Abstract
Objective
Delirium occurs frequently in adults, and is an independent predictor of mortality. However, the epidemiology and outcomes of pediatric delirium are not well-characterized. The primary objectives of this study were to describe the incidence of delirium in critically ill children, its duration, associated risk factors, and effect on in-hospital outcomes, including mortality. Secondary objectives included determination of delirium subtype, and effect of delirium on duration of mechanical ventilation (MV), and length of hospital stay (LOS).
Design
Prospective longitudinal cohort study.
Setting
Urban academic tertiary care pediatric intensive care unit (PICU).
Patients
All consecutive admissions from September 2014 through August 2015.
Intervention
Children were screened for delirium twice daily throughout their ICU stay.
Measurements and Main Results
Of 1547 consecutive patients, delirium was diagnosed in 267 (17%), and lasted a median of two days (IQR 1,5). Seventy-eight percent of children with delirium developed it within the first three PICU days. Most cases of delirium were of the hypoactive (46%) and mixed (45%) subtypes; only 8% of delirium episodes were characterized as hyperactive delirium. In multivariable analysis, independent predictors of delirium included age ≤2 years, developmental delay, severity of illness, prior coma, mechanical ventilation, and receipt of benzodiazepines and anticholinergics. PICU LOS was increased in children with delirium (adjusted relative LOS 2.3, CI= 2.1, 2.5, p<0.001), as was duration of MV (median 4 vs. 1 day, p<0.001). Delirium was a strong and independent predictor of mortality (adjusted OR 4.39, CI= 1.96–9.99, p<0.001).
Conclusions
Delirium occurs frequently in critically ill children and is independently associated with mortality. Some in-hospital risk factors for delirium development are modifiable. Interventional studies are needed to determine best practices to limit delirium exposure in at-risk children.
Keywords: delirium, pediatric, mortality, epidemiology, critical care, intensive care
Introduction
Delirium is a frequent and serious complication of critical illness, and has been linked to increased mortality, prolongation and complication of hospitalization, and long-term disability(1,2). An extensive literature exists describing the incidence, duration, risk factors, subtypes, and outcomes of delirium in adults, but there are few prospective longitudinal studies in critically ill children that describe the natural history of pediatric delirium (PD)(3–6).
In this study, we describe a cohort of children admitted to a single pediatric intensive care unit (PICU) over a calendar year. Our objective was to screen each child for delirium daily, from PICU admission through discharge, to determine incidence of delirium, time to onset, duration and fluctuation of clinical manifestations, phenotype of delirium, associated risk factors, and effect of delirium on in-hospital outcome measures (including mortality, length of stay (LOS), and duration of mechanical ventilation (MV). This is one of several planned analyses involving this cohort of patients, and all data described here are novel and have not been published elsewhere. A subset of these patients were included in an analysis of the effect of PD on hospital costs(7).
Materials and Methods
The Weill Cornell Medical College Institutional Review Board approved this observational, minimal risk study with waiver of requirement for informed consent. This prospective longitudinal study took place in an urban, academic, tertiary care, mixed PICU. All patients admitted to the PICU service for at least 24 hours between September 1, 2014 and August 31, 2015 were included.
Consistent with our PICU standard, each child was screened for delirium twice daily by the bedside nurse, at 6 am and 6 pm, using the Cornell Assessment of Pediatric Delirium (CAPD)(8). This is also consistent with the recent position paper published by the European Society of Paediatric and Neonatal Intensive Care (ESPNIC), recommending use of CAPD to assess for pediatric delirium (grade of recommendation = A) every 8–12 hours(9). The CAPD is a well-validated observational 8-item tool that can reliably distinguish between pain, agitation, residual sedation, and delirium(8,9). A CAPD score of 9 or higher represents a positive screen; the diagnosis is then confirmed by the physician(8). Any developmentally delayed child who screened positive for delirium on the CAPD subsequently had the diagnosis of delirium confirmed (or refuted) by an intensivist or psychiatrist prior to being classified as delirious. (The clinician had to establish an alteration from mental status at pre-hospital baseline, in order to ensure that static encephalopathy – i.e.: the underlying developmental delay – was not confused with delirium).
Each child was assigned a daily status: “comatose” (patients with a Richmond Agitation Sedation Scale (RASS) score of −4 or −5, who are unarousable to verbal stimulation(10,11), and therefore impossible to assess for delirium), “delirious” (CAPD score ≥9 with diagnosis confirmed by physician), or “normal” mental status (i.e.: delirium-free and coma-free (DFCF)). If an assessment opportunity was missed (due to noncompliance with screening protocol), status for that day was designated as “unknown”.
All patients who were diagnosed with delirium at least once during their PICU stay were designated “ever delirious” and compared to those patients who were never delirious. Time to onset of delirium was defined as the number of days from PICU admission to the first diagnosis of delirium. Duration of delirium was defined as the number of days spent delirious during the PICU stay. Recurrent delirium (which frequently is a warning sign of new inter-current illness) was defined as a second episode of delirium during a single hospital admission, following a minimum of 24 hours spent with normal mental status (DFCF).
Delirium subtype was determined by psychomotor activity and level of alertness, as assessed by the RASS, over the 24 hour period(11, 12). The frequency of RASS assessment was dependent on patient’s acuity level, and ranged from hourly to every 4 hours. The RASS score ranges from −5 (unarousable), through 0 (alert and calm), to +4 (combative)(10). Delirious children with RASS scores from 0 to −3 were designated as having hypoactive delirium. Delirious children with RASS scores from 0 to +4 were designated as having hyperactive delirium. Children with RASS scores crossing zero (including both negative and positive numbers) were designated as having mixed delirium.
Demographic data, including age, sex, primary diagnosis, pre-existing medical conditions, severity of illness (as measured by Pediatric index of Mortality-3 (PIM3) score(13), and divided into tertiles), and developmental status were collected on admission. As developmentally delayed children are at increased risk for developing delirium during critical illness, it is important to include these children in delirium research (8). “Developmental delay” was defined as a Pediatric Cerebral Performance Category (PCPC) of 4 (severe disability; conscious but dependent on others for daily support because of impaired brain function) at pre-hospital baseline (14).
Individual patient data, including Pediatric Logistic Organ Dysfunction 2 (PELOD-2) score(15) (after excluding neurologic component so as not to allow the presence of delirium to affect daily organ dysfunction score), RASS scores(10, 12), CAPD scores(8), need for respiratory support, and exposure to medications by categories (including narcotics, benzodiazepines, corticosteroids, anticholinergics, vasoactive medications, and neuroleptics), as well as other variables were collected daily. Upon discharge from hospital, mortality, duration of MV, PICU LOS, and hospital LOS were recorded.
Statistical Analysis
Demographic and clinical data were reported as N (%) and median (IQR) for categorical and continuous variables, respectively. Bivariate relationships between relevant variables and delirium development (ever vs. never delirious) were analyzed by Chi-Square/Fisher’s Exact tests, as appropriate. A conservative approach was taken towards unknown days, presuming them to be non-delirious, in order to avoid overestimating delirium presence. Multivariable logistic regression, modeling delirium development (ever/never), was constructed from bi-directional stepwise selection based on Akaike information criterion (AIC) of all factors from bivariate analyses that reached significance of 0.1. A multivariable linear regression was constructed to model PICU LOS, using same approach of the following relevant risk factors: delirium development, mechanical ventilation, probability of mortality, age, developmental delay, and pre-existing medical condition. Because LOS was skewed, LOS was log-transformed prior to modeling. A final multivariable logistic regression was constructed to assess the independent effect of delirium development on in-hospital mortality after controlling for severity of illness on admission. Statistical tests were two-sided with significance evaluated at 0.05 alpha level. Analyses were performed with R version 3.2.4 for Windows 64-bit.
Results
Descriptives of Patient Population
Our cohort included 1,547 unique admissions, and 7,591 study days. Demographic and clinical patient information is presented in Table 1. Fifty-seven percent of patients were male, 59% were under age five, and 21% were developmentally delayed. Forty-six percent were admitted with a primary diagnosis of respiratory failure, and 31% were admitted for post-operative care. Forty-two percent of patients were mechanically ventilated during their PICU stay. Forty-three percent were prescribed narcotics, 29% benzodiazepines, and 41% corticosteroids during their PICU course. Only seven percent of patients were on vasoactive medications. The average probability of mortality (POM) as calculated by PIM3 was 2%, with a median of 1%. Median PICU LOS was three days.
Table 1.
Patient Characteristics (N=1547)
| Characteristic | Descriptive | Overall Cohort (N=1547), N (%) | Never Delirious (N=1280), N (%) | Ever Delirious (N=267), N (%) | p-value |
|---|---|---|---|---|---|
| Sex | Male | 880 (56.88%) | 725 (56.64%) | 155 (58.05%) | 0.722 |
| Female | 667 (43.12%) | 555 (43.36%) | 112 (41.95%) | ||
|
| |||||
| Age at Admission (years) | 0–2 | 592 (38.27%) | 478 (37.34%) | 114 (42.70%) | 0.031 |
| >2–5 | 317 (20.49%) | 253 (19.77%) | 64 (23.97%) | ||
| >5–13 | 350 (22.62%) | 298 (23.28%) | 52 (19.48%) | ||
| >13 | 288 (18.62%) | 251 (19.61%) | 37 (13.86%) | ||
|
| |||||
| Probability of Mortality1 | <=0.8% | 518 (33.48%) | 465 (36.33%) | 53 (19.85%) | <0.001 |
| >0.8% & <= 1.4% | 509 (32.90%) | 440 (34.38%) | 69 (25.84%) | ||
| >1.4% | 520 (33.61%) | 375 (29.30%) | 145 (54.31%) | ||
|
| |||||
| Admitting Diagnosis Category | Respiratory insufficiency/failure | 709 (45.83%) | 589 (46.02%) | 120 (44.94%) | 0.222 |
| Infectious/inflammatory | 176 (11.38%) | 144 (11.25%) | 32 (11.99%) | ||
| Cardiac disease | 127 (8.21%) | 96 (7.50%) | 31 (11.61%) | ||
| Neurologic disorder | 366 (23.66%) | 304 (23.75%) | 62 (23.22%) | ||
| Hematologic/Oncologic disorder | 68 (4.40%) | 60 (4.69%) | 8 (3.00%) | ||
| Renal/Metabolic disorder | 101 (6.53%) | 87 (6.80%) | 14 (5.24%) | ||
|
| |||||
| Pre-Existing Medical Condition | No | 479 (30.96%) | 423 (33.05%) | 56 (20.97%) | <0.001 |
| Yes | 1068 (69.04%) | 857 (66.95%) | 211 (79.03%) | ||
|
| |||||
| Developmental Delay2 | No | 1228 (79.38%) | 1073 (83.83%) | 155 (58.05%) | <0.001 |
| Yes | 319 (20.62%) | 207 (16.17%) | 112 (41.95%) | ||
|
| |||||
| Ever in Coma3 | No | 1410 (91.14%) | 1236 (96.56%) | 174 (65.17%) | <0.001 |
| Yes | 137 (8.86%) | 44 (3.44%) | 93 (34.83%) | ||
|
| |||||
| Ever Mechanically Ventilated | No | 905 (58.50%) | 825 (64.45%) | 80 (29.96%) | <0.001 |
| Yes | 642 (41.50%) | 455 (35.55%) | 187 (70.04%) | ||
|
| |||||
| Benzodiazepines4 | Never | 1104 (71.36%) | 1025 (80.08%) | 79 (29.59%) | <0.001 |
| Ever | 443 (28.64%) | 255 (19.92%) | 188 (70.41%) | ||
|
| |||||
| Narcotics4 | Never | 883 (57.08%) | 806 (62.97%) | 77 (28.84%) | <0.001 |
| Ever | 664 (42.92%) | 474 (37.03%) | 190 (71.16%) | ||
|
| |||||
| Vasoactive Medications4 | Never | 1438 (92.95%) | 1232 (96.25%) | 206 (77.15%) | <0.001 |
| Ever | 109 (7.05%) | 48 (3.75%) | 61 (22.85%) | ||
|
| |||||
| Corticosteroids4 | Never | 908 (58.69%) | 778 (60.78%) | 130 (48.69%) | <0.001 |
| Ever | 639 (41.31%) | 502 (39.22%) | 137 (51.31%) | ||
|
| |||||
| Anti-Cholinergics4 | Never | 405 (26.18%) | 373 (29.14%) | 32 (11.99%) | <0.001 |
| Ever | 1142 (73.82%) | 907 (70.86%) | 235 (88.01%) | ||
|
| |||||
| In-hospital Mortality | Never | 1521 (98.32%) | 1268 (99.06%) | 253 (94.76%) | <0.001 |
| Ever | 26 (1.68%) | 12 (0.94%) | 14 (5.24%) | ||
Description of the 1547 admissions to the PICU during the study period. In bivariate analyses, pre-existing factors associated with diagnosis of delirium included age ≤2 years, developmental delay, pre-existing medical condition, and severity of illness. PICU-related factors linked to the development of delirium included mechanical ventilation (MV), coma, and receipt of benzodiazepines, narcotics, corticosteroids, anticholinergics, and vasoactive medications.
Probability of Mortality as determined by the PIM3 score.
Developmental delay defined as Pediatric Cerebral Performance Category 4 (see text for details).
Coma defined as unarousable to verbal stimulation, generally as a result of pharmacologic sedation (see text for details).
Medication categories: “Ever” includes patients who ever received a medication in this class.
Delirium Incidence and Associated Risk Factors
Delirium was diagnosed in 267 patients, for an incidence of 17.3%. Of the 7,591 patient study days, 1,259 were days with delirium, for a prevalence of 16.6% in this cohort. 566 patient days (7.5%) were classified as days with coma (almost always medication-induced), and 5671 patient days (74.7%) as days with normal mental status (DFCF). Only 95 patient days (<2%) were classified as unknown, due to missed opportunities for delirium screening.
In bivariate analyses(Table 1), pre-existing factors associated with diagnosis of delirium included age ≤2 years, developmental delay, pre-existing medical condition, and severity of illness. PICU-related factors linked to the development of delirium included mechanical ventilation (MV), coma, and receipt of benzodiazepines, narcotics, corticosteroids, anticholinergics, and vasoactive medications.
In multivariable modeling, independent predictors of delirium included age ≤2 years, developmental delay, severity of illness, mechanical ventilation, ever coma, and administration of benzodiazepines and anticholinergics(Table 2). After step-wise selection, narcotics fell out of the final model. The adjusted odds for delirium diagnosis were more than five times greater in patients who ever received benzodiazepines, as compared to those who never received benzodiazepines (adjusted OR=5.2, CI=3.7,7.5, p<0.001)(Table 2).
Table 2.
Multivariable Logistic Regression Analysis Predicting Ever Delirious (N=1547)
| Characteristic | Odds Ratio (95% CI) | p Value | |
|---|---|---|---|
| Age at Admission (years) | 0–2 | (reference) | |
| >2–5 | 0.606 (0.389–0.936) | 0.026 | |
| >5–13 | 0.412 (0.260–0.646) | <0.001 | |
| >13 | 0.399 (0.244–0.641) | <0.001 | |
| Probability of Mortality1 | <=0.8% | (reference) | |
| >0.8% & <=1.4% | 1.274 (0.824–1.978) | 0.277 | |
| >1.4% | 1.531 (1.016–2.318) | 0.043 | |
| Developmental Delay2 | 3.314 (2.328–4.724) | <0.001 | |
| Ever Mechanically Ventilated | 1.634 (1.155–2.314) | 0.006 | |
| Ever in Coma3 | 4.244 (2.684–6.783) | <0.001 | |
| Benzodiazepines4 | 5.245 (3.710–7.455) | <0.001 | |
| Anti-Cholinergics4 | 2.169 (1.412–3.416) | 0.006 |
In multivariable modeling, independent predictors of delirium included age ≤2 years, developmental delay, severity of illness, mechanical ventilation, ever coma, and administration of benzodiazepines and anticholinergics.
Developmental delay defined as Pediatric Cerebral Performance Category 4 (see text for details).
Coma defined as unarousable to verbal stimulation (see text for details).
Medication categories: includes patients who ever received a medication in this class.
Description of Delirium
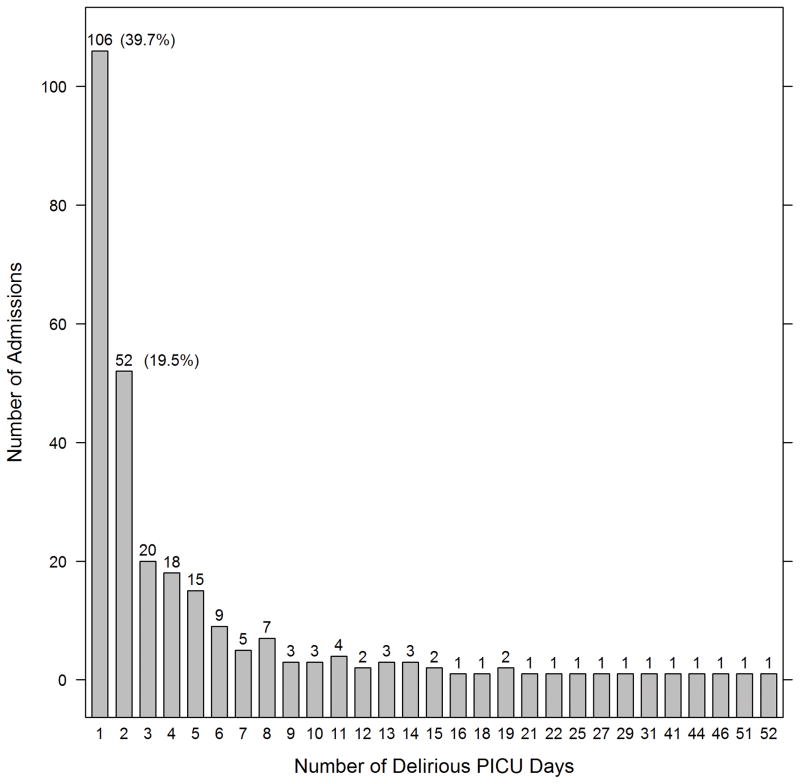
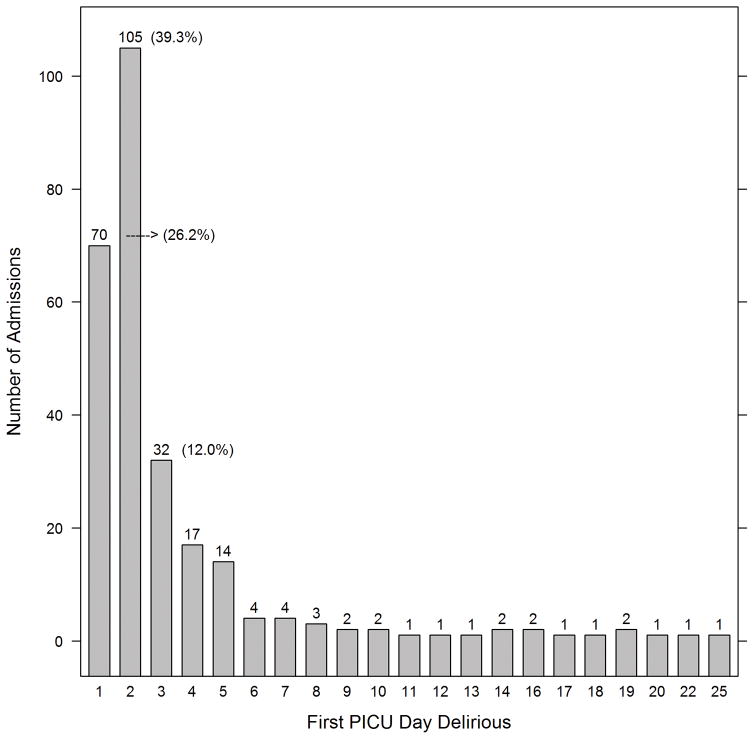
In the 267 patients who were delirious, duration of delirium ranged from one to 52 days, with an interquartile range of 1–5 days, and a median of two days(Figure 1a). Of those who were ever delirious, 77.5% were diagnosed with delirium within the first three PICU days, and 65.5% within the first 48 hours of admission(Figure 1b). Twenty-seven percent experienced recurrent delirium, with 71 patients experiencing at least 2 discrete episodes of delirium (range 2–14 delirium episodes in patients with recurrent delirium).
FIGURE 1.
FIGURE 1a: Duration (in days) of delirium (n=267 patients)
FIGURE 1b: Time (in PICU days) to onset of delirium (n=267 patients)
When assessed by phenotype, only eight percent (8.4%) of patient days with delirium were hyperactive. Forty-six percent (46.4%) of patient days with delirium were hypoactive, and 45.2% were mixed-type.
PELOD Scores (even after discounting neurologic component) were significantly higher on days with delirium, as compared to PELOD scores on days without delirium (mean 3.9 vs 2.2, p<0.001).
Effect on In-Hospital Outcomes
PICU and hospital LOS were both increased in children with delirium (median 7 vs. 3 days, p<0.001, and 8 vs. 3 days, p<0.001, respectively). After controlling for relevant confounders, including probability of mortality and mechanical ventilation, children with delirium demonstrated a more than two-times longer PICU LOS (adjusted relative LOS=2.3, CI=2.1,2.5, p<0.001)(Table 3). Duration of MV was associated with delirium status as well (median 4 vs. 1 day(s) for those ever delirious as compared to MV children who were never delirious(p<0.001)(Table 1). In-hospital mortality was significantly greater for children with delirium (5.24% vs. 0.94%, p<0.001), even after controlling for POM on admission, with adjusted OR for mortality of 4.39 (CI=1.96,9.99) for those ever delirious(p<0.001).
Table 3.
Multivariable Linear Regression Analysis Predicting PICU length of stay (N=1547)
| Characteristic | Relative Days (95% CI) | p Value | |
|---|---|---|---|
| Probability of Mortality1 | <=0.8% | reference | |
| >0.8% & <=1.4% | 1.022 (0.946–1.103) | 0.585 | |
| >1.4% | 1.313 (1.214–1.419) | <0.001 | |
| Ever Mechanically Ventilated | 1.224 (1.146–1.308) | <0.001 | |
| Ever Delirious | 2.309 (2.116–2.518) | <0.001 |
After controlling for relevant confounders, including probability of mortality and mechanical ventilation, children with delirium demonstrated a more than two-times longer PICU LOS.
Results are from a multivariable linear regression with log-transformed PICU days as outcome. Ratios >1.0 for relative days and their respective 95% confidence intervals indicate increased days over the reference group.
Probability of Mortality as determined by the PIM3 score.
Discussion
To our knowledge, this is the most comprehensive description of delirium in critically ill children to date, and the only study to follow each child throughout his/her PICU stay, with prospective daily assessment of delirium status and delirium subtype.
Monitoring for Pediatric Delirium
Consistent with the adult and pediatric literature, this study shows that delirium occurs frequently in critically ill children, and is linked to poor outcomes(1,3–5,16–18). There are clearly identifiable demographic risk factors associated with PD, such as young age and developmental delay(19–21). This perhaps parallels the adults at highest risk: those in the geriatric age group, and those with dementia(22,23). The extremely young and extremely old, and those with pre-existing cognitive dysfunction, seem to be most vulnerable to developing delirium when exposed to the physiologic stress of critical illness. Consistent with previous work by Schieveld, et al., severity of illness is associated with development of delirium(19). We have also identified several modifiable risk factors – deep sedation (represented here as coma status), and prescribing patterns that include benzodiazepines and anticholinergics. As conceptualized by Inouye, et al., in a seminal work on geriatric delirium, the interaction between predisposing and precipitating risk factors can lead to delirium. By avoiding precipitating factors, particularly in high-risk subgroups, we may be able to decrease the delirium burden in children(24).
It is important to note that, as in adults, only a minority of delirium was of the easily recognizable hyperactive subtype(1,25,26). In adults, hypoactive delirium is thought to be the most severe form of delirium, with worse prognostic implications(27). In addition, hypoactive subtype of delirium may be less likely to respond to treatment with antipsychotics (28). Further study is needed to determine whether this is true in children as well.
Consistent with the literature, many children were delirious during their first few PICU days. Without routine screening, these children, as well as children with hypoactive delirium, are likely underdiagnosed(29). This is of great clinical significance, as screening facilitates detection which allows for earlier intervention, and may lead to a decrease in delirium duration(30).
Benzodiazepines and Pediatric Delirium
In our cohort, delirium incidence was five times as likely in children who received benzodiazepines. While this is consistent with existing literature, where benzodiazepines have clearly been implicated in the development delirium in adults, it would be premature to assume the same in pediatrics(31–34). Before concluding that benzodiazepines contribute to the development of delirium, it is essential to assess the clinical order of events, and carefully account for the intricate relationship between benzodiazepine use, narcotic use, mechanical ventilation, and delirium in children. Standard approaches to adjusting for confounding may be affected by exposure to time-dependent covariates. Granular data collection regarding daily exposure to benzodiazepines and narcotics, including specific drugs administered, route of administration, number of doses, cumulative unit/kg/day of individual drugs, and of drug classes, is necessary(33,35). This is beyond the scope of this study, but is necessary in order to systematically assess the probability of transitioning to delirium with benzodiazepine usage. Plans for such a study are underway.
Pediatric Delirium and Mortality
In this cohort, there is a strong and independent relationship between ever being delirious, and in-hospital mortality. It is important to stress that this is merely an association; we do not attempt to show causality in this observational study. Nonetheless, it is interesting to note that delirium was a stronger predictor of mortality (OR 4.4) than the well-validated and widely-used PIM3 score (OR 3.2 for patients in the highest tertile compared to the lowest)(13). This is consistent with the findings in adults, where delirium has subsequently been included in prognostic scores(36,37). It is important that pediatric critical care practitioners pay attention to delirium, as it is an early-warning sign for patients who may fare worse.
Delirium Pathophysiology and Phenotype
It is noteworthy that despite a prolific amount of research on delirium in adults, with thousands of articles published in peer-reviewed journals over the past decade, the pathophysiology is still incompletely understood, and there are few evidence-based treatment strategies(1). Some studies have shown that antipsychotics shorten the duration of delirium; others have shown that antipsychotics are associated with transition to delirium(35,38,39). There is research that indicates that corticosteroids may increase the risk of delirium in geriatric patients, and additional research that suggests that corticosteroids are not harmful, and may be protective against the development of delirium after cardiopulmonary bypass (40–42). It is likely that although the final common pathway leading to delirium may be the same (alteration in neurotransmission, which results in an acute and fluctuating change in awareness and cognition), there are many disparate etiologies that can trigger the delirium cascade(43). This complex pathophysiology makes a one-size-fits-all approach to treatment unrealistic. We believe that it is necessary to identify discrete etiologies of delirium in order to determine how best to treat. When a child is diagnosed with delirium, the question should not be only: “How do I treat this delirium?”, but also “Why is this child delirious?”. With an understanding of the precipitating factors in the particular child, one can design an effective intervention(44–46).
Although as yet there are few evidence-based treatment strategies for delirium, we suggest the following three-pronged approach: investigating for underlying illness, iatrogenic causes, and abnormal environment. When delirium is diagnosed, the clinician should first assess for underlying illness that may have precipitated the delirium (as examples: a new urinary tract infection, or hypoxia). Then, investigation into iatrogenic causes is necessary (for instance, does the patient have evidence of opiate withdrawal?). Finally, environmental causes should be recognized and modified as able (mobilization of a bed-bound patient, attempts at creating an environment that promotes sleep, etc…) (6, 21, 45). For those children who remain delirious after the clinician has addressed these factors, pharmacologic treatment may be considered. The atypical antipsychotics (in particular, quetiapine) have been used safely for treatment of delirium in critically ill children (46–48).
This single-center study, with only 267 children with delirium, was not powered to allow for subgrouping and determination of precipitating causes. However, three important classification systems are suggested by these results. The first is the classification of delirium by subtype (hyperactive, hypoactive, and mixed), which has been intensively investigated in the adult literature(1,26,27). The second classification is delirium by time to event– early vs. late onset. We suspect that early-onset delirium (within 72 hours of admission) may have different etiologies than delirium that occurs later in the PICU course (as was true for 22% of patients). This has direct implications on the development of treatment strategies. With further study of late-onset delirium, we may be able to identify hospital-associated risk factors that are modifiable(21,49,50). The third classification is delirium by duration. A majority of children are delirious for only 1–2 days. It is likely that children with longer duration of delirium have a more severe form, with different causes, treatments, and heightened effect on outcomes(36,51,52). Large-scale multi-institutional studies will be necessary to further investigate these (and other) delirium classification systems.
Limitations
This study has several limitations. Notably, it is an observational study and can only determine associations. We attempted to control for many factors that could explain differences found between children with and without delirium, but it is likely that others were missed, and we do not intend to imply causality in regard to outcome variables. Additionally, this is a single-center study, in a PICU that is highly attuned to delirium, so the incidence and prevalence of delirium reported here may not be widely generalizable. Over the past several years, with increased awareness of PD in our unit, we have modified the way we care for children (with respect to prescribing practices, sedation strategies, and environmental modifications). This has decreased the overall incidence of delirium in our PICU, as compared with delirium rates in other units(21, 53). The medical staff were aware of the patients’ delirium status, and when delirium was diagnosed, it was acted upon, likely shortening its duration. It is likely that in PICUs that do not screen for delirium, its incidence may be higher and duration longer. Further multi-institutional studies of PD are necessary.
Conclusion
Delirium is common in critically ill children and is independently associated with in-hospital mortality. Several in-hospital risk factors for delirium development are modifiable, and interventional studies are needed to determine best practices to limit delirium exposure in at-risk children.
Acknowledgments
Funding: Supported by the Empire Clinical Research Investigator Program, and the Clinical Translational Science Center, grant number UL1-TR000457-06.
Footnotes
Name of institution where work performed: NY Presbyterian Hospital, Weill Cornell Medical College
Address for reprints: Reprints will not be ordered.
Disclosures: All authors have no relevant conflicts of interest to disclose.
Copyright form disclosure: Dr. Traube received support for article research from the National Institutes of Health. Dr. Greenwald received funding from legal firms for expert testimony. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Chani Traube, Department of Pediatrics, Weill Cornell Medical College, New York, NY.
Gabrielle Silver, Department of Psychiatry, Weill Cornell Medical College, New York, NY.
Linda M. Gerber, Department of Healthcare Policy and Research, Weill Cornell Medical College, New York, NY.
Savneet Kaur, Department of Pediatrics, Weill Cornell Medical College, New York, NY.
Elizabeth A. Mauer, Department of Healthcare Policy and Research, Weill Cornell Medical College, New York, NY.
Abigail Kerson, (no department – medical student), Weill Cornell Medical College, New York, NY.
Christine Joyce, Department of Pediatrics, New York Presbyterian Hospital, New York, NY.
Bruce M. Greenwald, Department of Pediatrics, Weill Cornell Medical College, New York, NY.
References
- 1.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013 Jan;41(1):278–80. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Association; 2013. [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 4.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010 Jul;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001 Dec;27(12):1892–900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith HAB, Brink E, Fuchs DC, et al. Pediatric Delirium: Monitoring and Management in the Pediatric Intensive Care Unit. Crit Care Pediatr Patient. 2013 Jun;60(3):741–60. doi: 10.1016/j.pcl.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Traube C, Mauer EA, Gerber LM, Kaur S, Joyce C, Kerson A, et al. Cost Associated With Pediatric Delirium in the ICU. Critical Care Medicine. 2016 Dec;44(12):e1175–9. doi: 10.1097/CCM.0000000000002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: A Valid, Rapid, Observational Tool for Screening Delirium in the PICU. Crit Care Med. 2014 Mar;42(3):656–63. doi: 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris J, Ramelet A-S, van Dijk M, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016 Jun;42(6):972–86. doi: 10.1007/s00134-016-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: Validity and Reliability in Adult Intensive Care Unit Patients. Am J Respir Crit Care Med. 2002 Nov 15;166(10):1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 12.Kerson AG, DeMaria R, Mauer E, et al. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. Journal of Intensive Care [Internet] 2016 Dec;4(1) doi: 10.1186/s40560-016-0189-5. [cited 2016 Oct 27] Available from: http://jintensivecare.biomedcentral.com/articles/10.1186/s40560-016-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straney L, Clements A, Parslow RC, et al. Paediatric Index of Mortality 3: An Updated Model for Predicting Mortality in Pediatric Intensive Care. Pediatr Crit Care Med. 2013 Sep;14(7):673–81. doi: 10.1097/PCC.0b013e31829760cf. [DOI] [PubMed] [Google Scholar]
- 14.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000 Jul;28(7):2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 15.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: An Update of the PEdiatric Logistic Organ Dysfunction Score. Crit Care Med. 2013 Jul;41(7):1761–73. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 16.Schieveld JN, Janssen NJ. Delirium in the Pediatric Patient: On the Growing Awareness of Its Clinical Interdisciplinary Importance. JAMA Pediatr. 2014;168(7):595–596. doi: 10.1001/jamapediatrics.2014.125. [DOI] [PubMed] [Google Scholar]
- 17.Schieveld JN, Leentjens AF. Delirium in severely ill young children in the pediatric intensive care unit (PICU) J Am Acad Child Adolesc Psychiatry. 2005;44(4):392–4. doi: 10.1097/01.chi.0000153231.64968.1a. discussion 395. [DOI] [PubMed] [Google Scholar]
- 18.Smith HAB, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med. 2011 Jan;39(1):150–7. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieveld JNM, Lousberg R, Berghmans E, et al. Pediatric illness severity measures predict delirium in a pediatric intensive care unit. Crit Care Med. 2008 Jun;36(6):1933–6. doi: 10.1097/CCM.0b013e31817cee5d. [DOI] [PubMed] [Google Scholar]
- 20.Smith HAB, Gangopadhyay M, Goben CM, et al. The Preschool Confusion Assessment Method for the ICU: Valid and Reliable Delirium Monitoring for Critically Ill Infants and Children. Crit Care Med. 2015 Nov;1 doi: 10.1097/CCM.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver G, Traube C, Gerber LM, et al. Pediatric Delirium and Associated Risk Factors: A Single-Center Prospective Observational Study. Pediatr Crit Care Med. 2015 May;16(4):303–9. doi: 10.1097/PCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106(5):565–573. doi: 10.1016/s0002-9343(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Morandi A, Davis D, Bellelli G, et al. The Diagnosis of Delirium Superimposed on Dementia: An Emerging Challenge. J Am Med Dir Assoc [Internet] 2016 Sep; doi: 10.1016/j.jamda.2016.07.014. [cited 2016 Sep 29]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S1525861016302924. [DOI] [PMC free article] [PubMed]
- 24.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 25.Peritogiannis V, Bolosi M, Lixouriotis C, et al. Recent Insights on Prevalence and Corelations of Hypoactive Delirium. Behav Neurol. 2015;2015:1–11. doi: 10.1155/2015/416792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004;2(2):171–9. doi: 10.1017/s1478951504040234. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JF, Pun BT, Dittus RS, et al. Delirium and Its Motoric Subtypes: A Study of 614 Critically Ill Patients: DELIRIUM SUBTYPES IN THE CRITICALLY ILL. J Am Geriatr Soc. 2006 Mar;54(3):479–84. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 28.Hermus IPM, Willems SJB, Bogman ACCF, Brabers L, Schieveld JNM. “Delirium” Is No Delirium: On Type Specifying and Drug Response. Critical Care Medicine. 2015 Dec;43(12):e589. doi: 10.1097/CCM.0000000000001251. [DOI] [PubMed] [Google Scholar]
- 29.Silver G, Traube C, Kearney J, et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med. 2012 Mar 10;38(6):1025–31. doi: 10.1007/s00134-012-2518-z. [DOI] [PubMed] [Google Scholar]
- 30.Corona A, Colombo R, Catena E. Early Identification of Subsyndromal Delirium in the Critically Ill: Don’t Let the Delirium Rise! Crit Care Med. 2016 Mar;44(3):644–5. doi: 10.1097/CCM.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 31.Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015 Dec;41(12):2130–7. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 32.Pisani MA, Murphy TE, Araujo KLB, et al. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009 Jan;37(1):177–83. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandharipande PA, Peterson J, Pun B, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–6. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 35.Pisani MA, Araujo KLB, Murphy TE. Association of Cumulative Dose of Haloperidol With Next-Day Delirium in Older Medical ICU Patients. Critical Care Medicine. 2015 May;43(5):996–1002. doi: 10.1097/CCM.0000000000000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010 Dec;38(12):2311–8. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 37.Scarpi E, Maltoni M, Miceli R, et al. Survival prediction for terminally ill cancer patients: revision of the palliative prognostic score with incorporation of delirium. The oncologist. 2011;16(12):1793–1799. doi: 10.1634/theoncologist.2011-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: The MIND randomized, placebo-controlled trial. Crit Care Med. 2010 Feb;38(2):428–37. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010 Feb;38(2):419–27. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and Transition to Delirium in Patients With Acute Lung Injury. Crit Care Med. 2014 Jun;42(6):1480–6. doi: 10.1097/CCM.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic Corticosteroids and Transition to Delirium in Critically Ill Patients. Crit Care Med. 2015 Dec;43(12):e585–8. doi: 10.1097/CCM.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 42.Mu JL, Lee A, Joynt GM. Pharmacologic Agents for the Prevention and Treatment of Delirium in Patients Undergoing Cardiac Surgery: Systematic Review and Metaanalysis. Crit Care Med. 2015 Jan;43(1):194–204. doi: 10.1097/CCM.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 43.Maldonado JR. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am J Geriatr Psychiatry. 2013 Dec;21(12):1190–222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Karnik NS, Joshi SV, Paterno C, et al. Subtypes of pediatric delirium: a treatment algorithm. Psychosomatics. 2007;48(3):253–257. doi: 10.1176/appi.psy.48.3.253. [DOI] [PubMed] [Google Scholar]
- 45.Schieveld JNM, Leroy PLJM, Os J, et al. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007 Apr 25;33(6):1033–40. doi: 10.1007/s00134-007-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traube C, Augenstein J, Greenwald B, et al. Neuroblastoma and pediatric delirium: A case series: Neuroblastoma and Delirium. Pediatr Blood Cancer. 2014 Jun;61(6):1121–3. doi: 10.1002/pbc.24917. [DOI] [PubMed] [Google Scholar]
- 47.Groves A, Traube C, Silver G. Detection and Management of Delirium in the Neonatal Unit: A Case Series. PEDIATRICS. 2016 Mar 1;137(3):1–4. doi: 10.1542/peds.2015-3369. cgi/doi/10.1542/peds.2015-3369. [DOI] [PubMed] [Google Scholar]
- 48.Joyce C, Witcher R, Herrup E, Kaur S, Mendez-Rico E, Silver G, et al. Evaluation of the Safety of Quetiapine in Treating Delirium in Critically Ill Children: A Retrospective Review. Journal of Child and Adolescent Psychopharmacology. 2015 Nov;25(9):666–70. doi: 10.1089/cap.2015.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois MJN, Dumont M, Dial S, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 50.Zaal IJ, Devlin JW, Peelen LM, et al. A Systematic Review of Risk Factors for Delirium in the ICU. Crit Care Med. 2015 Jan;43(1):40–7. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 51.Anderson BJ, Mikkelsen ME. Duration of Delirium and Patient-Centered Outcomes: Embracing the Short- and Long-Term Perspective. Crit Care Med. 2014 Jun;42(6):1558–9. doi: 10.1097/CCM.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med. 2012 Jul;40(7):2182–9. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traube C, Silver G, Reeder RW, et al. Pediatric Delirium in Critically-Ill Children: An International Point Prevalence Study. Critical Care Medicine. 2016 doi: 10.1097/CCM.0000000000002250. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]