Abstract
Background
Cardiovascular disease (CVD) is an emerging concern for HIV-infected patients. Hyperlipidemia is a risk factor for CVD and a complication of protease-inhibitor-based antiretroviral therapy, but little is known about its incidence and risk factors in treated patients in resource-limited settings (RLS).
Methods
We conducted a secondary analysis of ACTG A5230 trial in which HIV-infected adults from India, Malawi, Tanzania, Thailand and South Africa, with virologic relapse on first line therapy were initiated on lopinavir/ritonavir (LPV/r) monotherapy. Hyperlipidemia was, a Grade 2+ elevated fasting total cholesterol (FTC≥240 mg/dl) or fasting triglycerides (FTG≥500 mg/dl) or calculated low density lipoprotein cholesterol (LDL≥160 mg/dl) based on measurements at weeks 12, 24, 48, 68 and 104. We evaluated factors potentially associated with quantitative lipid changes from baseline to week 12. These were age, sex, race, site, and baseline body mass index, CD4 cell count, HIV-1 RNA level, and lipids.
Results
106 participants without hyperlipidemia at baseline started LPV/r; median age 39 years, 68% black African, 55% female. The cumulative incidence of hyperlipidemia at week 104 was 48% (95% CI: 36–58%). At week 12, there were significant mean increases from baseline in FTC (17 mg/dL, P<0.001) and FTG (104 mg/dL, P<0.001). In multivariable analysis, higher baseline FTC (P=0.044), FTG (P=0.025), Thai (P<0.001) or Indian sites (P=0.020) versus African sites were associated with increased risk of hyperlipidemia.
Conclusion
In HIV-infected adults in RLS initiating LPV/r, hyperlipidemia was common. Baseline lipid measurements and routine monitoring should be recommended in individuals starting LPV/r-based treatments with borderline high lipids.
INTRODUCTION
Non-infectious complications of HIV infection are an increasing concern globally. In resource-rich countries, non-communicable diseases including cardiovascular disease (CVD) have become leading causes of morbidity and mortality in persons living with HIV/AIDS. Similarly, as HIV care and treatment becomes more widely available, CVD has become an emerging epidemic in resource-limited settings [1].
Hyperlipidemia is a recognized risk factor for CVD and is also a complication of antiretroviral therapy (ART). [2 – 7] Hence, improved understanding of risk factors for hyperlipidemia among HIV-infected patients taking ART is important. Current HIV treatment guidelines for resource-limited settings recommend the use of a pharmacokinetically boosted protease inhibitor (PI) in combination with two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) for second-line therapy. [8] Lopinavir (LPV) boosted with low-dose ritonavir (LPV/r) is widely used for treatment of HIV in antiretroviral (ART)-experienced patients failing on a first-line ART regimen in resource-limited settings. [9] However, in resource-rich countries, LPV/r-containing regimens, compared to other protease inhibitors (PIs), have been associated with greater increases in lipid parameters. [10 – 12] Several studies have shown that Grade 3 or 4 elevations in total cholesterol and in triglycerides were more common in patients receiving LPV/r than in those receiving other PIs. [10, 12]
With the rising number of people starting second line ART regimens in resource-limited settings, hyperlipidemia due to protease inhibitors might be expected to be an increasing concern. [13] However, there are few data describing the risk of hyperlipidemia in resource-limited settings. We therefore set out to determine the incidence and risk factors of hyperlipidemia in LPV/r-treated patients in resource-limited settings.
METHODS
STUDY DESIGN
This was a retrospective analysis of data from the AIDS Clinical Trials Group (ACTG) A5230 clinical trial. A5230 study was an open-label, single arm pilot trial to explore the safety and efficacy associated with LPV/r monotherapy in antiretroviral-experienced, protease inhibitor-naïve participants experiencing virologic relapse on a non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing regimen. [14, 15] The study was conducted at five ACTG Clinical Research Sites, including three sites in Africa (Lilongwe, Malawi; Moshi, Tanzania; and Johannesburg, South Africa) and two sites in Asia (Chiang Mai, Thailand; and Chennai, India). Participants were followed for 104 weeks. Participants received LPV/r (400/100 mg twice daily) which was supplied as co-formulated Aluvia™ tablets through 104 weeks. Plasma HIV-1 RNA levels were measured in real time. Since A5230 was a pilot study and LPV/r monotherapy was a non-standard ART regimen, close monitoring of plasma HIV-1 RNA levels was conducted for the first 24 weeks of follow-up. For participants experiencing virologic failure, emtricitabine/tenofovir (FTC/TDF) (200/300 mg once daily), co-formulated as Truvada, was added to their treatment. Virologic failure was met with either of the following two conditions: (i) failure to suppress HIV-1 RNA to < 400 copies/mL by week 24, or (ii) confirmed HIV-1 RNA ≥ 400 copies/mL after confirmed HIV-1 RNA < 400 copies/mL. As reported previously, Forty seven (47) participants developed virologic relapse while on LPV/r monotherapy and 41 (33%) had their treatment intensified with TDF/FTC. [14]
STUDY POPULATION
Study participants were HIV-infected men and women, 18 years or older, experiencing virologic relapse while receiving a NNRTI-containing three-drug combination continuously for at least 6 months prior to study entry with screening plasma HIV-1 RNA of 1000 – 200,000 copies/mL. Entry criteria included estimated creatinine clearance >60mL/minute, AST, ALT and alkaline phosphatase <3 times the upper limit of normal, total bilirubin <2.5 times the upper limit of normal, a hemoglobin >8mg/dL, and platelets >50,000/mm3. Persons with prior PI use, detectable hepatitis B surface antigen (HBsAg), active substance abuse, serious medical condition within the previous 14 days were excluded, as were pregnant or breastfeeding women. In this analysis, participants with pre-existing hyperlipidemia (defined below) at study entry or who were on lipid lowering treatment at study entry were excluded. A5230 was approved by all relevant local and US institutional review boards and ethics committees, and all participants provided written informed consent.
DEFINITIONS AND DATA COLLECTION
Fasting total cholesterol (FTC), triglycerides (FTG) and high density lipoprotein (FHDL) were measured at study entry and at weeks 12, 24, 48, 68, and 104 during follow-up while receiving LPV/r monotherapy. Low density lipoprotein (LDL) was calculated using the Freidewald equation: LDL = [FTC − (FHDL − FTG/5)]. Hyperlipidemia was defined as a Grade 2 or higher elevation of one or more fasting lipid parameters or commencement of lipid lowering treatment without a grade 2 or higher lipid elevation. The Division of AIDS (DAIDS) table for Grading the Severity of Adult Adverse Events (DAIDS AE Grading Table), Version 1.0, December 2004 was used to grade hyperlipidemia. FTC ≥240 mg/dl, FTG ≥500 mg/dl and calculated LDL ≥160 mg/dl were defined as abnormal (i.e. Grade 2 or higher) using this classification. Grade 2 or higher lipid elevations by the DAIDS grading table translate to moderate to severe lipid elevations which in most cases warrant medical treatment with lipid lowering agents unlike milder lipid elevations which can be managed with life style modifications. Baseline plasma HIV-1 RNA level and CD4 cell count were defined as the measurements immediately prior to starting LPV/r monotherapy. Baseline body mass index BMI was similarly defined, calculated as weight (kg) divided by height (m) squared.
STATISTICAL ANALYSIS
Data analysis was restricted to the period during which participants remained on LPV/r monotherapy. The period ended when participants had confirmed virologic failure and intensified treatment through addition of FTC/TDF, when they permanently discontinued study treatment, or when they started lipid lowering treatment. The cumulative incidence of hyperlipidemia was calculated as the proportion of participants who developed hyperlipidemia while on LPV/r monotherapy at weeks 12, 24, 48, 68 and 104 using cumulative incidence estimates with 95% confidence limits. Proportional hazards models were used to evaluate factors associated with the hazard of hyperlipidemia while on LPV/r monotherapy. Risk factors of interest included age (18 – 29, 30 – 39, 40 – 49 and ≥ 50 years), sex, race/site (African, Indian and Thai); baseline BMI (<18.5, 18.5 – 25 and ≥25 kg/m2), CD4 cell count (<50, 50 – 199, 200 – 349 and ≥350 cells/mm3), HIV-1 RNA level (3.00 – 3.99, 4.00 – 4.99, 5.00 – 5.29 and ≥5.30 log10 copies/mL), and baseline lipids. Linear and mixed regression models were used to estimate unadjusted and adjusted mean changes in lipids 12 weeks after initiation of LPV/r monotherapy and associated 95% confidence intervals. SAS version 9.2 (Cary, NC) was used for statistical analysis.
RESULTS
ENROLLMENT AND FOLLOW-UP
A total of 123 participants were enrolled in the A5230 study and started LPV/r monotherapy. Nine participants were excluded from this analysis because they had pre-existing hyperlipidemia and a further eight were excluded because they had no fasting lipid measurements before starting LPV/r. The study population therefore included 106 participants, of whom 59 completed 104 weeks of follow-up while on LPV/r monotherapy. Among the 47 participants who discontinued LPV/r monotherapy before 104 weeks, 41 intensified treatment with TDF/FTC, 4 died and 2 were lost to follow-up.
BASELINE CHARACTERISTICS
The 106 participants were enrolled at 5 sites, three in Africa (Malawi [36 participants], Tanzania [20] and South Africa [16]), Thailand (22) and India (12). Table 1 describes characteristics of participants prior to starting LPV/r monotherapy in the overall study population and by site. The median age was 39 years, 55% were female, 68% were African and the median BMI was 22.8 kg/m2. Median CD4 count varied significantly by site (P<0.001) ranging from 104 cells/mm3 in Malawi to 242 cells/mm3 in South Africa. Median HIV-1 RNA also varied significantly by site (P=0.045), ranging from 4.09 log10 copies/mL in Thailand to 4.65 log10 copies/mL in Malawi.
Table 1.
Baseline Characteristics of Participants on LPV/r Monotherapy in A5230
| Characteristic | Total (N=106) |
WITS Helen Joseph (WITS HJH) CRS (N=16) |
Chiang Mai Univ. ACTG CRS (N=22) |
Site Chennai Antiviral Research and Treatment (N=12) |
Malawi CRS (N=36) |
Kilimanjaro Christian Medical CRS (N=20) |
P-Value | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | Median | 39 | 38.50 | 34.50 | 39.50 | 37 | 41 | 0.186* |
| Q1, Q3 | 32, 44 | 30, 44 | 30, 42 | 38.00, 44.50 | 32, 44 | 36.50, 48.00 | ||
| 18–29 | 14 (13%) | 4 (25%) | 5 (23%) | 0 (0%) | 5 (14%) | 0 (0%) | 0.262** | |
| 30–39 | 44 (42%) | 6 (38%) | 8 (36%) | 6 (50%) | 16 (44%) | 8 (40%) | ||
| 40–49 | 37 (35%) | 6 (38%) | 7 (32%) | 6 (50%) | 10 (28%) | 8 (40%) | ||
| 50+ | 11 (10%) | 0 (0%) | 2 (9%) | 0 (0%) | 5 (14%) | 4 (20%) | ||
| Sex | Male | 48 (45%) | 8 (50%) | 10 (45%) | 9 (75%) | 11 (31%) | 10 (50%) | 0.101** |
| Female | 58 (55%) | 8 (50%) | 12 (55%) | 3 (25%) | 25 (69%) | 10 (50%) | ||
| Race/Ethnicity | Thai | 22 (21%) | 0 (0%) | 22 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | <.001** |
| Black African | 72 (68%) | 16 (100%) | 0 (0%) | 0 (0%) | 36 (100%) | 20 (100%) | ||
| Indian (Native of India) |
12 (11%) | 0 (0%) | 0 (0%) | 12 (100%) | 0 (0%) | 0 (0%) | ||
| Weight | Median | 63.80 | 69.75 | 58.40 | 72.05 | 57.90 | 68 | 0.006* |
| Q1, Q3 | 54.90, 71.70 |
64.40, 79.00 |
52.30, 63.80 |
56.75, 75.10 | 52.40, 65.60 |
56.30, 73.00 | ||
| BMI | Median | 22.76 | 25.17 | 22.00 | 24.10 | 22.49 | 22.48 | 0.064* |
| Q1, Q3 | 21.13, 25.63 |
22.70, 28.28 |
20.07, 24.31 |
22.89, 26.34 | 20.96, 24.80 |
21.20, 25.86 | ||
| <18.5 | 11 (10%) | 0 (0%) | 2 (9%) | 2 (17%) | 6 (17%) | 1 (5%) | 0.528** | |
| 18.5 – 25.5 | 59 (56%) | 9 (56%) | 12 (55%) | 6 (50%) | 22 (61%) | 10 (50%) | ||
| >25.5 | 25 (24%) | 7 (44%) | 3 (14%) | 4 (33%) | 7 (19%) | 4 (20%) | ||
| 11 (10%) | 0 (0%) | 5 (23%) | 0 (0%) | 1 (3%) | 5 (25%) | |||
| CD4 cell count (cells/mm3) |
Median | 163.25 | 242.25 | 145.75 | 211 | 104.25 | 217.50 | <.001* |
| Q1, Q3 | 91.50, 268.00 |
195.25, 330.25 |
113.00, 230.50 |
150.75, 300.50 |
43.25, 155.00 |
130.75, 326.25 |
||
| <50 | 18 (17%) | 0 (0%) | 1 (5%) | 1 (8%) | 12 (33%) | 4 (20%) | 0.006** | |
| 50 – 199 | 43 (41%) | 4 (25%) | 13 (59%) | 4 (33%) | 17 (47%) | 5 (25%) | ||
| 200 – 349 | 34 (32%) | 9 (56%) | 7 (32%) | 6 (50%) | 5 (14%) | 7 (35%) | ||
| >=350 | 11 (10%) | 3 (19%) | 1 (5%) | 1 (8%) | 2 (6%) | 4 (20%) | ||
| HIV-1 RNA (copies/mL)) | Median | 4.34 | 4.23 | 4.09 | 4.40 | 4.65 | 4.24 | 0.045* |
| Q1, Q3 | 3.86, 4.92 | 3.70, 4.46 | 3.67, 4.82 | 3.87, 4.96 | 4.12, 5.05 | 3.61, 4.81 | ||
| <3.00 | 4 (4%) | 1 (6%) | 1 (5%) | 0 (0%) | 2 (6%) | 0 (0%) | 0.038** | |
| 3.00 – 3.99 | 30 (28%) | 4 (25%) | 9 (41%) | 4 (33%) | 4 (11%) | 9 (45%) | ||
| 4.00 – 4.99 | 53 (50%) | 11 (69%) | 11 (50%) | 7 (58%) | 17 (47%) | 7 (35%) | ||
| 5.00 – 5.29 | 13 (12%) | 0 (0%) | 1 (5%) | 0 (0%) | 8 (22%) | 4 (20%) | ||
| >=5.30 | 6 (6%) | 0 (0%) | 0 (0%) | 1 (8%) | 5 (14%) | 0 (0%) | ||
| Total cholesterol (mg/dL) | Median | 168.02 | 170.15 | 172 | 168 | 158.50 | 190.84 | 0.046* |
| Q1, Q3 | 140, 199 | 146.95, 203.02 |
158, 207 | 140.00, 186.50 |
134.50, 179.50 |
165.12, 208.04 |
||
| Triglycerides (mg/dL) | Median | 122.23 | 97.43 | 126.50 | 179.50 | 112.50 | 123.56 | 0.141* |
| Q1, Q3 | 88.57, 200.00 |
79.71, 132.86 |
79, 279 | 162.00, 199.50 |
84.00, 215.50 |
95.21, 209.91 |
||
| LDL cholesterol (mg/dL) | Median | 92.42 | 100.54 | 93.50 | 115 | 75 | 98.61 | 0.026* |
| Q1, Q3 | 72.31, 117.00 |
77.34, 125.68 |
81, 119 | 92.50, 125.00 |
65.50, 105.00 |
77.15, 124.71 |
||
| HDL cholesterol (mg/dL) | Median | 42.54 | 52.20 | 44 | 37 | 39.50 | 57.42 | 0.002* |
| Q1, Q3 | 34, 56 | 42.54, 58.01 |
33.40, 57.00 |
30, 42 | 30, 45 | 42.34, 63.03 |
Kruskal-Wallis Test
Chi-Square Test
At entry, median FTC and LDL varied significantly across sites (P=0.046 and P=0.026); median FTC ranged from 159 mg/dL in Malawi to 191 mg/dL in Tanzania and median LDL ranged from 75 mg/dL in Malawi to 115 mg/dL in India (Table 1).
INCIDENCE OF HYPERLIPIDEMIA
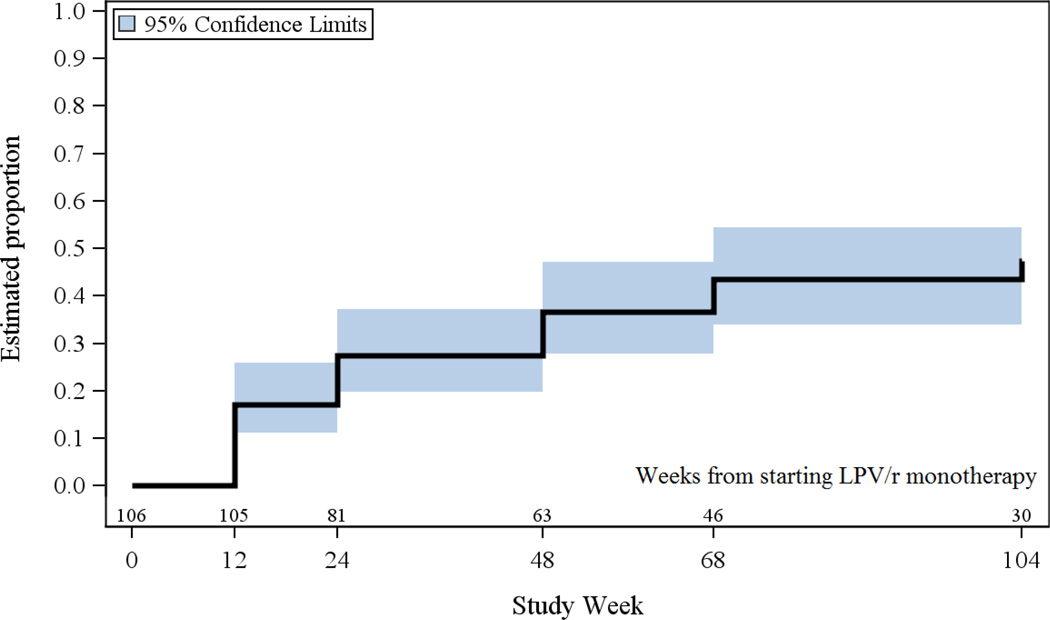
A total of 43 participants developed hyperlipidemia while on LPV/r monotherapy during follow-up, of whom 27 (63%), 15 (35%) and 12 (28%) developed Grade 2+ FTC, FTG and LDL at study-scheduled measurement times, respectively. Five (12%) of the 43 participants who developed hyperlipidemia started lipid lowering agents without having a Grade 2+ lipid level at a scheduled measurement time. Figure 1 shows the cumulative incidence of hyperlipidemia over time. At week 12, the time of the first evaluation of lipids in the study, 17% had developed hyperlipidemia. Thereafter there was an ongoing incidence of hyperlipidemia with a cumulative incidence by 104 weeks of 48% (95% CI: 36 – 58%) while on LPV/r monotherapy.
Figure 1.
Cumulative Incidence of Hyperlipidemia among Participants while on LPV/r Monotherapy
RISK FACTORS FOR HYPERLIPIDEMIA
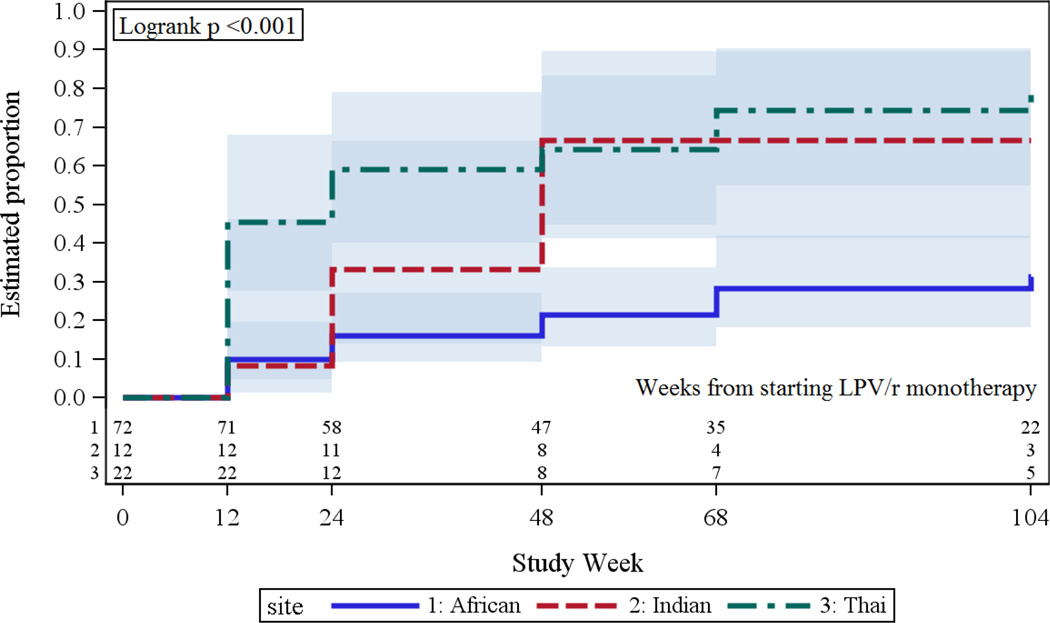
In univariable analyses, higher baseline FTC (P<0.001), FTG (P<0.001) and LDL (P=0.002) were associated with increased risk of hyperlipidemia. In addition, race (P<0.001) and site (P=0.001), but not sex, age, CD4 count, HIV-1 RNA, BMI or weight, were significantly associated with risk of hyperlipidemia. The variation by site reflected higher incidence at the Thai site and at the Indian site versus the African sites (and hence the potential difference according to race) (Figure 2); there was no significant difference among the three African sites. The unadjusted hazard ratio comparing risk among participants at the Thai site versus those at the African sites was 3.97 (95% confidence interval [CI]: 2.04 – 7.74; P<0.001) and among those at the Indian site versus those at the African sites was 2.69 (95% CI: 1.17 – 6.19; P=0.020). In terms of cumulative incidence by 104 weeks, these differences translated into proportions of 31% (95% CI: 19 –45%) at the African sites, 67% (31—87%) at the Indian site and 80% (53—92%) at the Thai site.
Figure 2.
Cumulative Incidence of Hyperlipidemia by Site among Participants on LPV/r Monotherapy
In multivariable analysis, adjusted for baseline FTC, FTG and LDL, the differences by site persisted: the adjusted hazard ratio comparing participants at the Thai site to those at African sites was 3.42 (95% CI: 1.67–7.00; P<0.001) and comparing participants at the site in India to those at African sites was 3.51 (1.22–10.1; P=0.020). There was no significant difference among the three African sites. In this multivariable analysis, risk of hyperlipidemia remained significantly associated with higher baseline FTC (P=0.044) and with higher baseline FTG (P=0.025) but not with baseline LDL (P=0.70). When sex, BMI, weight, CD4 count or HIV-1 RNA was added to this multivariable model, none of these variables was significantly associated with risk of hyperlipidemia and the differences among the sites persisted. Thus differences in risk of hyperlipidemia among the sites were not explained by any differences among sites in baseline lipid levels, sex, weight, BMI, CD4 count or HIV-1 RNA.
CHANGES IN LIPIDS
Because a large proportion of participants experienced hyperlipidemia and started lipid-lowering agents during follow-up, analysis of quantitative changes in lipid levels focused on the acute change between baseline and the first scheduled measurement after 12 weeks of LPV/r monotherapy. Of the 106 participants, 101 were included in this analysis; 5 participants were excluded because they did not have fasting week 12 lipid measurements. At week 12, there were significant mean increases from baseline in FTC (17 mg/dl; P<0.001) and FTG (104 mg/dl, P<0.001) but not LDL (P=0.58) (Table 2).
Table 2.
Week 12 Mean Lipid Changes Among Participants On LPV/r Monotherapy
| Study Week | ||||
|---|---|---|---|---|
| Characteristic | 0 (N=106) |
12 (N=99) |
P-Value* | |
| Total Cholesterol (mg/dl) |
N | 106 | 99 | <.001 |
| Mean (s.d.) | 0 (0) | 16.77 (31.38) | ||
| Triglycerides (mg/dl) |
N | 106 | 96 | <.001 |
| Mean (s.d.) | 0 (0) | 104.19 (168.14) | ||
| HDL Cholesterol (mg/dl) |
N | 106 | 99 | 0.004 |
| Mean (s.d.) | 0 (0) | −3.28 (10.96) | ||
| LDL Cholesterol (mg/dl) |
N | 106 | 96 | 0.580 |
| Mean (s.d.) | 0 (0) | −0.35 (30.49) | ||
In univariable linear regression analyses, associations of change in each of FTC, FTG and LDL from baseline to week 12 with sex, age, race, site, and baseline weight, BMI, CD4 count, HIV-1 RNA, FTC, FTG and LDL were evaluated but few significant associations were found. Specifically, higher baseline FTG level was significantly associated with greater increases in both FTC and FTG (P<0.001 for both). In addition, higher baseline FTC was significantly associated with greater increase in FTG (P=0.048). There was also significant variation among sites and among racial groups in change in FTG (P=0.049 and P=0.021, respectively). None of the variables considered was significantly associated with change in LDL.
There were variations in lipid levels by site location in both univariable analysis and multivariable analysis adjusted for baseline FTC, FTG and LDL. The variation among sites in change in FTG primarily reflected greater mean increase in FTG among participants at the Thai site (243 mg/dl) compared with those at the African sites (90 mg/dl) or the Indian site (79 mg/dl). Thus, compared with participants at the African sites, participants at the Thai site experienced mean increases in FTG which were greater by 153 mg/dl, and those at the Indian site experienced mean increases which were smaller by 11 mg/dl. These differences persisted in multivariable analysis adjusted for baseline FTC, FTG and LDL levels: greater by142 mg/dl among Thai participants and by 2 mg/dl among Indian participants.
DISCUSSION
In our analysis of a diverse population of individuals on LPV/r monotherapy from 5 different countries in resource-limited settings, the incidence of hyperlipidemia was high. It was more common among Thai and Indian participants compared to the African participants in both univariable and multivariable analyses. In addition, participants with higher baseline FTC and FTG were at higher risk of hyperlipidemia. Mean increases in FTG and FTC during the first 12 weeks of LPV/r monotherapy were significantly greater among participants with higher baseline FTG, and mean increase in FTG was also significantly higher among Thai participants than among Indian or African participants.
The incidence of hyperlipidemia was the highest among Thai participants at 80% followed by Indian participants at 67% and was least among African participants at 31%. These findings are consistent with previous studies which showed that the prevalence of hyperlipidemia in patients receiving PI-based ART ranged from 28% to 80%. [16, 17] Comparing with other studies that used LPV/r in combination with other antiretroviral drugs, we found similar rates in India and Thailand as found in Spain (65%) [18] and Italy (82%) [19], but lower rates in the African sites than in Zimbabwe (85%)3. Conversely, we found a similar rate of hyperlipidemia compared to a study in South Africa which reported a rate of 29%. [20] The differences among countries in our study were not explained by any differences among race/sites in baseline lipid levels, sex, weight, BMI, CD4 count or HIV-1 RNA levels.
The differences between the studies could be due to differences in the definitions of hyperlipidemia and differences in study designs as most of the studies were cross sectional. We looked at the rate of hyperlipidemia solely among participants on LPV/r monotherapy while other studies looked at the rate of hyperlipidemia in participants on LPV/r-based HAART regimens of which some of the lipid elevations may have been due to NRTI use. [1, 21] The majority of individuals (73%) in the A5230 study were on an NVP-based regimen at the time of screening. [22] For first-line NNRTI based ART treatment, RLS treatment programs mainly rely on NRTI backbone of either zidovudine (AZT) or stavudine (d4T) and lamivudine (3TC) or, more recently, tenofovir (TDF)NRTIs are known to have a mild effect on lipids with a high degree of heterogeneity in lipid response. [23] We excluded individuals with significant lipid elevations at the time of entry from the analysis but those with borderline elevations due to first-line treatment were included and this may have influenced lipid responses to LPV/r monotherapy. Conversely, TDF is a NRTI that has been reported to have a modest lipid lowering effect. [24, 25]
Race/ethnicity has been reported as a significant predictor of hyperlipidemia in the United States. [21, 26] Genetic susceptibility may greatly influence the risk for development of hyperlipidemia in HIV-infected patients. [27] African Americans were found to have the least increase in FTG compared to Latinos and Whites. [21] Foulkes et al found an association between apolipoprotein C3-related to rs10892151 (genotype) polymorphism and serum triglyceride levels which led to the belief that genetic differences may play a major role in HIV ART-induced dyslipidemia. [26] In another study, it was reported that patients who are heterozygous or homozygous for the apolipoprotein E-2 genotype have higher plasma triglyceride levels and may have more drastic changes in levels of cholesterol and triglycerides when receiving a PI-based therapy. [27] We found that Thai and Indian participants had higher risk for hyperlipidemia compared to Africans. Reasons for racial differences are not well understood but could beattributed to other factors which were not measured in this study such as genetic differences, differences in diet, life style factors and baseline lipoatrophy that have been found to have an association with dyslipidemias and metabolic syndrome. [27, 28, 29]. In our study, adjustments for weight and BMI did not explain the differences among countries.
PI-based treatment has been associated with increases in FTG and FTC levels. [3, 10, 12, 30 – 34] Similarly, among participants with hyperlipidemia in our study, most had Grade 2+ elevations in FTC (63%), while fewer had Grade 2+ elevations in FTG (35%) or LDL (28%). High baseline FTG and FTC were associated with hyperlipidemia among participants on LPV/r monotherapy in both univariable and multivariable analyses, as previously reported. [27] It is understandable that individuals with borderline high baseline lipid levels would require smaller changes in lipid levels to rise to levels that would meet the definition of hyperlipidemia and in this case, LPV/r exacerbated the already high lipid levels. Similarly, other studies on LPV/r used in combination with NRTIs reported high level of FTC and FTG as risk factors for increase in FTC and FTG. [2, 19, 27]
Our analyses for quantitative changes in lipid levels revealed that FTC and FTG significantly increased by 17 mg/dL and 104 mg/dL after 12 weeks on LPV/r monotherapy but not LDL. In a study by Shaffer involving women from 7 sub-Saharan African countries, there were significant mean increases of 29 mg/dL, 22 mg/dL and 15 mg/dL in FTC, FTG and LDL respectively after 48 weeks on LPV/r combined with Truvada. [2] There was a large variability in FTG change in our study. However, we found similar mean increases in FTG compared to a study conducted in Spain (73mg/dL). [18] The larger difference in FTG change in our study compared to Shaffer’s study cannot be clearly explained but could due to the lipid lowering effect of TDF or to differences by sex or race. [24, 25] Since Africans are generally known to have low lipid levels, the greater change in FTG levels at the Thai site may have contributed to high overall mean change in FTG in our study compared to Shaffer’s study. There was no significant evidence that the increases in FTC varied by race/site, however, a similar pattern as with FTG was observed. There was also no significant evidence that changes in LDL varied by race. Since LDL levels were calculated rather than measured directly, the decrease in LDL especially among the Thai participants, largely reflect the marked mean increase in FTG at that site.
The primary strengths of our study are in the relatively large sample size on LPV/r monotherapy as well as the long-term follow up. The diversity of the study population, including participants from different sites and of different races, allowed us to make comparisons among different sites in resource-limited settings. As there is little information about lipid changes in individuals on LPV/r monotherapy, this study will help to fill an important knowledge gap. Our study had several limitations. First, LDL levels were calculated rather than measured, and there may be discrepancies between calculated and measured LDL values particularly in the setting of increased FTG levels as found in our study. [35, 36] There could also be some differences among countries that we were not able to measure which might explain the differences between countries. Monotherapy, including that with LPV/r, is no longer recommended for standard treatment in management of HIV [8] and this may limit the generalizability of our results. However, our results likely remain relevant for individuals who require or may benefit from NRTI sparing regimens with PIs. [37]
In conclusion, we found that hyperlipidemia was common among participants on LPV/r monotherapy which was started as a second line therapy. Hyperlipidemia was more common among Thai and Indian participants compared to the African participants and among participants with high baseline FTG and FTC. Baseline lipid measurements should be recommended in individuals starting LPV/r-based treatments and routine monitoring for lipid changes should be conducted on individuals with borderline high lipids, especially FTG and FTC. Therefore, efforts to prevent and treat hyperlipidemia and other CVD risk factors will be important in reducing CVD-related morbidity and mortality in HIV-infected persons in resource-limited settings.
Acknowledgments
We thank all 5230 participants and sites that participated in this study.
Source of Funding: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mitch Matoga received funding from the ACTG Leadership and Operations Center, UM1 AI068636. Mina Hosseinipour received funding from University of North Carolina project (site 12001) CTU grant number 2UM1AI069423.
Michael Hughes, Evgenia Aga and Heather Ribaudo received funding from the ACTG Statistical and Data Management Center, UM1 AI068634. Nagalingeshwaran Kumarasamy received funding from YRGCARE Medical Centre (Site 11701) CTU Grant number AI069432. Dr. Bartlett receives salary support from the US National Institutes of Health Awards P30AI64518, U01AI067854, D43CA153722, and D43TW06732, and from the Health Resources and Services Administration Award T84HA21123.
Footnotes
Disclosure Statement - Conflict of Interest: We do not have any conflicts of interest to report.
REFERENCES
- 1.Shaffer D, Hughes M, Sawe F, et al. Cardiovascular Disease Risk Factors in HIC-Infected Women After Initiation of Lopinavir/Ritonavir-and Nevirapine-Based Antiretroviral Therapy in Sub-Saharan Africa: A5208 (OCTANE) Journal of Acquired Immune Deficiency Syndrome. 2014;66(2):155–163. doi: 10.1097/QAI.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazisa Hejazi, Rajikan R, Choong C, et al. Metabolic abnormalities in adult HIV infected population on antiretroviral medication in Malaysia: a cross-sectional survey. BMC Public Health. 2013;13:758. doi: 10.1186/1471-2458-13-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomo Z, Hakim J, Walker S, et al. the DART Team. Impact of second-line antiretroviral regimens on lipid profiles in an African setting: the DART trial sub-study AIDS Research and Therapy. 2014;11:32. doi: 10.1186/1742-6405-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palacios R, Santos J, Ruiz J, et al. Short-term lipid changes associated with highly active antiretroviral therapy in naive HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:249–251. doi: 10.1097/00126334-200310010-00021. [DOI] [PubMed] [Google Scholar]
- 5.Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 6.Riddler S, Smit E, Cole S, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 7.Aberg JA. Cardiovascular complications in HIV management: past, present, and future. J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):54–64. doi: 10.1097/QAI.0b013e31818ceaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Consolidated Guidelines on the use of Antiretroviral Drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2013 Jun; [PubMed] [Google Scholar]
- 9.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach: 2010 reveision. [PubMed] [Google Scholar]
- 10.Walmsley S, Bernstein B, King M, et al. Lopinavir–ritonavir versus Nelfinavir for the initial treatment of HIV infection. The New England Journal of Medicine. 2002;346(No 26):2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Seneviratne T, Noor M, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. National Institute of Health Public Access AIDS. 2004 Mar 5;18(4):641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madruga J, Berger D, Mc Murchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomized controlled phase III trial. The Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 13.WHO. UNAIDS; 2008. Report on the global AIDS epidemic. [Google Scholar]
- 14.Kumarasamy N, Madhavan V, Venkatesh K, et al. Lopinavir/Ritonavir Monotherapy as Second-line Antiretroviral Treatment in Resource-Limited Settings: Week 104 Analysis of AIDS Clinical Trials Group (ACTG) A5230. Clin Infect Dis. 60(10):1552–1558. doi: 10.1093/cid/civ109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett J, Ribaudo H, Wallis C, et al. Lopinavir/ritonavir monotherapy after virologic failure of first-line antiretroviral therapy in resource-limited settings. AIDS. 26(11):1345–1354. doi: 10.1097/QAD.0b013e328353b066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calza L, Manfredi R, Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. Journal of Antimicrobial Chemotherapy. 2004;53:10–14. doi: 10.1093/jac/dkh013. [DOI] [PubMed] [Google Scholar]
- 17.Hiransuthikul N, Hiransuthikul L, kannasook Y. Lipid Profiles of THAI Adult HIV-Infected Patients Receiving Protease Inhibitors. Southeast Asian Journal of Tropical Medicine and Public Health. 2007 Jan;38(1):69. [PubMed] [Google Scholar]
- 18.Montes M, Pulido F, Barros C, et al. Lipid disorders in antiretroviral-naïve patients treated with lopinavir/ritonavir-based HAART: frequency, characterization and risk factors. Journal of Antimicrobial Chemotherapy. 2005;55:800–804. doi: 10.1093/jac/dki063. [DOI] [PubMed] [Google Scholar]
- 19.Bongiovanni M, Bini T, Cicconi P, et al. Predictive factors of hyperlipidemia in HIV-infected subjects receiving lopinavir/ritonavir. AIDS Res Hum Retroviruses. 2006;22:132–138. doi: 10.1089/aid.2006.22.132. [DOI] [PubMed] [Google Scholar]
- 20.Sinxadi P, McIlleron H, Dave J, et al. Association of lopinavir concentrations with plasma lipid or glucose concentrations in HIV-infected South Africans: a cross sectional study. AIDS Research and Therapy. 2012;9:32. doi: 10.1186/1742-6405-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibert C, Shlay J, Sharma S, et al. Racial differences in changes of metabolic parameters and body composition in antiretroviral therapy-naive persons initiating antiretroviral therapy. JAIDS. 2009;50(1):44–53. doi: 10.1097/QAI.0b013e31818ce808. [DOI] [PubMed] [Google Scholar]
- 22.Wallis C, Aga E, Ribaudo H, et al. Drug Susceptibility and Resistance Mutations After First-Line Failure in Resource Limited Settings. Clin Infect Dis. 2014 Sep 1;59(5):706–715. doi: 10.1093/cid/ciu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estrada V, Portilla J. Dyslipidemia Related to Antiretroviral Therapy. AIDS Rev. 2011;13:49–56. [PubMed] [Google Scholar]
- 24.Tungsiripat M, Kitch D, Glesby M, et al. A Pilot Study to Determine the Impact on Dyslipidemia of Adding Tenofovir to Stable Background Antiretroviral Therapy: ACTG 5206. National Institute of Health Public Access AIDS. 2010;24(11):1781–1784. doi: 10.1097/QAD.0b013e32833ad8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massimiliano F, Bracciale L, Doino M, et al. Lipid-lowering effect of tenofovir in HIV-infected patients. Journal of Antimacrobial and Chemotherapy. 2011;464:682–683. doi: 10.1093/jac/dkq464. [DOI] [PubMed] [Google Scholar]
- 26.Foulkes A, Woh D, Frank I, et al. Associations among race/ethnicity, ApoC-III genotypes, and lipids in HIV-1-infected individuals on antiretroviral therapy. PLoS Med. 2006;3(3):0337–0347. doi: 10.1371/journal.pmed.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez E, Domingo P, Galindo M, et al. Risk of Metabolic Abnormalities in Patients Infected with HIV Receiving Antiretroviral Therapy that Contains Lopinavir-Ritonavir. Clin Infect Dis. 2004;38:1017–1023. doi: 10.1086/382531. [DOI] [PubMed] [Google Scholar]
- 28.Misra A, Kalpana Luthra, Vikram NK. Dyslipidemia in Asian Indians: determinants and significance. JAPI. 2004;52:137–142. [PubMed] [Google Scholar]
- 29.Lee D, Xuemei S, Church T, et al. Changes in Fitness and Fatness on the Development of Cardiovascular Disease Risk Factors: Hypertension, Metabolic Syndrome, and Hypercholesterolemia. J Am Coll Cardiol. 2012;59(7):665–672. doi: 10.1016/j.jacc.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpentier A, Patterson B, Uffelman K, et al. Mechanism of highly active anti-retroviral therapy-induced hyperlipidemia in HIV-infected individuals. Atherosclerosis. 2005;178(1):165–172. doi: 10.1016/j.atherosclerosis.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Shahmanesh M, Das S, Stolinski M, et al. Antiretroviral treatment reduces very-low-density lipoprotein and intermediate-density lipoprotein apolipoprotein B fractional catabolic rate in human immunodeficiency virus-infected patients with mild dyslipidemia. Clin Endocrinol Metab. 2005;90(2):755–760. doi: 10.1210/jc.2004-1273. [DOI] [PubMed] [Google Scholar]
- 32.Reeds D, Yarasheski K, Fontana L, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006;290(1):E47–E53. doi: 10.1152/ajpendo.00236.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lenhard J, Croom D, Weiel J, et al. Atherosclerosis and Lipoproteins: HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol. 2000;20(12):2625–2629. doi: 10.1161/01.atv.20.12.2625. [DOI] [PubMed] [Google Scholar]
- 34.Petit J, Duong M, Duvillard L, et al. LDL-receptors expression in HIV-infected patients: relations to antiretroviral therapy, hormonal status, and presence of lipodystrophy. Am J Physiol EndocrinolMetab. 2002;32(5):354–359. doi: 10.1046/j.1365-2362.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- 35.Lindsey C, Graham M, Johnston T, et al. A clinical comparison of calculated versus direct measurement of low-density lipoprotein cholesterol level. Pharmacotherapy. 2004 Feb;24(2):167–172. doi: 10.1592/phco.24.2.167.33142. [DOI] [PubMed] [Google Scholar]
- 36.Choi S, Park H, Kim M, et al. Difference between calculated and direct-measured low-density lipoprotein cholesterol in subjects with diabetes mellitus or taking lipid-lowering medications. J Clin Lipidol. 2012 Mar-Apr;6(2):114–120. doi: 10.1016/j.jacl.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 37.La Rosa AM, Harrison LJ, Taiwo B, et al. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. The Lancet HIV. 2016;3(6):e247–e258. doi: 10.1016/S2352-3018(16)30011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]