Abstract
The 5-HT6 receptor has been implicated in a variety of cognitive processes including habitual behaviors, learning, and memory. It is found almost exclusively in the brain, is expressed abundantly in striatum, and localizes to neuronal primary cilia. Primary cilia are antenna-like, sensory organelles found on most neurons that receive both chemical and mechanical signals from other cells and the surrounding environment; however, the effect of 5-HT6 receptor function on cellular morphology has not been examined. We confirmed that 5-HT6 receptors were localized to primary cilia in wild-type (WT) but not 5-HT6 knockout (5-HT6KO) in both native mouse brain tissue and primary cultured striatal neurons then used primary neurons cultured from WT or 5-HT6KO mice to study the function of these receptors. Selective 5-HT6 antagonists reduced cilia length in neurons cultured from wild-type mice in a concentration and time-dependent manner without altering dendrites, but had no effect on cilia length in 5-HT6KO cultured neurons. Varying the expression levels of heterologously expressed 5-HT6 receptors affected the fidelity of ciliary localization in both WT and 5-HT6KO neurons; overexpression lead to increasing amounts of 5-HT6 localization outside of the cilia but did not alter cilia morphology. Introducing discrete mutations into the third cytoplasmic loop of the 5-HT6 receptor greatly reduced, but did not entirely eliminate, trafficking of the 5-HT6 receptor to primary cilia. These data suggest that blocking 5-HT6 receptor activity reduces the length of primary cilia and that mechanisms that regulate trafficking of 5-HT6 receptors to cilia are more complex than previously thought.
Keywords: 5-HT6 receptors, Primary Cilia, Striatum, Serotonin, Serotonin-6, Primary cultured neurons
1. Introduction
Almost all mammalian cells, neurons included, have a primary cilium at some point during their life cycle (Bishop et al, 2007; Singla and Reiter, 2006; Wheatley et al, 1996). Primary cilia are microtubule-supported organelles that stem from the basal body and extend beyond the cell body into the extrasynaptic space (Arellano et al, 2012; Avasthi and Marshall, 2012; Louvi and Grove, 2011). Originally identified by the Swiss anatomist K.W. Zimmerman in 1898, primary cilia were neglected in biological research for many years and even misclassified as a vestigial organelle (Praetorius and Spring, 2003; Whitfield, 2004). Often referred to as “non-motile” cilia because they are not involved in motion generation, primary cilia are rich with receptors and receive both chemical and mechanical signals from the surrounding environment (Davenport and Yoder, 2005; Pazour and Witman, 2003; Praetorius and Spring, 2003). Signaling within primary cilia is transduced by a discrete set of membrane-bound receptors specifically localized to the primary cilium; while some receptors are found in both cilia and dendrites, others are exclusive to cilia and represent an interesting category of extra-synaptic receptor-mediated signals from the extracellular environment. Many studies have investigated the developmental impact of neuronal primary cilia, but their role in normal cognitive function is not well understood and there is some evidence that ciliary signaling throughout the lifespan modulates neuronal function and cognition (Berbari et al, 2008; Davenport and Yoder, 2005).
There is a rising interest in the function and significance of primary cilia, especially with regard to their role in a variety of disorders now known as “ciliopathies” (Ainsworth, 2007; Novarino et al, 2011). Some of the best characterized ciliopathies arise from mutations in or deletions of genes responsible for formation and function of primary cilia (Kim et al, 2010; Lee and Gleeson, 2011). Ciliopathies frequently involve malformations of primary cilia such as shortened, elongated or total ablation of the primary cilia (Avasthi et al, 2012; Marley and von Zastrow, 2012; Ou et al, 2009). Although, little is known about the function of primary cilia on neurons, common symptoms of ciliopathies include brain deformation and cognitive impairments (Green et al, 2012; Louvi and Grove, 2011). Even less is known about more subtle alterations in the signaling events that occur within cilia and how they affect health and disease, especially after early development.
The 5-HT6 receptor is the only serotonin (5-HT) receptor that localizes to primary cilia in neurons (Berbari et al, 2007; Brailov et al, 2000). 5-HT6 receptors are present during development and regulate neuronal migration (Dayer et al, 2015; Duhr et al, 2014; Grimaldi et al, 1998). These GαS-coupled metabotropic receptors couple to adenylyl cyclase and stimulate accumulation of cyclic adenosine monophosphate (cAMP) which, in most neurons, mediates a downstream excitatory effect (Duhr et al, 2014; Jacobshagen et al, 2014; Kohen et al, 2001). However, additional signal-transduction mechanisms distinct from the canonical G-protein signaling cascades have been recently described (Dayer et al, 2015; Duhr et al, 2014; Yun and Rhim, 2011; Yun et al, 2007). 5-HT6 receptors are expressed almost exclusively in the brain, especially densely in striatum, and have substantial similarities of sequence identity and pharmacological properties between rats and humans (Gerard et al, 1997; Hirst et al, 2003; Mitchell et al, 2007; Monsma Jr. et al, 1993; Ruat et al, 1993). 5-HT6 receptors are considered to be important targets for modulating learning, memory, and reward-motivated behavior (Codony et al, 2011; Mitchell and Neumaier, 2005). Increased expression of 5-HT6 receptors in neurons of the ventral striatum of rats blocks the reward-learning processes associated with cocaine (Ferguson et al, 2008). The specific cell type that expresses these receptors is critical as both compulsive behaviors and the sensitivity to cocaine reinforcement are altered by increasing 5-HT6 expression in indirect pathway medium spiny neurons (Brodsky et al, 2016). Together these studies implicate 5-HT6 receptors as a possible therapeutic target for drug addiction and possibly other cognitive disorders (Eskenazi et al, 2015a; Ferguson et al, 2011; Fukuo et al, 2010; Wilkinson et al, 2014).
5-HT6 receptors, along with most other cilia localizing GPCRs, have a five amino acid long ciliary targeting sequence (CTS) within their third intracellular (i3) loop that has been thought to be critical for trafficking into primary cilia (Berbari et al, 2008; Nachury et al, 2010; Nagata et al, 2013). Heterologously expressed 5-HT6 receptors have been reported to alter cilia length and dendritic morphology (Duhr et al, 2014; Guadiana et al, 2013) but endogenous 5-HT6 receptors in native neurons (such as medium spiny neurons) have not been examined in this manner.
In the present study, we asked whether the morphological properties of neuronal primary cilia are modulated by 5-HT6 receptor localization and function. In particular we evaluated whether manipulating 5-HT6 receptor activity and expression in striatal neurons alters neuronal primary cilia morphology and signaling. In both native brain tissue and primary cultured striatal neurons, 5-HT6 receptors were localized to cilia in wild-type (WT) but not 5-HT6 knockout mice (5-HT6KO). Treatment of cultured neurons with a selective 5-HT6 receptor antagonist reduced cilia length in striatal neurons in a concentration- and time-dependent manner, but agonism and antagonism of the endogenous receptor did not affect dendritic arborization. The level of heterologous expression of 5-HT6 receptors modified the fidelity with which 5-HT6 receptors localized to primary cilia in cultured neurons, but had no effect on primary cilia length. We targeted mutant 5-HT6 receptors containing mutations within the CTS decreased, but failed to eliminate, targeting to the primary cilia or changes in primary cilia length. Suggesting, that the CTS is not exclusively responsible for 5-HT6 receptor trafficking to primary cilia. Taken together, our findings suggest a role for endogenous 5-HT6 receptor activity in the dynamic regulation of striatal primary cilia and indicate that regulation of cilia trafficking of 5-HT6 receptors is more complex than previously thought.
2. Results
2.1 5-HT6 receptors localize to WT mouse neuronal primary cilia both in vivo and in vitro
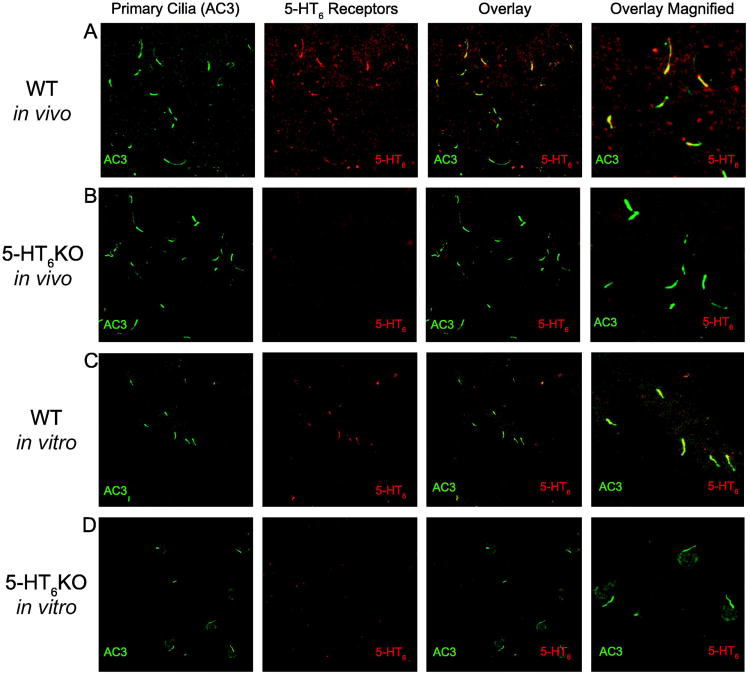
Previous studies have described endogenous 5-HT6 receptors on “cilia-like” processes in rats with electron microscopy and peroxidase staining (Brailov et al, 2000; Hamon et al, 1999). Here we confirmed that endogenous 5-HT6 receptors co-localize with adenylyl cyclase 3 (AC3), an established marker for primary cilia, (Bishop et al, 2007; Wang et al, 2009), in sections of WT mouse striatum (Figure 1A) and CA1 of WT mouse hippocampus (Supplemental Figure 1A). Further, 5-HT6 receptorimmunostaining was absent in 5-HT6KO sections despite continued presence AC3-positive cilia in striatum (Figure 1B) and CA1 of hippocampus (Supplemental Figure 1B). 5-HT6 receptors also localized to primary cilia in WT primary cultured striatal neurons following dissociation and 10 days growth in vitro (Figure 1C); as expected, primary cultured striatal neurons from 5-HT6 KO mice had cilia but lacked 5-HT6 immunostaining altogether (Figure 1D).
Figure 1. 5-HT6 receptors localize to mouse striatal primary cilia both in vivo and in vitro.
A) Images of WT mouse striatum stained against cilia marker AC3 (green), 5-HT6 receptors (red), and both (also shown at higher magnification). B) Images of 5-HT6 receptors mouse striatum stained against cilia marker AC3, 5-HT6 receptors, and both (also shown at higher magnification). C) Images of WT primary mouse striatal neurons stained against cilia marker AC3, 5-HT6 receptors, and both (also shown at higher magnification). D) Images of 5-HT6 receptor KO primary mouse striatal neurons stained against cilia marker AC3, 5-HT6 receptors, and both (also shown at higher magnification).
2.2 5-HT6 receptor antagonists shorten primary cilia of striatal neurons
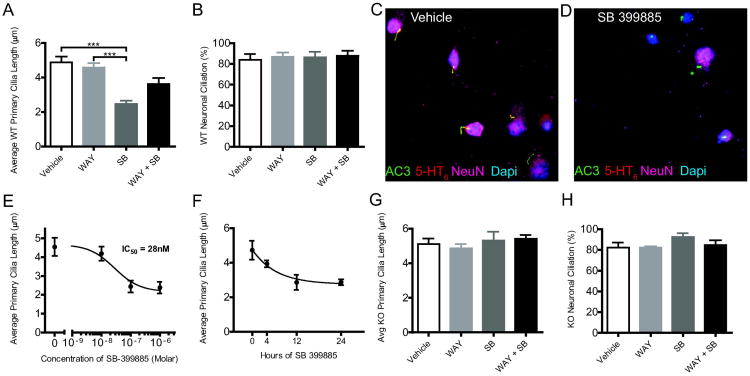
To examine the effect of endogenous 5-HT6 receptor activity on neuronal primary cilia morphology, we used primary cultured striatal neurons to facilitate precise control of drug concentration and ready imaging and measurement of individual primary cilia. Treatment of WT cultured striatal neurons with 1 μM of selective 5-HT6 receptor agonist (WAY-208466) for 24 hours had no effect on primary cilia length, but treatment with the 5-HT6-selective receptor antagonist (SB-399885) for 24 hours significantly reduced the length of primary cilia (Figure 2A, F(3,58) = 16.84; p<0.0001). Treatment with both 5-HT6 receptor agonist and antagonist together had no effect. These drug treatments did not alter the percentage of primary neurons with primary cilia (ciliation) on the striatal neurons (Figure 2B, F(3,33) = 0.113; p=0.95). Representative images of primary cilia on WT neurons treated with either vehicle or SB 399885 are shown in Figure 2C and 2D, respectively. Treatment with SB-399885 reduced the average primary cilia length in a concentration-dependent manner with an IC50 value of 28 nM (Figure 2E), which is similar to the previously reported Ki for this drug (Hirst et al, 2006). The effect of 1 μM SB-399885 treatments on WT cultured striatal neurons at different time intervals (0, 4, 12, and 24 hours) established that the antagonist-induced primary cilia shortening is also time dependent (Figure 2F). We observed a similar reduction in cilia length using another 5-HT6-selective antagonist, SB-258585 (1 μM, data not shown).
Figure 2. 5-HT6 receptor antagonism shortens primary cilia of striatal neurons.
A) Effect of 5-HT6 receptor-selective drugs on primary cilia lengths (Mean ± SEM) of WT primary striatal neurons in culture. Vehicle (n=18 coverslips), WAY (n=17 coverslips), SB (n=18 coverslips) WAY+SB (n=8 coverslips). B) Effect of 5-HT6 receptor-selective drugs on percentage of WT striatal neurons with primary cilia (Mean ± SEM). Vehicle (n=10 coverslips), WAY (n=10 coverslips), SB (n=9 coverslips) WAY+SB (n=8 coverslips). C) Primary cilia length in response to different concentrations of antagonist SB-399885 (Mean ± SEM); Vehicle (n=25 cilia across 5 coverslips), 10 nM (n=36 cilia across 5 coverslips), 100 nM (n=29 cilia across 5 coverslips) and 1 μM (n=30 cilia across 5 coverslips). D) Time-dependent reduction of cilia length by SB-399885 (Mean ± SEM); 0 (n=6 coverslips), 4 (n=7 coverslips), 12 (n=5 coverslips), and 24 hours (n=7 coverslips). * = P < 0.05, ** = P < 0.001, *** = P < 0.0001; one-way ANOVA followed by post hoc Tukey's HSD test. Effect of SB-399885 on primary cilia length of striatal neurons is 5-HT6 receptor specific. G) Effect of 5-HT6 receptor selective drugs on primary cilia lengths (Mean ± SEM) of 5-HT6 KO primary striatal neurons in culture. Vehicle (n=5 coverslips), WAY (n=6 coverslips), SB (n=8 coverslips) WAY+SB (n=10 coverslips). H) Effect of 5-HT6 receptor selective drugs on percentage of 5-HT6KO striatal neurons with primary cilia (Mean ± SEM). Vehicle (n=5 coverslips), WAY (n=5 coverslips), SB (n=5 coverslips) WAY+SB (n=5 coverslips). *P < 0.05, **P < 0.01; one-way ANOVA followed by post hoc Tukey's HSD test.
2.3 Effect of SB-399885 of primary cilia on striatal neurons is 5-HT6 receptor specific
We confirmed that SB-399885 reduced cilia length via 5-HT6 receptors using primary striatal neurons cultured from 5-HT6KO mice; there was no effect of 5-HT6-selective agonist (1 μM WAY-208466), antagonist (1 μM SB-399885) or both drugs together; none of these treatments altered primary cilia length (Figure 2G, F(3,26) = 0.46; p=0.71) or ciliation (Figure 2H, F(3,16) = 1.53; p=0.25).
2.4 5-HT6 agonists and antagonists do not affect dendritic arborization
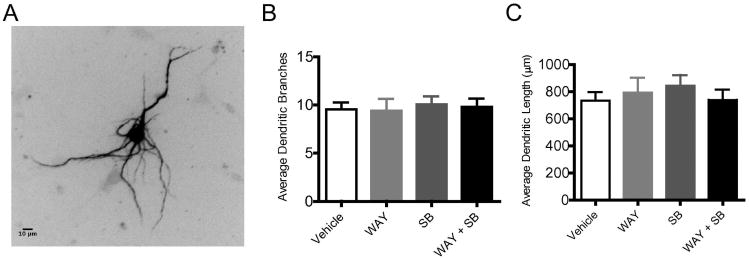
To assess the effect of endogenous 5-HT6 receptor activity on general neuronal morphology, primary cultured striatal neurons from WT mice were transfected with microtubule- associated- protein-2B fused to red fluorescent protein (Map2B-RFP) which labels the somato-dendritic domain (Figure 3A). Treatment with 5-HT6-selective agonist (1 μM WAY-208466), antagonist (1 μM SB-399885) or both drugs together had no effect on either the average number of dendritic branches (Figure 3B, F(3,68) = 0.09; p=0.96) nor the average total dendritic length (Figure 3C, 0. F(3,67) = 36; p=0.78) of the primary cultured striatal neurons.
Figure 3. 5-HT6 receptor agonists and antagonists do not affect dendritic arborization.
Primary striatal cultures were transfected on DIV7 with Map2B-RFP plasmid to identify dendritic branching, and treated with vehicle, 5-HT6 agonist (1μm Way-181187), 5-HT6 antagonist (1μm SB399885), or 1μm of Both from DIV9-10, fixed, and imaged. A) Representative photomicrograph of Map2B-RFP transfected neuron. B) Average number of dendritic branches (Mean ± SEM); Vehicle (n=24 neurons), WAY (n=17 neurons), SB (n=14 neurons) WAY+SB (n=17 neurons). *P < 0.05, **P < 0.01; one-way ANOVA followed by post hoc Tukey's HSD test. C) Average total dendritic length (Mean ± SEM); Vehicle (n=24 neurons), WAY (n=17 neurons), SB (n=14 neurons) WAY+SB (n=17 neurons). *P < 0.05, **P < 0.01; one-way ANOVA followed by post hoc Tukey's HSD test.
2.5 High levels of heterologous expression increased nonspecific localization of 5-HT6 receptors
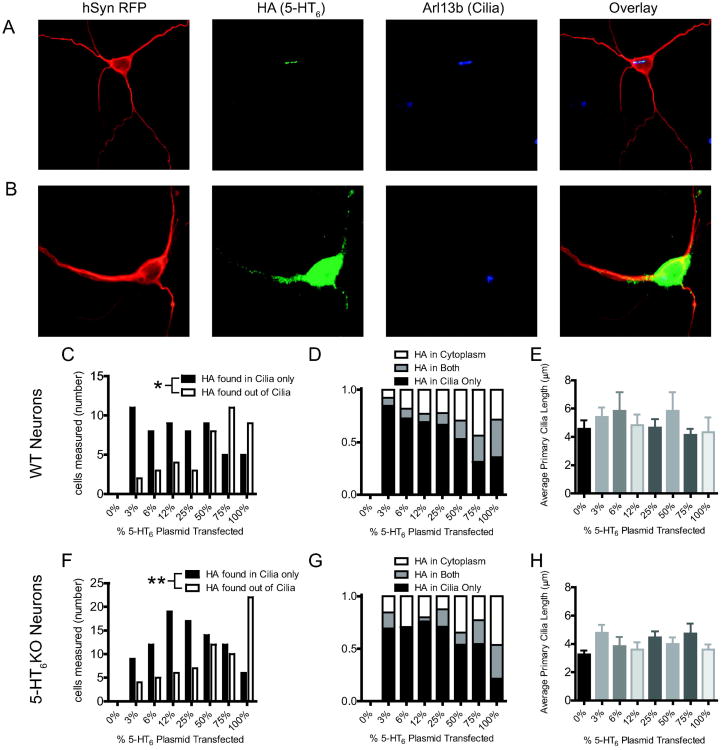
Previous studies of subcellular localization of 5-HT6 receptors to primary cilia used heterologous expression of 5-HT6 receptors (Berbari et al, 2008; Guadiana et al, 2013; Mahjoub and Stearns, 2012). HA-tagged 5-HT6 receptor plasmid was transfected into primary striatal neurons along with a second plasmid encoding hSYN-RFP and an empty vector comprising a total of 1 μg plasmid DNA per well (except the 100% 5-HT6 condition which received an extra 250ng hSYN-RFP per well). Immunostaining of the transfected neurons revealed that the heterologous 5-HT6 receptor protein did not always localize to neuronal primary cilia (Figure 4A), and sometimes localized throughout the cytoplasm (Figure 4B). This finding led us to vary the amount of 5-HT6 plasmid to evaluate the extent to which increased levels of overexpression affected subcellular localization of the HA-5-HT6 receptor (Susa et al, 2008). Each well of WT or 5-HT6KO neurons was transfected with a total of 1 μg of total plasmid DNA containing varying proportions of the 5-HT6 receptor plasmid. At low levels of expression, most of the immunostaining for 5-HT6 receptor was in the primary neuronal cilia, but as the amount of 5-HT6 receptor plasmid increased, the percentage of transfected neurons with HA-tagged receptor localized outside of primary cilia increased significantly in both WT neurons (Figure 4C, χ2(7, N=111) = 14.13, p=0.048) and 5-HT6KO neurons (Figure 4F, χ2(7, N=169) = 22.26, p=0.0023) neurons. Proportions of neurons where receptor was found only in primary cilia, only outside of primary cilia, or both were evaluated for both transfected WT (Figure 4D) and 5-HT6KO (Figure 4G) neurons. Primary cilia length did not change with different amounts of transfected plasmid in either WT (Figure 4E, 1-way ANOVA, p = 0.76) or 5-HT6KO (Figure 4H, 1-way ANOVA, p = 0.12) cultured striatal neurons.
Figure 4. High levels of heterologous expression increases nonspecific localization of 5-HT6 receptors.
A) Representative images of heterologous 5-HT6 receptor expressed in neuronal primary cilia. Red = hSynRFP (marker for neuronal specific transfection), Green = HA (HA-tagged 5-HT6 receptors), Blue = Arl13b (marker for primary cilia). B) Representative image of transfected 5-HT6 receptor expressed outside of neuronal primary cilia and cytoplasmically. Red = hSynRFP (marker for neuronal specific transfection), Green = HA (HA-tagged 5-HT6 receptors), Blue = Arl13b (marker for primary cilia). C) Receptor localization from gene-dose transfection of WT 5-HT6 receptor in WT primary culture neurons (counts). 0% (n=15 cells), 3% (n=13 cells), 6% (n=11 cells), 12% (n=13 cells), 25% (n=12 cells), 50% (n=17 cells), 75% (n=16 cells), 100% (n=14 cells). D) Proportion of total WT neurons transfected with varied amount of 5-HT6 receptor plasmid where HA is found either in primary cilia, outside of primary cilia, or both. E) Average primary cilia lengths resulting from gene-dose transfection of WT 5-HT6 receptor in WT primary culture neurons 0% (n=29 cells), 3% (n=10 cells), 6% (n=10 cells), 12% (n=10 cells), 25% (n=17 cells), 50% (n=6 cells), 75% (n=28 cells), 100% (n=13 cells). F) Receptor localization gene-dose transfection of 5-HT6 receptor in 5-HT6KO primary culture neurons (count). 0% (n=15 cells), 3% (n=13 cells), 6% (n=17 cells), 12% (n=25 cells), 25% (n=24 cells), 50% (n=26 cells), 75% (n=21 cells), 100% (n=28 cells). G) Proportion of total 5-HT6KO neurons transfected with varied amount of 5-HT6 receptor plasmid where HA is found either in primary cilia, outside of primary cilia, or both. H) Average primary cilia lengths resulting from gene-dose transfection of 5-HT6 receptor in 5-HT6KO primary culture neurons 0% (n=54 cells), 3% (n=19 cells), 6% (n=16 cells), 12% (n=20 cells), 25% (n=43 cells), 50% (n=17 cells), 75% (n=20 cells), 100% (n=29 cells). *P < 0.05, **P < 0.01; Chi-square Test.
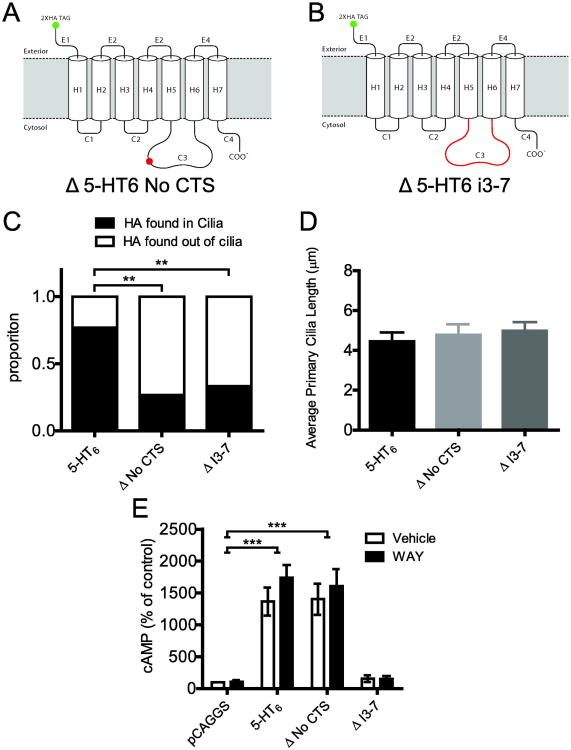
2.6 Mutations to the CTS reduced cilia targeting
Previous studies of the 5-HT6 and other GPCRs have suggested that a five amino acid long consensus sequence in the i3 loop acts as a cilia targeting sequence (CTS) (Berbari et al, 2008; Domire et al, 2011; Nagata et al, 2013). We took advantage of the CTS on the rat 5-HT6 receptor and cloned two mutant forms of receptor: one in which the five amino acid CTS was removed (Δ5-HT6 No CTS, Figure 5A) and one in which the entire i3 loop was replaced with that of the 5-HT7 receptor (Δ5-HT6 i3-7) (Figure 5B). The 5-HT7 receptor was chosen because it is also a Gαs-coupled receptor, but does not localize to primary cilia (Berbari et al, 2008). We transfected WT 5-HT6, Δ5-HT6 No CTS, and Δ5-HT6 i3-7 (25% of total 1 μg DNA transfected per well) into 5-HT6KO cultured neurons to test localization in the absence of endogenous 5-HT6 receptors (to preclude possible WT-mutant heteromerization and thus prevent potential co-trafficking to primary cilia). These two mutants had significantly reduced trafficking to primary cilia but a modest proportion of the mutant receptors were still localized to primary cilia (Figure 5C, Fisher's Exact test; Δ5-HT6 No CTS, p=0.001; Δ5-HT6 i3-7, p=0.007). The mutant 5-HT6 receptors did not affect the average length of primary cilia (Figure 5D, 1-way ANOVA; F(2,109)=0.007, p = 0.6836). Since it was possible that the functional properties of these mutants affected localization or cilia morphology, we tested whether these mutants were still able to activate adenylyl cyclase using an enzyme-linked immunosorbent assay to measure cAMP accumulation in IMCD3 cells, which do not express 5-HT6 receptors endogenously (data not shown). Both the WT 5-HT6 and Δ5-HT6 No CTS receptor showed constitutive activity relative to the empty vector control (Figure 5E, F(3,16)=46.20, p<0.001). Both the WT 5-HT6 and Δ5-HT6 receptor demonstrated agonist associated increased cAMP compared to their empty vector control (Figure 5E, F(3,16)=46.20, p<0.001); however, the addition of agonist did not further increase cAMP compared to vehicle for either of these receptors. However, the Δ5-HT6 i3-7 receptor failed to activate cAMP accumulation. Thus, the Δ5-HT6 i3-7 mutant was non-functional, at least in terms of the ability to increase adenylyl cyclase activity.
Figure 5. Mutations to the CTS reduces cilia targeting.
A) Representative diagram of mutation on 5-HT6 receptor to create a receptor without the primary cilia targeting sequence (Δ5-HT6 No CTS). B) Representative diagram of mutation on 5-HT6 receptor to create a receptor with 5-HT7 receptor intracellular loop 3 (IC3) (Δ5-HT6 i3-7). C) Proportion of receptor localized in and out of primary cilia in 5-HT6KO cultured neurons transfected with 25% receptor plasmid: WT 5-HT6 (n=39 neurons), Δ5-HT6 No CTS (n=15 neurons), or Δ5-HT6 i3-7 (n=18 neurons). D) Average primary cilia lengths when the mutant 5-HT6 receptors localize to cilia: WT 5-HT6 (n=43 neurons), Δ5-HT6 No CTS (n=28 neurons), or Δ5-HT6 i3-7 (n=41 neurons). E) Relative levels of cAMP accumulation in IMCD3 cells transfected with empty vector (pCAGGS), WT 5-HT6, Δ5-HT6 No CTS, or Δ5-HT6 i3-7; both in the presence and absence of WAY-208846 (n=3 independent biological replicates). * = P < 0.05, ** = P < 0.001, *** = P < 0.0001; 2-way ANOVA followed by post hoc Bonferroni test.
3. Discussion
The 5-HT6 receptor has become an important target for drug development (Fone, 2008) and at least one 5-HT6 antagonist is in later stages of development for the treatment of Alzheimer's disease (Wilkinson et al, 2014). However, the implications that 5-HT6 receptors localize to primary neuronal cilia has received scant attention. Primary cilia are enriched with signaling proteins such as receptors and second messenger systems that allow them to function as neuronal sensory organelles that sample the extrasynaptic space (Koemeter-Cox et al, 2014; Louvi and Grove, 2011; Whitfield, 2004). This could be an important mechanism for integrating extracellular signals over a different time scale than that associated with synaptic neurotransmission. 5-HT6 receptors on neuronal primary cilia are well positioned for detecting the extrasynaptic effects of 5-HT (Whitfield, 2004), rendering them unique targets for modulating neuronal plasticity without interfering with momentary synaptic events. Here, we demonstrate that 5-HT6 receptors preferentially localize to primary cilia and regulate primary cilia morphology. We also find that the level of 5-HT6 receptor expression dictates receptor localization, and that the mechanism leading to the unique neuronal primary cilia localization is more complicated than previously described.
We focused on striatal neurons since nearly all striatal medium spiny neurons express 5-HT6 receptors in rats (Helboe et al, 2015) although, this has not been directly measured in mouse brain, and due to the importance of 5-HT6 receptors in drug reward and procedural learning mechanisms (Brodsky et al, 2016; Eskenazi et al, 2015b). We observed no effect of the addition of a 5-HT6 agonist on primary cilia length, but 5-HT6 antagonists reduce striatal primary cilia length in a time- and concentration-dependent manner. There are at least two possible explanations for this. First, the 5-HT6 receptor has been reported to have constitutive activity (Brouard et al, 2015; Duhr et al, 2014; Jacobshagen et al, 2014; Sebben et al, 1994) and there may be a “ceiling effect” in which additional receptor activation produces no further elongation of primary cilia. The lack of effect of 5-HT6 overexpression or added agonist on cilia length is consistent with this hypothesis. Second, the primary neurons were cultured in the presence of B27 supplement and NeurobasalA, which can contain a small amount of serotonin. Because 5-HT6 receptors have moderately high affinity for 5-HT (Monsma Jr. et al, 1993), they might be activated by trace levels of serotonin. While we observed constitutive activity in our functional assay of cAMP accumulation following heterologous overexpression of the receptor, constitutive activity at 5-HT6 receptors has been described even in native tissues recently (Deraredj Nadim et al, 2016).
Regulation of cilia length is thought to modulate the impact of ciliary signaling (Marley and von Zastrow, 2012), with longer cilia allowing greater signaling capacity by the receptor systems within. The effect of 5-HT6 receptors on primary cilia morphology is particularly interesting because altered primary cilia length is regulated during key stages in development and is often associated with genetic ciliopathies (Armato et al, 2013; Chakravarthy et al, 2012; Pan and Snell, 2007). Another phenotype often seen in ciliopathies is a reduction in the number of neurons possessing primary cilia (Kulaga et al, 2004; Lee and Gleeson, 2011; Novarino et al, 2011; Ross et al, 2005). 5-HT6 receptor antagonism did not change the proportion of neurons bearing primary cilia nor did it affect dendritic outgrowth. This lack of effect of 5-HT6 on dendritic morphology differs from results in a previous report (Duhr et al, 2014), perhaps because of different culturing conditions and that expression of endogenous 5-HT6 receptors is significantly less than the dramatic exogenous overexpression in prior studies.
Using cultured 5-HT6KO neurons, 5-HT6 drugs did not alter cilia length, indicating that the effects of SB-399885 were dependent upon endogenous 5-HT6 receptors on the WT neurons. Although we were able to confirm that the antagonist effects were absent in striatal neurons cultured from 5-HT6KO mice, these germ-line null mutants had normal cilia length, perhaps due to developmental compensation that has been described for 5-HT6 effects on neuronal migration (Dayer et al, 2015). These data implicate 5-HT6 receptors in the dynamic regulation of neuronal primary cilia length, and provide another target, along with lithium, for studying inducible changes to primary cilia morphology (Miyoshi et al, 2009; Ou et al, 2009).
Heterologous expression of 5-HT6 receptors via in utero electroporation of embryonic neocortical neurons was previously reported to dramatically elongate primary cilia and change dendritic morphology (Guadiana et al, 2013). We expressed heterologous 5-HT6 receptors in both WT and 5-HT6KO striatal primary neurons after seven days of culture using lipofection and found the subcellular distribution of heterologously expressed 5-HT6 receptors was dependent on the amount of receptor plasmid introduced to the neurons, while primary cilia length was unaffected. The previous report studied neocortical neurons that were transfected by electroporation in utero and then cultured; they found that even mutant, nonfunctional receptors produced elongation of up to several hundred percent, along with other morphological and phenotypic anomalies such as branching cilia and a loss of AC3 expression. We did not observe these effects and others have shown that loss of AC3 markedly elongates olfactory cilia (Challis et al, 2016). Therefore, it is possible that altering 5-HT6 receptor expression alone has more modest effects on cilia morphology than disrupting GαS signaling in cilia altogether.
We found that transfection with increasing amounts of exogenous 5-HT6 receptor led to localization outside of primary cilia in both WT and 5-HT6KO cultured striatal neurons. Other investigators have used heterologous overexpression of ciliary receptors to study the effects on primary cilia, but to our knowledge the observation of gene dose-dependent mis-localization of protein has not been previously addressed. Here, we find that moderate to high levels of expression impact the fidelity of ciliary localization in primary neurons, perhaps due to overloading of the ciliary protein trafficking machinery. This is consistent with the notion that the extent of localization to primary cilia is more dependent on the trafficking machinery than on the total amount of protein expressed (McGlashan et al, 2010). Additionally, cilia length was unaffected by reintroducing 5-HT6 receptors into 5-HT6KO striatal neurons or increasing 5-HT6 expression in WT neurons. It is possible that cilia localization depends on the maturity of the neurons, and so studying them in neonatal neurons grown in culture for 10-14 days provides new perspective on 5-HT6 trafficking and signaling, as we did not observe the lengthening that others saw even with receptors deemed as nonfunctional. It is also possible that the effect of overexpression of 5-HT6 receptors in primary cilia may have cell type-specific effects, as we did not observe disrupted cilia morphology in striatal neurons as has been observed in neocortical neurons.
A five amino acid consensus sequence on the i3 loop, denoted the CTS, has been found in many GPCRs that traffic into primary cilia (Berbari et al, 2008; Nachury et al, 2010; Nagata et al, 2013). The 5-HT6 receptor is the only serotonin receptor that contains this motif and no other serotonin receptors have been reported to traffic to cilia. Based on these findings, we designed two mutant 5-HT6 receptors that disrupted the CTS in different ways and were predicted to block cilia trafficking in order to study the effect of 5-HT6 receptors signaling inside or outside of neuronal primary cilia. By deleting the CTS or by swapping the i3 loop from a non-cilia localizing receptor into the 5-HT6 receptor, we found that these changes significantly reduced, but did not entirely abolish, trafficking of the receptor to cilia. Additionally, cilia length was unaffected by reintroducing these mutant 5-HT6 receptors into 5-HT6KO striatal neurons. One possible explanation for how mutant receptors might gain access to cilia is through dimerization with endogenous 5-HT6 receptors; however, trafficking of the mutant 5-HT6 receptors in 5-HT6KO neurons was not substantially different than in WT neurons, so dimerization (at least with endogenous 5-HT6 receptors) is unlikely to explain trafficking to cilia. It remains possible that 5-HT6 receptors heterodimerize with some other cilia-localizing GPCR but that seems less likely since receptors heterodimerize rather freely but very few receptors gain access to cilia.
3.1 Conclusions
Together, our results implicate a role of 5-HT6 receptor signaling in primary cilia morphology. Our findings suggest that 5-HT6 receptor research should consider relative levels of receptor expression when evaluating their impact on primary cilia function. Future studies can also examine how 5-HT6 receptor signaling alters neuronal function and interacts with signaling via other cilia-localizing receptors; together such studies may provide novel avenues for treating a variety of neuropsychiatric diseases (Marley and von Zastrow, 2012).
4. Experimental Procedures
4.1 Animals
All animal procedures were approved by the University of Washington's Institutional Animal Care and Use Committee and were carried out in accordance with National Institutes of Health guidelines of the “Principles of Laboratory Animal Care” (NIH publication no. 86–23, 1996). Care was taken to minimize animal discomfort. 5-HT6KO mice on a C57BL/6 background (generously provided by Dr. Lawrence Tecott) (Bonasera et al, 2006) or WT C57BL/6 mice (Jackson Labs, Sacramento, CA) were housed for breeding with two females and one male per cage with access to food and water available ad libitum. 5-HT6KO mice were genotyped by polymerase chain reaction performed on tail DNA utilizing 5′GCCATGCTGAACGCGCTG as the forward primer upstream of the Htr6 null mutation and either 5′GCACCCAGGATGAGCGC or 5′TGCCCCAAAGGCCTACCCGCTTCC as the reverse primer for WT or 5-HT6KO, respectively.
4.2 Neuronal cell culture
Primary dissociated striatal cultures containing a small number of cortical neurons were generated from either postnatal day 0–1 C57BL/6 mice or postnatal day 0–1 5-HT6 KO mice (Bonasera et al, 2006). Dissociation and culture method were adapted from previously described protocols (Brewer, 1997; Lesiak et al, 2015; Pratt et al, 2011). Cells were plated at a density of 7 × 104 cells per cm2 on culture dishes pre-coated with poly-L-lysine (Sigma; molecular weight 300,000). Cultures were maintained in growth media consisting of Neurobasal-A (NBA) medium (Life Technologies, Carlsbad, CA) supplemented with B27 and Glutamax (Life Technologies) throughout treatment days. From the fourth day in vitro (DIV) until homogenization or fixation, culture medium was supplemented with 1 μM Ara-C (Sigma), a mitotic inhibitor. This results in cultures consisting of approximately 70% neurons and 30% glia. Cultures were maintained at 37°C in 5% CO2 from DIV0 until homogenization or fixation.
4.3 Drugs and Drug Treatments
Selective 5-HT6 receptor agonist WAY-208466 (N-[2-[3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-1-yl]ethyl]-N,N-dimethylamine) and selective 5-HT6 receptor antagonists SB-399885 (N-[3,5-dichloro-2-(methoxy)phenyl]-4-(methoxy)-3-(1-piperazinyl)benzenesulfonamide) HCl and SB-258585 (4-Iodo-N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]benzenesulfonamide) were obtained from Tocris Biosciences. Unless noted otherwise, drugs were diluted in warm neuronal growth medium and administered directly to primary neuronal cultures on DIV9.
4.4 Transfection/Plasmids
Cultured neurons were transfected on DIV7 using Lipofectamine 2000 (Thermo Fisher Scientific). Lipofectamine 2000 was added to warm NBA media (0.02 μL/μL) and incubated for 5 minutes before combining with pre-mixed plasmid DNA (50 μL/well). Total plasmid DNA for transfections always consisted of 1 μg/well (24-well plate), unless otherwise noted. The percentage of total plasmid transfected for each plasmid-combination used in the experiments was as follows: 25% 5-HT6 receptor or mutant 5-HT6 receptors (unless specified otherwise), 25% fluorescent protein reporter (hSyn-RFP or Clover), and for each condition empty plasmid vector (pCAGGS) was added to the plasmid mix to reach 100% of total transfected plasmid. For dendritic analysis pCAGGS-Map2B-RFP plasmid was used in place of fluorescent reporter to identify dendritic branching. Lipofectamine 2000/NBA/DNA mix was incubated at room temperature for 20 minutes, while native culture media was collected and kept at 37°C and replaced with growth medium. Lipofectamine 2000/NBA/DNA mix was added to plates (50 μL/well), and incubated for 35–40 minutes before transfection media was aspirated and replaced with original culture media. The plasmids containing the varied 5-HT6 receptors (Figure 5) were all tagged with a hemagglutinin (HA) epitope tag on the N-terminus of the receptor sequence via PCR cloning (Ferguson et al, 2008; Mitchell et al, 2007), and express under the CMV promoter.
4.5 Immunohistochemistry
Cultured neurons were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) / PHEMS (Sigma-Aldrich) buffer on DIV10 for 20 minutes at room temperature. Neurons were then permeabilized with 1×PBS/0.5% Triton-×100 for 10 minutes at room temperature, and blocked in 5% Bovine Serum Albumen (BSA) in PBS for 1 hour at 4°C. Cultured cells were incubated overnight at 4 °C with either of the following primary antibodies in PBS with 1% BSA: anti-adenylyl cyclase III (AC3) rabbit polyclonal antibody (sc-588; Santa Cruz Biotechnology, Santa Cruz, CA), used at 1:1000, anti- ADP-ribosylation factor-like protein 13B (Arl13b) mouse monoclonal antibody (73-287; NeuroMab, Davis, CA), used at 1:1000, anti-SR-6 (5-HT6) (A-20) raised in goat (sc-26668; Santa Cruz Biotechnology, Santa Cruz, CA), used at 1:1000, or anti-HA (1:1000, rabbit, Cell Signaling). Next, coverslips were rinsed briefly four times in PBS and incubated in species-appropriate Alexa 488 (green) and/or Alexa 568 (red)-conjugated donkey secondary antibodies (1:400, Invitrogen) for 1-2 hours at room temperature. Coverslips were washed 3 times in PBS, mounted on slides and using ProLong Gold Antifade media containing DAPI (Invitrogen, Carlsbad, CA).
Floating sections (40 μm) from adult mice (12-16 weeks old) were washed in 0.5% Triton-X/PBS for 10 min, then blocked in 10% normal goat serum (NGS)-Triton-X/PBS for 1 h. Sections were then incubated in 5% NGS-Triton-X/PBS containing anti-adenylyl cyclase III (ACIII) rabbit polyclonal antibody (sc-588; Santa Cruz Biotechnology, Santa Cruz, CA), used at 1:400 and anti-SR-6 (5-HT6) (A-20) raised in goat (sc-26668; Santa Cruz Biotechnology, Santa Cruz, CA), used at 1:400 with gentle agitation at 4°C overnight. Next, sections were rinsed 4 times in PBS and incubated with Alexa 488 or Alexa 568, conjugated goat anti-rabbit secondary antibody (1:250, Invitrogen, Carlsbad, CA) for 2 h. Sections were washed 3 times in PBS, mounted on slides and cover-slipped with ProLong Gold Antifade mounting medium.
4.6 Image Analysis
Immunostained primary cultured neurons or brain sections were imaged as stacks using either a Leica inverted widefield fluorescence microscope and associated Metamorph software or a Leica TCS SL Confocal at the University of Washington Keck Microscopy Facility. Stacked images were analyzed as maximum z-projections and images were analyzed using the NeuronJ plugin of the ImageJ software for cilia length or dendritic branching (Schneider et al, 2012; Wayman et al, 2012). Unless noted otherwise, primary cilia measurements from neurons were averaged from neurons on each culture coverslip and those coverslip averages were used as each biological replicate.
4.7 Cyclic AMP analysis
Cyclic adenosine monophosphate (cAMP) accumulation was measured using IMCD-3 kidney cells (ATCC, Manassas, VA) as they possess primary cilia (Mai et al, 2005); they were maintained in Dulbecco's modified eagle medium: nutrient mixture F-12 (DMEM/F12) media supplemented with 10% FBS, 1.2 g/l of sodium bicarbonate, and 0.5 mM sodium pyruvate (Invitrogen). Cells were plated on 6-well plates at 100,000 cells per well and allowed to grow to overnight. On the second day IMCD3 cells were transfected using lipofectamine 2000 with plasmids containing 5-HT6 receptors or our mutants and allowed to grow in the absence of serum for two days. The cells were then lysed with 0.1 M HCl, and then cellular cAMP levels were measured using an enzyme-linked immunosorbent assay-based detection kit (Cayman Chemicals, Ann Arbor, MI) according to the manufacturer's directions.
4.8 Data Analysis
Statistical analysis, non-linear regression modeling, and IC50 calculations were performed using GraphPad Prism. Significance for multiple comparisons was assessed with one-way analyses of variance (ANOVA) followed by Tukey post hoc tests. For analysis involving only two samples we used a two-tailed t-test. Chi-square (χ2) and Fisher's exact test were used where appropriate to evaluate contingency tables of categorical data done in GraphPad Prism. For all comparisons, we used an alpha value of 0.05.
Supplementary Material
Supplemental Figure 1. 5-HT6 receptors localize to mouse hippocampal primary cilia in vivo. A) AC3 immunostaining (green, left panel) in CA1 hippocampal neurons from wild-type mouse brains is restricted to cilia, and immunostaining of endogenous 5-HT6 receptors is present in a subset of these cilia (red, second panel). Overlayed images show that 5-HT6 immunostaining colocalizes with AC3 to cilia (third panel); the right panel shows the overlay at higher magnification. Note that there is no 5-HT6 immunostaining of cell bodies in hippocampal neurons, as is the case in striatum (Figure 1). B) While AC3 immunostaining of cilia is apparent in CA1 hippocampal neurons from 5-HT6KO, there is no 5-HT6 receptor detected despite normal distribution of AC3.
5-HT6 receptor antagonists reduce the length of primary cilia in striatal neurons.
High levels of heterologous expression impact trafficking of 5-HT6 receptors to cilia.
Mutations in the 5-HT6 receptor's third cytoplasmic loop alter cilia trafficking.
Acknowledgments
Support for this work was funded by NIDA Training Grant T32-DA00007278 (MB, AL), R01 DA030807 (JFN) and R21 DA035577. The authors gratefully acknowledge the technical support offered by Michele Kelly, Mike Alhquist, and Connor Nathe. We thank Laurence Tecott for providing the 5-HT6 receptor knock out mice.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT6
5-hydroxytryptamine6
- AC3
Adenylyl Cyclase 3 (marker of primary cilia)
- cAMP
Cyclic adenosine Monophosphate
- hSyn
Human Synapsin-1 promoter
- RFP
Red fluorescence protein
- WT
Wild-type
- 5-HT6KO
5-HT6 knockout
- CMV
Cytomegalovirus promoter
- pcDNA
Plasmid cytomegalovirus promoter deoxyribonucleic acid
- CTS
Ciliary targeting sequence
- i3 loop
Third intracellular loop
- DIV
Days in vitro
- NBA
Neurobasal-A medium
- PBS
Phosphate buffered saline
- BSA
Bovine serum albumin
- Arl13b
ADP-ribosylation factor-like protein 13B (marker of primary cilia)
- HA
Hemagglutinin tag
- Δ5-HT6 No CTS
5-HT6 receptor mutated to remove CTS
- Δ5-HT6 i3-7
5-HT6 receptors mutated to swap i3 loop with that of 5-HT7 receptor
- IMCD3
Inner medullary collecting duct
- FBS
Fetal bovine serum
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth C. Tails of the unexpected. Nature. 2007;448:638–641. doi: 10.1038/448638a. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR. Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. 2012;520:848–73. doi: 10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armato U, Chakravarthy B, Pacchiana R, Whitfield JF. Alzheimer's disease: An update of the roles of receptors, astrocytes and primary cilia (Review) Int J Mol Med. 2013;31:3–10. doi: 10.3892/ijmm.2012.1162. [DOI] [PubMed] [Google Scholar]
- Avasthi P, Marley A, Lin H, Gregori-Puigjane E, Shoichet BK, von Zastrow M, et al. A chemical screen identifies class a g-protein coupled receptors as regulators of cilia. ACS Chem Biol. 2012;7:911–9. doi: 10.1021/cb200349v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2012;83:S30–42. doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Bishop GA, Askwith CC, Lewis JS, Mykytyn K. Hippocampal Neurons Possess Primary Cilia in Culture. 2007;1100:1095–1100. doi: 10.1002/jnr.21209. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of Ciliary Localization Sequences within the Third Intracellular Loop of G Protein-coupled Receptors. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop Ga, Berbari NF, Lewis JS, Mykytyn K. Type III Adenylyl Cyclase Localizes to Mouse Brain. J Comp Neurol. 2007;571:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Chu HM, Brennan TJ, Tecott LH. A null mutation of the serotonin 6 receptor alters acute responses to ethanol. Neuropsychopharmacology. 2006;31:1801–13. doi: 10.1038/sj.npp.1301030. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Vergé D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–5. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Gibson AW, Smirnov D, Nair SG, Neumaier JF. Striatal 5-HT6 Receptors Regulate Cocaine Reinforcement in a Pathway-Selective Manner. Neuropsychopharmacology. 2016;41:2377–2387. doi: 10.1038/npp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard JT, Schweimer JV, Houlton R, Burnham KE, Quérée P, Sharp T. Pharmacological Evidence for 5-HT 6 Receptor Modulation of 5-HT Neuron Firing in Vivo. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00061. 150410150506007. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B, Gaudet C, Ménard M, Brown L, Atkinson T, LaFerla FM, et al. Reduction of the immunostainable length of the hippocampal dentate granule cells' primary cilia in 3×AD-transgenic mice producing human Aβ1-42 and tau. Biochem Biophys Res Commun. 2012;427:218–222. doi: 10.1016/j.bbrc.2012.09.056. [DOI] [PubMed] [Google Scholar]
- Challis RC, Tian H, Yin W, Ma M. Genetic ablation of type III adenylyl cyclase exerts region-specific effects on cilia architecture in the mouse nose. PLoS One. 2016;11:4–11. doi: 10.1371/journal.pone.0150638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codony X, Vela JM, Ramírez MJ. 5-HT6 receptor and cognition. Curr Opin Pharmacol. 2011;11:94–100. doi: 10.1016/j.coph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–69. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Jacobshagen M, Chaumont-Dubel S, Marin P. 5-HT6 Receptor: A New Player Controlling the Development of Neural Circuits. ACS Chem Neurosci. 2015 doi: 10.1021/cn500326z. [DOI] [PubMed] [Google Scholar]
- Deraredj Nadim W, Chaumont-Dubel S, Madouri F, Cobret L, De Tauzia ML, Zajdel P, et al. Physical interaction between neurofibromin and serotonin 5-HT 6 receptor promotes receptor constitutive activity. Proc Natl Acad Sci. 2016;113:12310–12315. doi: 10.1073/pnas.1600914113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domire JS, Green Ja, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci. 2011;68:2951–60. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr F, Déléris P, Raynaud F, Séveno M, Morisset-Lopez S, Mannoury la Cour C, et al. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol. 2014;10:590–7. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- Eskenazi D, Brodsky M, Neumaier JF. Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience. 2015a;299:97–106. doi: 10.1016/j.neuroscience.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi D, Brodsky M, Neumaier JF. Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience. 2015b;299:97–106. doi: 10.1016/j.neuroscience.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–4. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry. 2008;63:207–13. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Fone KCF. An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology. 2008;55:1015–1022. doi: 10.1016/j.neuropharm.2008.06.061. [DOI] [PubMed] [Google Scholar]
- Fukuo Y, Kishi T, Yoshimura R, Kitajima T, Okochi T, Yamanouchi Y, et al. Serotonin 6 receptor gene and mood disorders: case-control study and meta-analysis. Neurosci Res. 2010;67:250–5. doi: 10.1016/j.neures.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Gerard C, Martres M, Lefevre K, Verge D, Lanfumey L, Doucet E, et al. Immunolocalization of serotonin 5-HT 6 receptor-like material in the rat central nervous system. 1997:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- Green Ja, Gu C, Mykytyn K. Heteromerization of ciliary G protein-coupled receptors in the mouse brain. PLoS One. 2012;7:e46304. doi: 10.1371/journal.pone.0046304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bonnin A, Fillion MP, Ruat M, Traiffort E, Fillion G. Characterization of 5-ht6 receptor and expression of 5-ht6 mRNA in the rat brain during ontogenetic development. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:393–400. doi: 10.1007/pl00005184. [DOI] [PubMed] [Google Scholar]
- Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, et al. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci. 2013;33:2626–38. doi: 10.1523/JNEUROSCI.2906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Ph D, Doucet E, Ph D, Lefèvre K, Ph D, et al. Antibodies and Antisense Oligonucleotide for Probing the Distribution and Putative Functions of Central 5-HT 6 Receptors. Neuropsychopharmacology. 1999;21:68–76. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Helboe L, Egebjerg J, de Jong IEM. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: a double in situ hybridization study. Neuroscience. 2015;310:442–454. doi: 10.1016/j.neuroscience.2015.09.064. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, et al. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, et al. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553:109–19. doi: 10.1016/j.ejphar.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Jacobshagen M, Niquille M, Chaumont-Dubel S, Marin P, Dayer A. The serotonin 6 receptor controls neuronal migration during corticogenesis via a ligand-independent Cdk5-dependent mechanism. Development. 2014;141:3370–3377. doi: 10.1242/dev.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–51. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koemeter-Cox AI, Sherwood TW, Green Ja, Steiner Ra, Berbari NF, Yoder BK, et al. Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A. 2014;111:10335–40. doi: 10.1073/pnas.1403286111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Fashingbauer LA, Heidmann DEA, Guthrie CR, Hamblin MW. Cloning of the mouse 5-HT 6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Mol Brain Res. 2001;90:110–117. doi: 10.1016/s0169-328x(01)00090-0. [DOI] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 2011;3:1–9. doi: 10.1186/gm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak AJ, Brodsky M, Neumaier JF. RiboTag is a flexible tool for measuring the translationalstate of targeted cells in heterogeneous cell cultures. Biotechniques. 2015;58:308–317. doi: 10.2144/000114299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Grove Ea. Cilia in the CNS: the quiet organelle claims center stage. Neuron. 2011;69:1046–60. doi: 10.1016/j.neuron.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub MR, Stearns T. Supernumerary Centrosomes Nucleate Extra Cilia and Compromise Primary Cilium Signaling. Curr Biol. 2012;22:1628–1634. doi: 10.1016/j.cub.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai W, Chen D, Ding T, Kim I, Park S, Cho S, et al. Inhibition of Pkhd1 Impairs Tubulomorphogenesis of Cultured IMCD Cells. Mol Biol Cell. 2005;16:4398–4409. doi: 10.1091/mbc.E04-11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PLoS One. 2012;7:e46647. doi: 10.1371/journal.pone.0046647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, et al. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–33. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–30. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009;388:757–62. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata A, Hamamoto A, Horikawa M, Yoshimura K, Takeda S, Saito Y. Characterization of ciliary targeting sequence of rat melanin-concentrating hormone receptor 1. Gen Comp Endocrinol. 2013;188:159–165. doi: 10.1016/j.ygcen.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–9. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn Fa. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–17. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–7. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–20. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14:1112–4. doi: 10.1038/nn.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, et al. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun. 1993;193:268–76. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. HISTORICAL commentary NIH Image to ImageJ : 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebben M, Ansanay H, Bockaert J, Dumuis A. 5-HT6 receptors positively coupled to adenylyl cyclase in striatal neurones in culture. Neuroreport. 1994;5:2553–2557. doi: 10.1097/00001756-199412000-00037. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Susa T, Kato T, Kato Y. Reproducible transfection in the presence of carrier DNA using FuGENE6 and Lipofectamine 2000. Mol Biol Rep. 2008;35:313–319. doi: 10.1007/s11033-007-9088-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li V, Chan GCK, Phan T, Nudelman AS, Xia Z, et al. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:1–11. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, et al. PCB-95 Modulates the Calcium-Dependent Signaling Pathway Responsible for Activity-Dependent Dendritic Growth. Environ Health Perspect. 2012;120:1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN, Wang aM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- Whitfield JF. The neuronal primary cilium--an extrasynaptic signaling device. Cell Signal. 2004;16:763–7. doi: 10.1016/j.cellsig.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Windfeld K, Colding-Jorgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13:1092–1099. doi: 10.1016/S1474-4422(14)70198-X. [DOI] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim HJ, Kostenis E, Kim J, II, Seong JY, et al. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]
- Yun HM, Rhim H. The serotonin-6 receptor as a novel therapeutic target. Exp Neurobiol. 2011;20:159–68. doi: 10.5607/en.2011.20.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. 5-HT6 receptors localize to mouse hippocampal primary cilia in vivo. A) AC3 immunostaining (green, left panel) in CA1 hippocampal neurons from wild-type mouse brains is restricted to cilia, and immunostaining of endogenous 5-HT6 receptors is present in a subset of these cilia (red, second panel). Overlayed images show that 5-HT6 immunostaining colocalizes with AC3 to cilia (third panel); the right panel shows the overlay at higher magnification. Note that there is no 5-HT6 immunostaining of cell bodies in hippocampal neurons, as is the case in striatum (Figure 1). B) While AC3 immunostaining of cilia is apparent in CA1 hippocampal neurons from 5-HT6KO, there is no 5-HT6 receptor detected despite normal distribution of AC3.