Abstract
Purpose
Physical activity is associated with decreased adiposity-related inflammation in adults. Whether this association is independent of central obesity is unknown but important for understanding the mechanisms associated with reducing cardiometabolic disease risk through physical activity. This study examined whether associations of physical activity and obesity-related inflammatory markers were independent of central adiposity.
Methods
Between 2002 and 2005, 1970 participants from the Multi-Ethnic Study of Atherosclerosis completed detailed health history and physical activity questionnaires, underwent physical measurements including computed tomography to quantify abdominal visceral and subcutaneous fat, and measurements of adiponectin, leptin, interleukin-6, tumor necrosis factor-alpha, and resistin. Statistical analyses included analysis of covariance and multivariable-adjusted regression.
Results
The mean (range) age of participants was 64.7 (55-84) years and 50% were female. After adjustment for age and sex, and compared to the lowest quartile, inflammatory markers in the highest quartile of moderate-to-vigorous physical activity were 16% higher for adiponectin and 30%, 26% and 9% lower for leptin, interleukin-6, and resistin, respectively (p<0.05 for all). In linear regression adjusted for demographics, dyslipidemia, hypertension, diabetes, smoking, glomerular filtration rate, renin and aldosterone, each standard deviation increment of moderate-to-vigorous physical activity was associated with significantly higher levels of adiponectin (β=0.04) and lower levels of leptin (β=−0.06), interleukin-6 (β=−0.08), and resistin (β=−0.05, p<0.05 for all). The associations with leptin, interleukin-6, and resistin were independent of total and central adiposity (p<0.05), whereas the association between moderate-to-vigorous physical activity and adiponectin was attenuated by central adiposity (p>0.05). There were no significant interactions by race/ethnicity or sex.
Conclusions
Moderate-to-vigorous physical activity was associated with a more favorable profile of inflammatory markers, independent of relevant cardiometabolic disease risk factors including central obesity.
Keywords: Exercise, adiponectin, interleukin-6, resistin, obesity
Introduction
It is well established that physical activity is associated with decreased cardiovascular morbidity and mortality (14). Despite this robust relationship, the mechanisms involved are not completely understood. It has been hypothesized that the protective effect of physical activity on cardiometabolic health may be attributed to an anti-inflammatory effect (5). In this regard, cross-sectional data from large population cohorts suggest that physical activity is inversely associated with markers of inflammation (5,8,20), with C-reactive protein (CRP) being the inflammatory marker most studied. However, significant relationships between physical activity and markers of inflammation are not consistently reported in the literature and may vary across sex and ethnicity/race (5,15,20). More specifically, results from the Multi-Ethnic Study of Atherosclerosis (MESA) show that CRP was inversely related to physical activity in Hispanic men but not in men of other races or in women (20). Similarly, the Health, Aging, and Body Composition study reported inverse associations between physical activity and interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and CRP in older adults, but differences by race and sex were not examined (8).
Although central adipose tissue is a key source of pro-inflammatory cytokines (13), some research suggests the effects of physical activity on inflammation may be independent of changes in this tissue depot (4,5,8). Notably, central adiposity is comprised of both visceral and subcutaneous adipose tissue with the former being located within the abdominal cavity, and the latter under the skin but external to the muscle enveloping the visceral cavity. Compared to subcutaneous adipose tissue, visceral adipose tissue has greater inflammatory activity and secretes more inflammatory markers to the portal circulation (9,17). The majority of studies investigating the independent effects of physical activity on inflammation used indirect estimates of obesity, such as body mass index (BMI) and waist circumference (5,20), which cannot distinguish between fat depots. To our knowledge, only one study to date (8) investigated the associations between physical activity and inflammation with valid measures of abdominal visceral adipose tissue by computed tomography. In this study, adjustment for visceral adipose tissue attenuated the associations between physical activity and TNF-α and CRP, but the association between physical activity and IL-6 remained significant. Notably, only three inflammatory markers were studied.
Because there are ethnic differences in regional fat deposits (7) and levels of inflammatory markers (20), associations between physical activity and inflammation may vary by ethnic group. Thus, evaluation of a racially and ethnically diverse population using a valid measure isolating abdominal visceral adipose from subcutaneous adipose tissue located in the abdominal region would help to elucidate the association between physical activity and inflammation. Therefore, the purpose of the current study was to examine, in a multi-ethnic cohort, the associations of physical activity with adipokines known to contribute to cardiometabolic health (adiponectin, leptin, resistin, TNF-α, IL-6) and to determine if the associations were independent of abdominal visceral and subcutaneous fat. We hypothesized that moderate-to-vigorous physical activity would be associated with the individual adipokines, independent of abdominal visceral and subcutaneous fat.
Methods
Participants
The MESA is a longitudinal cohort study of adults drawn from six regions of the US. The overall design of the MESA study has been published (6). In brief, the cohort included a total of 6814 men and women aged 45-84 years who were free from clinically apparent cardiovascular disease at the time of enrollment (July 2000 to August 2002). Participant racial/ethnic groups included African American, Chinese American, Hispanic and non-Hispanic white. Individuals with a history of diagnosed angina, heart attack, heart failure, stroke or transient ischemic attack, or having undergone an invasive procedure for cardiovascular disease (coronary artery bypass graft, angioplasty, valve replacement or pacemaker placement) were excluded from the study. Participants who were enrolled in the study returned for follow-up clinic visits approximately 2, 4, and 6 years after the baseline clinic visit.
At clinic visits 2 and 3 (from 2002 to 2005), a random subset of 1970 participants were enrolled in an ancillary study where abdominal computed tomography (CT) scans were obtained and subsequently used to quantify visceral and subcutaneous fat mass. The data obtained on these participants comprise the sample for the current study. Approximately half of the 1970 participants had their abdominal CT scan at visit 2 and the other half at visit 3. To make the measurements contemporaneous, demographic, physical activity and adipokine data obtained during visit 2 or 3 were used in this study. The MESA studies were approved by the Institutional Review Board of each study site and all participants provided written informed consent.
Data collection
At all study visits, standard questionnaires were used to obtain information on participant sociodemographics, ethnicity and health history. Cigarette smoking was defined as current, former, or never smoker. Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively, with participants wearing minimal clothing and no shoes. BMI was calculated as weight divided by height squared. Waist and hip circumferences were measured using a standard flexible, tension-regulated tape measure. Blood pressure was measured with an automated monitor after 5 minutes of seated rest, with the last two of three readings averaged and recorded. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or taking antihypertensive medication.
Physical Activity Assessment
At each clinic visit, participants self-reported their physical activity using the Typical Week Physical Activity Survey (TWPAS). This survey was adapted from the Cross-Cultural Activity Participation Study (2) and designed to identify the frequency of and time spent in various physical activities during a typical week within the past month. The survey includes 28 items in categories of household chores, yard/lawn/garden/farm care, care of children/adults, transportation, walking (not at work), dancing and sport activities, conditioning activities (e.g., aerobics, cycling, jogging, rowing, and swimming), leisure activities, and occupational and volunteer activities. Where appropriate, questions differentiated between light-, moderate-, and heavy-intensity activities. Respondents were first asked whether they participated in each category of activity. If they answered yes, they were asked questions regarding the average number of days per week and time per day engaged in these activities. Minutes of activity were summed for each discrete activity type and multiplied by the metabolic equivalent (MET) level (3). Survey responses were quantified into MET-minutes per week of total intentional physical activity (walking for exercise, dancing, sport, and conditioning activities), conditioning activity (conditioning activities) and moderate-to-vigorous physical activity (moderate and vigorous activities from all categories).
Laboratory
At each clinic visit, venous blood was collected after a 12-hour fast and shipped to the MESA central laboratory for analysis of total and HDL cholesterol, triglycerides, glucose, and creatinine concentration. Stored fasting blood samples from visits 2 or 3 were analyzed for insulin, CRP, fibrinogen, renin, aldosterone, adiponectin, leptin, TNF-α, IL-6 and resistin. Adipokines were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Average analytic coefficients of variation across several control samples ranged from 6.0% to 13.0%. Dyslipidemia was defined as a total cholesterol/HDL-cholesterol ratio >5.0 or if the participant was taking medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥ 126 mg·dL−1 or use of hypoglycemic medication. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease-Epi equation (19).
Visceral and subcutaneous adiposity
Electron-beam CT scanners were used at Northwestern University and University of California, Los Angeles (Imatron C-150, Imatron Inc., South San Francisco, California), with the following settings: collimation 3 mm, slice thickness 6 mm, reconstruction using 25 6-mm slices with 35-cm field of view and normal kernel. Multidetector CT scanners were used at Columbia University, Wake Forest University, and University of Minnesota (Sensation 64 [Siemens, Malvern, Pennsylvania] and GE Lightspeed [GE Healthcare, Waukesha, Wisconsin], Siemens S4 Volume Zoom, and Siemens Sensation 16, respectively). Abdominal slices from these scans were processed using MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD) that measured fat, lean and total tissue using a semi-automated method. Subcutaneous adipose tissue was defined as the fat outside of the visceral cavity, not including the fat located within the muscular fascia. Fat tissue was identified as being between −190 and −30 Hounsfield units (HU), whereas lean tissue was identified as being between 0 and 100 HU. Densities outside of these ranges were labeled as undefined tissue type. Six transverse cross sectional slices were analyzed at the following spine levels: 2 at L2/L3, 2 at L3/L4 and 2 at L4/L5. Areas of interest were identified and a density value was assigned to each pixel. Using the pixel densities and the HU criteria above, total, fat and lean areas of the area of interest were calculated. CT imaging was interpreted blinded to clinical information.
Statistics
Analyses for this report utilized data from clinic visits 2 and 3 and were completed in 2016. All data were examined for outliers and normal distribution using plots and Kolmogorov-Smirnov tests of normality. Variables that were not normally distributed (e.g., the adipokines) were natural log (ln) transformed and rechecked for normality. Total intentional physical activity, total conditioning physical activity, and moderate-to-vigorous physical activity were examined as continuous variables (per 1 standard deviation [SD] increment) and as categorical variables (i.e. quartiles).
Total intentional physical activity and moderate-to-vigorous physical activity were grouped into quartiles and total conditioning physical activity into two groups (no conditioning versus conditioning). ANCOVA was used to determine the means of adipokines and inflammatory markers by group of total intentional physical activity, total conditioning physical activity and moderate-to-vigorous physical activity.
Cross-sectional multivariable linear regression was used to determine the associations between the independent variables (total intentional physical activity, total conditioning physical activity, and moderate-to-vigorous physical activity) and dependent variables (individual adipokines). The initial model (Model 1) was adjusted for age, sex, and race/ethnicity. Model 2 included Model 1 plus BMI. Model 3 included Model 2 plus dyslipidemia, hypertension, diabetes, smoking, glomerular filtration rate, renin and aldosterone. Model 4 included Model 3 plus height and abdominal subcutaneous and visceral fat (minus BMI). Multivariable interactions between race/ethnicity and physical activity for the different adipokines were assessed. All statistical analyses were conducted using STATA (Version 13; StatCorp) and a p-value of 0.05 was used to determine statistical significance.
Results
The study cohort characteristics are presented in Table 1. Forty percent of participants were non-Hispanic white, 21% were African American, 26% were Hispanic/Latino, and 13% were Chinese American. The mean age was 64.7 years and 50% were female. On average, participants were overweight, with a mean BMI and waist circumference of 28.2 kg·m−2 and 98.4 cm, respectively. Thirty-one percent of participants had a BMI greater than 30 kg·m−2. Almost half (46%) of participants were never smokers, 48% were hypertensive, 40% were dyslipidemic, and 15% had diabetes mellitus.
Table 1.
Characteristics of the study cohort (n=1970)
| Characteristic | Mean (SD)/Frequency (%) |
|---|---|
| Age (years, M [SD]) | 64.7 (9.7) |
| Female (% [Freq]) | 971 (50%) |
| Ethnicity (% [Freq]) | |
| Caucasian | 784 (40.1) |
| Chinese-American | 253 (12.9) |
| African-American | 408 (20.9) |
| Hispanic | 210 (26.1) |
| Ever Smoker (% [Freq]) | 1060 (54.2) |
| BMI (M [SD]) | 28.2 (5.2) |
| Dyslipidemia (% [Freq]) | 760 (39.8) |
| Diabetes (% [Freq]) | 285 (14.6) |
| Hypertension (% [Freq]) | 920 (47.5) |
| Waist circumference (cm, M [SD]) | 98.4 (14.1) |
| Hip circumference (cm, M [SD]) | 104.5 (10.9) |
| Waist-to-hip ratio | 0.94 (0.07) |
| Subcutaneous fat area (cm2, M [SD]) | 254 (117.7) |
| Visceral fat area (cm2, M [SD]) | 148 (69.2) |
| Glucose (mg·dL−1, M [SD]) | 98 (27.8) |
| Triglycerides (mg·dL−1, M [SD]) | 134 (94.9) |
| High-density lipoprotein (mg·dL−1, M [SD]) | 51 (15.1) |
| High sensitivity CRP (mg·L−1, Mdn [IQR] | 1.5 (0.7-3.3) |
| Adiponectin (mg·mL−1, Mdn [IQR]) | 17.4 (11.8-26.3) |
| Leptin (ng·mL−1, Mdn [IQR]) | 13.5 (5.6-28.3) |
| Resistin (ng·mL−1, Mdn [IQR]) | 15.0 (11.9-19.1) |
| Interleukin-6 (pg·mL−1, Mdn [IQR]) | 1.9 (1.2-2.9) |
| TNF-α (pg·mL−1, Mdn [IQR]) | 4.6 (3.4-6.4) |
| Total Conditioning Exercise (MET-min·wk−1, Mdn [IQR]) |
0 (0-495) |
| Total Intentional Exercise (MET-min·wk−1, Mdn [IQR]) |
823 (109-1888) |
| Moderate-to-Vigorous Physical Activity (MET-min·wk−1, Mdn [IQR]) |
3562 (1830-6373) |
M, mean; SD, standard deviation; %, percent; Freq, frequency; BMI, body mass index; CRP, C-reactive protein; TNF-α, tumor necrosis factor alpha; MET, metabolic equivalents
Characteristics by quartiles of moderate-to-vigorous physical activity
Participants who were younger and male reported higher levels of moderate-to-vigorous physical activity (Table 2). After adjusting for age and sex, mean levels of BMI, waist circumference, waist-to-hip ratio, abdominal subcutaneous fat, visceral fat, triglycerides, leptin, resistin, IL-6, fibrinogen, and CRP decreased and adiponectin and HDL-cholesterol increased across increasing quartiles of moderate-to-vigorous physical activity (p<0.05). There did not appear to be a trend for TNF-α (p>0.05). The prevalence of diabetes and dyslipidemia decreased across increasing quartiles of moderate-to-vigorous physical activity (p<0.05), but there were no differences in hypertension or smoking across these quartiles. When comparing the lowest to the highest quartile of moderate-to-vigorous physical activity, there was a 5.9% (15 cm2) and 10.9% (17 cm2) decrease in abdominal subcutaneous and visceral fat, respectively (p<0.05). Similarly, adiponectin was 16% (2.9 ng·mL−1) higher and leptin, IL-6, and resistin were 30% (7.3 ng·mL−1), 26% (0.7 pg·mL−1), and 9% (1.6 ng·mL−1) lower in the highest compared to the lowest quartile of moderate-to-vigorous physical activity.
Table 2.
Adjusted mean characteristics by quartile of MET-minutes of moderate-to-vigorous physical activity per week.
| Moderate-to-Vigorous Physical Activity | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic (unit) |
Q1
(n=489) |
Q2
(n=493) |
Q3
(n=491) |
Q4
(n=491) |
p-value |
| Age (years) | 66.7 | 66.0 | 63.8 | 61.2 | <0.01 |
| BMI (kg·m-2) a | 28.4 | 27.9 | 27.5 | 27.2 | <0.01 |
| Waist (cm) a | 99.4 | 97.0 | 95.8 | 95.1 | <0.01 |
| Waist-to-hip ratio a | 94.8 | 93.3 | 93.2 | 93.2 | <0.03 |
| Subcutaneous fat area (cm2) a | 258 | 251 | 238 | 243 | <0.01 |
| Visceral fat area (cm2) a | 152 | 140 | 136 | 135 | >0.01 |
| Insulin (pmol·L−1) a | 317.3 | 286.3 | 270.7 | 259.2 | 0.07 |
| Glucose (mg·dL−1) a | 103 | 98 | 96 | 98 | 0.20 |
| Triglycerides (mg·dL−1) a | 139 | 136 | 131 | 126 | <0.01 |
| HDL cholesterol (mg·dL−1) a | 51 | 51 | 52 | 53 | <0.01 |
| Adiponectin (mg·mL−1) a | 18.1 | 19.7 | 20.2 | 21.0 | <0.01 |
| Leptin (ng·mL−1) a | 24.4 | 20.8 | 19.0 | 17.1 | <0.01 |
| Resistin (ng·mL−1) a | 17.3 | 16.2 | 16.1 | 15.7 | 0.02 |
| Interleukin-6 (pg·mL−1) a | 2.7 | 2.3 | 2.3 | 2.0 | <0.01 |
| TNF-α (pg·mL−1) a | 6.0 | 5.9 | 5.7 | 5.1 | 0.50 |
| High sensitivity CRP (mg·L−1) a | 4.1 | 2.8 | 3.1 | 2.3 | <0.01 |
| Fibrinogen (mg·dL−1) a | 447 | 436 | 438 | 422 | <0.01 |
| Diabetes (%) a | 21.1 | 13.9 | 13.6 | 13.1 | <0.01 |
| Dyslipidemia (%) a | 41.6 | 35.2 | 42 | 35.1 | 0.03 |
| Hypertension (%) a | 51.6 | 45.1 | 48.3 | 45.2 | 0.11 |
| Current smoker (%) a | 66.8 | 56.8 | 62.0 | 57.4 | 0.06 |
Quartile cutpoints (MET-minutes·week−1): Q1=<1830, Q2=1830-3561, Q3=3562-6370, Q4=>6370
Adjusted for age and sex; Q, quartile; BMI, body mass index; HDL, high-density lipoprotein; TNF-α, tumor necrosis factor alpha; CRP, C-reactive protein.
Multivariate linear regression models
Multivariable-adjusted linear regression models were used to determine the independent associations between physical activity and adipokines (Table 3). With adjustment for age, sex, and race/ethnicity, a one standard deviation (1-SD) increment in moderate-to-vigorous physical activity was associated with a higher adiponectin (6.7%) but lower leptin (9.5%), resistin (5.2%), TNF-α (4.9%), and IL-6 (9.9%, p<0.05 for all). The associations of moderate-to-vigorous physical activity with leptin, resistin, and IL-6 were slightly attenuated but remained significant with the addition of all covariates including abdominal subcutaneous and visceral fat (model 4: 4.9%, 5.3%, 6.8%, respectively, p<0.05 for all). The association with TNF-α was attenuated to non-significance with addition of BMI to model 1 (>0.05). Similarly, the association with adiponectin remained significant in models 2 and 3 but became non-significant when abdominal visceral and subcutaneous fat were entered into the model (model 4, 3.5%, p=0.13).
Table 3.
Multivariable-adjusted linear regression models for the associations between physical activity (per 1 SD increment) and adipokines. Data are reported as standardized betas.
| Model | Ln Adiponectin | Ln Leptin | Ln Resistin | Ln TNF-α | Ln IL-6 |
|---|---|---|---|---|---|
| MVPA (4771 MET-min·week−1) | |||||
| 1 | 0.067* | −0.095* | −0.052* | −0.049* | −0.099* |
| 2 | 0.054* | −0.067* | −0.049* | −0.045 | −0.083* |
| 3 | 0.044* | −0.062* | −0.046 | −0.010 | −0.076* |
| 4 | 0.035 | −0.049* | −0.053* | −0.017 | −0.068* |
| Total Intentional Exercise (1817 MET-min·week−1) | |||||
| 1 | 0.050* | −0.069* | −0.027 | −0.022 | −0.101* |
| 2 | 0.030 | −0.027 | −0.022 | −0.016 | −0.077* |
| 3 | 0.025 | −0.042* | −0.021 | −0.012 | −0.055* |
| 4 | −0.002 | −0.025 | −0.027 | −0.030 | −0.059* |
| Total Conditioning Exercise (1023 MET-min·week−1) | |||||
| 1 | 0.028 | −0.028 | 0.004 | 0.005 | −0.088* |
| 2 | 0.022 | −0.015 | 0.006 | 0.007 | −0.080* |
| 3 | 0.016 | −0.039 | 0.001 | <0.001 | −0.062* |
| 4 | −0.005 | −0.012 | 0.005 | 0.002 | −0.067* |
Ln = natural log; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; MVPA, moderate-to-vigorous physical activity; Model 1 = adjusted for age, sex, race/ethnicity; Model 2 = Model 1 + BMI; Model 3 = Model 2 + dyslipidemia, hypertension, diabetes, smoking, glomerular filtration rate, renin and aldosterone; Model 4 = Model 3 plus height and subcutaneous and visceral fat (minus BMI);
p<0.05
Total intentional exercise was not as robustly associated with the adipokines (Table 3). Specifically, with adjustment for age, sex, and race/ethnicity, a 1-SD increment in total intentional exercise was associated with a higher adiponectin (5%, p<0.05) and lower leptin and IL-6 (6.9% and 10%, respectively, p<0.01 for both). When the models were additionally adjusted for BMI, the associations with adiponectin and leptin were attenuated (p>0.05). The association between total intentional exercise and IL-6 was slightly attenuated but remained significant with the addition of all covariates (p=0.02). Total intentional exercise was not independently related to resistin or TNF-α (p>0.05).
We conducted similar analyses with total conditioning exercise and results indicated total conditioning exercise was independently and inversely related to IL-6 after adjustment for all covariates (p<0.01, Table 3). However, there were no independent associations between total conditioning exercise and adiponectin, leptin, resistin, or TNF-α (p>0.05 for all models).
Physical activity quartiles using linear regression
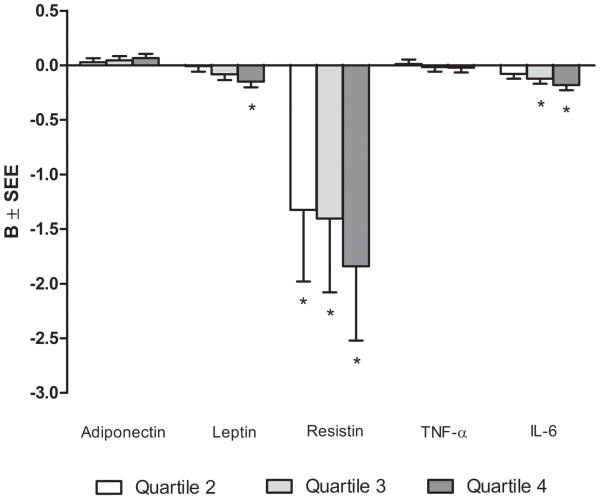
Multivariable-adjusted regression analysis was performed using quartiles of moderate-to-vigorous physical activity to determine if the associations were stronger by level of moderate-to-vigorous physical activity or if there were threshold effects (Figure 1). After adjustment for age, sex, and race/ethnicity, and compared to the first quartile, the second, third and fourth quartile of moderate-to-vigorous physical activity were associated with significantly higher levels of adiponectin and lower levels of leptin, resistin, and IL-6 (p<0.05). After adjustment for all covariates including abdominal subcutaneous and visceral fat, the second, third and fourth quartiles of moderate-to-vigorous physical activity were associated with significantly lower levels of resistin while the third and fourth quartile were associated with significantly lower levels of IL-6 (p<0.05). Only the fourth quartile of moderate-to-vigorous physical activity was associated with a significantly lower level of leptin (p<0.01) and nearly significantly higher levels of adiponectin (p=0.08).
Figure 1.
Multivariable-adjusted associations between quartiles of moderate-to-vigorous physical activity and levels of adipokines. Referent category: Quartile 1 – Quartile cutpoints (MET-minutes/week): Q1=<1830, Q2=1830-3561, Q3=3562-6370, Q4=>6370. Adjusted for age, sex, race/ethnicity, dyslipidemia, hypertension, diabetes, smoking, glomerular filtration rate, renin, aldosterone, height, and subcutaneous and visceral fat. B=slope; SEE, standard error of the estimate; Ln, natural logarithm, TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; *p<0.05
Differences in race/ethnicity
Using multiplicative interaction terms, we tested for significant differences in the magnitudes of the associations between the different types of physical activity (as continuous variables) and each inflammatory marker, by race/ethnicity and sex. There were no significant differences across race/ethnicity or sex.
Discussion
In this study of a large, multi-ethnic population-based cohort from multiple sites across the United States, higher levels of moderate-to-vigorous physical activity were associated with significantly higher levels of adiponectin and lower levels of leptin, IL-6, and resistin. The associations of moderate-to-vigorous physical activity with leptin, IL-6, and resistin were independent of relevant covariates including measures of total and central adiposity, whereas the association with adiponectin was attenuated by central adiposity. Notably, the magnitude of the associations did not vary by race/ethnic group or sex. These results suggest that physical activity may positively influence levels of selected adiposity-associated inflammatory markers, irrespective of total and/or central adiposity.
There is a growing body of evidence that higher levels of physical activity are associated with decreased levels of inflammatory markers. Cross-sectional data indicate that higher levels of physical activity are associated with lower levels of CRP (1,8,10,18,21,23), IL-6 (8,21,23), and leptin (23). However, data are inconsistent as to whether these relationships are independent or attenuated by estimates of body fat, including BMI, waist circumference, and waist-to-hip ratio. Furthermore, few studies investigated multiple markers of adiposity-related inflammation (8,21,23).
One mechanism in which physical activity is hypothesized to reduce inflammation is through a reduction in central adiposity (26). Obesity is associated with systemic inflammation, which in turn is associated with development of type 2 diabetes and cardiovascular disease (16). Central adipose tissue is known to produce pro-inflammatory cytokines, whereas low total body fat is associated with higher levels of adiponectin (9,13). Whether the effects of physical activity on adipokines levels are independent of central adiposity is currently unknown, but important for understanding the mechanisms associated with a reduced cardiometabolic disease risk with physical activity.
Our results extend the findings that physical activity is associated with lower systemic inflammation, to a large multiethnic cohort of middle-aged to older adults. Additionally, we used a measure of central adiposity that is a more precise measure than BMI or waist circumference (i.e., visceral fat by computed tomography). We found this measure significantly attenuated the relationship between physical activity and adiponectin but not leptin, resistin or IL-6. Moreover, our findings are consistent with longitudinal exercise training studies that reported significant decreases in leptin and resistin (12) and CRP (4) independent of changes in body composition. This suggests that physical activity may reduce pro-inflammatory cytokines independent of changes in visceral adiposity or fat loss. Together these findings suggest a direct effect of physical activity on specific markers of inflammation; however, the mechanism is still not clear. It has been suggested that exercise may regulate the synthesis of pro-inflammatory cytokines by acutely increasing levels of IL-6, which in turn stimulates production of anti-inflammatory cytokines IL-1 receptor antagonist and IL-10, and suppressing TNF-α through IL-6 dependent and independent pathways (22).
An interesting finding from our study is that the association between physical activity and adiponectin was attenuated by central adiposity but not total adiposity, as estimated by BMI. Adiponectin is secreted from adipose tissue and increasing adiposity typically results in lower levels of adiponectin (25). However, visceral adipose tissue produces less adiponectin than subcutaneous adipose tissue (11,24), so a lower proportion of total fat in the visceral depot makes increases in adiponectin more likely. Of note, previous studies have shown significant changes in body composition are needed to see increases in adiponectin with physical activity (25). In this cohort, as physical activity increased from the first to fourth quartile abdominal subcutaneous and visceral fat decreased by 5.9% and 10.9%, respectively, which corresponded with a 16% increase in adiponectin. Together these findings suggest that decreases in central fat may be more important than decreases in total fat loss for improving adiponectin with exercise.
Our findings suggest that there may be a dose response relationship between physical activity and markers of inflammation. As physical activity increased from the first to fourth quartile, markers of inflammation improved in a linear manner. Indeed, inflammatory markers in the highest quartile of moderate-vigorous physical activity were 16% higher for adiponectin and 30%, 26%, and 9% lower for leptin, IL-6, and resistin, respectively, compared to the lowest quartile. This trend was evident in all of the inflammatory markers measured except TNF-α. The association between moderate-to-vigorous physical activity and TNF-α was attenuated by body mass index, suggesting that TNF-α may be less sensitive to changes in physical activity and more sensitive to changes in body composition, which is consistent with previous research on TNF-α (8,21) and it’s soluble receptors (23).
It is becoming increasingly clear that physical activity is associated with a reduction in inflammatory markers, but many studies have examined only leisure time physical activity (21,23). Our study included household, transportation, occupational, and volunteer physical activity, in addition to intentional exercise, and provides evidence that accumulation of moderate-to-vigorous physical activity, regardless of whether or not the activity is intentional, contributes to lowering inflammation in middle-aged and older adults.
The strengths of the current study include a relatively large, multi-ethnic sample of men and women, the use of objective measures of abdominal subcutaneous and visceral obesity via CT scan, and inflammatory markers that were analyzed at a central laboratory, with a high level of reproducibility. Limitations include self-reported measures of physical activity which may be less accurate than objective measures of physical activity. However, our physical activity questionnaire included household, occupational, volunteer, and transportation activity to capture different types of moderate-to-vigorous physical activity as opposed to only intentional exercise. The study was cross sectional which does not provide information with regard to a possible causal relationship between physical activity and inflammation.
In summary, our findings suggest that moderate-to-vigorous physical activity was associated with a more favorable profile of inflammatory markers, independent of relevant risk factors for cardiometabolic disease, including abdominal subcutaneous and visceral adiposity. These results provide additional evidence for promoting physical activity as a modifiable risk factor for cardiometabolic disease.
Acknowledgements
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of Interest
The authors report no conflicts of interest. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–92. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Women’s Health Gend Based Med. 1999;8(6):805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exer. 2000;32(suppl9):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Arikawa AY, Thomas W, Schmitz KH, Kurzer MS. Sixteen weeks of exercise reduces c-reactive protein levels in young women. Med Sci Sports Exer. 2011;43(6):1002–9. doi: 10.1249/MSS.0b013e3182059eda. [DOI] [PubMed] [Google Scholar]
- 5.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clinica Chimica Acta. 2010;411:785–93. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JF, Chiapa AL, Rodriguez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 8.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. JAGS. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 10.Geffken DF, Cushman M, Burke G, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 11.Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes. 2011;6(suppl 1):13–20. doi: 10.3109/17477166.2011.604326. Epub 2011/09/16. doi: 10.3109/17477166.2011.604326. [DOI] [PubMed] [Google Scholar]
- 12.Gondim OS, Nunes de Camargo VT, Gutierrez FA, et al. Benefits of regular exercise on inflammatory and cardiovascular risk markers in normal weight, overweight, and obese adults. PLOS One. 2015;10(10):e0140596. doi: 10.1371/journal.pone.0140596. doi: 10.1371/journal.pone.0140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–41. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 14.Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events: inflammatory and metabolic mechanisms. Med Sci Sports Exerc. 2009;41(6):1206–11. doi: 10.1249/MSS.0b013e3181971247. [DOI] [PubMed] [Google Scholar]
- 15.Hellgren MI, Larsson CA, Daka B, Petzold M. C-reactive protein concentrations and level of physical activity in men and women with normal and impaired glucose tolerance. A cross-sectional population-based study in Sweden. J Phys Act Health. 2016;13:625–31. doi: 10.1123/jpah.2015-0168. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(14):860–67. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 17.Kralova LI, Kralova A, Cejkova S, et al. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J Transl Med. 2016;14(1):208. doi: 10.1186/s12967-016-0962-1. Epub 2016/07/13. doi: 10.1186/s12967-016-0962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie ME, Rabasa-Lhoret R, Doucet E, et al. Association between physical activity energy expenditure and inflammatory markers in sedentary overweight and obese women. Int J Obesity. 2010;34:1387–95. doi: 10.1038/ijo.2010.55. [DOI] [PubMed] [Google Scholar]
- 19.Levery AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–27. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mijka DS, Chang RW, Vu THT, et al. Physical activity and high-sensitivity c-reactive protein: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2009;36(1):56–62. doi: 10.1016/j.amepre.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA study. Preventive Medicine. 2005;40:432–7. doi: 10.1016/j.ypmed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 23.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reducted plasma levels of obesity-related inflammatory markers. Obesity Research. 2003;11(9):1055–64. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 24.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18(5):884–9. doi: 10.1038/oby.2009.443. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- 25.Simpson KA, Singh MAF. Effects of exercise on adiponectin: a systemic review. Obesity (Silver Spring) 2008;16:241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 26.Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–18. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]