Abstract
Combat-related blast trauma results in massive tissue injury and tends to involve multiple systems. Further, an acute measure of injury severity based on underlying biological mechanisms may be important for the triage and treatment of these types of patients. We hypothesized that urinary biomarkers (UBs) would reflect severity of injury and that they would be elevated for blast injuries compared to gunshot wounds (GSW) in a cohort of combat casualties. We also postulated that UBs would be higher in patients with burns compared to patients with non-burn trauma in a civilian cohort. Among 80 service members who sustained combat-related injuries, we performed generalized estimating equations to compare differences in log-transformed concentrations of the UBs by both (1) injury severity and (2) injury mechanism. Among 22 civilian patients, we performed Kruskal-Wallis tests to compare differences for the UBs stratified by burn and non-burn trauma. In the military cohort, with the exception of IL-18, all UBs were significantly (p<0.05) higher for patients with a severe combat-related injury (Injury Severity Score≥25). In addition, all crude UBs concentrations were significantly higher for blast vs. GSW patients (p<0.05). After adjusting for injury severity score and time of UB draw, KIM-1 (2.80 vs. 2.31; p=0.03) and LFABP (−1.11 vs. −1.92; p=0.02) were significantly higher for patients with a blast mechanism of injury. There were no significant differences in UBs between burn and non-burn civilian trauma patients. Future studies are needed to understand the physiologic response to trauma and the extent that UBs reflect these underlying processes.
Keywords: biomarkers, military personnel, explosive, burn, trauma
Introduction
Risk prediction in combat casualties is challenging, particularly in the forward deployed, austere combat environment. Two commonly used risk prediction scores are the injury severity score (ISS) (1) and the revised trauma score (2). However, because the determination of an ISS requires trained coders, risk prediction in the forward deployed setting is limited to vital signs [which can quantify risk in the form of the revised trauma score (RTS)] and clinical intuition. While urinary biomarkers (UBs) have generally been thought to be renal specific, they may reflect the overall injury severity in the setting of trauma. The kidneys receive a large portion of cardiac output (3) and are exquisitely sensitive to ischemic injury, making them promising markers for global hypo-perfusion. Indeed, UBs have been demonstrated to correlate with poor outcomes in combat casualties (4). Given the ready availability of urine, even in an austere environment complicated by prolonged evacuation times, UBs have the potential to be a deployable solution to quantify severity of injury and predict risk. Furthermore, because they are markers of organ injury, they have the potential to provide insight on the pathophysiologic response to combat injury.
Explosive injuries and gunshot wounds (GSWs) are the most common causes of combat trauma, accounting for 74.4% and 19.9% of all injuries, respectively (5). These injury patterns differ in both the mechanism and distribution of trauma. Compared to GSWs, where penetrating injuries predominate, explosive injuries are caused by the shock waves of the explosion, fragmentation, structural collapse and thermal injuries (6). This wider distribution and diverse pathology may not be apparent on initial evaluation by the triage clinician, reflected in initial vital signs or accounted for by anatomically based trauma scoring systems like the ISS.
Recent research has concentrated on the ability of UBs to predict acute kidney injury (AKI), need for renal replacement therapy (RRT) and mortality (4, 7, 8). However, most of these biomarkers are not only associated with AKI, but also with the underlying condition causing the AKI (8). For example, both plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) are elevated in patients with septic vs. non-septic AKI (9). Given the different patterns of injury seen in patients with explosive vs. GSWs, it is therefore plausible that UB elevations would differ between these groups. This could provide meaningful clinical information that is not provided by traditional trauma scoring systems or clinical intuition.
Combat-related blast trauma presents a unique mechanism of injury without a clear civilian equivalent. Given the drawdown of large scale combat operations, civilian studies will be needed to prepare for medical care in future conflicts. As explosive injuries are traditionally much less frequent in non-combat zones, an equivalent injury to evaluate the utility of UBs in civilian trauma patients is desirable. Burn injury was coined the “universal trauma model” by Dr Basil Pruitt (10). Notably, both serum and urine NGAL levels correlate with burn severity (11, 12). Comparing UB elevations in civilian populations with and without burn injury, and comparing UB patterns to combat casualties with explosive injuries, would provide evidence that the burn population can serve as a surrogate for future studies.
The purpose of our study was three-fold. Firstly, we sought to examine the relationship between UBs and severity of injury as determined by ISS in a cohort of patients with combat injury. Secondly, we sought to evaluate differences in UB levels between mechanisms of injury (explosive vs. GSW), also in a cohort of combat injured service members. Lastly, given the low prevalence of explosive injury in the civilian population, we sought to evaluate burn injury as a potential surrogate in a mixed civilian population with either burns or other traumatic injuries.
Methods
Study Sample and Ethical Approval
Recruitment and analysis of the military cohort has been previously described (4). In brief, the initial military cohort consisted of 89 injured active duty personnel that met the inclusion criteria from October 2012 to December 2013 admitted to the ICU in Craig Joint Theater Hospital in Afghanistan. Patients were excluded if they did not have a Foley catheter or if more than 48 hours had elapsed since their injury. Urine samples were collected within 3 hours of admission and then daily at 0800. The samples were centrifuged, and frozen prior to shipment to the United States for UB measurement. In order to test the hypothesis for the military cohort, an additional nine service members that did not sustain an injury from either an explosion or GSW (e.g. motor vehicle crash, crush) were excluded. Thus, the final analytic sample size was 80 injured active duty personnel. Service members had between one and four UB samples taken; therefore, a total of 146 observations were used in the analysis.
In order to test the hypothesis using the civilian cohort, we used data from a civilian cohort of 22 patients admitted to San Antonio Military Medical Center for burn or trauma. Urine samples were collected within 24 hours of admission and processed in the same laboratory as the downrange samples. Both the military (M-10238) and civilian (H-10-029) protocols were reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board.
Dependent Variables
Based on the data available at the time of study conception (13), there were five UB dependent variables of interest: (1) NGAL, (2) cystatin C (CyC), (3) kidney injury molecule-1 (KIM-1), (4) interleukin (IL)-18, and (5) liver-type fatty acid-binding protein (LFABP). As others have done (14, 15), biomarker levels were normalized for urine concentration by dividing by the creatinine concentration. Three of the biomarkers (CyC, LFABP, NGAL) were measured in nanograms per milligram of creatinine (ng/mg) and two biomarkers (IL-18, KIM-1) were measured in picograms per milligram of creatinine (pg/mg).
Independent Variables
The two primary independent variables of interest were (1) severe combat-related injuries and (2) mechanism of injury. A severe combat-related injury was a dichotomous variable defined as having an ISS ≥25 (yes vs. no). Patients were also categorized into a dichotomous variable based on mechanism of injury (explosive vs. GSW). Descriptive data on demographic variables (age, race, and sex), time to first UB draw following injury, mean arterial pressure (MAP) at ICU admission, AKI, RRT, and mortality were also reported. AKI was defined using an accepted creatinine based criteria (16) as previously described (4).
Statistical Methods
Descriptive statistics on demographic variables (age and sex), time to first UB draw, AKI, RRT, and mortality were reported. The median and interquartile ranges were reported for continuous variables. The number and proportion were reported for categorical variables. Patients’ baseline characteristics were compared by mechanism of injury (explosive vs. GSW). For continuous variables, the Kruskal-Wallis test was performed and for categorical variables either the Chi-square test or Fisher’s exact test was performed, as appropriate. In order to normalize the data, all UBs were natural log-transformed. The hypothesis for the military cohort was that levels of UBs would be significantly elevated for service members with both severe injuries and an explosive-mechanism of injury. To test this hypothesis, generalized estimating equations (GEE) with a normal distribution and exchangeable covariance structures were used to perform repeated measures regression analysis. There were three GEE models performed for each of the five log-transformed UBs. The first model regressed the UB concentration on severe combat-related injury status (yes vs. no) while adjusting for UB draw time. The second model regressed the UB concentration on mechanism of injury. The third and final model regressed UB concentration on mechanism of injury while adjusting for injury severity score and draw time. For each of the five UBs, log-transformed least squares (LS) means and 95% confidence intervals were reported.
The hypothesis for the civilian cohort was that compared to patients with non-burn trauma, levels of UBs would be significantly elevated for patients with burn-related trauma. In order to test this hypothesis, the Kruskal-Wallis test was performed. A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
The baseline characteristics of the military cohort are presented in Table 1. They were primarily young (median: 26 years; IQR: 23–30), male (n=77; 93%) and were injured by an explosion (n=55, 69%). The median ISS of the cohort was 18 (IQR: 12–41) with 44% sustaining a severe combat-related injury (n=35) defined as an ISS ≥25. The median first time to UB draw was 17 hours (IQR: 10–27) after injury. Approximately one-third of these patients had AKI (n=29; 36%) and a small proportion required RRT (n=6; 8%). Eight patients (10%) died of wounds. Compared to patients with GSW, patients with explosive injuries had higher median ISS (26 vs. 16; p=0.04) and a longer median interval from injury to UB sampling (21 hours vs. 12 hours; p=0.04).
Table 1.
Baseline Characteristics of Military Cohort
| Full Cohort | Explosive | GSW | p value* | |
|---|---|---|---|---|
| N | 80 | 55 | 25 | – |
| Age, median (IQR) | 26 (23–30) | 26 (23–29) | 28 (24–31) | 0.14 |
| Male, N (%) | 77 (93) | 52 (95) | 25 (100) | 0.55 |
| Injury Severity Score, median (IQR) | 18 (12–41) | 26 (14–50) | 16 (9–25) | 0.04 |
| Injury Severity Score ≥25, N (%) | 35 (44) | 28 (51) | 7 (28) | 0.06 |
| Urine draw time from injury in hours, median (IQR) | 17 (10–27) | 21 (11–28) | 12 (10–19) | 0.04 |
| Mean Arterial Pressure (mmHg) | 86 (76–101) | 84 (72–100) | 96 (79–101) | 0.15 |
| Acute Kidney Injury, N (%) | 29 (36) | 18 (33) | 11 (44) | 0.33 |
| Renal Replacement Therapy, N (%) | 6 (8) | 5 (9) | 1 (4) | 0.66 |
| Mortality, N (%) | 8 (10) | 6 (11) | 2 (8) | 1.00 |
GSW= Gunshot wound
Comparing explosive to gunshot wound
Compared to patients with less severe injury, patients with severe combat-related injury (ISS≥25) were more likely to develop AKI (48.6% vs. 26.7%, p=0.04) and were more likely to die (22.9% vs. 0%, p<0.01). No differences were observed in age, gender, time to first urine collection, MAP at ICU admission or RRT requirement.
Patients with severe combat-related trauma had statistically higher concentrations of all UBs except IL-18 (0.08 vs. −0.50; p=0.08) (Figure 1). Compared to patients with GSWs, patients with an explosive mechanism of injury had statistically higher concentrations of all five UBs (Figure 2). After adjusting for draw-time and ISS, only KIM-1 (2.80 vs. 2.31; p=0.03) and LFABP (−1.11 vs. −1.92; p=0.02) were significantly higher for patients with an explosive mechanism of injury (Figure 3).
Figure 1.
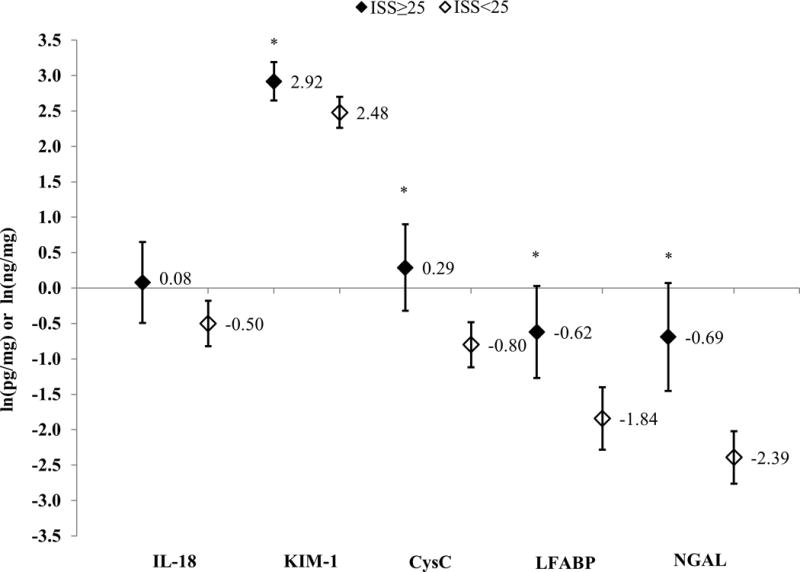
Log-Transformed Least Squares Means and 95% Confidence Intervals for Urinary Biomarker Concentrations, Stratified by Severe Combat-Injury Status; IL-18 and KIM-1 measured in pg/mg of creatinine; CysC, LFABP, and NGAL measured in ng/mg of creatinine; All least-squares means adjusted for time to urinary biomarker draw time; *p<0.05
Figure 2.
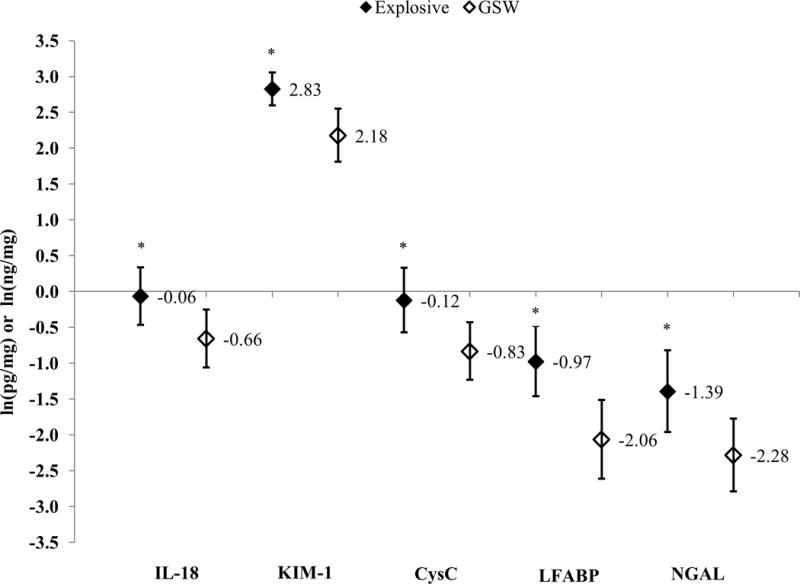
Log-Transformed Least Squares Means and 95% Confidence Intervals for Urinary Biomarker Concentrations, Stratified by Mechanism of Injury; IL-18 and KIM-1 measured in pg/mg of creatinine; CysC, LFABP, and NGAL measured in ng/mg of creatinine; *p<0.05
Figure 3.
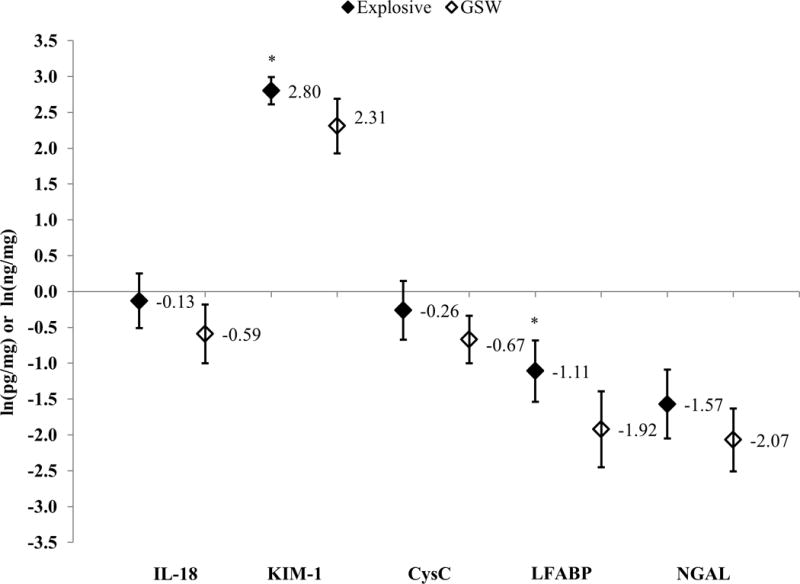
Log-Transformed Least Squares Means and 95% Confidence Intervals for Urinary Biomarker Concentrations, Stratified by Mechanism of Injury; IL-18 and KIM-1 measured in pg/mg of creatinine; CysC, LFABP, and NGAL measured in ng/mg of creatinine; All least-squares means adjusted for injury severity score and time to urinary biomarker draw time; *p<0.05
The civilian cohort (n=22) consisted of predominantly middle-aged (median: 48 years; IQR: 33–58), male (n=16; 73%) patients. Exactly half of the patients sustained a burn-related trauma (n=11; 50%) and approximately one-third of patients died (n=7; 32%). Both the burn and trauma patients had similar median ages (47 years vs. 48 years, respectively) and were predominantly male (64% vs. 82%, respectively). Of the patients with trauma, seven were injured in motor vehicle accidents, two patients were injured by GSWs, one person was injured in a physical assault and one patient was injured as the result of a fall. In the patients with burn injury, the median total body surface area burned (TBSA) was 26.5% (IQR: 19.6%–51.0%, range: 9.0%–77.0%). Compared to the trauma civilian patients, none of the UBs were significantly higher for burned civilian patients (Table 2).
Table 2.
Log-Transformed Urinary Biomarker Concentration of Civilian Patients, Stratified by Burn Status
| Urinary Biomarker | Burn (n=11) | Trauma (n=11) | p-value* |
|---|---|---|---|
| IL-18 (median, IQR) | 1.15 (0.33–1.99) | 0.36 (−0.14–0.76) | 0.16 |
| KIM-1 (median, IQR) | 3.61 (3.24–3.72) | 3.44 (2.85–4.41) | 0.87 |
| CysC (median, IQR) | 0.01 (−1.23–0.97) | −0.87 (−2.16–0.01) | 0.36 |
| LFABP (median, IQR) | −0.91 (−2.69–0.96) | −1.47 (−2.99–0.03) | 0.48 |
| NGAL (median, IQR) | −1.26 (−1.76–0.49) | −1.54 (−2.11–1.06) | 0.37 |
p-value for Kruskal-Wallis test
Discussion
This study had three primary findings: 1) with the exception of IL-18, concentrations of UBs were significantly higher for service members with severe combat-related injuries; 2) concentrations of all five UBs were higher for service members with an explosive mechanism of injury; and 3) even after adjusting for overall injury severity, two biomarkers (KIM-1 and LFABP) were significantly higher for service members with explosive mechanisms of injury. We did not observe differences in civilian patients with burn and non-burn trauma, however the sample size was admittedly underpowered for the civilian cohort (n=22).
The patients in the cohort with severe combat-related injury (defined as ISS≥25) were clearly severely injured and at high risk for complications. These patients had a higher mortality rate (22.9% vs. 0% for those with ISS<25) and were more likely to develop AKI (48.6% vs. 26.7%). We found that four of the UBs studied, KIM-1, CysC, LFABP and NGAL, were associated with severe combat-related injury. This demonstrates the feasibility of using UBs for estimating injury severity in combat casualties. When these findings are put in the context of our prior work in this cohort showing that UBs predict the combined endpoint of mortality or need for RRT (4), they further the concept that UBs can be used for the estimation of injury severity and risk stratification in the forward-deployed combat environment. In this setting UBs could be used for the purposes of triage and prioritization of evacuation. However, an understanding of the timing of UB collection in conjunction with different types of resuscitation is also important to meet this goal. For example, even after adjustment for draw time and ISS, we found that all five UBs were significantly elevated for patients that received a massive transfusion (defined as 10 units of packed red blood cells in the first 24 hours, data not shown). However, our inferences regarding these results are limited because of the variable timing between resuscitation and UB sample collection.
We found that all five UBs studied were higher in patients with explosive injuries. However, this association remained significant only for LFABP and KIM-1 after adjustment for draw time and ISS. There are two biologically plausible mechanisms for differences in UB patterns among patients with explosive vs. GSW injuries. Firstly, the hemodynamic effects of explosive injury which are independent of blood loss, include bradycardia, hypotension, and myocardial depression without a compensatory increase in peripheral vascular resistance (17). This response is likely driven by a vagal reflex (18) and overproduction of nitric oxide (19). These effects, which would not be accounted for by an anatomically based scoring system like the ISS, might result in prolonged ischemia to the kidneys with resultant increase in UB concentrations. The second plausible mechanism is the effect of blast injury at the tissue level. Blast injury has been shown to cause elevations in IL-6, E-selectin, tumor necrosis factor-α (TNF-α), hypoxia inducible factor-1 (HIF-1), thrombomdulin and platelet derived growth factor (20, 21), a process that may be mediated by endothelial damage (20). KIM-1 is a transmembrane glycoprotein that is undetectable in healthy kidneys, but becomes highly expressed and detectable in urine within hours after renal ischemic injury; it is thought to promote apoptotic and necrotic cell clearance as well as to facilitate remodeling in the injured epithelium (22). The shedding of KIM-1 into the urine is mediated by matrix metalloproteinase, a process which is upregulated by TNF-α (23). LFABP is expressed in the kidneys, as well as the liver, intestine, pancreas, lungs, and stomach. Urinary levels increase after an acute ischemia-reperfusion injury and are highly correlated with ischemic time (24). LFABP expression in the setting of hypoxia is likely driven by HIF-1 (25). The tissue level effects of blast injury could therefore increase KIM-1 and LFABP in the urine via increased levels of TNF-α and HIF-1, respectively. This damage at the cellular level is not accounted for by traditional scoring systems for trauma. While further work is clearly required before UBs can be operationalized, our findings provide further evidence that UBs have the potential to revolutionize risk prediction in critically injured combat casualties.
Beyond risk prediction, how can this knowledge be used to advance combat casualty care? As recently reviewed (26), LFABP is thought to be a marker for renal hypoxia and vulnerability to further renal insults. KIM-1 may play a central role in renal recovery and thus, when it appears in the urine, the optimal treatment strategy may be one focused on enhancing renal recovery (26). In this context, our findings imply that avoiding further nephrotoxic insults and developing treatments that aide renal recovery may be of greater benefit to patients with explosive compared to GSW injuries.
Other groups have looked at both serum and urine biomarkers in the setting of burn or traumatic injury. Urinary NGAL has been associated with AKI in critically ill trauma patients (27). Both plasma and urine NGAL have also been associated with AKI in patients with burn injury (11, 12). Serum NGAL has been associated with mortality and renal failure in combat casualties (28). Serum LFABP has been examined as a predictor of severe abdominal injuries (defined as an abdominal Abbreviated Injury Score≥3) (29). These investigators found that LFABP level drawn at admission predicted the subsequent diagnosis of a severe abdominal injury with 80% sensitivity and 75% specificity (29). While the intention of our study was not to examine the utility of UBs for the purposes of diagnosing an intra-abdominal injury, these findings underscore the potential of biomarkers in the forward deployed/prolonged field care setting where advanced diagnostics (e.g. CT scan) may not be available.
Given the rarity of explosive injuries in the civilian setting and the continued withdrawal of US military forces from combat zones, new models of military trauma will need to be studied to optimize healthcare in future conflicts. Burn injury has been coined the “universal trauma model” (10). We therefore hypothesized that burn injury would result in higher UB concentrations compared with non-burn trauma. If the UB patterns in burn patients were similar to that seen in explosive injury, it would suggest that burn injured patients could serve as a model for explosive injury in biomarker research. While we did not find that UBs were elevated in burn patients, our sample size was small (n=22) which limited our statistical power. Future studies with a more robust sample size and rich clinical data would allow for a more thorough investigation of burns as a potential civilian proxy for military-related explosive injuries.
This study had several limitations. The small number of patients enrolled, in both the combat and civilian settings, limits the statistical power of the study. This was particularly problematic for the study of burn patients. This exploratory sub-study was designed to generate hypotheses and begin to establish war parallels to a civilian patient population. We are currently enrolling burn patients in a separate study to examine this association further. Differences in baseline characteristics, including ISS and timing of urine draw, were observed between patients with explosive and GSW mechanisms of injury. While we attempted to account for these in our models, we cannot rule out bias. For example, if biomarkers are elevated closer to the point of injury, then biomarkers for the explosive injuries maybe biased towards the null because patients with explosive injuries had a longer time to urinary biomarker measurement. Furthermore, a lack of serum samples does not allow us to test our hypotheses with respect to endothelial damage. While this study does not have the capability to elucidate mechanisms, it suggests such work on mechanisms will be important in the future. Given both the unique patient population and unique patterns of combat injury, this work will be difficult to do in civilian trauma populations. In preparation for the next conflict, the military research community should consider a large scale, prospective study involving protocolized urine and serum collection from combat injured patients. This could be accomplished by leveraging existing systems, which already collects data on combat casualties. Such an effort would provide invaluable insights into the pathophysiology of combat trauma. This knowledge could be used to define accurate risk prediction models, develop novel treatments for combat trauma and ultimately improve outcomes.
In conclusion, we found that UBs were associated with severity of injury and found differences in UB concentrations based on mechanism of injury. In the context of our prior work demonstrating that UBs predicted mortality or the need for RRT (4), these findings provide more evidence for the role of UBs in the forward deployed setting. Current techniques, which utilize enzyme-linked immunosorbent assays, are not suitable for deployment in austere settings. However, if further developed into rapid and deployable tests (30), future studies utilizing serial measurements across the spectrum of care could be done to assess the predictive power of biomarkers at all phases of injury. In the austere, forward deployed environment this information could be used to triage patients and prioritize aeromedical evacuation. In the future, a better understanding of the physiologic response to trauma and the extent that UBs reflect these underlying processes, could be used to develop tailored approaches for combat casualties based not only on severity, but also mechanism of injury.
Acknowledgments
The authors would like to thank Dr. Kristen R. Glass, Dr. Benjamin D. Morrow, Dr. Wayne Latack, Dr. Kristin K. Saenz, Catherine Rauschendorfer, Javance Tercero and the members of the United States Central Command Joint Combat Casualty Research Team who assisted with data collection.
Source of Funding:
The study was funded by a grant from the Air Force Medical Support Agency and by an appointment to the Internship/Research Participation Program at the United States Army Institute of Surgical Research (USAISR), administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA. Additional funding was also provided by the Clinical Trials Task Area, USAISR.
Footnotes
Conflicts of interest
Dr. Edward Siew reports a consultancy with Alere. Otherwise, the authors have no potential conflicts of interest to disclose.
Disclaimer:
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army the Department of the Air Force, the Department of Defense or the U.S. Government.
Authorship Statement
All of the authors have met one or more of the International Committee of Medical Journal Editors criteria for authorship.
References
- 1.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96. [PubMed] [Google Scholar]
- 2.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the Trauma Score. J Trauma. 1989;29(5):623–9. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Botti RE, Razzak MA, MacIntyre WJ, Pritchard WH. The relationship of renal blood flow to cardiac output in normal individuals as determined by concomitant radioisotopic measurements. Cardiovasc Res. 1968;2(3):243–6. doi: 10.1093/cvr/2.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Stewart IJ, Glass KR, Howard JT, Morrow BD, Sosnov JA, Siew ED, Wickersham N, Latack W, Kwan HK, Heegard KD, Diaz C, Henderson AT, Saenz KK, Ikizler TA, Chung KK. The potential utility of urinary biomarkers for risk prediction in combat casualties: a prospective observational cohort study. Crit Care. 2015;19:252. doi: 10.1186/s13054-015-0965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmont PJ, Jr, McCriskin BJ, Sieg RN, Burks R, Schoenfeld AJ. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J Trauma Acute Care Surg. 2012;73(1):3–12. doi: 10.1097/TA.0b013e318250bfb4. [DOI] [PubMed] [Google Scholar]
- 6.Plurad DS. Blast injury. Mil Med. 2011;176:276–82. doi: 10.7205/milmed-d-10-00147. [DOI] [PubMed] [Google Scholar]
- 7.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–73. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D’Amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36(3):452–61. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt B., Jr The universal trauma model. Bull Am Coll Surg. 1985;70(10):2–13. [Google Scholar]
- 11.Yavuz S, Anarat A, Acarturk S, Dalay AC, Kesiktas E, Yavuz M, Acarturk TO. Neutrophil gelatinase associated lipocalin as an indicator of acute kidney injury and inflammation in burned children. Burns. 2014;40(4):648–54. doi: 10.1016/j.burns.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Yang HT, Yim H, Cho YS, Kym D, Hur J, Kim JH, Chun W, Kim HS. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit Care. 2014;18(4):R151. doi: 10.1186/cc13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein SL. Acute kidney injury biomarkers: renal angina and the need for a renal troponin I. BMC medicine. 2011;9(1):1. doi: 10.1186/1741-7015-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D’amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive care medicine. 2010;36(3):452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 15.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase–associated lipocalin for diagnosing acute kidney injury. Annals of internal medicine. 2008;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eknoyan G, Lameire N, Eckardt K, Kasiske B. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney international Supplements. 2012;2:1–138. [Google Scholar]
- 17.Irwin RJ, Lerner MR, Bealer JF, Brackett DJ, Tuggle DW. Cardiopulmonary physiology of primary blast injury. J Trauma. 1997;43:650–5. doi: 10.1097/00005373-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Irwin RJ, Lerner MR, Bealer JF, Mantor PC, Brackett DJ, Tuggle DW. Shock after blast wave injury is caused by a vagally mediated reflex. J Trauma. 1999;47:105–10. doi: 10.1097/00005373-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Zunic G, Pavlovic R, Malicevic Z, Savic V, Cernak I. Pulmonary blast injury increases nitric oxide production, disturbs arginine metabolism, and alters the plasma free amino acid pool in rabbits during the early posttraumatic period. Nitric Oxide. 2000;4(2):123–8. doi: 10.1006/niox.2000.0276. [DOI] [PubMed] [Google Scholar]
- 20.Spear AM, Davies EM, Taylor C, Whiting R, Macildowie S, Kirkman E, Midwinter M, Watts SA. Blast Wave Exposure to the Extremities Causes Endothelial Activation and Damage. Shock. 2015;44(5):470–8. doi: 10.1097/SHK.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning JL, Mo LW, Lu KZ, Lai XN, Wang ZG, Ma D. Lung injury following lower extremity blast trauma in rats. J Trauma Acute Care Surg. 2012;73(6):1537–44. doi: 10.1097/TA.0b013e318266013a. [DOI] [PubMed] [Google Scholar]
- 22.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29:1301–11. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim AI, Chan LY, Lai KN, Tang SC, Chow CW, Lam MF, Leung JC. Distinct role of matrix metalloproteinase-3 in kidney injury molecule-1 shedding by kidney proximal tubular epithelial cells. Int J Biochem Cell Biol. 2012;44(6):1040–50. doi: 10.1016/j.biocel.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 25.Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T, Portilla D, Sugaya T. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296(4):F669–79. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10(1):147–55. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, Rizos D, Baltopoulos G, Haliassos A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47(1):79–82. doi: 10.1515/CCLM.2009.004. [DOI] [PubMed] [Google Scholar]
- 28.Mellor AJ, Woods D. Serum neutrophil gelatinase-associated lipocalin in ballistic injuries: a comparison between blast injuries and gunshot wounds. J Crit Care. 2012;27:419.e1–5. doi: 10.1016/j.jcrc.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Voth M, Holzberger S, Auner B, Henrich D, Marzi I, Relja B. I-FABP and L-FABP are early markers for abdominal injury with limited prognostic value for secondary organ failures in the post-traumatic course. Clin Chem Lab Med. 2015;53(5):771–80. doi: 10.1515/cclm-2014-0354. [DOI] [PubMed] [Google Scholar]
- 30.Sato R, Suzuki Y, Takahashi G, Kojika M, Inoue Y, Endo S. A newly developed kit for the measurement of urinary liver-type fatty acid-binding protein as a biomarker for acute kidney injury in patients with critical care. J Infect Chemother. 2015;21:165–9. doi: 10.1016/j.jiac.2014.10.017. [DOI] [PubMed] [Google Scholar]


