Abstract
Background
Choosing the appropriate endpoint for a trauma hemorrhage control trial can determine the likelihood of its success. Recent Phase 3 trials and observational studies have used 24-hour and/or 30-day all-cause mortality as the primary endpoint and some have not used exception from informed consent (EFIC), resulting in multiple failed trials. Five recent high-quality prospective studies among 4,064 hemorrhaging trauma patients provide new evidence to support earlier primary endpoints.
Methods
The goal of this project was to determine the optimal endpoint for hemorrhage control trials using existing literature and new analyses of previously published data.
Results
Recent studies among bleeding trauma patients show that hemorrhagic deaths occur rapidly, at a high rate, and in a consistent pattern. Early preventable deaths among trauma patients are largely due to hemorrhage and the median time to hemorrhagic death from admission is 2.0-2.6 hours. Approximately 85% of hemorrhagic deaths occur within 6 hours. The hourly mortality rate due to traumatic injury decreases rapidly after enrollment from 4.6% per hour at 1 hour post-enrollment to 1% per hour at 6 hours to <0.1% per hour by 9 hours and thereafter. Early primary endpoints (within 6 hours) have critically important benefits for hemorrhage control trials, including being congruent with the median time to hemorrhagic death, biologic plausibility, and enabling the use of all-cause mortality, which is definitive and objective.
Conclusions
Primary endpoints should be congruent with the timing of the disease process. Therefore, if a resuscitation/hemorrhage control intervention is under study, a primary endpoint of all-cause mortality evaluated within the first 6 hours is appropriate. Before choosing the timing of the primary endpoint for a large multicenter trial, we recommend performing a Phase 2 trial under EFIC to better understand the effects of the hemorrhage control intervention and distribution of time to death. When early primary endpoints are used, patients should be monitored for multiple subsequent secondary safety endpoints, including 24 hour and 30 day all-cause mortality as well as the customary safety endpoints.
Keywords: outcomes, trauma, resuscitation, transfusion, clinical trial
The epidemiology of trauma supports that injury is one of the largest public health problems in the world today. In 2010, there were 5.1 million deaths from injuries,1 greater than the number of deaths due to HIV, tuberculosis and malaria combined (3.8 million). Worldwide the number of deaths from injuries increased by 24% between 1990 and 2010.2 In the US, trauma deaths increased by 23% between 2000 and 2010, while the US population increased only 9.7%.3 Additionally, at least 20% of all trauma deaths are the result of survivable injuries and are therefore preventable with optimal care.1,4
In February 2008, physicians, ethicists, statisticians, and research scientists from the military, academia, industry, the Food and Drug Administration (FDA), and the National Heart, Lung and Blood Institute (NHLBI) gathered to discuss the obstacles confronting the trauma community in their efforts to improve patient outcomes. The consensus was that more discussion was needed and that consideration of new endpoints for clinical trials in emergency trauma research was a worthwhile and necessary goal. At the time, few prospective studies of bleeding patients had been performed and the timing of endpoints was therefore not well described or understood.5
Since that meeting, five major prospective studies have been published providing data on time to death among injured and hemorrhaging patients, including a large clinical trial that provides data relevant to endpoint issues. In this commentary, we review the new literature and perform a secondary analysis of data from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial to determine the optimal endpoints for hemorrhagic trauma trials.
What has been learned from recent hemorrhage control studies?
Four large multicenter randomized clinical trials and one observational study among hemorrhaging trauma patients have been published since the endpoint meeting of 2008. In 2010, the CONTROL trial of recombinant activated factor VII (rFVIIa) among patients with active hemorrhage caused by trauma was published with an enrollment of 573 subjects having a 30-day mortality of 11.6%.6 This trial’s publication did not present time to hemorrhagic death and was ended early due to futility.
In a 2011 trial of prehospital resuscitation of patients with hypovolemic shock comparing hypertonic saline vs. normal saline, 30-day mortality was 26.9% among 853 patients enrolled.7 A pooled analysis of both cohorts in the trial (traumatic brain injury [TBI] and hypovolemic shock) reported that the median time to death was 2 hours in the shock cohort, 29 hours in the TBI cohort and 4 hours in patients with both. Additionally, sepsis and multiple organ dysfunction accounted for only 2% of deaths.8
The prospective, randomized, prehospital PolyHeme trial, also published in 2011, reported a 30-day mortality of 11.5% among the 714 patients enrolled with acute blood loss.9,10 In this study, approximately 50% of patients who did not receive PolyHeme (controls) died within 2 hours according to that manuscript’s first figure. Interestingly, these three prospective randomized studies with 3 different hemostatic interventions showed no significant differences between experimental treatment and controls in mortality at 28-30 days.
Among 1245 patients enrolled, the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study reported an in-hospital 30-day mortality of 25% and that 94% of hemorrhagic deaths occurred with 24 hours of hospital admission.11 The median time to hemorrhagic death in this study was 2.6 hours (Interquartile range [IQR]: 1.7-5.4). PROMMTT likely included a mix of low to moderately bleeding patients as well as severely bleeding patients due to the eligibility criterion that patients were required to receive at least one unit of RBCs within six hours of admission.
Lastly, the PROPPR trial enrolled 680 patients at 12 centers over 15 months, and studied the early administration of plasma, platelets and red blood cells (RBCs) in a 1:1:1 ratio compared with a 1:1:2 ratio.12 While the investigators attempted to apply lessons learned from previously published studies, PROPPR did not result in significant differences in mortality at 24 hours or at 30 days, the co-primary endpoints. However, more patients in the 1:1:1 group achieved hemostasis and fewer experienced death due to exsanguination by 24 hours. Additionally, no other differences in safety-related outcomes were identified between the two groups despite the increased transfusion of plasma and platelets in the 1:1:1 group.12 From admission, the median time to hemorrhagic death was 2.4 hours (IQR: 1.2-4.0) and the median time to hemostasis was 2.3 hours (IQR: 1.6-3.6).
In summary, the median time to hemorrhagic death was 2.0-2.6 hours in the four prospective resuscitation trauma studies that reported it (Table 1). Additionally, mortality at 30 days varied among the 5 studies (n=4,064) from 11.5-26.9%, which is likely a reflection of hemodynamic instability and injury severity among each study’s enrolled patient population. Only PROMMTT and PROPPR reported 24-hour mortality rates, which were 11.9% and 14.9% respectively. The 4 studies reporting information about hemorrhagic death (n = 3491) consistently indicate that severe hemorrhage in trauma patients resolves or causes death within 3 hours for at least 50% of patients.
Table 1.
Summary of time to death measure
| Study | N | Year | Time to hemorrhagic death (hrs) |
All-cause mortality at 24 hrs |
All-cause mortality at 30 days |
|---|---|---|---|---|---|
| rFVIIa7 | 573 | 2010 | NA | NA | 11.6% |
| HSD shock5 | 852 | 2011 | 2* | NA | 26.9%** |
| PolyHeme6 | 714 | 2011 | 2 | NA | 11.5% |
| PROMMTT8 | 1245 | 2013 | 2.6 | 11.9%*** | 20.9%*** |
| PROPPR9 | 680 | 2015 | 2.4 | 14.9% | 24.1% |
Time to all-cause death in shock cohort
All-cause mortality at 28 days
In-hospital mortality
Additional evidence from post hoc analyses of PROPPR
Previously unpublished results from PROPPR show that at three hours after randomization there was a significant difference in all-cause mortality between groups (Table 2). At three hours, there were 20 deaths (5.9%) in the 1:1:1 group vs 38 deaths (11.1%) in 1:1:2 (p = 0.02 with Mantel-Haenszel adjusting for site) and the increased mortality in the 1:1:2 group was predominantly due to exsanguination deaths. From 3 hours through 30 days, there were 106 additional deaths, 55 (17.3%) in the 1:1:1 group and 51 (16.8%) in the 1:1:2 group (p = NS). The causes of death were similar in both groups during this time period. TBI (n = 55, median time to death = 41 hours) and multiple organ failure (MOF) (n = 18, median time to death = 221 hours) accounted for the majority of deaths after 3 hours and were not different between groups. At three hours the difference in mortality between the two groups was 5.2% and at 30 days the difference decreased to 3.7%, without crossing of hazards and likely due to competing risks.
Table 2.
PROPPR mortality at various endpoints
| 1:1:1 | 1:1:2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time since randomization |
Cumulative number of deaths (% of total) |
Number of new deaths since the previous interval |
Causes of death for new cases in the interval |
N (% of new deaths)* |
Cumulative number of deaths (% of total) |
Number of new deaths since the previous interval |
Causes of death for new cases in the interval |
N % of new deaths)* |
P-value for overall mortality** |
Adjusted*** RR (95% CI) |
| 1 hour | 11 (3.3) | 11 | Hemorrhage | 9 (82) | 20 (5.9) | 20 | Hemorrhage | 18 (90) | 0.11 | 0.55 |
| TBI | 1 (9) | TBI | 4 (20) | (0.27-1.13) | ||||||
| MI | 1 (9) | Other | 2 (10) | |||||||
| Other | 1 (9) | |||||||||
| 3 hours | 20 (5.9) | 9 | Hemorrhage | 9 (100) | 38 (11.1) | 18 | Hemorrhage | 17 (94) | 0.02 | 0.53 |
| TBI | 2 (11) | (0.32-0.89) | ||||||||
| MI | 1 (9) | |||||||||
| PE | 1 (9) | |||||||||
| Other | 1 (9) | |||||||||
| 6 hours | 33 (9.8) | 13 | Hemorrhage | 11 (85) | 46 (13.5) | 8 | Hemorrhage | 8 (100) | 0.13 | 0.72 |
| TBI | 3 (23) | Other | 1 (13) | (0.48-1.10) | ||||||
| Other | 1 (8) | |||||||||
| 12 hours | 38 (11.2) | 5 | Hemorrhage | 2 (40) | 51 (14.9) | 5 | Hemorrhage | 3 (60) | 0.16 | 0.75 |
| TBI | 3 (60) | TBI | 3 (60) | (0.51-1.11) | ||||||
| Other | 1 (20) | |||||||||
| 18 hours | 40 (11.8) | 2 | TBI | 1 (50) | 56 (16.4) | 5 | Hemorrhage | 4 (80) | 0.11 | 0.72 |
| Other | 1 (50) | TBI | 1 (20) | (0.50-1.05) | ||||||
| Stroke | 1 (20) | |||||||||
| 24 hours | 43 (12.7) | 3 | TBI | 3 (100) | 58 (17.0) | 2 | TBI | 2 (100) | 0.12 | 0.75 (0.52-1.08) |
| 30 days | 75 (22.4) | 32 | Hemorrhage | 5 (16) | 89 (26.1) | 31 | TBI | 23 (74) | 0.26 | 0.86 |
| TBI | 16 (50) | Sepsis | 2 (6) | (0.65-1.12) | ||||||
| Sepsis | 1 (3) | MOF | 8 (26) | |||||||
| MOF | 10 (31) | MI | 1 (3) | |||||||
| Stroke | 2 (6) | Other | 3 (10) | |||||||
| Other | 5 (16) | |||||||||
Percentages may sum to more than 100% because patients may have had multiple reported causes of death
Calculated using the Mantel-Haenszel test for binary outcomes measured from randomization, adjusting for site
Adjusted for site
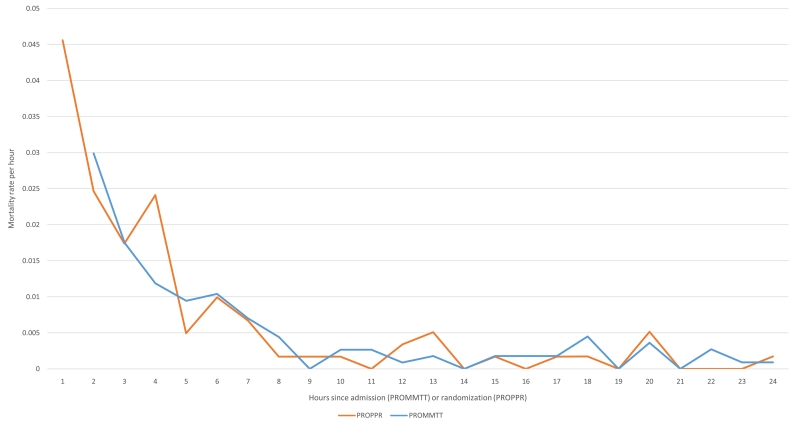
Examining the hourly mortality rates ascertained in PROMMTT and PROPPR gives another perspective (Figure 1). Since PROMMTT was observational and excluded patients who died in the first 30 minutes, the mortality rate at 1 hour underestimates the mortality rate in all bleeding trauma patients and these data were excluded from the graph. However, curves generated from both studies are surprisingly similar- starting high and rapidly decreasing to 1% per hour around 5-6 hours and to nearly 0 approximately 8-9 hours after study enrollment. Referring back to Table 2, the proportion of death due to hemorrhage decreases after 6 hours and the proportion of death due to other causes, particularly TBI, increases.
Figure 1. Hourly mortality rates from PROMMTT and PROPPR.
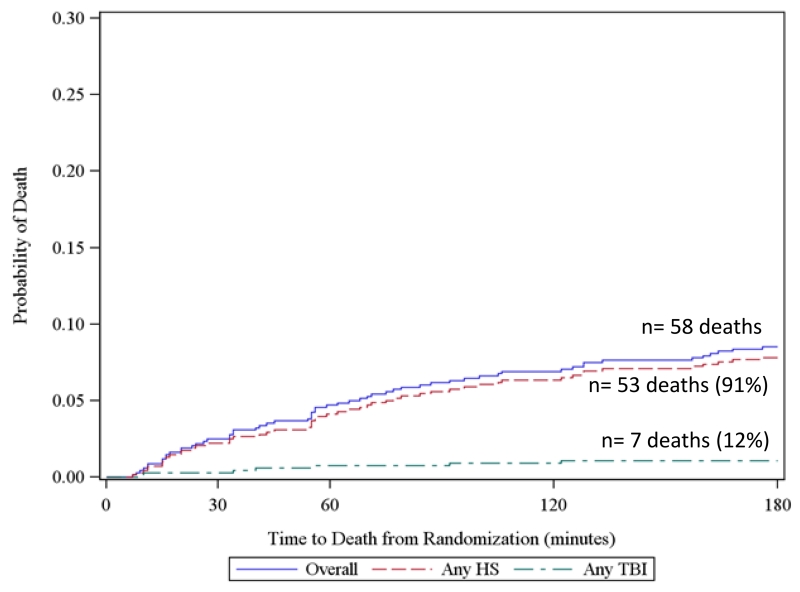
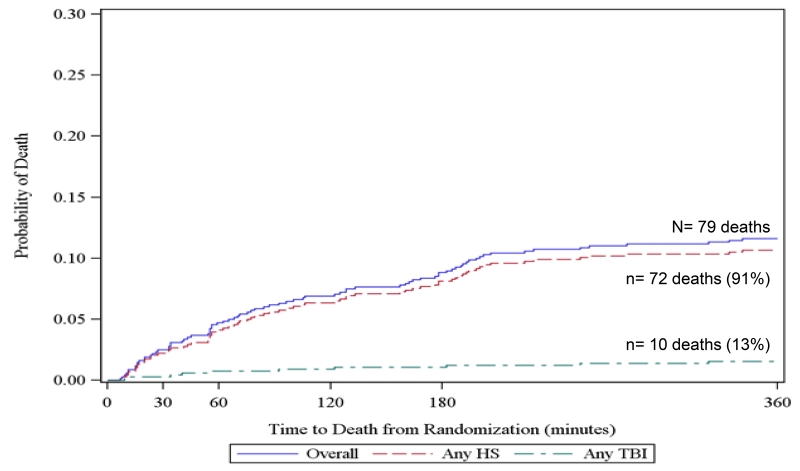
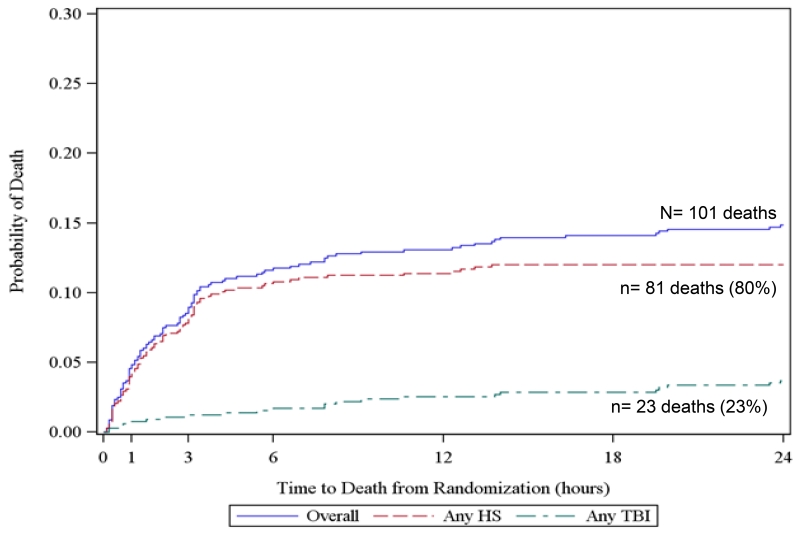
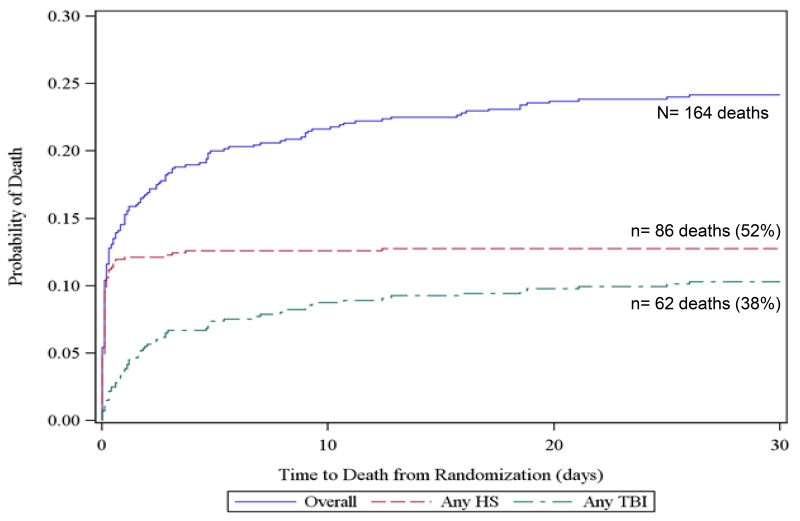
To further elucidate competing risks of death due to hemorrhage and TBI, we created all-cause Kaplan Meier failure curves overlaid with cause-specific curves for exsanguination and TBI for all subjects at 3 hours, 6 hours, 24 hours, and 30 days from the PROPPR trial. For cause-specific curves, deaths other than the specified cause are censored. These figures show that at 3 and 6-hours, hemorrhage accounted for 91% of all deaths. However at 24 hours and 30 days, TBI accounted for 23 and 38% of deaths respectively. These figures suggest that within 6 hours of admission, over 90% deaths were hemorrhagic and therefore cause-specific mortality mirrors all-cause mortality in this population.
PROPPR showed that rapid enrollment and randomization of patients in hemorrhagic shock is very achievable under exception from informed consent (EFIC). Patients were enrolled into the study at a median of 28 minutes after hospital arrival, enrollment at the 12 North American sites progressed much faster than expected (up to 90 patients a month), and the study concluded 6 months ahead of schedule.13 But the results also raise questions about whether the presence of severe traumatic brain injuries in enrolled patients was a reason why the trial did not achieve significance at either of the co-primary endpoints. In an ideal study design for resuscitation and hemorrhage control studies, patients with severe (non-survivable) head injury should be excluded. In reality it is difficult, if not impossible, to exclude all patients with severe head injuries because severely bleeding patients often have an altered sensorium and must be enrolled before CT scans are done. Additionally, other indicators of severe head injury such as depressed Glasgow Coma Scale often occur in hemorrhagic shock patients who are intubated. Based on physical exam at hospital arrival, PROPPR was able to minimize the number of patients with non-survivable brain injuries enrolled in the study. Although 23% of patients had an AIS-head>2, only 1.8% had isolated head injuries (AIS-head>2 and AIS<3 in all other body regions). Polytrauma patients who were at risk of death due to both hemorrhage and traumatic brain injury were enrolled by design and 30% of enrolled patients died with brain injury as a cause of death. For these reasons, competing risk of death due to head injury will continue to be an issue in hemorrhage control studies until earlier diagnostic technologies become available.
Discussion
Trauma deaths occur rapidly, at a high rate, and in a consistent pattern. Early preventable deaths are largely from hemorrhage, and recent studies show a median time to hemorrhagic death of 2-2.6 hours, despite differences in interventions and populations enrolled. In addition, the hourly mortality rate due to traumatic injury decreases rapidly over 6 hours. After 24 hours, small numbers of deaths occur daily due to various causes (pulmonary embolism, myocardial infarction, stroke, MOF, TBI, withdrawal of support, etc). Death due to these other causes, including MOF, is a relatively uncommon daily event, over 30 days (< 5%) compared to the major causes of death, exsanguination and TBI. However, about 25% of all deaths still occur after 3 days (24% in PROPPR), so these late deaths are an important component of overall mortality, but are delayed and very heterogeneous.
Over the last 5 years additional data relevant to resuscitation and hemorrhage control trials have become available, thus a new data-driven and biology-based discussion of endpoints is warranted. Traditional endpoints for resuscitation and hemorrhage control studies are 24-hour and/or 30-day all-cause mortality. However, these endpoints are merely convenient, and these arbitrary points on clocks and calendars may not be appropriate for all trauma studies. Knowledge of the biology of the disease and the mechanism of the intervention in question is key to choosing the timing of a primary endpoint and designing the trial. For example, the CONTROL trial, which examined the effect of rFVIIa on mortlaity at 30 days, was not performed under EFIC in the US and therefore the mean time from injury to first dose of rFVIIa was 4-5 hours, which later studies would show is after the median time to death due to hemorrhage (2.0-2.6 hours). Additionally, patients were required to receive between 4 and 8 units of RBCs prior to randomization. Because of this enrollment criterion and delay in obtaining consent, the CONTROL trial preferentially selected patients who were likely to survive, resulting in lower 30-day mortality in enrolled patients, and directly leading to the early termination of the trial for futility.
For an early endpoint to be warranted, the intervention must either definitively control hemorrhage or help keep the patient alive long enough or create better surgical conditions so that definitive hemorrhage control can occur. The authors of the CONTROL trial posited that rFVIIa would act as an adjunct to other interventions to control coagulopathic bleeding.6 However, they used a tiered endpoint that did not reflect the timing of hemorrhage: 1) superiority in all-cause 30 day or 2) non-inferiority in all-cause 30 day mortality and superiority on durable morbidity (pulmonary or renal dysfunction at day 30). Similarly in PROPPR, co-primary endpoints of all-cause 24-hour and 30-day mortality did not reflect the timing of hemorrhage. Some interventions may be efficacious within 5 minutes (e.g. tourniquets), and some within 2-6 hours (e.g. blood products). A small vanguard phase, pilot study or separate Phase 2 trial should be performed prior to a large multisite trial to help define the appropriate timing of the endpoint. Single arm Phase 2 futility designs can also be utilized to help determine whether an intervention should continue to a large multicenter Phase 3. Most importantly, these initial studies should be performed under EFIC in the US or comparable regulations in other countries in order to enroll patients at risk of death due to hemorrhage. The downside of using EFIC is that the investigator must submit an investigational new drug or investigational device exemption application to the FDA, which could add an extra year to the study timeline.
Using all-cause mortality as the endpoint for resuscitation and hemorrhage control trials is ideal because the endpoint is definitive and objective. Using cause-specific mortality in hemorrhage control trials adds subjectivity and potential misclassification due to the clinical determination of the primary cause of death in multiply injured patients. For example, if a patient dies very early in their hospital stay with indications of shock and hemorrhage, physicians likely will not have had time to send the patient to CT and therefore it may be unknown whether the patient’s death was due to both hemorrhage and severe TBI. Full autopsies are infrequently performed on trauma decedents, and if they are, the actual primary anatomic cause of death is not often noted. Including misclassified TBI deaths as exsanguination deaths will dilute the observable effect of interventions targeting hemorrhage.
Using PROPPR data, we found that if shorter time periods after hospital admission are used as the endpoint for a hemorrhage-control study (within 6 hours using PROPPR data), all-cause mortality is nearly equivalent to cause-specific mortality (death due to hemorrhage). In PROPPR, 80% of all deaths due to hemorrhage occurred by 4.8 hours after admission. Therefore, early primary endpoints (1-6 hours) have critically important benefits, including being congruent with the median time to the expected effect of the experimental intervention and hemorrhagic death, biologic plausibility, and enabling the definitive and objective all-cause mortality to be used instead of the more subjective cause-specific mortality. Later primary endpoints (e.g. 24 hours and 30 days) require the use of cause-specific mortality and additional analysis of competing causes of death in studies of hemorrhage control interventions. It should be noted that even in studies using early endpoints, patients should continue to be followed through hospital discharge or day 30 (or longer) to monitor secondary safety endpoints such as later mortality and morbidity such as VTE, MOF, sepsis and other relevant complications.
Another important aspect of this discussion is sample size estimates, which vary depending on the timing of the endpoint, whether the endpoint is all-cause or cause-specific mortality and the event rate assumptions. Using PROPPR data, sample size estimates for varying absolute differences in proportions, timing of endpoint and type of endpoint were estimated and are shown in Table 3. The sample sizes range from nearly 20,000 overall for a 2% difference in proportion using 30-day all-cause mortality to 286 overall for a 12% difference in proportions using 2-hour cause-specific mortality. For the differences seen in PROPPR, the required sample size is 1,246 for 3 hour all-cause mortality (4.5%) or 5,826 for 30 day all-cause mortality (3%). Importantly, event rates for the endpoint of interest can vary across populations: in the hemorrhage control studies discussed previously, 30-day mortality rates ranged between 12 and 27%. For this reason, it is appropriate to perform a small pilot, vanguard phase, or Phase 2 futility study to verify the event rates used in the sample size calculations.
Table 3.
Sample size estimates for varying absolute difference in proportions, given the follow-up window and mortality outcome
| Computed Total N (2-Group Trial) | Absolute Difference in Proportions | SS Needed in PROPPR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up window |
Mortality Outcome |
Reference Proportion |
0.02 | 0.04 | 0.05 | 0.07 | 0.08 | 0.1 | 0.12 | |
| 30-day | Overall | 0.22 | 19232 | 4950 | 3212 | 1682 | 1302 | 854 | 604 | 5826 |
| Any HS | 0.11 | 11466 | 3068 | 2026 | 1096 | 862 | 580 | 422 | 3008 | |
| 24-hour | Overall | 0.13 | 13074 | 3458 | 2272 | 1218 | 954 | 638 | 462 | 2968 |
| Any HS | 0.09 | 9772 | 2656 | 1766 | 966 | 764 | 520 | 382 | 1558 | |
| 6-hour | Overall | 0.1 | 10630 | 2864 | 1898 | 1032 | 814 | 550 | 402 | 3264 |
| Any HS | 0.09 | 9772 | 2656 | 1766 | 966 | 764 | 520 | 382 | 2570 | |
| 4-hour | Overall | 0.09 | 9772 | 2656 | 1766 | 966 | 764 | 520 | 382 | 2252 |
| Any HS | 0.08 | 8892 | 2440 | 1630 | 900 | 714 | 488 | 360 | 2084 | |
| 3-hour | Overall | 0.06 | 7068 | 1996 | 1348 | 760 | 608 | 422 | 316 | 1246 |
| Any HS | 0.05 | 6122 | 1764 | 1202 | 686 | 552 | 388 | 292 | 1290 | |
| 2-hour | Overall | 0.05 | 6122 | 1764 | 1202 | 686 | 552 | 388 | 292 | 2722 |
| Any HS | 0.047 | 5834 | 1694 | 1158 | 664 | 536 | 378 | 286 | 2502 | |
Ref proportion from PROPPR (1:1:1 group)
Fixed scenario elements: asymptotic normal distribution, normal approximation method, alpha (adjusted for 3 looks) = 0.044, nominal power=0.9, 2 sided hypothesis test, 0 null proportion difference
Another potential endpoint that has been only occasionally discussed in the literature is hemostasis, which may be the most direct endpoint for hemorrhage control trials. However, hemostasis has not been used as a primary endpoint in any trauma resuscitation study to date, and therefore little data exists regarding how it would perform. Determining whether a patient has achieved hemostasis requires physician judgement, so it is more subjective than all-cause mortality and may be perceived to be especially problematic in unblinded studies. Hemostasis was an ancillary endpoint in PROPPR and occurred at a median of 2.3 hours from admission (or 1.7 hours from randomization). Time to control of acute hemorrhage was also rated favorably as a potential end point in a survey of members of the American Association for the Surgery of Trauma.14 Because of the importance of hemorrhage control, we recommend that future trials continue to develop and evaluate hemostasis as a secondary endpoint.
Recommendations
We recommend that primary endpoints be congruent with the biology and timing of the disease process. Therefore, if a resuscitation/hemorrhage control intervention is under study, a primary endpoint of all-cause mortality evaluated within the first 6 hours may be appropriate. Before choosing the timing of the primary endpoint for a large multicenter trial, we recommend performing a small (1-3 center) vanguard phase or a separate phase 2 trial under EFIC to better understand the biologic effects of the new hemorrhage control intervention and the mortality in the target population. Single arm Phase 2 futility designs can also be utilized to help determine whether an intervention should continue to a large multicenter Phase 3. When early primary endpoints are used, patients should be monitored for multiple subsequent secondary safety endpoints, including 24 hour and 30 day all-cause mortality as well as the more traditional safety endpoints.
Figure 2. Kaplan Meier failure curves of cause-specific (hemorrhagic shock [HS] and traumatic brain injury [TBI]) and all-cause mortality using PROPPR data.
a. 3-hour mortality
Includes 4 subjects with both HS and TBI and 2 subjects with neither
b. 6-hour mortality
Includes 5 subjects with both HS and TBI and 2 subjects with neither
c. 24-hour mortality
Includes 7 subjects with both HS and TBI and 4 subjects with neither
d. 30-day mortality
Includes 8 subjects with both HS and TBI and 24 subjects with neither
Acknowledgments
Contributors (to be indexed as contributors in PubMed)
Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group:
Clinical Coordinating Center, University of Texas Health Science Center at Houston: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee co-Chair); Jeanette M. Podbielski, RN; Angela M. Beeler, BS.
Data Coordinating Center, University of Texas Health Science Center at Houston: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Stacia M. DeSantis, PhD; Hongjian Zhu, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; Michael O. Gonzalez, MS.
Core Laboratory, University of Texas Health Science Center at Houston: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; David Clark, BS.
Resuscitation Outcomes Consortium, University of Washington: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; Kellie Sheehan, BSN.
Systems Biology Committee, University of California, Berkeley: Alan Hubbard, PhD (co-Chair); Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD, MPH (co-Chair, University of Washington); Jeannie L. Callum, MD (co-Chair, Sunnybrook Health Sciences Centre)
Anesthesiology Committee: Jean-Francois Pittet, MD (Chair, University of Alabama at Birmingham)
Emergency Medicine Committee: Christopher N. Miller, MD (Chair, University of Cincinnati);
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees co-Chair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Helen Hancock, BS, MT(ASCP); Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research - Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr, DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Patrick L. Bosarge, MD; Albert T. Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS; Marisa B. Marques, MD .
Oregon Health and Science University: Martin A. Schreiber, MD ; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD ; Richard M. Scanlan, MD; Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Sciences Centre: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; Connie Colavecchia, BSc, MLT.
Source of Funding/ Role of Sponsors: This work was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863) and the U.S. Department of Defense, as well as Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health (CRR-120612). NHLBI and the DoD were consulted regarding study design only. No sponsors were involved in the collection, management, or analysis of the data; preparation of the manuscript; or the decision to submit the manuscript for publication. The content is the sole responsibility of the authors and is not to be construed as official or as reflecting the views of any sponsor.
Footnotes
Conflicts of Interest: No conflicts of interest have been declared by any author in regards to this manuscript.
Trial Registration: Clinicaltrials.gov, NCT01545232
References
- 1.National Academies of Sciences, Engineering, and Medicine . A national trauma care system: Integrating military and civilian trauma systems to achieve zero preventable deaths after injury. The National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 2.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 3.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 4.Kwon AM, Garbett NC, Kloecker GH. Pooled preventable death rates in trauma patients : Meta analysis and systematic review since 1990. Eur J Trauma Emerg Surg. 2014;40(3):279–285. doi: 10.1007/s00068-013-0364-5. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb JB, Weiskopf R, Champion H, Gould SA, Sauer RM, Brasel K, Bochicchio G, Bulger E, Cotton BA, Davis D, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 6.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 7.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–590. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard AC, Moore EE, Moore FA, Hides GA, Guthrie BJ, Omert LA, Gould SA, Rodman GH, Jr., PolyHeme Study Group Postinjury resuscitation with human polymerized hemoglobin prolongs early survival: a post hoc analysis. J Trauma. 2011;70(5 Suppl):S34–37. doi: 10.1097/TA.0b013e31821a586e. [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, Duane TM, Weireter LJ, Jr., Gomez GA, Cipolle MD, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208(1):1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baraniuk S, Tilley BC, del Junco DJ, Fox EE, van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation. Injury. 2014;45(9):1287–1295. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alawadi ZM, LeFebvre E, Fox EE, Del Junco DJ, Cotton BA, Wade CE, Holcomb JB. Alternative end points for trauma studies: A survey of academic trauma surgeons. Surgery. 2015;158(5):1291–1296. doi: 10.1016/j.surg.2015.03.030. [DOI] [PubMed] [Google Scholar]