Abstract
Patients with chronic kidney disease (CKD) are at increased risk for cardiovascular disease and death, yet little data exists regarding the comparative efficacy of coronary revascularization procedures in CKD patients with multivessel disease. We created a cohort of 4,687 adults who underwent cardiac catheterization, had a serum creatinine value measured within 30 days, and had more than one vessel with ≥50% stenosis. We used Cox proportional hazard regression modeling weighted by the inverse probability of treatment to examine the association between four treatment strategies (medical management, percutaneous coronary intervention [PCI] with bare metal stent, PCI with drug-eluting stent, and coronary artery bypass grafting [CABG]) and mortality among patients across categories of estimated glomerular filtration rate (eGFR); secondary outcome was a composite of mortality, MI, or revascularization. Compared with medical management, CABG was associated with a reduced risk of death for patients of any non-dialysis CKD severity (HR range 0.43–0.59). There were no significant mortality differences between CABG and PCI, except a decreased death risk in CABG-treated severe CKD patients (HR range 0.54–0.55). Compared with medical management and PCI, CABG was associated with a lower risk of death, MI, or revascularization in non-dialysis CKD patients (HR range 0.41–0.64). There were similar associations between eGFR decrease and mortality increase across all multivessel CAD patient treatment groups. When accounting for treatment propensity, surgical revascularization was associated with improved outcomes in patients of all CKD severities. A prospective randomized trial in CKD patients is required to confirm our findings.
Keywords: coronary artery disease, end-stage renal disease, chronic kidney disease, percutaneous coronary intervention, coronary artery bypass surgery, survival, mortality
Approximately 26 million Americans have chronic kidney disease (CKD), and these patients are at high risk for cardiovascular disease-related mortality, acute myocardial infarction, and poor long term outcomes.1-2 For patients with both CKD and multivessel CAD, the optimal revascularization strategy is not known. Some observational studies of CKD patients compared outcomes after coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), but many of these analyses produced mixed results, and mostly include observations prior to the introduction of newer drug-eluting stents (DES).3-5 Furthermore, overall mortality after CABG has declined over the last 20 years as a result of process improvement interventions, enhanced perioperative care, and secondary prevention.6-8 Thus, revascularization efforts in the modern era may have a differential effect on outcomes in patients with CKD. Unfortunately, CKD patients have been significantly underrepresented in cardiovascular disease therapeutic trials, including those aimed at revascularization techniques.9-13 The present study examines the comparative effectiveness of CABG, PCI with bare metal stents (BMS), PCI with DES, and medical management alone, in patients with multivessel disease and varying degrees of renal dysfunction.
Methods
The Duke Databank for Cardiovascular Disease is a registry that includes patient demographic information, clinical history, laboratory data, cardiac catheterization results, and coronary revascularization procedures in patients who have undergone cardiac catheterization at Duke University Medical Center. Patients are contacted six and twelve months after their enrollment catheterization, and then annually afterwards for follow-up regarding survival and cardiovascular events. An independent mortality committee reviewed follow-up results to confirm deaths, while follow up MI was based on clinical diagnosis assigned by the patient's physician.
Our study population included patients who underwent left heart catheterization between January 1, 2003 and December 31, 2010. We included patients who had both serum creatinine measured within 30 days prior to the procedure, and stenosis ≥50% in more than one major coronary artery. Figure 1 illustrates the selection process used to determine the final study population. We included patients whose initial procedure was affiliated with our medical center and we excluded patients with a history of prior PCI, prior CABG, primary valvular heart disease, non-ischemic indication for catheterization, non-significant CAD, and significant life-limiting comorbidities such as metastatic cancer and acquired immune deficiency syndrome. Out of 9,825 procedures that met initial inclusion criteria, we identified 8,811 unique patients after excluding repeat procedures. The final population was obtained after excluding patients with single vessel disease, patients who received PCI other than stenting, and those without a serum creatinine measured within 30 days. Medically managed patients who died within five days of their catheterization were excluded in order to minimize an early mortality bias. We did not exclude patients who presented with acute myocardial infarction. We assigned the treatment category as the first intervention received as intention-to-treat within 30 days of the index procedure. The final sample contained 4,687 patients divided into the following treatment categories: 985 in the medical management arm; 1,012 in the BMS arm; 1,278 in the DES arm; and 1,412 in the CABG arm. The institutional review board for Duke University Medical Center approved the study. Due to the nature of the study, a waiver of informed consent was obtained.
Figure 1. Study population flow diagram.
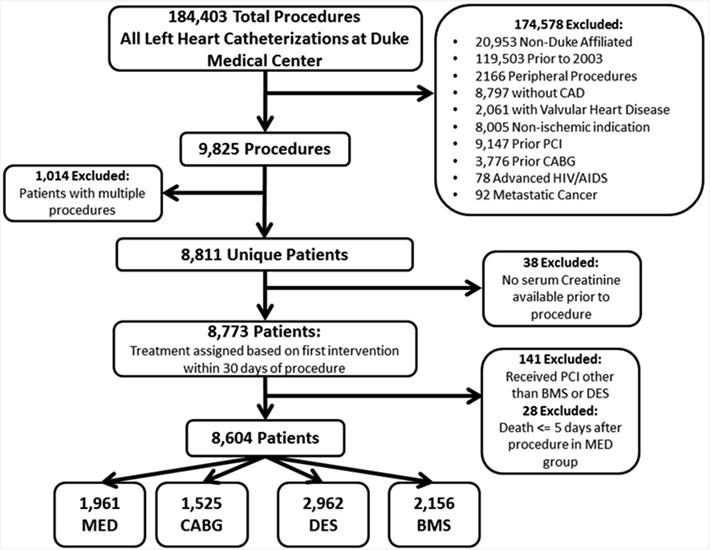
This figure displays a flow diagram of the study population, from the initial cohort, through exclusions, to the final study population.
BMS = bare metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; DES = drug-eluting stent; HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; MED = medical management; PCI = percutaneous coronary intervention
Patients were classified into five groups according to their eGFR, which was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation that is based on serum creatinine, age, sex, and race.14 The most recently measured serum creatinine prior to the day of the procedure was used in the estimation equation in order to classify patients according to the following eGFR groups: normal (≥60 ml/min/1.73m2), mild CKD (45–59 ml/min/1.73m2), moderate CKD (30–44 ml/min/1.73m2), severe CKD (<30 ml/min/1.73m2), and end-stage renal disease (i.e., patient receiving chronic dialysis). The primary clinical endpoint was death from any cause. The secondary endpoint was a composite of death, MI, or revascularization.
The Duke Databank for Cardiovascular Diseases contains both demographic data and comorbid conditions of the patients at the time of cardiac catheterization. In addition to age, race, and gender, we assessed our study population for the following: history of hypertension, diabetes mellitus, tobacco abuse, eGFR, MI, stroke, peripheral vascular disease, cerebral vascular disease, hyperlipidemia, congestive heart failure disease (New York Heart Association [NYHA] Class), left ventricular ejection fraction, heart rate, and extent of CAD.
We compared baseline characteristics of patients by treatment strategy using the Kruskal-Wallis test for continuous variables and the Pearson chi-square test for categorical variables. Estimates of the hazard ratios of death, as well as the composite of death, MI, or revascularization comparing medical management, PCI with BMS, and PCI with DES with CABG across CKD categories, were obtained using Cox proportional hazards models weighted by the inverse probability of treatment received.15 The same method was used to estimate the hazard of the outcomes of interest per 10 unit decrease of continuous eGFR (from 0 to 85 ml/min/1.73m2) for each treatment group. Continuous eGFR was truncated at a maximum of 85 ml/min to satisfy linearity. The interactions between CKD and treatment, as well as continuous eGFR and treatment, were also assessed. Kaplan-Meier curves were weighted by the inverse probability of treatment received.
For the development of a propensity score model for medical management versus PCI with BMS versus PCI with DES versus CABG, a generalized logit model was fit-adjusted for clinically chosen patient characteristics before treatment. Missing data was handled using a single imputation strategy. After imputation, continuous variables were evaluated for linearity and cubic splines were utilized, if necessary. The following variables were chosen to be included in the propensity model: age, gender, race, history of hypertension, smoking, diabetes, cerebral vascular disease, peripheral vascular disease, hyperlipidemia, history of MI, stroke, heart rate, number of diseased vessels, NYHA class, and ejection fraction. Interactions between CKD group and all of the covariates were evaluated using backwards selection to determine inclusion into the propensity model. Significant interactions (p<0.05) were included in the propensity model in order to achieve balance across the CKD groups. The final propensity model included all of the aforementioned independent variables, as well as CKD group, CKD group*age, CKD group*heart rate, and CKD group*NYHA class. The adequacy of the propensity model was assessed by checking the distribution of the propensity scores by treatment for reasonable overlap and checking the pre- and post-inverse probability of treatment weighting balance of the covariates.16 P-values <0.05 were used to determine statistical significance. All analyses were performed using SAS versions 8.2 and 9.2 (SAS Institute, Inc., Cary, NC).
Results
A total of 4,687 patients met inclusion criteria and were included in the analysis (Table 1); of these, 3,144 had normal kidney function (eGFR ≥60 ml/min/1.73m2), 760 had mild CKD (eGFR 45–59 ml/min/1.73m2), 408 had moderate CKD (eGFR 30–44 ml/min/1.73m2), 240 had severe CKD (eGFR <30 ml/min/1.73m2), and 135 patients were on dialysis. The overall sample was mostly male (67.9%, 3,185/4,687), and the median age was 64 years (interquartile range 55–72). Median time of follow-up was 5.1 years (3.1–7.3). The prevalence of diabetes mellitus in the study population was 32.5% (1,523/4,687). The prevalence of hypertension, NYHA Class III/IV heart failure, and diabetes mellitus was higher in both the medically managed and CABG groups, compared with the BMS and DES groups. With the exception of smoking history, the prevalence of all the variables in Table 1 varied significantly across the different treatment groups.
Table 1. Demographics and baseline characteristics.
| Variable | Medical | PCI-BMS | PCI-DES | CABG | p-value |
|---|---|---|---|---|---|
| (N = 985) | (N = 1012) | (N = 1278) | (N = 1412) | ||
| Age (years) | <0.0001 | ||||
| Median (25th, 75th) | 67 (58,76) | 62 (53,71) | 62 (54,70) | 64 (56,72) | |
| White | 636/976 (65.2%) | 676/1005 (67.3%) | 921/1268 (72.6%) | 1083/1403 (77.2%) | |
| Black | 292/976 (29.9%) | 267/1005 (26.6%) | 256/1268 (20.2%) | 252/1403 (18.0%) | |
| Native American | 24/976 (2.5%) | 34/1005 (3.4%) | 57/1268 (4.5%) | 40/1403 (2.9%) | |
| Other | 24/976 (2.5%) | 28/1005 (2.8%) | 34/1268 (2.7%) | 28/1403 (2.0%) | |
| Men | 617/985 (62.6%) | 682/1012 (67.4%) | 870/1278 (68.1%) | 1016/1412 (72.0%) | <0.0001 |
| Hypertension | 710/985 (72.1%) | 664/1012 (65.6%) | 864/1278 (67.6%) | 998/1412 (70.7%) | 0.005 |
| Diabetes mellitus | 417/985 (42.3%) | 251/1012 (24.8%) | 384/1278 (30.0%) | 471/1412 (33.4%) | <0.0001 |
| History of Myocardial Infarction | 415/985 (42.1%) | 712/1012 (70.4%) | 598/1278 (46.8%) | 590/1412 (41.8%) | <0.0001 |
| Cerebrovascular disease | 155/985 (15.7%) | 69/1012 (6.8%) | 89/1278 (7.0%) | 129/1412 (9.1%) | <0.0001 |
| Peripheral vascular disease | 123/985 (12.5%) | 50/1012 (4.9%) | 84/1278 (6.6%) | 124/1412 (8.8%) | <0.0001 |
| Stroke | 86/985 (8.7%) | 74/1012 (7.3%) | 76/1278 (5.9%) | 144/1412 (10.2%) | 0.001 |
| Smoker | 396/985 (40.2%) | 392/1012 (38.7%) | 521/1278 (40.8%) | 612/1412 (43.3%) | 0.131 |
| Hyperlipidemia | 544/985 (55.2%) | 505/1012 (49.9%) | 697/1278 (54.5%) | 830/1412 (58.8%) | <0.0001 |
| NYHA Class | <0.0001 | ||||
| None | 708/961 (73.7%) | 911/1000 (91.1%) | 1127/1246 (90.4%) | 1134/1355 (83.7%) | |
| I | 21/961 (2.2%) | 15/1000 (1.5%) | 29/1246 (2.3%) | 24/1355 (1.8%) | |
| II | 56/961 (5.8%) | 23/1000 (2.3%) | 26/1246 (2.1%) | 75/1355 (5.5%) | |
| III | 117/961 (12.2%) | 32/1000 (3.2%) | 41/1246 (3.3%) | 74/1355 (5.5%) | |
| IV | 59/961 (6.1%) | 19/1000 (1.9%) | 23/1246 (1.8%) | 48/1355 (3.5%) | |
| eGFR(ml/min) | <0.0001 | ||||
| N | 985 | 1012 | 1278 | 1412 | |
| Median (25th, 75th) | 63 (44, 83) | 76 (57, 95) | 74 (57,89) | 72 (55, 89) | |
| Kidney function (ml/min/1.73m2) | <0.0001 | ||||
| Normal (≥60) | 535/985 (54.3%) | 715/1012 (70.7%) | 911/1278 (71.3%) | 983/1412 (69.6%) | |
| Mild (45–59) | 188/985 (19.1%) | 160/1012 (15.8%) | 206/1278 (16.1%) | 206/1412 (14.6%) | |
| Mod (30–44) | 122/985 (12.4%) | 79/1012 (7.8%) | 93/1278 (7.3%) | 114/1412 (8.1%) | |
| Severe (<30) | 74/985 (7.5%) | 37/1012 (3.7%) | 50/1278 (3.9%) | 79/1412 (5.6%) | |
| Dialysis | 66/985 (6.7%) | 21/1012 (2.1%) | 18/1278 (1.4%) | 30/1412 (2.1%) | |
| Ejection fraction | <0.0001 | ||||
| N | 772 | 758 | 998 | 1407 | |
| Median (25th, 75th) | 55 (37, 61) | 54 (44, 60) | 57 (47,64) | 51 (43, 61) | |
| Heart rate | <0.0001 | ||||
| N | 985 | 1012 | 1278 | 1411 | |
| Median (25th, 75th) | 72 (63,84) | 72 (62, 86) | 70 (61, 81) | 71 (62, 82) | |
| Number of diseased vessels | <0.0001 | ||||
| Two | 491/985 (49.8%) | 662/1012 (65.4%) | 904/1278 (70.7%) | 319/1412 (22.6%) | |
| Three | 494/985 (50.2%) | 350/1012 (34.6%) | 374/1278 (29.3%) | 1093/1412 (77.4%) | |
| Follow-up time (years) | <0.0001 | ||||
| N | 985 | 1012 | 1278 | 1412 | |
| Median (25th, 75th) | 3.5 (1.2,6.0) | 4.2 (3.0,6.0) | 6.5 (3.8,8.1) | 5.9 (3.7,8.0) |
CABG = coronary artery bypass grafting; eGFR = estimated glomerular filtration rate; PCI-BMS = percutaneous coronary intervention with bare metal stent; PCI-DES = percutaneous coronary intervention with drug-eluting stent; NYHA = New York Heart Association
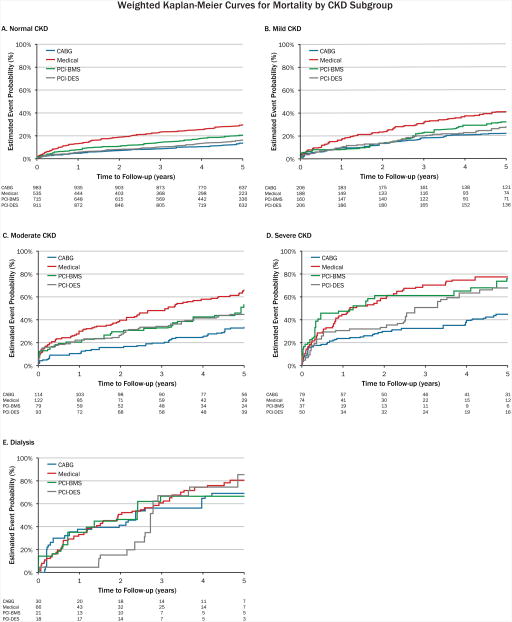
Initially, there was an imbalance of covariates within each treatment group. However, this markedly improved after inverse probability weighting for both categorical and continuous variables (Figure 2a and 2b). As expected within each treatment strategy, there was a graded increase in mortality with lower levels of kidney function (Figure 3, weighted Kaplan-Meier mortality curves). Stratified by CKD subgroup, we then compared the inverse probability weighted hazard ratios and 95% confidence intervals for CABG mortality with the various treatment strategies (Table 2). In patients with mild, moderate, and severe CKD, there was a significantly lower mortality risk after CABG compared with medical management. CABG was associated with a lower risk of death compared with PCI with BMS in patients with mild, moderate, and severe CKD, but this association was only statistically significant in those with severe CKD. Compared with patients with mild, moderate, and severe CKD who received PCI with DES, there was a trend towards lower mortality with CABG, but none of these differences reached statistical significance. Among dialysis patients, there were no significant differences in mortality between CABG and medical management, PCI with BMS, or PCI with DES. The overall interaction of treatment by CKD stage was not significant (p=0.18). Table 3 shows the hazard of death for each 10-ml/min/1.73m2 decrement in eGFR irrespective of revascularization strategy. The interaction of eGFR by treatment was statistically significant (p=0.04).
Figure 2. Balance of variables.
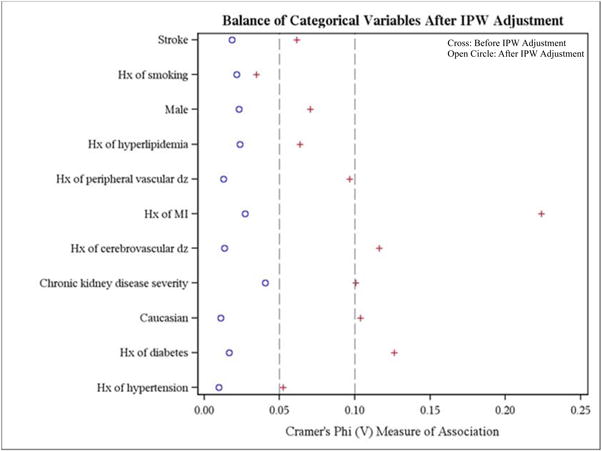
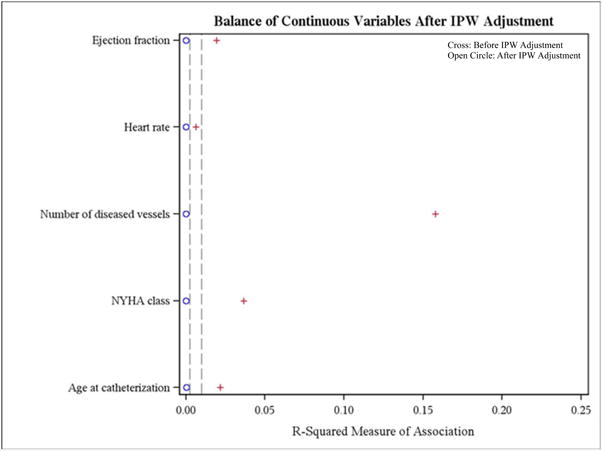
This figure displays of the balance of: A) categorical variables; and B) continuous variables, before (cross) and after (open circle) IPW adjustment
Hx = history; IPW = inverse probability weighting; MI = myocardial infarction; NYHA = New York Heart Association
Figure 3. Weighted Kaplan-Meier curves for mortality.
This figure displays the weighted Kaplan-Meier curves for mortality for the following CKD subgroups: A) normal CKD; B) mild CKD; C) moderate CKD; D) severe CKD; and E) dialysis. Number at risk is un-weighted. Observations are weighted by the inverse probability of type of treatment administered using a propensity model which included the following variables: age, gender, race (white/non-white), history of hypertension, smoking, diabetes, cerebral vascular disease, peripheral vascular disease, hyperlipidemia, MI, stroke, heart rate, number of diseased vessels, NYHA class, ejection fraction CKD group, CKD group*age, CKD group*pulse, and CKD group*NYHA class.
CABG = coronary artery bypass grafting; CKD = chronic kidney disease; MI = myocardial infarction; NYHA = New York Heart Association; PCI-BMS = percutaneous coronary intervention with bare metal stent; PCI-DES = percutaneous coronary intervention with drug-eluting stent
Table 2. Mortality: hazard ratios and confidence intervals for mortality after coronary –artery bypass surgery compared to each treatment, stratified by chronic kidney disease category.
| eGFR (ml/min/1.73m2) | CABG vs. MED | CABG vs. BMS | CABG vs. DES |
|---|---|---|---|
| Normal CKD (≥60) | 0.47 (0.37, 0.60) | 0.79 (0.60, 1.04) | 0.99 (0.77, 1.27) |
| Mild CKD (45–59) | 0.59 (0.40, 0.86) | 0.67 (0.44, 1.02) | 0.79 (0.54, 1.14) |
| Moderate CKD (30–44) | 0.43 (0.29, 0.63) | 0.67 (0.42, 1.06) | 0.65 (0.42, 1.0) |
| Severe CKD (<30) | 0.44 (0.28, 0.68) | 0.42 (0.24, 0.76) | 0.65 (0.41, 1.02) |
| Dialysis | 0.81 (0.43, 1.53) | 0.94 (0.37, 2.38) | 0.86 (0.45, 1.63) |
BMS = bare metal stent; CKD = chronic kidney disease; DES = drug-eluting stent; MED = medical management; All other abbreviations can be found in Table 1.
Table 3. Mortality: hazard ratios and confidence intervals for estimated glomerular filtration rate (per 10 ml/min/1.73m2 decrease).
| Medical | PCI-BMS | PCI-DES | CABG | |
|---|---|---|---|---|
| Continuous eGFR <85 (ml/min/1.73m2) | 1.25 (1.2, 1.3) | 1.31 (1.23, 1.4) | 1.36 (1.31, 1.42) | 1.29 (1.23, 1.35) |
All abbreviations can be found in Table 1.
The composite endpoint hazards were significantly lower in patients with normal, mild, moderate, and severe kidney disease following CABG when compared to medical management, PCI with BMS, and PCI with DES (Table 4). Among dialysis patients, there was no difference in the composite outcome after comparing CABG with the other treatment strategies. Table 5 shows the hazard of the composite endpoint for each 10-ml/min/1.73m2 decrement in eGFR. The interaction of continuous eGFR by treatment was not statistically significant (p=0.091).
Table 4. Composite of death, myocardial infarction, or revascularization: hazard ratios and confidence intervals for coronary artery bypass surgery compared to each treatment, stratified by chronic kidney disease category.
| eGFR (ml/min/1.73m2) | CABG vs. MED | CABG vs. BMS | CABG vs. DES |
|---|---|---|---|
| Normal CKD (≥60) | 0.48 (0.40, 0.59) | 0.46 (0.37, 0.56) | 0.64 (0.53, 0.77) |
| Mild CKD (45–59) | 0.48 (0.34, 0.68) | 0.57 (0.39, 0.83) | 0.57 (0.41, 0.80) |
| Moderate CKD (30–44) | 0.50 (0.35, 0.71) | 0.53 (0.34, 0.82) | 0.63 (0.42, 0.95) |
| Severe CKD (<30) | 0.41 (0.28, 0.61) | 0.54 (0.31, 0.92) | 0.55 (0.35, 0.86) |
| Dialysis | 0.65 (0.38, 1.12) | 1.09 (0.46, 2.56) | 0.92 (0.52,1.62) |
Table 5. Composite of death, myocardial infarction, or revascularization: hazard ratios and confidence intervals for estimated glomerular filtration rate (per 10 ml/min/1.73m2 decrease).
| Medical | PCI-BMS | PCI-DES | CABG | |
|---|---|---|---|---|
| Continuous eGFR <85 (ml/min/1.73m2) | 1.17 (1.13, 1.21) | 1.09 (1.03, 1.15) | 1.17 (1.13, 1.22) | 1.18 (1.13, 1.23) |
Discussion
In a population of patients with multivessel CAD, CABG was associated with a lower risk of death when compared with medical management for those with mild, moderate, and severe CKD. There was a trend towards lower mortality with CABG compared with PCI, either with BMS or DES, among patients with mild, moderate, and severe CKD, but the majority of these associations did not reach statistical significance. Meaningful differences were only statistically significant when comparing CABG to PCI with BMS in patients with severe CKD. When we examined the composite outcome of death, MI, or revascularization, we found that CABG was associated with a significantly lower risk of the composite compared with medical management, PCI with BMS, and PCI with DES among those with any severity of CKD, but not those on dialysis. In the current DES era, these findings have important implications for the optimal management of patients with CKD and multivessel CAD.
A prior study using the Duke Databank compared CABG and PCI in years 1995-2000; this study found a mortality benefit of CABG over PCI in patients with moderate and severe CKD.3 Other retrospective studies have found a lower risk of death or MI with CABG compared with PCI, but many of these studies did not differentiate between the type of stent deployed, and some included percutaneous coronary angioplasty.3-5 Although other studies have shown conflicting results after comparing outcomes associated with the type of stent (BMS or DES) used in CKD patients,17,18 while studies with longer-term follow-up have found more consistent benefits with CABG compared with PCI.19-21 Although our analyses found mostly similar effects between DES and BMS when compared with CABG across the CKD groups, we cannot definitively conclude whether or not there was a differential effect on outcomes based on the type of stent deployed. Finally, recent randomized trials have also demonstrated improved outcomes with CABG compared with PCI with DES for complex multivessel disease.22-24 Nevertheless, few patients with CKD were included, thereby limiting the generalizability of these findings among those with moderate to severe CKD. However, since patients with CKD have more complex and diffuse CAD, our study results in the context of these recent trials suggest that CKD patients may preferentially benefit in the short- and long-term from CABG rather than PCI.
To more appropriately determine whether outcomes differ following CABG or PCI in CKD patients, we leveraged our analyses by focusing on only contemporary time points (2003– 2010) when DES had become available, and after a nationwide decline in post-operative mortality associated with bypass surgery. Other retrospective analyses include patients who underwent PCI during time points prior to the year 2003, which may not be reflective of contemporary practice.3,4,25 Despite differences in the population composition, our findings were similar to those of a study from the Canadian province of Ontario that observed improved outcomes following CABG when compared with DES.20
Our study offers several other important advantages over prior analyses. First, our single center databank provides a realistic cohort, and afforded a more complete long-term follow-up for outcomes evaluation. Second, our chosen time period allowed fair comparison between the available revascularization strategies, since the equal opportunity for BMS and DES better reflects current practice. We were able to stratify based on type of stent received as opposed to grouping BMS and DES recipients together. Finally, our registry is unique in that it captures anatomic information from each catheterization, which was important for estimating the propensity for treatment in our model.
Importantly, we did not observe any differences in our primary (all-cause mortality) or secondary (composite of death, MI, or revascularization) outcomes when comparing CABG to other strategies in dialysis patients. Our study only had a total of 135 patients on dialysis; this small sample size limited our ability to discern any differences in outcomes among the different treatment groups. Larger registry analyses have found a long-term mortality benefit associated with CABG compared with PCI for multivessel disease in dialysis patients.26,27 Nonetheless, the small population of dialysis patients in our final sample precludes making any firm conclusions. The number of patients with severe CKD was also much lower compared with other CKD subgroups, which may provide a possible explanation for a lack of significant differences in mortality with PCI compared with CABG, where there was an overall trend towards higher mortality with PCI. Also, while repeat revascularization rates may differ between the various strategies, the revascularization rates for de-novo lesions following both PCI and CABG are clinically significant and warrant inclusion in outcomes analyses.28 Also, our databank did not include the indication or urgency of the catheterization procedures, calculated SYNTAX scores, or data on adherence to medical therapy following catheterization. Finally, since this was a retrospective analysis of a non-randomized cohort, we must acknowledge the presence of selection bias or any unmeasured variables that were not captured in the propensity model.
Highlights.
Examined CV outcomes in all strata of CKD patients with multivessel CAD
-
For pre-dialysis CKD patients, CABG compared to PCI was associated with:
Trend towards a reduced risk of all-cause mortality and
Reduced risk of the composite outcome of death, MI, and revascularization
No observed differences in outcomes among patients on dialysis
Acknowledgments
Duke O'Brien Center for Kidney Research and the Duke Clinical Research Institute. A poster presentation based on this manuscript was presented at ASN Kidney Week 2014 in Philadelphia, PA.
Sources of funding: Dr. Roberts was supported/funded by the Duke Training Grant in Nephrology (T32DK007731). Research reported in this publication was supported by the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30DK096493.
Footnotes
Conflict of interest disclosures: Dr. Marroquin reports grant support from Abbott Vascular
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Kidney Foundation. About chronic kidney disease. National Kidney Foundation web site; [Accessed September 23, 2016]. http://www.kidney.org/kidneydisease/aboutckd.cfm. [Google Scholar]
- 2.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- 3.Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, Smith MS, Califf RM, Mark DB, Owen WF., Jr Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–80. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 4.Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106:2207–2211. doi: 10.1161/01.cir.0000035248.71165.eb. [DOI] [PubMed] [Google Scholar]
- 5.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–1119. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL STS National Database Committee. A decade of change--risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: a report from the STS National Database Committee and the Duke Clinical Research Institute Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480–490. doi: 10.1016/s0003-4975(01)03339-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SH, Theurer PF, Bell GF, Maresca L, Leyden T, Prager RL Michigan Society of Thoracic and Cardiovascular Surgeons. A statewide quality collaborative for process improvement: internal mammary artery utilization. Ann Thorac Surg. 2010;90:1158–1164. doi: 10.1016/j.athoracsur.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Williams JB, Delong ER, Peterson ED, Dokholyan RS, Ou FS, Ferguson TB, Jr Society of Thoracic Surgeons and the National Cardiac Database. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation. 2011;123:39–45. doi: 10.1161/CIRCULATIONAHA.110.981068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 10.Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, Schönberger JP, Buller N, Bonser R, Disco C, Backx B, Hugenholtz PG, Firth BG, Unger F. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez AE, Baldi J, Fernández Pereira C, Navia J, Rodriguez Alemparte M, Delacasa A, Vigo F, Vogel D, O'Neill W, Palacios IF ERACI II Investigators. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II) J Am Coll Cardiol. 2005;46:582–588. doi: 10.1016/j.jacc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 12.Booth J, Clayton T, Pepper J, Nugara F, Flather M, Sigwart U, Stables RH SoS Investigators. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS) Circulation. 2008;118:381–388. doi: 10.1161/CIRCULATIONAHA.107.739144. [DOI] [PubMed] [Google Scholar]
- 13.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green SM, Selzer F, Mulukutla SR, Tadajweski EJ, Green JA, Wilensky RL, Laskey WK, Cohen HA, Rao SV, Weisbord SD, Lee JS, Reis SE, Kip KE, Kelsey SF, Williams DO, Marroquin OC. Comparison of bare-metal and drug-eluting stents in patients with chronic kidney disease (from the NHLBI Dynamic Registry) Am J Cardiol. 2011;108:1658–1664. doi: 10.1016/j.amjcard.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai TT, Messenger JC, Brennan JM, Patel UD, Dai D, Piana RN, Anstrom KJ, Eisenstein EL, Dokholyan RS, Peterson ED, Douglas PS. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. 2011;58:1859–1869. doi: 10.1016/j.jacc.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan W, Ivanov J, Ko D, Fremes S, Rao V, Jolly S, Cantor WJ, Lavi S, Overgaard CB, Ruel M, Tu JV, Džavik V. Clinical outcomes of treatment by percutaneous coronary intervention versus coronary artery bypass graft surgery in pateints with chronic kidney disease undergoing index revascularization in Ontario. Circ Cardiovasc Interv. 2015;8:e001973. doi: 10.1161/CIRCINTERVENTIONS.114.001973. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi H, Morimoto T, Furukawa Y, Nakagawa Y, Tazaki J, Sakata R, Okabayashi H, Hanyu M, Shimamoto M, Nishiwaki N, Komiya T, Kimura T CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Comparison of five-year outcome of percutaneous coronary intervention with coronary artery bypass grafting in triple-vessel coronary artery disease (from the coronary revascularization demonstrating outcome study in Kyoto PCI/CABG Registry Cohort-2) Am J Cardiol. 2015;116:59–65. doi: 10.1016/j.amjcard.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Fanari Z, Weiss SA, Zhang W, Sonnad SS, Weintraub WS. Comparison of percutaneous coronary intervention with drug eluting stents versus coronary artery bypass grafting in patients with multivessel coronary artery disease: meta-analysis of six randomized controlled trials. Cardiovasc Revasc Med. 2015;16:70–77. doi: 10.1016/j.carrev.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Choo SJ, Chung CH, Lee JW, Cohen DJ, Yeung AC, Hur SH, Seung KB, Ahn TH, Kwon HM, Lim DS, Rha SW, Jeong MH, Lee BK, Tresukosol D, Fu GS, Ong TK BEST Trial Investigators. Trial of everoimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–1212. doi: 10.1056/NEJMoa1415447. [DOI] [PubMed] [Google Scholar]
- 24.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 25.Chang TI, Leong TK, Kazi DS, Lee HS, Hlatky MA, Go AS. Comparative effectiveness of coronary artery bypass grafting and percutaneous coronary intervention for multivessel coronary disease in a community-based population with chronic kidney disease. Am Heart J. 2013;165:800–808. doi: 10.1016/j.ahj.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shroff GR, Solid CA, Herzog CA. Long-term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug-eluting and bare metal stents in the United States. Circulation. 2013;127:1861–1869. doi: 10.1161/CIRCULATIONAHA.112.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parasca CA, Head SJ, Milojevic M, Mack MJ, Serruys PW, Morice MC, Mohr FW, Feldman TE, Colombo A, Dawkins KD, Holmes DR, Jr, Kappetein PA SYNTAX Investigators. Incidence, Characteristics, Predictors, and Outcomes of Repeat Revascularization After Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: The SYNTAX Trial at 5 Years. JACC Cardiovasc Intervention. 2016;9:2493–2507. doi: 10.1016/j.jcin.2016.09.044. [DOI] [PubMed] [Google Scholar]