t test or Welch’s t test. There were no ROIs that showed significant volume differences between patients with schizophrenia and healthy controls once Bonferroni correction was used for multiple comparisons.
There were also no significant differences in FA between two groups (p < 0.000735). Using a Bonferroni correction for multiple tests, patients with schizophrenia showed significantly increased TR compared with controls in 4 ROIs (mean ± SD of control vs. patients): 1) left bank of the superior temporal sulcus (2.46 ± 0.0713*10−3 vs. 2.56 ± 0.0201*10−3, t=−3.773, p=0.0004); 2) right inferior temporal gyrus (2.53 ± 0.0682*10−3 vs. 2.62 ± 0.107*10−3, t=−3.929, p=0.0002); 3) right inferior parietal gyrus (2.56 ± 0.0795*10−3 vs. 2.65 ± 0.122*10−3, t=−3.657, p=0.0005); and, 4) right middle temporal gyrus (2.64 ± 0.0188*10−3 vs. 2.76 ± 0.0184*10−3, t=−3.994, p=0.0002) (Table 2).
Table 2.
Comparisons of volume and diffusion measures in significantly different ROIs between groups
| Volume (ml) | Statistical analyses† | TR (*10−3 mm2/s) | Statistical analyses | AD (*10−3 mm2/s) | Statistical analyses | RD (*10−3 mm2/s) | Statistical analyses | |
|---|---|---|---|---|---|---|---|---|
| Left bank of superior temporal sulcus
| ||||||||
| Healthy | 2.76±0.41 | t=1.76, p=0.083 | 2.46±0.071 |
t=−3.77 p=0.0004‡ |
0.94±0.025 |
t=−3.95 p=0.000 |
0.76±0.026 |
t=−3.53 p=0.000 |
| Patients | 2.54±0.46 | 2.56±0.020 | 0.97±0.045 | 2‡ | 0.79±0.046 | 8‡ | ||
| Right inferior temporal gyrus | ||||||||
| Healthy | 9.03±1.67 | t=−0.48, p=0.63 | 2.53±0.068 |
t=−3.93 p=0.0002‡ |
0.99±0.024 |
t=−3.71 p=0.000 |
0.77±0.024 |
t=−3.29 p=0.001 |
| Patients | 9.13±1.43 | 2.62±0.11 | 1.02±0.039 | 4‡ | 0.80±0.036 | 6 | ||
| Right inferior parietal gyrus | ||||||||
| Healthy | 14.19±1.49 | t=1.58, p=0.12 | 2.56±0.080 |
t=−3.66 p=0.0005‡ |
0.99±0.032 |
t=−2.52 p=0.014 |
0.79±0.026 |
t=−3.88 p=0.000 |
| Patients | 13.48±1.69 | 2.65±0.12 | 1.02±0.046 | 0.82±0.040 | 3‡ | |||
| Right middle temporal gyrus | ||||||||
| Healthy | 10.73±1.34 | t=0.80, p=0.42 | 2.64±0.019 |
t=−3.99 p=0.0002 |
1.01±0.030 |
t=−3.50 p=0.000 |
0.81±0.028 |
t=−4.17 p=0.000 |
| Patients | 10.43±1.23 | 2.76±0.018 | 1.05±0.044 | 8 | 0.8±0.04 | 1 | ||
Abbreviations: TR (Trace), AD (Axial Diffusion), RD (Radial Diffusion)
Student t test after correction for estimated intra cranial volume
Unequal variance, analyzed by Welch t test
3.3. Correlation between Trace and clinical data
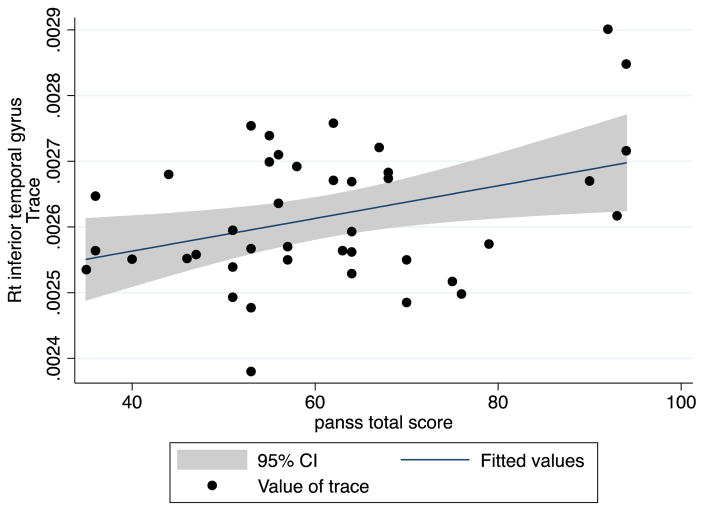
In the patient group, we evaluated the correlation between TR and clinical data for the ROIs that showed significant TR increase. TR values in the right inferior temporal gyrus were significantly correlated with total PANSS scores (correlation coefficient: 0.3720, p=0.0166) and general PANSS score (correlation coefficient: 0.3439, p=0.0277) but not significantly correlated with positive PANSS score (Spearman’s rho: 0.2286, p=0.1506) or negative PANSS score (Spearman’s rho: 0.2640, p=0.0954) (Figure 1).
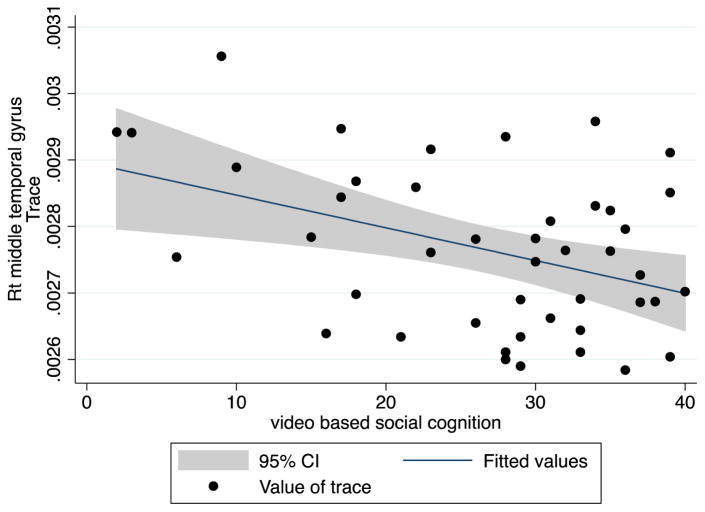
Fig. 1.
Correlation between trace and video based social cognition in right middle Temporal gyrus
VISC was negatively correlated with TR value for the right middle temporal gyrus (correlation coefficients: −0.4131, p=0.0053). There were no significant correlations between duration of illness and TR in these ROIs (Figure 2).
Fig. 2.
Correlation between PANSS total score and mean trace in right inferior temporal gyrus
4. Discussion
Our study shows that patients with schizophrenia are characterized by increased diffusivity in the left bank of the superior temporal sulcus, the right inferior parietal, the right inferior temporal, and the right middle temporal gyri, when compared with healthy controls. In addition, higher diffusivity in the right middle temporal gyrus was associated with lower function of social cognition in patients with schizophrenia. Patients with higher diffusivity in the right inferior temporal gyrus showed higher PANSS total score and PANSS general score.
Few previous studies have reported GM diffusivity changes in schizophrenia. Lee et al. showed increased diffusivity in manually segmented superior temporal gyrus in chronic schizophrenia (Lee et al., 2009). A study by Moriya et al. (Moriya et al., 2010) reported a significant increase in the mean diffusivity in the left parahippocampal cortex, the left insula, and the right anterior cingulate cortex using voxel based analysis. Further, Park et al. analyzed three GM ROIs from FreeSurfer’s parcellation and reported that patients showed increased axial and radial diffusivities in the prefrontal cortex and temporal cortex. Additionally, axial and radial diffusivities were significantly correlated with positive symptom scores in all regions of interest (Park et al., 2014). While none of those studies analyzed entire brain parcellations, the direction of changes reported previously (i.e., increased diffusivity in GM) is in line with our current findings.
The inferior parietal gyrus is known to be one of the regions of the default mode network and plays a major role in sensory integration, body image, concept of self, and executive function (Guo et al., 2014; Torrey, 2007; van den Heuvel and Hulshoff Pol, 2010). According to previous studies, patients with schizophrenia show decreased volume, surface area, thickness, and/or gyrification in this region of GM (Palaniyappan and Liddle, 2012; Ren et al., 2013) and abnormal function in fMRI studies (Liu et al., 2006; Ren et al., 2013; Zhang et al., 2016). In our study, diffusivity in the right inferior parietal gyrus was increased in patients. However, we did not find a significant correlation between social cognition and diffusivity in this area.
In our study, several parts of the temporal lobe – the left bank of the superior temporal sulcus, and the right middle and inferior temporal gyrus - also showed increased diffusivity in patients with schizophrenia. According to a recent review, superior temporal sulcus (STS) is associated with social cognition including social perception and understanding of others’ actions and mental states (Deen et al., 2015; Ethofer et al., 2013). Social cognitive deficits are core symptoms of schizophrenia. They are correlated with poor functional outcome and prognosis and severe negative symptoms (Fett et al., 2011; Lincoln et al., 2011; Sparks et al., 2010; Ventura et al., 2011). Furthermore, they are apparent prior to diagnosis and are found in familial high risk subjects (Eack et al., 2010; Gibson et al., 2010; Green et al., 2012) and in remitted patients (Bora et al., 2009). Due to these characteristic features, social cognitive dysfunction has been suggested as an endophenotype or a core psychopathology for schizophrenia (Gur et al., 2007). In line with these results, our study showed that the increased diffusivity in the right middle temporal gyrus in schizophrenia was associated with social cognitive dysfunction as measured by VISC.
We found the correlation between the social cognition assessed using the visual scale for social cognition (VISC) and the diffusivity in the right, but not the left middle temporal gyrus. According to a review on the neurobiology of social cognition (Adolphs, 2001; Van Overwalle, 2009), the right hemisphere has an important role in the processing of emotional and social information. In addition, there are several studies that show the important role of the right hemisphere during the processing of visual information (De Renzi et al., 1994; Le Grand et al., 2003). Findings of right but not left middle temporal gyral findings are thus in keeping with what others have reported regarding social cognition and right hemisphere findings in areas thought to be involved in the processing of emotional and social information.
In addition to being involved in social cognition, the middle and the inferior temporal gyri are also involved in several cognitive functions: language (Binder et al., 1997), visual perception (Peelen et al., 2006), and multimodal sensory integration. Abnormalities in this area have been shown to be associated with thought disorder (Shenton et al., 1992), psychotic symptoms (Barta et al., 1990; Sumich et al., 2005), and prognosis (Turetsky et al., 1995). In our study, we demonstrated that diffusion abnormalities in the right inferior temporal gyrus were correlated with the severity of psychotic symptoms (higher PANSS total score and PANSS general score).
Patients with schizophrenia often also show decreased thickness, volume, and functional activity in the temporal lobe (Hoffman et al., 2008; Kasai et al., 2003; Onitsuka et al., 2004; Picchioni et al., 2015; Shenton et al., 2001; 1992; Turetsky et al., 1995). Regarding diffusivity in the temporal lobe in schizophrenia, most studies have reported a significant decrease of FA and an increase in diffusivity in the frontal and temporal white matter and in fiber bundles connecting these regions including the cingulum, arcuate fasciculus, and uncinate fasciculus (Ellison- Wright and Bullmore, 2009; Kubicki et al., 2007). In line with these reports, our study showed a correlation between diffusivity in the right temporal lobe and with social cognition as well as total PANSS score.
Existing structural volumetric studies focusing on temporal lobe commonly report interactions between side and diagnosis, where changes are often left lateralized (Crow, 1990; Hirayasu et al., 1998; Honea et al., 2005; Kasai et al., 2003; Onitsuka et al., 2004; H. Park et al., 2004; Turetsky et al., 1995). However, abnormalities in diffusivity in recent onset and first episode schizophrenia are more often reported to be localized to the right, rather than to the left temporal lobe (Federspiel et al., 2006; Garver et al., 2008; Hao et al., 2006; Peters et al., 2009). These previous results and our findings – no significant changes in GM volumes and right-lateralization of diffusivity in the middle and the inferior temporal gyrus - suggest that structural changes in GM might be subtle and follow the pattern of WM, rather than GM pathology.
Our study showed the abnormal changes of GM in dMRI, not in T1 image. dMRI is fundamentally based on the movement of water molecules, which is influenced by cellular or nervous membranes (Flynn et al., 2003), axonal diameter, myelin thickness, and volume of the extracellular space (Assaf and Pasternak, 2007). While white matter abnormalities in schizophrenia are often associated with abnormalities in myelin and oligodendrocytes (Flynn et al., 2003; Hakak et al., 2001; Uranova et al., 2001; 2004), the biological underpinnings of diffusion changes in GM are less understood. Candidate processes might include alterations in intrinsic connections (Lewis and Gonzalez- Burgos, 2000), loss of dendritic spines (Bennett, 2011; Glausier and Lewis, 2013; Konopaske et al., 2014) or even increased volume of extracellular water due to inflammation (Doorduin et al., 2009; Pasternak et al., 2012; Potvin et al., 2008).
We could not find the significant changes in FA image, but in TR image. Because FA is a measure of directionality and TR is a measure of magnitude of water movement in a voxel, they can be independent to each other, depending on the location where they are measured. (Wieshmann et al., 1999) Therefore, increased TR and no change of FA, like our findings, might be related to the increased water content (such as in cases of edema) (Alexander et al., 2007), or to the subtle loss of neuronal processes (Costa et al., 2001; Glantz and Lewis, 2000; Lewis and Gonzalez- Burgos, 2000).
The effects of antipsychotic medications on dMRI are thus far not clear. Several papers have reported significant correlations between medication dosage and diffusion changes (Bartzokis et al., 2007; Minami et al., 2003), while others have not found such effects (i.e., Lee et al., 2013). We did not find a significant relationship between total duration and equivalent dose of antipsychotic medication and volumetric or diffusion abnormalities.
This study has several methodological limitations. Firstly, even though we did not find correlations between TR and CPZ, medication effects cannot be entirely ruled out. A small number of studies have reported an association between medication and DTI measures (Minami et al., 2003; Okugawa et al., 2004), while other studies have not reported such effects (Foong, 2000; Kanaan et al., 2009; Lee et al., 2013). Reports about the effect of medication on diffusivity are inconsistent (Kubicki et al., 2005). Secondly, our study may be underpowered due to the small sample size. Thirdly, the effect of sex on white matter microstructure has been suggested (Bora et al., 2012; Herting et al., 2012). While there was no significant difference in gender ratio in our study, we could not exclude the possibility of gender effect. We could not perform sex stratified analysis due to the small sample size.
This study demonstrates high sensitivity of dMRI to subtle pathology in gray matter in recent onset schizophrenia, as well as an association between increased diffusivity in temporal GM and abnormalities in social cognition and exacerbation of psychiatric symptoms.
Supplementary Material
Acknowledgments
Funding source
This study was supported by the National Research Foundation of Korea (NRF-2012R1A1A1006514, JSL), Korean Society for Schizophrenia Research, National Institute of Mental Health (R01 MH102377, R01 MH074794, MK), and a VA merit Award (MES).
Footnotes
Contributors
JungSun Lee, Chang-Yoon Kim and Yeon Ho Joo designed the study and wrote the protocol. JungSun Lee, Chang-Yoon Kim, and Yeon Ho Joo managed the recruitment and collected the clinical information of participants. MRI data were processed by JungSun Lee and Domick Newell. All statistical analyses were done by JungSun Lee and Sylvain Bouix. JungSun Lee wrote the first draft of the manuscript. JungSun Lee, Sylvain Bouix, Martha E. Shenton, Marek Kubicki supervised the statistical analyses and edited multiple drafts of this manuscript. All authors contributed to and have improved the final manuscript.
Conflict of interest
All authors declare that they have no conflict of financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Rahman MF, Qiu A, Woon PS, Kuswanto C, Collinson SL, Sim K. Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS ONE. 2012;7:e29315. doi: 10.1371/journal.pone.0029315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, Field AS. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connectivity. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review. J Mol Neurosci. 2007;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. American Journal of Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J. Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophrenia research. 2007;93:13–22. doi: 10.1016/j.schres.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Progress in Neurobiology. 2011;95:275–300. doi: 10.1016/j.pneurobio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychological Medicine. 2012;42:295–307. doi: 10.1017/S0033291711001450. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: Meta-analysis. Schizophrenia research. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Camchong J, Lim KO, Sponheim SR, MacDonald AW. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients’ nonpsychotic relatives. Front Hum Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic Spine Hypoplasticity and Downregulation of Reelin and GABAergic Tone in Schizophrenia Vulnerability. Neurobiology of Disease. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Temporal Lobe Asymmetries as the Key to the Etiology of Schizophrenia. Schizophrenia bulletin. 1990;16:433. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Perani D, Carlesimo GA, Silveri MC, Fazio F. Prosopagnosia can be associated with damage confined to the right hemisphere--an MRI and PET study and a review of the literature. Neuropsychologia. 1994;32:893–902. doi: 10.1016/0028-3932(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional Organization of Social Perception and Cognition in the Superior Temporal Sulcus. - PubMed - NCBI. Cereb. Cortex. 2015;25:4596–4609. doi: 10.1093/cercor/bhv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, Sweeney JA, Keshavan MS. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia bulletin. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia research. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Wiethoff S, Bisch J, Schlipf S, Wildgruber D, Kreifelts B. Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. Neuroimage. 2013;76:45–56. doi: 10.1016/j.neuroimage.2013.02.064. [DOI] [PubMed] [Google Scholar]
- Federspiel A, Begré S, Kiefer C, Schroth G, Strik WK, Dierks T. Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis. 2006;22:702–709. doi: 10.1016/j.nbd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez MDG, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Foong J. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. Journal of Neurology, Neurosurgery & Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Presented at the The American journal of psychiatry, American Psychiatric Association; 2010. pp. 686–693. [DOI] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. International Journal of Neuropsychopharmacology. 2008;11:49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Prinstein MJ, Perkins DO, Belger A. Social skill and social cognition in adolescents at genetic risk for psychosis. Schizophrenia research. 2010;122:179–184. doi: 10.1016/j.schres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased Dendritic Spine Density on Prefrontal Cortical Pyramidal Neurons in Schizophrenia. Archives of general psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. REVIEW DENDRITIC SPINE PATHOLOGY IN SCHIZOPHRENIA. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JG, Hyun MH, Kim CY. The Assessment of Social Cognitive Ability for Schizophrenia. Korean Journal of health Psychology. 2008;13:461–480. [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Kee K, Kern RS, Lee J, Sergi MJ, Subotnik KL, Sugar CA, Ventura J, Yee CM, Nuechterlein KH. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophrenia bulletin. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kendrick KM, Yu R, Wang HLS, Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp. 2014;35:123–139. doi: 10.1002/hbm.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, Zhao J. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neuroscience Letters. 2012;531:5–9. doi: 10.1016/j.neulet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophrenia bulletin. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. PNAS. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, Kuang F, Xu L, Yi Y, Zhang Z. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. NeuroReport. 2006;17:23–26. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun- Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. American Journal of Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Anderson AW, Varanko M, Gore JC, Hampson M. Time course of regional brain activation associated with onset of auditory/verbal hallucinations. The British journal of psychiatry: the journal of mental science. 2008;193:424–425. doi: 10.1192/bjp.bp.107.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hohenberg CC, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TUW, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton M. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophrenia bulletin. 2014;40:895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional Deficits in Brain Volume in Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry Studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophrenia research. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Jang J. Relation of Social Cognition and Psychosocial Function in Chronic Schizophrenia Patients. University of Ulsan College of Medicine; 2007. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P. White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br J Psychiatry. 2009;194:236–242. doi: 10.1192/bjp.bp.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RAA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biological psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton M, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive Decrease of Left Superior Temporal Gyrus Gray Matter Volume in Patients With First-Episode Schizophrenia. American Journal of Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincses ZT, Tóth E, Bankó N, Veréb D, Szabó N, Csete G, Faragó P, Király A, Bencsik K, Vécsei L. Grey matter atrophy in patients suffering from multiple sclerosis. Ideggyogy Sz. 2014;67:293–300. [PubMed] [Google Scholar]
- Knöchel C, Oertel-Knöchel V, Schönmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DEJ. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park H, Maier S, Kikinis R, Jolesz FA, Shenton M. A review of diffusion tensor imaging studies in schizophrenia. Journal of psychiatric research. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton M. Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study. The American journal of psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C-F, McCarley RW, Shenton M. The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N Y Acad Sci. 2005;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton M. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Expert face processing requires visual input to the right hemisphere during infancy. Nat Neurosci. 2003;6:1108–1112. doi: 10.1038/nn1121. [DOI] [PubMed] [Google Scholar]
- Lee K, Yoshida T, Kubicki M, Bouix S, Westin CF, Kindlmann G, Niznikiewicz M, Cohen A, McCarley RW, Shenton M. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophrenia research. 2009;108:33–40. doi: 10.1016/j.schres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton M. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Iimaging (DTI) study. Schizophrenia research. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Research Bulletin. 2000;52:309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Mehl S, Kesting ML, Rief W. Negative symptoms and social cognition: identifying targets for psychological interventions. Schizophrenia bulletin. 2011;37(Suppl 2):S23–32. doi: 10.1093/schbul/sbr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. NeuroReport. 2006;17:19. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, Ha- Kawa S, Ikeda K, Kinoshita T. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moriya J, Kakeda S, Abe O, Goto N, Yoshimura R, Hori H, Ohnari N, Sato T, Aoki S, Ohtomo K, Nakamura J, Korogi Y. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophrenia research. 2010;116:196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Muñoz Maniega S, Lymer GKS, Bastin ME, Marjoram D, Job DE, Moorhead TWJ, Owens DG, Johnstone EC, McIntosh AM, Lawrie SM. A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophrenia research. 2008;106:132–139. doi: 10.1016/j.schres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Minami T, Tamagaki C, Takase K, Sugimoto T, Sawada S, Kinoshita T. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton M, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. The American journal of psychiatry. 2004;161:1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Dissociable morphometric differences of the inferior parietal lobule in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;262:579–587. doi: 10.1007/s00406-012-0314-y. [DOI] [PubMed] [Google Scholar]
- Park H, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton M. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Park H, Kim D, Kim J. Positive symptoms and water diffusivity of the prefrontal and temporal cortices in schizophrenia patients: a pilot study. Psychiatry Research. 2014;224:49–57. doi: 10.1016/j.pscychresns.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin C-F, Bouix S, Seidman LJ, Goldstein JM, Woo TUW, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton M, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32:17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. Patterns of fMRI Activity Dissociate Overlapping Functional Brain Areas that Respond to Biological Motion. Neuron. 2006;49:815–822. doi: 10.1016/j.neuron.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Peters BD, Schmitz N, Dingemans PM, van Amelsvoort TA, Linszen DH, de Haan L, Majoie CB, Heeten den GJ. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophrenia research. 2009;111:192–193. doi: 10.1016/j.schres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Picchioni M, Toulopoulou T, Rijsdijk F, McDonald C, Kane F, Kalidindi S, Murray R, McGuire P. Genetic and Environmental Influences On Brain Function in Schizophrenia. an FMRI Study of the Maudsley Twin and Family Cohorts. European Psychiatry. 2015;30:1740. [Google Scholar]
- Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:585–587. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan M, Lee SH, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, Mesholam-Gately RI, Goldstein JM, McCarley RW, Shenton M, Levitt JJ. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophrenia research. 2013;145:1–10. doi: 10.1016/j.schres.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Lui S, Deng W, Li F, Li M, Huang X, Wang Y, Li T, Sweeney JA, Gong Q. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. The American journal of psychiatry. 2013;170:1308–1316. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- Shenton M, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton M, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the Left Temporal Lobe and Thought Disorder in Schizophrenia. The New England journal of medicine. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sparks A, McDonald S, Lino B, O’Donnell M, Green MJ. Social cognition, empathy and functional outcome in schizophrenia. Schizophrenia research. 2010;122:172–178. doi: 10.1016/j.schres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Sumich A, Chitnis XA, Fannon DG, O’ ‘Ceallaigh S, Doku VC, Faldrowicz A, Sharma T. Unreality symptoms and volumetric measures of Heschl’s gyrus and planum temporal in first-episode psychosis. Biological psychiatry. 2005;57:947–950. doi: 10.1016/j.biopsych.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Tardif E, Clarke S. Intrinsic connectivity of human auditory areas: a tracing study with DiI. Eur J Neurosci. 2001;13:1045–1050. doi: 10.1046/j.0953-816x.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. https://www.R-project.org/ [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophrenia research. 2007;97:215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Archives of general psychiatry. 1995;52:1061–1070. doi: 10.1001/archpsyc.1995.03950240079013. [DOI] [PubMed] [Google Scholar]
- Uluğ AM, Moore DF, Bojko AS, Zimmerman RD. Clinical use of diffusion-tensor imaging for diseases causing neuronal and axonal damage. American Journal of Neuroradiology. 1999;20:1044–1048. [PMC free article] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Research Bulletin. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia research. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Hum Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Wood RC, Hellemann GS. Symptom Domains and Neurocognitive Functioning Can Help Differentiate Social Cognitive Processes in Schizophrenia: A Meta-Analysis. Schizophrenia bulletin. 2011;39:102–111. doi: 10.1093/schbul/sbr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston PSJ, Simpson IJA, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimer’s Research & Therapy. 2015;7:1–8. doi: 10.1186/s13195-015-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshmann UC, Clark CA, Symms MR, Franconi F, Barker GJ, Shorvon SD. Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magnetic Resonance Imaging. 1999;17:1269–1274. doi: 10.1016/s0730-725x(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, Schuur M, Haik S, Collins SJ, Jansen GH, Stokin GB, Pimentel J, Hewer E, Collie D, Smith P, Roberts H, Brandel JP, van Duijn C, Pocchiari M, Begue C, Cras P, Will RG, Sanchez-Juan P. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Picchioni M, Allen P, Toulopoulou T. Working Memory in Unaffected Relatives of Patients with Schizophrenia: A Meta-Analysis of Functional Magnetic Resonance Imaging Studies. Schizophrenia bulletin. 2016:sbv221. doi: 10.1093/schbul/sbv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.