Abstract
BACKGROUND:
There are conflicting findings from observational studies of the arrhythrogenic potential of azithromycin. Our aim was to quantify the association between azithromycin use and the risk of ventricular arrhythmia.
METHODS:
We conducted a nested case–control study within a cohort of new antibiotic users identified from a network of 7 population-based health care databases in Denmark, Germany, Italy, the Netherlands and the United Kingdom for the period 1997–2010. Up to 100 controls per case were selected and matched by age, sex and database. Recency of antibiotic use and type of drug (azithromycin was the exposure of interest) at the index date (occurrence of ventricular arrhythmia) were identified. We estimated the odds of ventricular arrhythmia associated with current azithromycin use relative to current amoxicillin use or nonuse of antibiotics (≥ 365 d without antibiotic exposure) using conditional logistic regression, adjusting for confounders.
RESULTS:
We identified 14 040 688 new antibiotic users who met the inclusion criteria. Ventricular arrhythmia developed in 12 874, of whom 30 were current azithromycin users. The mean age of the cases and controls was 63 years, and two-thirds were male. In the pooled data analyses across databases, azithromycin use was associated with an increased risk of ventricular arrhythmia relative to nonuse of antibiotics (adjusted odds ratio [OR] 1.97, 95% confidence interval [CI] 1.35–2.86). This increased risk disappeared when current amoxicillin use was the comparator (adjusted OR 0.90, 95% CI 0.48–1.71). Database-specific estimates and meta-analysis confirmed results from the pooled data analysis.
INTERPRETATION:
Current azithromycin use was associated with an increased risk of ventricular arrhythmia when compared with nonuse of antibiotics, but not when compared with current amoxicillin use. The decreased risk with an active comparator suggests significant confounding by indication.
Azithromycin is a widely prescribed broad-spectrum macrolide used mainly for the treatment of respiratory and urinary tract bacterial infections. Concerns have been raised recently regarding its arrhythmogenic potential, a risk already known to be associated with the first marketed macrolide, erythromycin.1–6 Several case reports have described QT-interval prolongation,7–9 torsades de pointes10–12 and polymorphic ventricular tachycardia13 following use of azithromycin. Many observational studies have reported conflicting results about the association between azithromycin use and cardiovascular death.14–21 Because the known azithromycin-related cardiac events are related to QT-interval prolongation, torsades de pointes and ventricular arrhythmia,18,19 these observational studies are limited by the broad category of cardiovascular death used as an outcome, which likely only partially captured cardiac risk associated with azithromycin use. To date, only 1 observational study has investigated the association between azithromycin use and ventricular arrhythmia specifically.22
Given the conflicting findings regarding this widely used antibiotic and the lack of data on ventricular arrhythmia specifically, we aimed to quantify the association between azithromycin use and the risk of ventricular arrhythmia using data from a network of 7 health care databases from 5 European countries.
Methods
Study design and setting
We conducted a nested case–control study within a cohort of new antibiotic users identified from a network of 7 population-based health care databases in 5 European countries that participated in the ARITMO (Arrhythmogenic Potential of Drugs) study.23 The ARITMO study was conducted from Jan. 1, 1997, to Dec. 31, 2010, and the databases covered a total population of about 28 million.
The individual databases were the Health Search Database Cegedim Strategic Data Longitudinal Patient Database (Italy), the Integrated Primary Care Information Database (the Netherlands), The Health Improvement Network (United Kingdom), the PHARMO Database Network (the Netherlands), the Aarhus University Hospital Database (northern and central regions of Denmark), the German Pharmacoepidemiological Research Database (Germany) and the Emilia-Romagna Database (Emiglia-Romagna region of Italy) (Table 1). Harmonized data extraction, quality assurance and analyses when combining multiple health care databases for drug safety studies have been described elsewhere.24
Table 1:
Features of the databases included in the study
| Database | Country | Type of database and setting | Study period | Diagnosis coding | Prescription coding | Source population |
|---|---|---|---|---|---|---|
| AARHUS | Denmark | NHS-linked regional database (northern and central regions); community, and in- and outpatient hospital settings | 2000–2010 | ICD-10 | ATC | 1 559 718 |
| ERD | Italy | NHS-linked regional database (Emiglia-Romagna region); community setting | 2005–2010 | ICD-9-CM | ATC | 6 079 798 |
| HSD-CSD-LPD | Italy | Nationwide general practice database; community setting | 2000–2010 | ICD-9-CM and free text | ATC | 1 240 561 |
| GePaRD | Germany | Nationwide general practice database; community and hospital settings | 2005–2009 | ICD-10-GM | ATC | 7 285 935 |
| IPCI | Netherlands | Nationwide general practice database; community setting | 1998–2010 | ICPC and free text | ATC | 1 016 632 |
| PHARMO | Netherlands | Nationwide record linkage system; community setting | 1999–2009 | ICD-9-CM | ATC | 4 625 868 |
| THIN | United Kingdom | Nationwide general practice database; community setting | 1997–2010 | Read codes and free text | BNF/multilex | 6 951 894 |
Note: AARHUS = Aarhus University Hospital Database; ATC = Anatomic Therapeutic Chemical drug classification; BNF = British National Formulary codes; ERD = Emilia-Romagna Database; GePaRD = German Pharmacoepidemiological Research Database; HSD-CSD-LPD = Health Search Database Cegedim Strategic Data Longitudinal Patient Database; ICD-9-CM = International Classification of Diseases, ninth edition, clinical modification; ICD-10 = International Classification of Diseases, 10th edition; ICD-10-GM = International Classification of Diseases, 10th edition, German modification; ICPC = International Classification of Primary Care; IPCI = Integrated Primary Care Information Database; NHS = National Health Service; THIN = The Health Improvement Network.
The databases in the ARITMO network have been used previously in pharmacoepidemiologic studies,25 and trends in antibiotic use across databases have been investigated.26 The ARITMO studies are registered in the ENCePP (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance) registry of studies held at the European Medicines Agency (www.encepp.eu/encepp/viewResource.htm?id=4669).
Study population
We included patients 85 years of age or younger who were new antibiotic users (no antibiotic use in the prior year). Cohort entry occurred on the date of the first recorded antibiotic prescription among patients with at least 1 year of database history.
We excluded patients over 85 years of age to avoid immortal time bias. We excluded patients with malignant cancer because they may have been admitted to hospital for long periods, which would preclude full capture of their medical history. In addition, we excluded, wherever possible, people with a record of hospital admission.
We followed patients from the first antibiotic prescription to the earliest of the following: end of study, occurrence of ventricular arrhythmia, transfer out of database, diagnosis of malignant cancer, 85th birthday or death.
Cases and controls
For cases, we identified patients with the primary outcome of ventricular arrhythmia (the case definition and coding algorithms are reported in Appendix 1, eFigure 1 and eTable1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.160355/-/DC1). Outcome validation was conducted in every database for a random sample of 200 cases through independent manual review of medical records by 2 experts per database who were blind to drug exposure. In the Integrated Primary Care Information database, manual validation of all automatically detected cases was performed because of extensive use of unstructured, free-text patient notes. A positive predictive value of 90% or higher was achieved.24 We excluded cases if they were current users of azithromycin and another antibiotic concomitantly; we also excluded cases if they were not current, recent or past users of azithromycin.
We selected up to 100 controls per case and matched them by year of birth (within 1 yr), sex, index date and database using incidence density sampling.
Exposure
We obtained data on antibiotics (Anatomic Therapeutic Chemical classification codes J01*) from the electronic drug prescription/dispensing records in the study databases. We calculated the exposure period by dividing the total number of units prescribed or dispensed by the daily number of units taken (or the defined daily dose if the prescribed dosage was unavailable).
Exposure to antibiotics was categorized as current, recent or past. Current exposure was defined as the period covering the index date (occurrence of ventricular arrhythmia), or ending within 7 days before the index date to account for lack of compliance with treatment or late registration of ventricular arrhythmia (i.e., to reduce exposure misclassification and outcome misclassification). Recent exposure was defined as the period ending between 7 and 89 days before the index date (complications of severe infections or delayed effects of antibiotic exposure may have an effect on the occurrence of the primary outcome). Past exposure was defined as the period ending between 90 and 364 days before the index date (both antibiotic exposure and infection are likely to have no effect on occurrence of the outcome). To take into account the baseline risk of ventricular arrrhythmia, we defined a non-exposure category as no antibiotic exposure within 365 days before the index date.
Covariates
We considered the following risk factors of ventricular arrhythmia as covariates: age and sex; cardiovascular diseases; metabolic diseases; other diseases potentially related to increased risk of ventricular arrhythmia (e.g., atrial fibrillation or flutter and cardiomyopathies); prior use of anti-arrhythmia drugs, and concomitant use (defined as use within 90 d before index date) of medications known to induce hypokalemia or QT-interval–prolonging drugs (i.e., drugs with established torsadogenic risk according to the CredibleMeds list27). (The covariates and coding algorithms are reported in Appendix 1, eTables 2–5, using the harmonization process that has been described before.24)
Statistical analysis
Data were extracted locally and transformed into a simple common data model according to a process described previously.28 Data were then transformed locally from the common input files into fully anonymized data sets with use of the custom-built JAVA-based software Jerboa.29
We calculated crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) using conditional logistic regression analysis for current, recent and past use of azithromycin, with current use of amoxicillin as the primary comparator. We also calculated ORs for current, recent and past use of azithromycin, amoxicillin and other antibiotics using non-exposure to antibiotics as a secondary comparator.
Confounders included in the final models were selected through a stepwise approach. Well-known risk factors for ventricular arrhythmia were included a priori as confounders in the final multivariate model (Appendix 1, eTable 2). Other potential confounders were included if they had a prevalence greater than 5% among the controls and were associated with the outcome in the univariate model. All analyses were conducted in each database, and a meta-analysis of risk estimates from the individual databases was carried out. Heterogeneity across databases was estimated with use of the Cochran Q statistic. The I2 statistic was then used to express the percentage variation across the databases due to heterogeneity, with values greater than 75% considered to denote a high level of heterogeneity. As a conservative approach, we used a 2-stage random-effects model for the meta-analysis of pooled data.30 Only databases with 3 or more cases currently using azithromycin were included in the meta-analysis.
We performed 2 sensitivity analyses. In one, we changed the definition of current antibiotic exposure by excluding the 7-day carry-over period that was used to account for lack of compliance or late registration of ventricular arrhythmia. In another, we excluded all patients who had a diagnosis of acute myocardial infarction reported within 15 days before the index date, to test for confounding owing to recent acute myocardial infarction.
Ethics approval
This study was not conducted with direct patient involvement, and all data were analyzed retrospectively. Prior ethics approval for the study was obtained in line with national regulations.
Results
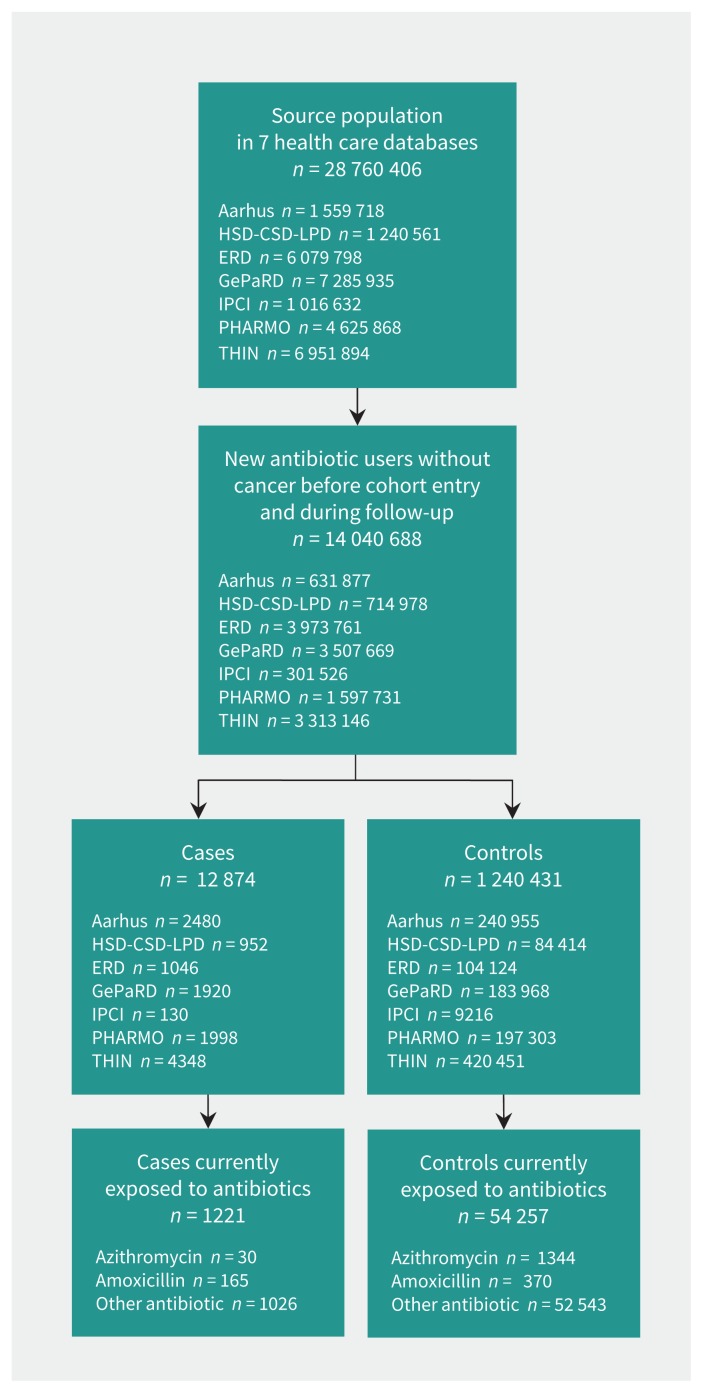
In the source population of 28 760 406 people, we identified 14 040 688 (48.8%) new antibiotic users who met the inclusion criteria. Ventricular arrhythmia developed in 12 874 (0.1%) and 1 240 431 (8.8%) were selected as matched controls (Figure 1). The mean age of the cases and controls was 63 years, and two-thirds were male (Table 2). Well-known risk factors for ventricular arrhythmia were all more frequent among cases than among controls.
Figure 1:
Selection of study cohort from 7 databases. AARHUS = Aarhus University Hospital Database, ERD = Emilia-Romagna Database, GePaRD = German Pharmacoepidemiological Research Database, HSD-CSD-LPD = Health Search Database Cegedim Strategic Data Longitudinal Patient Database, IPCI = Integrated Primary Care Information, PHARMO = PHARMO Database Network, THIN = The Health Improvement Network.
Table 2:
Demographic and clinical characteristics of ventricular arrhythmia cases and matched controls in an inception cohort of new users of antibiotics across all databases (1-stage pooling)
| Characteristic | Group; no. (%) of patients* | p value | |
|---|---|---|---|
| Cases† n = 12 874 |
Controls n = 1 240 431 |
||
| Age, yr, mean ± SD | 63.5 ± 15.3 | 63.6 ± 15.4 | Matching factor |
| Age group, yr | |||
| < 60 | 4210 (32.7) | 405 621 (32.7) | |
| 60–79 | 7106 (55.2) | 678 516 (54.7) | |
| ≥ 80 | 1558 (12.1) | 156 294 (12.6) | |
| Sex | Matching factor | ||
| Male | 8561 (66.5) | 824 887 (66.5) | |
| Female | 4313 (33.5) | 415 544 (33.5) | |
| Well-known risk factors for ventricular arrhythmia | |||
| Atrial fibrillation/flutter | 2332 (18.1) | 61 844 (5.0) | < 0.001 |
| Cardiomyopathy | 1097 (8.5) | 10 887 (0.9) | < 0.001 |
| Cerebrovascular event | 1539 (12.0) | 81 444 (6.6) | < 0.001 |
| Coronary artery disease | 5919 (46.0) | 229 811 (18.5) | < 0.001 |
| Electrolyte imbalance | 1100 (8.5) | 57 663 (4.6) | < 0.001 |
| Heart failure | 2913 (22.6) | 54 114 (4.4) | < 0.001 |
| Hypertension | 10 254 (79.6) | 730 389 (58.9) | < 0.001 |
| Peripheral arterial disease | 626 (4.9) | 26 970 (2.2) | < 0.001 |
| Prior use of anti-arrhythmic drugs | 1009 (7.8) | 13 865 (1.1) | < 0.001 |
| Concomitant use‡ of medications known to induce hypokalemia | 4661 (36.2) | 211 284 (17.0) | < 0.001 |
| Concomitant use‡ of QT-interval–prolonging drugs§ | 2089 (16.2) | 75 177 (6.1) | < 0.001 |
| Potential risk factors for ventricular arrhythmia¶ | |||
| Alcohol abuse | 343 (2.7) | 12 157 (1.0) | < 0.001 |
| Chronic liver disease | 683 (5.3) | 43 325 (3.5) | < 0.001 |
| Chronic respiratory disease | 5310 (41.2) | 390 364 (31.5) | < 0.001 |
| Conduction disorders | 1146 (8.9) | 27 911 (2.3) | 0.001 |
| Congenital heart disease | 151 (1.2) | 2968 (0.2) | < 0.001 |
| Diabetes mellitus | 2395 (18.6) | 140 208 (11.3) | < 0.001 |
| Hyperthyroidism | 300 (2.3) | 16 195 (1.3) | < 0.001 |
| Hypothyroidism | 1033 (8.0) | 68 233 (5.5) | < 0.001 |
| Acute and chronic renal failure | 802 (6.2) | 23 324 (1.9) | < 0.001 |
| Lipid metabolism disorders | 5906 (45.9) | 351 428 (28.3) | < 0.001 |
| Obesity | 1223 (9.5) | 71 835 (5.8) | < 0.001 |
| Other cardiac arrhythmias** | 1394 (10.8) | 34 920 (2.8) | < 0.001 |
| Cardiac valve disorders | 1012 (7.9) | 28 970 (2.3) | < 0.001 |
Note: SD = standard deviation.
Unless stated otherwise.
Current users of both azithromycin and other antibiotics were not included in the analysis.
Within 3 mo before index date.
Use of drugs with established torsadogenic risk according to CredibleMeds list.27
Information on smoking status was partly available only in 2 databases and therefore was not included in the final multivariate models.
Except for atrial fibrillation/flutter, conduction disorders, QT-interval prolongation, ventricular arrhythmia and sudden cardiac death.
Of the cases, 30 were current azithromycin users, matched to 1344 controls. In the primary analysis, we found no significant increase in risk of ventricular arrhythmia associated with current use of azithromycin versus current use of amoxicillin (adjusted OR 0.94, 95% CI 0.50–1.77) (Table 3). The risk was decreased among recent and past users of azithromycin versus amoxicillin (adjusted OR 0.58, 95% CI 0.38–0.87, and 0.52, 95% CI 0.37–0.73, respectively).
Table 3:
Risk of ventricular arrhythmia associated with current, recent and past use of azithromycin compared with current use of amoxicillin (1-stage pooling)
| Exposure* | No. (%) of cases† n = 479 |
No. (%) of controls n = 1848 |
OR (95% CI) | |
|---|---|---|---|---|
| Crude‡ | Adjusted§ | |||
| Current use of amoxicillin | 165 (34.4) | 370 (20.0) | 1.00 (ref) | 1.00 (ref) |
| Use of azithromycin | ||||
| Current | 30 (6.3) | 88 (4.8) | 1.05 (0.63–1.74) | 0.94 (0.50–1.77) |
| Recent | 107 (22.3) | 483 (26.1) | 0.60 (0.44–0.83) | 0.58 (0.38–0.87) |
| Past | 177 (37.0) | 907 (49.1) | 0.49 (0.36–0.66) | 0.52 (0.37–0.73) |
Note: CI = confidence interval, OR = odds ratio, ref = reference category.
Current = exposure period covered the index date (occurrence of ventricular arrhythmia) or ended within 7 days before the index date; recent = exposure period ended between 7 and 89 days before the index date; past = exposure period ended between 90 and 364 days before the index date.
Current users of both azithromycin and other antibiotics were not included in the analysis.
Crude ORs were estimated for matched case–control pairs and cannot be calculated directly from the values in this table.
Adjusted for risk factors of ventricular arrhythmia: atrial fibrillation/flutter, cardiomyopathy, coronary artery disease, cerebrovascular disorders, chronic obstructive pulmonary disease, electrolytic imbalance, heart failure, hypertension, diabetes mellitus, lipid disorder, peripheral arterial disease, hypothyroidism, prior use of antiarrhythmic drugs, concomitant use of drugs known to cause hypokalemia, and concomitant use of drugs known to prolong QT interval.
Compared with nonuse of antibiotics, current use of azithromycin was associated with an increased risk of ventricular arrhythmia, which was decreased substantially but remained significant after adjustment for potential confounders (adjusted OR 1.97, 95% CI 1.35–2.86) (Table 4). There was no increased risk of ventricular arrhythmia among recent and past users of azithromycin compared with nonuse of antibiotics (adjusted OR 1.12, 95% CI 0.92–1.37, and 1.10, 95% CI 0.95–1.28, respectively). Use of other antibiotics (excluding azithromycin and amoxicillin) was associated with an increased risk of ventricular arrhythmia compared with nonuse of antibiotics (adjusted OR 1.83, 95% CI 1.71–1.97). An increased risk was also associated with recent and past use of other antibitiocs, but the effect was smaller (adjusted OR 1.32, 95% CI 1.25–1.39, for recent use, and 1.11, 95% CI 1.06–1.16, for past use).
Table 4:
Risk of ventricular arrhythmia associated with current, recent and past use of azithromycin and other antibiotics compared with nonuse of antibiotics (1-stage pooling)
| Exposure* | No. (%) of cases† n = 12 874 |
No. (%) of controls n = 1 240 431 |
OR (95% CI) | |
|---|---|---|---|---|
| Crude‡ | Adjusted§ | |||
| Nonuse of antibiotics | 5060 (39.3) | 601 049 (48.5) | 1.00 (ref) | 1.00 (ref) |
| Use of azithromycin | ||||
| Current use | 30 (0.2) | 1344 (0.1) | 2.83 (1.97–4.08) | 1.97 (1.35–2.86) |
| Recent use | 109 (0.8) | 8315 (0.7) | 1.65 (1.36–2.00) | 1.12 (0.92–1.37) |
| Past use | 187 (1.5) | 18 000 (1.5) | 1.28 (1.10–1.48) | 1.10 (0.95–1.28) |
| Use of other antibiotics¶ | ||||
| Current use | 1026 (8.0) | 52 543 (4.2) | 2.43 (2.27–2.60) | 1.83 (1.71–1.97) |
| Recent use | 2617 (20.3) | 193 154 (15.6) | 1.69 (1.61–1.77) | 1.32 (1.25–1.39) |
| Past use | 3845 (29.9) | 366 026 (29.5) | 1.29 (1.23–1.34) | 1.11 (1.06–1.16) |
Note: CI = confidence interval, OR = odds ratio, ref = reference category.
Current = exposure period covered the index date (occurrence of ventricular arrhythmia) or ended within 7 days before the index date; recent = exposure period ended between 7 and 89 days before the index date; past = exposure period ended between 90 and 364 days before the index date.
Current users of both azithromycin and other antibiotics were not included in the analysis.
The crude ORs are estimated for matched case-control pairs and cannot be calculated directly from the table above.
Adjusted for risk factors of ventricular arrhythmia: atrial flutter/fibrillation, cardiomyopathy, coronary artery disease, cerebrovascular disorders, chronic obstructive pulmonary disease, electrolytic imbalance, heart failure, hypertension, diabetes mellitus, lipid disorders, peripheral arterial disease, hypothyroidism, prior use of antiarrhythmic drugs, concomitant use of drugs known to cause hypokalemia, and concomitant use of drugs known to prolong QT interval.
Does not include amoxicillin.
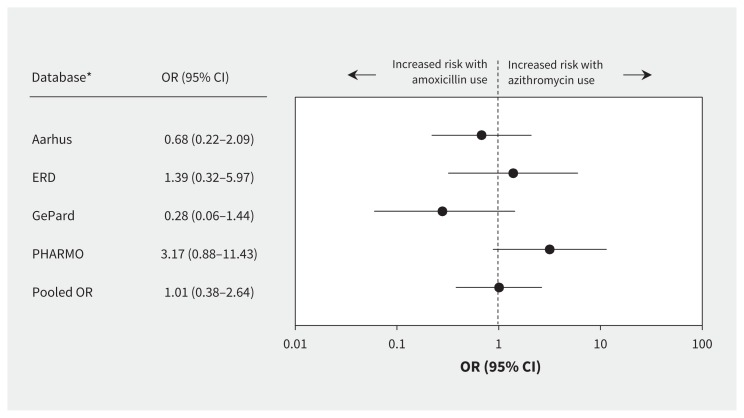
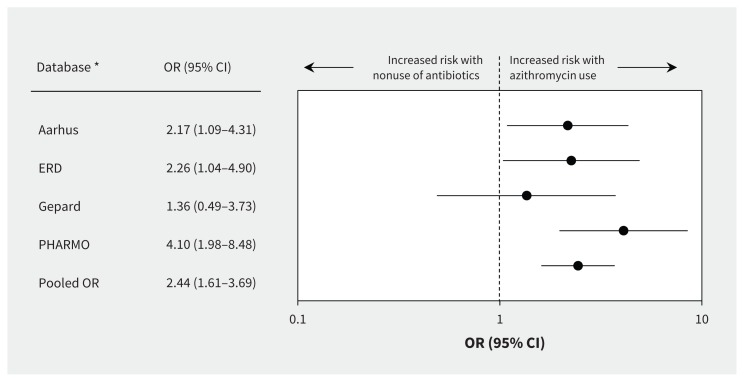
For the 2-stage meta-analysis, we used data from only the PHARMO Database Network, the Aarhus University Hospital Database, the German Pharmacoepidemiological Research Database and the Emilia-Romagna Database, because the other databases had fewer than 3 cases who were current azithromycin users. Heterogeneity among databases was low and nonsignificant (Q statistic 3.39; p = 0.3; I2 = 11%). The comparison of current azithromycin use and current amoxicillin use showed no increased risk of ventricular arrhythmia (OR 1.01, 95% CI 0.38–2.64) (Figure 2). However, the pooled estimate showed an increased risk of ventricular arrhythmia associated with current azithromycin use compared with nonuse of antibiotics (OR 2.44, 95% CI 1.61–3.69) (Figure 3).
Figure 2:
Database-specific risk of ventricular arrhythmia associated with current use of azithromycin compared with current use of amoxicillin (2-stage random-effects model). Values greater than 1.0 indicate an increased risk of ventricular arrhythmia with use of azithromycin (values plotted on logarithmic scale). Cochran Q = 0.08; p = 0.1; I2 = 51%. CI = confidence interval, OR = odds ratio. *Databases with fewer than 3 exposed cases were not included in the model.
Figure 3:
Database-specific risk of ventricular arrhythmia associated with current use of azithromycin versus nonuse of antibiotics (2-stage random-effects model). Values greater than 1.0 indicate an increased risk of ventricular arrhythmia with use of azithromycin (values plotted on logarithmic scale). Cochran Q = 3.4; p = 0.3; I2 = 11.4%. CI = confidence interval, OR = odds ratio. *Databases with fewer than 3 exposed cases were not included in the model.
The findings were similar to those of the primary analysis when we excluded the 7-day carry-over period: compared with nonuse of antibiotics, current azithromycin use and current amoxicillin use were associated with a similar increased risk of ventricular arrhythmia (adjusted OR 1.9, 95% CI 1.1–3.1, and 1.9, 95% CI 2.5–2.3, respectively). When we excluded patients with a diagnosis of acute myocardial infarction within 15 days before the index date, both azithromycin and amoxicillin use were associated with an increased risk of ventricular arrhythmia compared with nonuse of antibiotics (adjusted OR 1.7, 95% CI 1.2–2.5, and 1.9, 95%CI 1.7–2.2, respectively).
Interpretation
Our study showed an increased risk of ventricular arrhythmia associated with current azithromycin use compared with nonuse of antibiotics. Confounding by indication likely played a major role in the assessment of these associations, because the increased risk disappeared when current amoxicillin use was the comparator. This result was consistent across separate databases as well as in the 1- and 2-stage pooled analyses. Confounding by indication in the context of current azithromycin use versus non-use of antibiotics refers to the increased baseline risk of ventricular arrhythmia associated with the indication of the antibiotic use (i.e., the infection) rather than the exposure itself (i.e., azithromycin). The comparison between amoxicillin and azithromycin is more likely a true reflection of the risk of ventricular arrhythmia. The rationale behind our use of amoxicillin as a control is therefore twofold: (a) it is not expected to have an increased risk of ventricular arrhythmia; and (b) it would avoid introducing confounding by indication because both amoxicillin and azithromycin have a similar indication of use and spectrum of action. The above is not true of a comparison between azithromycin use and nonuse of antibiotics. Amoxicillin has been used as a comparator in similar studies previously.14,17
Given the upper limit of the 95% CI and the crude incidence rate of ventricular arrhythmia in the general population in all 7 databases, we would expect at most 8.07 excess cases of ventricular arrhythmia to be associated with azithromycin use per 100 000 person-years compared with nonuse of antibiotics.
There are conflicting results from large epidemiologic studies reporting the risk of cardiovascular death and, more rarely, cardiac arrhythmia. A US cohort study reported a 2.5- and 2.9-fold increased risk of cardiovascular death compared with amoxicillin and nonuse of antibiotics, respectively, among Medicare beneficiaries.14 Following publication of these data, the US Food and Drug Administration issued a safety warning about azithromycin-related cardiovascular death.15 A subsequent Danish study showed that the increased risk of cardiovascular death associated with azithromycin use compared with nonuse of antibiotics (rate ratio 2.85, 95% CI 1.13–7.24) disappeared when use of penicillin V was the comparator (rate ratio 0.93, 95% CI 0.56–1.55).16 More recently, a cohort study using US Veterans’ Affairs data reported an almost twofold increased risk of serious arrhythmia associated with current azithromycin use compared with current amoxicillin use.17
In 2014, a US study reported that, among older patients admitted to hospital with pneumonia, azithromycin use was associated with a small increased risk of myocardial infarction among azithromycin users compared with nonusers, but no effect on cardiac arrhythmias was observed.20
The findings among outpatients from the US studies14,17,20 conflict with our findings and those reported by Svanström and colleagues.16 A possible explanation for this is that Medicaid beneficiaries and retired veterans may have a more marked presence of multiple confounders such as older age, lower socioeconomic status, increased number of comorbidities, obesity, current smoking status and disability, which may make them more vulnerable to the cardiovascular adverse effects of azithromycin than other populations, or increase the impact of residual confounding.
Our findings are in agreement with those from a cohort study involving older patients in Canada (specifically Ontario), which found no increased risk of ventricular arrhythmia associated with macrolide use compared with no macrolide use.21 The study was based on the assumption that an increased risk of ventricular arrhythmia is a class effect, potentially masking the risk of ventricular arrhythmia associated with individual agents.
That the risk of ventricular arrhythmia for macrolides as a class does not necessarily reflect the risk of individual macrolide antibiotics was illustrated by Chou and colleagues,22 who conducted the only published cohort study to date investigating the association between ventricular arrhythmia and azithromycin use, set in the Taiwan National Health Insurance Database. Electrophysiologic evidence supports our findings and suggests that azithromycin lacks arrhythmogenic potential at therapeutic doses.31,32 Fever itself may be a risk factor for arrhythmia, and therefore confounding by indication is pronounced when antibiotics are used in patients with high fever.33
Strengths and limitations
Main strengths of our study include the size of the database network used, since ventricular arrhythmia is a rare outcome, and the common methodology used. All of the databases except the Emiglia-Romagna Database and the Aarhus University Hospital Database are representative of the countries from which data were drawn. In addition, exposure to individual antibiotics was identified in such a way that only users of a single antibiotic were included in the analyses.
Results from the 1-stage pooling were confirmed in the meta-analysis (2-stage pooling) of risk estimates from the individual databases. Taken together, these factors substantially increase the reliability of the study findings. Although it may be considered a limitation that 3 of the databases were excluded from the 2-stage pooling analysis owing to the low number of cases, all 7 databases were included in the 1-stage pooling analysis.
Our study has some other limitations. Exposure misclassification was possible if patients did not fill their prescriptions or take their medications. However, risk estimates were consistent across databases, which suggests a negligible effect of this bias. In addition, exclusion of the 7-day carry-over period to take into account lack of compliance yielded results similar to those of the primary analysis.
The exclusion of cancer patients and those 85 years and older may be considered a limitation; however, the latter approach has been used previously.34,35
Findings from our study may not be directly extrapolated to the hospital setting, because the health status of patients and the nature of antibiotic use in the community setting is likely to be very different.
Conclusion
In this large population-based study of data from 7 European countries, current azithromycin use was associated with an increased risk of ventricular arrhythmia when compared with nonuse of antibiotics, but not when compared with current amoxicillin use. The decreased risk with an active comparator suggests significant confounding by indication.
Footnotes
Competing interests: Tania Schink works in departments that occasionally perform studies funded by pharmaceutical industries (Bayer, Celgene, GlaxoSmithKline, Mundipharma, Novartis, Purdue Pharma, Sanofi-Aventis, Sanofi Pasteur MSD and STADA). Miriam Sturkenboom heads a research unit that holds unconditional research contracts with some pharmaceutical companies (Eli Lilly, Pfizer, AstraZeneca Novartis, Boehringer, GlaxoSmithKline and Servier), none of which are related to this study. Mariam Molokhia has received grants from Pfizer, Astra-Zeneca and the International Serious Adverse Reactions Events Consortium for work unrelated to this study. Edeltraut Garbe has in the past run a department that occasionally performed studies for pharmaceutical industries (Bayer, Celgene, GlaxoSmithKline, Mundipharma, Novartis, Sanofi, Sanofi Pasteur MSD and STADA) and has been a consultant to Bayer, Nycomed, Teva, GlaxoSmithKline, Schwabe and Novartis. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Gianluca Trifirò and Miriam Sturkenboom contributed substantially to the study concept and design. Giampiero Mazzaglia, Irene Bezemer, Edeltraut Garbe, Elisabetta Poluzzi, Trine Frøslev and Miriam Sturkenboom contributed to data acquisition. Gianluca Trifirò, Maria de Ridder, Janet Sultana, Alessandro Oteri, Peter Rijnbeek, Serena Pecchioli, Tania Schink, Elisabetta Poluzzi, Trine Frøslev, Mariam Molokhia, Igor Diemberger and Miriam Sturkenboom contributed to the analysis and interpretation of data. Gianluca Trifirò and Janet Sultana drafted the article, and all of the authors revised it critically for important intellectual content. All of the authors approved the final version to be published and agreed to act as guarantors of the work.
Funding: This study was funded by the European Community’s Seventh Framework Programme under grant agreement number 241679 — the ARITMO project.
References
- 1.Vogt AW, Zollo RA. Long Q-T syndrome associated with oral erythromycin used in preoperative bowel preparation. Anesth Analg 1997;85:1011–3. [DOI] [PubMed] [Google Scholar]
- 2.Tschida SJ, Guay DR, Straka RJ, et al. QTc-interval prolongation associated with slow intravenous erythromycin lactobionate infusions in critically ill patients: a prospective evaluation and review of theliterature. Pharmacotherapy 1996;16:663–74. [PubMed] [Google Scholar]
- 3.De Ponti F, Poluzzi E, Montanaro N. QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol 2000; 56:1–18. [DOI] [PubMed] [Google Scholar]
- 4.Drici MD, Knollmann BC, Wang WX, et al. Cardiac actions of erythromycin: influence of female sex. JAMA 1998;280:1774–6. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis 2002;35:197–200. [DOI] [PubMed] [Google Scholar]
- 6.Koh TW. Risk of torsades de pointes from oral erythromycin with concomitant carbimazole (methimazole) administration. Pacing Clin Electrophysiol 2001; 24:1575–6. [DOI] [PubMed] [Google Scholar]
- 7.Matsunaga N, Oki Y, Prigollini A. A case of QT-interval prolongation precipitated by azithromycin. N Z Med J 2003;116:U666. [PubMed] [Google Scholar]
- 8.Samarendra P, Kumari S, Evans SJ, et al. QT prolongation associated with azithro-mycin/amiodarone combination. Pacing Clin Electrophysiol 2001;24:1572–4. [DOI] [PubMed] [Google Scholar]
- 9.Russo V, Puzio G, Siniscalchi N. Azithromycin-induced QT prolongation in elderly patient. Acta Biomed 2006;77:30–2. [PubMed] [Google Scholar]
- 10.Arellano-Rodrigo E, García A, Mont L, et al. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome [article in Spanish]. Med Clin (Barc) 2001;117:118–9. [DOI] [PubMed] [Google Scholar]
- 11.Kezerashvili A, Khattak H, Barsky A, et al. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol 2007;18:243–6. [DOI] [PubMed] [Google Scholar]
- 12.Huang BH, Wu CH, Hsia CP, et al. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol 2007;30:1579–82. [DOI] [PubMed] [Google Scholar]
- 13.Kim MH, Berkowitz C, Trohman RG. Polymorphic ventricular tachycardia with a normal QT interval following azithromycin. Pacing Clin Electrophysiol 2005;28:1221–2. [DOI] [PubMed] [Google Scholar]
- 14.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA statement regarding azithromycin (Zithromax) and the risk of cardiovascular death. Silver Spring (MD): US Food and Drug Administration; 2012. Available: www.fda.gov/Drugs/DrugSafety/ucm304372.htm (accessed 2015 June 24). [Google Scholar]
- 16.Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013;368:1704–12. [DOI] [PubMed] [Google Scholar]
- 17.Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med 2014;12:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–64. [DOI] [PubMed] [Google Scholar]
- 19.Marcus FI, Cobb LA, Edwards JE, et al. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am J Cardiol 1988;61:8–15. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen EM, Halm EA, Pugh MJ, et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 2014;311:2199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trac MH, McArthur E, Jandoc R, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 2016;188:E120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis 2015;60:566–77. [DOI] [PubMed] [Google Scholar]
- 23.Molokhia M, De Ponti F, Behr ER, et al. Academic output from EU-funded health research projects [letter]. Lancet 2012;380:1903–4. [DOI] [PubMed] [Google Scholar]
- 24.Avillach P, Coloma PM, Gini R, et al. Harmonization process for the identification of medical events in eight European healthcare databases: the experience from the EU-ADR project. J Am Med Inform Assoc 2013;20:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mor A, Frøslev T, Thomsen RW, et al. Antibiotic use varies substantially among adults: a cross-national study from five European countries in the ARITMO project. Infection 2015;43:453–72. [DOI] [PubMed] [Google Scholar]
- 26.Diemberger I, Oteri A, Rijnbeek P, et al. Epidemiology of ventricular arrhythmias in five European countries [abstract submitted to World Congress in Cardiac Electrophysiology and Cardiac Techniques, June 18–21, 2014 — Nice France. Europace 2014;16(Suppl 2). [Google Scholar]
- 27.CredibleMeds [homepage]. Available: https://crediblemeds.org (accessed 2014 Jan. 15).
- 28.Coloma PM, Schuemie MJ, Trifirò G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf 2011;20:1–11. [DOI] [PubMed] [Google Scholar]
- 29.Trifirò G, Coloma PM, Rijnbeek PR, et al. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: Why and how? J Intern Med 2014;275:551–61. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohara H, Nakamura Y, Watanabe Y, et al. Azithromycin can prolong QT interval and suppress ventricular contraction, but will not induce Torsade de Pointes. Cardiovasc Toxicol 2015;15:232–40. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima T, Kaneko Y, Kurabayashi M. Unveiling specific triggers and precipitating factors for fatal cardiac events in inherited arrhythmia syndromes. Circ J 2015;79:1185–92. [DOI] [PubMed] [Google Scholar]
- 33.Pasquié JL, Sanders P, Hocini M, et al. Fever as a precipitant of idiopathic ventricular fibrillation in patients with normal hearts. J Cardiovasc Electrophysiol 2004;15:1271–6. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Díaz S, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer 2007;120:1565–72. [DOI] [PubMed] [Google Scholar]
- 35.García Rodríguez LA, Cea Soriano L, Hill C, et al. Increased risk of stroke after discontinuation of acetylsalicylic acid: a UK primary care study. Neurology 2011;76:740–6. [DOI] [PubMed] [Google Scholar]