Abstract
Contralateral suppression of otoacoustic emissions (OAEs) is frequently used to assess the medial olivocochlear (MOC) efferent system, and may have clinical utility. However, OAEs are weak or absent in hearing-impaired ears, so little is known about MOC function in the presence of hearing loss. A potential alternative measure is contralateral suppression of the auditory steady-state response (ASSR) because ASSRs are measurable in many hearing-impaired ears. This study compared contralateral suppression of both transient-evoked otoacoustic emissions (TEOAEs) and ASSRs in a group of ten primarily older adults with either normal hearing or mild sensorineural hearing loss. Responses were elicited using 75-dB peak sound pressure level clicks. The MOC was activated using contralateral broadband noise at 60 dB sound pressure level. Measurements were made concurrently to ensure a consistent attentional state between the two measures. The magnitude of contralateral suppression of ASSRs was significantly larger than contralateral suppression of TEOAEs. Both measures usually exhibited high test–retest reliability within a session. However, there was no significant correlation between the magnitude of contralateral suppression of TEOAEs and of ASSRs. Further work is needed to understand the role of the MOC in contralateral suppression of ASSRs.
I. INTRODUCTION
Assessment of auditory efferent function holds promise for clinical applications, some of which include identifying the source of hearing-in-noise difficulties (Tokgoz-Yilmaz et al., 2013), predicting susceptibility to noise-induced hearing loss (Maison and Liberman, 2000), and detecting the onset of presbycusis (Zhu et al., 2007). Auditory efferent function is typically assessed using contralateral suppression of cochlear responses called otoacoustic emissions (OAEs) (Collet et al., 1990). However, contralateral suppression measured in this way is often small in magnitude and may not be measurable in ears with hearing loss. As a result, little is known about the status of efferent function when there is damage to the peripheral auditory system. This study was developed to determine whether contralateral suppression of a measure of neural response, the auditory steady-state response (ASSR), might provide a more robust indication of auditory efferent activity than the more typically measured changes in OAEs.
The auditory efferent system modifies peripheral hearing function to improve sound detection in noise and to protect the periphery from acoustic trauma (for comprehensive reviews, see Guinan, 2006, 2011). The medial olivocochlear (MOC) branch of the auditory efferent system consists of fibers that predominately project from the medial superior olive to synapse on the outer hair cells (OHCs) of the opposite cochlea (Warr and Guinan, 1979). When the MOC system is stimulated by sound, MOC fibers release acetylcholine into the synaptic cleft, hyperpolarizing the OHCs. Because OHC motility forms the basis of the cochlear amplifier (Dallos, 1992), hyperpolarization of OHCs reduces the motility and thus the gain of the cochlear amplifier. In the case of transient sounds in the presence of background noise, the efferent-mediated reduction in cochlear amplifier gain reduces auditory nerve fiber responses to the continuous noise more than the responses to transient sounds, thus enhancing the detection of transient sounds in background noise (Winslow and Sachs, 1987; Guinan and Gifford, 1988; Kawase et al., 1993).
In normal-hearing individuals, the amount of MOC activity is moderately correlated with the ability to understand speech in the presence of background noise (Giraud et al., 1997; Kumar and Vanaja, 2004; de Boer and Thornton, 2008; Abdala et al., 2014; Mishra and Lutman, 2014; Bidelman and Bhagat, 2015), lending further support to the hypothesis that the efferent system aids with hearing in noise. Clinical populations, such as hearing-impaired individuals, often experience significant difficulties communicating in background noise. It has been suggested that some hearing-in-noise problems could be due at least in part to compromised MOC function (Keppler et al., 2010; Lisowska et al., 2014). However, little is known about how the MOC system functions in ears with hearing loss, primarily due to methodologic barriers.
Nearly all previous studies have used OAEs to assess MOC function in humans. Presumably, OAEs are sounds generated as a by-product of cochlear amplification (Brownell, 1990), and can be noninvasively measured using a miniature probe microphone and loudspeaker placed in the external ear canal (Kemp, 1978). Activation of the MOC bundle reduces cochlear-amplifier gain, which is exhibited as decreased OAE levels (reviewed in Guinan, 2006). Contralateral suppression of OAEs is typically used to assess MOC activity, wherein OAEs are measured without and with contralateral acoustic stimulation (CAS) that activates the contralateral MOC pathway. Contralateral suppression has been described for all types of OAEs, including spontaneous otoacoustic emissions (SOAEs) (e.g., Mott et al., 1989; Zhao and Dhar, 2010), stimulus frequency otoacoustic emissions (SFOAEs) (e.g., Guinan et al., 2003; Lilaonitkul and Guinan, 2009; Zhao et al., 2015), distortion-product otoacoustic emissions (DPOAEs) (e.g., Siegel and Kim, 1982; Moulin et al., 1993; Abdala et al., 2009; Deeter et al., 2009), and transient-evoked otoacoustic emissions (TEOAEs) (e.g., Collet et al., 1990; Hood et al., 1996; Mertes and Goodman, 2016). Unlike DPOAEs, which are generated in the cochlea by two mechanisms, TEOAEs elicited by low to moderate stimulus levels are generated by only one cochlear mechanism (Shera and Guinan, 1999), which simplifies the interpretation of the TEOAE magnitude changes observed in the presence of CAS. Use of higher stimulus levels (which may be necessary for eliciting TEOAEs in hearing-impaired ears) can generate short-latency TEOAE components that may be due to nonlinear distortion (Moleti et al., 2012) and/or basal reflections (Goodman et al., 2011), but these components can be eliminated from analyses through time windowing procedures, which was the approach taken in the current study. Further, TEOAEs have the added advantage of being more easily measured in humans than SFOAEs, and TEOAEs are present in nearly all ears with normal hearing, unlike SOAEs (Kapadia and Lutman, 1997). Because of these notable advantages, TEOAEs were selected for examination in the current study.
Although contralateral suppression of OAEs is a convenient method for studying MOC function in normal-hearing ears, OAEs are often weak or absent in ears with hearing loss (Prieve et al., 1993; Gorga et al., 1997; Konrad-Martin et al., 2002), which has limited studies of human MOC function to individuals with normal or near-normal hearing. The result is that little is known about the MOC system in ears with hearing loss. Alternative assessments of MOC function in hearing-impaired ears are therefore warranted. One potentially feasible measure of MOC activity is contralateral suppression of the auditory steady state response (ASSR). The ASSR is a measure of neural phase locking in response to modulations in the amplitude and/or frequency of a stimulus (Galambos et al., 1981). The ASSR is typically measured using scalp electrodes and is exhibited as a peak in the electroencephalography (EEG) spectrum that corresponds precisely to that of the stimulus modulation frequency. The ASSR is generated by subcortical structures and can also include contributions from cortical structures at stimulus modulation rates less than approximately 60 Hz (Kuwada et al., 2002).
There are several reasons why contralateral suppression of ASSRs may be a promising tool for studying MOC activity. First, ASSRs can be measured at suprathreshold stimulus levels in ears with significant hearing loss and demonstrate similar amplitudes as normal-hearing individuals (Rodriguez et al., 1986; Vander Werff and Brown, 2005; Leigh-Paffenroth and Murnane, 2011). Second, ASSR amplitudes decrease in the presence of contralateral noise (Maki et al., 2009; Kawase et al., 2012; Kiyokawa et al., 2012; Usubuchi et al., 2014), which may be due at least in part to MOC activity. Third, contralateral suppression of auditory neural responses is often larger than contralateral suppression of OAEs (Puria et al., 1996; Chabert et al., 2002; Lichtenhan et al., 2016). Larger contralateral suppression values may be more easily detected in hearing-impaired ears than smaller changes and could therefore be more useful clinically, relative to the smaller contralateral suppression values observed with OAE measures.
The extent to which contralateral suppression of the ASSR involves the MOC system is not known at this time. A previous study found that 40-Hz, but not 80-Hz, ASSR detection thresholds were significantly elevated in the presence of CAS (Maki et al., 2009). The authors argued that if the MOC were involved, it would have affected both the 40- and 80-Hz ASSRs because they are both generated by subcortical structures that should be inhibited by MOC activation. Alternatively, it is possible that an effect was only seen for 40-Hz ASSRs because the response amplitudes and signal-to-noise ratios (SNRs) are larger than at 80-Hz (Purcell and Dajani, 2008), which are important considerations for being able to detect small MOC-induced changes in response amplitude (Goodman et al., 2013). Additionally, Maki et al. (2009) did not assess MOC activity via OAEs, so the extent of MOC activation in their subjects cannot be ascertained.
As an initial step toward examining the role of the MOC in contralateral suppression of ASSRs, the current study compared contralateral suppression of ASSRs with contralateral suppression of TEOAEs in response to suprathreshold stimuli in a group of adults with normal hearing or with mild hearing loss. Because ASSRs can be generated in response to a click train, where the ASSR occurs at the frequency corresponding to the click rate (Galambos and Makeig, 1992), ASSRs and TEOAEs were elicited with the same click stimuli. Additionally, ASSRs and TEOAEs were measured concurrently using an interleaving paradigm to ensure that subject attention and alertness were identical across the two measurements and to allow for verification that the MOC was activated in individual subjects (via contralateral suppression of TEOAEs). Because MOC activity can be modulated by changes in arousal and attention (Froehlich et al., 1993; Maison et al., 2001; de Boer and Thornton, 2007; Smith and Cone, 2015), it was important to ensure that there were no differences in subject state between the two types of measurements by using a concurrent data-collection protocol.
It was hypothesized that both response measures would demonstrate high test–retest reliability within a session, based on previous studies of MOC test–retest reliability (Mishra and Lutman, 2013; Mertes and Goodman, 2016). It was further postulated that contralateral suppression of ASSRs would be significantly larger than contralateral suppression of TEOAEs, as was demonstrated for contralateral suppression of auditory nerve responses (Puria et al., 1996; Chabert et al., 2002; Lichtenhan et al., 2016). Finally, it was hypothesized that the MOC-based modifications in both measures would be significantly correlated. A larger reduction in OHC motility (i.e., larger contralateral suppression of TEOAEs) should diminish the stimulation to progressively higher auditory centers, thus resulting in greater contralateral suppression of the ASSR.
II. METHODS
A. Subjects
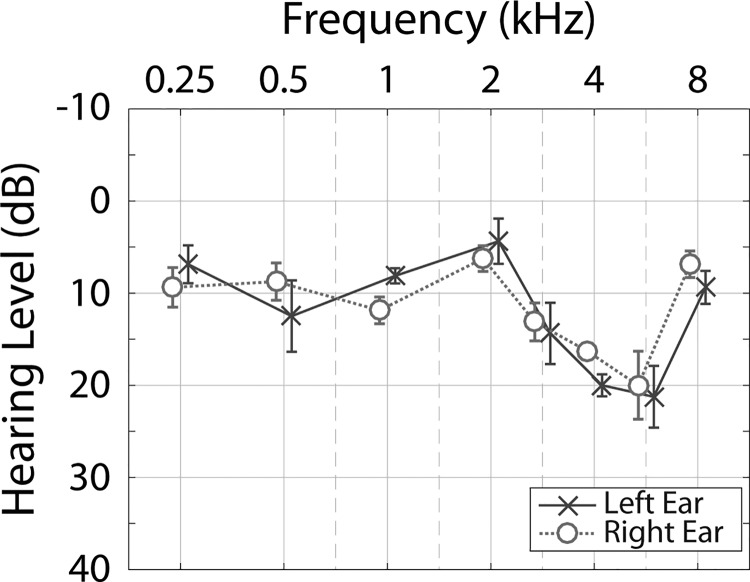
Ten adult subjects (nine males) were recruited from the VA Loma Linda Healthcare System and the surrounding community. Subject ages ranged from 34 to 70 yrs [mean = 54.3 yrs, standard deviation (SD) = 12.9]. All subjects had an unremarkable otoscopic examination, immittance audiometry results within normal clinical limits, three-frequency (500, 1000, and 2000 Hz) pure-tone averages ≤25 dB hearing level (HL), no air-bone gaps >10 dB at two or more frequencies, and no history of conductive hearing disorders. Mean audiometric thresholds and standard errors of the mean (SEMs) for the left and right ears are shown in Fig. 1. Before beginning the experiment, all subjects demonstrated measurable TEOAEs and ASSRs with an SNR of >6 dB. The study protocol was approved by the VA Loma Linda Healthcare System's Institutional Review Board and written informed consent was obtained from all subjects prior to their enrollment in the study. All subjects received monetary compensation for their participation.
FIG. 1.
(Color online) Mean audiometric thresholds for the left and right ears. Error bars represent ±1 SEM. Results for the left and right ears are offset from each other at a given frequency to aid visualization.
B. Equipment
All testing was conducted in a double-walled sound-attenuating booth (Industrial Acoustics Company, Bronx, NY) with subjects seated comfortably in a recliner. Pure-tone air-conduction thresholds were measured at octave frequencies from 0.25 to 8 kHz as well as interoctave frequencies of 3 and 6 kHz using an Astera2 audiometer (GN Otometrics, Taastrup, Denmark). Immittance measures were performed using a GSI TympStar immittance bridge (Grason-Stadler, Eden Prairie, MN).
For TEOAE and ASSR testing, stimulus presentation and response acquisition were achieved using an RZ6 I/O processor [Tucker-Davis Technologies (TDT), Alachua, FL] interfacing with a WS4 workstation (TDT) controlled by custom code written in the Matlab (MathWorks, Natick, MA) and RPvdsEx (TDT) programming languages. Digitally-generated stimuli were routed from the processor to two PA5 programmable attenuators (TDT) and then to a pair of ER-2 insert earphones (Etymōtic Research, Elk Grove Village, IL). The left earphone was inserted in the left ear and the sound tubing of the right earphone was connected to an ER-10B+ OAE probe microphone assembly (Etymōtic Research) that was inserted in the right ear. The ER-10B+ microphone amplifier was set to +40 dB gain. The microphone signal was sampled at a rate of 24414.0625 Hz (the default sampling rate of the RZ6 processor). ASSR recordings were implemented using scalp electrodes connected to an RA4LI headstage (TDT) and an RA4PA preamplifier (TDT). Single-channel ASSR recordings were made with an active electrode placed on the high forehead (Fz), a reference electrode placed on the right mastoid (M2), and a ground electrode placed on the low forehead (Fpz). The EEG signal was sampled at a rate of 939 Hz.
C. Experimental stimuli
TEOAEs and ASSRs were measured concurrently using the RZ6 processor and RPvdsEx software. A cartoon of the measurement setup is shown in Fig. 2. TEOAEs and ASSRs were elicited by 80 -μs clicks presented at 75 dB peak sound pressure level (pSPL) at a rate of 39.0625/s (selected to yield an integer number of samples based on the sampling rate, but nominally referred to hereafter as 40 Hz). Click stimuli were generated by the RZ6 I/O processor.
FIG. 2.
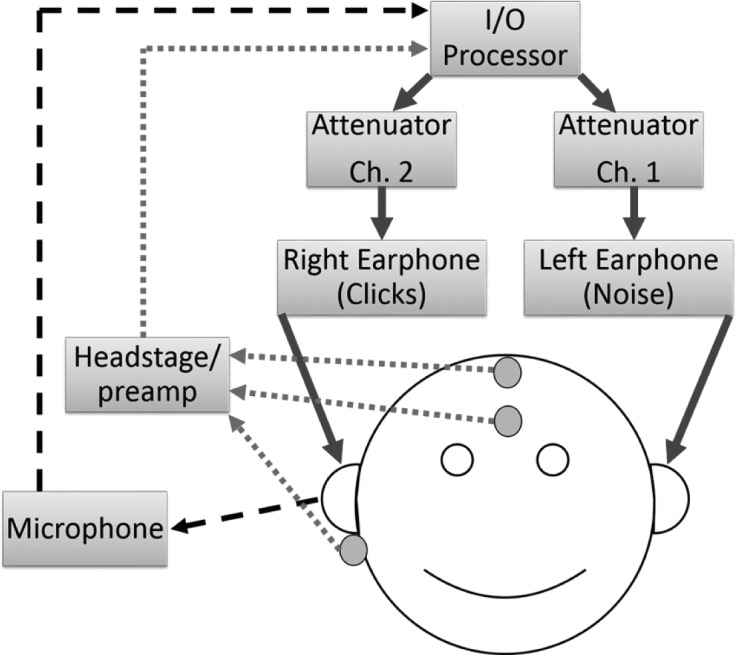
(Color online) Schematic of the equipment setup for concurrent measurement of contralateral suppression of ASSRs and of TEOAEs. Stimulus outputs are represented by solid arrows. Input from the ER-10B+ microphone is represented by a dashed black arrow. Inputs from the electrode headstage and preamplifier are represented by the dotted arrows.
MOC activation was achieved using broadband Gaussian noise generated by the RZ6 I/O processor at the sampling rate noted above. The noise was presented to the ear opposite to the click-stimulated ear, and the noise will be referred to as CAS to be consistent with previous literature. The CAS was presented at an overall root-mean-square (RMS) level of 60 dB(A) sound pressure level (SPL). This level was selected based on previous work demonstrating that it is an effective activator of the MOC (e.g., Guinan et al., 2003; Mertes and Goodman, 2016) while minimizing elicitation of the middle-ear muscle reflex (MEMR), which can confound the interpretation of changes in both OAE levels and stimulus levels (Goodman et al., 2013) as discussed below. The SPL of the CAS was calibrated in an AEC202 2-cc coupler (Larson Davis, Depew, NY).
D. TEOAE and ASSR recording procedure
Prior to each recording, all electrode impedances were verified to be ≤5 kΩ, with no more than a 2-kΩ difference among electrode impedances. Additionally, the click-stimulus levels were calibrated in subjects' ear canals to be within ±0.3 dB of the target level of 75 dB pSPL before each recording began. In each subject, the clicks were presented to the right ear while the CAS was presented to the left ear, because larger MOC-induced changes in OAE magnitudes have been demonstrated in this configuration, relative to presenting clicks in the left ear and CAS in the right ear (Khalfa et al., 1997). To remove low-frequency acoustic noise during the recording prior to saving the data to disk, the ER-10B+ microphone signal was high-pass filtered with a second-order Butterworth filter with a cutoff frequency of 250 Hz.
Each recorded set of TEOAEs and ASSRs consisted of waveforms obtained in two conditions: without CAS and with CAS (referred to hereafter as no CAS and CAS, respectively). Because the index of MOC function was the magnitude differences between the two conditions, it was crucial to minimize potential differences between waveform amplitudes in the two conditions not due to CAS (e.g., drift in stimulus levels, TEOAE levels, and/or ASSR levels across time; Goodman et al., 2013). Therefore, the two conditions were interleaved across the recording duration so that any drifts would be distributed across measurements made in both conditions.
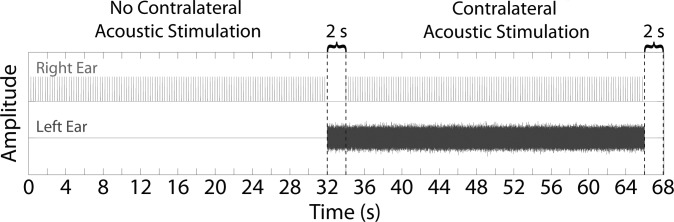
A schematic of an interleaved stimulus recording is shown in Fig. 3. The no CAS condition (i.e., only clicks presented in the right ear) came first, followed by 2 s of the contralateral noise to allow for the full onset of the MOC reflex (Backus and Guinan, 2006). The CAS condition (i.e., clicks presented in the right ear and broadband noise presented in the left ear) was then presented, followed by 2 s of silence to permit a full offset of the MOC reflex (Backus and Guinan, 2006) before the sequence was repeated.
FIG. 3.
(Color online) Schematic of one interleaved presentation of stimuli. Click trains are shown in the top half of the panel for the right ear and broadband contralateral noise is shown in the bottom half of the panel for the left ear. The number of clicks displayed is reduced by a factor of 10 to aid visualization of individual click stimuli. Two stimulus conditions (no CAS and CAS) are shown, separated by a 2-s noise-alone interval (first pair of vertical dashed lines). The two stimulus conditions were repeated continuously, with 2 s of silence between repetitions (second pair of vertical dashed lines). Note that TEOAEs and ASSRs were measured concurrently for the duration of the recording.
The stimulus presentation represented in Fig. 3 was repeated 10 times, for a total test time of 11.3 min for one recording set. The click-stimulus levels, as measured in the ear canal using the ER-10B+ microphone, were monitored visually by the experimenter in real-time to ensure stimulus stability. After the first recording set was completed, the earphones were removed and the subject was provided with a 5-min break, followed by a second recording set in order to compute within-session test–retest reliability. The experimenter attempted to place the ER-10B+ probe in a similar location in the ear canal for both measurements.
To ensure that subjects had a consistent attentional state during the recordings, subjects participated in a visual-attention task described by Mertes and Goodman (2016). Specifically, subjects watched a computer screen inside the sound booth and were instructed to quietly click a mouse button as soon as possible when the computer screen turned blue, which happened every 1–4 s, selected from a random uniform distribution. The ER-10B+ microphone cable was situated away from subjects' bodies to reduce measured vibrations that may have occurred due to subjects' use of the mouse during recordings. Subjects were provided with visual feedback regarding their reaction times during the task so they could monitor their performance. The experimenter also monitored subject performance so that any changes in performance across time were identified (which may have been due to drifts in attention), and which alerted the experimenter to provide the subject with a short break. In addition to allowing for monitoring of a subject attentional state, the visual task was implemented because work in both humans and animals suggest that MOC activity is increased during a visual task (Puel et al., 1988; de Boer and Thornton, 2007; Delano et al., 2007), which should increase the detectability of an MOC-induced change in TEOAE levels relative to no task. The visual task was implemented throughout the entire recording so that subjects were actively engaged during both the no CAS and CAS conditions; therefore, differences in attention between the two conditions were minimized.
E. Middle-ear muscle reflex check
Prior to processing and analyzing the ASSR and TEOAE waveforms, it was important to determine if there was evidence of the activation of the MEMR caused by the introduction of CAS. If the MEMR is activated when the CAS is turned on, the impedance characteristics of the middle-ear system are altered, which can in turn modify OAE levels and measured stimulus levels (Whitehead et al., 1991). If the MEMR is stimulated in an OAE-based test of MOC function, it is difficult to ascertain whether the change in OAE level was due to activation of the MOC, of the MEMR, or a combination (Guinan et al., 2003; Goodman et al., 2013). Studies have detected activation of the MEMR by comparing the stimulus level measured in the ear canal in the CAS versus no CAS conditions (Guinan et al., 2003; Zhao and Dhar, 2010; Abdala et al., 2013; Goodman et al., 2013; Boothalingam and Purcell, 2015; Lichtenhan et al., 2016; Mertes and Goodman, 2016). Changes in stimulus level exceeding a criterion amount were attributed to alterations in middle-ear impedance caused by MEMR activation induced by the CAS. In the current study, MEMR activation was identified if the absolute value of the mean difference in stimulus levels between the no CAS and CAS exceeded 0.14% (Abdala et al., 2013). However, no subjects were identified as having MEMR activation and therefore the results of the MEMR check will not be discussed further. It must be noted that this MEMR check cannot be used to detect if the click stimuli themselves were eliciting the MEMR, so it is possible that there was MEMR activation in both the no CAS and CAS conditions due to the clicks, but not due to the introduction of CAS.
F. Data pre-processing
After the MEMR check was completed, the recorded ASSR and TEOAE waveforms were each sorted to form two matrices of waveforms in the no CAS and CAS conditions, each 320 s in duration (recall that there were 10 interleaves consisting of 32 s in each condition). The time vector associated with the individual waveform buffers was set so that time zero was in reference to the time corresponding to the maximum amplitude of the click stimulus. Both ASSR matrices were reshaped into 320 buffers that were 1 s in duration, and both TEOAE matrices were reshaped into 12 500 buffers that were 20 ms in duration. The first 3.5 ms of the TEOAE waveforms were zeroed out to remove stimulus artifact. To reduce frequency splatter in the frequency domain analysis, all waveforms were ramped on and off with raised-cosine ramps (ASSRs: duration = 50 ms; TEOAEs: duration = 2.5 ms). For the TEOAE waveforms, the onset ramps were applied beginning at 3.5 ms post-stimulus onset. Waveforms were bandpass filtered digitally using a Hann window-based filter design (ASSRs: passband = 30–50 Hz, filter order = 1024; TEOAEs: passband = 1000–4000 Hz, filter order = 256). Artifact rejection was performed post hoc to remove individual buffers with excessively high or low amplitudes. Any ASSR buffer in which the peak amplitude exceeded ±10 μV was rejected. Any ASSR or TEOAE buffer in which the peak and RMS amplitudes fell outside 1.5× the interquartile range were also rejected (Goodman et al., 2009). Following artifact rejection, each ASSR matrix was reshaped into 20 buffers that were each 16 s in duration, allowing for increased frequency resolution relative to 1-s buffers. To obtain estimates of the signal and the noise floor, the matrices of the ASSR and TEOAE waveforms in both the no CAS and CAS conditions were divided into two equally-sized buffers, A and B (odd- and even-numbered waveforms, respectively). The estimate of the signal was obtained as , and the noise floor estimate was computed as (Kemp et al., 1990).
G. Quantification of contralateral suppression
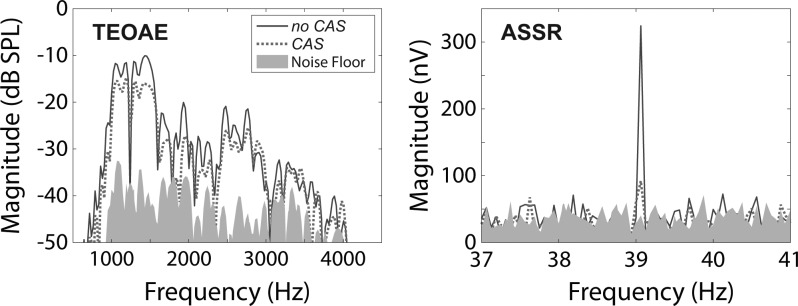
Contralateral suppression of ASSRs and of TEOAEs was analyzed in the frequency domain. An example of TEOAE and ASSR spectra obtained in the no CAS and CAS conditions is shown in Fig. 4. The two replicates of each measurement for each subject were averaged, and fast Fourier transforms (FFTs) were computed on the mean signal and noise floor waveforms for ASSRs and TEOAEs in the no CAS and CAS conditions. The bin widths of the FFTs were 0.0625 Hz for ASSRs and 11.92 Hz for TEOAEs. To compare the size of contralateral suppression of ASSRs to that of TEOAEs, contralateral suppression of each measure was expressed as a single value in the same unit of measurement. TEOAE levels are typically expressed in dB SPL, whereas ASSR amplitudes are routinely expressed in nV. The relative change in magnitude between the no CAS and CAS conditions was expressed in decibels for both measures. TEOAEs are observed across a broad frequency range, but the ASSR occurs at a single frequency (39.0625 Hz); therefore, the magnitude change in TEOAEs was reduced to a single value by converting magnitude to linear units (mPa) and summing the magnitude from 1000–4000 Hz. The magnitude at a given TEOAE frequency was included in the summing operation only if the SNR at that frequency was >6 dB in both the no CAS and CAS conditions. The summed TEOAE amplitudes for the no CAS and CAS conditions were converted to decibels, and contralateral suppression was computed by subtracting the summed magnitudes in dB (no CAS-CAS). For ASSRs, the magnitude values were converted from nV to dB, and contralateral suppression was computed by subtracting the magnitudes in dB (no CAS-CAS). In the example shown in Fig. 4, contralateral suppression of TEOAEs was 3.75 dB and contralateral suppression of ASSRs was 10.92 dB. A larger value of contralateral suppression indicated a greater reduction in response magnitude in the presence of CAS.
FIG. 4.
(Color online) Recorded TEOAE and ASSR spectra (left and right panels, respectively) for one representative subject. Solid lines represent responses obtained in the no CAS condition. Dotted lines represent responses obtained in the CAS condition. The filled gray regions represent the recording noise floors.
III. RESULTS
A. TEOAE and ASSR magnitudes
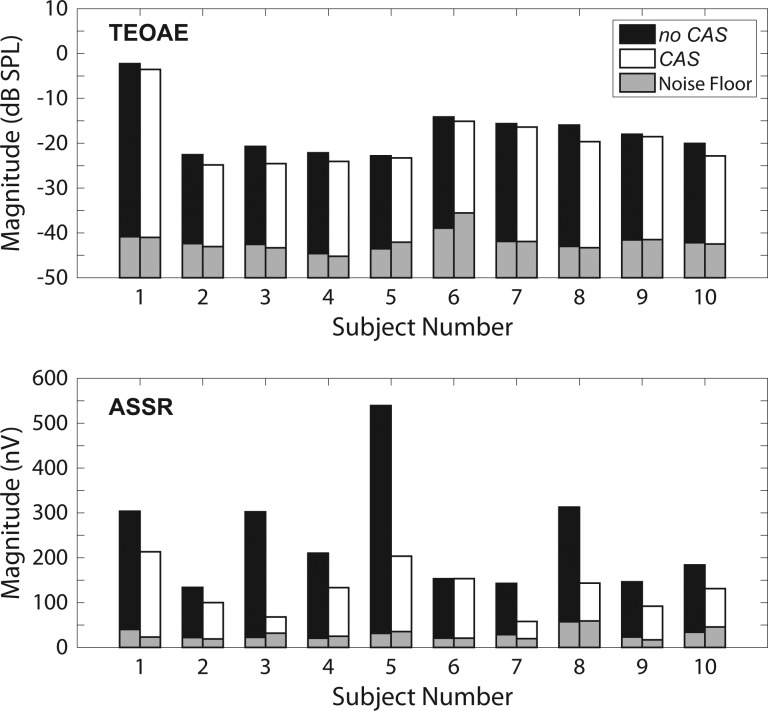
Figure 5 shows TEOAE and ASSR response magnitudes in the no CAS and CAS conditions for all individual subjects. For each subject, the mean signal and noise floor magnitudes were computed across the two measurements. For this figure, the magnitudes are expressed in the typical units for each measurement (dB SPL for TEOAEs, nV for ASSRs). Tables I and II show descriptive statistics (means, SDs, minima, and maxima) for TEOAE and ASSR group data, respectively. In both the no CAS and CAS conditions, all subjects had measurable TEOAEs and ASSRs, as evidenced by SNRs >6 dB. Additionally, these SNRs were sufficiently high for detecting small magnitude changes due to CAS (Goodman et al., 2013).
FIG. 5.
Magnitudes of TEOAEs and ASSRs (top and bottom panels, respectively) for each individual subject. Responses in the no CAS and CAS conditions are represented by black and white bars, respectively. The recording noise floors are represented by gray bars; noise floors in the no CAS and CAS conditions are on the left and right side, respectively, for each individual subject. The magnitudes displayed here represent the mean computed across the two replicate measurements obtained from each subject.
TABLE I.
Descriptive statistics for TEOAE group data for the parameters of signal magnitude, noise-floor magnitude, and SNR. Results are displayed to facilitate comparison between the no CAS and CAS conditions for each parameter. Means, minima, and maxima are in dB SPL. SNRs and SDs are in dB.
| Parameter | Condition | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Signal | no CAS | −17.4 | 6.2 | −22.8 | −2.3 |
| CAS | −19.3 | 6.5 | −24.8 | −3.6 | |
| Noise Floor | no CAS | −42.2 | 1.5 | −44.6 | −38.9 |
| CAS | −41.9 | 2.5 | −45.2 | −35.6 | |
| SNR | no CAS | 24.7 | 5.4 | 19.8 | 38.6 |
| CAS | 22.7 | 5.7 | 18.2 | 37.4 |
TABLE II.
Descriptive statistics for ASSR group data for the parameters of signal magnitude, noise-floor magnitude, and SNR. Table format is identical to that of Table I, except that all values are in nV.
| Parameter | Condition | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Signal | no CAS | 242.9 | 126.4 | 133.8 | 539.3 |
| CAS | 129.7 | 52.2 | 58.0 | 213.4 | |
| Noise floor | no CAS | 30.1 | 11.6 | 20.6 | 57.4 |
| CAS | 29.7 | 13.7 | 16.9 | 59.1 | |
| SNR | no CAS | 212.8 | 122.3 | 111.8 | 507.7 |
| CAS | 100.0 | 50.8 | 36.1 | 190.2 |
B. Test–retest reliability of contralateral suppression
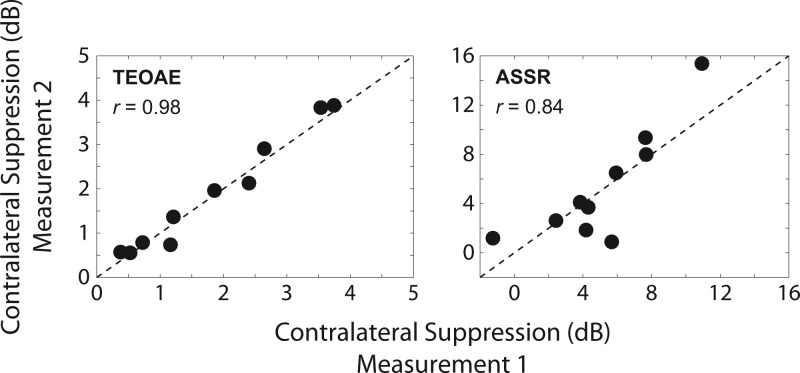
To more directly compare the size of contralateral suppression of TEOAEs to that of ASSRs, contralateral suppression of both measures was expressed in dB. Within-session test–retest reliability of contralateral suppression was analyzed for both measures by computing the correlation between contralateral suppression at the first and second measurements. Results shown in Fig. 6 revealed high test–retest reliability as evidenced by a strong correlation for contralateral suppression of TEOAEs, r = 0.98, p < 0.01, and of ASSRs, r = 0.84, p < 0.01. These results were consistent with the hypothesis that test–retest reliability would be high for both measures.
FIG. 6.
Test–retest reliability of contralateral suppression of TEOAEs (left panel) and of ASSRs (right panel). Results are plotted as the contralateral suppression value obtained in the second measurement against that of the first measurement (note the different ordinate scales). The filled circles represent data from each individual subject. The dashed line represents a 1:1 correspondence between the results at each measurement. Both measures exhibited high test–retest reliability within a session.
It must be noted that Subject 6 showed poor test–retest reliability for contralateral suppression of the ASSR. The magnitude increased by 1.24 dB at the first measurement (opposite of the expected direction) and decreased by 1.22 dB at the second measurement (the expected direction). No other subjects demonstrated this inconsistency in contralateral suppression of ASSRs. Additional repeated measures in this subject (not shown) continued to exhibit inconsistent magnitude increases and decreases of the ASSR. The reason for this variability in results was unclear because this subject demonstrated sufficient ASSR magnitudes and SNRs (see right panel of Fig. 5), and also showed consistent CAS-induced suppression of TEOAEs. Despite these inconsistencies, this subject's results were included so that the group data were representative of all subjects tested.
C. Relative magnitude of contralateral suppression
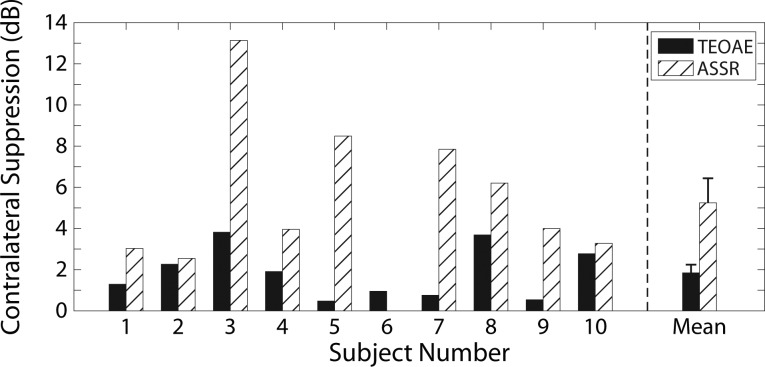
Because there was high test–retest reliability in contralateral suppression of TEOAEs and of ASSRs, the mean contralateral suppression value for the two repeated measurements was computed for each subject and experimental task, and this mean value will be reported hereafter. Comparisons of contralateral suppression of TEOAEs and of ASSRs are shown in Fig. 7 for each individual subject and for the group data. As expected, all subjects showed a decrease in the magnitudes of TEOAEs and of ASSRs in the presence of CAS (with the exception of Subject 6, as described above). Contralateral suppression of TEOAEs ranged from 0.5 to 3.8 dB [mean = 1.8 dB, SD = 1.3], while contralateral suppression of ASSRs ranged from −0.01 to 13.1 dB [mean = 5.2 dB, SD = 3.8]. It can be seen from Fig. 7 that there was considerable intersubject variability regarding the size of the difference between contralateral suppression of TEOAEs and of ASSRs. However, a paired-samples t-test indicated that contralateral suppression of ASSRs was significantly larger in magnitude than contralateral suppression of TEOAEs, t(9) = −3.0441, p < 0.05, consistent with the hypothesized result.
FIG. 7.
Contralateral suppression of TEOAEs (black bars) and of ASSRs (hatched bars). Individual subject data are plotted to the left of the vertical dashed line. The mean data (+1 SEM) are plotted to the right of the dashed line. Note that Subject 6 showed close to 0 dB (−0.01 dB) of contralateral suppression of the ASSR and thus no hatched bar is apparent for this subject.
D. Association between contralateral suppression of TEOAEs and of ASSRs
Although contralateral suppression of ASSRs was larger than of TEOAEs, it was of interest to examine the association between the two measures. A strong correlation would suggest that both measures assess MOC activity. Furthermore, it might suggest that contralateral suppression of ASSRs could be used in place of contralateral suppression of TEOAEs, which would be useful in ears with sensorineural hearing loss, which demonstrate absent TEOAEs (e.g., Prieve et al., 1993), but measurable ASSRs (e.g., Vander Werff and Brown, 2005). The association between contralateral suppression of TEOAEs and of ASSRs is shown in Fig. 8. There was a trend of increasing contralateral suppression of ASSRs with increasing contralateral suppression of TEOAEs, but there was no significant correlation between the two measures, r = 0.34, p = 0.33, contrary to the hypothesized result.
FIG. 8.
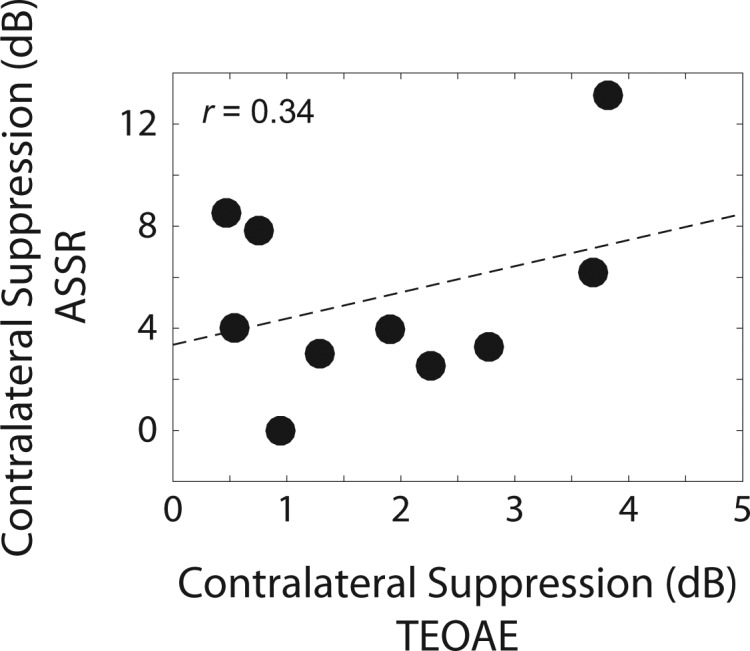
Association between contralateral suppression of TEOAEs and of ASSRs. The filled circles represent data from individual subjects. The dashed line represents a least-squares fit to the data. The correlation between the two measures was not significant.
IV. DISCUSSION
A. Feasibility of concurrent measurements
The purpose of this study was to determine if contralateral suppression of the ASSR can be used as a metric of MOC activity. Concurrent measurements were made to reduce the effects of subject attention, which can impact the strength of MOC activity (Maison et al., 2001; de Boer and Thornton, 2007). This study demonstrated that concurrent measurements are feasible in a relatively short duration of 11 min. Several studies have described methods for concurrently measuring DPOAEs and ASSRs (Purcell et al., 2003; Oswald et al., 2006; Rosner et al., 2011; Wittekindt et al., 2014). However, to the authors' knowledge, the present study is the first to report concurrent measurements of TEOAEs and of ASSRs for the purpose of examining contralateral suppression.
B. Contralateral suppression of cochlear versus neural responses
Contralateral suppression of TEOAEs was measurable in all subjects, as expected. The range of values for contralateral suppression of TEOAEs was consistent with previous reports (e.g., Collet et al., 1990; Hood et al., 1996; de Ceulaer et al., 2001; Goodman et al., 2013). Test–retest reliability was also high, as expected based on previous work (Mishra and Lutman, 2013; Mertes and Goodman, 2016), although further work is needed to establish test–retest reliability across longer time periods. Contralateral suppression of ASSRs was measured reliably in nine of ten subjects.
It was unclear why Subject 6 demonstrated large inconsistencies in how the ASSR changed in the presence of CAS (i.e., both magnitude decreases and increases were exhibited across repeated measures). Previous studies have reported that a minority of subjects can exhibit TEOAE magnitude increases, rather than decreases, in the presence of CAS (e.g., Hood et al., 1996; Goodman et al., 2013). Additionally, subcortically-generated envelope following responses can demonstrate magnitude enhancements in the presence of contralateral noise (Bharadwaj et al., 2015). Suppression of OAE levels in the presence of enhancements in ASSR levels could be reflective of more complicated processes contributing to the ASSR, relative to the TEOAEs. However, these magnitude increases would be expected to be consistent across repeated measures (Mertes and Goodman, 2016), but Subject 6 demonstrated inconsistent magnitude increases and decreases across six repeated measures (not shown). These variable results could not be attributed to poor hearing thresholds (all thresholds were ≤25 dB HL), lack of contralateral suppression of TEOAEs, poor ASSR SNRs, equipment problems, or variable attentional states during measurements. It is possible, but unlikely, that some factor not accounted for by the inclusion/exclusion criteria, perhaps related to medical history, could have contributed to the variable results.
When associating MOC activity with auditory perception, the most relevant effect is on the neural output (Guinan, 2014). The finding of larger contralateral suppression of ASSRs than of TEOAEs appears consistent with previous reports showing that the MOC can exhibit larger effects on neural responses than on OAEs (Puria et al., 1996; Chabert et al., 2002; Lichtenhan et al., 2016). These previous studies examined the effect of MOC stimulation on the compound action potential (CAP) in humans or in animals. Larger changes in the neural responses suggest that OAE-based measures of the MOC may underestimate the magnitude of the MOC effect on the neural response.
While it may therefore appear to be advantageous to use the CAP to assess MOC activity, these measurements have been acknowledged to require long data-collection times (10 h) to detect an MOC-induced amplitude change (Lichtenhan et al., 2016). The concurrent measurements of TEOAEs and of ASSRs made in the current study have the advantage of being obtained in a matter of minutes rather than hours. Faster data collection times are also advantageous to reduce attentional drifts and changes in probe position over time (Goodman et al., 2013).
C. The role of the MOC in contralateral suppression of the ASSR
Contralateral suppression of the 40-Hz ASSR has been demonstrated previously (Galambos and Makeig, 1992; Maki et al., 2009; Kawase et al., 2012; Kiyokawa et al., 2012; Usubuchi et al., 2014). However, the physiologic source of the contralateral-suppression effect has yet to be determined. Several studies have argued that the suppression is a result of neural centers above the level of the brainstem because CAS did not inhibit the wave V amplitude of the auditory brainstem response (Galambos and Makeig, 1992), nor did it alter detection thresholds of the 80-Hz ASSR (Maki et al., 2009), which are both generated by subcortical structures (Møller, 1998; Kuwada et al., 2002). However, other studies have shown that CAS alters the amplitude and latency of wave V in humans (Sininger and Cone-Wesson, 2006; Schochat et al., 2012). Furthermore, animal studies have shown that MOC stimulation can alter activity of the cochlear nucleus, superior olivary complex, and inferior colliculus (Desmedt, 1962; Starr and Wernick, 1968; Mulders et al., 2008; Seluakumaran et al., 2008), demonstrating that the MOC can affect brainstem function.
In the current study, the correlation between contralateral suppression of TEOAEs and of ASSRs was weak and non-significant, suggesting that contralateral suppression of the ASSR may not serve as a substitute measure for contralateral suppression of TEOAEs to assess MOC activity in hearing-impaired ears. It is possible that the non-significant finding was due to low statistical power. It is also possible that contralateral suppression of TEOAEs and of ASSRs tap into different aspects of MOC activity. As discussed above, contralateral suppression of OAEs may underestimate the effect of MOC activity on neural responses, but previous studies (Puria et al., 1996; Lichtenhan et al., 2016) did not report the correlation between the magnitude of contralateral suppression of DPOAEs and CAPs.
Another potential complication involves MOC collaterals to the cochlear nucleus. Because these collaterals lie beyond the OHCs, it is possible that MOC effects on neural function would be exhibited differently from MOC effects on cochlear function and thus not be correlated. MOC collaterals have been demonstrated in non-human animals such as cats and guinea pigs (Brown et al., 1988; Mulders et al., 2009). However, such collaterals have not been observed in humans (Moore and Osen, 1979), so this does not appear to explain the discrepant results between contralateral suppression of ASSRs and of TEOAEs. Complex level-dependent effects of MOC activation on inner hair cell activity have been described recently (Guinan, 2012), so the hypothesis that contralateral suppression of TEOAEs and of ASSRs are linearly correlated may have been too simplistic to characterize the relationship between MOC effects on the peripheral and central auditory system.
Another potential explanation for the weak, non-significant correlation is that contralateral suppression of the 40-Hz ASSR represents an effect such as central masking (Zwislocki, 1972), which is argued by Maki et al. (2009) to not involve the MOC system. However, even the role of the MOC in central masking is controversial. Some psychophysical work in humans and in animals supports the involvement of the MOC in central masking (Smith et al., 2000; Aronoff et al., 2015), but electrophysiologic work in animals with selective blockade of MOC efferents suggests that the MOC is not involved in central masking (Aran et al., 2000). More work is needed to determine the extent to which the MOC contributes to contralateral suppression of the ASSR before it can be used as a metric of MOC activity.
D. Potential limitations
A click rate of 39.0625/s was utilized in the current study in order to elicit ASSRs close to 40 Hz and also to elicit TEOAEs. However, this click rate likely elicited the ipsilateral MOC pathway (Boothalingam and Purcell, 2015). In this case, the size of the magnitude of change that can be detected between the no CAS and CAS conditions would be reduced, because there would be partial MOC activation in the no CAS condition (Guinan, 2006). However, if correct, ipsilateral MOC activation did not prevent the measurement of robust contralateral suppression seen in all subjects in this study. Additionally, no evidence of MEMR activation due to CAS was uncovered in any subject, but it cannot be determined from the current measurements whether the click stimuli themselves elicited the MEMR in both the no CAS and CAS conditions.
Increasing the click rate to measure 80-Hz ASSRs would increase the likelihood of ipsilateral MOC activation and MEMR activation (Boothalingam and Purcell, 2015), so it may not be feasible to use a click-evoked paradigm as in the current study to assess the 80-Hz ASSR. A click rate slower than 40 Hz [e.g., 20 Hz, where robust contralateral suppression of ASSRs can be obtained (Usubuchi et al., 2014)] could be used to avoid ipsilateral MOC activation and MEMR while still allowing for concurrent measurement of TEOAEs and of ASSRs. However, relative to the 40-Hz ASSR, amplitudes of ASSRs <40 Hz are lower and the noise floors are higher (Picton et al., 2003), which could require longer data-collection times than those seen in the current study. It may be that different stimulus and recording paradigms from those used here are needed to assess contralateral suppression with ASSRs less than or greater than 40 Hz.
The present study implemented a visual attention task designed to minimize drifts in attention across the recording period. It has been demonstrated that visual attention by itself can modulate OAE and ASSR amplitudes in the absence of CAS (e.g., Puel et al., 1988; Wittekindt et al., 2014), presumably due to corticofugal activity involving the MOC. Therefore, engaging subjects in the current visual attention task may have elicited MOC activity in addition to the MOC activity caused by the CAS. As mentioned previously, subjects were engaged in the task during both conditions with and without CAS, so attention-based MOC effects should be similar between conditions and any response amplitude differences between conditions should therefore have been due primarily to MOC activation caused by CAS. Additionally, the effects of visual attention (approximately 0.2 dB; Wittekindt et al., 2014) would be smaller than the effects observed in the present study. Furthermore, de Boer and Thornton (2007) found no significant difference in contralateral suppression of TEOAEs in a condition with CAS and passive listening relative to a condition with CAS and visual attention. Therefore, it is likely that the observed amplitude changes in this study were due primarily to CAS, with a smaller contribution from the visual attention task.
The concurrent measurement paradigm used in this study ensured that any drift in stimulus levels across time would impact both the TEOAEs and ASSRs. Additional post hoc analysis of stimulus stability, expressed as the maximum percent change in amplitude between the initial click and all remaining clicks after rejecting artifacts (Glattke and Robinette, 2007), showed high stability in all subjects (mean = 92.1%, SD = 3.1%, range = 85.7%–96.6%). However, methods to reduce stimulus drift could be implemented as well. Post hoc detrending of stimulus levels is one method to reduce drift, but Goodman et al. (2013) found that detrending in most cases did not alter whether a TEOAE amplitude change was statistically significant, relative to no detrending. In the present study, stimulus levels were calibrated in the ear canal prior to each recording, but it is possible that periodic re-calibration of stimulus levels during recording (e.g., after a subject moves or swallows) could minimize drift and should be explored in future studies. In the present study, a single click stimulus level of 75 dB pSPL was used to elicit TEOAEs and ASSRs. MOC activity has a stronger effect on responses to lower stimulus levels (Hood et al., 1996) and thus a lower stimulus level may have shown larger CAS effects. Preliminary testing for this study showed that a click level of 65 dB pSPL did not elicit measurable ASSRs in some subjects, which could be a result of their age and/or hearing status (recall that mainly older adults, some with mild hearing loss, were tested in this study). Future research could incorporate measurements at multiple stimulus levels to compute “effective attenuation” (e.g., Collet et al., 1990; Puria et al., 1996; de Boer and Thornton, 2007; Lichtenhan et al., 2016), which is the difference in stimulus level with versus without CAS that is needed to achieve the same TEOAE or ASSR magnitude. This is in contrast to the current study, in which the stimulus level was constant and the change in TEOAE and ASSR levels with versus without CAS was computed. Effective attenuation may be a more sensitive metric of MOC activity than contralateral suppression at a single stimulus level. For example, de Boer and Thornton (2007) found no significant effect of CAS on TEOAE levels measured at a single stimulus level, but found a significant effect of CAS when the effect was expressed as effective attenuation. The current study has established that contralateral suppression of TEOAEs and of ASSRs can be measured simultaneously, laying the groundwork for future studies comparing parameters such as effective attenuation.
V. CONCLUSIONS
Contralateral suppression of TEOAEs and of ASSRs could be measured concurrently, usually with high test–retest reliability. The magnitude of contralateral suppression of ASSRs was nearly always greater than that of TEOAEs obtained at the same stimulus level. Contralateral suppression of TEOAEs and of ASSRs was not significantly correlated, suggesting that contralateral suppression of ASSRs may not serve as a valid substitute measure for contralateral suppression of TEOAEs in hearing-impaired ears. Future work is needed to determine the role of the MOC in contralateral suppression of ASSRs.
ACKNOWLEDGMENTS
This research was supported by NIH Grant No. F32 DC015149 (I.B.M.), NIH Grant No. R01 DC000626 (M.R.L.), and a U.S. Department of Veterans Affairs RR&D Senior Research Career Scientist Award C4042L (M.R.L.). Support was also provided by the Loma Linda Veterans Association for Research and Education to one of the authors (I.B.M.). The authors thank Erin C. Wilbanks and Barden B. Stagner for their assistance with data collection. The authors also thank Dr. Brenda L. Lonsbury-Martin and Dr. Glen K. Martin for their comments regarding the manuscript and Dr. Robert F. Burkard for helpful discussions regarding this research.
Portions of this work were presented at the 39th Annual MidWinter Meeting of the Association for Research in Otolaryngology, San Diego, California, February 20–24, 2016, and at the Annual Scientific and Technology Conference of the American Auditory Society, Scottsdale, Arizona, March 3–5, 2016.
References
- 1. Abdala, C. , Dhar, S. , Ahmadi, M. , and Luo, P. (2014). “ Aging of the medial olivocochlear reflex and associations with speech perception,” J. Acoust. Soc. Am. 135, 754–765. 10.1121/1.4861841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdala, C. , Mishra, S. , and Garinis, A. (2013). “ Maturation of the human medial efferent reflex revisited,” J. Acoust. Soc. Am. 133, 938–950. 10.1121/1.4773265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdala, C. , Mishra, S. K. , and Williams, T. L. (2009). “ Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex,” J. Acoust. Soc. Am. 125, 1584–1594. 10.1121/1.3068442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aran, J.-M. , Pajor, A.-M. , de Sauvage, R. C. , and Erre, J.-P. (2000). “ Role of the efferent medial olivocochlear system in contralateral masking and binaural interactions: An electrophysiological study in guinea pigs,” Audiology 39, 311–321. 10.3109/00206090009098012 [DOI] [PubMed] [Google Scholar]
- 5. Aronoff, J. M. , Padilla, M. , Fu, Q.-J. , and Landsberger, D. M. (2015). “ Contralateral masking in bilateral cochlear implant patients: A model of medial olivocochlear function loss,” PLoS One 10, e0121591. 10.1371/journal.pone.0121591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Backus, B. C. , and Guinan, J. J., Jr. (2006). “ Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. 119, 2889–2904. 10.1121/1.2169918 [DOI] [PubMed] [Google Scholar]
- 7. Bharadwaj, H. , Pardo, C. , Shera, C. , and Shinn-Cunningham, B. (2015). “ Olivocochlear efferent effects on neural temporal coding of sounds in humans,” Assoc. Res. Otolaryngol. Abstr. 38, PS669. [Google Scholar]
- 8. Bidelman, G. M. , and Bhagat, S. P. (2015). “ Right-ear advantage drives the link between olivocochlear efferent ‘antimasking’ and speech-in-noise listening benefits,” Neuroreport 26, 483–487. 10.1097/WNR.0000000000000376 [DOI] [PubMed] [Google Scholar]
- 9. Boothalingam, S. , and Purcell, D. W. (2015). “ Influence of the stimulus presentation rate on medial olivocochlear system assays,” J. Acoust. Soc. Am. 137, 724–732. 10.1121/1.4906250 [DOI] [PubMed] [Google Scholar]
- 10. Brown, M. C. , Liberman, M. C. , Benson, T. E. , and Ryugo, D. K. (1988). “ Brainstem branches from olivocochlear axons in cats and rodents,” J. Comp. Neurol. 278, 591–603. 10.1002/cne.902780410 [DOI] [PubMed] [Google Scholar]
- 11. Brownell, W. E. (1990). “ Outer hair cell electromotility and otoacoustic emissions,” Ear Hear. 11, 82–92. 10.1097/00003446-199004000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chabert, R. , Magnan, J. , Lallemant, J. G. , Uziel, A. , and Puel, J. L. (2002). “ Contralateral sound stimulation suppresses the compound action potential from the auditory nerve in humans,” Otol. Neurotol. 23, 784–788. 10.1097/00129492-200209000-00029 [DOI] [PubMed] [Google Scholar]
- 13. Collet, L. , Kemp, D. T. , Veuillet, E. , Duclaux, R. , Moulin, A. , and Morgon, A. (1990). “ Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects,” Hear. Res. 43, 251–261. 10.1016/0378-5955(90)90232-E [DOI] [PubMed] [Google Scholar]
- 14. Dallos, P. (1992). “ The active cochlea,” J. Neurosci. 12, 4575–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Boer, J. , and Thornton, A. R. (2007). “ Effect of subject task on contralateral suppression of click evoked otoacoustic emissions,” Hear. Res. 233, 117–123. 10.1016/j.heares.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 16. de Boer, J. , and Thornton, A. R. (2008). “ Neural correlates of perceptual learning in the auditory brainstem: Efferent activity predicts and reflects improvement at a speech-in-noise discrimination task,” J. Neurosci. 28, 4929–4937. 10.1523/JNEUROSCI.0902-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Ceulaer, G. , Yperman, M. , Daemers, K. , Van Driessche, K. , Somers, T. , Offeciers, F. E. , and Govaerts, P. J. (2001). “ Contralateral suppression of transient evoked otoacoustic emissions: Normative data for a clinical test set-up,” Otol. Neurotol. 22, 350–355. 10.1097/00129492-200105000-00013 [DOI] [PubMed] [Google Scholar]
- 18. Deeter, R. , Abel, R. , Calandruccio, L. , and Dhar, S. (2009). “ Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 126, 2413–2424. 10.1121/1.3224716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delano, P. H. , Elgueda, D. , Hamame, C. M. , and Robles, L. (2007). “ Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas,” J. Neurosci. 27, 4146–4153. 10.1523/JNEUROSCI.3702-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desmedt, J. E. (1962). “ Auditory-evoked potentials from cochlea to cortex as influenced by activation of the efferent olivo-cochlear bundle,” J. Acoust. Soc. Am. 34, 1478–1496. 10.1121/1.1918374 [DOI] [Google Scholar]
- 21. Froehlich, P. , Collet, L. , and Morgon, A. (1993). “ Transiently evoked otoacoustic emission amplitudes change with changes of directed attention,” Physiol. Behav. 53, 679–682. 10.1016/0031-9384(93)90173-D [DOI] [PubMed] [Google Scholar]
- 22. Galambos, R. , and Makeig, S. (1992). “ Physiological studies of central masking in man. I: The effects of noise on the 40-Hz steady-state response,” J. Acoust. Soc. Am. 92, 2683–2690. 10.1121/1.404383 [DOI] [PubMed] [Google Scholar]
- 23. Galambos, R. , Makeig, S. , and Talmachoff, P. J. (1981). “ A 40-Hz auditory potential recorded from the human scalp,” Proc. Natl. Acad. Sci. U.S.A. 78, 2643–2647. 10.1073/pnas.78.4.2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giraud, A. L. , Garnier, S. , Micheyl, C. , Lina, G. , Chays, A. , and Chéry-Croze, S. (1997). “ Auditory efferents involved in speech-in-noise intelligibility,” Neuroreport 8, 1779–1783. 10.1097/00001756-199705060-00042 [DOI] [PubMed] [Google Scholar]
- 25. Glattke, T. J. , and Robinette, M. S. (2007). “ Transient evoked otoacoustic emissions in populations with normal hearing sensitivity,” in Otoacoustic Emissions: Clinical Applications, edited by Robinette M. S. and Glattke T. J. ( Thieme, New York: ), pp. 87–105. [Google Scholar]
- 26. Goodman, S. S. , Fitzpatrick, D. F. , Ellison, J. C. , Jesteadt, W. , and Keefe, D. H. (2009). “ High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans,” J. Acoust. Soc. Am. 125, 1014–1032. 10.1121/1.3056566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodman, S. S. , Mertes, I. B. , Lewis, J. D. , and Weissbeck, D. K. (2013). “ Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects,” J. Assoc. Res. Otolaryngol. 14, 829–842. 10.1007/s10162-013-0409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman, S. S. , Mertes, I. B. , and Scheperle, R. A. (2011). “ Delays and growth rates of multiple TEOAE components,” in What Fire is in Mine Ears: Progress in Auditory Biomechanics, edited by Shera C. A. and Olson E. S. ( AIP, Melville, New York: ), pp. 279–285. [Google Scholar]
- 29. Gorga, M. P. , Neely, S. T. , Ohlrich, B. , Hoover, B. , Redner, J. , and Peters, J. (1997). “ From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss,” Ear Hear. 18, 450–455. 10.1097/00003446-199712000-00003 [DOI] [PubMed] [Google Scholar]
- 30. Guinan, J. J., Jr. (2006). “ Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. 27, 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- 31. Guinan, J. J., Jr. (2011). “ Physiology of the medial and lateral olivocochlear systems,” in Auditory and Vestibular Efferents, edited by Ryugo D. K., Fay R. R., and Popper A. N. ( Springer Science+Business Media, LLC, New York: ), pp. 39–81. [Google Scholar]
- 32. Guinan, J. J., Jr. (2012). “ How are inner hair cells stimulated? Evidence for multiple mechanical drives,” Hear. Res. 292, 35–50. 10.1016/j.heares.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guinan, J. J., Jr. (2014). “ Olivocochlear efferent function: Issues regarding methods and the interpretation of results,” Front. Syst. Neurosci. 8, 1–5. 10.3389/fnsys.2014.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guinan, J. J., Jr. , Backus, B. C. , Lilaonitkul, W. , and Aharonson, V. (2003). “ Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–540. 10.1007/s10162-002-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guinan, J. J., Jr. , and Gifford, M. L. (1988). “ Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions,” Hear. Res. 33, 97–114. 10.1016/0378-5955(88)90023-8 [DOI] [PubMed] [Google Scholar]
- 36. Hood, L. J. , Berlin, C. I. , Hurley, A. , Cecola, R. P. , and Bell, B. (1996). “ Contralateral suppression of transient-evoked otoacoustic emissions in humans: Intensity effects,” Hear. Res. 101, 113–118. 10.1016/S0378-5955(96)00138-4 [DOI] [PubMed] [Google Scholar]
- 37. Kapadia, S. , and Lutman, M. E. (1997). “ Are normal hearing thresholds a sufficient condition for click-evoked otoacoustic emissions?,” J. Acoust. Soc. Am. 101, 3566–3567. 10.1121/1.418317 [DOI] [PubMed] [Google Scholar]
- 38. Kawase, T. , Delgutte, B. , and Liberman, M. C. (1993). “ Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve responses to masked tones,” J. Neurophysiol. 70, 2533–2549. [DOI] [PubMed] [Google Scholar]
- 39. Kawase, T. , Maki, A. , Kanno, A. , Nakasato, N. , Sato, M. , and Kobayashi, T. (2012). “ Contralateral white noise attenuates 40-Hz auditory steady-state fields but not N100m in auditory evoked fields,” Neuroimage 59, 1037–1042. 10.1016/j.neuroimage.2011.08.108 [DOI] [PubMed] [Google Scholar]
- 40. Kemp, D. T. (1978). “ Stimulated acoustic emissions from within the human auditory system,” J. Acoust. Soc. Am. 64, 1386–1391. 10.1121/1.382104 [DOI] [PubMed] [Google Scholar]
- 41. Kemp, D. T. , Ryan, S. , and Bray, P. (1990). “ A guide to the effective use of otoacoustic emissions,” Ear Hear. 11, 93–105. 10.1097/00003446-199004000-00004 [DOI] [PubMed] [Google Scholar]
- 42. Keppler, H. , Dhooge, I. , Corthals, P. , Maes, L. , D'haenens, W. , Bockstael, A. , Philips, B. , Swinnen, F. , and Vinck, B. (2010). “ The effects of aging on evoked otoacoustic emissions and efferent suppression of transient evoked otoacoustic emissions,” Clin. Neurophysiol. 121, 359–365. 10.1016/j.clinph.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 43. Khalfa, S. , Morlet, T. , Micheyl, C. , Morgon, A. , and Collet, L. (1997). “ Evidence of peripheral hearing asymmetry in humans: Clinical implications,” Acta Otolaryngol. 117, 192–196. 10.3109/00016489709117767 [DOI] [PubMed] [Google Scholar]
- 44. Kiyokawa, H. , Kawase, T. , Oshima, H. , Maki, A. , and Kobayashi, T. (2012). “ Frequency characteristics of contralateral sound suppression of 40-Hz auditory steady-state response,” Eur. Arch. Otorhinolaryngol. 269, 791–797. 10.1007/s00405-011-1734-4 [DOI] [PubMed] [Google Scholar]
- 45. Konrad-Martin, D. , Neely, S. T. , Keefe, D. H. , Dorn, P. A. , Cyr, E. , and Gorga, M. P. (2002). “ Sources of DPOAEs revealed by suppression experiments, inverse fast Fourier transforms, and SFOAEs in impaired ears,” J. Acoust. Soc. Am. 111, 1800–1809. 10.1121/1.1455024 [DOI] [PubMed] [Google Scholar]
- 46. Kumar, U. A. , and Vanaja, C. S. (2004). “ Functioning of olivocochlear bundle and speech perception in noise,” Ear Hear. 25, 142–146. 10.1097/01.AUD.0000120363.56591.E6 [DOI] [PubMed] [Google Scholar]
- 47. Kuwada, S. , Anderson, J. S. , Batra, R. , Fitzpatrick, D. C. , Teissier, N. , and D'angelo, W. R. (2002). “ Sources of the scalp-recorded amplitude-modulation following response,” J. Am. Acad. Audiol. 13, 188–204. [PubMed] [Google Scholar]
- 48. Leigh-Paffenroth, E. D. , and Murnane, O. D. (2011). “ Auditory steady state responses recorded in multitalker babble,” Int. J. Audiol. 50, 86–97. 10.3109/14992027.2010.532512 [DOI] [PubMed] [Google Scholar]
- 49. Lichtenhan, J. T. , Wilson, U. S. , Hancock, K. E. , and Guinan, J. J., Jr. (2016). “ Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses,” Hear. Res. 333, 216–224. 10.1016/j.heares.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lilaonitkul, W. , and Guinan, J. J., Jr. (2009). “ Reflex control of the human inner ear: A half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking,” J. Neurophysiol. 101, 1394–1406. 10.1152/jn.90925.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lisowska, G. , Namyslowski, G. , Orecka, B. , and Misiolek, M. (2014). “ Influence of aging on medial olivocochlear system function,” Clin. Interv. Aging 9, 901–914. 10.2147/cia.s61934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maison, S. , Micheyl, C. , and Collet, L. (2001). “ Influence of focused auditory attention on cochlear activity in humans,” Psychophysiology 38, 35–40. 10.1111/1469-8986.3810035 [DOI] [PubMed] [Google Scholar]
- 53. Maison, S. F. , and Liberman, M. C. (2000). “ Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20, 4701–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maki, A. , Kawase, T. , and Kobayashi, T. (2009). “ Effects of contralateral noise on 40-Hz and 80-Hz auditory steady-state responses,” Ear Hear. 30, 584–589. 10.1097/AUD.0b013e3181acfb57 [DOI] [PubMed] [Google Scholar]
- 55. Mertes, I. B. , and Goodman, S. S. (2016). “ Within- and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions,” Ear Hear. 37, e72–e84. 10.1097/AUD.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 56. Mishra, S. K. , and Lutman, M. E. (2013). “ Repeatability of click-evoked otoacoustic emission-based medial olivocochlear efferent assay,” Ear Hear. 34, 789–798. 10.1097/AUD.0b013e3182944c04 [DOI] [PubMed] [Google Scholar]
- 57. Mishra, S. K. , and Lutman, M. E. (2014). “ Top-down influences of the medial olivocochlear efferent system in speech perception in noise,” PLoS One 9, e857656. 10.1371/journal.pone.0085756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moleti, A. , Botti, T. , and Sisto, R. (2012). “ Transient-evoked otoacoustic emission generators in a nonlinear cochlea,” J. Acoust. Soc. Am. 131, 2891–2903. 10.1121/1.3688474 [DOI] [PubMed] [Google Scholar]
- 59. Møller, A. R. (1998). “ Neural generators of the brainstem auditory evoked potentials,” Semin. Hear. 19, 11–27. 10.1055/s-0028-1082955 [DOI] [Google Scholar]
- 60. Moore, J. K. , and Osen, K. K. (1979). “ The cochlear nuclei in man,” Am. J. Anat. 154, 393–418. 10.1002/aja.1001540306 [DOI] [PubMed] [Google Scholar]
- 61. Mott, J. B. , Norton, S. J. , Neely, S. T. , and Warr, W. B. (1989). “ Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear,” Hear. Res. 38, 229–242. 10.1016/0378-5955(89)90068-3 [DOI] [PubMed] [Google Scholar]
- 62. Moulin, A. , Collet, L. , and Duclaux, R. (1993). “ Contralateral auditory stimulation alters acoustic distortion products in humans,” Hear. Res. 65, 193–210. 10.1016/0378-5955(93)90213-K [DOI] [PubMed] [Google Scholar]
- 63. Mulders, W. H. , Paolini, A. G. , Needham, K. , and Robertson, D. (2009). “ Synaptic responses in cochlear nucleus neurons evoked by activation of the olivocochlear system,” Hear. Res. 256, 85–92. 10.1016/j.heares.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 64. Mulders, W. H. A. M. , Seluakumaran, K. , and Robertson, D. (2008). “ Effects of centrifugal pathways on responses of cochlear nucleus neurons to signals in noise,” Eur. J. Neurosci. 27, 702–714. 10.1111/j.1460-9568.2008.06046.x [DOI] [PubMed] [Google Scholar]
- 65. Oswald, J. A. , Rosner, T. , and Janssen, T. (2006). “ Hybrid measurement of auditory steady-state responses and distortion product otoacoustic emissions using an amplitude-modulated primary tone,” J. Acoust. Soc. Am. 119, 3886–3895. 10.1121/1.2197789 [DOI] [PubMed] [Google Scholar]
- 66. Picton, T. W. , John, M. S. , Dimitrijevic, A. , and Purcell, D. W. (2003). “ Human auditory steady-state responses,” Int. J. Audiol. 42, 177–219. 10.3109/14992020309101316 [DOI] [PubMed] [Google Scholar]
- 67. Prieve, B. A. , Gorga, M. P. , Schmidt, A. , Neely, S. T. , Peters, J. , Schultes, L. , and Jesteadt, W. (1993). “ Analysis of transient-evoked otoacoustic emissions in normal-hearing and hearing-impaired ears,” J. Acoust. Soc. Am. 93, 3308–3319. 10.1121/1.405715 [DOI] [PubMed] [Google Scholar]
- 68. Puel, J.-L. , Bonfils, P. , and Pujol, R. (1988). “ Selective attention modifies the active micromechanical properties of the cochlea,” Brain Res. 447, 380–383. 10.1016/0006-8993(88)91144-4 [DOI] [PubMed] [Google Scholar]
- 69. Purcell, D. W. , and Dajani, H. R. (2008). “ The stimulus-response relationship in auditory steady-state response testing,” in The Auditory Steady-State Response: Generation, Recording, and Clinical Applications, edited by Rance G. ( Plural Publishing, Inc., San Diego, CA: ), pp. 55–82. [Google Scholar]
- 70. Purcell, D. W. , John, M. S. , and Picton, T. W. (2003). “ Concurrent measurement of distortion product otoacoustic emissions and auditory steady state evoked potentials,” Hear. Res. 176, 128–141. 10.1016/S0378-5955(02)00770-0 [DOI] [PubMed] [Google Scholar]
- 71. Puria, S. , Guinan, J. J., Jr. , and Liberman, M. C. (1996). “ Olivocochlear reflex assays: Effects of contralateral sound on compound action potentials versus ear-canal distortion products,” J. Acoust. Soc. Am. 99, 500–507. 10.1121/1.414508 [DOI] [PubMed] [Google Scholar]
- 72. Rodriguez, R. , Picton, T. , Linden, D. , Hamel, G. , and Laframboise, G. (1986). “ Human auditory steady state responses: Effects of intensity and frequency,” Ear Hear. 7, 300–313. 10.1097/00003446-198610000-00003 [DOI] [PubMed] [Google Scholar]
- 73. Rosner, T. , Kandzia, F. , Oswald, J. A. , and Janssen, T. (2011). “ Hearing threshold estimation using concurrent measurement of distortion product otoacoustic emissions and auditory steady-state responses,” J. Acoust. Soc. Am. 129, 840–851. 10.1121/1.3531934 [DOI] [PubMed] [Google Scholar]
- 74. Schochat, E. , Matas, C. G. , Samelli, A. G. , and Carvallo, R. M. M. (2012). “ From otoacoustic emission to late auditory potentials P300: The inhibitory effect,” Acta Neurobiol. Exp. 72, 296–308. [DOI] [PubMed] [Google Scholar]
- 75. Seluakumaran, K. , Mulders, W. H. A. M. , and Robertson, D. (2008). “ Unmasking effects of olivocochlear efferent activation on responses of inferior colliculus neurons,” Hear. Res. 243, 35–46. 10.1016/j.heares.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 76. Shera, C. A. , and Guinan, J. J., Jr. (1999). “ Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- 77. Siegel, J. H. , and Kim, D. O. (1982). “ Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity,” Hear. Res. 6, 171–182. 10.1016/0378-5955(82)90052-1 [DOI] [PubMed] [Google Scholar]
- 78. Sininger, Y. S. , and Cone-Wesson, B. (2006). “ Lateral asymmetry in the ABR of neonates: Evidence and mechanisms,” Hear. Res. 212, 203–211. 10.1016/j.heares.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 79. Smith, D. W. , Turner, D. A. , and Henson, M. M. (2000). “ Psychophysical correlates of contralateral efferent suppression. I. The role of the medial olivocochlear system in ‘central masking’ in nonhuman primates,” J. Acoust. Soc. Am. 107, 933–941. 10.1121/1.428274 [DOI] [PubMed] [Google Scholar]
- 80. Smith, S. B. , and Cone, B. (2015). “ The medial olivocochlear reflex in children during active listening,” Int. J. Audiol. 54, 518–523. 10.3109/14992027.2015.1008105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Starr, A. , and Wernick, J. S. (1968). “ Olivocochlear bundle stimulation: Effects on spontaneous and tone-evoked activities of single units in cat cochlear nucleus,” J. Neurophysiol. 31, 549–564. [DOI] [PubMed] [Google Scholar]
- 82. Tokgoz-Yilmaz, S. , Kose, S. K. , Turkyilmaz, M. D. , and Atay, G. (2013). “ The role of the medial olivocochlear system in the complaints of understanding speech in noisy environments by individuals with normal hearing,” Auris Nasus Larynx 40, 521–524. 10.1016/j.anl.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 83. Usubuchi, H. , Kawase, T. , Kanno, A. , Yahata, I. , Miyazaki, H. , Nakasato, N. , Kawashima, R. , and Katori, Y. (2014). “ Effects of contralateral noise on the 20-Hz auditory steady state response–magnetoencephalography study,” PLoS One 9, e99457. 10.1371/journal.pone.0099457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vander Werff, K. R. , and Brown, C. J. (2005). “ Effect of audiometric configuration on threshold and suprathreshold auditory steady-state responses,” Ear Hear. 26, 310–326. 10.1097/00003446-200506000-00007 [DOI] [PubMed] [Google Scholar]
- 85. Warr, W. B. , and Guinan, J. J., Jr. (1979). “ Efferent innervation of the organ of corti: Two separate systems,” Brain Res. 173, 152–155. 10.1016/0006-8993(79)91104-1 [DOI] [PubMed] [Google Scholar]
- 86. Whitehead, M. L. , Martin, G. K. , and Lonsbury-Martin, B. L. (1991). “ Effects of the crossed acoustic reflex on distortion-product otoacoustic emissions in awake rabbits,” Hear. Res. 51, 55–72. 10.1016/0378-5955(91)90007-V [DOI] [PubMed] [Google Scholar]
- 87. Winslow, R. L. , and Sachs, M. B. (1987). “ Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise,” J. Neurophysiol. 57, 1002–1021. [DOI] [PubMed] [Google Scholar]
- 88. Wittekindt, A. , Kaiser, J. , and Abel, C. (2014). “ Attentional modulation of the inner ear: A combined otoacoustic emission and EEG study,” J. Neurosci. 34, 9995–10002. 10.1523/JNEUROSCI.4861-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao, W. , Dewey, J. B. , Boothalingam, S. , and Dhar, S. (2015). “ Efferent modulation of stimulus frequency otoacoustic emission fine structure,” Front. Syst. Neurosci. 9, 1–12. 10.3389/fnsys.2015.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao, W. , and Dhar, S. (2010). “ The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions,” J. Assoc. Res. Otolaryngol. 11, 53–67. 10.1007/s10162-009-0189-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhu, X. , Vasilyeva, O. N. , Kim, S. , Jacobson, M. , Romney, J. , Waterman, M. S. , Tuttle, D. , and Frisina, R. D. (2007). “ Auditory efferent feedback system deficits precede age-related hearing loss: Contralateral suppression of otoacoustic emissions in mice,” J. Comp. Neurol. 503, 593–604. 10.1002/cne.21402 [DOI] [PubMed] [Google Scholar]
- 92. Zwislocki, J. J. (1972). “ A theory of central auditory masking and its partial validation,” J. Acoust. Soc. Am. 52, 644–659. 10.1121/1.1913154 [DOI] [Google Scholar]