Abstract
Higher red cell distribution width (RDW) has been associated with poor prognosis in patients with heart failure (HF). RDW is also closely associated with iron deficiency. However, the mechanism underlying this association is unclear. The relationship between left ventricular end-diastolic pressure (LVEDP) and RDW has not been studied, especially in those without HF. We examined the relationship between LVEDP and RDW in 1,084 consecutive stable patients who underwent elective coronary angiography. We observed that 38% had high LVEDP (>16mmHg) and 29% had history of HF. The median RDW was 13.4%, which was higher with increasing LVEDP (p<0.0001) and significantly higher among patients with HF (p<0.0001). Baseline RDW were independently associated with high LVEDP even after multivariable logistic regression analysis (adjusted odds ratio [OR] per unit change: 1.14, 95% CI: 1.0–1.29, p=0.044). Interestingly, result were stronger in non-HF cohort (adjusted OR per unit change: 1.37, 95% CI: 1.13–1.67, p=0.001). In addition, elevated (third versus first tertiles) RDW levels were independently a predictor of high LVEDP and were associated with a 4.8-fold increased 5-year mortality risk (adjusted hazard ratio: 4.11, 95% CI: 2.12–7.96, p<0.0001), even with the addition of B-type natriuretic peptide to the model (adjusted OR for LVEDP: 2.25, 95% CI: 1.0–5.05, p=0.05; adjusted HR for mortality: 3.79, 95% CI: 1.033–13.89, p=0.044, respectively). In conclusion, high RDW levels were observed in patients with or without HF and independently associated with high LVEDP and with mortality.
Keywords: Heart Failure, Left Ventricular Diastolic Dysfunction, Prognosis, red cell distribution width, Iron Deficiency
Previous studies have reported the strong association of elevated red cell distribution width (RDW) levels and adverse prognosis in patients with chronic1 and acute decompensated heart failure (HF)2,3. Interestingly, this association was independent of hemoglobin (HGB) and the presence of anemia. However, the mechanism underlying of the association between elevated RDW levels and adverse prognosis in patients with HF is unclear. Increased RDW indicates variability in the size of circulating erythrocytes (anisocytosis) and may be helpful in the differential diagnosis of anemia4. Classically RDW is increased in iron deficiency5. Iron plays a key role in oxygen metabolism, energy production and oxidative metabolism in both skeletal myocytes and cardiomyocytes6. Experimental animal models have shown that iron deficiency results in abnormalities in myocardial function, mitochondrial dysfunction, myocardial remodeling and can cause left ventricular (LV) diastolic dysfunction7,8. Because the above mechanisms may impact RDW, it is possible that the relationship of iron deficiency and LV diastolic dysfunction and clinical outcomes are reflected by RDW. However, the relationship between RDW and elevated LV end-diastolic pressure (LVEDP) measurements, reflecting the degree of ventricular stiffness and diastolic dysfunction, has not yet been carefully explored. Herein, we aimed to examine the relationship between RDW and LVEDP and long-term clinical prognosis in stable patients who underwent elective diagnosis coronary angiography, with or without a history of HF.
METHODS
This is a single-center prospective cohort study. We included 1,084 adult patients (ages ≥ 18 years) who underwent elective coronary angiography at our tertiary care center with available completed data of invasive catheter-derived LVEDP and documented RDW measured by a central core research laboratory. The first data set (n=869) included patients recruited between 2001 and 2004. All-cause mortality was prospectively tracked for 5 years as part of a standardized protocol that includes telephone/postcard contact, manual chart review, and confirmation by Social Security Death Index until 2011. A second data set (n=215) included patients recruited between 2012 and 2014. We excluded patients who had an acute coronary syndrome, revascularization within 30 days before enrollment, or history of heart transplantation. HF was defined as a documented history of HF by treating board-certified cardiology staff at the Cleveland Clinic in the electronic medical record and confirmed by trained research personnel at the time of enrollment. Fasting plasma samples were collected at the time of cardiac catheterization after arterial sheath placement, but before the catheterization procedure or any drug administration. All participants gave written informed consent and the study was approved by the Cleveland Clinic Institutional Review Board.
We reviewed cardiac catheterization reports from the electronic medical record and Syngo Dynamics cardiovascular imaging software (Siemens medical solutions, USA) for collected LVEDP data. The LVEDP was performed during left-sided heart catheterization procedures with 4–6 French pigtail catheter inserted into the distal LV apex via the femoral, brachial or radial approach. LVEDP was recorded during coronary angiography procedure and before intervention procedure (if applicable).
Hematologic analyses were performed with an ADVIA 120 hematology analyzer (Siemens, New York, NY). All hematology measurements were generated automatically by the analyzer during routine performance of complete blood count as previously described9. Anemia was defined according to World Health Organization criteria: HGB <13 g/dL for men or <12 g/dL for women. Routine laboratory tests were performed and samples measured on the Abbott Architect platform (Abbott Laboratories, Abbott Park, Illinois). An estimated glomerular filtration rate (eGFR, mL/min/1.73m2) was calculated using the Modification of Diet in Renal Disease study equation10. LV ejection fraction (LVEF) was determined via comprehensive transthoracic echocardiography by the Cleveland Clinic Echocardiography Laboratory, reviewed by board-certified cardiologists, and collected by reviewing electronic medical records and Syngo Dynamics cardiovascular imaging software (Siemens medical solutions, USA). Continuous data are presented as mean ± standard deviation or median (interquartile range [IQR]).
Comparisons among three or more groups were evaluated by one way analysis of variance or the Kruskal-Wallis test according to whether or not the distribution was normal. Categorical variables are presented as number (%) and compared between groups with chi-square tests. All variables were tested for normal distribution using the Kolmogorov-Smirnov test. Spearman’s rank correlation was used as non-parametric measure of association between RDW and other variables of interest. On the basis of previous studies, a LVEDP>16 mmHg was chosen as a cutoff value for definitive objective evidence of increased LV filling pressure and considered as providing definite evidence of LV diastolic dysfunction11. Univariate and multivariate logistic regression analysis was used to determine independent predictors of high LVEDP according to RDW as a continuous variable, as well as according to tertiles with respect to the 1st tertiles (odds ratios=1). Kaplan-Meier analysis with Cox proportional hazard regression were used to determine hazard ratios (HR) and 95% confidence interval (95% CI) for 5-year all-cause mortality stratified according to RDW tertiles. Variables entered into the multivariable model as potential confounders of the relationship of RDW to study outcomes were based on individual traditional risk variables (including age, gender, systolic blood pressure [BP], low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and diabetes mellitus), eGFR, LVEF, as well as hematologic indices that have previously shown to be a potential prognosis in heart failure12,13 (including HGB, mean corpuscular hemoglobin concentration [MCHC] and anemic status) to predict both high LVEDP and all-cause mortality risks. In the subset (n=326), B-type natriuretic peptide (BNP) levels were an additional covariate. All analyses were performed using JMP Pro version 10 (SAS Institute, Cary, North Carolina). A p value<0.05 was considered statistically significant.
Results
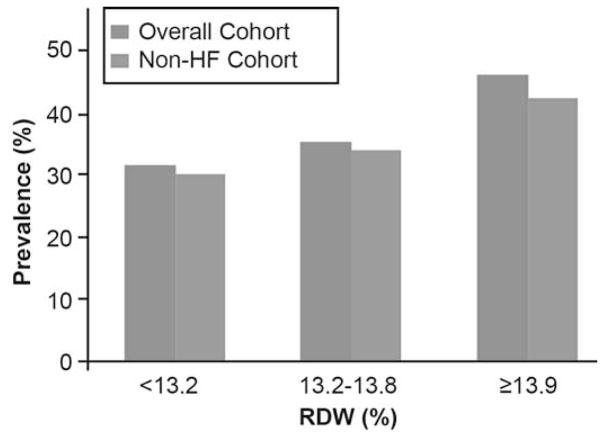
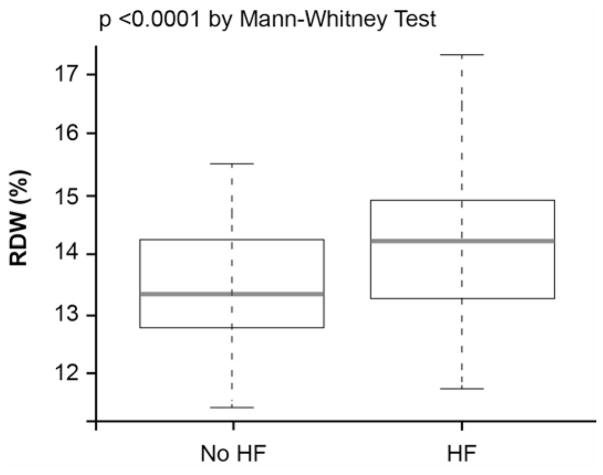
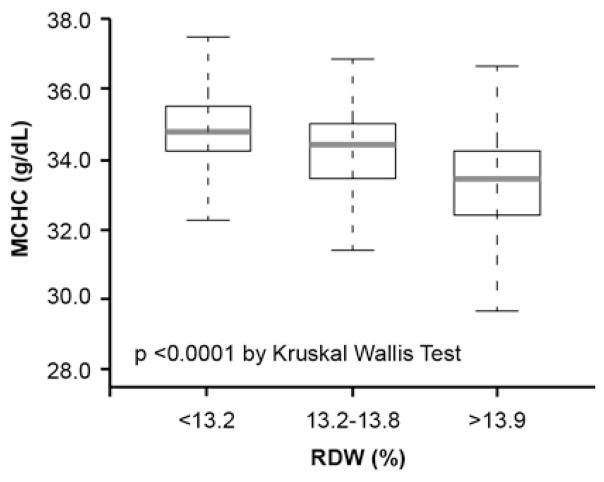
The baseline characteristics of the overall 1,084 patients are shown in Table 1. Overall, the cohort is representative of stable patients who underwent elective coronary angiography: 290 (29%) had history of chronic HF, 620 (58%) had history of coronary artery disease (CAD) and 370 (34%) had anemia. A higher RDW level was associated with a history of diabetes mellitus, HF, anemia and a higher percentage of patients with LVEF≤40%. The prevalence of high LVEDP was 37.6% (408/1,084) in the overall cohort and 34.8% (276/794) in the non-HF cohort. The prevalence of high LVEDP progressively increased with increasing RDW tertiles even in the non-HF cohort (Figure 1). The median RDW was 13.4% (IQR 13.0%–14.2%), which was higher among patients with HF than without HF (13.9% [13.3%–14.9%] versus 13.4% [12.8%–13.9%], p<0.0001) (Figure 2A). The RDW levels were significantly inversed correlated with MCHC (r=−0.5, p<0.0001) (Table 2), and the MCHC levels were significantly lower with increasing RDW tertiles (Figure 4B).
Table 1.
Baseline Characteristics
| Red Cell Distribution Width Tertiles
|
|||||
|---|---|---|---|---|---|
| Overall (n=1,084) | (<13.2%) (n=369) | (13.2–13.8%) (n=349) | (≥13.9%) (n=366) | p Value | |
| Age, (year) | 63±10 | 62±9 | 63±10 | 64±11 | <0.001 |
|
| |||||
| Men | 65% | 69% | 65% | 60% | 0.044 |
|
| |||||
| Body-mass index, (kg/m2) | 29.2 (26–32.7) | 28.1 (25.4–31.2) | 29.2 (26.3–32.6) | 30.1 (26.7–34.6) | <0.001 |
|
| |||||
| Systolic blood pressure, (mmHg) | 131 (118–146) | 130 (121–143) | 131 (119–147) | 131 (115–146) | 0.75 |
|
| |||||
| History of heart failure | 29% | 16% | 27% | 44% | <0.0001 |
|
| |||||
| History of coronary artery disease | 58% | 57% | 54% | 63% | 0.033 |
|
| |||||
| Anemia | 34% | 23% | 30% | 48% | <0.001 |
|
| |||||
| Diabetes mellitus | 26% | 18% | 23% | 36% | <0.001 |
|
| |||||
| Hypertension | 76% | 67% | 75% | 85% | <0.001 |
|
| |||||
| Low-density lipoprotein cholesterol, (mg/dl) | 88 (68–111) | 89.5 (70–113) | 92 (72–117) | 80 (61–102) | <0.001 |
|
| |||||
| High-density lipoprotein cholesterol, (mg/dl) | 48 (40–60) | 49 (41–60.2) | 49 (41–61) | 47 (38–58) | <0.001 |
|
| |||||
| Estimated glomerular filtration rate, (ml/min/1.73 m2) | 91.7 (70.7–115.2) | 95 (72.6–116) | 97 (75.5–123.7) | 83.3 (65.1–107.3) | <0.001 |
|
| |||||
| Left ventricular end-diastolic pressure, (mmHg) | 15 (11–20) | 14 (10–18) | 15 (11–19) | 16 (12–21) | <0.001 |
|
| |||||
| Left ventricular ejection fraction | 55% (45–60) | 55% (46–60) | 55% (50–60) | 55% (45–60) | 0.11 |
|
| |||||
| Left ventricular ejection fraction ≤ 40% | 19% | 16% | 17% | 23% | 0.04 |
|
| |||||
| Medication: | |||||
| Aspirin Angiotensin-converting enzyme inhibitors or angiotensin receptor Statin blocker Beta-blocker | 68% | 77% | 69% | 60% | <0.001 |
| 47% | 47% | 43% | 52% | 0.06 | |
| 65% | 64% | 67% | 63% | 0.47 | |
| 61% | 60% | 59% | 64% | 0.29 | |
|
| |||||
| Hemoglobin, (g/dL) | 13.1 (12.1–14.2) | 13.6 (12.5–14.5) | 13.1 (12.1–14.2) | 12.6 (11.6–13.8) | <0.001 |
|
| |||||
| Mean corpuscular hemoglobin concentration, (g/dL) | 34.2 (33.3–35.1) | 34.8 (34.2–35.5) | 34.4 (33.6–35.1) | 33.5 (32.4–34.2) | <0.001 |
|
| |||||
| Red cell distribution width | 13.4% (13.0–14.2) | 12.7% (12.5–12.9) | 13.4% (13.2–13.6) | 14.6% (14.2–15.4) | <0.001 |
|
| |||||
| B-type natriuretic peptide, (pg/mL) | 104 (40–252) | 63 (50–268) | 122 (50–27) | 250 (103–485) | <0.0001 |
Figure 1.
- Chi-Square, p<0.0001 in the Overall Cohort (n=1,084)
- Chi-Square, p=0.018 in the Non-HF Cohort (n=794)
Figure 2.
Comparison of Red Cell Distribution Width (RDW) Levels Between Patients With and Without History of Heart Failure (HF) (A) and the Relationship Between RDW Levels and Left Ventricular End-Diastolic Pressure in the Overall Cohort (B)
Table 2.
Univariate Correlation between Red Cell Distribution Width Levels, Left Ventricular End-Diastolic Pressure and Laboratory for the Overall Cohort and the Non-Heart Failure Cohort
| Variable | Overall Cohort (n=1,084) | Non-Heart Failure Cohort (n=794) | ||
|---|---|---|---|---|
| Spearman Correlation | p Value | Spearman Correlation | p Value | |
| Left ventricular end- diastolic pressure | 0.16 | <0.0001 | 0.15 | <0.0001 |
| Left ventricular ejection fraction | −0.07 | 0.032 | 0.02 | 0.63 |
| Hemoglobin | −0.26 | <0.0001 | −0.2 | <0.0001 |
| Mean corpuscular hemoglobin concentration | −0.5 | <0.0001 | −0.48 | <0.0001 |
| Mean corpuscular volume | −0.1 | 0.001 | −0.13 | 0.08 |
| Estimated glomerular filtration rate | −0.13 | <0.0001 | 0.3 | <0.0001 |
| B-type natriuretic peptide | 0.38 | <0.0001 | 0.3 | <0.0001 |
Figure 4.
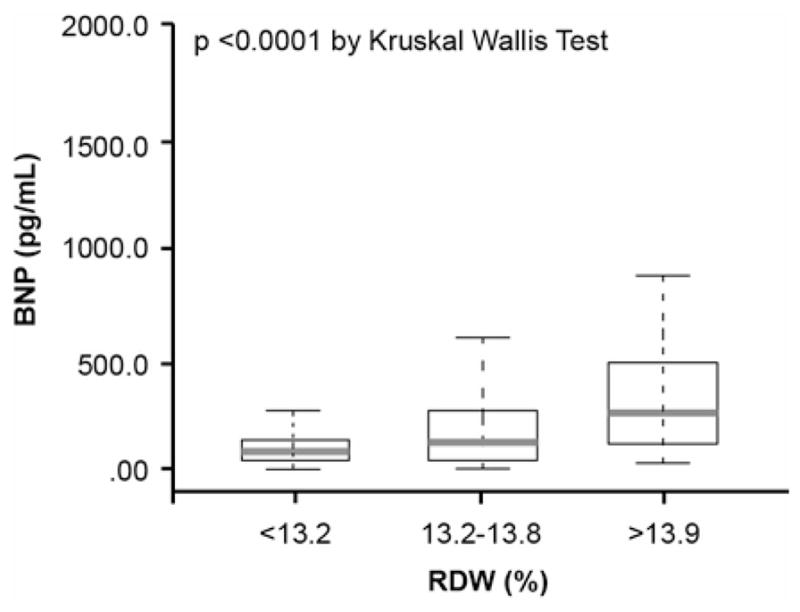
Relationship between B-type Natriuretic Peptide (BNP) (A), Mean Corpuscular Hemoglobin Concentration (MCHC) (B) and Red Cell Distribution Width (RDW) Levels in the Overall Cohort
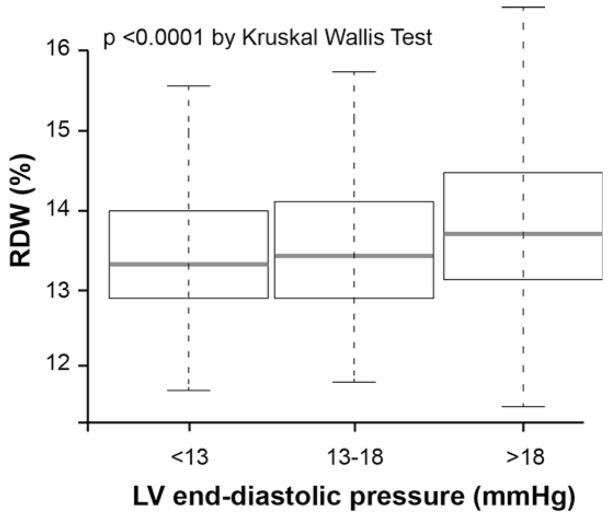
The relationship between RDW levels with LVEDP, laboratory and echocardiographic parameters are presented in Table 2. The RDW levels were modest but significantly correlated with LVEDP (r=0.16, p<0.0001), and significantly higher with increasing LVEDP (p<0.0001) (Figure 2B). In univariate logistic regression analysis, the RDW showed a significant association with high LVEDP (LVEDP>16mmHg), as well as HGB and anemia, but not MCHC (Table 3). In multivariate logistic regression analysis with all variables, RDW remained a significant predictor of high LVEDP. Interestingly, although HGB, anemic status and MCHC that previously were shown to be a potential prognosis in HF12,13, they were no longer significant predictors of high LVEDP after adjustment with the multivariable model (Table 3). In further examination of this relationship in the non-HF cohort (n=794), the correlation with RDW remained significantly particular for LVEDP (p<0.0001) but no longer associated in LVEF (p=0.63) (Table 2), and RDW levels were significantly higher with increasing LVEDP (p=0.002). In univariate logistic regression analysis, the RDW showed a significant association with high LVEDP. In multivariate logistic regression analysis with all variables, RDW remained a significant predictor of high LVEDP (Table 3).
Table 3.
Univariate and Multivariate Logistic Regression for Independent Prediction of High Left Ventricular End-Diastolic Pressure (>16 mmHg)
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | OR, 95% CI | p Value | OR, 95% CI | p Value |
| Overall Cohort (n=1,084) | ||||
|
| ||||
| Red cell distribution width | 1.18 (1.08–1.3) | 0.001 | 1.14 (1.00–1.29) | 0.044 |
|
| ||||
| Mean corpuscular hemoglobin concentration | 0.93 (0.85–1.02) | 0.11 | 1.03 (0.91–1.16) | 0.62 |
|
| ||||
| Hemoglobin | 0.91 (0.84–0.99) | 0.022 | 1.06 (0.92–1.23) | 0.43 |
|
| ||||
| Anemia | 1.43 (1.11–1.85) | 0.006 | 1.47 (0.95–2.26) | 0.08 |
|
| ||||
| Age | 1.00 (0.99–1.01) | 0.94 | 1.00 (0.98–1.02) | 0.82 |
|
| ||||
| Men | 0.82 (0.63–1.06) | 0.13 | 0.65 (0.45–0.95) | 0.028 |
|
| ||||
| Systolic blood pressure | 1.00 (1.0–1.01) | 0.42 | 1.00 (1.0–1.01) | 0.21 |
|
| ||||
| Diabetes mellitus | 1.7 (1.28–2.25) | <0.0001 | 1.36 (0.98–1.88) | 0.064 |
|
| ||||
| High–density lipoprotein cholesterol | 1.0 (0.99–1.01) | 0.61 | 1.00 (0.99–1.01) | 0.99 |
|
| ||||
| Low–density lipoprotein cholesterol | 1.0 (0.99–1.00) | 0.33 | 1.00 (0.99–1.04) | 0.85 |
|
| ||||
| Estimated glomerular filtration rate | 1.00 (1.0–1.03) | 0.85 | 1.00 (1.0–1.01) | 0.43 |
|
| ||||
| Left ventricular ejection fraction | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | 0.002 |
|
| ||||
| Non-Heart Failure Cohort (n=794) | ||||
|
| ||||
| Red cell distribution width | 1.24 (1.08–1.43) | 0.002 | 1.37 (1.13–1.67) | 0.001 |
|
| ||||
| Mean corpuscular hemoglobin concentration | 0.96 (0.86–1.07) | 0.46 | 1.09 (0.94–1.28) | 0.24 |
|
| ||||
| Hemoglobin | 0.92 (0.84–1.02) | 0.09 | 1.0 (0.83–1.21) | 0.98 |
|
| ||||
| Anemia | 1.26 (0.92–1.72) | 0.15 | 1.19 (0.7–2.01) | 0.52 |
|
| ||||
| Age | 0.99 (0.98–1.01) | 0.46 | 0.99 (0.97–1.01) | 0.31 |
|
| ||||
| Men | 0.86 (0.64–1.16) | 0.33 | 0.79 (0.49–1.26) | 0.32 |
|
| ||||
| Systolic blood pressure | 1.00 (0.99–1.01) | 0.35 | 1.01 (0.99–1.01) | 0.20 |
|
| ||||
| Diabetes mellitus | 1.38 (0.98–1.95) | 0.07 | 1.22 (0.81–1.84) | 0.35 |
|
| ||||
| High-density lipoprotein cholesterol | 1.0 (0.99–1.01) | 0.74 | 1.00 (0.99–1.02) | 0.51 |
|
| ||||
| Low-density lipoprotein cholesterol | 1.0 (0.99–1.00) | 0.85 | 1.00 (0.99–1.01) | 0.95 |
|
| ||||
| Estimated glomerular filtration rate | 0.99 (0.99–1.00) | 0.70 | 0.99 (0.99–1.01) | 0.68 |
|
| ||||
| Left ventricular ejection fraction | 0.99 (0.98–1.01) | 0.39 | 0.99 (0.98–1.01) | 0.63 |
We further examined the ability of RDW to predict high LVEDP from multivariate logistic regression analysis across 1st, 2nd and 3rd tertiles of RDW levels. Importantly, elevated RDW levels (tertiles 3 versus 1) were a significantly predictor of high LVEDP (Table 4). Interestingly, after multivariable adjustment for traditional risk variables, elevated RDW levels remained a significant predictor of high LVEDP.
Table 4.
Odds Ratios (OR) for the Prediction of High Left Ventricular End-Diastolic Pressure (>16 mmHg) and Cox Proportional Hazards Ratio (HR) for Predicting Risk of 5-Year All-Cause Death, Third versus First Red Cell Distribution Width Tertiles in the Overall Cohort (n=1,084)
| High Left Ventricular End-Diastolic Pressure | 5-Year All-Cause Death | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | HR (95% CI) | p Value | |
| Overall Cohort (n=1,084) | ||||
| Unadjusted | 1.82 (1.35–2.46) | <0.0001 | 4.8 (2.83–7.98) | <0.0001 |
| Adjusted model 1 | 1.88 (1.28–2.77) | 0.0014 | 4.11 (2.12–7.96) | <0.0001 |
| Adjusted model 2 | 2.25 (1.0–5.06) | 0.05 | 3.79 (1.03–13.89) | 0.044 |
Adjusted model 1: adjusted for traditional risk factors (age, sex, systolic blood pressure , low-density lipoprotein, high-density lipoprotein cholesterol and diabetes mellitus), estimated glomerular filtration rate, left ventricular ejection fraction and hematologic indices (including hemoglobin, mean corpuscular hemoglobin concentration and anemic status).
Adjusted model 2: Model 1 + B-type natriuretic peptide (n=326).
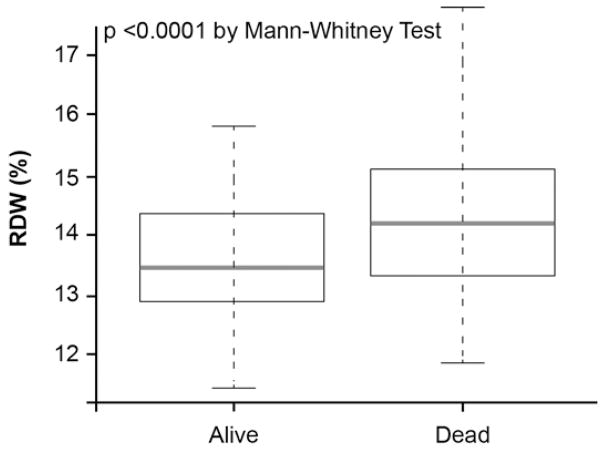
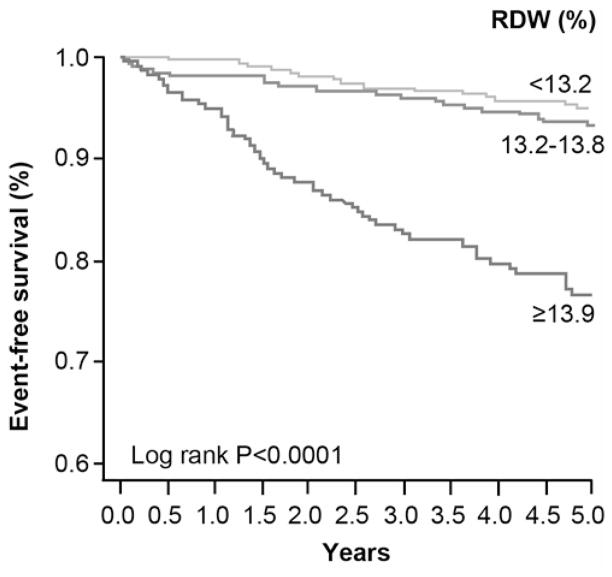
Over the 5-year follow-up, 97 deaths occurred in our study cohort. Ten patients were lost to follow-up, and 215 patients were not reached at 5-year follow-up because of varying dates of enrollment. The death rate was significantly higher among those with higher baseline RDW levels (p<0.0001) (Figure 3A). As illustrated in the Kaplan-Meier analyses show in Figure 3B, a graded increase in risk of death was observed with increasing RDW levels stratified by tertiles, with log-rank, p<0.0001. Elevated RDW levels were associated with a 4.8-fold increase in risk of 5-year all-cause death. Importantly, after multivariable adjustment as described previously, elevated RDW levels remained independently associated with an increased risk of death (Table 4).
Figure 3.
Baseline Red Cell Distribution Width (RDW) Levels between Alive and Dead Patients at 5-Year Follow-Up (A) Kaplan-Meier Estimates for 5-Year All-Cause Death with RDW Levels Stratified as Tertiles in the Overall Cohort (B)
We also found a significantly correlation between RDW and BNP levels (r=0.38, p<0.0001) and modest but significant inverse correlation with eGFR (r=−0.13, p<0.0001) (Table 2). BNP levels were significantly higher with increasing RDW tertiles (p<0.0001) (Figure 4A). We further examined the association between RDW and cardiorenal indexes by added BNP to the multivariable adjustment as described previously. In this model, elevated RDW levels were still independently associated with increased risk of 5-year all-cause death and a trend to significant predictor of high LVEDP (Table 4).
DISCUSSION
The key findings of the present study cohort are the following. First, the prevalence of high LVEDP was 37.6% in the overall cohort and 34.8% in the non-HF cohort. Thus, an invasive LVEDP measurement to a large cohort of stable cardiology patients allowed identification of a large number of individuals with subclinical high LVEDP. Second, RDW levels were independent predictor of high LVEDP after multivariable adjustment for other prognosis factors including HGB and anemic status. Furthermore, HGB and anemic status were no longer associated with high LVEDP when RDW were added to the multivariable logistic regression model. Third, elevated RDW levels were significant predictor of high LVEDP and portend poorer long-term mortality even when adjusted for traditional risk factors, LVEF, other hematologic indices (HGB, anemic status, and MCHC) and cardiorenal indexes (eGFR and BNP).
Our results are consistent with recent studies on the predictive role of elevated RDW levels and mortality risk in patients with HF1–3, in patients with CAD14 and in an unselected population referred for coronary angiography15. The relationship between RDW and echocardiographic parameters of diastolic dysfunction has been reported16. However, the association between RDW and direct invasive LVEDP measurement, which is the most reliable evidence of increased LV filling pressure and the main physiologic consequence of LV diastolic dysfunction11,17, has not been well explored. Thus, this study demonstrated for the first time that elevated RDW levels can be used to predict high LVEDP. Importantly, RDW was strongly correlated with BNP (r=0.38, p<0.0001), which is a well-established correlate with LV filling pressure. Moreover, elevated LV filling pressure is a strong predictor of poor prognosis and sudden death regardless of LVEF18. Therefore, our findings provide a potential underlying mechanism of elevated RDW levels and portend poor prognosis in patients with HF.
The mechanistic link between elevated RDW levels and high LVEDP in our study might be explained by iron deficiency since several studies have demonstrated the consistent association between elevated RDW levels and iron deficiency. Moreover, previous studies have report that elevated RDW closely associated with lower serum iron and ferritin levels, impaired iron mobilization, and increased serum soluble transferrin receptor in patients with HF19,20. In addition, a change of RDW levels after initiation of iron therapy can be used to distinguish iron deficiency from other hypochromic anemias21. MCHC is one of the several hematologic indices in detecting iron deficiency22. Okonko et al demonstrated that low MCHC is an attenuated functional iron status23. Simbaqueba et al recently reported that relative hypochromia (as reflected by low MCHC levels) portends a poor prognosis in patients with chronic HF with reduced ejection fraction (HFrEF)13. Interestingly, our study showed a strongly significant inversed correlation between RDW and MCHC (r=−0.5, p<0.0001). Taken together, since both RDW and MCHC were closely associated with underlying iron deficiency and portend poor prognosis, elevated RDW levels in this study might be explained by ineffective red cell production or immaturity of erythrocytes in the underlying iron deficiency.
Importantly, iron deficiency can lead to LV diastolic dysfunction since iron plays a key role in oxygen metabolism and energy production in cardiomyocytes6 and is associated with reduced cardiac energetic reserve24. Recently, a large study showed that the presence of iron deficiency portends poor prognosis in patients with HF, independent of the presence of anemia23,25. Furthermore, administration of intravenous iron has been associated with improved symptoms, functional capacity and quality-of-life, regardless of anemic status26. Therefore, the strong relationship between elevated RDW levels and LV diastolic dysfunction, which was defined by high LVEDP might be explained by the pathophysiological underlying iron deficiency although, HF is associated with inflammation and activation of pro-inflammatory cytokines in the early and late stages of HF27. However, the consideration of inflammatory or oxidative markers is less attractive, since previous studies showed the inconsistent association between RDW and inflammatory cytokines in patients with HF2,3,13,19.
Despite the different cardiac phenotypes in HFrEF versus HF with preserved ejection fraction (HFpEF), elevated LV filling pressure is their underlying mechanism17,28. Interestingly, our study demonstrated an independent association between elevated RDW levels and high LVEDP in a mixed patient population who underwent elective coronary angiography. Therefore, our findings provide the mechanistic link between elevated RDW levels and high LVEDP in patients with HF, which is generalized to both HFrEF and HFpEF. Another interesting findings in the present study is one-third (34.8%) of patients in the non-HF cohort had evidence of high LVEDP, and RDW remained an independent predictor of high LVEDP. In particular, elevated RDW levels in stable cardiology patients may help in early identification of patient with subclinical high LVEDP or “silent HF”, and may identify a subset of patients who may have underlying iron deficiency. Importantly, according to the lack of proven therapy in HFpEF, the role iron deficiency and LV diastolic dysfunction in HFpEF remains unclear. Further study is needed to determine whether improved RDW with interventions such as iron therapy can improve LV diastolic dysfunction and prognosis in patients with HF (both HFrEF and HFpEF) or even in stable cardiology patients.
Our study was in a single tertiary care center that recruited patients at the point of cardiac catheterization; therefore we cannot exclude selection bias for patients undergoing elective coronary angiography. The lack of echocardiographic parameters regarding diastolic dysfunction and our cohort is relatively representative of a mixed patient population with and without history of HF. However, we using direct invasive measurement of LVEDP, which is the gold standard for established evidence of elevated LV filling pressure in patients with HF. We also did not have information regarding blood iron studies to established diagnosis of iron deficiency. Nevertheless, the consistent correlation between iron deficiency and RDW in prior studies and the strong association between RDW and MCHC in our study cohort can integrate strong indirect evidence of iron deficiency. Because RDW were measured at only a single time-point, we were unable to determine the prognostic value and impact of LV dysfunction of changing RDW. The relatively low mortality rate (n=97) may have limited our multivariate analysis.
Acknowledgments
Funding This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements: P01HL076491, P01HL098055, R01HL103931, R01DK106000, R01HL103866, P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr. Hazen is also partially supported by a gift from the Leonard Krieger endowment and by the Foundation LeDucq.
Footnotes
Disclosures Dr. Hazen is named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: Abbott Laboratories, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., P&G, and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., P&G, Pfizer Inc., and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott Laboratories, Inc., Cleveland Heart Lab., Siemens, Esperion, Frantz Biomarkers, LLC, Liposcience Inc. and P&G. All other authors have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 2.van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL., Jr Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail. 2010;12:129–136. doi: 10.1093/eurjhf/hfp179. [DOI] [PubMed] [Google Scholar]
- 3.Pascual-Figal DA, Bonaque JC, Redondo B, Caro C, Manzano-Fernandez S, Sanchez-Mas J, Garrido IP, Valdes M. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11:840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- 4.Romero Artaza J, Carbia CD, Ceballo MF, Diaz NB. Red cell distribution width (RDW): its use in the characterization of microcytic and hypochromic anemias. Medicina (B Aires) 1999;59:17–22. [PubMed] [Google Scholar]
- 5.van Zeben D, Bieger R, van Wermeskerken RK, Castel A, Hermans J. Evaluation of microcytosis using serum ferritin and red blood cell distribution width. Eur J Haematol. 1990;44:106–109. doi: 10.1111/j.1600-0609.1990.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 6.Cairo G, Bernuzzi F, Recalcati S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006;1:25–39. doi: 10.1007/BF02829934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong F, Zhang X, Culver B, Chew HG, Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 2005;109:277–286. doi: 10.1042/CS20040278. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Tsujino T, Matsumoto M, Sakoda T, Ohyanagi M, Masuyama T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol. 2009;296:H585–593. doi: 10.1152/ajpheart.00463.2008. [DOI] [PubMed] [Google Scholar]
- 9.Brennan ML, Reddy A, Tang WH, Wu Y, Brennan DM, Hsu A, Mann SA, Hammer PL, Hazen SL. Comprehensive peroxidase-based hematologic profiling for the prediction of 1-year myocardial infarction and death. Circulation. 2010;122:70–79. doi: 10.1161/CIRCULATIONAHA.109.881581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 12.O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 13.Simbaqueba C, Shrestha K, Patarroyo M, Troughton RW, Borowski AG, Klein AL, Tang WH. Prognostic implications of relative hypochromia in ambulatory patients with chronic systolic heart failure. Congest Heart Fail. 2013;19:180–185. doi: 10.1111/chf.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 15.Cavusoglu E, Chopra V, Gupta A, Battala VR, Poludasu S, Eng C, Marmur JD. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010;141:141–146. doi: 10.1016/j.ijcard.2008.11.187. [DOI] [PubMed] [Google Scholar]
- 16.Oh J, Kang SM, Hong N, Choi JW, Lee SH, Park S, Shin MJ, Jang Y, Chung N. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J Card Fail. 2009;15:517–522. doi: 10.1016/j.cardfail.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC. The treatment targets in acute decompensated heart failure. Rev Cardiovasc Med. 2001;2(Suppl 2):S7–S12. [PubMed] [Google Scholar]
- 19.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF., Jr Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akarsu S, Taskin E, Yilmaz E, Yilmaz H, Kilic M, Aygun AD. Treatment of iron deficiency anemia with intravenous iron preparations. Acta Haematol. 2006;116:51–57. doi: 10.1159/000092348. [DOI] [PubMed] [Google Scholar]
- 22.Bovy C, Gothot A, Delanaye P, Warling X, Krzesinski JM, Beguin Y. Mature erythrocyte parameters as new markers of functional iron deficiency in haemodialysis: sensitivity and specificity. Nephrol Dial Transplant. 2007;22:1156–1162. doi: 10.1093/ndt/gfl765. [DOI] [PubMed] [Google Scholar]
- 23.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 27.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:723–727. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 28.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]