Abstract
Cochlear implants (CIs) provide children with access to speech information from a young age. Despite bilateral cochlear implantation becoming common, use of spatial cues in free field is smaller than in normal-hearing children. Clinically fit CIs are not synchronized across the ears; thus binaural experiments must utilize research processors that can control binaural cues with precision. Research to date has used single pairs of electrodes, which is insufficient for representing speech. Little is known about how children with bilateral CIs process binaural information with multi-electrode stimulation. Toward the goal of improving binaural unmasking of speech, this study evaluated binaural unmasking with multi- and single-electrode stimulation. Results showed that performance with multi-electrode stimulation was similar to the best performance with single-electrode stimulation. This was similar to the pattern of performance shown by normal-hearing adults when presented an acoustic CI simulation. Diotic and dichotic signal detection thresholds of the children with CIs were similar to those of normal-hearing children listening to a CI simulation. The magnitude of binaural unmasking was not related to whether the children with CIs had good interaural time difference sensitivity. Results support the potential for benefits from binaural hearing and speech unmasking in children with bilateral CIs.
I. INTRODUCTION
For normal-hearing (NH) listeners, binaural hearing provides advantages for speech reception in noise and for signal detection (Webster, 1951; Schubert, 1956; Carhart et al., 1967; Bronkhorst and Plomp, 1988; Lavandier and Culling, 2010). One advantage, referred to as “binaural unmasking,” can be observed when one compares listeners' performance in a diotic condition in which the target has the same interaural configuration as the noise, to a dichotic condition in which the target and noise have different interaural configurations. For example, listeners have better speech reception thresholds when speech has a non-zero interaural time difference (ITD) and the masker has a 0-μs ITD compared to when both stimuli have a 0-μs ITD. Binaural unmasking can be observed by presenting stimuli through headphones, which allows for the manipulation of ITDs in the absence of differences between the ears in the signal-to-noise ratio (SNR) cause by monaural “head shadow,” or interaural level differences (ILDs). Furthermore, those same binaural processing mechanisms are thought to contribute to signal detection and speech reception benefits in free-field listening situations involving spatial separation of sound sources (Carhart, 1965; Hawley et al., 2004). Binaural unmasking of tones in noise arises from the interaural decorrelation (i.e., reduced similarity of the signals between the ears, or the introduction of dynamic ITDs and ILDs) that occurs from the combination of the target and masker (Domnitz and Colburn, 1976). Interaural decorrelation is thought to provide the listener with information about the presence of the signal to be detected or the spectral structure of target (Akeroyd and Summerfield, 2000).
Binaural unmasking was studied here in children who are profoundly deaf and received cochlear implants (CIs) in both ears. CIs are the standard of care for children and adults with severe-to-profound hearing loss, with the goal of providing access to sound for these individuals. It is now common for individuals to receive bilateral CIs, which have the potential to provide some of the binaural hearing benefits that NH listeners receive from having access to sound in two ears. Bilateral CIs have been shown to improve reception of speech in noise in free field when there is spatial separation of sound sources. For most CI listeners, the benefit occurs mainly from monaural head shadow (e.g., Schleich et al., 2004; Litovsky et al., 2006a; Litovsky et al., 2009; Loizou et al., 2009; Misurelli and Litovsky, 2012). However, CI users show little, if any, evidence of a benefit from binaural processing (i.e., auditory processing involving interaural comparisons) for speech unmasking, which has been examined in free field conditions by comparing unilateral and bilateral performance or minimizing head shadow advantages through the use of symmetrical masker locations. When stimuli are presented via direct audio input to the processors, binaural advantages for speech unmasking have also not been found (van Hoesel et al., 2008; Loizou et al., 2009). Thus, controlling inputs at the level of individual electrodes may be required for achieving binaural unmasking, and was the goal of the present study in children, as described in greater detail below.
Studies on binaural unmasking in adults with bilateral CIs have focused on those with post-lingual onset of deafness (Long et al., 2006; Lu et al., 2010, 2011). Many children with CIs are pre-lingually deaf and thus, have had limited access to acoustic binaural hearing prior to receiving their CIs (Grieco-Calub and Litovsky, 2010; Niparko et al., 2010). Furthermore, some children with CIs experience a period of unilateral stimulation prior to being implanted. Results from psychophysical experiments with children with CIs suggest that limited experience with bilateral hearing results in deficits in binaural hearing abilities, specifically ITD sensitivity (Salloum et al., 2010; Gordon et al., 2014). The hypothesis that auditory deprivation affects ITD sensitivity is supported by physiological experiments examining the effect of auditory deprivation on ITD representations in cats (Hancock et al., 2010; Tillein et al., 2010). Even with several years of bilateral hearing experience, it is uncertain that children with CIs process binaural cues in the same way as NH children. Therefore, children with CIs should not be expected to perform the same as post-lingually deafened adults with CIs on measures of binaural processing. Binaural processing in children with bilateral CIs was thus examined in this study in order to understand the extent to which binaural unmasking can be observed in children who have undergone auditory deprivation early in life, followed by bilateral stimulation. It is important to note that the stimulation mode in today's clinical processors does not preserve binaural cues with fidelity (Kan and Litovsky, 2015), and results from this work are interpreted in that context.
Children with bilateral CIs have shown binaural unmasking of tones in 50-Hz bandwidth noise when presented temporal envelope modulations at single pairs of electrodes, i.e., one active electrode in each ear (Van Deun et al., 2009). Binaural unmasking in children with CIs suggests that children with CIs are sensitive to interaural decorrelation of temporal envelope in dichotic listening conditions. Such a result is noteworthy given that some children with bilateral CIs do not show sensitivity to ITDs and suggests that children with CIs were able to use the dynamic ILDs which comprise interaural decorrelation of temporal envelope. Thus for children with CIs, binaural unmasking measures the use of a binaural cue (i.e., interaural envelope decorrelation) which they could potentially use to obtain better speech reception in noise.
Many of the previous studies using single-electrode pairs have demonstrated binaural sensitivity in CI listeners (e.g., van Hoesel and Tyler, 2003; Long et al., 2006; van Hoesel et al., 2009). With a single pair of electrodes there is absence of stimulation from neighboring electrodes. However, everyday stimulation involves multiple electrode pairs to take advantage of the tonotopic organization of the auditory system. It is unknown how binaural information spread across multiple electrodes would change overall binaural hearing performance. On one hand, it is possible that there would be improvement in binaural unmasking with multi-electrode stimulation compared to single-electrode stimulation if listeners are able to integrate signal information across different stimulation sites (i.e., neural populations stimulated by individual electrodes). Studies on NH adults suggest that listeners are able to integrate information across separate places along the cochlea. Diotic signal detection accuracy has been found to improve when the number of signals increases even when the signals occur in separate critical bands (Green, 1958; Buus et al., 1986). There is also evidence of integration with dichotic signal detection. Langhans and Kohlrausch (1992) found that dichotic signal detection thresholds improved similar to diotic signal detection thresholds (i.e., binaural unmasking remained constant) as the number of spectral components outside of any one critical band increased. This was the case even across a frequency range in which dichotic signal detection thresholds are unaffected by signal frequency (Kohlrausch, 1986). There is also evidence of integration across channels with monaurally presented sinusoidally amplitude-modulated pulse trains in adults with CIs (Galvin et al., 2014).
On the other hand, performance may suffer with multi-electrode stimulation due to channel interactions (i.e., stimulation of overlapping populations of auditory nerve fibers by different electrodes) (Shannon, 1983; Abbas et al., 2003; Cohen et al., 2003). Lu et al. (2011) examined binaural unmasking with multi-electrode stimulation in adults and found poorer performance when neighboring electrodes were stimulated. Overlap in stimulated neural populations by neighboring electrodes could interfere with binaural unmasking for a number of reasons. First, channel interactions may reduce the interaural decorrelation in dichotic conditions at the level of the auditory nerve. This would likely occur when the modulations presented by neighboring electrodes are uncorrelated (monaurally), since in this situation channel interactions could effectively reduce the depth of the temporal envelope fluctuations and therefore reduce the fluctuations of the ILD. Second, channel interactions could also be detrimental if they are asymmetrical between the ears (van der Heijden and Trahiotis, 1998), which would be expected, for example, when electrodes between the ears are placed at different distances from the neural tissue or when there are different extents of neural survival between the ears. Binaural asymmetries in the extents of overlapping stimulation between electrodes could reduce interaural correlation in diotic conditions at the level of the auditory neurons, since places of stimulation that are anatomically matched across the ears would receive inputs that are different. A reduction in interaural correlation in diotic conditions would make signal detection based on interaural correlation more difficult (Gabriel and Colburn, 1981).
In the current study, we sought to examine binaural unmasking with multi-electrode stimulation while minimizing the effect of channel interactions. Lu et al. (2011) found that binaural unmasking performance in adults was worse when there was greater overlap in stimulated neural populations, suggesting that the negative effect of channel interactions can be reduced with wide spacing of electrodes. This is consistent with the finding that ITD discrimination performance in adults with amplitude-modulated pulse trains is maintained with dual versus single electrode-pair stimulation when electrodes are widely spaced (Ihlefeld et al., 2014). Thus in this study, stimulation sites were widely spaced to minimize overlap in stimulated neural populations. In addition, in this study, for any stimulus interval, electrodes in the multi-electrode condition were all presented with the same sample of noise and the same signal. Van Deun et al. (2011) found that diotic and dichotic detection thresholds of three adults with CIs were similar between stimulation with a single electrode and stimulation with three adjacent electrodes when electrodes presented the same sample of noise and the same signal for each stimulus interval. In contrast, when different samples of noise and different signals were presented to the adjacent electrodes, thresholds markedly increased. This supports the idea that the presentation of the same stimuli to different electrodes reduces the effect of channel interactions on binaural unmasking likely due to monaurally correlated temporal envelope modulation. While the presentation of exactly the same envelope to different channels would not naturally occur, this manipulation can be informative regarding binaural processing of speech which tends to have redundant modulations across channels. Furthermore, it is informative regarding whether binaural hearing performance can be improved by stimulating a greater neural population with the same modulations, which may be informative to future signal processing strategies.
The purpose of this study was to examine the relationship between single- and multi-electrode stimulation, for diotic and dichotic signal detection in children with bilateral CIs. Diotic and dichotic signal detection thresholds were measured using three stimulation sites individually and combined. We hypothesized that if information is integrated across sites, performance would improve with multi-electrode stimulation compared to single-electrode stimulation for both diotic and dichotic signal detection. Alternatively, if listeners fully use information from the best site and no integration occurs, performance with multi-electrode stimulation would be similar to the best performance with single-electrode stimulation. Additionally, performance on the binaural unmasking task was examined relative to performance on an ITD discrimination task (Ehlers et al., 2015), to examine whether binaural unmasking reflects aspects of auditory processing not captured by ITD sensitivity.
II. METHOD
A. Participants and equipment
Participants included 11 children (five male, six female) with bilateral CIs between the ages of 11 and 17 years [average = 14.1, standard deviation (s.d.) = 1.7]. All of the children had Nucleus device types manufactured by Cochlear Ltd. Table I shows demographic information and the electrode pairs used for the current study. Higher numbered electrodes are located more towards the apical end of the array. Stimuli were delivered from a personal computer using the Nucleus Implant Communicator and bilaterally synchronized L34 processors (Cochlear Ltd.).
TABLE I.
Demographics of the participants with CIs. Electrode pairs used for testing are shown along with the proportion of the dynamic range in current units used for presenting the signal detection stimuli.
| Participant | Age (years) | Hearing history | Etiology | Age at 1st activation (years) | Age at 2nd activation (years) | Internal device (L, R) | Base pair (L, R) | Middle pair (L, R) | Apical pair (L, R) | Base prop. DR (L, R) | Middle prop. DR (L, R) | Apex prop. DR (L, R) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIDX | 11.6 | Pro.a HLb ID at birth | Connexin 26 | 1.43 | 2.59 | CI24R(CS), CI24R(CS) | 4, 4 | 12, 12 | 20, 18 | 0.87, 0.87 | 0.77, 0.89 | 0.88, 0.88 |
| CIDQ | 12.2 | ID at birth | Connexin 26 | .82 | 4.34 | CI24RE, CI24R(CS) | 4, 4 | 12, 12 | 20, 20 | 0.77, 0.60 | 0.76, 0.66 | 0.72, 0.66 |
| CIDJ | 13.1 | ID at 12 months | Hereditary | 1.62 | 5.04 | CI24RE, CI24R(CS) | 6, 6 | 12, 12 | 20, 18 | 0.75, 0.86 | 0.77, 0.84 | 0.73, 0.85 |
| CIEV | 13.2 | Progressed from sev.c to pro. by 2–3 years | Hereditary | 2.67 | 10.95 | CI24RE, CI24R(CA) | 4, 6 | 12, 14 | 20, 20 | 0.83, 1 | 0.80, 1 | 0.79, 0.94 |
| CIAG | 13.3 | Progressed from mod.d–sev. (L) and sev.-pro. (R) to pro. by 15 months | Connexin 26 | 1.72 | 3.12 | CI24R(CS), CI24R(CS) | 4, 4 | 12, 12 | 20, 20 | 0.68, 0.76 | 0.74, 0.71 | 0.81, 0.63 |
| CIAW | 13.6 | Pro. HL ID at 3 months | Congenital CMV | 1.21 | 5.46 | CI24RE, CI24R(CS) | 4, 4 | 12, 8 | 20, 22 | 0.5, 0.82 | 0.66, 0.73 | 0.58, 0.81 |
| CIBO | 14.2 | Progressed from mod. to pro. HL by 28 months | EVAS/ Pendred syndrome | 2.83 | 3.90 | CI24R(CS), CI24R(CS) | 4, 4 | 12, 12 | 20, 18 | 0.93, 0.97 | 0.92, 1 | 0.93, 1 |
| CIAP | 14.7 | Progressed from mild to sev.-pro. by 3 years | Unknown | 3.47 | 5.10 | CI24R(CA),CI24R(CA) | 4, 4 | 12, 10 | 20, 16 | 1, 1 | 1, 0.95 | 1, 1 |
| CIBK | 15.2 | ID at 17 months | Connexin 26 | 2.14 | 7.11 | CI24RE, CI24R(CS) | 4, 4 | 12, 12 | 20, 18 | 0.76, 0.82 | 0.71, 0.85 | 0.72, 0.84 |
| CIEU | 16.2 | Progressed from sev. to pro. by age 4 (R) and 8 (L) years | Hereditary | 4.28 | 10.45 | CI24RE, CI24R(CS) | 4, 4 | 12, 12 | 18, 18 | 0.75, 0.73 | 0.63, 0.78 | 0.65, 0.84 |
| CIAQ | 17.5 | Pro. HL ID at 13 months | Connexin 26 | 4.00 | 8.21 | CI24R, CI24R | 4, 4 | 12, 13 | 20, 19 | 0.89, 0.9 | 0.88, 0.91 | 0.89, 0.89 |
Profound.
Hearing loss.
Severe.
Moderate.
Nine NH adults (one male, eight female) and nine NH children (seven male, two female) also participated in this study. The ages of the NH adults ranged from 18 to 25 years (average = 20.8, s.d. = 2.5). The ages of the NH children ranged from 11 to 13 years (average = 12.2, s.d. = 0.9), a range which corresponded to the lower end of the age range of the children with CIs, selected because younger children were more likely to show performance different from that of the NH adults. Tables II and III show the ages and pure tone thresholds in dB sound pressure level (SPL) of the NH children and adults, respectively. Stimuli were delivered by a personal computer connected to a Tucker-Davis Technologies system (System 3 with RP2.1, HB7, PA5 units) and ER-2 insert earphones (Etymotic Research, Inc.). The study was approved by the University of Wisconsin-Madison institutional review board.
TABLE II.
Age and pure tone thresholds in dB SPL of the NH children.
| Participant | Age (years) | 3650 Hz (L, R) |
|---|---|---|
| CUV | 11.2 | 8.1, 14 |
| CVH | 11.2 | 11.3, 13.3 |
| CRO | 11.6 | 6.3, 9.5 |
| CRP | 11.6 | 1.7, 0.9 |
| CLC | 12.1 | 11.8, 11.3 |
| CQQ | 12.3 | 8.8, 6.6 |
| CPU | 12.8 | 10.1, 12.9 |
| CRK | 13.1 | 11.4, 16.8 |
| CSK | 13.9 | 8.4, 12.3 |
TABLE III.
Age and pure tone thresholds in dB SPL of the NH adults.
| Participant | Age (years) | 3650 Hz (L, R) | 6922 Hz (L, R) | 13 014 Hz (L, R) |
|---|---|---|---|---|
| TEF | 18 | 7.4, 9.4 | −4.0, −10.9 | 6.8, 7.0 |
| TDP | 19 | 15.8, 20.5 | 16.6, 2.7 | 10.0, 9.7 |
| TDY | 19 | 15.2, 22.8 | 2.5, 7.1 | 6.8, 10.6 |
| TEA | 19 | 2.6, 1.1 | −0.3, −0.9 | 3.2, 7.4 |
| TEB | 19 | 10.8, 5.9 | 6.1, 6.6 | 3.4, 6.9 |
| TDZ | 22 | 12.4, 8.2 | −6.3, 5.7 | 11.7, 30.8 |
| TAF | 23 | 5.5, 17.3 | 7.3, 8.2 | 6.7, 10.5 |
| TAW | 23 | 14.7, 15.2 | 2.2, 9.2 | 8.5, 11.3 |
| TDQ | 25 | 19.6, 28.0 | 2.1, 10.8 | 0.9, 2.3 |
B. Stimuli used with CI participants
Electrical stimuli were trains of biphasic pulses presented in MP1+2 (monopolar) mode. Each phase had a duration of 25 μs and the inter-phase gap was 8 μs. Electrodes in the right and left ears were selected based on an interaural pitch matching task. The stimuli used for pitch matching were 300-ms constant amplitude biphasic pulse trains presented at a rate of 100 pulses per second (pps), and at levels that were determined by the participants to be comfortable. One hundred pps was used for pitch matching as it matched the rate of the stimuli used for the ITD discrimination task. ITD discrimination was measured at a low pulse rate of 100 pps because performance has been shown to degrade with increasing pulse rates beyond 200 pps (van Hoesel et al., 2009). Stimuli used for loudness mapping of the diotic and dichotic signal detection stimuli were 400-ms pulse trains presented at 1000 pps, matching the duration and pulse rate of the diotic and dichotic signal detection stimuli. Diotic and dichotic signal detection were measured at 1000 pps, because dichotic signal detection and interaural correlation discrimination has been shown to be poorer at low rates (Todd et al., 2014; Goupell and Litovsky, 2015). Loudness mapping was conducted using both unilateral and bilateral presentations of stimuli. Bilateral presentation of stimuli allowed for level adjustments which aimed to achieve perceptually balanced levels across ears and is atypical of today's clinical loudness mapping procedures.
The electric stimuli used to measure diotic and dichotic signal detection were based on digital representations of acoustic stimuli which were generated at a sampling rate of 44 100 Hz. Samples of Gaussian noise were generated with a center frequency (CF) of 500 Hz, bandwidth of 50 Hz, and a duration of 400 ms created in the frequency domain. The target signal was a 300-ms, 500-Hz tone which, when presented, was temporally centered in the noise. Both the tone and the noise had 50-ms onset and offset ramps created with a Hanning window. The tone and noise were either interaurally in-phase (NoSo), or the noise was in-phase and the tone was interaurally out-of-phase (NoSπ). The SNR of the tone and noise varied between 20 dB and −32 dB in steps of 2 dB. Stimuli were pre-generated and consisted of 35 independent noise samples.
The Hilbert envelopes of the acoustic stimuli were calculated and were normalized to the average amplitude. The envelopes were then resampled at a rate of 1000 Hz. The envelopes were compressed between participants' thresholds and maximum levels (found during mapping with the multi-electrode stimulation) using the compression function used by Long et al. (2006). The envelopes were used to modulate the amplitude of 400-ms electrical pulse trains at 1000 pps. Timing of pulses on left and right sides were synchronized; therefore, the interaural decorrelation in the dichotic condition was introduced only in the temporal envelope (i.e., a dynamic ILD). For one participant, CIDQ, envelopes were resampled at 1800 Hz and were used to modulate pulse trains at 1800 pps which was a rate used in that participant's clinical map. This was done because the participant had a considerably smaller dynamic range at 1000 pps than the other participants and difficulty with the signal detection task during the familiarization with the stimuli at 1000 pps. The three panels of Fig. 1 show examples of an NoSπ stimulus, at SNRs of 10, 0, and −10 dB, from top to bottom, respectively. The modulations appearing in Fig. 1 are those of the temporal envelope of the combination of noise and tone. The temporal fine structure of the noise and tone are not represented in the modulations due to the way in which the temporal envelope was calculated.
FIG. 1.
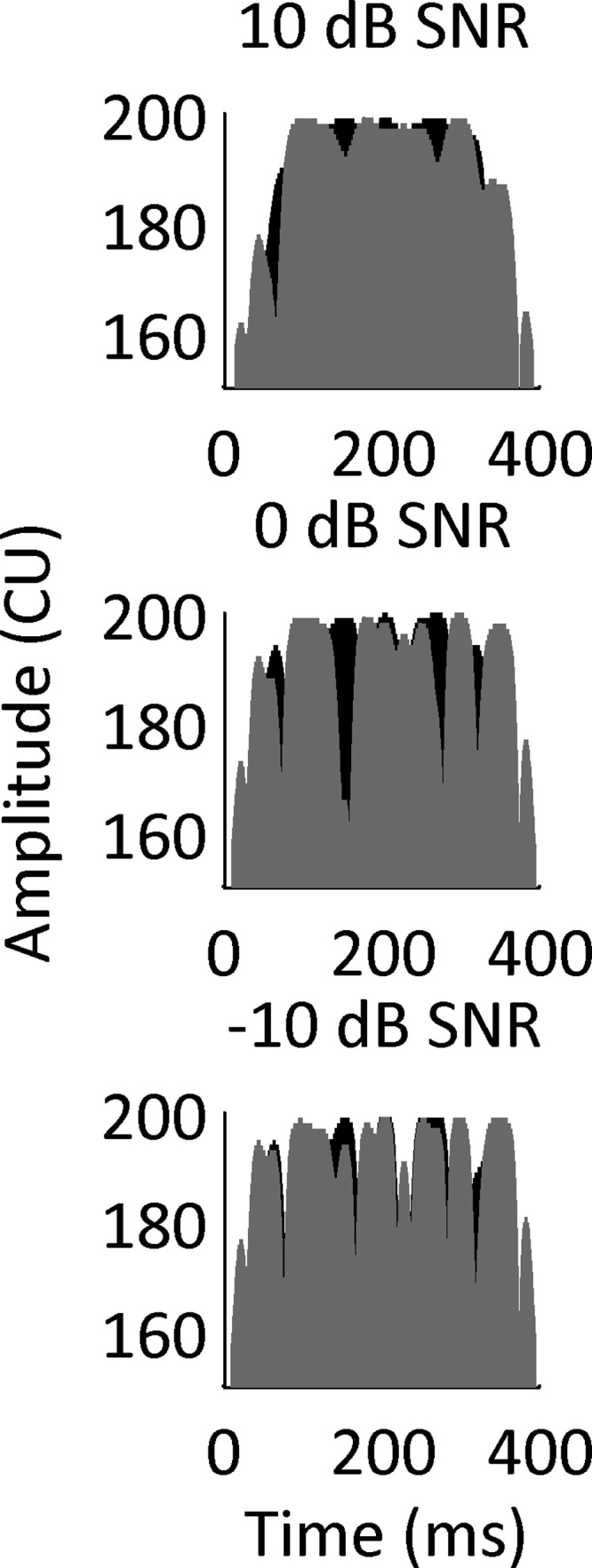
Amplitude in current units (CU) as a function of time (ms) for the envelope of an electrical NoSπ stimulus at 10 dB SNR (top), 0 dB SNR (middle), and −10 dB SNR (bottom). The stimuli were generated by calculating the Hilbert envelope of a tone in 50-Hz bandwidth noise and applying a compression function. The range of the scale of the ordinate was arbitrarily chosen. The left and right channels are shown in black and gray, respectively.
In the single-electrode conditions, stimuli were presented to a single bilateral pair of electrodes, located at either the basal, middle, or apical regions of the electrode arrays. In the multi-electrode conditions stimuli were presented to all three electrode pairs. When all three electrode pairs were active, electrodes on each side (left or right) were activated sequentially with 333 μs between pulse onsets from different electrodes, and the time synchrony of electrodes across the ears was maintained. For any stimulus interval in the multi-electrode condition, the temporal envelope from the same sample of noise was provided to the compression function that mapped the envelopes between thresholds and maximum stimulation levels for each of the three electrode pairs. When the target was presented, it had the same interaural phase relationship (NoSo or NoSπ) for all three electrodes.
C. Stimuli used with NH participants
In order to compare the performance of NH listeners and CI listeners, we utilized an acoustic CI simulation presented to NH children and adults. The acoustic stimuli used with the NH participants were designed to simulate the electrical stimuli used with CI users. The original stimuli were the same as those of the participants with CIs, except that they were created at a sampling rate of 50 000 Hz. The Hilbert envelopes of these stimuli were used to modulate the amplitude of trains of Gaussian-shaped pulses (Lu et al., 2007; Goupell et al., 2010; Goupell et al., 2013). Each pulse train had a CF of either 3650, 6922, or 13 014 Hz, to simulate electrodes at the apical, middle, and basal regions of an electrode array. These CFs were calculated to have a spacing of 4.5 mm along the basilar membrane according to the Greenwood function (Greenwood, 1990). This spacing is somewhat smaller than the approximate 6 mm (0.75 mm between electrodes × 8 electrodes) between electrodes used with the participants with CIs. However, this spacing was necessary to avoid frequencies which were too low and would result in sensitivity to temporal fine structure, and to avoid frequencies which were too high and have lower audibility. The Greenwood function (1990) was used to calculate bandwidths equivalent to 1.5 mm along the basilar membrane. Accordingly, pulse trains were set to have equivalent rectangular bandwidths equal to 788, 1467, and 2732 Hz for the 3650, 6922, and 13 014 Hz CFs, respectively. Unlike the 1000-pps stimuli used with the CI participants, a pulse rate of 300 pps was used to maintain a modulation depth >99% between pulses. If the modulation depth were reduced, the pulse train bandwidth would decrease, and thus would introduce a confound and be a less realistic simulation of monopolar stimulation. Note, Goupell (2012) did not find an effect of pulse rate on NoSo and NoSπ thresholds in adult NH listeners; therefore, the use of a lower pulse rate for the NH participants was not expected to change performance and thus produce a confound with the 1000-pps electrical pulse trains presented to the children with CIs. Pulse trains were normalized to have equal spectral-peak energy (Goupell et al., 2013). Like the CI participants, for the NH adults, stimuli were presented to either a single CF or to all three CFs. When stimuli were presented to all three CFs, the normalized pulse trains were summed together with a 333–μs delay between the three pulse trains of different CFs. The multi-site pulse trains were presented at a level of 67 dB-A. The pulse trains at 3650, 6922, and 13 014 Hz were presented at levels of 66, 58, and 43 dB-A, respectively. For the NH children, stimuli were presented to a single CF (3650 Hz) with the aim of examining overall performance differences between the NH children and CI children. Interaurally uncorrelated pink noise was presented from DC to 20 kHz at 60 dB SPL to mask possible combination tones.
D. Procedure
1. CI loudness mapping
a. Stimuli used to measure pitch-matching and ITD discrimination.
Comfortable levels were found for each of the even numbered electrodes. This was done by increasing the level of the stimulus until the participant indicated that the sound was comfortable and out of the quiet range.
b. Stimuli used to measure diotic and dichotic signal detection.
Thresholds and maximum acceptable loudness levels were measured through experimenter adjustments for each of the six electrodes (three bilateral pairs) to be used for the diotic and dichotic signal detection task. Thresholds were levels that provided a consistent response from participants on ascending tracks. Maximum acceptable loudness levels were measured by slowly and carefully increasing the stimulus level, until the participant indicated that it was the highest level still within the comfortable range. At least two measures of maximum acceptable loudness levels were obtained, and the average was the final value used for each electrode.
In order to ensure levels were comfortable in the multi-electrode condition of the signal detection task, maximum levels (used for the compression function) were adjusted with the following procedure using the noise stimuli of the signal detection task. Noise was used for setting maximum levels as it was similar to the stimuli the listener would be hearing during the experimental task, which was either noise or a combination of noise and tone. For each of the left and right sides, maximum levels for the three electrodes were lowered relative to the maximum acceptable loudness level by 25 current units on average, which was a reduction of the dynamic range to approximately 60%. The three electrodes were then stimulated concurrently, and maximum levels were raised in small steps until the participant indicated the loudness was at the high end of the perceived comfortable range. Maximum levels were adjusted by changing the maximum levels for each electrode by the same number of current units. Each side (stimulating three electrodes concurrently) was then stimulated sequentially and the maximum levels of one side were further adjusted so that the two sides were as close to equal loudness as possible.
Subsequently, each individual left-right pair was stimulated using diotic noise to evaluate whether the auditory image was perceived by the participant to be approximately centered in the head. If the participant indicated that the auditory image was not approximately centered, small adjustments in the maximum levels were made to bring the image towards center. Table I shows the proportion of the dynamic range (in current units) for each electrode that were used to present stimuli.
2. CI interaural pitch matching
Interaurally pitch-matched electrode pairs were determined prior to testing, using methods similar to those of previous studies (Litovsky et al., 2010; Litovsky et al., 2012; Kan et al., 2013). This was done under the assumption that electrodes in left and right ears that elicit similar pitch percepts deliver place-matched information to neurons in the brainstem. The process for determining pitch-matched electrodes was initiated with a pitch-rating task in which all active, even-numbered electrodes from both sides were stimulated individually in a random order. The participants rated the perceived pitch of each stimulus by selecting a location on a visual analog scale. Responses to 10 trials were collected for each of the electrodes tested (approximately 11 electrodes in each ear, and 22 total). If this task had been performed at a previous visit to the lab, it was not repeated at the visit at which the other measures of the study were collected. Second, the results from the pitch-rating task were used to select electrodes in the two ears, for a direct interaural pitch comparison task. The pitch comparison task was completed in order to find three pitch-matched pairs, spaced along the electrode array at apical, middle, and basal regions. Six electrodes on the right were chosen for each of three comparison electrodes on the left (typically L4, L12, and L20), thus a total of 18 comparisons. Each set of six electrodes typically consisted of consecutive even-numbered electrodes in the region (apex, mid, or base) of the corresponding electrode on the left. For the experimental task, one of the three electrodes on the left was stimulated, followed by one of the six corresponding electrodes on the right. The participant indicated whether the second sound was much higher, higher, the same, lower, or much lower in pitch than the first sound. Responses to 20 trials were collected for each comparison. Stimuli were presented in a random order. Typically, the electrode which had the highest number of same responses was chosen as the match. If there were two or more electrodes with equal numbers of same responses, electrodes which had closer numbers of higher and lower responses were given preference.
3. NH pure tone thresholds
For the NH participants, tone detection thresholds were measured at the CFs of the stimuli (3650, 6922, and 13 014 Hz for the adults; 3650 Hz for the children) using a two-down, one-up adaptive procedure, which ended after ten reversals. The step size of the adaptive procedure changed from 3 to 1.5 dB after the first reversal and to 0.5 dB after the second reversal. Typically, a single track was collected per CF for each ear. Thresholds were calculated by averaging the last six reversals of each track. Thresholds are shown in Table II for the children and Table III for the adults.
4. CI interaural time differences
The NoSo and NoSπ thresholds obtained in this study are evaluated below in the context of ITD sensitivity examined by Ehlers et al. (2015). ITD just noticeable differences (JNDs) were measured in the CI participants on the same visit as the diotic and dichotic signal detection measurements were made. ITD discrimination was not measured for participants CIAW or CIAG and was only tested on the middle electrode pair for participant CIDX due to time limitations. Stimuli consisted of 100-pps constant-amplitude pulse trains delivered at comfortable levels. Testing was conducted using a method of constant stimuli. The ITDs tested were adjusted based on the participant's responses, and the data were then used to create psychometric functions with at least four points, each point consisting of at least 40 trials. ITDs greater than 1600 μs were not tested. Participants heard two intervals and responded by indicating whether the second sound was perceived to be to the left or right of the first sound. The ITD of the second sound was equal in magnitude but opposite in direction to the ITD of the first sound. Psychometric curves of accuracy as a function of ITD (on a linear scale) were constructed from the data using an established method (Wichmann and Hill, 2001a,b). ITD JNDs were the estimated ITDs at which participants' responses were 70.7% correct based on the psychometric curve. If ITD JNDs could not be determined or were estimated to be 1600 μs they were classified as ≥1600 μs. As discussed below, the ITD data were used here to examine the relationship between ITD sensitivity and binaural unmasking.
5. CI and NH NoSo and NoSπ signal detection
The signal detection task consisted of a three-interval two-alternative forced-choice task in which the target interval (NoSo or NoSπ) occurred in either the second or the third interval, and was randomly chosen on each trial. Non-target intervals consisted of diotic noise (No). Each interval contained a different noise sample, which was randomly selected without replacement. Inter-stimulus intervals were 300 ms. Participants were instructed to select the interval (second or third) in which the stimulus was different. Based on prior research and pilot testing, participants were given some information about how the sounds might be perceived. For the NoSo stimuli, participants were told that the stimulus that was different might have a percept of a sound that was “smoother” in nature (Goupell and Litovsky, 2015). For the NoSπ stimuli, they were told that the stimulus that was different might additionally have a perceived “width” or “movement” in the head. Correct answer feedback was always provided.
The SNR of the tone and noise were varied using a two-down one-up adaptive procedure beginning at 20 dB SNR. Initially the step size was 8 dB and changed to 4 dB after one reversal and 2 dB after three reversals. The adaptive track stopped after ten reversals. Stimuli were presented in blocks in which the target was either NoSo or NoSπ. This was done to reduce the number of times participants would have to switch the cue(s) to which they were attending. Within each block, one track for each of the four stimulation conditions (three single-electrode-pair conditions + one multi-electrode-pair condition) was presented in a newly randomized order. The total number of conditions was eight (four stimulation conditions × two interaural phase conditions). Typically four complete tracks were completed for each condition.
A probe stimulus, to assess whether the participant was paying attention, was played in the case that there were three or more consecutive increases in signal level and the adaptive track had reached a level of 12 dB SNR or greater. The probe stimulus was at 20-dB SNR. If the participant's response to the probe was correct, the track continued, otherwise another probe was presented. After three incorrect responses to the probe (or any stimulus at 20-dB SNR), the track was stopped and was not used in the analysis. This procedure was modified when a participant showed consistently poor performance, such that the probe was still presented but the participant needed a greater number (i.e., 12) of incorrect responses for the track to end.1
Prior to measuring signal detection thresholds, participants were familiarized with the stimuli. Familiarization typically consisted of the signal detection task at 12-dB SNR for the NoSo condition and 0-dB SNR for the NoSπ condition. Participants completed at least ten trials for each condition prior to testing. The testing procedure for the NH participants followed that of the CI participants, except that the NH children were tested only at the simulated apical electrode (i.e., 3650-Hz CF).
For each condition, threshold estimates were averaged across adaptive tracks. The NoSo and NoSπ thresholds were fit to linear mixed-effects model which had random intercepts for participants. F-tests were conducted by comparing models with and without the specific predictor variable of interest. Predictor variables included phase (NoSo and NoSπ), place (apex, middle, and base), adaptive track order, and age. Furthermore, t-tests were conducted to compare single- and multi-site diotic and dichotic signal detection thresholds. Binaural masking level differences (BMLDs) were calculated by subtracting NoSπ from NoSo thresholds.
III. RESULTS
A. NoSo and NoSπ thresholds
1. CI participants
Figure 2 shows NoSo and NoSπ thresholds for each of the children with CIs for each of the single- and multi-electrode conditions. NoSπ thresholds were lower than NoSo thresholds [F1,74 = 49.67, p < 0.0001]. The mean NoSo threshold was 2.4 dB (s.d. = 7.4 dB) and the mean NoSπ was −3.8 dB (s.d. = 4.7 dB), with a mean BMLD of 6.3 dB (s.d. = 6.1 dB).2 The mean BMLD for each stimulation condition is shown in Table IV.
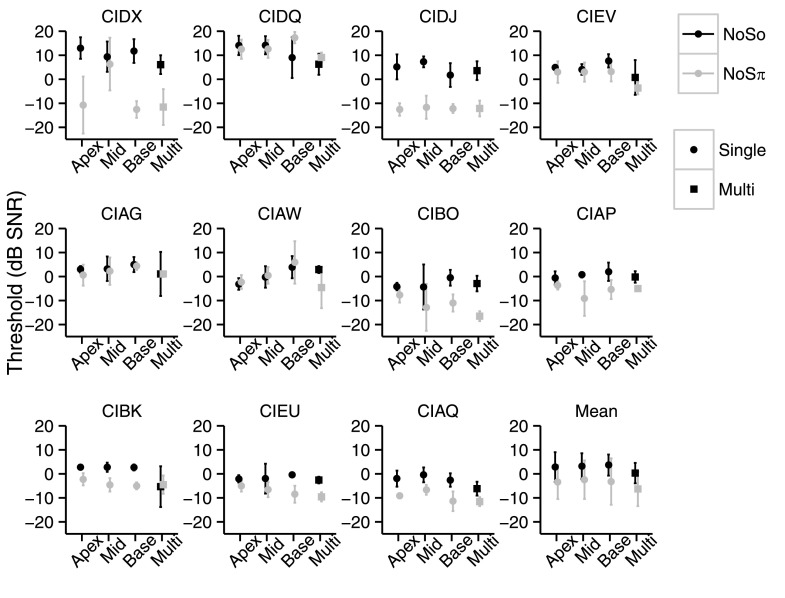
FIG. 2.
NoSo (black) and NoSπ (gray) thresholds (dB SNR) of the children with CIs for each stimulation condition. Each panel shows the data of an individual child except for the last panel which shows the mean thresholds. Single-electrode-pair stimulation is shown by circles and multi-electrode-pair stimulation is shown by squares. Error bars represent ±1 standard deviation.
TABLE IV.
Mean BMLD and standard deviations.
| CI children | NH children | NH adults | |
|---|---|---|---|
| Apex | 6.2 (7.6) | 9.9 (3.6) | 8.8 (6.4) |
| Middle | 5.6 (5.6) | NA | 5.2 (6.5) |
| Base | 6.9 (8.5) | NA | 8.7 (9.7) |
| Multi | 6.6 (6.7) | NA | 10.7 (9) |
| Mean | 6.3 (6.1) | NA | 8.3 (7.1) |
The effect of place of stimulation (apex, middle, base) [F2,52 = 0.13, p = 0.87] and the phase × place interaction [F2,50 = 0.11, p = 0.89] were not significant. The effect of track order was examined to determine whether the results were affected by learning or fatigue. The effect of order [F1,331 = 0.26, p = 0.60] and the phase × order interaction [F1,330 = 0.050, p = 0.82] were not significant. The three-way interaction of phase × order × place was also not significant [F3,318 = 0.34, p = 0.79].
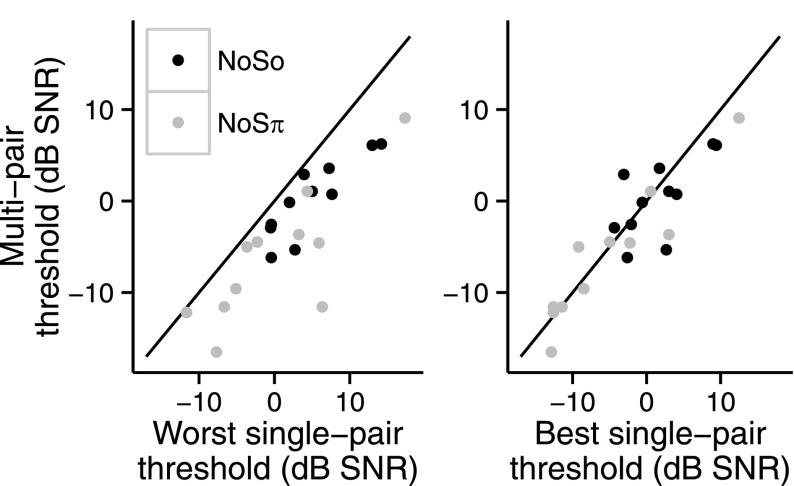
Performance was compared between single- and multi-electrode stimulation conditions. Stimulation sites were ranked as best, second best, and worst based on thresholds in each of the diotic and dichotic conditions separately. Of interest was whether the multi-electrode was better than the best single-electrode performance which would suggest integration of information across stimulation sites. Performance in the multi-electrode condition that was not better than the best single-electrode performance but better than the worst or second best could suggest that the participants were relying on better performing stimulation sites. Holm-Bonferroni corrections were made for NoSo and NoSπ separately. For both NoSo and NoSπ, thresholds in the multi-electrode condition were better than the worst single-electrode thresholds [NoSo: t30 = 5.86, p < 0.0001; NoSπ: t30 = 5.43, p < 0.0001]. NoSo thresholds in the multi-electrode condition were better than the second best single-electrode NoSo thresholds [t30 = 3.55, p = 0.0026]. The multi-electrode thresholds were not different from the second best single-electrode NoSπ threshold [t30 = 2.18, p = 0.073] or the best single-electrode thresholds for both configurations [NoSo: t30 = 1.58, p = 0.12; NoSπ: t30 = 0.84, p = 0.40]. Figure 3 (left panel) shows thresholds from the multi-electrode conditions, as a function of the worst single-electrode threshold for each child. All points fall at or below the identity line indicating that, in general, the multi-electrode thresholds were better than at least one of the single-electrode thresholds. Figure 3 (right panel) shows the multi-electrode thresholds as a function of the best single-electrode threshold with points falling on both sides of the identity line, suggesting that multi-electrode thresholds were similar to the best single-electrode thresholds.
FIG. 3.
Multi-electrode-pair NoSo and NoSπ thresholds (dB SNR) as a function of the worst single-electrode-pair threshold (dB SNR) of the children with CIs (left panel). Multi-electrode-pair NoSo and NoSπ thresholds (dB SNR) as a function of the best single-electrode-pair threshold (dB SNR) of the children with CIs (right panel). There are two points per child: one for the NoSo condition (black) and the other for the NoSπ condition (gray).
2. NH adults
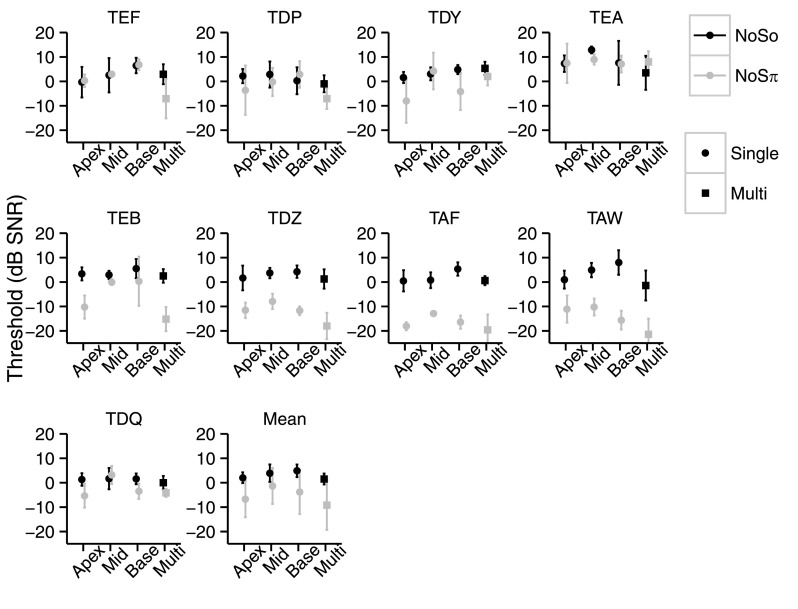
Figure 4 shows the NoSo and NoSπ thresholds of each of the NH adults as a function of place of stimulation. NoSπ thresholds were lower than NoSo thresholds [F1,59 = 56.62, p < 0.0001]. The mean NoSo threshold was 3.1 dB SNR (s.d. = 2.0 dB) and the mean NoSπ was − 5.3 dB SNR (s.d. = 8.0 dB). The mean BMLD was 8.3 dB (s.d. = 7.1) and can be seen for each stimulation condition in Table IV. Using a Holm-Bonferroni correction for NoSo and NoSπ separately, the multi-site thresholds were found to be better than the worst single-site thresholds [NoSo: t24 = 5.36, p < 0.0001; NoSπ: t24 = 5.74, p < 0.0001]. The NoSπ multi-site thresholds were also better than the second best single-site NoSπ threshold [t24 = 3.38, p = 0.0049]. The multi-site thresholds were not significantly different from the second best single-site NoSo threshold [t24 = 2.23, p = 0.069] or the best single-site thresholds [NoSo: t24 = 0.35, p = 0.73; NoSπ: t24 = 1.27, p = 0.22].
FIG. 4.
NoSo (black) and NoSπ (gray) thresholds (dB SNR) of the NH adults for each stimulation condition. Each panel shows data from an individual listener except for the last panel which shows the mean performance. Single-site stimulation is shown by circles and multi-site stimulation is shown by squares. Error bars represent ±1 standard deviation.
The effect of place (CF) was significant [F2,40 = 3.31, p = 0.046]. Post hoc pairwise contrasts using a Holm-Bonferroni correction (for three contrasts) showed that the middle CF produced higher NoSπ thresholds than the apical CF [t40 = −2.56, p = 0.042]. There was no significant difference in NoSπ thresholds between the apical and basal CFs [t40 = −1.37, p = 0.35] or the middle and basal CFs [t40 = 1.18, p = 0.35]. There were no significant differences between places for the NoSo condition. The phase × place interaction was not significant [F2,40 = 0.92, p = 0.41].
3. NH children
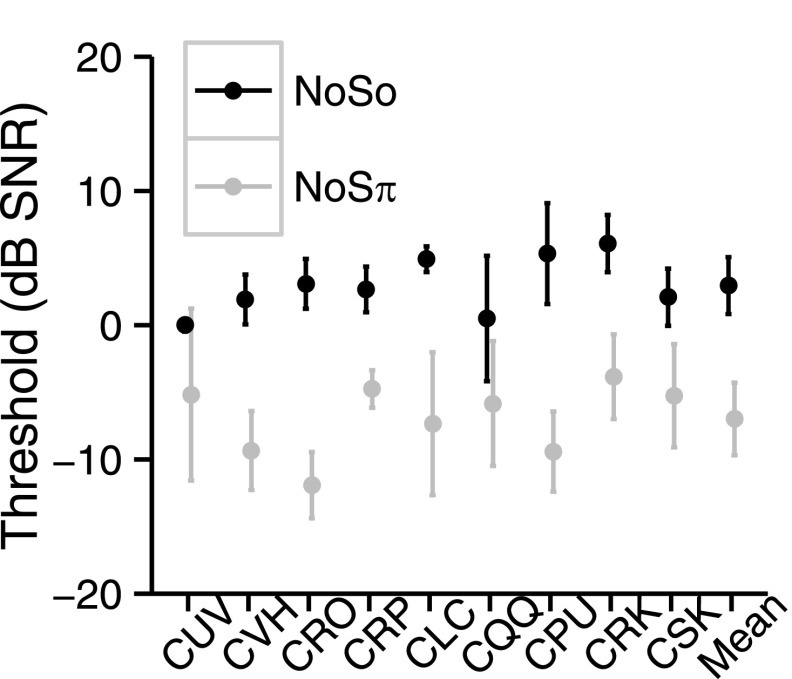
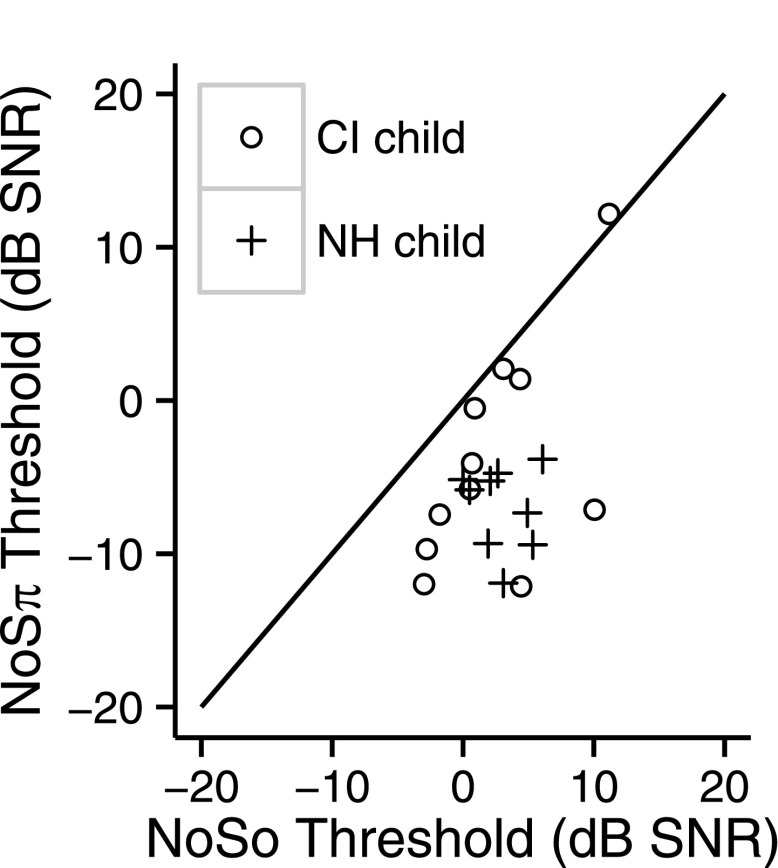
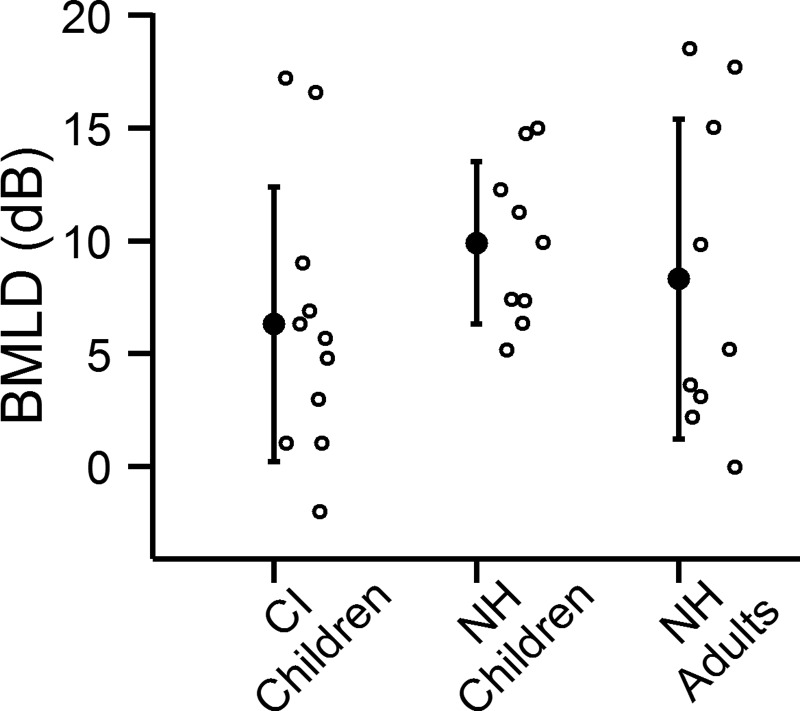
Figure 5 shows the NoSo and NoSπ thresholds for the NH children (CF = 3650 Hz). For the NH children, NoSπ thresholds were lower than NoSo thresholds [F1,8 = 75.26, p < 0.0001]. The mean NoSo threshold (CF = 3650 Hz) was 3.0 dB SNR (s.d. = 2.1 dB) and the mean NoSπ (CF = 3650 Hz) was −7.0 dB SNR (s.d. = 2.7 dB), with a mean BMLD of 9.9 dB (s.d. = 3.6). Figure 6 shows the NoSo and NoSπ thresholds of the children with CIs (averaged across thresholds of all conditions) relative to the children with NH. For the NoSo thresholds, the two groups overlap, with some children with CIs performing either better or worse than the children with NH. For the NoSπ, seven of the children with CIs performed as well as the NH children and four performed worse. Figure 7 shows the BMLDs of each of the three groups (i.e., children with CIs, children with NH, and adults with NH). There were no significant differences between the children with CIs and the children with NH [NoSo: t14 = 0.27, p = 0.79, NoSπ: t13 = − 1.29, p = 0.22, BMLD: t17 = 1.62, p = 0.12]. There were also no significant differences between the NH children and the NH adults [NoSo: t16 = −0.11, p = 0.91, NoSπ: t10 = −0.6, p = 0.56, BMLD: t12 = 0.59, p = 0.57].
FIG. 5.
NoSo (black) and NoSπ (gray) thresholds (dB SNR) of the NH children and the mean performance. Error bars represent ±1 standard deviation.
FIG. 6.
NoSπ thresholds as a function of NoSo thresholds. Thresholds of the children with CIs are shown by circles. Thresholds of the children with NH are shown by plus signs. The diagonal line is the line of equality.
FIG. 7.
Average BMLDs (dB) for the children with CIs, NH children, and NH adults are shown by the filled points. BMLDs of individual participants are shown by unfilled points. Error bars represent ±1 standard deviation.
B. Relationship between ITD sensitivity and binaural unmasking
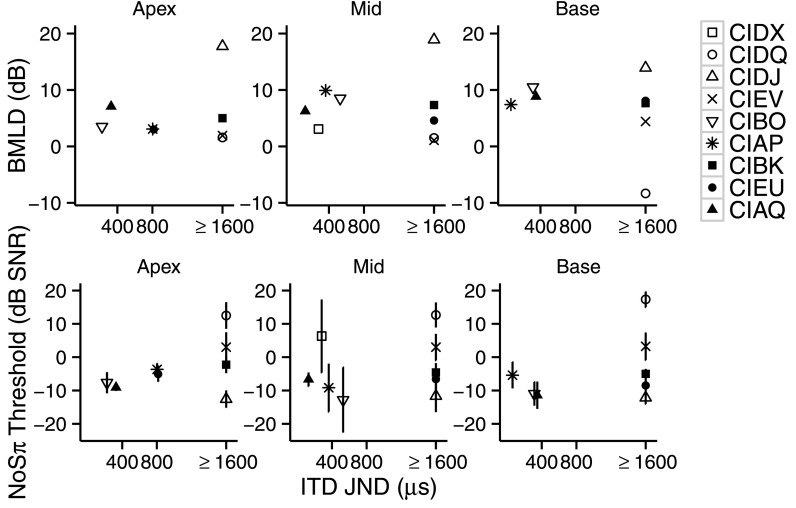
Five children (out of nine tested) showed ITD sensitivity (<1600 μs) for at least one electrode pair. The relationship between ITD sensitivity and BMLDs is shown in the top panels of Fig. 8, and the relationship between ITD sensitivity and NoSπ thresholds is shown in the bottom panels of Fig. 8. Data are split into panels corresponding to places of stimulation for each child with CIs. We were interested in whether NoSπ stimuli produced lower thresholds (i.e., participants were more sensitive to the binaural cue) in participants with ITD sensitivity given that ITD processing may aid in binaural unmasking if interaural differences in the temporal modulations of the NoSπ stimuli can be processed as temporal envelope ITDs. If ITD processing is involved with binaural unmasking, we would expect less unmasking from children who are insensitive to ITDs. This issue was of particular interest because a relationship has been found between ITD discrimination and NoSπ thresholds in adults with CIs (Goupell and Litovsky, 2015).
FIG. 8.
Top: BMLDs (dB) as a function of ITD JNDs (μs) of the children with CIs. Bottom: NoSπ (dB SNR) thresholds as a function of ITD JNDs (μs) of the children with CIs. Panels from left to right show performance at the apical, middle, and basal places of stimulation, respectively. Each child is represented with a unique symbol. Error bars represent ±1 standard deviation.
Figure 8 (top) shows that the BMLDs of the children with ITD sensitivity are within the range of those of the children without ITD sensitivity. For the children without ITD sensitivity, BMLDs ranged from −8.3 to 18.9 dB. The BMLDs of the children with ITD sensitivity ranged from 3.0 to 10.5 dB. Similarly, it can be seen in Fig. 8 (bottom) that the NoSπ thresholds of the children with ITD sensitivity are within the range of those of the children without ITD sensitivity. For the children without ITD sensitivity, NoSπ thresholds ranged from −12.6 to 17.3 dB SNR. Similarly, the NoSπ thresholds of the children with ITD sensitivity ranged from −12.8 to 6.3 dB SNR.
C. Effect of age
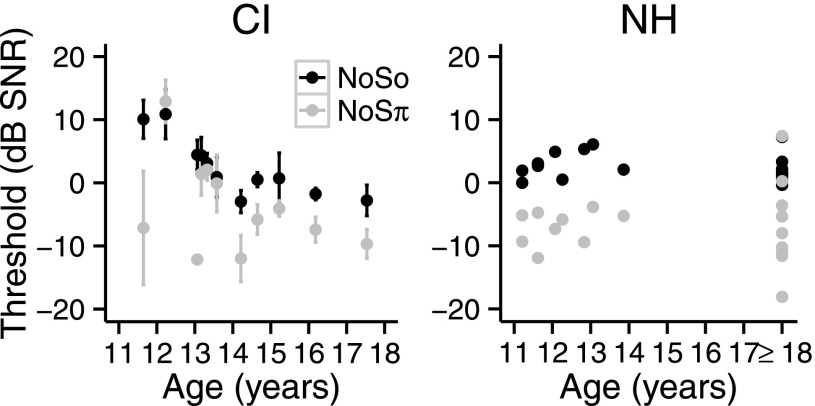
One of the questions addressed in this study was the effect of age of children with CIs on diotic and dichotic signal detection thresholds. Studies of NH children have found that NoSo thresholds, NoSπ thresholds, and BMLDs improve with age (Hall and Grose, 1990). Figure 9 (left) shows NoSo and NoSπ thresholds averaged across conditions for each child with CIs as a function of age. The effect of age was examined by comparing models with and without the variable age. Thresholds were lower for children with higher ages [F1,9 = 6.96, p = 0.026]. The interaction between phase and age was not significant [F1,8 = 0.54, p = 0.48]. NoSo and NoSπ data were examined separately in order to evaluate whether the effect of age could be found with each. The effect of age was significant for NoSo [F1,10 = 21.48, p = 0.00093], but not for NoSπ [F1,10 = 2.078, p = 0.18]. Figure 9 (right) shows NoSo and NoSπ thresholds for the children with NH as a function of age with the data of the NH adults (shown as ≥ 18) for the 3650-Hz CF included for comparison. For the NH children, the effect of age was not significant [F1,7 = 2.22, p = 0.18], and the interaction between phase and age was not significant [F1,6 = 0.0002, p = 0.98].
FIG. 9.
Left: NoSo (black) and NoSπ (gray) thresholds (dB SNR) of the children with CIs as a function of age (years). Right: NoSo (black) and NoSπ (gray) thresholds (dB SNR) of the children with NH as a function of age (years). Thresholds (at the 3650-Hz CF) of adults with NH are shown at ≥18 years. Error bars show ±1 standard deviation.
IV. DISCUSSION
Research on children with bilateral CIs has shown that binaural unmasking can be observed when a limited number of electrode pairs in the cochlear array is stimulated (Van Deun et al., 2009; Van Deun et al., 2011). However, everyday listening with CIs involves multi-electrode stimulation in order for spectrally complex information to be transmitted to the listener. Bilateral CIs provide benefits over unilateral CIs for speech reception in noise in situations involving spatial separation of sources. However, findings with both adults and children suggest the benefit depends mainly on monaural head shadow. Benefits from binaural processing (i.e., processing involving interaural comparisons) are limited (van Hoesel et al., 2008; Loizou et al., 2009; Misurelli and Litovsky, 2015), indicating a need to understand binaural hearing under multi-electrode stimulation in listeners with CIs. In this study, we examined binaural unmasking in children with bilateral CIs in conditions that involved single- and multi-electrode stimulation. Children with bilateral CIs are a unique population in that many of them are congenitally deaf, and the only auditory input that they have received consists of stimuli that have been processed through the CI and are degraded at the level of binaural presentation. Furthermore, many of them have experienced a period of unilateral stimulation prior to being implanted. This situation raises questions about the extent to which development of binaural abilities in this population can be within the range of what is seen in children with NH. Measures of binaural unmasking are of interest in this population as binaural unmasking may represent processes different from processes represented by measures of ITD discrimination.
In this study, we examined diotic (NoSo) and dichotic (NoSπ) signal detection with multi-electrode-pair stimulation (i.e., three pairs). We compared performance to that with single-electrode-pair stimulation at each of the sites that were used for the multi-electrode stimulation. Of interest was whether performance for the children with CIs would be better in the multi-electrode condition relative to any of the single-electrode conditions. In addition, performance on binaural unmasking was compared between children with ITD sensitivity and those without ITD sensitivity.
A. Signal detection thresholds and BMLDs
Most of the children with CIs in this study showed non-zero BMLDs (Fig. 7) with the average BMLD being 6.3 dB. That is, most of the children with CIs showed better signal detection thresholds in dichotic conditions than in diotic conditions. The only other study to date to examine diotic and dichotic signal detection thresholds in a sizable group of children with bilateral CIs was by Van Deun et al. (2009), 3 who found an average BMLD of 6.4 dB with single-electrode stimulation in a group of 7- to 15-year-olds, which is nearly identical to the average BMLD found in this study. Interestingly, seven of the 11 children with CIs in this study had thresholds in the range of the children with NH listening to a CI simulation (Fig. 6), suggesting that, with the stimuli used in this study, there is not a strong influence of acoustic deprivation on binaural unmasking. These results are consistent with the finding of a binaural interaction components measured in brainstem activity of children with bilateral CIs (e.g., Gordon et al., 2012). The stimuli in this study differed from those used in most previous studies on binaural unmasking of listeners with CIs in that the stimuli were not “transposed” (van de Par and Kohlrausch, 1997; Long et al., 2006; Van Deun et al., 2009). That is, the half-wave-rectified temporal fine structure of the unprocessed stimuli did not appear as temporal envelope modulations of the electric pulse trains. The interaural differences that occurred in the dichotic stimuli of this study were due to the effect of the target signal on the temporal envelope of the noise, which depends on the phase of the signal. Presumably, sensitivity to the interaural differences present in the dichotic stimuli in this study would depend largely on the processing of dynamic ILDs. This idea is supported by the finding that children without ITD sensitivity demonstrated positive BMLDs. In fact, they had BMLDs and dichotic detection thresholds that were similar to the children who were sensitive to ITDs (Fig. 8), providing no evidence that ITD sensitivity is required for or promotes binaural unmasking when only the temporal envelope of the unprocessed stimulus is presented. However, despite the similarity in performance between the children with CIs and with NH, it is uncertain that the children with CIs, given their atypical hearing histories, were using the same cues or processing the stimuli in the same way as the children with NH. Since children with CIs tend to show positive BMLDs, it is also possible that adults with early-onset deafness who receive bilateral implants in adulthood would also show BMLDs. This conjecture results from the fact that these populations are similar in that both groups had little to no access to acoustic hearing early in life. However, adult CI users with early-onset deafness might not show BMLDs if showing BMLDs depends on implantation early in life. Goupell (2015) examined the performance of adult CI users on interaural envelope correlation discrimination which is thought to rely on the same binaural processing mechanisms as the dichotic signal detection measure used in this study. One of three adults with early-onset deafness showed sensitivity to temporal envelope decorrelation. Examination of larger numbers of individuals with early-onset deafness and late implantation would be needed to assess the effect of auditory deprivation early in life (Laback et al., 2015).
There was no systematic effect of place of stimulation (apical, middle, and basal) for the children with CIs, which is consistent with studies in adults with CIs that have not found an effect of place on ITD discrimination (van Hoesel et al., 2009; Litovsky et al., 2010). Similar to the results of the children with CIs in this study, Goupell (2015) found no effect of CF for interaural-correlation discrimination using a high-frequency CI-simulation in NH adults. In the current study, NH participants showed poorer NoSπ thresholds at the 6922 Hz CF compared to the 3650 Hz CF, but it is unclear whether this result is meaningful especially given that a difference was not found between the 3650 Hz CF and the 13 014 Hz CF.
For the children with CIs, performance with single-electrode-pair stimulation in this study may have been poorer than what it would have been had higher levels of stimulation been used. The maximum level for the single-electrode conditions was typically lower than what it could have been, because for each electrode the same levels were used for both the single- and multi-electrode conditions. Since multi-electrode simulation is louder than single-electrode stimulation, lower levels were necessary. Studies on NH participants have shown that sensitivity to envelope ITDs and ILDs decreases at lower levels, with the effect being greater for ITDs than ILDs (Dietz et al., 2013). Furthermore, it is possible that had we conducted a loudness-balancing procedure across the different stimulation sites prior to the experimental task, there would have been less across-site variability. However, loudness balancing between stimulation sites that produce different pitch and quality percepts is not straightforward, because it is difficult to be sure that only the loudness cue is used in the judgment regarding whether the stimuli at the different sites of stimulation are perceived to be similar. Additionally, in this study loudness mapping involved bilateral stimulation, which is often unavailable with current clinical loudness mapping programs. Future research is needed to assess the benefit of bilateral mapping on binaural hearing of CI users.
For the children with CIs, thresholds for the NoSo stimuli were found to be related to chronological age (Fig. 9). This group of children ranged in age from 11 to 17 years. From this sample of children, it appeared that NoSo thresholds decreased as a function of age from 11 to 14 years and thereafter almost plateaued. This developmental trend is in contrast to findings with NH children studied by Hall and Grose (1990), for whom NoSo (and NoSπ) thresholds reached the same level of performance seen in adults, by six to seven years of age. However, there are substantial differences between the populations of children studied and the stimuli used in the two studies. Hall and Grose (1990) used acoustic stimuli which were not processed to simulate CI signal processing. In addition, Hall and Grose (1990) held the level of their noise constant and varied the level of the signal. In the current study, we held the overall level of the combination of noise and signal constant but varied the SNR. This may have resulted in a more difficult task since the level cue was removed requiring the participants to listen for differences in the temporal envelope fluctuations to distinguish the target from the reference. However, the front-end signal processing used in the current study may not have made much difference; in the current study when a CI simulation was used to minimize differences between the stimuli presented to the children with CIs and the NH children, there was no effect of age for NH children from 11 to 13 years of age (Fig. 9). Despite the use of a CI simulation, the electrical stimulation that the children with CIs received was fundamentally different from to the acoustic stimulation of the NH children. Furthermore, the condition of the peripheral auditory system was likely different between the two groups of children. Thus, the children with CIs likely received a more degraded auditory signal than the NH children, and performance on the signal detection task may have benefitted from the greater auditory experience and cognitive functioning of the older children with CIs. Additionally, it is possible that the auditory histories of the children with CIs contribute to a slower auditory development. The auditory histories of the children with CIs comprise a period of auditory deprivation and experience with only degraded speech signals, which potentially may affect the rate of auditory development. The developmental trend in this study is similar to the finding of a relationship between age (or CI experience) and speech detection/discrimination thresholds in noise in children with CIs (Chadha et al., 2011; Killan et al., 2015).
NoSπ thresholds showed a less consistent pattern with age for the children with CIs. In fact, some of the younger children with CIs showed the best NoSπ thresholds. It may be that the binaural cues were more salient to these children, involving greater activation of the central binaural system, for reasons other than chronological age. Despite the lack of relationship between age and NoSπ thresholds in this study, experience may play a role in the development of binaural hearing for children with CIs. Studies have shown that some children with CIs improve over time with performance on localization accuracy and acuity suggesting that experience can help to improve binaural hearing for children with CIs (Litovsky et al., 2006b; Grieco-Calub and Litovsky, 2010; Zheng et al., 2015).
B. Single- vs multi-electrode thresholds
Children with CIs showed positive BMLDs in both the single-electrode and the multi-electrode conditions. In the latter, for both diotic and dichotic thresholds, the children with CIs showed performance that was better than their worst single-electrode thresholds and was similar to their best single-electrode threshold (Fig. 3). In this study the three stimulation sites were spaced widely along the array, and the temporal envelope was presented using the same samples of noise. Presenting the same samples of noise was expected to reduce the effect of interference between electrodes because any neural populations that were stimulated by more than one electrode would receive relatively correlated information from each electrode. Furthermore, wide spacing of electrodes was expected to reduce the number of neural fibers that were stimulated by more than one electrode. Van Deun et al. (2011) tested three children with stimuli presented from three adjacent electrodes and found that all children had positive BMLDs. However, diotic and dichotic signal detection thresholds were higher and BMLDs were smaller in the multi-electrode condition compared to the single-electrode condition, likely due to overlap in the neural populations stimulated by each electrode.
In the present study, there was no change in thresholds for the multi-electrode condition compared to the best single-electrode condition, for either the children with CIs or the NH adults (Fig. 4). It may be the case that improvements in the multi-electrode condition were too small to be observed in the present study. It is also possible that had a larger number of electrodes been used in the multi-electrode condition, an integration effect may have been observed. Furthermore, the comparison of the multi-electrode threshold to the best single-site threshold has inherent limitations, as selecting the best single-site threshold may have involved bias in favor of the single-electrode threshold. These results should also be considered in light of the fact that redundant modulation information was presented across stimulation sites. It may be the case that with different samples of noise presented across remote stimulation sites, an integration effect may have been observed. However, the results suggest that presenting redundant information at separate spectral locations did not result in reduced performance or interference. Despite the general pattern of thresholds in the multi-electrode condition being similar to thresholds in the best single-electrode condition, for a number of individual children the best performance was in the multi-electrode conditions. However, this pattern of results did not typically occur for both the diotic and dichotic conditions, as can be seen in Fig. 2. For example, participant CIBK's performance was best in the multi-electrode condition only for NoSo, which eliminated the BMLD in the multi-electrode condition. Participant CIAW's performance was best in the multi-electrode condition, but only for NoSπ; thus, a BMLD was only observed in the multi-electrode condition. Participant CIEV was the only child that demonstrated a BMLD in the multi-electrode condition and performed better in the multi-electrode condition for both NoSo and NoSπ. The variable performance of the participants with CIs suggests there may be certain circumstances in which integration of information across stimulation sites is more likely, and the reason for this requires further investigation.
In summary, the results provide further support for the hypothesis that children with bilateral CIs are able to show binaural unmasking for signal detection. Binaural unmasking was demonstrated even in children who did not show sensitivity to ITDs. Children with CIs showed binaural unmasking with multi-electrode stimulation that was similar in magnitude to binaural unmasking with single-electrode stimulation at the best performing stimulation site suggesting that the children were able to use information from the best performing stimulation site in a multi-electrode context. Some of the children with CIs showed performance that was similar to the performance of NH children listening to a single-channel CI simulation. The results are encouraging in that they suggest that despite limited experience with typical bilateral hearing, children with CIs are sensitive to binaural cues, which they could potentially use for binaural unmasking of speech.
ACKNOWLEDGMENTS
This work was supported by NIH Grant No. R01DC008365 and R01DC003083 to R.Y.L, NIH Grant No. F31DC013238 to A.E.T., and NIH Grant No. R01DC014948 to M.J.G. Support was also provided by Grant No. NIH P30HD03352 to the Waisman Center. We would like to thank Shelly Godar for help with recruitment and testing of the children with cochlear implants. Thanks also go to Brianna Vandyke for help with testing the normal-hearing participants.
Portions of this work were presented in “Sensitivity to interaural level differences is more prevalent than interaural timing differences in children who use bilateral cochlear implants,” at the 38th Midwinter Meeting of the Association for Research in Otolaryngology, Baltimore, MD, 2015 and in “Sensitivity to interaural timing differences in children with bilateral cochlear implants,” at the Conference on Implantable Auditory Prostheses, Tahoe, CA, 2015.
Footnotes
Participant CIAW had difficulty completing a full track for the base pair (NoSπ), and only three complete tracks were obtained on that condition. Participant CIDX had difficulty completing full tracks for the middle pair (NoSo) and the base pair (NoSo) mid-way and near the end of testing, respectively, which may have been due to fatigue effects. However, four thresholds for this participant were obtained for all conditions. For participant CIDQ, the criteria for a track to end early were modified because the participant showed consistent, poor performance. For this participant, tracks were sometimes limited by the highest SNR available in the adaptive track (20 dB SNR) and therefore thresholds reported can be considered underestimates.
If the data are considered without the results of participant CIDQ, who had considerable difficulty with the task, then the mean NoSo threshold was 1.65 (s.d. = 3.99 dB) and the mean NoSπ threshold was −5.5 dB SNR (s.d. = 5.23) with a mean BMLD of 7.15 dB (s.d. = 5.73).
Van Deun et al. (2011) also examined diotic and dichotic signal detection thresholds in three children with CIs. The three children had taken part in the study by Van Deun et al. (2009).
References
- 1. Abbas, P. J. , Hughes, M. L. , Brown, C. J. , Miller, C. A. , and South, H. (2003). “ Channel interaction in cochlear implant users evaluated using the electrically evoked compound action potential,” Audiol. Neurootol. 9, 203–213. 10.1159/000078390 [DOI] [PubMed] [Google Scholar]
- 2. Akeroyd, M. A. , and Summerfield, A. Q. (2000). “ Integration of monaural and binaural evidence of vowel formants,” J. Acoust. Soc. Am. 107, 3394–3406. 10.1121/1.429410 [DOI] [PubMed] [Google Scholar]
- 3. Bronkhorst, A. W. , and Plomp, R. (1988). “ The effect of head-induced interaural time and level differences on speech intelligibility in noise,” J. Acoust. Soc. Am. 83, 1508–1516. 10.1121/1.395906 [DOI] [PubMed] [Google Scholar]
- 4. Buus, S. , Schorer, E. , Florentine, M. , and Zwicker, E. (1986). “ Decision rules in detection of simple and complex tones,” J. Acoust. Soc. Am. 80, 1646–1657. 10.1121/1.394329 [DOI] [PubMed] [Google Scholar]
- 5. Carhart, R. (1965). “ Monaural and binaural discrimination against competing sentences,” Int. Audiol. 4, 5–10. [Google Scholar]
- 6. Carhart, R. , Tillman, T. W. , and Johnson, K. R. (1967). “ Release of masking for speech through interaural time delay,” J. Acoust. Soc. Am. 42, 124–138. 10.1121/1.1910541 [DOI] [PubMed] [Google Scholar]
- 7. Chadha, N. K. , Papsin, B. C. , Jiwani, S. , and Gordon, K. A. (2011). “ Speech detection in noise and spatial unmasking in children with simultaneous versus sequential bilateral cochlear implants,” Otol. Neurotol. 32, 1057–1064. 10.1097/MAO.0b013e3182267de7 [DOI] [PubMed] [Google Scholar]
- 8. Cohen, L. T. , Richardson, L. M. , Saunders, E. , and Cowan, R. S. C. (2003). “ Spatial spread of neural excitation in cochlear implant recipients: Comparison of improved ECAP method and psychophysical forward masking,” Hear. Res. 179, 72–87. 10.1016/S0378-5955(03)00096-0 [DOI] [PubMed] [Google Scholar]
- 9. Dietz, M. , Bernstein, L. R. , Trahiotis, C. , Ewert, S. D. , and Hohmann, V. (2013). “ The effect of overall level on sensitivity to interaural differences of time and level at high frequencies,” J. Acoust. Soc. Am. 134, 494–502. 10.1121/1.4807827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Domnitz, R. H. , and Colburn, H. S. (1976). “ Analysis of binaural detection models for dependence on interaural target parameters,” J. Acoust. Soc. Am. 59, 598–601. 10.1121/1.380904 [DOI] [PubMed] [Google Scholar]
- 11. Ehlers, E. , Godar, S. , Kan, A. , Todd, A. , and Ltivosky, R. (2015). “ Sensitivity to interaural level differences is more prevalent than interaural timing differences in children who use bilateral cochlear implants,” in 38th MidWinter Meeting of the Association for Research in Otolaryngology, Baltimore, MD. [Google Scholar]
- 12. Gabriel, K. J. , and Colburn, H. S. (1981). “ Interaural correlation discrimination: I. Bandwidth and level dependence,” J. Acoust. Soc. Am. 69, 1394–1401. 10.1121/1.385821 [DOI] [PubMed] [Google Scholar]
- 13. Galvin, J. J., 3rd , Oba, S. , Fu, Q. J. , and Baskent, D. (2014). “ Single- and multi-channel modulation detection in cochlear implant users,” PLoS One 9, e99338. 10.1371/journal.pone.0099338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon, K. A. , Deighton, M. R. , Abbasalipour, P. , and Papsin, B. C. (2014). “ Perception of binaural cues develops in children who are deaf through bilateral cochlear implantation,” PLoS One 9, e114841. 10.137/journal.pone.0114841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon, K. A. , Salloum, C. , Toor, G. S. , van Hoesel, R. , and Papsin, B. C. (2012). “ Binaural interactions develop in the auditory brainstem of children who are deaf: Effects of place and level of bilateral electrical stimulation,” J. Neurosci. 32, 4212–4223. 10.1523/JNEUROSCI.5741-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goupell, M. J. (2012). “ The role of envelope statistics in detecting changes in interaural correlation,” J. Acoust. Soc. Am. 132, 1561–1572. 10.1121/1.4740498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goupell, M. J. (2015). “ Interaural envelope correlation change discrimination in bilateral cochlear implantees: Effects of mismatch, centering, and onset of deafness,” J. Acoust. Soc. Am. 137, 1282–1297. 10.1121/1.4908221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goupell, M. J. , and Litovsky, R. Y. (2015). “ Sensitivity to interaural envelope correlation changes in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 137, 335–349. 10.1121/1.4904491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goupell, M. J. , Majdak, P. , and Laback, B. (2010). “ Median-plane sound localization as a function of the number of spectral channels using a channel vocoder,” J. Acoust. Soc. Am. 127, 990–1001. 10.1121/1.3283014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goupell, M. J. , Stoelb, C. , Kan, A. , and Litovsky, R. Y. (2013). “ Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening,” J. Acoust. Soc. Am. 133, 2272–2287. 10.1121/1.4792936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green, D. M. (1958). “ Detection of multiple component signals in noise,” J. Acoust. Soc. Am. 30, 904–910. 10.1121/1.1909400 [DOI] [Google Scholar]
- 22. Greenwood, D. D. (1990). “ A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- 23. Grieco-Calub, T. M. , and Litovsky, R. Y. (2010). “ Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing,” Ear Hear. 31, 645–656. 10.1097/AUD.0b013e3181e50a1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall, J. W. III , and Grose, J. H. (1990). “ The masking-level difference in children,” J. Am. Acad. Audiol. 1, 81–88. [PubMed] [Google Scholar]
- 25. Hancock, K. E. , Noel, V. , Ryugo, D. K. , and Delgutte, B. (2010). “ Neural coding of interaural time differences with bilateral cochlear implants: Effects of congenital deafness,” J. Neurosci. 30, 14068–14079. 10.1523/JNEUROSCI.3213-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hawley, M. L. , Litovsky, R. Y. , and Culling, J. F. (2004). “ The benefit of binaural hearing in a cocktail party: Effect of location and type of interferer,” J. Acoust. Soc. Am. 115, 833–843. 10.1121/1.1639908 [DOI] [PubMed] [Google Scholar]
- 27. Ihlefeld, A. , Kan, A. , and Litovsky, R. (2014). “ Across-frequency combination of interaural time difference in bilateral cochlear implant listeners,” Front. Syst. Neurosci. 8, 1–10. 10.3389/fnsys.2014.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kan, A. , and Litovsky, R. Y. (2015). “ Binaural hearing with electrical stimulation,” Hear. Res. 322, 127–137. 10.1016/j.heares.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kan, A. , Stoelb, C. , Litovsky, R. Y. , and Goupell, M. J. (2013). “ Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users,” J. Acoust. Soc. Am. 134, 2923–2936. 10.1121/1.4820889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Killan, C. F. , Killan, E. C. , and Raine, C. H. (2015). “ Changes in children's speech discrimination and spatial release from masking between 2 and 4 years after sequential cochlear implantation,” Cochlear Implants Int. 16, 270–276. 10.1179/1754762815Y.0000000001 [DOI] [PubMed] [Google Scholar]
- 31. Kohlrausch, A. (1986). “ The influence of signal duration, signal frequency and masker duration on binaural masking level differences,” Hear. Res. 23, 267–273. 10.1016/0378-5955(86)90115-2 [DOI] [PubMed] [Google Scholar]
- 32. Laback, B. , Egger, K. , and Majdak, P. (2015). “ Perception and coding of interaural time differences with bilateral cochlear implants,” Hear. Res. 322, 138–150. 10.1016/j.heares.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 33. Langhans, A. , and Kohlrausch, A. (1992). “ Spectral integration of broadband signals in diotic and dichotic masking experiments,” J. Acoust. Soc. Am. 91, 317–326. 10.1121/1.402774 [DOI] [PubMed] [Google Scholar]
- 34. Lavandier, M. , and Culling, J. F. (2010). “ Prediction of binaural speech intelligibility against noise in rooms,” J. Acoust. Soc. Am. 127, 387–399. 10.1121/1.3268612 [DOI] [PubMed] [Google Scholar]
- 35. Litovsky, R. , Parkinson, A. , Arcaroli, J. , and Sammeth, C. (2006a). “ Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Litovsky, R. Y. , Goupell, M. J. , Godar, S. , Grieco-Calub, T. , Jones, G. L. , Garadat, S. N. , Agrawal, S. , Kan, A. , Todd, A. , Hess, C. , and Misurelli, S. (2012). “ Studies on bilateral cochlear implants at the University of Wisconsin's Binaural Hearing and Speech Laboratory,” J. Am. Acad. Audiol. 23, 476–494. 10.3766/jaaa.23.6.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Litovsky, R. Y. , Johnstone, P. M. , Godar, S. , Agrawal, S. , Parkinson, A. , Peters, R. , and Lake, J. (2006b). “ Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle,” Ear Hear. 27, 43–59. 10.1097/01.aud.0000194515.28023.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Litovsky, R. Y. , Jones, G. L. , Agrawal, S. , and van Hoesel, R. (2010). “ Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans,” J. Acoust. Soc. Am. 127, 400–414. 10.1121/1.3257546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litovsky, R. Y. , Parkinson, A. , and Arcaroli, J. (2009). “ Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loizou, P. C. , Hu, Y. , Litovsky, R. , Yu, G. , Peters, R. , Lake, J. , and Roland, P. (2009). “ Speech recognition by bilateral cochlear implant users in a cocktail-party setting,” J. Acoust. Soc. Am. 125, 372–383. 10.1121/1.3036175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Long, C. J. , Carlyon, R. P. , Litovsky, R. Y. , and Downs, D. H. (2006). “ Binaural unmasking with bilateral cochlear implants,” J. Assoc. Res. Otolaryngol. 7, 352–360. 10.1007/s10162-006-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu, T. , Carroll, J. , and Zeng, F. G. (2007). “ On acoustic simulations of cochlear implants,” in Conference on Implantable Auditory Prostheses, Lake Tahoe, CA. [Google Scholar]
- 43. Lu, T. , Litovsky, R. , and Zeng, F. G. (2010). “ Binaural masking level differences in actual and simulated bilateral cochlear implant listeners,” J. Acoust. Soc. Am. 127, 1479–1490. 10.1121/1.3290994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu, T. , Litovsky, R. , and Zeng, F. G. (2011). “ Binaural unmasking with multiple adjacent masking electrodes in bilateral cochlear implant users,” J. Acoust. Soc. Am. 129, 3934–3945. 10.1121/1.3570948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Misurelli, S. M. , and Litovsky, R. Y. (2012). “ Spatial release from masking in children with normal hearing and with bilateral cochlear implants: Effect of interferer asymmetry,” J. Acoust. Soc. Am. 132, 380–391. 10.1121/1.4725760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Misurelli, S. M. , and Litovsky, R. Y. (2015). “ Spatial release from masking in children with bilateral cochlear implants and with normal hearing: Effect of target-interferer similarity,” J. Acoust. Soc. Am. 138, 319–331. 10.1121/1.4922777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Niparko, J. K. , Tobey, E. A. , Thal, D. J. , Eisenberg, L. S. , Wang, N. Y. , Quittner, A. L. , and Fink, N. E. (2010). “ Spoken language development in children following cochlear implantation,” JAMA 303, 1498–1506. 10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salloum, C. A. , Valero, J. , Wong, D. D. , Papsin, B. C. , van Hoesel, R. , and Gordon, K. A. (2010). “ Lateralization of interimplant timing and level differences in children who use bilateral cochlear implants,” Ear Hear. 31, 441–456. 10.1097/AUD.0b013e3181d4f228 [DOI] [PubMed] [Google Scholar]
- 49. Schleich, P. , Nopp, P. , and D'Haese, P. (2004). “ Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant,” Ear Hear. 25, 197–204. 10.1097/01.AUD.0000130792.43315.97 [DOI] [PubMed] [Google Scholar]
- 50. Schubert, E. D. (1956). “ Some preliminary experiments on binaural time delay and intelligibility,” J. Acoust. Soc. Am. 28, 895–901. 10.1121/1.1908508 [DOI] [Google Scholar]
- 51. Shannon, R. V. (1983). “ Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction,” Hear. Res. 12, 1–16. 10.1016/0378-5955(83)90115-6 [DOI] [PubMed] [Google Scholar]
- 52. Tillein, J. , Hubka, P. , Syed, E. , Hartmann, R. , Engel, A. K. , and Kral, A. (2010). “ Cortical representation of interaural time difference in congenital deafness,” Cereb. Cortex 20, 492–506. 10.1093/cercor/bhp222 [DOI] [PubMed] [Google Scholar]
- 53. Todd, A. E. , Goupell, M. J. , and Litovsky, R. Y. (2014). “ Binaural unmasking with temporal envelope and fine structure in cochlear implant listeners,” in Association for Research in Otolaryngology 37th MidWinter Meeting, San Diego, CA. [Google Scholar]
- 54. van de Par, S. , and Kohlrausch, A. (1997). “ A new approach to comparing binaural masking level differences at low and high frequencies,” J. Acoust. Soc. Am. 101, 1671–1680. 10.1121/1.418151 [DOI] [PubMed] [Google Scholar]
- 55. van der Heijden, M. , and Trahiotis, C. (1998). “ Binaural detection as a function of interaural correlation and bandwidth of masking noise: Implications for estimates of spectral resolution,” J. Acoust. Soc. Am. 103, 1609–1614. 10.1121/1.421295 [DOI] [PubMed] [Google Scholar]
- 56. Van Deun, L. , van Wieringen, A. , Francart, T. , Buchner, A. , Lenarz, T. , and Wouters, J. (2011). “ Binaural unmasking of multi-channel stimuli in bilateral cochlear implant users,” J. Assoc. Res. Otolaryngol. 12, 659–670. 10.1007/s10162-011-0275-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Deun, L. , van Wieringen, A. , Francart, T. , Scherf, F. , Dhooge, I. J. , Deggouj, N. , Desloovere, C. , Van de Heyning, P. H. , Offeciers, F. E. , De Raeve, L. , and Wouters, J. (2009). “ Bilateral cochlear implants in children: Binaural unmasking,” Audiol. Neurootol. 14, 240–247. 10.1159/000190402 [DOI] [PubMed] [Google Scholar]
- 58. van Hoesel, R. J. M. , Bohm, M. , Pesch, J. , Vandali, A. , Battmer, R. D. , and Lenarz, T. (2008). “ Binaural speech unmasking and localization in noise with bilateral cochlear implants using envelope and fine-timing based strategies,” J. Acoust. Soc. Am. 123, 2249–2263. 10.1121/1.2875229 [DOI] [PubMed] [Google Scholar]
- 59. van Hoesel, R. J. M. , Jones, G. L. , and Litovsky, R. Y. (2009). “ Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse rate, modulation rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Hoesel, R. J. M. , and Tyler, R. S. (2003). “ Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- 61. Webster, F. A. (1951). “ The influence of interaural phase on masked thresholds. I. The role of interaural time-deviation,” J. Acoust. Soc. Am. 23, 452–462. 10.1121/1.1906787 [DOI] [Google Scholar]
- 62. Wichmann, F. A. , and Hill, N. J. (2001a). “ The psychometric function: I. Fitting, sampling, and goodness of fit,” Percept. Psychophys. 63, 1293–1313. 10.3758/BF03194544 [DOI] [PubMed] [Google Scholar]
- 63. Wichmann, F. A. , and Hill, N. J. (2001b). “ The psychometric function: II. Bootstrap-based confidence intervals and sampling,” Percept. Psychophys. 63, 1314–1329. 10.3758/BF03194545 [DOI] [PubMed] [Google Scholar]
- 64. Zheng, Y. , Godar, S. P. , and Litovsky, R. Y. (2015). “ Development of sound localization strategies in children with bilateral cochlear implants,” PLoS One 10, e0135790. 10.1371/journal.pone.0135790 [DOI] [PMC free article] [PubMed] [Google Scholar]