Abstract
Background
There is a growing interest in the intersection of heart failure (HF) and frailty; however, estimates of the prevalence of frailty in HF vary widely. The purpose of this paper was to quantitatively synthesize published literature on the prevalence of frailty in HF and to examine the relationship between study characteristics (i.e. age and functional class) and the prevalence of frailty in HF.
Methods
The prevalence of frailty in HF, divided into Physical Frailty and Multidimensional Frailty measures, was synthesized across published studies using a random-effects meta-analysis of proportions approach. Meta-regression was performed to examine the influence of age and functional class (at the level of the study) on the prevalence of frailty.
Results
A total of 26 studies involving 6896 patients with HF were included in this meta-analysis. Despite considerable differences across studies, the overall estimated prevalence of frailty in HF was 44.5% (95% Confidence Interval, 36.2%–52.8%; z = 10.54; p < 0.001). The prevalence was slightly lower among studies using Physical Frailty measures (42.9%, z = 9.05; p < 0.001) and slightly higher among studies using Multidimensional Frailty measures (47.4%, z = 5.66; p < 0.001). There were no significant relationships between study age or functional class and prevalence of frailty.
Conclusions
Frailty affects almost half of patients with HF and is not necessarily a function of age or functional classification. Future work should focus on standardizing the measurement of frailty and on broadening the view of frailty beyond a strictly geriatric syndrome in HF.
Keywords: Heart Failure, Meta-Analysis, Aging, Frailty
1. Introduction
In recent years, frailty, often defined as “a biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes,” (1) (p. M146) has emerged as a significant area of research in heart failure (HF). Given the value of frailty in predicting worse clinical- and patient-oriented outcomes among older adults in general (1) and adults with HF in particular (2–7), there is now a substantial, worldwide literature base on frailty in HF. Indeed, HF is associated with accelerated biological aging (8) and, as a result, geriatric syndromes like frailty (9) are more likely to present irrespective of chronological age. Additionally, recent scientific statements have recommended a formal frailty assessment as a critical element in determining the care of adults with advanced HF (10), those being listed for heart transplant (11), and those in skilled nursing facilities (12).
A number of published studies on frailty in HF and several systematic reviews have provided insight into the overlap between frailty and HF, including proposed pathogenic mechanisms and recommended interventions to prevent or ameliorate frailty (9, 13–16). The overall prevalence and knowledge of factors that influence frailty in HF, however, are reported with considerable inconsistency across studies and have not been effectively synthesized through prior narrative reviews. The purpose of this meta-analysis was to quantitatively synthesize published literature on the prevalence of frailty in HF. In an effort to extend the perspective of frailty in HF beyond a strictly geriatric syndrome, we also examined the relationship between study characteristics (i.e. age and functional class of the sample) and prevalence of frailty in HF using meta-regression.
2. Methods
2.1 Data Sources and Study Eligibility
This study was a meta-analysis of published data-based studies on frailty in HF. Studies were considered eligible for inclusion if they met the following criteria: 1) sample or subsample consisted of HF patients, and 2) the prevalence (i.e. n or rate with denominator) of frailty in the sample or subsample of HF patients was available using any form of frailty assessment or portion of an assessment (e.g. gait speed or grip strength). Both observational and interventional studies (baseline data) were considered for inclusion. Non-English studies were excluded. We searched PubMed and CINAHL up until July 15, 2016 using the MeSH heading heart failure and either the keyword frail* or MeSH heading “Frail Elderly” or MeSH heading “Geriatric Assessment.” The term “geriatric assessment” was used because studies that include a frailty assessment are often categorized as a geriatric assessment. Abstracts were reviewed for the above criteria and reference lists were hand-searched for additional relevant studies not identified in the search engines. To minimize publication bias, abstracts were screened and known experts in the area of frailty in HF were approached at national meetings to identify potential works-in-progress. Full search strategies are presented within the PRISMA (17) flow diagram (Figure 1). Study screening and evaluation for eligibility for this meta-analysis was performed and validated by two members of the research team (Q.E.D. and C.S.L.). Reference list of excluded studies available upon request.
Figure 1. PRISMA Flow Diagram.
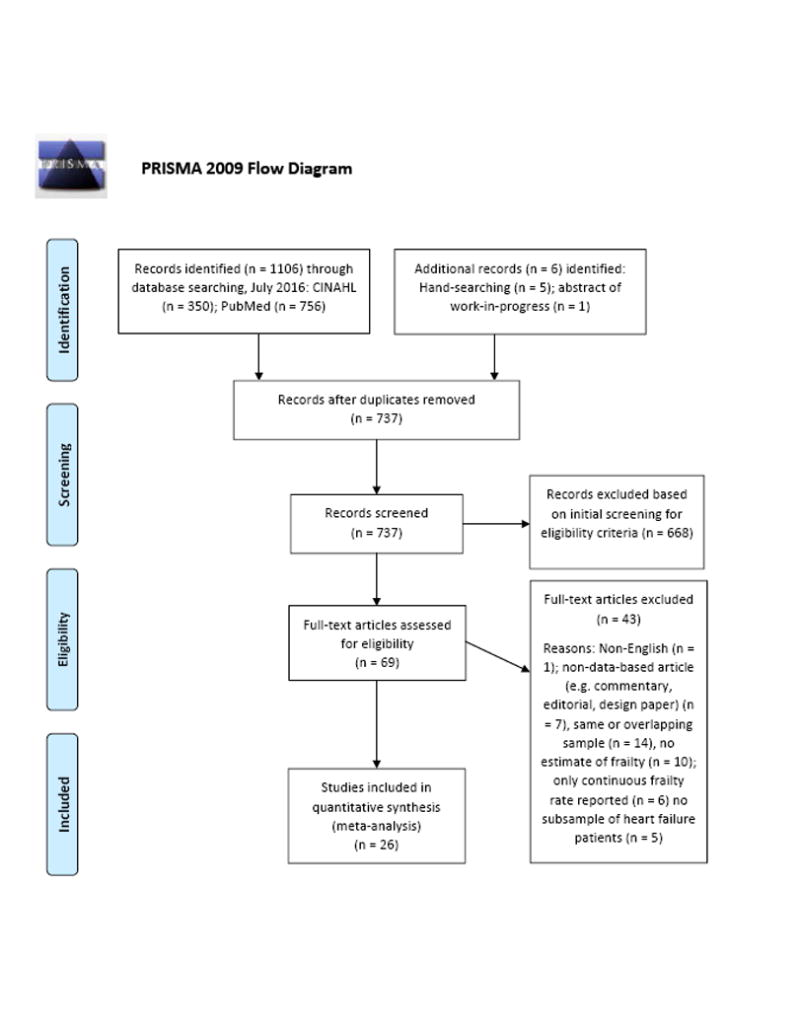
PRISMA flow diagram showing study identification, selection, eligibility, and inclusion. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097 For more information, visit www.prisma-statement.org.
2.2 Data Extraction
Data were extracted for the following variables: 1) study first author, 2) year and country of publication, 3) number of HF patients in sample or subsample, 4) description of frailty measure, 5) prevalence of frailty in sample, 6) mean or median age of sample, and 7) proportion of New York Heart Association (NYHA) functional class III/IV patients. If clarification on extracted findings was required, the corresponding author was contacted via electronic mail to request this information and also to query about any known pending work on frailty in HF. Extracted data were independently verified (i.e. double verification) by another member of the study team (S.K.). The authors conducted this meta-analysis in concordance with PRISMA standards of quality for reporting meta-analyses (17) and the guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (18).
2.3 Statistical Analysis
We used a random-effects meta-analysis of proportions approach (19) to quantify the prevalence of frailty in HF. Prevalence, or proportional, data assumes a binomial distribution (i.e. number of “events” versus number of “non-events” in a sample). Traditional meta-analytic approaches are problematic when prevalence proportions approach the limits of 0% or 100%. Thus, a few revised procedures, including the recently developed metaprop Stata command (19), have been developed to address this problem. The command metaprop pools proportions and uses the score statistic and the exact binomial method, with the option to incorporate the Freeman-Tukey double arcsine transformation, to compute 95% confidence intervals. A random-effects model, within metaprop, was chosen because of the considerable heterogeneity across studies in both the measurement of frailty and the samples studied. In random-effects models, the effect sizes of observed studies are considered to represent a distribution of possible effects; random-effects meta-analysis incorporates both within-study variance and between-study heterogeneity (20). Studies reporting frailty estimates were dichotomized into two groups according to measurement type: “Physical Frailty” and “Multidimensional Frailty.” The prevalence of frailty in HF was quantified overall and by measurement type. In addition to weighted estimates, the 95% confidence interval (CI) was reported along with z tests (weighted estimate divided by the standard error of the weighted estimate) and associated p values as metrics of precision.
Heterogeneity was quantified in this meta-analysis for the overall estimate and estimate by measurement type. Total dispersion in effect sizes across studies (Q) and the associated p value were calculated. We also calculated I2 as a “signal-to-noise” ratio of excess dispersion to total dispersion – ranges from 0% (indicating that all of the heterogeneity is spurious) to 100% (indicating that all of the heterogeneity is “real” and requires further examination and explanation) (21). Publication bias and bias associated with small study effects were assessed visually with funnel plots and Egger’s test, respectively (22).
In an effort to explain significant observed heterogeneity, a random-effects meta-regression was performed. Meta-regression assesses the relationship between study-level factors and the effect size (23, 24). Our main factors of interest were the average study age and proportion of NYHA Class III/IV patients. The predictor variable was examined for statistical significance using p values along with the slope coefficient ± the standard error. A p value < 0.05 was considered significant for all models. Comprehensive Meta-Analysis V3.3 and Stata MP 13.1 were used for these analyses.
3. Results
3.1 Included Studies
Results of study identification, screening, eligibility, and inclusion are outlined in the PRISMA flow diagram (Figure 1). Twenty-six published studies (2–7, 25–44), involving a total of 6896 patients with HF, were considered eligible and included in the meta-analysis (Table). Seventeen studies were classified as “Physical Frailty” as they used primarily physical frailty assessments such as the full Frailty Phenotype measure (1), portions of the Frailty Phenotype measure (e.g. gait speed or handgrip strength), or the Short Physical Performance Battery (45). Nine studies were classified as “Multidimensional Frailty” as they used multidimensional frailty measurements, including the Frailty Index (46), the Tilburg Frailty Indicator (47), and a geriatric assessment (also termed fragility assessment) that included multiple geriatric tests.
Table.
Characteristics of Studies
| First Author (Year; Country) | Sample Size | Frailty Measure | Prevalence of Frailty (%) | Age in years (M±SD) | NYHA Class III/IV (%) | HF Sample Characteristics |
|---|---|---|---|---|---|---|
| Abou-Raya (2009; Egypt) (28) | 83 (HF subsample) | Physical Frailty: Modified Frailty Phenotype Criteria | 29.0 | 69.9±4.5 | 49.0 | ≥65 years, with EF ≤40% |
| Boxer (2008; USA) (29) | 60 | Physical Frailty: Frailty Phenotype Criteria | 25.0 | 77.0±10.0 | 42.0 | ≥60 years, with EF ≤40% |
| Cacciatore (2005; Italy) (4) | 120 (HF subsample) | Multidimensional Frailty: Frailty Staging System | 15.0 (highest frailty grouping) | 75.9±6.7 | Continuous range reported | ≥65 years |
| Chaudhry (2013; USA) (34) | 758 | Physical Frailty: handgrip strength and gait speed | 41.8 (for both handgrip strength and gait speed) | 79.7±6.2 | 30.3 | CHS participants (≥65 years) newly diagnosed with HF during the study |
| Chiarantini (2010; Italy) (33) | 157 (baseline) | Physical Frailty: Short Physical Performance Battery | 50.9 (SPPB scores 0–4) | 80.0±13.8 | 59.9 | ≥65 years, admitted to hospital |
| Chung (2014; USA) (6) | 72 (baseline) | Physical Frailty: Handgrip strength <25% of total body weight | 22.0 | 59.0±2.0 | 99.0 | Patients undergoing LVAD (no age limit) |
| Dominguez-Rodriguez (2015; Spain) (7) | 102 (baseline) | Physical Frailty: Frailty Phenotype Criteria | 28.0 | 73.0±4.0 | 100 | ≥70 years, non-ischemic cardiomyopathy, undergoing CRT-D, with EF <30% |
| Dunlay (2014; USA) (8) | 99 | Multidimensional Frailty: Frailty Index | 61.6 (definition of Frailty Index>0.25) | 65.1±9.4 | None reported | Patients undergoing LVAD as DT (no age limit) |
| Eastwood (2016; Canada) (35) | 382 (both readmitted and non-readmitted) | Multidimensional Frailty: defined as >75 years of age, >3 comorbid conditions, and required assistance with activities of daily living | 35.0 (overall for both readmitted and non-readmitted) | None reported | None reported | Admitted to hospital with diagnosis of HF (age range 19–105) |
| Gastelurrutia (2014; Spain) (9) | 1314 | Multidimensional Frailty: Evaluation using multiple geriatric scales/tests | 44.2 | 66.7±12.4 | 32.9 | Outpatients (no age limit) |
| Jha (2016; Australia) (36) | 156 | Physical Frailty: Modified Frailty Phenotype Criteria | 33.0 | 53.0±13.0 | 100% | Referred for heart transplant (no age limit) |
| Joyce (2015; USA) (30) | 88 | Physical Frailty: Handgrip strength | 70.0 | 64.0±16.0 | None reported | ADHF requiring in-hospital treatment (no |
| Khandelwal (2012; India) (37) | 30 (HF subsample) | Physical Frailty: Frailty Phenotype Criteria | 76.7 | Not available for HF subsample | Not available for HF subsample | ≥60 years, admitted to hospital |
| Madan (2016; USA) (38) | 40 | Physical Frailty: Frailty Phenotype Criteria | 65.0 | 74.9±6.5 | 100 | ≥65 years, NYHA III or IV, with EF ≤ 35% |
| McNallan (2013; USA) (10) | 448 | Physical Frailty: Frailty Phenotype Criteria | 19.0 | 73.2±13.3 | None reported | Community-dwelling (no age limit) |
| Mylnarska (2016; Poland) (39) | 106 | Multidimensional Frailty: Canadian Study of Health & Aging Clinical Frailty Scale | 77.4 | 74.9±6.3 | Continuous range reported | ≥65 years, receiving CRT-D |
| Newman (2001; USA) (40) | 181 (HF subsample) | Physical Frailty: Frailty Phenotype Criteria | 23.2 | Not available for HF subsample | Not available for HF subsample | CHS participants (≥65 years) at baseline, community-dwelling |
| Newton (2016; Australia) (41) | 557 (at discharge, those that had frailty assessment) | Multidimensional Frailty: Survey of Health, Ageing and Retirement in Europe Frailty Index | 71.0 | 77.0±13.0 (entire sample, n = 811) | 38.0 (those at discharge, n = 706) | De novo or ADHF admitted to hospital, both HFrEF and HFpEF |
| Pilotto (2010; Italy) (42) | 376 | Multidimensional Frailty: Multidimensional Prognostic Index | 17.8 (highest group) | 80.5±7.3 | None reported | ≥65 years, admitted to the hospital with diagnosis of HF |
| Pulignano (2010; Italy) (31) | 173 | Multidimensional Frailty: Modified Frailty Score based on Frailty Index | 16.2 (highest group) | 77.4±5.9 | 61.8 | ≥70 years, after a hospitalization |
| Pulignano (2016; Italy) (43) | 331 | Physical Frailty: Gait speed | 26.6 | 78.0±5.2 | 51.4 | ≥70 years, community-dwelling HF with reduced or normal EF |
| Reeves (2016; USA) (44) | 27 (ADHF subsample) | Physical Frailty: Frailty Phenotype Criteria | 56.0 | 72.0±10.0 | None reported | ≥65 years, admitted with ADHF |
| Sanchez (2011; Spain) (32) | 211 | Physical Frailty: Frailty Phenotype Criteria | 40.8 | 81.6±5.0 | 23.6 | ≥75 years, admitted to hospital |
| Uchmanowicz (2016; Poland) (45) | 100 | Multidimensional Frailty: Tilburg Frailty Indicator | 89.0 | 67.2±10.2 | 37.0 | Diagnosis of HF (no age limit) |
| Vidan (2016; Spain) (46) | 416 | Physical Frailty: Frailty Phenotype Criteria | 76.0 | 80.0±6.1 | 25.5 | ≥70 years, hospitalized for HF |
| Woods (2005; USA) (47) | 509 | Physical Frailty: Frailty Phenotype Criteria | 45.6 | Not available for HF subsample | Not available for HF subsample | Women’s Health Initiative Observation Study participants at baseline (65–79 years) |
ADHF = acute decompensated heart failure; CHS = Cardiovascular Health Study; CRT-D = cardiac resynchronization therapy-defibrillator; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVAD = left ventricular assist device; M, = mean; NYHA = New York Heart Association; SD = standard deviation; SPPB = Short Physical Performance Battery; USA = United States of America.
3.2 Meta-Analysis
The overall estimated prevalence of frailty in HF was 44.5% (95% CI, 36.2%–52.8%; z = 10.54; p < 0.001) (Figure 2). Heterogeneity statistics (Q = 1438.78; p < 0.001, I2 = 98.3%) indicated there was significant and substantive variability in the prevalence of frailty in HF across studies. The estimated prevalence of frailty in HF as assessed by the Physical Frailty measures was 42.9% (95% CI, 33.6%–52.2%; z = 9.05; p < 0.001). The estimated prevalence of frailty in HF as assessed by Multidimensional Frailty measures was slightly higher at 47.4% (95% CI, 31.0%–63.8%; z = 5.66; p < 0.001). Effect sizes reported in studies were distributed symmetrically (see Supplemental Material), and there was no significant bias from small studies (Egger’s test p = 0.846). In sensitivity analyses, removing studies that included advanced HF patients immediately prior to heart transplant or ventricular assist device placement did not significantly change the estimated prevalence (45.3%; 95% CI, 36.4%–54.2%). Moreover, the estimated prevalence by community (39.0%; 95% CI, 28.4%–49.7%) versus hospitalized or recently hospitalized (48.7%; 95% CI, 35.7%–61.6%) was not substantially different.
Figure 2. Estimated Prevalence of Frailty in Heart Failure.
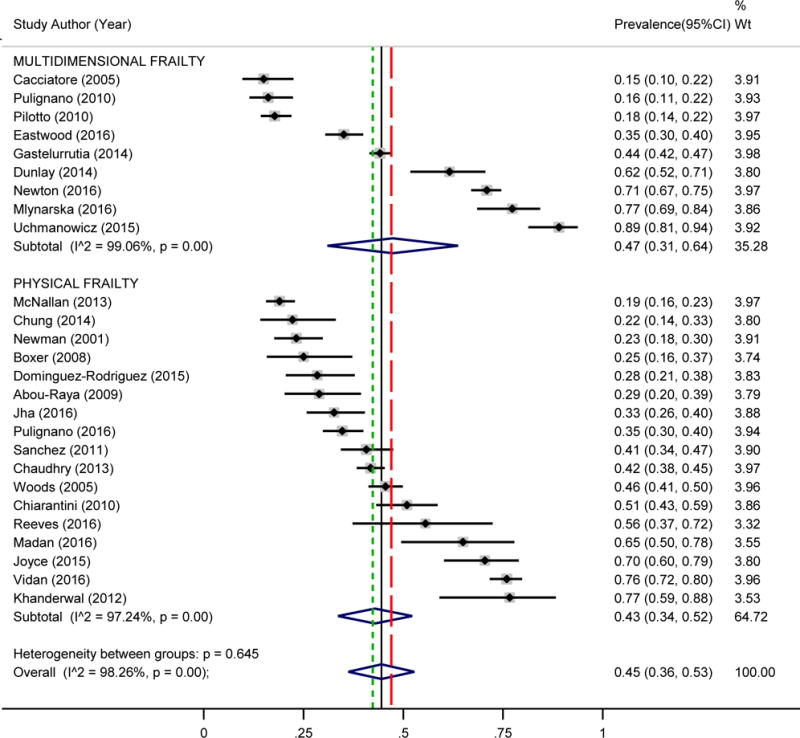
Random effects meta-analysis of prevalence of frailty in heart failure by measurement type (Physical Frailty (z = 9.05, p < 0.001; denoted by short-dashed line) and Multidimensional Frailty (z = 5.66 p < 0.001; denoted by long-dashed line) and overall (z = 10.54, p < 0.001; denoted by solid line)). CI = confidence interval; Wt = weight.
3.3 Meta-Regression
In preliminary analysis, average study age was significantly different across the 22 studies that reported average age (F = 120.36, p < 0.001); thus, we proceeded with examining age as a predictor variable in explaining variability in the prevalence of frailty in HF. There was no significant relationship, however, between average study age and prevalence of frailty in HF (see Supplemental Material; β = −0.001±0.007, t = −0.21, p = 0.838), indicating age does not explain the heterogeneity observed across studies. There was also no significant relationship between proportion of NYHA class III/IV patients in each study and prevalence of frailty in HF (see Supplemental Material; β = −0.263±0.198, t = −1.33, p = 0.204), indicating HF functional classification does not explain the heterogeneity observed across studies. In sensitivity analyses, removing studies that included advanced HF patients did not significantly change the age meta-regression results or the NYHA meta-regression results.
4. Discussion
Despite substantial variation across published studies, we derived a precise estimate of the prevalence of frailty in HF based on data from 26 published studies involving 6896 patients with HF worldwide. In this first known meta-analysis of the prevalence of frailty in HF, it is evident frailty affects almost one in every two adults with HF. Moreover, the prevalence of frailty in HF is not a function of age or functional classification but perhaps also reflective of other mechanisms. Finally, based on differences in measurement across studies, it is apparent there is a small, but meaningful, divide regarding the most appropriate measure that undoubtedly interferes with our ability to capture frailty in HF and integrate frailty into the clinical spectrum.
The high prevalence of frailty in HF indicates frailty is more common in HF than we may have previously thought, which has important implications for practitioners caring for adults with HF. In the landmark study by Fried et al. (1), overall prevalence of frailty was estimated at about 7% in a large sample of community-dwelling older adults aged 65–101 years in the United States. Prevalence of frailty increased with each 5-year age group with an estimate of about 23% in those over 90 years of age. As such, HF is associated with a rate of frailty (about 45% across a wide age range) that is substantially higher than what is seen among community-dwelling oldest-old adults.
In order to pinpoint study-level factors that would potentially explain the heterogeneity of reported prevalence rates across studies, we examined both age and functional classification of the sample. Neither age nor functional classification assessed at the study level were significantly associated with prevalence of frailty across studies. Frailty has traditionally been considered a geriatric syndrome; but, the lack of a relationship between age and frailty in HF indicates frailty in HF is not confined to older adults. In fact, frailty is high even among studies with younger HF patients (3, 33). As mentioned by previous reviews (16), the high prevalence of frailty in younger patients indicates we should consider frailty in HF at all ages rather than as a strict geriatric syndrome. In the subgroup of studies that reported NYHA class, functional classification of the sample was also not significantly associated with prevalence of frailty in HF across studies.
Thus, if neither an age-related variable nor a HF functional class-related variable are associated with frailty, then other mechanisms could explain variations in frailty in HF. The interrelationships between neurohormonal dysregulation, inflammation, and skeletal muscle dysfunction have been proposed as underlying pathogenic mechanisms of frailty,(48) which have been noted to also parallel the pathogenesis of HF.(13) The specific biological mechanisms of frailty in HF, however, have not been investigated in detail, but are a rich area of investigation given the interest in potentially reversing frailty through advanced HF therapies.(14) Most likely the gradient of frailty in HF is more granular than simply age or functional classification, and it may be due to the interaction of multiple age- and HF-related factors, including but not limited to chronological age, subtype of HF (reduced versus preserved ejection fraction), severity of HF from hemodynamic, neuroendocrine, and inflammatory dysregulation standpoints, and the comorbidities feeding into the condition of HF.
Ecological bias (i.e. bias due to aggregating data), however, must be taken into consideration in interpreting these non-significant findings.(49, 50) In examining study-level factors, we were limited to aggregate data (i.e. the average age and proportion of NYHA Class III/IV). As such, even though we did not observe a significant relationship, we are cautious in deducing conclusions about individuals given the limitations of using aggregate data. Future research could focus on pooling patient-level data,(49) which would potentially yield more nuanced information regarding a relationship between age and frailty and/or NYHA and frailty.
The lack of relationship between the two study-level factors and prevalence rate could also be a reflection of the substantial variability in how frailty was measured. Many studies have adopted a physical frailty perspective based primarily on the definition of frailty set forth by Fried and colleagues (1) (Table). In contrast, other studies incorporated other factors (e.g. social, cognitive, and psychological factors) into their definition and considered frailty to be the cumulative sum of all these factors. In this meta-analysis, we noted the prevalence of frailty in HF differed by the two perspectives. The wide dispersion in prevalence rates, however, even among similar measures and similar sub-populations of HF patients, highlights that an assessment of frailty is far from standardized. Thus, there is a need to reach consensus of how to measure frailty in HF so that differences in the prevalence of frailty can be attributed to differences in underlying disease processes and not to substantial differences in measurement.
4.1 Future Recommendations
Our meta-analysis highlights several opportunities to improve future research on frailty in HF. First and foremost, there is a need to standardize the measurement of frailty in HF. The major benefit of using a unified measure is that we can make comparisons across studies to better learn from cumulative science and design interventions based on results from multiple studies. Based on the preponderance of evidence, we propose that the full Frailty Phenotype be adopted as a measure of physical frailty in HF as it could easily be used across research and practice settings at this time. Moving forward, more research is needed to rigorously test and refine frailty measures to generate the most precise and accurate measure of frailty in HF. There is also a need to disambiguate the relationship between physical frailty and related concepts such as cognitive function and psychosocial health in order to improve the predictive ability of frailty, as recently studied by Jha et al. (33).
Second, the clinical implications of studying frailty in HF are considerable. Given the finding that younger patients still had a high prevalence of frailty in HF, we should broaden our view of frailty as a strictly geriatric syndrome to encompass the entire chronological age spectrum in HF. Similar to recent guidelines (10–12), we recommend that an assessment of frailty be incorporated into clinical practice for all patients with HF; however, appropriate interventions to mitigate frailty in HF across the lifespan have yet to be determined. Of note, the majority of studies in this meta-analysis included only older HF patients, and thus, more research is needed to better understand the prevalence of frailty in younger HF patients. Moreover, given the wide variety of settings in which frailty was assessed (e.g. outpatients or acute decompensated HF), more research is needed to examine frailty in various sub-populations of HF, which may yield important information about the pathogenesis of frailty in HF.
Finally, there is a need to examine shared biological pathways and manifestations of frailty and HF, including frailty in HF as a result of both primary aging and HF itself. Goldwater and Pinney (15) recently explicated a difference between frailty related to primary aging and frailty related to the progression of HF. Even though there is noted considerable overlap in pathological mechanisms (e.g. systemic inflammation, oxidative stress) (13) between aging-related frailty and HF-related frailty, there may be subtle differences in the ability to mitigate frailty in these two groups with interventions such as ventricular assist devices (14).
4.2 Limitations
The findings of this meta-analysis have several limitations. First, due to the integration of studies that used different measures of frailty, our findings demonstrated considerable heterogeneity that should be acknowledged along with our estimate of the prevalence of frailty in HF. Second, although we made every effort to identify completed or on-going studies of frailty in HF, it is possible that we inadvertently missed published or unpublished research in this area. Finally, we selected NYHA functional classification a priori as a HF-related factor because this variable was figured to be the most commonly reported across studies. It is uncommon to know all the available study-level factors a priori, and thus, we were unable to tease apart other study-level factors (e.g. percentage of patients HF with reduced ejection fraction) that would potentially explain differences in reported prevalence.
5. Conclusions
In this meta-analysis of frailty in HF, our findings demonstrate frailty affects almost one in every two patients with HF. These results point to the importance of studying frailty in HF across a patient’s lifespan and broadening our view of frailty beyond a strictly geriatric syndrome. As such, there is a need to critically examine all aspects of frailty in HF, including standardizing the measurement of frailty in HF, understanding the underlying pathological mechanisms, and mitigating the effects of frailty in HF.
Supplementary Material
Figure A. Funnel plot for assessment of publication bias; Egger’s test for bias of small studies effects: t = −0.20, p = 0.846.
Figure B1. Meta-regression of the influence of average study age on prevalence of frailty in heart failure. Each circle represents a study; the size of the circle indicates sample size.
Figure B2. Meta-regression of the influence of proportion of New York Heart Association functional class III/IV patients in the study on prevalence of frailty in heart failure. Each circle represents a study; the size of the circle indicates sample size.
Highlights.
Almost half of all heart failure patients are considered frail
Prevalence of frailty in heart failure was not related to age or functional class
There is significant heterogeneity in the measurement of frailty in heart failure
Future work should focus on standardizing a measure of frailty in heart failure
Broaden our view of frailty beyond a strictly geriatric syndrome in heart failure
Acknowledgments
The authors would like to thank Jonathan Auld, RN, MS, CNL, MAT for assisting with data verification.
Funding
This work was supported by the National Institutes of Health/National Institute of Nursing Research (NIH/NINR) Ruth L. Kirschstein National Research Service Award (1F31NR015936-01; Denfeld), the National Hartford Centers of Gerontological Nursing Excellence (NHCGNE) Patricia G. Archbold Scholar Program (Denfeld), and an ARCS Scholar Award (Denfeld). Current post-doctoral funding for Quin Denfeld provided by NIH/National Heart, Lung, and Blood Institute (NIH/NHLBI) at Oregon Health & Science University Knight Cardiovascular Institute (2T32HL094294; Thornburg). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NINR, NIH/NHLBI, or the NHCGNE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of Interest
None
References
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D’Ambrosio D, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35(12):723–30. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung CJ, Wu C, Jones M, Kato TS, Dam TT, Givens RC, et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J Card Fail. 2014;20(5):310–5. doi: 10.1016/j.cardfail.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez-Rodriguez A, Abreu-Gonzalez P, Jimenez-Sosa A, Gonzalez J, Caballero-Estevez N, Martín-Casañas FV, et al. The impact of frailty in older patients with non-ischaemic cardiomyopathy after implantation of cardiac resynchronization therapy defibrillator. Europace. 2015;17(4):598–602. doi: 10.1093/europace/euu333. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33(4):359–65. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastelurrutia P, Lupon J, Altimir S, de Antonio M, Gonzalez B, Cabanes R, et al. Fragility is a key determinant of survival in heart failure patients. Int J Cardiol. 2014;175(1):62–6. doi: 10.1016/j.ijcard.2014.04.237. [DOI] [PubMed] [Google Scholar]
- 7.McNallan SM, Singh M, Chamberlain AM, Kane RL, Dunlay SM, Redfield MM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1(2):135–41. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong LSM, van der Harst P, de Boer RA, Huzen J, van Gilst WH, van Veldhuisen DJ. Aging, telomeres and heart failure. Heart Fail Rev. 2010;15(5):479–86. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodson JA, Chaudhry SI. Geriatric conditions in heart failure. Curr Cardiovasc Risk Rep. 2012;6(5):404–10. doi: 10.1007/s12170-012-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin-Adams M, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21(6):519–34. doi: 10.1016/j.cardfail.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Jurgens CY, Goodlin S, Dolansky M, Ahmed A, Fonarow GC, Boxer R, et al. Heart failure management in skilled nursing facilities: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circulation. 2015;8(3):655–87. doi: 10.1161/HHF.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 13.Boxer RS, Shah KB, Kenny AM. Frailty and prognosis in advanced heart failure. Curr Opin Support Palliat Care. 2014;8(1):25–9. doi: 10.1097/SPC.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 14.Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circulation. 2012;5(2):286–93. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwater DS, Pinney SP. Frailty in advanced heart failure: a consequence of aging or a separate entity? Clin Med Insights Cardiol. 2015;9:39–46. doi: 10.4137/CMC.S19698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jha SR, Ha HSK, Hickman LD, Hannu M, Davidson PM, Macdonald PS, et al. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20(5):553–60. doi: 10.1007/s10741-015-9493-8. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486–504. [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 24.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Raya S, Abou-Raya A. Osteoporosis and congestive heart failure (CHF) in the elderly patient: double disease burden. Arch Gerontol Geriatr. 2009;49(2):250–4. doi: 10.1016/j.archger.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Boxer R, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56(3):454–61. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 27.Joyce E, Gopal DM, Luk A, Groarke JD, Shah SP, Stewart GC, et al. Grip strength assessment and early outcomes in hospitalized acute decompensated heart failure: a prospective study. J Card Fail. 2015;21(8, Supplement):S117. [Google Scholar]
- 28.Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, Gregori D, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med (Hagerstown) 2010;11(10):739–47. doi: 10.2459/JCM.0b013e328339d981. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez E, Vidán MT, Serra JA, Fernández-Avilés F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97(19):1602–6. doi: 10.1136/hrt.2011.227504. [DOI] [PubMed] [Google Scholar]
- 30.Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16(5):390–5. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61(6):635–42. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eastwood CA, Quan H, Howlett JG, King-Shier KM. Factors associated with 7-Day rehospitalization after heart failure admission. J Cardiovasc Nurs. 2016 doi: 10.1097/JCN/0000000000000347. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Jha SR, Hannu MK, Gore K, Chang S, Newton P, Wilhelm K, et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplant. 2016;35(9):1092–100. doi: 10.1016/j.healun.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Khandelwal D, Goel A, Kumar U, Gulati V, Narang R, Dey AB. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J Nutr Health Aging. 2012;16(8):732–5. doi: 10.1007/s12603-012-0369-5. [DOI] [PubMed] [Google Scholar]
- 35.Madan SA, Fida N, Barman P, Sims D, Shin J, Verghese J, et al. Frailty assessment in advanced heart failure. J Card Fail. 2016;22(10):840–4. doi: 10.1016/j.cardfail.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Mlynarska A, Mlynarski R, Biernat J, Sosnowski M, Golba KS. Frailty syndrome in heart failure patients who are receiving cardiac resynchronization. Pacing Clin Electrophysiol. 2016;39(4):370–4. doi: 10.1111/pace.12800. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 38.Newton PJ, Davidson PM, Reid CM, Krum H, Hayward C, Sibbritt DW, et al. Acute heart failure admissions in New South Wales and the Australian Capital Territory: the NSW HF Snapshot Study. Med J Aust. 2016;204(3):113 e1–8. doi: 10.5694/mja15.00801. [DOI] [PubMed] [Google Scholar]
- 39.Pilotto A, Addante F, Franceschi M, Leandro G, Rengo G, D’Ambrosio P, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail. 2010;3(1):14–20. doi: 10.1161/CIRCHEARTFAILURE.109.865022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, et al. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights from the IMAGE-HF study. JACC Heart Fail. 2016;4(4):289–98. doi: 10.1016/j.jchf.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Reeves GR, Whellan DJ, Patel MJ, O’Connor CM, Duncan P, Eggebeen JD, et al. Comparison of frequency of frailty and severely impaired physical function in patients >/=60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117(12):1953–8. doi: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchmanowicz I, Gobbens RJ. The relationship between frailty, anxiety and depression, and health-related quality of life in elderly patients with heart failure. Clin Interv Aging. 2015;10:1595–600. doi: 10.2147/CIA.S90077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18(7):869–75. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- 44.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative observational study. J Am Geriatr Soc. 2005;53(8):1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 45.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 46.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. The Scientific World Journal. 2001;1:323–36. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. The tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–55. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient-versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21(3):371–87. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]
- 50.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A. Funnel plot for assessment of publication bias; Egger’s test for bias of small studies effects: t = −0.20, p = 0.846.
Figure B1. Meta-regression of the influence of average study age on prevalence of frailty in heart failure. Each circle represents a study; the size of the circle indicates sample size.
Figure B2. Meta-regression of the influence of proportion of New York Heart Association functional class III/IV patients in the study on prevalence of frailty in heart failure. Each circle represents a study; the size of the circle indicates sample size.


