Abstract
Background
Anatomic, physiologic, and behavioral studies in animals suggest that spinally released oxytocin should produce analgesia in humans and may also protect from chronic pain after injury. Here we report preclinical toxicity screening of oxytocin for intrathecal delivery.
Methods
Intrathecal oxytocin, 11 μg (6 IU) or vehicle, was injected intrathecally in 24 rats, followed by frequent behavioral assessment and histologic examination of spinal contents 2 or 14 days after injection. In 3 dogs, a range of intrathecal oxytocin doses (18-550 μg in 0.5 mL) was injected followed by physiologic, biochemical, and behavioral assessments. Ten dogs were then randomized to receive 5 daily injections of intrathecal oxytocin, 550 μg in 0.5 mL, or vehicle with similar assessments and, 2 days later, necropsy and histologic analysis.
Results
In rats intrathecal oxytocin resulted in transient scratching and itching behaviors, without other differences from vehicle. There was no behavioral, gross anatomic, or histologic evidence of neurotoxicity. Dose-ranging in dogs suggested mild effects on motor tone, blood pressure and heart rate at the 550 μg dose. Repeated boluses in dogs did not produce behavioral, biochemical, neurological, gross anatomic, or histologic evidence of neurotoxicity.
Conclusions
Substances, including natural neurotransmitters, may be toxic when administered in pharmacologic doses in the spinal cord. This preclinical toxicity screen in two species suggests that bolus injections of oxytocin in concentrations up to 1100 μg/mL is unlikely to cause neurotoxicity. They also support cautious clinical application of intrathecal oxytocin under regulatory supervision.
Introduction
The nonapeptide oxytocin, contained in neurons of the hypothalamic paraventricular nucleus (PVN), is released from the pituitary into the blood where it induces uterine contractions, protects the fetal brain during delivery, and facilitates lactation.1 Importantly, circulating oxytocin re-enters the central nervous system to an exceedingly limited extent.2 Other neurons in the PVN project within the central nervous system to alter many aspects of behavior, especially related to social engagement and bonding, in both sexes across the life cycle.1
We recently observed that chronic pain from childbirth, including cesarean delivery, is remarkably rare3 and that recovery from hypersensitivity from peripheral nerve injury in rats is accelerated when injury occurs surrounding the time of delivery.4 This protective effect in the peripartum period is reversed by intrathecally administered oxytocin receptor antagonists,4 consistent with a role for spinal oxytocin in accelerating recovery from pain after injury. This hypothesis is consistent with recent anatomic and physiologic studies. As such, oxytocin-containing PVN neurons project to the superficial dorsal horn of the spinal cord,5,6 and cerebrospinal fluid (CSF) oxytocin concentrations increase in lumbar CSF in women during labor.7 PVN stimulation temporarily reverses spinal neuronal8,9 and behavioral10 hypersensitivity from nerve injury in a manner reversed by oxytocin receptor antagonists. These effects are mimicked by intrathecal injection of oxytocin itself.10,11 Oxytocin acts in the spinal cord to inhibit nociceptive neurotransmission in part by stimulating γ-amino-butyric acid release12 and increasing potassium channel conductance in lamina II neurons.13 The above observations suggest that intrathecal oxytocin may treat established neuropathic pain and potentially prevent chronic pain after surgery.
These studies provide the rationale for clinical investigation of intrathecal oxytocin for the prevention and treatment of pain, but do not provide evidence of safety. Administering large doses of oxytocin systemically to achieve central nervous system penetration is not feasible, given QT prolongation and hypotension that would ensue.14,15 Oxytocin is an endogenously produced compound not implicated to cause cell necrosis, apoptosis, or inflammation, but other endogenously produced peptides do cause neurotoxicity when administered intrathecally,16,17 as does stimulation of some spinal neurotransmitter receptors by intrathecally administered agonists.18 In addition, although oxytocin has a higher affinity for oxytocin receptors than vasopressin receptors,19 its agonist activity at higher concentrations on vasopressin receptors could lead to spinal cord ischemia locally or hypertension and hyponatremia by systemic absorption. For these reasons, formal preclinical toxicity testing is mandatory.20 We report here preclinical toxicity of intrathecal oxytocin in validated approaches using rats and dogs,17,21-24 focusing on local effects, physiologic actions, and spinal histopathology as guidance for subsequent clinical introduction for investigation.
Materials and Methods
The studies were approved by the Animal Care and Use Committee at the University of California, San Diego for dog studies and Wake Forest School of Medicine for rat studies.
Drug
Since marketed formulations of oxytocin contain neurotoxic preservatives and are only available in low concentrations, we obtained USP® oxytocin powder from GRINDEKS (Riga, Latvia), a Food and Drug Administration certified facility which manufactures oxytocin for clinical use. It was manufactured according to Good Manufacturing Practices guidelines. Information on synthesis methods, composition and other characteristics that define this powder are maintained by GRINDEKS and filed with the Food and Drug Administration.
Rat Studies
Twenty four male Sprague-Dawley rats (Harlan-Industries, Indianapolis, IN), weighing 238-306 g, were studied. Animals were maintained in individual cages with ad libitum food and water on a 12-h light/dark cycle.
Drug administration
Following acclimation to the laboratory and testing procedures, animals were briefly anesthetized with inhalational isoflurane and a direct lumbar puncture was performed between the L5 and L6 vertebrae using a 30 gauge, ½ inch needle which is at the level of the cauda equina. Previous dye distribution studies in rats have demonstrated that a 10 μl injection volume spreads to above the L3-4 level following lumbar puncture at this site.25 Proper needle location was identified by a brief tail flick. In control experiments using motor block from injection of lidocaine as a measure of success, the success rate in our laboratory exceeds 95%. Following lumbar puncture, rats received an injection of 10 μL sterile saline alone, or containing 11 μg (6 IU) oxytocin (n=12 per group). Oxytocin was dissolved in sterile saline and terminally sterilized by passage through a sterile 0.22 micron filter. The dose and concentration of oxytocin used and timing of experiments were determined in consultation with Food and Drug Administration staff and the concentration used was the same as that used in the study in beagle dogs.
Behavioral assessment
Each animal received a single bolus injection. Half of the animals (n=6 with saline and 6 with oxytocin injection) were sacrificed 2 days following injection and the other half (n=6 with saline and 6 with oxytocin injection) were sacrificed 14 days following injection. Arousal, motor coordination and motor tone were assessed in all animals 0.5, 1, 2, and 4 hr after intrathecal injection, then daily until 2 days after treatment. These assessments were also made at 7 and 14 days after treatment in the longer observation group. Arousal scores were assessed on a 7 point scale as previously described,26 ranging from -3 (comatose) to +3 (maximal excitation). Motor coordination was evaluated using a 4 point scale, ranging from normal symmetric posture (0) to lack of righting response (-3) and motor tone using a 7 point scale ranging from extreme rigidity (-3) to normal (0) to no postural tone (3). In addition, since intrathecal oxytocin causes scratching behavior in mice,27 we also assessed grooming and scratching behavior, summing episodes of these behaviors during a 2 min period.
Anatomic assessment
On completion of the study 2 or 14 days after intrathecal injection, animals were deeply anesthetized with pentobarbital. The left ventricle was cannulated and perfused with phosphate buffer, followed by 4% paraformaldehyde. After removal of the vertebral column, the spinal cords were removed. After fixation in formalin, blocks were embedded in paraffin, sectioned at 10 μm thickness and stained with hematoxylin-eosin for general examination and Luxol fast blue for myelin. Coded sections from the lower lumbar region of the spinal cord (L4-6) rostral to the injection site were examined by an investigator in random order for all animals, without knowledge of drug treatment group. Particular attention was given to the presence or absence of inflammation and demyelination in the spinal cord parenchyma.
Dog studies
Thirteen adult, destination bred beagles (Marshall Farms USA, North Rose, NY) were studied. Animals were housed in a dedicated facility with fluorescent lighting on a daily 12-hour light/dark cycle, with free access to dry dog food and water. A dose ranging study was performed in 3 male dogs followed by a repeated bolus study in 5 male and 5 female dogs.
Dose ranging study
Surgery
Food was withheld on the evening prior to surgery. An intrathecal catheter was inserted as previously described.22 Sulfamethoxazole-trimethoprim (240 mg tablet, 15-25 mg/kg, oral, twice daily) was given 48 h prior to surgery and for 48 h after surgery. Following sedation with xylazine, 1.5 mg/kg, intramuscular, anesthesia was induced with mask isoflurane, the trachea intubated, and anesthesia maintained with 1.0-2.0% isoflurane and 60% N20/40% O2 with spontaneous ventilation. Animals were continuously monitored for oxygen saturation, inspired and end tidal values of isoflurane, carbon dioxide, N2O, heart and respiratory rate. Following skin preparation with chlorhexadine, a midline neck incision and dissection were performed, the cisterna magnum exposed, a 1-2 mm incision made in the dura, and a sterilized polyurethane intrathecal catheter (0.61 mm OD, fabricated at ReCathCo Inc, Allison Park, PA) inserted and passed caudally a distance of approximately 40 cm. Confirmation of appropriate catheter placement in the intrathecal space was judged by the free outflow of CSF. The catheter was then tunneled subcutaneously and caudally to exit at the midline scapular region. Dexamethasone sodium phosphate (0.25 mg/kg, intramuscular) was administered just after catheter placement, and butorphanol tartrate, 0.04 mg/kg, intramuscular, and carprofen, 4.5 mg/kg, SQ were administered to relieve post-operative pain and inflammation.
Drug administration
Each animal received a series of intrathecal injections of escalating doses of oxytocin (18, 55, 180, and 550 μg, corresponding to 10, 30, 100, and 300 IU, in an 0.5 mL volume followed by 0.3 mL saline flush) beginning 3 days after intrathecal catheter insertion and with doses separated by 2-5 days. Based on tolerability results, one of the animals then received the 550 μg / 0.5 mL intrathecal dose at approximately 24 h intervals for 5 days.
Behavioral, physiologic, and biochemical measures
Levels of arousal, muscle tone, and coordination were assessed as previously described26 daily beginning 17 days prior to the first intrathecal dose, at 10, 20, 30, 60, 120, and 240 min after each dose and daily in between dosing. Animals were also assessed for general behavior, appetite, temperature, and defecation and urination on a daily basis. Blood pressure and heart rate were measured non-invasively, and respiratory rate by observation at the same times as the behavioral assessments. Prior to surgery, blood samples were taken for standard hematology and clinical chemistry testing, performed by a clinical laboratory service.
Plasma Oxytocin Sampling and Analysis
Cephalic vein blood samples (approximately 2 mL) were collected by venipuncture into EDTA tubes prior to intrathecal injection and at the same time as behavioral assessments. Blood samples were placed on wet ice, centrifuged, and the plasma stored frozen at -20±10°C until assayed. Oxytocin concentrations were measured by ELISA using a commercially available kit (Assay Designs, Oxytocin Enzyme Immunoassay Kit, Ann Arbor, MI), using the methods described in the commercial kit.
Repeated bolus study
Unless otherwise indicated, surgery and behavioral, physiologic, and biochemical measures were identical to the dose-ranging study.
Drug administration
Animals were randomized, using an unbalanced design to receive daily intrathecal injections of vehicle (2 males, 2 females) or oxytocin (3 males, 3 females) for 5 consecutive days. Based on the dose-ranging study, the daily oxytocin dose was 550 μg delivered in 0.5 mL.
Timing of assessments
Behavioral assessment, blood pressure, and heart and respiratory rate were determined prior to surgery and 15 min before and 60 min after each intrathecal dose. Neurologic examinations were performed by a veterinarian (PR) prior to placement of the intrathecal catheter, after intrathecal catheter placement but prior to initiation of the dosing regimen, and at the end of the dosing regimen. Blood was sampled for oxytocin analysis 60 min following the first and fifth intrathecal dose. In addition, blood and cisternal CSF were sampled before surgery and at necropsy for routine hematology and chemistry.
Anatomic assessment
Two days following the last intrathecal injection, the dog was sedated with sodium pentobarbital (35 –50 mg/kg), the chest was opened, the aorta catheterized, and blood was cleared by perfusion with approximately four liters of 0.9% saline, followed by approximately four liters of 10% formalin. The dura of the spinal canal was then exposed by laminectomy. Dye was injected through the catheter to visualize the catheter tip position and its spatial relationship to the cord. The cord was then cut into four sections: cervical, thoracic, lumbar (with catheter tip region) and lumbar, below the catheter tip region, removed and submitted for microscopic examination by a neuropathologist (MG). Gross necropsy evaluation was performed by a veterinarian (KO), including examination of skin, muscle, sciatic nerve, eye, ear, salivary glands and associated lymph nodes, oral cavity, tongue, larynx, trachea, bronchi, lungs, parabronchial lymph nodes, heart, thyroid, parathyroid, adrenal, pituitary, brain, kidneys, urinary bladder, reproductive system, liver, pancreas, spleen, esophagus, stomach, small and large intestine, and mesenteric lymph nodes. Tissues sampled and placed in formalin from all animals included spinal cord, dorsal root ganglia, eye (retina), sciatic nerve, cerebral cortex (dorsal, at midpoint between rostral and caudal poles), and brainstem. Sections of spinal cord with associated dura and dorsal root ganglion were examined by a neuropathologist from four levels (cervical, thoracic, upper lumbar, lumbar). Hematoxylin and eosin stained sections for each level and glial fibrillary acidic protein (GFAP) and Luxol fast blue stained sections of the upper lumbar block were examined for evidence of spinal cord compression, reactive gliosis, demyelination, inflammation, fibrosis, and any other injury. Histopathology was graded for each level based on the degree of inflammation and fibrosis. In brief, the degree of pathology (based on chronic and/or acute inflammation and fibrosis) was graded as 0 (normal), mild (1), moderate (2) or severe (3), as previously described.26. The veterinarians and neuropathologist were blinded as to treatment group.
Statistical analysis
Data are presented as mean ± SD or median [25th to 75th percentile] as appropriate. Only summary statistics are shown for the dose-ranging study in 3 animals. Repeated observations were analyzed using a repeated measures three way ANOVA followed by protected post-hoc testing. For single measures, the groups were compared by unprotected, two-way Student's t tests. P < 0.05 was considered significant.
Results
Rat study
Spinal injections were well tolerated by all rats and none of the animals exhibited pain behavior following injection of the drug. Intrathecal injection of oxytocin, but not vehicle, produced grooming and scratching behavior, lasting < 1 hr (p<0.001; Figure 1). Neither saline nor oxytocin produced changes in motor coordination or tone (all scores were normal at all times after injection). Arousal scores were increased in 2 of 12 animals receiving oxytocin (to 1 = intense grooming and mild hyperactivity) 30 min after injection, but all other scores were normal at all times after injection.
Figure 1.
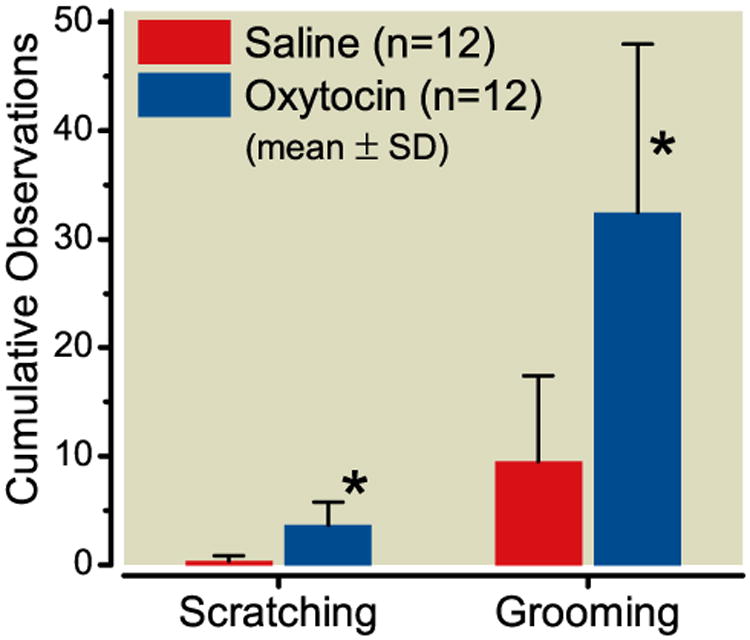
Cumulative observations of scratching and grooming behaviors over 2 min following intrathecal injection of saline (red) or oxytocin (blue) in rats. Data are mean ± SD in 12 animals. *P < 0.001 compared to saline.
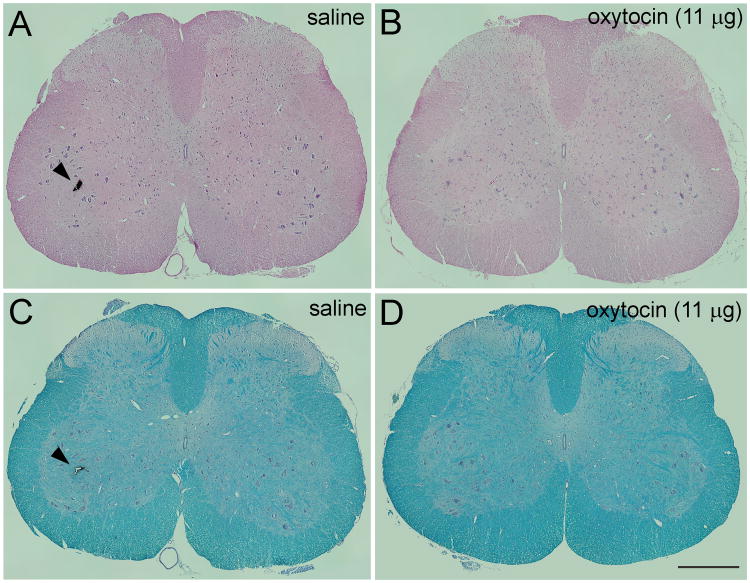
All rats survived treatments as scheduled and there were no abnormal behaviors observed on days of testing or thereafter. All animals showed normal gait and appeared well groomed throughout the study period. Body weight and weight gain did not differ between groups (data not shown). At the end of study, visual inspection of the spinal cord tissue and meninges revealed no gross abnormalities. Perfusion was adequate in all animals. Histologic examination revealed no signs of inflammation or necrosis in any of the treatment groups based on hematoxylin and eosin stain (Figure 2A,B) or white matter vacuolization or demyelination based on Luxol Fast Blue staining (Figure 2C,D).
Figure 2.
Histological assessment of lumbar rat spinal cord fourteen days following a single bolus intrathecal injection of saline (A, C) or 11 μg oxytocin (B, D). Histological examination revealed no signs of inflammation or necrosis in either treatment groups based on hematoxilin and eosin stain (A, B) or white matter vacuolization/demyelination based on Luxol Fast Blue staining (C, D). Some spinal cord tissue blocks were marked in the ventral horn with ink (arrowhead) for identification purposes after histological processing. Scale bar in D = 500 μ.
Dog dose ranging study
No untoward effects on behavior or health were noted during the course of the study. Arousal, muscle tone and coordination were all within normal range. No clear pattern of intrathecal oxytocin on blood pressure emerged, although there was a large effect size of reduced blood pressure (-28%) 10-20 min after injection of the lowest dose and a modest effect size of increased blood pressure (+27%) 20-60 min after injection of the largest dose (Figure 3A). Heart rate decreased 20-50% with a nadir 10-20 min after intrathecal injection in a dose-independent manner (Figure 3B). Plasma concentrations of oxytocin increased from a baseline of approximately 100 pg/ml in a dose-dependent fashion with a peak 20-30 min after intrathecal injection (Figure 4). The animal receiving 5 day repeated bolus injections demonstrated agitation during injection on the 3rd, 4th, and 5th days and hindlimb stiffness in posture 10-60 min after injection after the 4th and 5th injections. Mean arterial blood pressure increased 10-30% and heart rate decreased 10-45% 1 h after the daily injections. Pre-injection plasma oxytocin was numerically greater before the last injection (300 pg/ml) than the first (180 pg/ml), with peak concentrations of approximately 3000 pg/ml 20 min after injection on both days.
Figure 3.
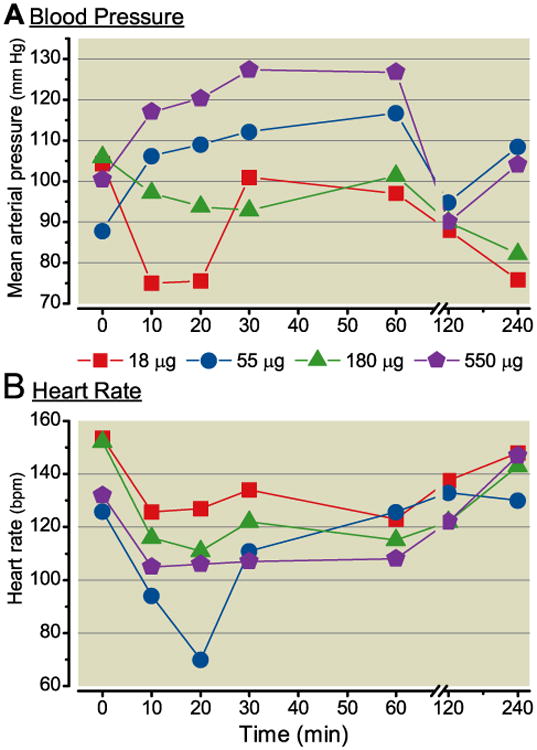
A) Mean arterial blood pressure and B) heart rate after intrathecal injection, at time 0, of oxytocin in 0.5 mL in 3 dogs. Data are median.
Figure 4.
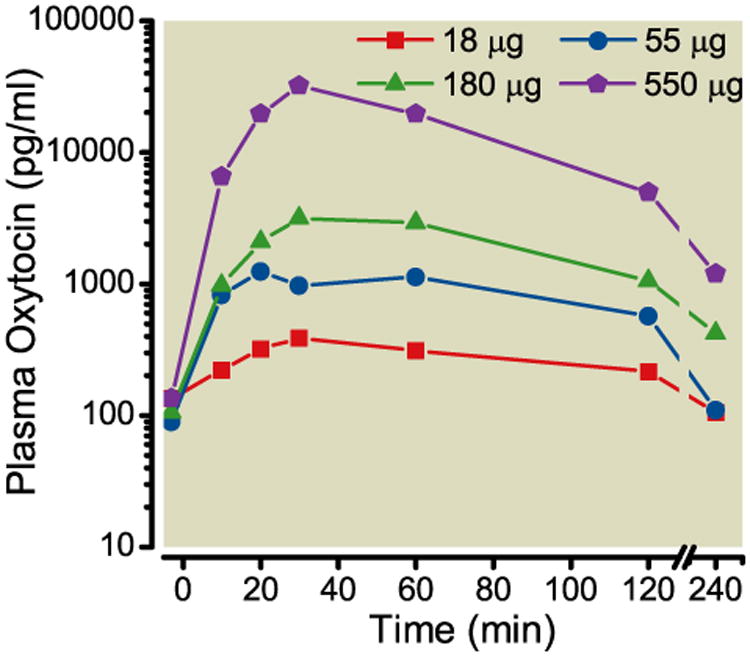
Plasma oxytocin concentrations after intrathecal injection, at time 0, of oxytocin in 3 dogs. Data are median.
Dog repeated bolus study
All animals survived to sacrifice. No untoward effects on general behavior, health, or body weight were noted during the course of study. Detailed neurological examinations after catheter placement and at sacrifice were unremarkable with the exception of two dogs, both in the intrathecal saline group, which exhibited a very mild hypermetria in the hind limbs after intrathecal catheter placement. The magnitude of the change was judged to be modest and acceptable to permit entry of the dogs in the study. Behavioral scores for arousal, muscle tone, and coordination were assessed over a range of 0 to ± 3 as previously described23 during the interval before and though 240 min after each of the 5 intrathecal injections. The sum of the behavioral scores for all of the vehicle dogs (N = 4) was 0, 0 and 0 and for the Oxytocin dogs (N= 6) was 0, 2, 0 for each of the three measures, respectively, indicating no treatment related changes after multiple intrathecal deliveries of oxytocin.
Body temperatures were within normal range of 37.2 - 39.0°C before and at 60 min after injection. Respiratory rates were within normal range of 18-34 breaths/minute before and at 60 min after injection without difference between groups. Groups did not differ in mean arterial pressure before or 1 hr after each injection (Figure 5A). In contrast, the groups differed with respect to heart rate (Figure 5B). In the saline group, there was no effect of day of injection or injection on heart rate. In contrast, in the oxytocin effect there was a significant effect of heart rate with injection (p=0.013) but not an effect of day or interaction between injection and day. This can be exemplified by collapsing the effect of injection on all days, with heart rate 111 ± 21 beats/min before and 111 ±24 beats/min after injection after saline treatment compared to 100 ± 27 beats/min before and 73 ± 33 beats/min after injection after oxytocin treatment.
Figure 5.
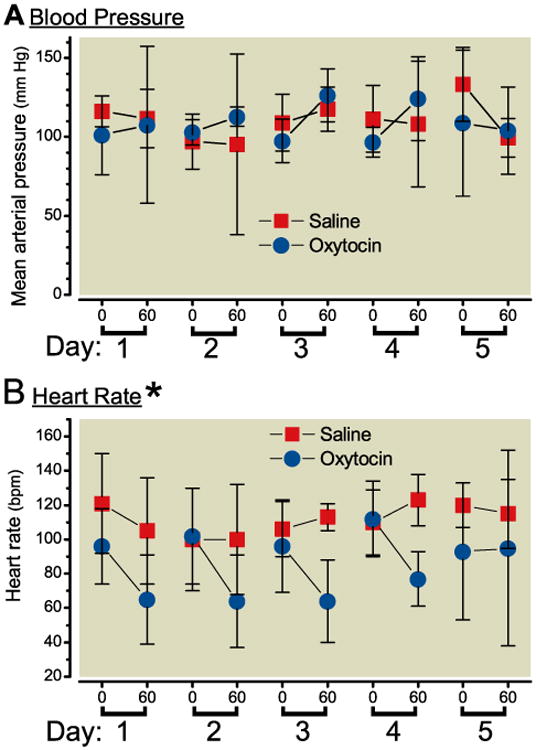
A) Mean arterial blood pressure (upper panel) and B) heart rate before and 60 min after intrathecal injection on 5 consecutive days of oxytocin, 550 μg in 0.5 mL in 6 dogs (blue circles) or saline in 4 dogs (red squares). Data are mean ± SD, *Groups differ by repeated measures ANOVA.
There were no clinically significant differences between treatment groups in pre and post treatment chemistries or hematology in CSF or blood, including sodium concentration (data not show). There was a large increase in CSF protein concentration, as previously observed in this intrathecal catheter preparation, from 19 ± 11 mg/dL before catheterization to 94 ± 47 mg/dL at necropsy in the oxytocin group and from 16 ± 1 to 101 ± 27 mg/dL respectively in the saline group. One animal from each group had white blood cell count increased in CSF at the end of treatment without local or systemic evidence of infection. Oxytocin concentrations in plasma 1 hr post injection on day 0 and day 4 were not different in animals receiving saline (1649 ± 1503 and 1188 ± 961 pg/mL respectively) or in those receiving oxytocin (19894 ± 6316 and 27260 ± 5046 pg/mL respectively).
At necropsy, organs and tissues were grossly normal, with the exception of small foci on the subcapsular and cut surface of both kidneys, consistent with minimal bilateral infarcts (oxytocin animal) and mild multifocal petechial in all lung lobes (saline animal).
Spinal histopathology revealed no systematic change in any treatment group, when the oxytocin treatment group was specifically compared with the saline treatment group (Table 1). Comparison of pathology scores across spinal block C (catheter tip, where drug concentrations are considered to be highest) revealed no statistical difference between oxytocin and vehicle treated animals. Comparisons of pathology scores were made across treatments for the A, B and D blocks and similarly, no difference were noted (Table 2). Further, comparisons between A, B, D vs C blocks revealed no trend for differences between those blocks which were distal to the catheter tip or proximal to the catheter tip, where drug concentration would be highest (Table 2). Representative images are presented in Figure 6.
Table 1. Summary of Spinal Cord Pathology in Dogs.
| Animal | Test / Control Article | Score C | Score A+B+C+D | A Cervical +DRG | B Thoracic +DRG | C Lumbar +DRG | D Lumbar +DRG |
|---|---|---|---|---|---|---|---|
| 543 2774 | Oxytocin | 0 | 0.75 | fibroblasts/macrophages | Un- remarkable | Un- remarkable | Un- remarkable |
| 502 8604 † | Oxytocin | 0.75 | 3.25 | compressed/sparse inflammation | fibroblasts/macrophages/sparse inflammation | minimal fibroblasts | minimal fibroblasts/macrophages |
| 542 9935 * | Oxytocin | 0.75 | 3.75 | Un- remarkable | fibroblasts/macrophages | lymphocytes/plasma cells/neutrophils/fibroblasts | fibroblasts/macrophages |
| 543 4297 * † | Saline | 0.75 | 1.5 | Un- remarkable | Un- remarkable | mild thickening/fibroblasts/macrophages | fibroblasts/macrophages/monocytes |
| 501 5219 * | Saline | 0.75 | 3.25 | thickening/fibroblasts/macrophage | fibroblasts/macrophages | focal thickening/fibroblasts | fibroblasts/macrophages |
| 501 5014 | Oxytocin | 1 | 2.75 | focal thickening/fibroblasts | Un- remarkable | focal thickening/fibroblasts/rare clusters macrophages/lymphocytes | multifocal fibroblasts/macrophages/ |
| 503 7565 * | Oxytocin | 1.25 | 5.25 | neutrophils/macrophage/lymphocytes | focal thickening/ | neutrophils/macrophages/lymphocytes/neutrophils | lymphocytes/plasma cells |
| 501 8501 | Saline | 2 | 6 | thickening/compressed/fibroblasts/macrophage | compressed/fibroblasts/macrophages | lymphocytes/plasma cells/macrophages | lymphocytes/plasma cells/macrophages |
| 543 0194 | Oxytocin | 2.25 | 10.25 | mild thickening/neutrophils/fibroblasts | mild compression/fibroblasts/macrophages | mild thickening/neutrophils/fibroblasts | mild thickening/neutrophils/fibroblasts |
| 502 9988 | Saline | 3 | 11 | thickening/compressed/fibroblasts/macrophage neutrophils/lymphocytes | compressed/fibroblasts/macrophages neutrophils/lymphocytes | macrophages neutrophils/lymphocytes/plasma cells/ | macrophages neutrophils/lymphocytes/plasma cells/ |
= representative hematoxylin and eosin
= representative glial fibrillary acidic protein
DRG – dorsal root ganglion
Table 2. Individual Histology Spinal Cord Scores in Dogs.
| Dog | Drug | A | B | C | D | Total |
|---|---|---|---|---|---|---|
| 543 2774, female | oxytocin | 0.75 | 0.00 | 0.00 | 0.00 | 0.75 |
| 502 8604, male | oxytocin | 1.00 | 1.25 | 0.75 | 0.75 | 3.75 |
| 542 9935, female | oxytocin | 0.75 | 1.25 | 0.75 | 1.00 | 3.75 |
| 503 7565, male | oxytocin | 1.00 | 2.00 | 1.25 | 1.00 | 5.25 |
| 501 8501, male | oxytocin | 0.75 | 1.00 | 2.00 | 2.25 | 6.00 |
| 543 0194, female | oxytocin | 3.00 | 2.00 | 2.25 | 3.00 | 10.25 |
| 543 4297, female | saline | 0.00 | 0.00 | 0.75 | 0.75 | 1.50 |
| 501 5219, male | saline | 0.75 | 0.75 | 0.75 | 1.00 | 3.25 |
| 501 5014, male | saline | 0.75 | 0.00 | 1.00 | 1.00 | 2.75 |
| 502 9988, male | saline | 3.00 | 3.00 | 3.00 | 2.00 | 11.00 |
| P value (oxytocin vs saline) | 0.2381 | 0.2381 | 0.4571 | 0.4571 | 0.3048 | |
| (Mann-Whitney one tailed) | U = 8.5 | U=8.0 | U = 11.0 | U = 11.5 | U = 9.5 |
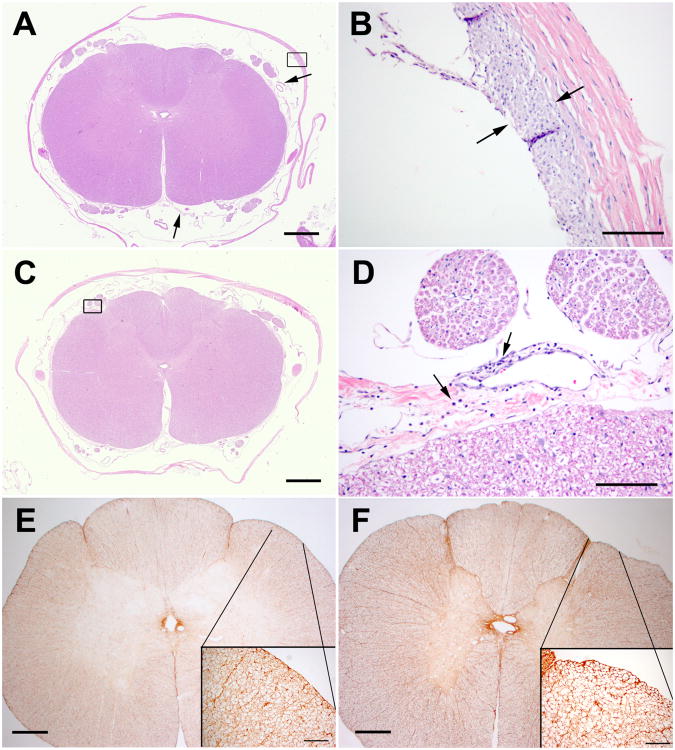
Figure 6.
A and B. Representative hematoxylin and eosin stained section of D block of dogs receiving 5 repeated intrathecal injections of saline. A). The dura is focally, mildly thickened adjacent to the catheter site (box, shown in higher magnification in B). The arachnoid has no inflammation, but has mild, multifocal fibrosis (arrows). There is no inflammation in spinal cord or nerve roots. B). The region of dural thickening has fibroblast proliferation and macrophage infiltration on the inner surface of the dura (between arrows). C and D. Representative hematoxylin and eosin stained section of D block of dogs receiving 5 repeated intrathecal injections of oxytocin (550 μg/0.5 mL). The arachnoid has a diffuse, but sparse infiltrate of lymphocytes and plasma cells, predominantly perivascular (boxed area shown at higher magnification in D). There is no inflammation in spinal nerve roots or spinal cord. D). Higher magnification of boxed area in C. There is sparse chronic inflammation, predominantly perivascular (arrows). E and F. Glial fibrillary acidic protein stains. Insets represent higher magnifications of indicated areas. E). Saline treated dog. F). Oxytocin treated dog. There is delicate astrocytic staining. No reactive astrocytes were noted after 5 daily intrathecal injections of either injectate. Magnification bars: A,C = 1 mm; B,D = 100μ; E,F = 600μ, insets = 100μ.
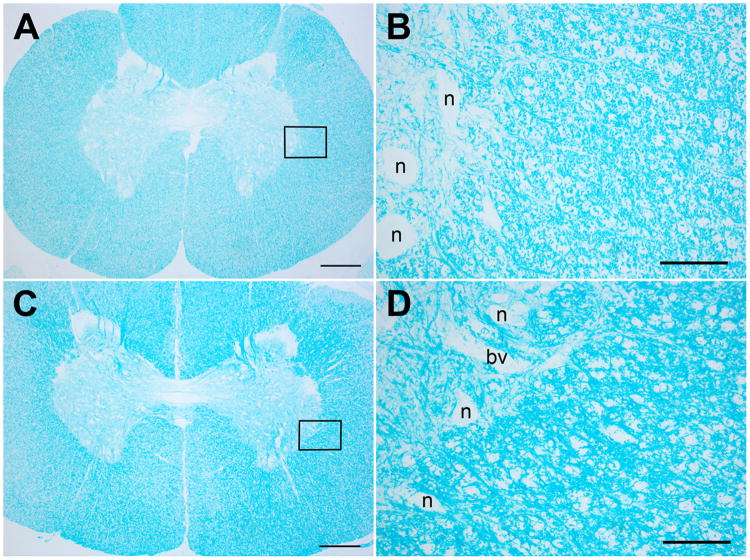
Most animals showed focal, minimal thickening of the dura with a mild fibroblast proliferation and macrophage infiltration, consistent with a reaction to the catheter. Most had a mild inflammatory infiltrate in dura (focal) and/or arachnoid (focal or diffuse). The inflammatory infiltrates were variable mixtures of macrophages, lymphocytes, plasma cells and neutrophils (see Table 1). Three animals had leptomeningeal inflammation in the section of medulla (two oxytocin, one saline); one of these also had leptomeningeal inflammation in the section of neocortex (saline). There was no inflammation in the spinal cord parenchyma. Several animals had focal, mild compression of the outer surface of the spinal cord in the region of the catheter, but none of these showed any evidence of parenchymal damage to the spinal cord by hematoxylin and eosin, GFAP or myelin stains. Two animals had minimal gliosis in the spinal cord gray matter, seen only with GFAP stains. This did not correlate with the presence or degree of spinal cord compression, meningeal inflammation, or other changes. Sections stained with Luxol fast blue showed no evidence of demyelination (Figure 7). Sections of peripheral nerve, dorsal root ganglia, retina and skeletal muscle were unremarkable.
Figure 7.
Representative Luxol fast blue stained sections of C blocks. A and B, Saline injected animal; C and D, oxytocin injected animal. The boxes in A and C indicate the regions shown at higher magnification in B and D. There is no evidence of demyelination. The larger unstained areas in B and D are neurons (n) and blood vessels (bv). Magnification bars: A, C = 600μ; B,D = 100μ.
Discussion
Pre-clinical assessment for clinical introduction of an agent for intrathecal administration includes effects at the site of injection and those distant from injection, whether due to systemic absorption or cephalad transport in CSF. The current study showed no evidence of drug related tissue toxicity, as measured by neurologic assessment, gross necropsy, histopathology examination, and CSF chemistry and hematology, in two species: the rat and the dog. In the rat both acute (2 day) and chronic (14 day) histopathology was screened after a single intrathecal injection. In the dog multiple intrathecal injections at 24 h intervals revealed no cumulative behavioral toxicity or histopathologic toxicity, including measures of focal astrocyte activation. Hemodynamic changes were consistent with effects on oxytocin and vasopressin receptors, which may be dose limiting in clinical application. The robustness and adequacy of this preclinical evaluation and its implications for clinical introduction deserve comment.
Intrathecal dosing and drug- tissue exposure
As the intrathecal space represents a relatively static fluid environment,28 the occurrence of local adverse events is considered to reflect primarily the local concentrations to which the adjacent tissue (spinal cord and meninges) are exposed and the duration of that exposure.29,30 With regard to concentration, clinicians typically measure intrathecal injections in terms of dose, but for local toxicity, both drug concentration and duration of exposure are considered to be of particular importance.30,31 The therapeutic intrathecal oxytocin dose in dogs is unknown, but in rats, it is approximately 0.1-1.0 μg,32 administered in a volume of 10 μL, or a concentration of 10-100 μg/μL. The highest concentration utilized in the present evaluation was 1100 μg/mL (∼ 600 IU/mL) was chosen in part because of mild behavioral and hemodynamic effects in the dog dose ranging study.
A further consideration regarding the local tissue drug exposure after bolus delivery reflects the local dilution of the intrathecal injectate and this dilution factor is proportional to the local volume in to which the drug is delivered. We have previous noted that the local volume at the cord level is conservatively proportional to the circumference of the cord which in humans and dogs represents a ratio of 3 to 1. Thus, for a given dose and volume, the human displays a dilution which is approximately three times that observed after lumbar intrathecal delivery the dog (see19). In the present case, we suggest that based on local dilution in the two spinal spaces, equivalent estimated concentrations would require 3300 μg/mL in the human to approach the same concentrations that were well tolerated in the dog. Finally we mimicked the planned clinical exposure of a bolus intrathecal injection (rather than by an infusion delivery), and to increase the robustness of the neurotoxicity exposure by repeated daily bolus dosing in the dog.
Projected safety of intrathecal oxytocin in humans
In humans, we have estimated that the projected maximum intrathecal dose should not exceed 150 μg/3 mL (50 μg/μL), given concerns of systemic side effects. Given these three parameters defining the robustness of the exposure, we would note that the projected human bolus dose would thus be 1/22 to 1/66 of the dose readily tolerated in dogs with and without consideration respectively of a dilution factor. Further, in contrast to the projected single dose exposure for human, the present work shows that even five repeated injections of this dose / concentration were without deleterious reactions when assessed by function and systematic spinal histopathology, even at the site of drug delivery where concentrations and exposure would be maximum.
Importantly, the current study provides evidence for safety at these concentrations and dosing regimens only, and other studies would be necessary to support sustained treatment or intrathecal infusion. Additionally, whether perineural or epidural administration of oxytocin is safe was not assessed by these studies.
Spinal inflammatory responses to the catheter
We note parenthetically that lack of inflammation in spinal cord histopathology in the current rodent study is in contrast to our previous work with toxicity assessment of neostigmine, adenosine, and ketorolac,26,33,34 in which inflammation and white matter vacuolation was present in drug- and vehicle treated animals. This was likely due to the presence of the spinal catheter in these other studies, as there was a rim of inflammatory cells surrounding the catheter track and inflammation of tissue adjacent to the track. We did not insert an intrathecal catheter in the current rodent studies of oxytocin, and did not observe such histopathologic changes. In the dog studies, we did observe minor changes after catheterization, consistent with previous reports.18,33,34 The transient scratching and grooming behavior observed in rats parallels that observed in mice and is consistent with oxytocin action on vasopressin 1a receptors in the spinal cord of rodents.27 No such behavior was observed in the dog toxicity study, but whether intrathecal oxytocin would induce itching in humans is unknown.
Physiological effects of intrathecal oxytocin
Changes in blood pressure and heart rate were suggested in the small dose ranging study and in heart rate were confirmed in the repeated bolus study in dogs. The time of peak effect in these changes coincided with the time of peak oxytocin concentration in plasma, suggestive of a systemic effect. Bolus intravenous doses of 2-10 IU (3.6-18 μg) oxytocin cause transient hypotension in women at cesarean delivery, and it is conceivable that the numerical reduction in blood pressure after intrathecal injection of 18 μg oxytocin in the dose ranging study reflected a similar action. In contrast, the peak plasma concentrations of 20-30 μM far exceed the affinity of oxytocin at vasopressin V1a receptors,19 which produce vasoconstriction and, with sustained stimulation, hyponatremia. Severe hypertension or hyponatremia were not observed in any animal at any dose, and the study was not adequately powered to observe smaller changes which might be clinically relevant in some patients, so blood pressure and serum sodium should be carefully monitored in clinical investigations of intrathecal oxytocin. The reduction in heart rate with intrathecal oxytocin dosing was more clearly evident in these dogs, which could reflect enhanced baroreflex actions of oxytocin on vasopressin35 or oxytocin.36 As with blood pressure and serum sodium, heart rate should be closely monitored in the clinical introduction of intrathecal oxytocin.
Limitations of preclinical toxicity assessment
As reviewed above, the present study represent a robust assessment of potential toxicity defining the lack of effect of doses 22-66 times those to be employed in humans and that of multiple versus a single administration. Nevertheless, it is important to recognize the limitations of preclinical toxicity screening such as in the current study. For practical and ethical reasons, studies are performed in a small number of animals, and even relatively common (30% incidence) catastrophic events cannot be observed with statistical certainty. Although this is counterbalanced to some degree by the large increase in dose and concentration beyond the planned clinical ones, it is still conceivable that local neurotoxicity could occur. There is also a large species difference in normal circulating oxytocin concentrations, with humans showing levels 1-5% of those in these dog studies,37 and it is conceivable that more pronounced systemic effects may occur clinically due to increased sensitivity in humans. Neither rat nor dog studies predicted severe nausea following intrathecal neostigmine in humans,26,38 although in this case one could anticipate an emetogenic effect by cholinergic stimulation of the area postrema following cephalad spread. Oxytocin, in contrast, is not associated with nausea in the absence of its use for post-partum hemorrhage where other factors may be involved. Finally, laboratory and case reports suggest that oxytocin can prolong QT interval14,39 and this was not assessed in the current study. Whether intrathecal oxytocin alters QT interval deserves study.
Summary
Robust studies reported here in two species (rats and dog) do not show signs of neurotoxicity from intrathecal bolus dosing of oxytocin in concentrations up to 1100 μg/mL and up to total doses of 11 μg in the rat and 550 μg in the dog. There is a suggestion that the highest dose of intrathecal oxytocin in dogs might decrease heart rate, but does not alter serum sodium concentration. These results resulted in Investigation New Drug approval from the Food and Drug Administration to begin clinical investigation of intrathecal oxytocin as a novel analgesic, and they guide the focus of safety assessments in those initial trials.
Acknowledgments
Supported in part by grant GM048085 from the National Institute of Health, Bethesda, MD.
We would like to acknowledge the expert assistance of Chuanyao Tong, M.D. at Wake Forest University School of Medicine, Winston-Salem, NC and Nicolle Tozier and Mary Ceccolini, B.S. at University of California, San Diego, CA.
Footnotes
The authors declare no competing interests.
References
- 1.Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: The great facilitator of life. Prog Neurobiol. 2009;88:127–51. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang YS, Park JH. Brain uptake and the analgesic effect of oxytocin--its usefulness as an analgesic agent. Arch Pharm Res. 2000;23:391–5. doi: 10.1007/BF02975453. [DOI] [PubMed] [Google Scholar]
- 3.Eisenach JC, Pan P, Smiley RM, Lavand'homme P, Landau R, Houle TT. Resolution of Pain after Childbirth. Anesthesiology. 2013;118:143–51. doi: 10.1097/ALN.0b013e318278ccfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez S, Liu B, Hayashida K, Houle TT, Eisenach JC. Reversal of Peripheral Nerve Injury-induced Hypersensitivity in the Postpartum Period: Role of Spinal Oxytocin. Anesthesiology. 2013;118:152–9. doi: 10.1097/ALN.0b013e318278cd21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 6.Condes-Lara M, Martinez-Lorenzana G, Rojas-Piloni G, Rodriguez-Jimenez J. Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Res. 2007;1160:20–9. doi: 10.1016/j.brainres.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Takeda S, Kuwabara Y, Mizuno M. Effects of pregnancy and labor on oxytocin concentrations in human plasma and cerebrospinal fluid. Endocrinol Japon. 1985;32:875–80. doi: 10.1507/endocrj1954.32.875. [DOI] [PubMed] [Google Scholar]
- 8.Condes-Lara M, Maie IA, Dickenson AH. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Res. 2005;1045:124–33. doi: 10.1016/j.brainres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Lorenzana G, Espinosa-Lopez L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, Condes-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140:265–73. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Miranda-Cardenas Y, Rojas-Piloni G, Martínez-Lorenzana G, Rodríguez-Jiménez J, López-Hidalgo M, Freund-Mercier MJ, Condés-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–9. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 11.DeLaTorre S, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Villanueva L, Condes-Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long-term potentiation in dorsal horn nociceptive neurons: Electrophysiological and behavioral evidence. Pain. 2009;144:320–8. doi: 10.1016/j.pain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in laminas I-II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breton JD, Poisbeau P, Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther. 2004;76:359–64. doi: 10.1016/j.clpt.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in Blood Pressure and Cardiac Output during Cesarean Delivery: The Effects of Oxytocin and Carbetocin Compared with Placebo. Anesthesiology. 2013;119:541–51. doi: 10.1097/ALN.0b013e31829416dd. [DOI] [PubMed] [Google Scholar]
- 16.Caudle RM, Isaac L. Intrathecal dynorphin(1-13) results in an irreversible loss of the tail-flick reflex in rats. Brain Res. 1987;435:1–6. doi: 10.1016/0006-8993(87)91579-4. [DOI] [PubMed] [Google Scholar]
- 17.Gaumann DM, Yaksh TL. Intrathecal somatostatin in rats: Antinociception only in the presence of toxic effects. Anesthesiology. 1988;68:733–42. [PubMed] [Google Scholar]
- 18.Yaksh TL, Tozier N, Horais KA, Malkmus S, Rathbun M, Lafranco L, Eisenach JC. Toxicology profile of N-methyl-D-aspartate antagonists delivered by intrathecal infusion in the canine model. Anesthesiology. 2008;108:938–49. doi: 10.1097/ALN.0b013e31816c902a. [DOI] [PubMed] [Google Scholar]
- 19.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–28. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaksh TL, Collins JG. Studies in animals should precede human use of spinally administered drugs. Anesthesiology. 1989;70:4–6. [PubMed] [Google Scholar]
- 21.Yaksh TL, Noueihed RY, Durant PA. Studies of the pharmacology and pathology of intrathecally administered 4-anilinopiperidine analogues and morphine in the rat and cat. Anesthesiology. 1986;64:54–66. doi: 10.1097/00000542-198601000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sabbe MB, Grafe MR, Pfeifer BL, Mirzai THM, Yaksh TL. Toxicology of baclofen continuously infused into the spinal intrathecal space of the dog. NT. 1993;14:397–410. [PubMed] [Google Scholar]
- 23.Sabbe MB, Grafe MR, Mjanger E, Tiseo PJ, Hill HF, Yaksh TL. Spinal delivery of sufentanil, alfentanil, and morphine in dogs: Physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920. doi: 10.1097/00000542-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Yaksh TL, Rathbun M, Jage J, Mirzai T, Grafe M, Hiles RA. Pharmacology and toxicology of chronically infused epidural clonidine·HCl in dogs. Fundam Appl Toxicol. 1994;23:319–35. doi: 10.1006/faat.1994.1112. [DOI] [PubMed] [Google Scholar]
- 25.Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: A novel method for the experimental study of opioid tolerance. Anesth Analg. 2006;103:714–20. doi: 10.1213/01.ane.0000226100.46866.ea. [DOI] [PubMed] [Google Scholar]
- 26.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–27. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higuchi H, Hirata J, Adachi Y, Kazama T. Influence of lumbosacral cerebrospinal fluid density, velocity, and volume on extent and duration of plain bupivacaine spinal anesthesia. Anesthesiology. 2004;100:106–14. doi: 10.1097/00000542-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Walker SM, Yaksh TL. Neuraxial analgesia in neonates and infants: A review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–62. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaksh TL. In: Spinal delivery and assessment of drug safety, Fundamental neuropathology for pathologists and toxicologists. Bolon BB, editor. Hoboken, NJ: John Wiley and Sons; 2011. pp. 451–62. [Google Scholar]
- 31.Eisenach JC, Yaksh TL. Safety in numbers: How do we study toxicity of spinal analgesics? Anesthesiology. 2002;97:1047–9. doi: 10.1097/00000542-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Yu SQ, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983:13–22. doi: 10.1016/s0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]
- 33.Chiari A, Yaksh TL, Myers RR, Provencher J, Moore L, Lee CS, Eisenach JC. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology. 1999;91:824–32. doi: 10.1097/00000542-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 34.Yaksh TL, Horais KA, Tozier N, Rathbun M, Meschter C, Richter P, Rossi S, Tong C, Cline MJ, Eisenach JC. Intrathecal ketorolac in dogs and rats. Toxicol Sci. 2004;80:322–34. doi: 10.1093/toxsci/kfh168. [DOI] [PubMed] [Google Scholar]
- 35.Liard JF. Vasopressin in cardiovascular control: Role of circulating vasopressin. Clin Sci (Lond) 1984;67:473–81. doi: 10.1042/cs0670473. [DOI] [PubMed] [Google Scholar]
- 36.Huang W, Sjoquist M, Skott O, Stricker EM, Sved AF. Oxytocin antagonist disrupts hypotension-evoked renin secretion and other responses in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R760–R765. doi: 10.1152/ajpregu.2001.280.3.R760. [DOI] [PubMed] [Google Scholar]
- 37.Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grunder G, Spreckelmeyer KN. Oxytocin plasma concentrations after single intranasal oxytocin administration - a study in healthy men. Neuropeptides. 2012;46:211–5. doi: 10.1016/j.npep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Uzun M, Yapar K, Uzlu E, Citil M, Erdogan HM. QT interval prolongation and decreased heart rates after intravenous bolus oxytocin injection in male and female conscious rabbits. Gen Physiol Biophys. 2007;26:168–72. [PubMed] [Google Scholar]