Abstract
Commercial preparations containing synthetic cannabinoids (SCBs) are rapidly emerging as drugs of abuse. Although often assumed to be “safe” and “legal” alternatives to cannabis, reports indicate that SCBs induce toxicity not often associated with the primary psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC). This chapter will summarize the evidence that use of SCBs poses greater health risks relative to marijuana and suggest that distinct pharmacological properties and metabolism of SCBs relative to Δ9-THC may contribute to this increased toxicity. Studies reviewed will indicate that in contrast to partial agonist properties of Δ9-THC typically observed in vitro, SCBs act as full CB1 and CB2 receptor agonists both in cellular assays and animal studies. Furthermore, unlike Δ9-THC metabolism, several SCB metabolites retain high affinity for and exhibit a range of intrinsic activities at CB1 and CB2 receptors. Finally, the potential for SCBs to cause adverse drug–drug interactions with other drugs of abuse, as well as with common therapeutic agents, will be discussed. Collectively, the evidence provided in this chapter indicates that SCBs should not be considered safe and legal alternatives to marijuana. Instead, the enhanced toxicity of SCBs relative to marijuana, perhaps resulting from the combined actions of a complex mixture of different SCBs present and their active metabolites that retain high affinity for CB1 and CB2 receptors, highlights the inherent danger that may accompany use of these substances.
1 Introduction
Synthetic cannabinoids (SCBs) have become popular recreational drugs among young adults in the USA. Use of these substances first emerged in Europe, where they were marketed as Spice, then quickly spread throughout the USA, where they were marketed as K2 [1]. SCBs are sometimes marketed for commercial distribution in the form of capsules, tablets, and powders but are most commonly laced onto herbal mixtures (e.g., potpourri or incense) to be smoked (as reviewed by Tai and Fantegrossi [2]). Commercial SCB products are widely available on the internet and, despite regulatory efforts to curtail their availability, remain accessible at “brick and mortar” establishments such as head shops and convenience stores. Their widespread distribution can be attributed to clever marketing tactics, which usually involve colorful packaging and mislabeling designed to portray a harmless herbal blend which is “not intended for human consumption.” These deceptive marketing tactics were adopted to circumvent legal ramifications for selling drugs of abuse, but it is unlikely that criminal proceedings stemming from the sale of prohibited substances would be impeded by arguments about product labels. Importantly, the marketing of these products appears to be specifically designed to give users the false assumption that these drugs are harmless, “natural,” legal alternatives to cannabis.
Examining the pharmacological similarities between SCBs and Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive constituent in marijuana, has been a topic of great interest among scientists and lawmakers. In this regard, SCBs have been reported to exhibit higher binding affinity at both CB1 and CB2 cannabinoid receptor (CBR) subtypes, and also to display varying intrinsic activity relative to Δ9-THC, both in cellular assays and animal studies [3–7]. Unlike Δ9-THC, which consistently exhibits partial agonist efficacy in vitro [4, 5], SCBs are fully efficacious at both CBRs across a range of in vitro and in vivo assays [4, 5, 8]. In addition, metabolites of SCBs often retain higher CBR affinity than Δ9-THC and may elicit pharmacological and toxicological effects distinct from those induced by Δ9-THC. These active metabolites could potentially explain the increased morbidity and mortality associated with SCB exposure, as compared to what is typically seen with marijuana. The remainder of this chapter will discuss the pharmacological and toxicological effects related to the metabolism of SCBs with a particular emphasis on their active metabolites and show that these substances are not safe, have a greater toxicological profile than has been reported with marijuana, and should not be considered a legal alternative to cannabis.
2 Synthetic Cannabinoid Metabolism
Metabolism of xenobiotics occurs through several biotransformation pathways which are conserved across species and quite old from an evolutionary perspective. The goal of drug metabolism is to detoxify potentially harmful compounds, removing them from the circulation and ultimately excreting them from the body altogether. The liver plays a major role in this detoxification process. In some cases, metabolism may activate inert compounds (the concept of a “pro-drug”) or produce metabolic intermediates which may themselves induce toxicity. In most cases, oxidative metabolism of xenobiotics first occurs via the hepatic cytochrome P450 (CYP) enzyme system at which point the metabolites are conjugated with a sugar moiety, glucuronic acid, by a class of enzymes called UDP-glucuronosyltransferases (UGTs). The resulting metabolites are then soluble enough to be removed from the body.
The metabolism of Δ9-THC has been a reference standard for understanding cannabinoid pharmacokinetics [9]. Metabolism of Δ9-THC by human hepatic micro-somes initially occurs via oxidation by CYP subtypes 2C9 and 3A4 [10]. In brief, hydroxylation of Δ9-THC by CPY2C9 produces 11-hydroxy-Δ9-THC, which is the only psychoactive metabolite of Δ9-THC [11, 12]. Further oxidation of the remaining hydroxyl groups of Δ9-THC produces carboxylic acids at several positions along the alkyl side chain, and these metabolites are devoid of biological effects. Further oxidation of the active metabolite 11-hydroxy-Δ9-THC abolishes pharmacological activity and leads to the production of 11-nor-9-carboxy-Δ9-THC, which is then conjugated at the carboxyl position to form O-ester glucuronide, the major metabolite excreted in human urine [13].
In the early 2000s, initial reports of SCB metabolism emerged with a focus on in vitro metabolism of CBR ligands (11R)-2-methyl-11-[(morpholin-4-yl)methyl]-3-(naphthalene-1-carbonyl)-9-oxa-1-azatricyclo[6.3.1.04,12]dodeca-2,4(12),5,7-tetraene (WIN-55,212-2) [14], 1-[2-(morpholin-4-yl)ethyl]-2-methyl-3-(4-methoxybenzoyl)-6-iodoindole (AM-630) [15], and (2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenyl-methanone (JWH-015) [16]. The subsequent recreational use of SCBs shifted the focus more towards in vivo metabolism of the commonly abused CBR ligand naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) and its analogs. Metabolites of JWH-018 were first identified in urine specimens obtained from three people who had smoked a commercial product containing this compound [17]. Predominant phase I metabolites are formed by oxidation of the indole ring or the N-alkyl chain to form mono-hydroxylated compounds. The phase I JWH-018 metabolites are excreted in urine almost exclusively in the form of phase II glucuronide conjugates, as determined by gas- and liquid-chromatography mass spectrometry (GC-MS and LC-MS/MS). Figure 1 depicts the metabolism of JWH-018 to its 4-hydroxyindole metabolite and corresponding glucuronide conjugate. Subsequent investigations demonstrated the formation of phase I mono-hydroxylated metabolites and phase II glucuronides in human hepatic microsomes incubated with JWH-018 [19], urine specimens obtained from individual users of Spice/K2 products, and from rats administered JWH-018 [20, 21], naphthalen-1-yl-(1-butylindol-3-yl)methanone (JWH-073) [22], or 2-(2-methoxyphenyl)-1-(1-pentylindol-3-yl)ethanone (JWH-250) [20]. Collectively, these studies confirmed that the primary urinary metabolites of these SCBs are excreted in the form of a single hydroxylation and subsequent glucuronidation.
Fig. 1.
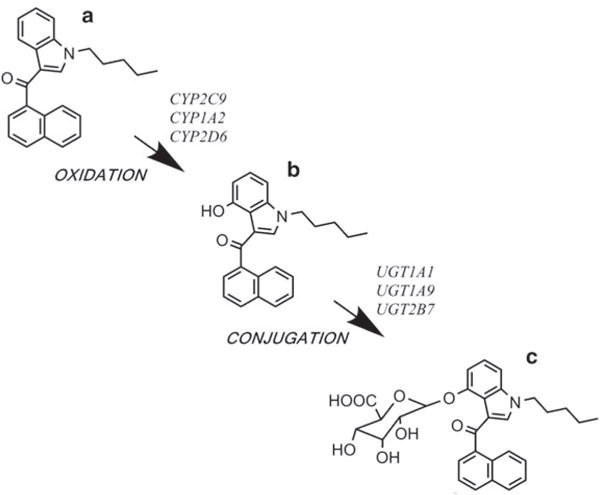
Metabolism of the synthetic cannabinoid (SCB) JWH-018. The parent compound JWH-018 (a) undergoes phase I oxidation by cytochrome P450 (CYP) enzymes to form the bioactive JWH-018 4-hydroxyindole metabolite (b) [5]. Phase II conjugation by UDP-glucuronosyltransferases (UGTs) forms the corresponding glucuronide conjugate (c) [18]. Specific CYP enzymes and UGTs responsible for these biotransformation reactions are noted
It was not until 2011 that reference standards for identifying specific JWH-018 and JWH-073 metabolites were developed allowing for both in vivo and in vitro quantitative measurements by LC-MS [18, 23, 24]. Human urine specimens were obtained from individuals who ingested JWH-018 or a mixture of JWH-018 and JWH-073. Analysis determined that the metabolites found in the urine were excreted in high concentrations and primarily in the form of glucuronic acid conjugates [18]. Several additional laboratories have replicated with these findings [20, 25–30].
It is well accepted that CYPs are involved in the biotransformation of SCBs in humans as recognized by the formation of hydroxylated metabolites [14, 17–19, 24, 29]. In vitro metabolism studies have determined that SCBs JWH-018 and its fluorinated analogue 1-[(5-fluoropentyl)-1H-indol-3-yl]-(naphthalen-1-yl)methanone (AM-2201) are metabolized via oxidation by hepatic CYP subtypes 2C9 and 1A2 [23]. Outside the liver, CYPs are ubiquitously expressed in the body in a tissue-specific manner. CYP2C9 is also highly expressed in the intestine [31], so it is likely that intestinal CYP2C9 is involved in the metabolism of SCBs when ingested orally. CYP1A2 is highly expressed in the lung and is likely responsible for the metabolism of smoked SCBs [32]. There is evidence of the involvement of CYP2D6 in the metabolism of JWH-018 and AM-2201 in the brain especially in brain regions that have a high expression of CB1Rs, including the cortex, hippocampus, and cerebellum. It is likely that CYP2D6 is involved in the management of brain concentrations of these SCBs and their active metabolites, more so than in the liver.
Urine specimens obtained from individuals who ingested SCBs contain high concentrations of glucuronide metabolites [17–19, 24], coupled with no traces in serum [33, 34], suggesting that glucuronic acid conjugation plays a key role in the excretion of these drugs in urine. In the liver, JWH-018 and JWH-073 metabolites are formed by major UGT isoforms UGT1A1, UGT1A9, and UGT2B7 [18] (see Fig. 1). In addition, extrahepatic UGT isoforms involved in metabolism include UGT1A7 expressed in lung, UGT1A3 and UGT2B7 expressed in brain (as well as liver), and UGT1A10. Importantly, human UGT1A3 and UGT2B7 are predominant isoforms responsible for generating the major metabolites of JWH-018 and JWH-073 found in urine [35]. Since UGT1A3 and UGT2B7 are also expressed in the brain, these UGT isoforms may play a role in regulating brain concentrations of SCBs and their active metabolites at the CB1R, similar to CYP2D6 (as previously discussed).
3 Synthetic Cannabinoid Cellular Signaling
The pharmacological profiling of Δ9-THC has led to the characterization of metabolites produced by SCBs JWH-018 and JWH-073 [36]. Like the phytocannabinoid Δ9-THC, both JWH-018 and JWH-073 have high affinity for the CB1 and CB2Rs [3–7]. Chemical structures for JWH-073 many of the related SBCs discussed in this chapter are shown in Fig. 2. Emerging SCBs found in commercial preparations also have high affinity for the CB1 and CB2Rs, including 2-[(1S,3R)-3-hydroxycyclo-hexyl]-5-(2-methyloctan-2-yl)phenol (CP-47,497) [37], (1-pentylindol-3-yl)naphtha-len-1-ylmethane (JWH-175) [38], 1-([(1E)-3-pentylinden-1-ylidine]methyl)naphthalene (JWH-176) [38], (1-pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)metha-none (UR-144) [39], naphthalen-1-yl-(1-pentylpyrrol-3-yl)methanone (JWH-030) [40], 2-(2-methoxyphenyl)-1-(1-pentylindol-3-yl)ethanone (JWH-250) [41], N-(1-adamantyl)-1-pentylindazole-3-carboxamide (APINACA, or AKB48) [42], and 1-[(N-methylpiperidin-2-yl)methyl]-3-(adamant-1-oyl)indole (AM-1248) [43]. Furthermore, most commercial SCB products display high potency and efficacy as CB1R agonists both in vitro and in vivo, including JWH-018 [5, 8], JWH-073 [4, 44], AM-1248 [39], CP-55,940 [40], WIN-55,512-2 [40], and (6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6H,6aH,7H,10H,10aH-benzo[c]isochro-men-1-ol (HU-210) [45].
Fig. 2.
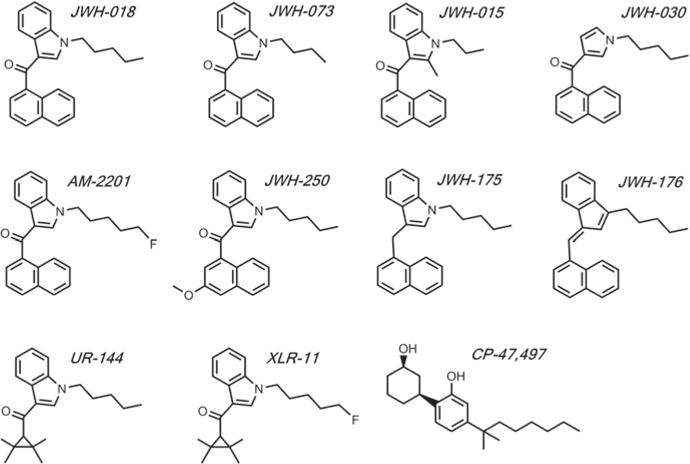
Chemical structures of SCBs discussed in this chapter. Abbreviations are as follows: JWH-018 naphthalen-1-yl-(1-pentylindol-3-yl)methanone, JWH-073 naphthalen-1-yl-(1-butylindol-3-yl)methanone, JWH-015 (2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone, JWH-030 naphthalen-1-yl-(1-pentylpyrrol-3-yl)methanone, AM-2201 1-[(5-fluoropentyl)-1H-indol-3-yl]-(naphthalen-1-yl)methanone, JWH-250 2-(2-methoxyphenyl)-1-(1-pentylindol-3-yl)ethanone, JWH-175 (1-pentylindol-3-yl)naphthalen-1-ylmethane, JWH-176 1-([(1E)-3-pentylinden-1-ylidine]methyl)naphthalene, UR-144 (1-pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone, XLR-11 (1-(5-fluoropentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone, CP-47,497 (2-[(1S,3R)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol
The abuse liability of SCB products may be attributed to the presence of high affinity and fully efficacious CB1 agonists in these commercial products [46]. For instance, the in vitro efficacy of Δ9-THC is partial relative to JWH-018 and JWH-073, which are fully efficacious [4, 8, 46]. Importantly, not all in vitro or ex vivo assessments can identify differences in efficacy between THC and SCBs. An interesting study by Hoffman et al. [47] demonstrated similar efficacy for SCBs and THC in an electrophysiological assay reflecting inhibition of transmitter release. Nevertheless, the often demonstrated low in vitro efficacy of Δ9-THC does not necessarily translate to partial agonism in vivo, and Δ9-THC often displays in vivo efficacy comparable to fully efficacious agonists [48]. In addition, abrupt discontinuation of chronic marijuana use or Δ9-THC administration produces a withdrawal syndrome in humans and rodents that is accompanied by a region-specific downregulation and desensitization of CB1Rs in the brain [49–51]. Thus, it is possible that commercial SCB products that contain high-efficacy agonists may intensify the adverse effects related to tolerance, dependence, and withdrawal of SCB abuse relative to Δ9-THC. For instance, the commercial SCB product “Spice Gold” produced a withdrawal phenomenon and dependence syndrome in humans that transpired after abrupt discontinuation of use in the form of drug craving, elevated blood pressure, nausea, tremor, profuse sweating, and nightmares [52]. The active constituents of this product were not forensically determined in the product itself or in fluids or tissue from the case subject, but contemporaneous laboratory studies determined that a mixture of the SCBs JWH-018 and CP-47,497 was present in this commercial smoking blend at the time and in the geographic area where the aforementioned case occurred [52].
Metabolism of Δ9-THC produces a single active metabolite (11-hydroxy-Δ9-THC) that exhibits reduced CB1R affinity compared with the parent compound [53]. On the other hand, metabolism of commercial SCBs including JWH-018, JWH-073, and AM-2201 produces numerous major mono-hydroxylated metabolites that retain nanomolar binding affinity for CB1Rs [4, 5, 23] (see Fig. 1), unlike their carboxylated metabolites, which do not bind to nor activate CB1Rs.
In addition to retaining high CB1R affinity, in vitro functional assays (G-protein activation) demonstrate that the major mono-hydroxylated metabolites of JWH-018, JWH-073, and AM-2201 exhibit partial to full efficacy at the CB1Rs similar to fully efficacious CP-55,940 [4, 5, 23]. Of further importance, the in vivo cannabimimetic effects of JWH-018 and JWH-073 mono-hydroxylated metabolites elicited profound hypothermic and locomotor depressant effects in mice [4]. These effects are attenuated by CB1R antagonist/inverse agonist 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide (AM-251), suggesting that these metabolites are mediating their effects through the CB1R, similar to the parent ligands. Moreover, the effects elicited by these metabolites may be associated with adverse effects of SCB use. For instance, it has been shown that several mono-hydroxylated metabolites of JWH-018, JWH-073, and AM-2201 retain high CB1R affinity and activity and may display additive or synergistic interactions with other SCBs [54]. Additional contributing factors to adverse effects of SCB use can be: (1) drug effects of non-cannabinoid-like ligands found in commercial SCB products, (2) variations in batch-to-batch preparations with differences in the concentration and content found in commercial SCB products, and (3) an exacerbation of SCB adverse effects in drug users with preexisting conditions [36, 55–58].
Although evidence suggests that mono-hydroxylated metabolites of JWH-018, JWH-073, and AM-2201 are active at CB1Rs in both in vitro and in vivo assays [54], it is also possible for some oxidized metabolites of SCBs to function as antagonists at CB1Rs. Despite the fact that the 7-hydroxyindole derivative of JWH-073 has not been detected in human urine, it has been shown to bind to CB1Rs with nanomolar affinity without eliciting any G-protein activation at concentrations that are pharmacologically relevant, up to 10 μM [4]. Furthermore, Schild analysis has shown that the 7-hydroxyindole derivative of JWH-073 competitively antagonizes G-protein activation in vitro. In mice, hypothermia induced by JWH-018 was attenuated by pretreatment with this oxidized derivative of JWH-073. Alternatively, JWH-018 induced analgesia, catalepsy, and locomotor activity were not altered by this metabolite. Overall, these in vitro and in vivo findings along with the lack of the 7-hydroxyindole derivative of JWH-073 detected in human urine suggest that this oxidized derivative of JWH-073 may not be formed in humans or readily cross the blood–brain barrier.
In addition to a human oxidation product of JWH-018 acting as a CB1R antagonist, it has also been shown that a major human glucuronidated metabolite of JWH-018 (5-hydroxypentyl-β-D-glucuronide) retains significant affinity for CB1Rs and displays CB1R antagonism in vitro [59]. Interestingly, this study also showed that a major structurally similar glucuronidated metabolite of THC (11-nor-9-carboxy-THC-β-D-glucuronide) lacked CB1R affinity and activity. Collectively, these findings demonstrate that both hydroxylated and glucuronidated metabolites of SCBs may retain significant CB1R affinity in the absence of intrinsic activity, which suggest that some SCB metabolites can produce physiologically relevant antagonism of effects of CB1Rs.
When discussing the pharmacological and toxicological effects of SCBs, the primary focus has been geared towards understanding CB1R-induced responses. Many SCBs not only have high affinity and significant intrinsic activity at the CB1R, they very often also have comparable binding and functional activity at the second major CBR subtype, CB2R [3, 6]. CB1Rs are primarily located within the CNS, while CB2Rs are most abundantly located on immune cells in peripheral regions [60] and are associated with immune functions, inflammation, and bone formation [61]. More recent studies have demonstrated that activation of low numbers of CB2R in the CNS can modulate the abuse-related properties of alcohol [62], nicotine [63], and cocaine [64]. In addition, mono-hydroxylated metabolites of JWH-018 and JWH-073 have also been shown to retain high CB2R affinity and efficacy [7].
Interestingly, findings have implicated the involvement of endocannabinoid signaling in the modulation of the serotonin system (as reviewed by Haj-Dahmane and Shen [65]. For instance, chronic activation of CB2Rs has been shown to produce an upregulation of 5-HT2A receptors in the prefrontal cortex of mice [66, 67]. CNS abnormalities in 5-HT2A function can lead to mental disorders, including anxiety [68] and psychosis [69]. Furthermore, 5-HT2A receptor signaling is a major site of action for hallucinogenic drugs [70]. Common adverse effects associated with SCB use are not often observed with Δ9-THC, such as anxiety and psychosis [71]. It is possible to speculate that SCB- or SCB metabolite-induced upregulation of 5-HT2A receptors, mediated via CB2R activation, might contribute to anxiety and psychosis that are observed after exposure to SCBs found in commercial abuse-ready preparations. In this regard, a case study reported on four patients hospitalized for psychosis who smoked a product containing the SCB AM-2201 while in the clinic. The authors described the appearance of new psychotic symptoms, and a marked worsening of mood and anxiety symptoms in four patients, and noted that even though they all ingested the same drug, the clinical picture differed markedly among the individual patients [72], perhaps implicating individual metabolism of AM-2201 in the diversity of effects observed. Thus, it is clearly important for future studies to explore both CB1R and CB2R signaling as it relates to the pharmacological and toxicological effects of SCBs and its metabolites. Furthermore, future studies should consider the capacity of these drugs to modulate the expression and function of other, non-CBR systems.
4 Synthetic Cannabinoid Drug–Drug Interactions
Commercial SCB preparations often contain multiple drugs in combination, and the concentrations of these specific SCBs vary widely from product to product, or even within a product from batch to batch [58, 59]. Therefore, it is possible for drug–drug interactions to exist both within and between these diverse mixtures of SCBs, which may contribute to abuse-related and adverse effects associated with the use of these drugs. As described above, mouse studies have shown that coadministration of JWH-018 and JWH-073 produced additive, synergistic, or antagonistic interactions compared to administration of either drug alone, depending on the specific endpoint examined and the drug dose ratio employed [54]. Evidence of synergistic effects with these two SCBs was demonstrated both in vivo and in vitro, with mouse assays of Δ9-THC discrimination and analgesia, and with displacement of radioligand binding from CB1Rs in a cellular model. In addition to synergism, SCB blended mixtures can influence the relative potency of both their subjective and adverse effects. Furthermore, polysubstance abuse may lead to unpredictable effects of SCBs that may contribute to even greater abuse-related and adverse effects. Future studies are needed to understand the drug–drug interactions among SCBs and co-exposure to other drugs of abuse.
Given their shared metabolism via P450 isoforms, combined use of SCBs with various prescription medications could also potentially result in adverse drug–drug interactions. Commonly prescribed drugs such as valproic acid (an anticonvulsant and mood stabilizer) and sertraline (an antidepressant) potently inhibit CYP2C9, while drugs such as ciprofloxacin (an antibiotic) and fluvoxamine (an antidepressant) strongly inhibit CYP1A2. Additionally, CYP2C9 is a major polymorphic enzyme [31] and is responsible for the metabolism of a number of clinically important drugs such as warfarin (a blood thinner), phenytoin (an anticonvulsant), tolbutamide (an antidiabetic agent), losartan (an antihypertensive), and ibuprofen (a nonsteroidal anti-inflammatory drug). Over five allelic variants of CYP2D6 have been identified, including two “loss of function” variants (CYP2C9*4 and CYP2C9*5) (Seng and Seng 2008). Similarly, CYP1A2 is responsible for the metabolism of numerous psychiatric medications including antipsychotics (olanzapine, clozapine, haloperidol, and thioridazine), antidepressants (imipramine, clomipramine, and fluvoxamine), and cholinesterase inhibitors used in the treatment of Alzheimer’s disease (tacrine) (Shirley et al. 2003), but this enzyme is well conserved without common functional polymorphisms (Hiratsuka 2012). Because SCBs are also substrates for these P450 isoforms, the possibility of drug–drug interactions is a serious consideration with the use of SCBs.
5 Conclusions
Commercial SCB products are not safe alternatives to cannabis and pose significant threats to public health. It is anticipated that morbidity and mortality rates will continue to increase in correlation to SCB exposure. Recent reports have demonstrated that SCBs present a pharmacological and toxicological profile that is distinct from Δ9-THC found in marijuana. For instance, SCBs primarily act as full CB1 and CB2R agonists both in vitro and in vivo, while Δ9-THC is a weak partial agonist. Furthermore, several SCB metabolites bind with high affinity at CB1 and CB2Rs, while displaying a range in intrinsic activities from neutral antagonists to partial agonists to full agonists, both in cellular assays and animal studies. These findings illustrate that commercial SCBs products are not safe and should not be considered an alternate form of marijuana. Rather, they produce greater toxicity relative to marijuana, which could be attributed to the combined actions of the varying SCBs or their metabolites present in these products. Still, these findings present the supporting evidence that the pharmacological and toxicological properties of SCBs pose a severe health risk.
Contributor Information
Sherrica Tai, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences College of Medicine, Mail Slot 638, 4301 West Markham Street, Little Rock, AR 72207, USA; Department of Pharmacology, University of Michigan Medical School, 2301 MSRB III, 1150 W. Medical Center Drive, Ann Arbor, MI 48109, USA.
William E. Fantegrossi, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences College of Medicine, Mail Slot 638, 4301 West Markham Street, Little Rock, AR 72207, USA
References
- 1.Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF. Hijacking of basic research: the case of synthetic cannabinoids. Methods Rep RTI Press. 2011;2011:17971. doi: 10.3768/rtipress.2011.op.0007.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai S, Fantegrossi W. Synthetic cannabinoids: pharmacology, behavioral effects, and abuse potential. Curr Addict Rep. 2014:1–8. doi: 10.1007/s40429-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB (1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 4.Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin CN, Murphy JW, Huffman JW, Kendall DA. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291:837–844. [PubMed] [Google Scholar]
- 7.Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269:100–108. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005;(168):657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Bornheim LM, Lasker JM, Raucy JL. Human hepatic microsomal metabolism of delta 1-tetrahydrocannabinol. Drug Metab Dispos. 1992;20:241–246. [PubMed] [Google Scholar]
- 12.Watanabe K, Matsunaga T, Yamamoto I, Funae Y, Yoshimura H. Involvement of CYP2C in the metabolism of cannabinoids by human hepatic microsomes from an old woman. Biol Pharm Bull. 1995;18:1138–1141. doi: 10.1248/bpb.18.1138. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto I, Watanabe K, Kuzuoka K, Narimatsu S, Yoshimura H. The pharmacological activity of cannabinol and its major metabolite, 11-hydroxycannabinol. Chem Pharm Bull. 1987;35:2144–2147. doi: 10.1248/cpb.35.2144. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G. In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphtha-lenyl) methanone mesylate, a cannabinoid receptor agonist. Drug Metab Dispos. 2002;30:1077–1086. doi: 10.1124/dmd.30.10.1077. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Ma P, Wang W, Cole RB, Wang G. Characterization of rat liver microso-mal metabolites of AM-630, a potent cannabinoid receptor antagonist, by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2004;39:672–681. doi: 10.1002/jms.640. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Ma P, Cole RB, Wang G. Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Anal Bioanal Chem. 2006;386:1345–1355. doi: 10.1007/s00216-006-0717-6. [DOI] [PubMed] [Google Scholar]
- 17.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, James LP, Moran JH, Radominska-Pandya A. Conjugation of synthetic cannabinoids JWH-018 and JWH-073, metabolites by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39:1967–1976. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wintermeyer A, Moller I, Thevis M, Jubner M, Beike J, Rothschild MA, Bender K. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem. 2010;398:2141–2153. doi: 10.1007/s00216-010-4171-0. [DOI] [PubMed] [Google Scholar]
- 20.Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2519–2526. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, Schanzer W, Thevis M. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2011;3:609–620. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- 22.Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, Nosyrev A, Vedenin A, Izotov B, Zabirova I, Rozhanets V. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1126–1136. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuck S, Moller I, Thomas A, Klose A, Schlorer N, Schanzer W, Thevis M. Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Anal Bioanal Chem. 2011;401:493–505. doi: 10.1007/s00216-011-4931-5. [DOI] [PubMed] [Google Scholar]
- 26.de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine – an Australian perspective. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;897:22–31. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Dowling G, Regan L. A method for CP 47, 497 a synthetic non-traditional cannabinoid in human urine using liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:253–259. doi: 10.1016/j.jchromb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.ElSohly MA, Gul W, Elsohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35:487–495. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- 29.Hutter M, Broecker S, Kneisel S, Auwarter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J Mass Spectrom. 2012;47:54–65. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- 30.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012;4:745–753. doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- 31.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavanello S, Fedeli U, Mastrangelo G, Rota F, Overvad K, Raaschou-Nielsen O, Tjonneland A, Vogel U. Role of CYP1A2 polymorphisms on lung cancer risk in a prospective study. Cancer Genet. 2012;205:278–284. doi: 10.1016/j.cancergen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45:1186–1194. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- 34.Kacinko SL, Xu A, Homan JW, McMullin MM, Warrington DM, Logan BK. Development and validation of a liquid chromatography-tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. J Anal Toxicol. 2011;35:386–393. doi: 10.1093/anatox/35.7.386. [DOI] [PubMed] [Google Scholar]
- 35.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381–6388. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seely KA, Prather PL, James LP, Moran JH. Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv. 2011;11:36–51. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huffman JW, Thompson AL, Wiley JL, Martin BR. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg Med Chem. 2008;16:322–335. doi: 10.1016/j.bmc.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of can-nabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- 39.Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD. Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J Med Chem. 2010;53:295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- 40.Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of canna-binoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther. 1998;285:553–560. [PubMed] [Google Scholar]
- 41.Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int. 2013;227:21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 43.Jankovics P, Varadi A, Tolgyesi L, Lohner S, Nemeth-Palotas J, Balla J. Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary. Forensic Sci Int. 2012;214:27–32. doi: 10.1016/j.forsciint.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47, 497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howlett AC, Champion TM, Wilken GH, Mechoulam R. Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 46.Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman AF, Lycas MD, Kaczmarzyk JR, Spivak CE, Baumann MH, Lupica CR. Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid ‘Spice’ compounds: comparison with Δ9-tetrahydrocannabinol. Addict Biol. 2016 doi: 10.1111/adb.12334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta-9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- 49.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 51.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kochanowski M, Kala M. Tetrahydrocannabinols in clinical and forensic toxicology. Przegl Lek. 2005;62:576–580. [PubMed] [Google Scholar]
- 54.Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE. Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther. 2013;346:350–361. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49:760–764. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patton AL, Chimalakonda KC, Moran CL, McCain KR, Radominska-Pandya A, James LP, Kokes C, Moran JH. K2 toxicity: fatal case of psychiatric complications following AM2201 exposure. J Forensic Sci. 2013;58:1676–1680. doi: 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seely KA, Brents LK, Radominska-Pandya A, Endres GW, Keyes GS, Moran JH, Prather PL. A major glucuronidated metabolite of JWH-018 is a neutral antagonist at CB1 receptors. Chem Res Toxicol. 2012;25:825–827. doi: 10.1021/tx3000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 61.Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr Med Chem. 2010;17:1393–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- 62.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haj-Dahmane S, Shen RY. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology. 2011;61:414–420. doi: 10.1016/j.neuropharm.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franklin JM, Carrasco GA. G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2A receptors. J Biol Chem. 2013;288:15712–15724. doi: 10.1074/jbc.M113.454843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 receptor- and beta arrestin 2-dependent upregulation of serotonin 2A receptors. Eur Neuropsychopharmacol. 2013;23:760–767. doi: 10.1016/j.euroneuro.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghisleni G, Kazlauckas V, Both FL, Pagnussat N, Mioranzza S, Rocha JB, Souza DO, Porciuncula LO. Diphenyl diselenide exerts anxiolytic-like effect in Wistar rats: putative roles of GABAA and 5HT receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1508–1515. doi: 10.1016/j.pnpbp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Morgan D, Kondabolu K, Kuipers A, Sakhuja R, Robertson KL, Rowland NE, Booth RG. Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: potential utility as antipsychotic medications. Neuropharmacology. 2013;72:274–281. doi: 10.1016/j.neuropharm.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21:320–326. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 72.Celofiga A, Koprivsek J, Klavz J. Use of synthetic cannabinoids in patients with psychotic disorders: case series. J Dual Diagn. 2014;10(3):168–173. doi: 10.1080/15504263.2014.929364. [DOI] [PubMed] [Google Scholar]


