Abstract
Introduction
We evaluated the potential utility of a new prototype noninvasive muscle oxygenation (MOx) measurement for the identification of shock severity in a population of patients admitted to the trauma resuscitation rooms of a Level I regional trauma center. The goal of this project was to correlate MOx with shock severity as defined by standard measures of shock: systolic blood pressure, heart rate, and lactate.
Methods
Optical spectra were collected from subjects by placement of a custom-designed optical probe over the first dorsal interosseous muscles on the back of the hand. Spectra were acquired from trauma patients as soon as possible upon admission to the trauma resuscitation room. Patients with any injury were eligible for study. MOx was determined from the collected optical spectra with a multi-wavelength analysis that used both visible and near-infrared regions of light. Shock severity was determined in each patient by a scoring system based on combined degrees of hypotension, tachycardia, and lactate. MOx values of patients in each shock severity group (mild, moderate, and severe) were compared using two-sample t-tests.
Results
In 17 healthy control patients, the mean MOx value was 91.0 ± 5.5%. A total of 69 trauma patients were studied. Patients classified as having mild shock had a mean MOx of 62.5 ± 26.2% (n = 33), those classified as in moderate shock had a mean MOx of 56.9 ± 26.9% (n = 25) and those classified as in severe shock had a MOx of 31.0 ± 17.1% (n = 11). Mean MOx for each of these groups was statistically different from the healthy control group (p<0.05).
Receiver operating characteristic (ROC) analyses show that MOx and shock index (heart rate/systolic blood pressure) identified shock similarly well (area under the curves (AUC) = 0.857 and 0.828, respectively). However, MOx identified mild shock better than shock index in the same group of patients (AUC = 0.782 and 0.671, respectively).
Conclusions
The results obtained from this pilot study indicate that MOx correlates with shock severity in a population of trauma patients. Noninvasive and continuous MOx holds promise to aid in patient triage and to evaluate patient condition throughout the course of resuscitation.
Keywords: Noninvasive monitoring, Traumatic shock, Near-infrared spectroscopy, Visible spectroscopy
Introduction
Severity of injury in patients following traumatic injury remains difficult to accurately assess, especially in the pre-hospital setting. Triage guidelines commonly include vital signs and anatomic injury, and rely heavily on individual judgment. Pre-hospital vital signs have been found to be both predictive (1, 2) and not predictive (3) of mortality. A recent study has shown that pre-hospital lactate ≥ 2.5 mmol/L was more predictive of the need for early resuscitative care than systolic blood pressures of ≤ 90 mm Hg (4). Clinical scoring systems have been developed that show promise for predicting in-hospital mortality or intensive care unit (ICU) admission (5–7), but no clear gold standard has emerged. There exists a need for a simple, noninvasive monitoring tool to rapidly identify patients in need of resuscitation.
We have developed a prototype of a noninvasive muscle oximeter based on optical spectroscopy that determines oxygen available at the cellular level, rather than just in the vascular space where oxygen is typically measured clinically. Our device measures spectra in the visible and near-infrared (NIR) wavelength regions, in contrast to near-infrared spectroscopy (NIRS) devices on the market that measure only a few selected wavelengths in the NIR. Peripheral muscle is an excellent tissue to monitor in impending shock since it is not a vital organ. During conditions of impaired oxygen delivery in the body, such as in trauma with significant blood loss, blood may be preferentially shunted away from the skin and skeletal muscles to maintain better perfusion to the vital organs. Thus, decreased muscle oxygenation (MOx) may be the first indicator of dysregulation of the cardiovascular system, signaling the early onset of shock.
We hypothesized that MOx is a sensitive measure of shock in patients with traumatic injuries and that it has potential as a triage tool. The goal of this project was to correlate noninvasive MOx with shock severity defined by systolic blood pressure (SBP), heart rate (HR), and lactate in a population of trauma patients upon admission to the emergency department (ED) at Harborview Medical Center (HMC) in Seattle, Washington, a major Level 1 regional trauma center in the Pacific Northwest.
Materials and Methods
Patients
Patients who were triaged into the ED trauma resuscitation rooms in a convenience sample during the study period (December 2009 to September 2011) were considered for enrollment. All trauma patients admitted to the ED trauma resuscitation rooms when our research team was present, regardless of injury type or severity, were candidates for the study. With a single prototype device, only one trauma patient could be studied at a time. When the device was available, all patients admitted to the trauma resuscitation rooms were approached for the study by our research team.
Patients with bilateral arm or hand injuries were excluded, as well as pregnant patients and prisoners. Patients were either initially approached for consent, or delayed consent was obtained following initial stabilization for subjects unable to provide consent with no immediate next of kin available. Data from subjects studied with delayed consent for whom consent was subsequently declined were purged and not included in the analyses. Our goal was to obtain initial optical data as soon as possible after admission to the ED in order to obtain MOx information before resuscitation was complete or definitive therapy provided. However, measurements made for this study did not interfere with the standard assessment and treatment provided to these patients. The study was approved by the Institutional Review Board at the University of Washington.
The control group was comprised of 17 healthy adult subjects with a mean age of 41.1 ± 9.5 years. The group included eight men and nine women. The control group had no history of hypertension, cardiovascular disease, pulmonary disease, diabetes, or deep vein thrombosis.
Optical System and Data Acquisition
Optical spectra were collected from control subjects and trauma patients with a custom-designed optical probe developed in our laboratory. The probe consists of a set of illuminating optical fibers arranged in an arc separated from a central set of detecting fibers arranged in a spot (Fiberoptic Systems, Inc., Simi Valley, CA). The distance from the illuminating to the detecting optical fibers was set at 9, 11 or 13 mm in three different probes. The choice of probe was based on physical characteristics of the subject and the resulting quality of optical spectra obtained on initial testing of each subject. Spectra were obtained using an imaging spectrometer (iHR320, Horiba Jobin Yvon, Edison, NJ) coupled with a 256 × 1024-pixel thermoelectrically cooled CCD detector (Synapse, Horiba Jobin Yvon, Edison, NJ). A custom-designed LED-based light source (Innovations in Optics, Inc., Woburn, MA) provided illumination in the visible and NIR wavelength regions (500–800 nm). Figure 1 is an illustration of the prototype device.
Figure 1.
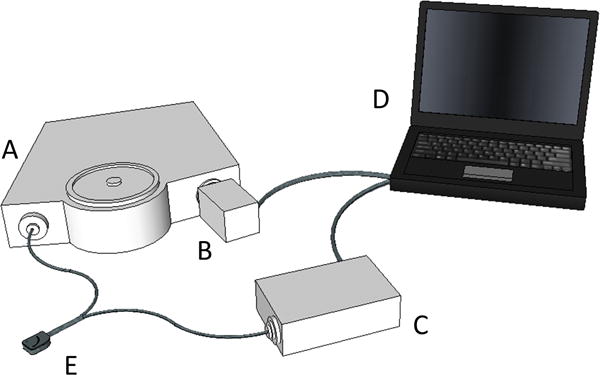
Prototype device to measure muscle oxygenation. A, Spectrometer; B, CCD camera; C, LED light source; D, computer; E, fiber optic probe. The probe is connected to the spectrometer and the light source via two discrete fiber bundles and is placed on the back of the hand during spectral acquisition.
Optical spectra were collected from all subjects by placement of an optical probe over the first dorsal interosseous (FDI) muscles on the back of the hand. The probe was held in place with tape or an elastic wrap. Optical spectra were collected every 2–5 s. In ED patients, spectra were acquired continuously for a period of 8–15 minutes. In control subjects, spectra were collected for 3 minutes at rest while breathing room air.
MOx was determined from the collected optical spectra using a multiwavelength analysis, Locally Weighted Regression (LWR), previously described (8) using MATLAB (R2014a, The MathWorks, Inc., Natick, MA) and PLS Toolbox (v7.8, Eigenvector Research, Inc., Wenatchee, WA). The pattern-matching algorithm was trained on a reference set of spectra obtained from 33 healthy subjects with a wide range of MOx values, body mass index (BMI), and skin tones. This group of healthy subjects had no overlap with the control group for the trauma study. This group ranged in age from 18 to 53 years with a mean of 39.2 ± 9.0 years. Each spectrum acquired from trauma patients and control subjects was applied to the LWR model to yield a MOx value. Mean MOx values were calculated for each patient or control subject from all spectra collected. Mean MOx was used to represent each subject in statistical analyses.
The quality of optical spectra obtained from study patients and control subjects was independently evaluated by a standard chemometric Q-residual test to eliminate spectra that could not appropriately be analyzed with the analysis algorithm. The Q-residual test determines if there is sufficient similarity in character between a given spectrum and those used in the training set (9). MOx values from study spectra were considered to be invalid if the Q-residuals were greater than the 95% confidence interval of the Q-residuals in the training set. Patients with invalid spectra were excluded from the study.
Shock Severity
Clinical data were collected from the medical record, which included all arterial blood gases, hematocrits, lactates, and vital signs during the one hour period after the start of the optical measurements. Patient hospital length of stay (LOS), use of mechanical ventilatory support, duration of ICU admission, and mortality during that hospitalization were also recorded.
Shock severity at the time of study was determined based on maximum HR, minimum SBP, and maximum lactate during the hour subsequent to the start of optical data collection. A scoring system to define shock severity was derived prior to all data acquisition and is shown in Table 1. This simple approach was chosen over established general scoring systems in order to most accurately assess clinical status of the patient at the time of the MOx measurement. Subjects were considered to have mild shock for scores from 0–2, moderate for scores of 3–5, and severe if 6 or greater.
Table 1.
Scoring System for Clinical Shock Severity
| 0 pts | 1 pt | 2 pts | 3 pts | 4 pts | 5 pts | |
|---|---|---|---|---|---|---|
| Maximum Heart Rate (beats/min) | < 100 | 100–120 | > 120 | |||
| Minimum Systolic Pressure (mm Hg) | ≥ 100 | 90–99 | 80–89 | < 80 | ||
| Maximum Lactate (mmol/L) | 0 – 0.9 | 1 – 1.9 | 2 – 3.9 | 4 – 7.9 | 8 – 11.9 | ≥ 12 |
|
Total score (pts) = Heart Rate + Systolic Pressure + Lactate Shock severity: Mild = 0–2 pts, Moderate = 3–5 pts; Severe = 6–10 pts | ||||||
Shock severity for each patient was not known during the optical spectral acquisition; it was determined post hoc with values taken from the medical record. Personnel who acquired optical spectra were not made aware of the parameters used in the shock severity scoring system. Injury severity scores (ISS) (10) were calculated by hospital systems already in place and were subsequently collected from the medical record for each subject.
Statistical Analysis
Results are summarized as mean ± standard deviation (SD). Two-sample Student’s t-tests were performed using Origin (v. 9.1.0, OriginLab Corp., Northampton, MA). Receiver operating characteristic (ROC) curves were constructed in R (v. 2.15.1, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Optical spectra were acquired from 107 patients upon admission to the ED trauma resuscitation rooms at HMC. The reasons for exclusion from the final data analysis are indicated in Figure 2. Four patients had uninterpretable spectra and 16 patients were excluded because of unacceptable Q-residuals from the acquired optical spectra. Seventeen subjects had no lactate drawn within the specified time period and one subject was subsequently determined to have liver failure without trauma. A total of 69 subjects remained for the final analysis. Subjects ranged from 9 to 91 years of age, with a mean age of 39.9 ± 20.5 years. There were 57 males and 12 female subjects in the final cohort.
Figure 2.
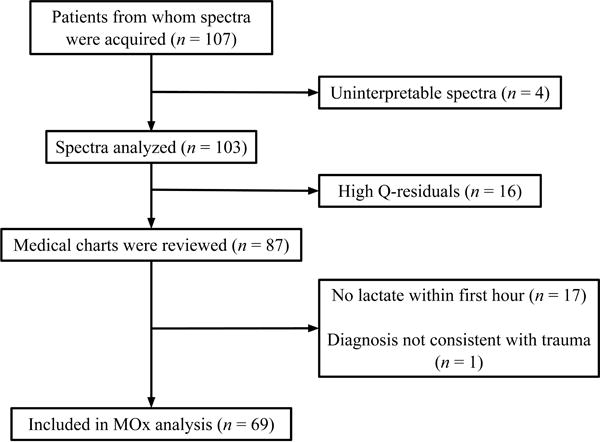
Patient enrollment. Muscle oxygenation (MOx) was measured from spectra acquired from trauma patients. Q-residuals measure the similarity between patient spectra and the spectra used to train the algorithm that measures MOx. High Q-residuals (> 95% confidence interval) indicate a level of dissimilarity that renders MOx measurements invalid.
The collection of uninterpretable spectra from four excluded patients was the result of operator error. Our device is a laboratory prototype and is not very user-friendly, especially in a chaotic trauma bay. Among the 16 excluded patients with high Q-residuals, there was no apparent pattern in shock severity: four were in mild shock, eight were in moderate shock, and four were in severe shock.
Mechanism of injury in the final cohort of patients was largely from motor vehicle collisions, pedestrians hit by motor vehicles, and falls. The distribution of the mechanisms of injury by shock severity category is shown in Table 2. Of the 69 patients, 54 were identified as having fractures, 36 had head or spinal injuries, 28 had associated pulmonary injuries including pneumothoraces or pulmonary contusions, and 17 had associated solid organ injuries. Eight patients did not survive to hospital discharge following their injury.
Table 2.
Demographics and Physiologic Parameters of Shock Groups
| Mild (n = 33) |
Moderate (n = 25) |
Severe (n = 11) |
|
|---|---|---|---|
| Sex | |||
| Male (%) | 27 (81.8) | 20 (80.0) | 10 (90.9) |
| Female (%) | 6 (18.2) | 5 (20.0) | 1 (9.1) |
| Age (yr) | 40.4 ± 22.4 | 42.8 ± 18.1 | 31.6 ± 18.1 |
| Primary Diagnosis | |||
| Motor Vehicle Collision (%) | 11 (33.3) | 9 (36.0) | 3 (27.3) |
| Fall (%) | 4 (12.1) | 6 (24.0) | 1 (9.1) |
| Car vs. Pedestrian, Bicycle, or Skateboard (%) | 9 (27.3) | 2 (8.0) | 3 (27.3) |
| Gun Shot Wound (%) | 0 | 4 (16.0) | 1 (9.1) |
| Bicycle or Skateboard Accident (%) | 4 (12.1) | 1 (4.0) | 0 |
| ATV Accident (%) | 1 (3.1) | 1 (4.0) | 1 (9.1) |
| Other (%) | 4 (12.1) | 2 (8.0) | 2 (18.1) |
| ISS | 22.5 ± 11.5 | 24.2 ± 14.4 | 29.7 ± 8.5* |
| Disposition | |||
| OR (%) | 5 (15.2) | 9 (36.0) | 6 (54.5) |
| ICU (%) | 20 (60.6) | 13 (52.0) | 4 (36.4) |
| Floor (%) | 4 (12.1) | 0 | 0 |
| Died in OR/ICU (%) | 4 (12.1) | 3 (12.0) | 1 (9.1) |
| Hospital Length of Stay (days) | 11.3 ± 11.2 | 16.9 ± 20.7 | 30.5 ± 27.8* |
| ICU Length of Stay (days) | 5.3 ± 9.5 | 8.4 ± 10.4 | 9.4 ± 6.3 |
| Time on Mechanical Ventilation (days) | 3.4 ± 8.1 | 5.0 ± 6.0 | 6.4 ± 5.9 |
| Maximum HR (bpm) | 84.9 ± 17.9 | 110.5 ± 28.9* | 123.9 ± 23.0* |
| Minimum SBP (mm Hg) | 123.4 ± 15.8 | 103.6 ± 27.0* | 78.9 ± 20.0* |
| Maximum Lactate (mmol/L) | 1.5 ± 0.7 | 3.4 ± 1.6* | 5.0 ± 3.3* |
| Shock Index | 0.70 ± 0.16 | 1.18 ± 0.45* | 1.65 ± 0.46* |
| Minimum SpO2 (%) | 97.2 ± 3.6 | 98.1 ± 2.6 | 93.6 ± 6.8* |
| MOx (%) | 62.5 ± 26.2 | 56.9 ± 26.9 | 31.0 ± 17.1* |
Significantly different from mild group, p < 0.05.
ATV, all-terrain vehicle; ISS, Injury Severity Score; OR, operating room; ICU, intensive care unit; HR, heart rate; SBP, systolic blood pressure; SpO2, arterial saturation by pulse oximetry; MOx, muscle oxygenation
Shock Severity, Clinical Outcomes, and Clinical Measures
Of the 69 patients included in the analyses, 33 patients were classified as having mild shock, 25 had moderate shock and 11 had severe shock, based on the clinical severity score described above and in Table 1. Trends in standard clinical measures and outcomes correlated with shock severity, as seen in Table 2. ISS increased from 22.5 ± 11.5 in the mild group to 24.2 ± 14.4 and 29.7 ± 8.5 in the moderate and severe groups, respectively. Similarly, LOS, length of ICU stay, and length of time on mechanical ventilation increased from mild through moderate to severe shock. The mean LOS almost tripled in severe shock (30.5 ± 27.8) relative to mild shock (11.3 ± 11.2). ICU length of stay and time on mechanical ventilation almost doubled in severe shock relative to mild.
Because they were components of the shock severity scoring system, it is not surprising that maximum HR and maximum lactate increased from mild through moderate to severe shock. Minimum SBP decreased from mild to severe shock. Accordingly, shock index, calculated as HR/SBP, increased from 0.70 ± 0.16 in mild shock to 1.18 ± 0.45 in moderate shock to 1.65 ± 0.46 in severe shock. Shock index has been proposed as a reliable marker of severe shock, with normal values between 0.5 and 0.7 (11).
Arterial saturation by pulse oximetry (SpO2) was not a sensitive measure of shock severity. SpO2 was indistinguishable in mild and moderate shock (97.2 ± 3.6% and 98.1 ± 2.6%, respectively) and was slightly lower in severe shock (93.6 ± 6.8%).
Shock Severity and MOx
Average MOx ranged from 4.1% to 100% in all control subjects and trauma patients. In the healthy control group, mean MOx for the 17 subjects was 91.0 ± 5.5% (Fig. 3). Patients in the mild shock category had a mean MOx of 62.5 ± 26.2%, those classified as having moderate shock had a mean MOx of 56.9 ± 26.9%, and mean MOx in the severe shock category was 31.0 ± 17.1%. Mean MOx for each of these groups was statistically different from the mean in the healthy control group, as were mild and moderate from severe, based on two-sample t-tests (p<0.05). Mean MOx for the mild and moderate shock groups were not statistically significantly different from each other.
Figure 3.
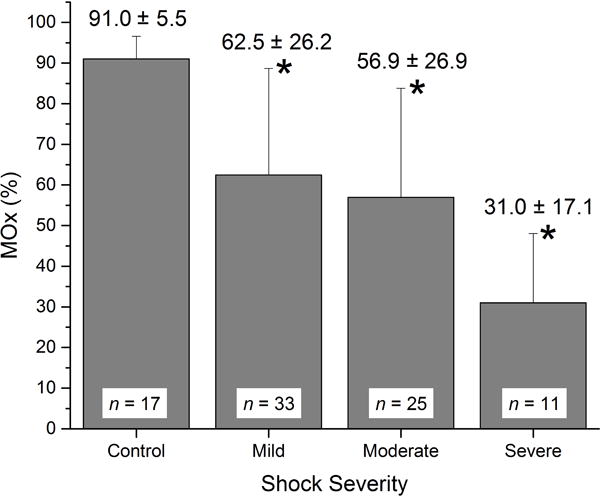
Mean MOx in the healthy control group and in the three shock severity groups. In all shock groups, mean MOx was statistically lower than in the healthy control group (*, p<0.05).
There was a trend toward lower MOx values for patients who died compared with survivors (44.6 ± 25.2%, n = 8 vs. 56.9 ± 27.4%, n = 61), for those who required ICU care compared with those who did not (54.5 ± 27.8%, n = 62 vs. 64.1 ± 21.8%, n = 7), and for those who received mechanical ventilator support compared with those that did not (53.5 ± 27.7%, n = 52 vs. 61.3 ± 25.7%, n = 17). None of these differences reached statistical significance in this small patient sample.
ROC curves were constructed to compare MOx measured in control subjects with MOx measured in all trauma patients together and in each individual shock severity group (Fig. 4A). MOx accurately predicted the presence of shock of all degrees (mild, moderate, and severe) with an area under the curve (AUC) of 0.857. MOx identified the presence of shock perfectly in the severe group (AUC = 1.000) and did very well in the moderate shock group (AUC = 0.892). In the mild shock group, MOx was a good indicator of the presence of shock with an AUC of 0.782.
Figure 4.
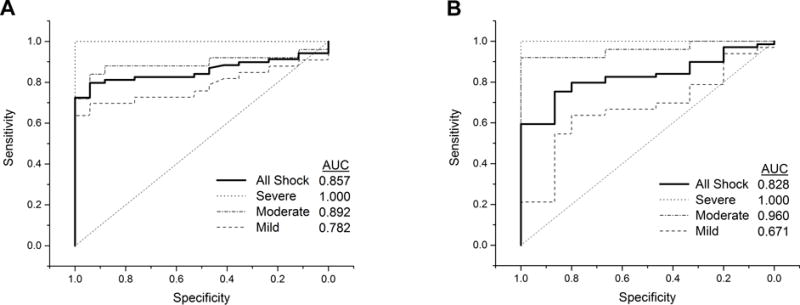
Receiver operating characteristic curves comparing MOx values and shock index values measured in healthy control subjects and trauma patients. Based on measurements of heart rate, systolic blood pressure, and lactate made within an hour of spectral acquisition, patients were categorized into shock severity groups of mild, moderate, and severe (Table 1). A) When all trauma patients were included in the test group (mild, moderate, and severe shock groups = ‘All Shock’), MOx identified the presence of shock well, with an area under the curve (AUC) of 0.857. MOx identified shock in the severe and moderate groups very well, and had considerable success with the identification of mild shock. B) The ability of shock index to identify shock in general (‘All Shock’) was comparable to that of MOx. Shock index successfully identified severe and moderate shock, but was not as successful as MOx at identifying mild shock.
Although not the primary goal of this study, one patient had continuous measurement of MOx during a period of time involving active resuscitation for hypotension. Figure 5 shows the time course of the MOx measurement for a 77-year-old male suffering from shock due to trauma resulting from a fall from a ladder, including concussion, facial fracture, and arm and hip contusions. This patient received two boluses of saline in quick succession for low blood pressure with intermittent measurement of mean arterial pressure (MAP). MAP showed significant improvement several minutes after the start of the second fluid bolus. MOx responded immediately to the first fluid bolus, but then dropped precipitously to a low value of ~28%. MOx increased back to normal levels in response to the second fluid bolus. Arterial saturation remained above 95% during this same time period.
Figure 5.
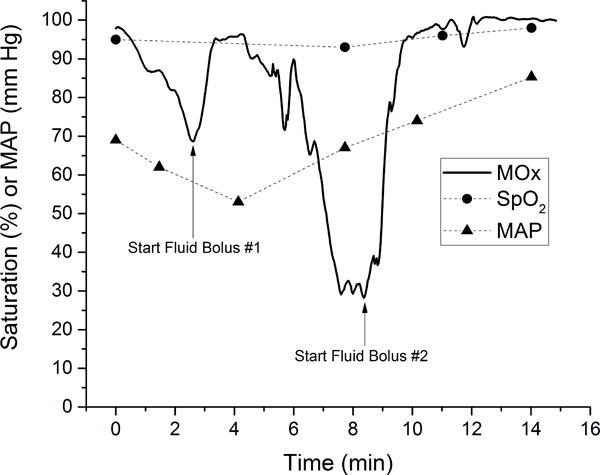
MOx during active resuscitation. MOx decreased prior to administration of a fluid bolus, and rapidly improved following fluid administration. MOx returned to the normal range after the second bolus. The patient’s mean arterial blood pressure (MAP) also improved following the fluid administration. Arterial oxygen saturation (SpO2) measured by pulse oximetry did not change appreciably.
Shock Severity and Shock Index
Shock index in healthy controls was 0.60 ±0.11. ROC curves were constructed to compare shock index in control subjects with shock index in all trauma patients together and in each individual shock severity group (Fig. 4B). Taking shock of all degrees together (mild, moderate, and severe), shock index identified the presence of shock well with an AUC of 0.828. Shock index perfectly identified the presence of shock in the severe group (AUC = 1.000) and was exceptional in the moderate group (AUC = 0.960). Shock index was not as successful as MOx at identifying shock in the mild group (AUC = 0.671).
Discussion
In this pilot study, MOx was a sensitive indicator of the presence of shock in trauma patients. Trauma patients were studied upon admission to the ED trauma resuscitation rooms of a Level I regional trauma center. Because MOx could distinguish patients in mild, moderate, and severe shock (defined by degrees of tachycardia, hypotension, and lactate levels as in Table 1), these preliminary results indicate the potential of noninvasive MOx measurement to be an effective triage tool and to aid in the assessment of a patient’s response to therapy.
All patients in our study were considered to have some degree of shock, as defined in Table 1. Trauma patients with injuries not serious enough to be admitted into the trauma resuscitation rooms were not included in the study. Also, 17 patients from whom spectra were acquired were excluded from the final analysis because no lactate was drawn within the first hour and shock severity could not be determined (Fig. 2). It is likely that exclusion of patients without an early lactate skewed our “mild” shock category toward the more severely injured side of a patient group with only slightly abnormal vital signs and lactates. Finally, many of the patients in our study received pre-hospital resuscitation, most commonly intravenous fluids. Pre-hospital treatment may have normalized HR and SBP somewhat, giving patients with significant injuries a low shock score (Table 1) upon admission to a trauma resuscitation room.
MOx proved to be sensitive to shock, from mild to severe (Fig. 3) in this pilot study. MOx has the potential to identify and stratify hemorrhagic shock because it is a direct indicator of tissue perfusion. Hemoglobin within arterioles, capillaries, and venules and myoglobin within muscle cells all contribute to the MOx measurement. MOx is a measure of the balance of oxygen supply and demand within muscle cells.
Shock index has been shown to be correlated retrospectively to LOS, length of ICU stay (12), mortality (12, 13), the need for massive blood transfusion (14) and the presence of severe septic shock (11). Figs. 4A and 4B show ROC analyses that compare the ability of MOx and shock index, respectively, to identify shock in general and to predict shock severity. The scoring system shown in Table 1 to classify shock severity was used in both sets of analyses; the same patients were classified as mild, moderate, and severe in Figs. 4A and 4B. MOx and shock index had comparable abilities to identify shock in general, with AUCs of 0.857 and 0.828, respectively. Shock index was very effective in identifying moderate and severe shock with AUCs ≥ 0.96 in these two groups. However, MOx was substantially better at identifying mild shock (AUC = 0.782) compared to shock index (AUC = 0.671).
MOx has potential value in identifying patients in mild shock who may otherwise be missed. The noninvasive nature of the MOx measurement will facilitate frequent patient assessments. A study of patients with initial normal vital signs demonstrated the importance of frequent evaluation in ED patients. Clinical deterioration in the first 24 hr was found to be associated with a mortality rate that was four times higher than in non-deteriorating patients (15).
Rapid and continuous MOx measurement may help to improve the timeliness of triage decisions. The goals of fluid resuscitation and blood transfusion are to maintain or improve tissue perfusion. Since excessive fluid resuscitation (16–18) and blood transfusion (19, 20) can be harmful, a direct measure of tissue perfusion, such as MOx, may help to determine when resuscitation is adequate. Figure 5 indicates the potential value of continuous MOx measurement during fluid resuscitation. Early in the time course, the patient was deteriorating with decreasing MOx and a low MAP. After two fluid boluses, the patient’s MOx was stable with improved MAP. Stable, high MOx values suggest that sufficient treatment had been administered to normalize tissue oxygenation.
In contrast to MOx, SpO2 did not distinguish patients in mild and moderate shock (Table 2). Abnormally low SpO2 was measured in some, but not all, of the patients in severe shock. Occult hypoperfusion (hypoperfusion in the presence of normal vital signs) is one of the more dangerous developments in trauma patients with normal vital signs and SpO2. Patients with occult hypoperfusion have increased LOS (21), and early identification and correction of occult hypoperfusion improves survival (22). With sensitivity to even mild shock, low MOx in the presence of normal vital signs may theoretically be able to signal occult hypoperfusion in trauma patients.
Tissue oxygenation has been of interest for several years as a means for assessing adequacy of the cardiovascular system (23–26). Several devices have been developed based on NIRS. Traditional NIRS has been used in trauma patients to measure tissue oxygen saturation (StO2) with a commercially available device. NIRS has limited value as a triage tool because it is not sensitive enough to identify mild or moderate hypovolemic shock (27). A recent large (n = 490) study of StO2 in trauma patients demonstrated an average difference in StO2 of only 3% between survivors and patients who died (82% vs. 79%) (28), a very small difference from a clinical perspective. In the present pilot study, although the difference did not reach statistical significance due to the small study size, the mean difference between survivors and those that died was 12% (57% vs. 45%). Future studies of MOx are needed to confirm this observed trend.
Our approach to measure MOx using optical spectroscopy differs substantially from traditional NIRS. In contrast to measuring 2–6 discrete wavelengths in the NIR region as traditional NIRS does, we measure and analyze full spectra in the visible and NIR regions. Our device uses a sculpted illuminating light to optimize the signal-to-noise ratio in our system. The large information content associated with full spectra, as opposed to the sparse information provided by a few wavelengths, allows accurate and sensitive MOx measurements from subjects with a wide variety of BMI and skin tones (8).
In this first clinical study with our laboratory prototype device, the exclusion of 16 patients on the basis of high Q-residuals suggested that improvements are needed to our approach. Spectra have high Q-residuals if there is a mismatch between the shapes of their spectra compared to the shapes of spectra in the training set. Interestingly, 12 of the 16 patients with high Q-residuals were outside of the age range of the healthy group who contributed training set spectra to the LWR model. At the extremes, four were 3–15 years old and four were 73–83 years old. It is likely that the small hand sizes of the young children made it difficult to collect spectra of adequate quality. In elderly patients, decreased muscle mass may have caused the ratio of muscle to bone in the tissue underlying the optical probe to be smaller from that ratio in the younger, healthy population that contributed spectra to the LWR model. The different tissue compositions may have caused spectra from elderly patients to be dissimilar to spectra from younger adults. These observations suggest that ultimately, training set spectra should be collected from subjects with a wide age range to accurately measure MOx in all patients.
Our study has several limitations. Because of the small sample size, data in the different shock categories had large standard deviations. While there was a trend that indicated that a higher mortality rate was correlated with lower MOx, the sample size was too small to show statistical significance. Second, we devised the shock grading system to represent a combination of HR, SBP, and lactate. Since the scoring system is not a standard one, our data may not be easily compared to data from other studies. Thirdly, blood sampling for lactate levels that contributed to the shock severity score was done up to an hour after optical spectra were acquired. We could not control the timing of the blood sampling. As lactate is a metabolic response to hypoperfusion, we presumed that lactates acquired within an hour of spectral acquisition were relevant to shock severity at the time of spectral acquisition. However, the mismatch in timing is a limitation of the study. Lastly, because our equipment is currently in early development and is not particularly user-friendly, it was only possible to make initial MOx measurements of trauma patients upon presentation in the ED trauma resuscitation rooms. We did not attempt to measure MOx as treatment progressed in each patient and we did not study the ability of MOx to guide resuscitation.
Conclusions
The results obtained from this study demonstrate that MOx correlated with clinical status at admission to the ED trauma resuscitation room in a population of trauma patients. This measurement of MOx holds promise to identify patients early in the development of shock, which may be particularly useful in trauma patients. Future work with a more clinically compatible version of this technology will be directed toward continuous monitoring. Larger studies using MOx will be needed in the future to determine the value of MOx as a triage tool and as a monitor of tissue perfusion during treatment.
Acknowledgments
Source of Funding:
This study was supported in part by NIH grant 1R21EB009099 and grants from the Seattle Medic One Foundation, the Wallace H. Coulter Foundation, the Washington State Life Sciences Discovery Fund, and the University of Washington Center for Commercialization.
Footnotes
Conflicts of Interest:
Drs. Arakaki and Schenkman and Mr. Ciesielski have a significant financial interest in Opticyte, Inc., which is not directly or significantly related to the research. None of the other authors have disclosed that they have any potential conflicts of interest.
References
- 1.Franklin GA, Boaz PW, Spain DA, Lukan JK, Carrillo EH, Richardson JD. Prehospital hypotension as a valid indicator of trauma team activation. J Trauma. 2000;48(6):1034–7. doi: 10.1097/00005373-200006000-00006. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 2.Woodford MR, Mackenzie CF, DuBose J, Hu P, Kufera J, Hu EZ, Dutton RP, Scalea TM. Continuously recorded oxygen saturation and heart rate during prehospital transport outperform initial measurement in prediction of mortality after trauma. J Trauma Acute Care Surg. 2012;72(4):1006–11. doi: 10.1097/TA.0b013e318241c059. [DOI] [PubMed] [Google Scholar]
- 3.Newgard CD, Rudser K, Hedges JR, Kerby JD, Stiell IG, Davis DP, Morrison LJ, Bulger E, Terndrup T, Minei JP, Bardarson B, Emerson S. A critical assessment of the out-of-hospital trauma triage guidelines for physiologic abnormality. J Trauma. 2010;68(2):452–62. doi: 10.1097/TA.0b013e3181ae20c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyette FX, Meier EN, Newgard C, McKnight B, Daya M, Bulger EM, Powell JL, Brasel KJ, Kerby JD, Egan D, Sise M, Coimbra R, Fabian TC, Hoyt DB. A comparison of prehospital lactate and systolic blood pressure for predicting the need for resuscitative care in trauma transported by ground. J Trauma Acute Care Surg. 2015;78(3):600–6. doi: 10.1097/TA.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barfod C, Lauritzen MM, Danker JK, Soletormos G, Forberg JL, Berlac PA, Lippert F, Lundstrom LH, Antonsen K, Lange KH. Abnormal vital signs are strong predictors for intensive care unit admission and in-hospital mortality in adults triaged in the emergency department – a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2012;20:28. doi: 10.1186/1757-7241-20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merz TM, Etter R, Mende L, Barthelmes D, Wiegand J, Martinolli L, Takala J. Risk assessment in the first fifteen minutes: a prospective cohort study of a simple physiological scoring system in the emergency department. Crit Care. 2011;15(1):R25. doi: 10.1186/cc9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonglet ML, Minon JM, Seidel L, Poplavsky JL, Vergnion M. Prehospital identification of trauma patients with early acute coagulopathy and massive bleeding: results of a prospective non-interventional clinical trial evaluating the Trauma Induced Coagulopathy Clinical Score (TICCS) Crit Care. 2014;18(6):648. doi: 10.1186/s13054-014-0648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arakaki LS, Schenkman KA, Ciesielski WA, Shaver JM. Muscle oxygenation measurement in humans by noninvasive optical spectroscopy and Locally Weighted Regression. Anal Chim Acta. 2013;785:27–33. doi: 10.1016/j.aca.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens H, Naes T. Multivariate Calibration. Chichester: John Wiley & Sons; 1989. [Google Scholar]
- 10.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96. [PubMed] [Google Scholar]
- 11.Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, Panacek E, Shapiro N. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14(2):168–74. doi: 10.5811/westjem.2012.8.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. A prehospital shock index for trauma correlates with measures of hospital resource use and mortality. Surgery. 2012;152(3):473–6. doi: 10.1016/j.surg.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Odom SR, Howell MD, Gupta A, Silva G, Cook CH, Talmor D. Extremes of shock index predicts death in trauma patients. J Emerg Trauma Shock. 2016;9(3):103–6. doi: 10.4103/0974-2700.185272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pottecher J, Ageron FX, Fauche C, Chemla D, Noll E, Duranteau J, Chapiteau L, Payen JF, Bouzat P. Prehospital shock index and pulse pressure/heart rate ratio to predict massive transfusion after severe trauma: Retrospective analysis of a large regional trauma database. J Trauma Acute Care Surg. 2016;81(4):713–22. doi: 10.1097/TA.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen DP, Brabrand M, Lassen AT. Prognosis and risk factors for deterioration in patients admitted to a medical emergency department. PLoS One. 2014;9(4):e94649. doi: 10.1371/journal.pone.0094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, Salim A. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;70(2):398–400. doi: 10.1097/TA.0b013e318208f99b. [DOI] [PubMed] [Google Scholar]
- 17.Roppolo LP, Wigginton JG, Pepe PE. Intravenous fluid resuscitation for the trauma patient. Curr Opin Crit Care. 2010;16(4):283–8. doi: 10.1097/MCC.0b013e32833bf774. [DOI] [PubMed] [Google Scholar]
- 18.Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, Chang HE, Chen SC, Lee CC. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies. Crit Care Med. 2014;42(4):954–61. doi: 10.1097/CCM.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 19.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 20.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettila V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Muller RG, Moller MH, Steensen M, Tjader I, Kilsand K, Odeberg-Wernerman S, Sjobo B, Bundgaard H, Thyo MA, Lodahl D, Maerkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. N Engl J Med. 2014 doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 21.Thom O, Taylor DM, Wolfe RE, Myles P, Krum H, Wolfe R. Pilot study of the prevalence, outcomes and detection of occult hypoperfusion in trauma patients. Emerg Med J. 2010;27(6):470–2. doi: 10.1136/emj.2009.073254. [DOI] [PubMed] [Google Scholar]
- 22.Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 1999;47(5):964–9. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 23.Iyegha UP, Conway T, Pokorney K, Mulier KE, Nelson TR, Beilman GJ. Low StO2 measurements in surgical intensive care unit patients is associated with poor outcomes. J Trauma Acute Care Surg. 2014;76(3):809–16. doi: 10.1097/TA.0b013e3182ab07a4. [DOI] [PubMed] [Google Scholar]
- 24.Khasawneh MA, Zielinski MD, Jenkins DH, Zietlow SP, Schiller HJ, Rivera M. Low tissue oxygen saturation is associated with requirements for transfusion in the rural trauma population. World J Surg. 2014;38(8):1892–7. doi: 10.1007/s00268-014-2505-3. [DOI] [PubMed] [Google Scholar]
- 25.Beekley AC, Martin MJ, Nelson T, Grathwohl KW, Griffith M, Beilman G, Holcomb JB. Continuous noninvasive tissue oximetry in the early evaluation of the combat casualty: a prospective study. J Trauma. 2010;69(Suppl 1):S14–25. doi: 10.1097/TA.0b013e3181e42326. [DOI] [PubMed] [Google Scholar]
- 26.Moore FA, Nelson T, McKinley BA, Moore EE, Nathens AB, Rhee P, Puyana JC, Beilman GJ, Cohn SM, St OSG. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcome. The Journal of trauma. 2008;64(4):1010–23. doi: 10.1097/TA.0b013e31816a2417. [DOI] [PubMed] [Google Scholar]
- 27.Crookes BA, Cohn SM, Bloch S, Amortegui J, Manning R, Li P, Proctor MS, Hallal A, Blackbourne LH, Benjamin R, Soffer D, Habib F, Schulman CI, Duncan R, Proctor KG. Can near-infrared spectroscopy identify the severity of shock in trauma patients? J Trauma. 2005;58(4):806–13. doi: 10.1097/01.ta.0000158269.68409.1c. discussion 13-6. [DOI] [PubMed] [Google Scholar]
- 28.Iyegha UP, Conway T, Pokorney K, Mulier KE, Nelson TR, Beilman GJ. Low StO2 measurements in surgical intensive care unit patients is associated with poor outcomes. J Trauma Acute Care Surg. 2014;76(3):809–16. doi: 10.1097/TA.0b013e3182ab07a4. [DOI] [PubMed] [Google Scholar]


