Abstract
Insulin secretion is critically dependent on the proper function of a complex molecular network. CaV2.3-knockout (CaV2.3–/–) and PKCλ-knockout (PKCλ–/–) mouse models now suggest that these 2 players, the Cav2.3 channel and PKCλ, are important constituents of this molecular network. Subsequent to glucose stimulation, insulin is released from the pancreatic β cell in a biphasic pattern, i.e., a rapid initial phase followed by a slower, more sustained phase. Interestingly, Ca2+ influx through the CaV2.3 channel regulates only the second phase of insulin secretion. PKCλ seems to enter the β cell nucleus and in turn modulates the expression of several genes critical for β cell secretory function. Studies by Hashimoto et al. and Jing et al. in this issue of the JCI set out to answer the question of why numerous isoforms of proteins with similar functions are present in the β cell. This is important, since it has been difficult to understand the modulatory and/or regulatory roles of different isoforms of proteins in defined subcellular compartments and at various times during the secretory process in both β cell physiology and pathophysiology.
The pancreatic β cell adequately and efficiently secretes insulin to maintain glucose homeostasis. Numerous players with distinct roles act in concert to precisely regulate the complex process of insulin secretion. The β cell relies on common mechanisms shared by other types of cells to execute exocytosis of insulin-containing granules, but also exhibits unique features. The β cell is exquisitely sensitive to glucose. Upon elevation of the plasma glucose level, the β cell efficiently takes up glucose through glucose transporters. Thereafter, subsequent glucose metabolism results in the activation of a series of signal transduction events. A well-known paradigm demonstrates that an increase in the ATP/ADP ratio derived from glucose metabolism closes ATP-sensitive K+ (KATP) channels, resulting in depolarization of the plasma membrane. The membrane depolarization in turn opens CaV channels, mediating Ca2+ influx. The resultant increase in [Ca2+]i triggers direct interactions between exocytotic proteins situated in the insulin-containing granule membrane and those localized in the plasma membrane. Eventually, the interaction between exocytotic proteins initiates the fusion of insulin-containing granules with the plasma membrane, i.e., insulin exocytosis (1). There is no doubt that this KATP channel–dependent pathway plays a central role in the β cell stimulus-secretion coupling. However, abolition of this pathway does not entirely block glucose-stimulated insulin secretion. This observation has led to several significant discoveries of novel mechanisms of glucose-stimulated insulin secretion, which constitute a KATP channel–independent pathway (2). For example, application of high glucose together with activators of PKA and PKC significantly stimulates insulin secretion from the β cell even under conditions where there is neither Ca2+ influx through the plasma membrane nor Ca2+ mobilization from intracellular stores (3). These KATP channel–dependent and KATP channel–independent mechanisms operate in a highly cooperative manner, always guaranteeing adequate release of insulin to maintain normoglycemia (4).
Dynamics of insulin secretion
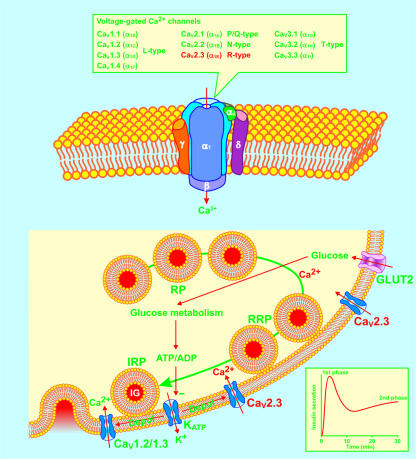
When the β cell is exposed to an abrupt and sustained increase in the concentration of glucose, it responds with a biphasic insulin secretory pattern (Figure 1, inset). This response is characterized by a rapid initial phase of insulin release, which is maintained for about 10 minutes, followed by a nadir, and subsequently, a gradually increasing second phase, which reaches a plateau after a further 25 to 30 minutes. In efforts to explain biphasic insulin secretion, several models have been developed, such as compartmental, feedback, and immediate and time-dependent effect models (5–7). It has been documented that some of the KATP channel–dependent mechanisms are involved in the first phase of insulin secretion. It has been suggested that the KATP channel–independent mechanisms underlie the second phase of insulin secretion (4, 8). Moreover, it has been generally accepted that [Ca2+]i regulates both first- and second-phase insulin secretion (8). The CaV channel–mediated Ca2+ influx significantly contributes to the [Ca2+]i increase in the β cell (1). Although the mouse β cell carries fewer types of CaV channels than the β cell from other species, it is still equipped with 4 types of identified CaV channels, designated CaV1.2, CaV1.3, CaV2.1, and CaV2.3 (Figure 1) (1). Indeed, these CaV channels form a complex molecular network with other proteins, enabling the β cell to delicately secrete insulin. However, complexity makes understanding the role of these different elements in β cell function difficult. In this issue of the JCI, Jing et al. (9) have successfully defined the distinct role of the CaV2.3 channel in second-phase insulin secretion.
Figure 1.
The functional CaV channel consists of pore-forming subunits CaVα1 and auxiliary subunits CaVβ, CaVα2/δ, and CaVγ. Four types of CaVα1 subunits, designated CaV1.2, CaV1.3, CaV2.1, and CaV2.3, conducting L-, P/Q-, and R-type Ca2+ currents, have been identified in the mouse β cell. Glucose-stimulated insulin secretion is characterized by a rapid first phase of insulin release for about 10 minutes, followed by a nadir, and subsequently a gradually increasing second phase reaching a plateau after 25 to 30 minutes (inset). Insulin-containing granules (IG) are functionally divided into three pools: the reserve pool (RP), the readily releasable pool (RRP), and the immediately releasable pool (IRP). The present consensus is that the KATP channel–dependent mechanisms trigger first-phase insulin secretion from the IRP by opening CaV1.2 and CaV1.3 channels. The KATP channel–independent mechanisms underlie second-phase insulin secretion by recruiting insulin-containing granules from RP and RRP to IRP. The Ca2+ influx through β cell CaV2.3 channels is now demonstrated to play a prominent role in second-phase insulin secretion. CaV1.2/1.3, CaV1.2 channels or CaV1.3 channels; CaV2.3, CaV2.3 channels; Depol, depolarization; GLUT2, glucose transporter 2.
Ca2+ influx through the CaV2.3 channel regulates second-phase insulin secretion
The CaV2.3 channel has been the most difficult subtype of CaV channels to evaluate the function of due to the lack of a selective blocker. The presence of the CaV2.3 channel in the mouse β cell was reported after the polypeptide SNX482, used as a CaV2.3 channel–selective blocker, was made available (10, 11). It is known that SNX482 is capable of blocking other types of CaV channels as well (12). Therefore, caution should be exercised when using this Ca2+ channel blocker to define the role of native CaV2.3 channels in cells containing multiple types of CaV channels. Jing et al. (9) combined genetic deletion of the CaV2.3 subunit gene and pharmacological ablation of the CaV2.3 channel with SNX482 in exploring the role of the CaV2.3 channel in biphasic insulin secretion. This combined approach can provide assurance that the observed effects are not nonspecific or due to an insufficient selectivity of SNX482.
The work by Jing et al. (9) confirms that the CaV2.3 channel is present in mouse β cells and that in CaV2.3-knockout (CaV2.3–/–) mice, impaired glucose homeostasis is caused by defective insulin secretion. This is in agreement with previous work from this group demonstrating that SNX482 significantly diminishes β cell CaV currents, causing a reduction in β cell exocytosis (11). It is of fundamental importance to determine the period when the CaV2.3 channel makes its contribution during biphasic insulin secretion. In this context, it is of particular interest to localize where the CaV2.3 channel plays its role in the complex molecular network enabling the β cell to delicately secrete insulin. It is within this framework that the study by Jing et al. sheds new light on the role of the CaV2.3 channel in dynamic insulin secretion. This study has been able to relate the CaV2.3 channel–mediated Ca2+ influx to second-phase insulin secretion, where the regulatory mechanisms at the molecular level are poorly understood.
The work by Jing et al. (9) draws our attention to several important points. First, unlike the β cell CaV1.2 and CaV1.3 channels, which are tightly coupled to the exocytotic machinery (13–15), the CaV2.3 channel seems to be distant from the areas in the β cell where insulin-containing granules fuse with the plasma membrane and release their contents. Neither the deletion of the CaV2.3 subunit gene nor the pharmacological ablation of the CaV2.3 channel altered the exocytotic profile of insulin-containing granules from the immediately releasable pool. These manipulations also did not affect first-phase insulin secretion. This demonstrates that the CaV2.3 channel does not regulate the exocytotic capacity of the β cell (Figure 1).
Second, Ca2+ influx through the CaV2.3 channel mediates second-phase insulin secretion by recruiting insulin-containing granules from other pools to the immediately releasable pool. This is strongly supported by the fact that a massive decrease in insulin release occurred selectively at the second phase from either the CaV2.3–/– islets or islets exposed to SNX482 (9). Recently, it has been hypothesized that there is 1 rate-limiting step between the 2 phases of insulin secretion (16). This key step controls the conversion of insulin-containing granules in the readily releasable pool to the immediately releasable pool. It has been postulated that the KATP channel–independent pathway regulates this key step. A variety of signaling molecules, e.g., cyclic AMP, diacylglycerol (DAG), GTP, and ATP are involved in this pathway, resulting in second-phase insulin secretion (16). Jing et al. have added a new important player, the CaV2.3 channel, to this pathway. It is attractive to speculate that the CaV2.3 channel may segregate its Ca2+ influx from the Ca2+ influx through the CaV1.2 and CaV1.3 channels by using unknown molecular barriers or selecting different cellular locations (Figure 1).
Third, the CaV2.3 channel plays a role in β cell [Ca2+]i oscillations. Jing et al. (9) showed that the CaV2.3–/– β cell not only displays a reduced increase in integral [Ca2+]i, but also a slower frequency of [Ca2+]i oscillations following high glucose stimulation. [Ca2+]i oscillations are involved in the regulation of a number of cellular processes, e.g., insulin secretion and gene expression (17, 18). Recently, we demonstrated that the fast [Ca2+]i oscillations arising from CaVβ3 gene deletion significantly enhance insulin secretion (17). Jing et al. (9) seem not to have considered that the decreased frequency of [Ca2+]i oscillations may also contribute to the decreased insulin secretion observed from CaV2.3–/– islets. We believe that both the amount of [Ca2+]i and the [Ca2+]i oscillation frequency affect insulin secretion. This is strongly supported by the data obtained by Jing et al., which showed a 20% decrease in integral [Ca2+]i, a 30% decrease in [Ca2+]i oscillation frequency, and a 50% decrease in insulin secretion from CaV2.3–/– islets. Additionally, it is of particular interest that CaV2.3–/– islets contain less-differentiated islet cells. This phenomenon may also correlate with the reduced frequency of [Ca2+]i oscillations in CaV2.3–/– islets. It is reasonable to assume that a proper frequency of [Ca2+]i oscillations drives the expression of some genes critical for β cell differentiation since [Ca2+]i oscillations have been shown to regulate gene expression (18). The adult pancreas maintains adaptive β cell growth via differentiation from pancreatic ductal cells and replication from already existing β cells (19). In vivo β cell regeneration has been demonstrated in human subjects (19). Understanding how the CaV2.3 channel–mediated [Ca2+]i oscillations contribute to β cell regeneration is therefore of interest. It may provide new knowledge regarding β cell growth and lead to the development of novel therapeutic approaches to diabetes that selectively manipulate CaV2.3 channel expression.
It should be pointed out that the reduction in integral [Ca2+]i and [Ca2+]i oscillation frequency in CaV2.3–/– islets observed by Jing et al. (9) might also decrease Ca2+-dependent adenylyl cyclase and phospholipase C activities (20, 21). The resultant decreases in the production of cAMP and DAG may in turn attenuate second-phase insulin secretion (16). It would be important to explore these possibilities in future studies.
PKC isoforms in β cells
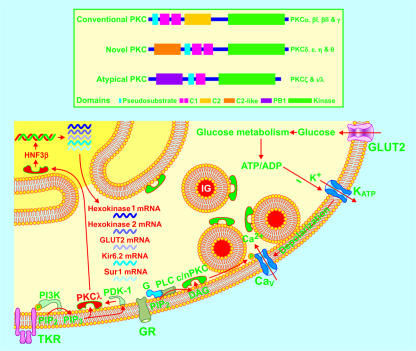
Ten isoforms of PKC with closely related kinase domains are classified into 3 subgroups in terms of the differences in their function and structure. The conventional PKC isoforms (PKCα, PKCβI, PKCβII, and PKCγ) are Ca2+ dependent and sensitive to both DAG and phosphatidylserine (PS). The novel PKC isoforms (PKCδ, PKCε, PKCη, and PKCθ) are sensitive to both DAG and PS, but Ca2+ independent. The atypical PKC isoforms (PKCζ and PKCι/λ) are Ca2+ independent and DAG insensitive, but sensitive to PS (Figure 2) (22). Two additional isoforms of PKC, PKCμ and PKCν, have also been cloned (22). The β cell is equipped with multiple PKC isoforms, which are involved in the regulation of insulin secretion via multiple targets (23–26). PKCλ is equipped with nuclear import and export signals. Therefore, activated PKCλ can quickly translocate to the nucleus (Figure 2) (27). This isoform has also been visualized in the β cell. In vitro experiments suggest that atypical PKC is involved in both glucose-stimulated insulin secretion and glucose-induced gene transcription (28, 29). However, the different isoforms of atypical PKC cannot be differentiated by available pharmacological reagents even in in vitro experiments (30). Neither the in vitro nor the in vivo function of PKCλ in the β cell is known.
Figure 2.
Ten isoforms of PKC with closely related kinase domains are classified into 3 subgroups: conventional PKC isoforms (PKCα, PKCβI, PKCβII, and PKCγ), novel PKC isoforms (PKCδ, PKCε, PKCη, and PKCθ), and atypical PKC isoforms (PKCζ and PKCι/λ). Multiple conventional PKC and novel PKC isoforms (c/n PKC) are involved in the regulation of insulin secretion through different targets. PKCλ in the β cell is now shown to participate in glucose-stimulated insulin secretion by modulating the expression of HNF3β, hexokinase 1, hexokinase 2, glucose transporter 2, Kir6.2, and Sur1 subunit genes critical for β cell function. G, GTP-binding protein; GR, GTP-binding protein–coupled receptors; P, phosphoryl group; PB1, Phox and Bem 1; PDK-1, phosphatidylinositol 3-kinase-dependent kinase–1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PLC, phospholipase C; TKR, tyrosine kinase receptor.
PKCλ modulates the expression of several genes critical for β cell secretory function
In this issue of the JCI, Hashimoto et al. (31) report that they have created βPKCλ–/– mice. Using these knockout mice, they show for the first time to our knowledge that, in vivo, PKCλ regulates glucose-stimulated insulin secretion by maintaining proper expression of some genes critical for β cell function. The work by Hashimoto et al. (31) demonstrates that the βPKCλ–/– mice, without compensatory increase of β cell PKCζ, another atypical PKC isoform, exhibit glucose intolerance with decreased glucose-stimulated insulin secretion without significant insulin resistance. The isolated βPKCλ–/– islets are characterized by an elevation in basal insulin secretion and a reduction in glucose-stimulated insulin secretion resembling the profile of insulin secretion from type 2 diabetic islets (7).
The question that immediately arises is how deletion of one single gene for PKCλ in the β cell causes complicated changes in insulin secretion, i.e., more basal insulin release and less glucose-stimulated insulin secretion. To address this question, Hashimoto et al. (31) examined possible changes in the function, morphology, and gene expression of the βPKCλ–/– islets with regard to insulin secretion. Interestingly, the βPKCλ–/– islets showed complicated alterations in expressions of several genes critical for β cell function, including glucose transporter 2, HNF3β, and hexokinase 1 and 2 as well as Kir6.2 and Sur1 subunits of KATP channels. Increases in hexokinase 1 and 2 expression may explain elevated basal insulin secretion. Decreases in expressions of glucose transporter 2, HNF3β, Kir6.2, and Sur1 subunits may consequently account for impaired glucose-stimulated insulin secretion (Figure 2). Importantly, introduction of the PKCλ gene in βPKCλ–/– islets normalized hexokinase 1, HNF3β, and Kir6.2 subunit expression and in turn reversed elevated basal insulin release as well as blunted glucose–stimulated insulin secretion to normal. The increased basal insulin release and blunted insulin response to high glucose also became normal following restoration of HNF3β expression. It is clear that PKCλ plays a crucial role in controlling the expression of multiple genes critical for β cell secretory function. Therefore, disturbed expression of the PKCλ gene may be involved in the development of the polygenetic disease type 2 diabetes. This is in line with the findings by Hashimoto et al. (31), namely that βPKCλ knockout caused a type 2 diabetes–like phenotype including a higher basal and lower glucose-stimulated insulin release as well as glucose intolerance. This may also suggest that the gene encoding PKCλ could serve as a potential therapeutic target.
It should be kept in mind that sometimes protein function is not directly proportional to protein abundance. For example, a very small percentage of KATP channels are kept open in the normal β cell at basal glucose levels (32). It is therefore crucial to evaluate the activity of hexokinases, glucose transporter 2, and KATP channels in PKCλ–/– β cells to clarify to what extent changes in the amounts of the actual mRNAs and proteins indeed correlate with alterations in their function. Furthermore, type 2 diabetes is characterized by a blunted first phase and a right-shifted second phase of insulin secretion. Therefore, it is of particular interest to further examine how βPKCλ knockout affects the kinetics of biphasic insulin secretion.
In general, the findings of these 2 studies are important since they reveal specific functions for defined protein isoforms in pancreatic β cell signal-transduction. This field of research will continue to be a significant challenge for us when trying to understand the roles of different protein isoforms in the dynamic and complex processes of both physiological and pathophysiological insulin secretion. This is of particular interest when trying to define novel glucose-dependent drugable targets in the treatment of type 2 diabetes. In terms of clinical relevance, the roles of CaV2.3 and PKCλ need to be verified in human β cell signal transduction.
Footnotes
See the related article beginning on page 138.
Nonstandard abbreviations used: CaV2.3–/–, CaV2.3-knockout; DAG, diacylglycerol; KATP, ATP-sensitive K+; PS, phosphatidylserine.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Yang S-N, Berggren P-O. β-Cell CaV channel regulation in physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2005;288:E16–E28. doi: 10.1152/ajpendo.00042.2004. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa T, Komatsu M, Asanuma N, Sato Y, Sharp GW. Glucose action ‘beyond ionic events’ in the pancreatic β cell. Trends Pharmacol. Sci. 1998;19:496–499. doi: 10.1016/s0165-6147(98)01273-5. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu M, Schermerhorn T, Aizawa T, Sharp GW. Glucose stimulation of insulin release in the absence of extracellular Ca2+ and in the absence of any increase in intracellular Ca2+ in rat pancreatic islets. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10728–10732. doi: 10.1073/pnas.92.23.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 5.Nesher R, Cerasi E. Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology. 1987;121:1017–1024. doi: 10.1210/endo-121-3-1017. [DOI] [PubMed] [Google Scholar]
- 6.Grodsky GM. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J. Clin. Invest. 1972;51:2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51:S53–S59. doi: 10.2337/diabetes.51.2007.s53. [DOI] [PubMed] [Google Scholar]
- 8.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the β-cell signals controlling insulin secretion. Eur. J. Clin. Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 9.Jing X, et al. Cav2.3 calcium channels control second-phase insulin release. J. Clin. Invest. 2005;115:146–154. doi:10.1172/JCI200522518. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newcomb R, et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 11.Schulla V, et al. Impaired insulin secretion and glucose tolerance in β cell-selective CaV1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo G, Aldea M, Fuentealba J, Albillos A, Garcia AG. SNX482 selectively blocks P/Q Ca2+ channels and delays the inactivation of Na+ channels of chromaffin cells. Eur. J. Pharmacol. 2003;475:11–18. doi: 10.1016/s0014-2999(03)02084-3. [DOI] [PubMed] [Google Scholar]
- 13.Yang S-N, et al. Syntaxin 1 interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji J, et al. Modulation of L-type Ca2+ channels by distinct domains within SNAP-25. Diabetes. 2002;51:1425–1436. doi: 10.2337/diabetes.51.5.1425. [DOI] [PubMed] [Google Scholar]
- 15.Wiser O, et al. The voltage sensitive LC-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. U. S. A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am. J. Physiol. Cell Physiol. 2004;287:C565–C571. doi: 10.1152/ajpcell.00079.2004. [DOI] [PubMed] [Google Scholar]
- 17.Berggren P-O, et al. Removal of Ca2+ channel β3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119:273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 19.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 20.Thore S, Dyachok O, Tengholm A. Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J. Biol. Chem. 2004;279:19396–19400. doi: 10.1074/jbc.C400088200. [DOI] [PubMed] [Google Scholar]
- 21.Fagan KA, Graf RA, Tolman S, Schaack J, Cooper DM. Regulation of a Ca2+-sensitive adenylyl cyclase in an excitable cell. Role of voltage-gated versus capacitative Ca2+ entry. J. Biol. Chem. 2000;275:40187–40194. doi: 10.1074/jbc.M006606200. [DOI] [PubMed] [Google Scholar]
- 22.Parker PJ, Murray-Rust J. PKC at a glance. J. Cell. Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 23.Arkhammar P, et al. Protein kinase C modulates the insulin secretory process by maintaining a proper function of the β-cell voltage-activated Ca2+ channels. J. Biol. Chem. 1994;269:2743–2749. [PubMed] [Google Scholar]
- 24.Eliasson L, et al. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic β cells. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- 25.Zaitsev SV, Efendic S, Arkhammar P, Bertorello AM, Berggren P-O. Dissociation between changes in cytoplasmic free Ca2+ concentration and insulin secretion as evidenced from measurements in mouse single pancreatic islets. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9712–9716. doi: 10.1073/pnas.92.21.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez CF, et al. Rapid association of protein kinase Cε with insulin granules is essential for insulin exocytosis. J. Biol. Chem. 2003;278:44753–44757. doi: 10.1074/jbc.M308664200. [DOI] [PubMed] [Google Scholar]
- 27.Perander M, Bjorkoy G, Johansen T. Nuclear import and export signals enable rapid nucleocytoplasmic shuttling of the atypical protein kinase Cλ. J. Biol. Chem. 2001;276:13015–13024. doi: 10.1074/jbc.M010356200. [DOI] [PubMed] [Google Scholar]
- 28.Harris TE, Persaud SJ, Jones PM. Atypical isoforms of pKc and insulin secretion from pancreatic beta-cells: evidence using Go 6976 and Ro 31-8220 as Pkc inhibitors. Biochem. Biophys. Res. Commun. 1996;227:672–676. doi: 10.1006/bbrc.1996.1567. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa N, et al. Possible involvement of atypical protein kinase C (PKC) in glucose-sensitive expression of the human insulin gene: DNA-binding activity and transcriptional activity of pancreatic and duodenal homeobox gene-1 (PDX-1) are enhanced via calphostin C-sensitive but phorbol 12-myristate 13-acetate (PMA) and Go 6976-insensitive pathway. Endocr. J. 1999;46:43–58. doi: 10.1507/endocrj.46.43. [DOI] [PubMed] [Google Scholar]
- 30.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol. Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto N, et al. PKCλ regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic β cells. J. Clin. Invest. 2005;115:138–145. doi:10.1172/JCI200522232. doi: 10.1172/JCI22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rorsman P, Berggren P-O, Bokvist K, Efendic S. ATP-regulated K+ channels and diabetes mellitus. News Physiol. Sci. 1990;5:143–147. [Google Scholar]