Abstract
Background
Childhood growth stunting is negatively associated with cognitive and health outcomes, claimed to be irreversible after age 2.
Aim
To estimate growth rates for children 2 ≤ age ≤ 7 who were stunted (sex-age standardized z-score [HAZ] <−2), marginally-stunted (−2≤ HAZ ≤ −1), or not-stunted (HAZ >−1) at baseline and tracked annually until age 11; frequency of movement among height categories; and variation in height predicted by early childhood height.
Participants/methods
We used a nine-year annual panel (2002–2010) from a native Amazonian society of horticulturalists-foragers (Tsimane’; n=174 girls; 179 boys at baseline) is used. We used descriptive statistics and random-effect regressions.
Results
We found some evidence of catch-up growth in HAZ but persistent height deficits. Children stunted at baseline improved 1 HAZ unit by age 11, and had higher annual growth rates than non-stunted children. Marginally-stunted boys had a 0.1 HAZ units higher annual growth rate than non-stunted boys. Despite some catch up, ~80% of marginally-stunted children at baseline remained marginally-stunted by age 11. The height deficit increased from age 2 to11. We found modest year-to-year movement between height categories.
Conclusions
The prevalence of growth faltering among the Tsimane’ has declined, but hurdles still substantially lock children into height categories.
Introduction
Childhood growth stunting is a public-health concern because in high-prevalence developing countries it is associated with higher mortality for children < age 5 years, lower educational attainment, and worse physical health, cognitive skills, and socioeconomic outcomes throughout life and, in some cases, across generations (Berkman et al. 2002, Crookston, et al. 2013, Dewey and Begum, 2011, Hoddinott et al., 2008, Hoddinott et al., 2013, Prendergast and Humphrey, 2014, Schott et al., 2013, Schwinger et al., 2016, Victora et al., 2008). Stunting, defined as being 2 standard deviations (SD) below the median age-sex standardized height of well-nourished populations (World Health Organization, 2006, 2015), is more prevalent in low-income nations and reflects disease and nutrition stress during infancy and childhood (Black et al., 2008, Victora et al., 2008). Stunting is thought by some to be irreversible after age 2 (see review in Crookston et al. (2013)), but some evidence suggests that it is reversible with improved living conditions or with public health interventions (Adair, 1999, Lundeen et al., 2014a, Prentice et al., 2013, Schott et al., 2013). These analyses have also raised the question of how to best assess catch-up growth in height based on longitudinal analyses (Cameron et al., 2005, Georgiadis et al., 2016, Leroy et al., 2015, Victora et al., 2014). Assessing the growth velocity of stunted children matters because it can provide insights into plasticity in human growth and the relative importance of environmental conditions in early life. Additionally, research suggests that growth rates during childhood may provide information about downstream health risks, including metabolic disease (Frisancho, 2003, Hoffman et al., 2000, Popkin et al., 1996).
Much of what we know about growth velocities and catch-up growth in low-income nations comes from public health trials, is confined to children <age 5, or lacks repeated annual measures from the same children for more than five consecutive years (Grantham-McGregor et al., 2007). Thus, we know little about whether children who are stunted at age 2 remain stunted through childhood, up to age 11 before the start of puberty.
Here we help fill the gaps by drawing on a nine-year annual panel (2002 – 2010) from a low-income, native Amazonian society of horticulturalists-foragers in Bolivia (Tsimane’). Our main aim is to characterize growth during ages 2–11. We focus on three sets of estimates: (1) growth rates of children 2 ≤ age ≤ 7 who were stunted (HAZ < −2), marginally-stunted (−2 ≤ HAZ ≤ −1), or not-stunted (HAZ > −1) at baseline (2002) and who were tracked annually until they reached age 11 or until the panel ended (2010); (2) the frequency of movement among the three height categories (stunted, marginally-stunted, and not-stunted); and (3) whether stunting at age 2 is predictive of subsequent short height at age 11..
Through the first set of estimates we try to answer the question of how fast children grow between ages 2 and 11 in a remote, low-income rural setting without public health interventions to redress growth faltering. We then compare growth velocities in raw units (cm/year) in our sample with growth velocities from international reference groups to explore the extent of catch-up growth and height deficits. The second set of estimates allows us to explore the amount of movement across height categories. Much has been written about the ability of stunted children to attain normal adult height, but less attention has been paid to downward movements in height categories of children who were marginally-stunted or who were not-stunted during childhood. During ages 2 through 11, are children once stunted always stunted, once not-stunted always not-stunted, or do they move among height categories in the long-term? Through the third set of estimates we explore if children at the eve of puberty are locked into their early childhood height and assess the room for public policies to redress growth faltering.
Study participants and methods
We used data from the Tsimane’ Amazonian Panel Study (TAPS), a biocultural anthropological study in which anthropometric and economic variables were measured annually from all residents in 13 villages along the Maniqui River, Department of Beni, between June and September from 2002 to 2010 (Leonard et al., 2015).
Previous studies have found that a substantial share of Tsimane’ children are growth-stunted, as are other native Amazonian societies (Godoy et al. 2010; Foster et al. 2005; Orr et al, 2001). Previous studies among Tsimane’ children have shown high levels of parasitic infections, with estimates of between 55% and 70% of children showing positive for hookworm infections (Tanner et al. 2009), and a substantial decrease in linear growth and body fat reserves from the activation of immune defenses as indicated by concentration of C-reactive protein (CRP) (McDade et al., 2008). Household-level analysis of food-use and consumption suggest that Tsimane’ meet daily energy and protein requirements (Godoy et al. 2005), so child stunting probably reflects high infectious diseases loads and marginal dietary quality and not limited food consumption. Additionally, evidence among Tsimane’ suggests that growth retardation in children has persistent effects in body composition later in life (Tanner et al., 2014).
The sample at the start of the panel (2002) included 633 adults (≥ age 16) and 820 children (< age 16). Methods of data collection have been described in earlier publications (Godoy et al., 2010, Tanner et al., 2014, Foster et al., 2005). We measured standing height following the protocol of Lohman et al. (1988), using a Seca 213 portable stadiometer, and calculated age- and sex-specific height-for-age z-scores using the World Health Organization (WHO) growth standards for children 24–60 months of age (de Onis et al., 2004, World Health Organization, 2006) and the WHO references for children > 60 months (de Onis et al., 2007). Following the WHO (2009) guidelines, HAZ measures beyond +/− 6 SDs were flagged as probably contaminated by large measurement errors and excluded from the analysis (n=6; 0.003% of total observations).
We limited the analysis to all children (girls=174, boys=179) 2 ≤ age ≤ 7 at baseline (2002) and no more than 11 years of age by 2010 because we wanted to estimate growth patterns before puberty. Restricting the upper baseline age to seven years had the advantage of ensuring that these older children were re-measured on several occasions before the end of the panel.
To analyze growth rates we computed annual changes in height in both cm and HAZ for two reasons. First, HAZ overstates the amount of catch-up growth in terms of height deficits because height standard deviations in the well-nourished reference population increase with age until middle or later adolescence (Leroy et al., 2015, Victora et al., 2014). Second, HAZ scores and height deficits had random measurement error (Godoy et al., 2008a); 10.6% of parents admitted not knowing their child’s exact age and had to guess. Most analyses of stunting consider only two height categories: stunted (HAZ < −2) and not stunted (−2 ≤ HAZ). Because we are interested in changes children might undergo between stunted and non-stunted categories over the life of the panel, we split the non-stunted category into two sub-categories: marginally-stunted (−2 ≤ HAZ ≤ −1) and not-stunted (HAZ >−1). The finer-grained sub-division allows for a more nuanced analysis of movement among height categories (Teivaanmäki et al., 2015).
For the analysis we used descriptive statistics and graphs to compare height categories at baseline with height categories later in childhood. We used the following OLS and random-effect regressions to estimate growth rates and catch-up growth:
| (Eq. 1; OLS) |
| (Eq. 2; random effects) |
where the subscripts stand for individual child (i), household (h), village (v), year (t), and baseline (b). In Eq. 1, Yihvt includes height in 2010 or at age 11 in cm and HAZ and annual change in height expressed in cm/year and HAZ/year, Hihvb indicates baseline height (cm or HAZ). Xihvt is a vector with covariates (child’s age, sex, survival or the number of times the child was measured in the panel, and village fixed-effects), and εihvt is an error term. In Eq. 2, Yihvt indicates the annual change in HAZ during 2002–2010, Sihvb is an indicator variable for baseline stunted, S′ihvb is an indicator variable for baseline marginally-stunted, Cihvt, Mhvt, Hhvt are vectors with covariates for the child (birth order, lagged weight, baseline age, gender, birth season, times the child was measured in the panel), mother (age, schooling, height, weight), and household (total children, income, wealth, area of forest cleared). In estimating Eq. 2 we use village fixed-effects. εihvt is an error term. Appendix A contains definitions of the explanatory variables used in the regressions.
Results
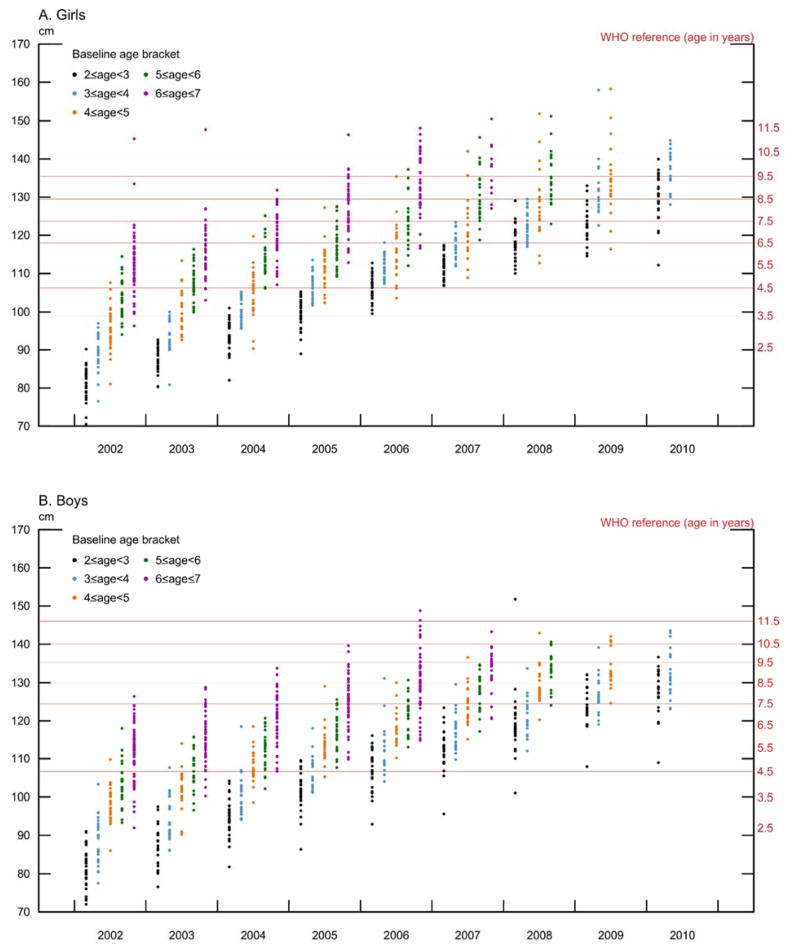
Figure 1 shows the age-based distribution of each cohort in a scatter plot, including WHO reference height at different ages for girls (Figure 1A) and boys (Figure 1B). The figures suggests that that girls and boys 2 ≤ age ≤ 7 at baseline grew steadily during the nine years of the panel, with no obvious tapering off in height for either sex or age bracket, except for the oldest children at baseline. While it is common to find growth canalization in the long-term, growth is non-linear and may show substantial variation in the short-term (Hermanussen et al. 2002). We examined this variation by estimating the correlations between heights at different ages and generally found that higher correlations between measurements at older age and shorter measurement intervals, consistent with previous findings (Cole, 1997) (Appendix B).
Figure 1.
Height Distribution for Tsimane’ children, 2 < age < 7 at baseline [2002] and no more than age 11 by 2010, measured annually.
Note: The WHO reference for age 2–5 comes from the WHO Child Growth Standards (World Health Organization, 2006; Chapter 3, Table 20, p. 43 for boys and Chapter 3, Table 29, p. 70 for girls). The WHO reference for age 5–11 comes from the 1977 National Center for Health Statistics/WHO reference data http://www.who.int/growthref/en/. The sample excludes children > 11 years of age.
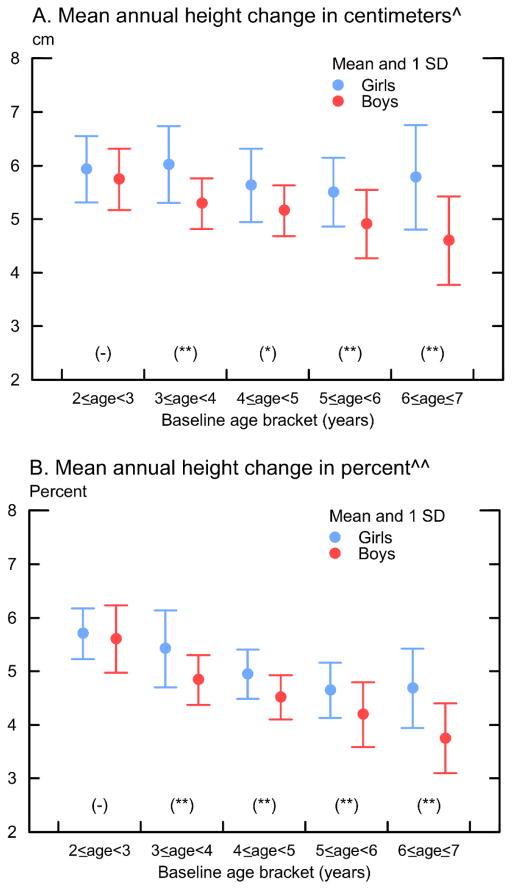
Figure 2 shows lower growth rates for older children and higher growth rates for girls than for boys. Depending on the child’s sex, older children who had been age 5–6 at baseline grew by 5.4–4.1 cm / year or 4.5–3.4% /year whereas children who had been age 2 at baseline grew by 6.1–5.8 cm / year or 5.9–5.6% /year. Over the entire period, girls grew by an average of 4.4 cm / year or 3.9% / year whereas boys grew by 3.9 cm /year or 3.5%/year (detailed standing height and annual height increments in cm during 2002–2010 by age are shown in Appendix B).
Figure 2.
Mean annual change in height for Tsimane’ children, 2 < age < 7 at baseline (2002) and no more than age 11 by 2010, measured annually
Notes: * Annual change in cm = (height at end-line –height in 2002) / number of years in between. ** Annual change in % = ln (height at end-line / height in 2002) / number of years in between. * and ** indicate significance of gender difference at < 5% and < 1%. The sample excludes children > 11 years of age.
Is the growth rate of Tsimane’ children enough to ensure convergence to international standards? To answer the question we compared growth velocities between our sample and a reference population and also estimate height deficits. In the USA, median annual growth rates between 2 ≤ age ≤ 11 were higher than among Tsimane’ children, and averaged 6.6 cm (range: 5.7 – 8.6 cm) among girls and 6.3 cm (range: 5.1 – 8.3 cm) among boys (Tanner and Davies, 1985). Thus, the difference in the average growth velocities for the two populations in favor of the reference population hints at more divergence in height at the end of the study rather than catch-up. We present further evidence for divergence in height during childhood by examining measurements of linear growth, in cm and HAZ, and height deficits from WHO reference growth tables at two and four years of age for middle-income countries from the Consortium of Health-orientated Research in Transitioning Societies (COHORTS) and the Tsimane’ (Appendix C). The results for the Tsimane’ are consistent with COHORTS countries. As the children aged, the height deficit compared to WHO standards increased, despite the overall decrease in the prevalence of stunting.
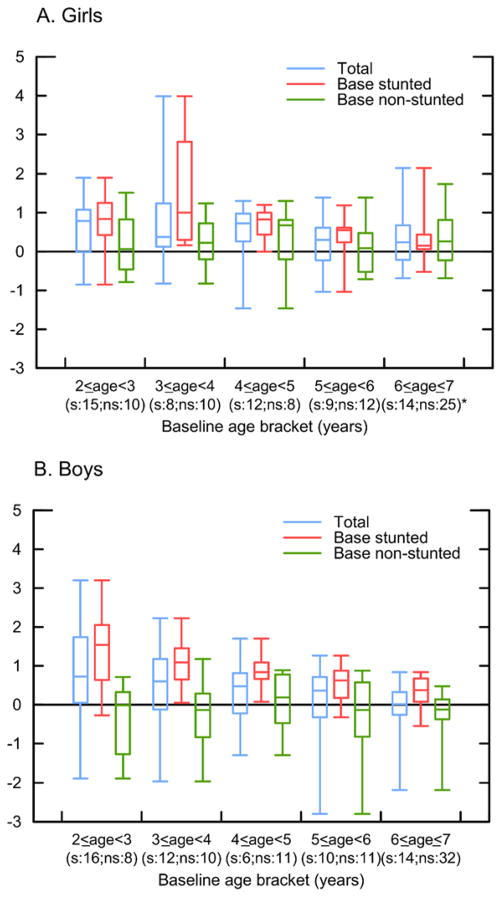
Figure 3 shows two noteworthy results. First, we find evidence of catch-up growth in HAZ (mean HAZ and annual change in HAZ during 2002–2010 by age are shown in Appendix D). The average stunted girl or boy in 2002 saw an improvement of ~1 HAZ unit by age 11 (Appendix D). The improvements in HAZ applied on average to girls and to boys of all ages. Second, Figure 3 shows that in some age brackets, children who had not been stunted at baseline experienced growth faltering on average, with more faltering among boys than among girls. The total change in HAZ between baseline and end-line (2010) grew wider (became negative) for some girls who had normal height at ages 2–3 and for some boys of normal height in all age groups. In the next section we examine within-child changes in height categories in more detail.
Figure 3.
Total change in HAZ (z-score) for Tsimane’ children, 2 < age < 7 at baseline (2002) and no more than age 11 by 2010, re-measured at end-line (A. girls, B. boys).
Notes: *s=number of observations in the base stunted category; ns=number of observations in the base non-stunted category. The box plots display the following statistics: minimum, first quartile, median, third quartile, and maximum. The sample excludes children > 11 years of age.
Table I shows growth rates and height at the end of the study for children 2 ≤ age ≤ 7 at baseline and ≤ 11 years of age during 2002 – 2010. Column A1 (section I) suggests that, for the sample of girls and boys combined, initially taller children grew at a lower rate than shorter children. Each additional cm of height at baseline was associated with a 0.02 cm / year lower annual growth rate (girls = −0.01 cm / year, p = 0.3; boys = −0.03 cm / year, p = 0.005). Column A2 (section I) suggests that baseline and end-line heights were positively associated; each additional cm of height at baseline was associated with 0.3 cm taller height at age 11 (girls = 0.3 cm, p = 0.001; boys = 0.3 cm, p = 0.001). Column B (section I) contains similar analysis, but with height expressed in HAZ. A 1-unit increase in baseline HAZ was associated with 0.07 SD / year lower growth rate in HAZ and with a 0.5 greater HAZ by age 11 or year 2010, consistent with the visual and descriptive results presented earlier. In section II of Table I we repeated the analysis of section I of the same table, but separately for each of the initial height categories. Column A1 (section II) suggests that growth rates did not vary by baseline height categories, with one exception. Among stunted children, each additional cm of baseline height was associated with a 0.03 cm / year lower growth rate. The same results appear when we express height as HAZ (Column B1, section II). A one unit higher baseline HAZ among stunted children was associated with a 0.08 SD lower HAZ growth rate (Appendix E shows a comparison of annual growth rates in the three categories). As before, we find a positive association between baseline height and height at the end of the study (Table I: Columns A2 and B2, section II). An additional cm of baseline height across any of the three height categories was associated with 0.1 cm taller height at the end of the study (column A2, section II), and an additional HAZ unit in baseline height across any of the three height categories was associated with 0.4 – 0.7 HAZ unit taller height at the end of the study.
Table I.
Height growth rates for Tsimane’ children 2≤age≤7 at baseline (2002) tracked until 2010 or until they reached age 11 (standard errors in parenthesis)
| Group | Baseline height expressed in: | Dependent variable is standing height in 2010 or at age 11 when exiting the panel; height expressed in: | |||
|---|---|---|---|---|---|
|
| |||||
| [A] Centimeters (cm) | [B] HAZ | ||||
|
|
|
||||
| [1] Change cm/year | [2] Height at the end of the study | [1] Change HAZ/year | [2] HAZ at the end of the study | ||
| [I] Pooled | ^ | ^ | |||
|
| |||||
| Centimeters | −0.02 (0.01)* | 0.33 (0.01)** | |||
|
| |||||
| R2 | 0.02 | 0.39 | ^ | ^ | |
|
| |||||
| N | 1619 | 1728 | |||
|
| |||||
| HAZ | ^ | ^ | −0.07 (0.01)** | 0.52 (0.02)** | |
|
| |||||
| R2 | ^ | ^ | 0.03 | 0.54 | |
|
| |||||
| N | 1619 | 1728 | |||
|
| |||||
| [II] Stunted, marginally-stunted, and not-stunted | |||||
|
| |||||
| Stunted | Centimeters or HAZ | −0.03 (0.01)** | 0.17 (0.02)** | −0.08 (0.03)** | 0.41 (0.05)** |
|
| |||||
| (HAZ < −2) | R2 | 0.03 | 0.21 | 0.05 | 0.28 |
|
| |||||
| N | 775 | 821 | 775 | 821 | |
|
| |||||
| Marginally-stunted | Centimeters or HAZ | −0.02 (0.02) | 0.17 (0.02)** | −0.08 (0.06) | 0.72 (0.10)** |
|
| |||||
| (−2 ≤ HAZ ≤ −1) | R2 | 0.03 | 0.28 | 0.02 | 0.22 |
|
| |||||
| N | 541 | 586 | 541 | 586 | |
|
| |||||
| Not-stunted | Centimeters or HAZ | −0.02 (0.03) | 0.15 (0.02)** | −0.07 (0.06) | 0.52 (0.05)** |
|
| |||||
| (HAZ > −1) | R2 | 0.09 | 0.71 | 0.14 | 0.67 |
|
| |||||
| N | 303 | 321 | 303 | 321 | |
Notes: OLS regressions include a child’s age, sex, and survival (number of times the child was measured in the panel), village fixed effects, constant, and robust standard errors (shown in parenthesis).
= variable intentionally left out.
significant at < 5% and < 1%. For regressions in column [B], section II, baseline height is expressed in HAZ. N denotes the number of children in each category. To account for possible age-specific non-linear changes in growth and growth velocity, we re-run all the regressions in Table I including a quadratic term for age. The results of the regressions did not change, except for an increase of 0.01 in the coefficient for not-stunted (column A1 and A2, panel [II]) which does not change our conclusions.
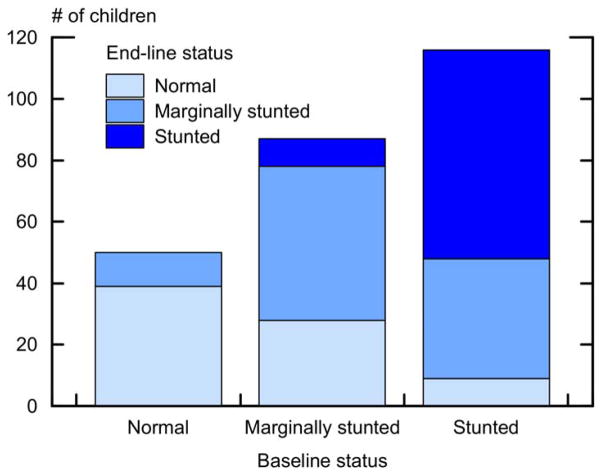
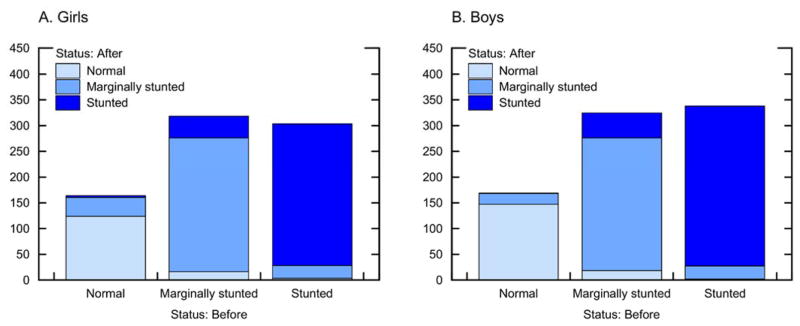
In Figure 4 we summarize changes in height category between baseline height categories when children were 2 ≤ age ≤ 7 and height categories by the end of the panel in 2010, or when the same children had reached age 11 (the specific numbers in each height category at baseline and end-line are shown in Appendix F). On average, children were more likely to recover from growth faltering when younger. For example, 32% (28 / 87) of children who had been marginally-stunted at baseline had attained normal HAZ by age 11, and 42% of children who had been stunted at baseline had become marginally-stunted (34%) or attained not-stunted HAZ (8%) by the time they had reached age 11. However, 7.9% of the sample saw either a deterioration in their HAZ status from not-stunted to marginally-stunted (n =11, 4.3% of the sample) or from marginally-stunted to stunted (n = 9, 3.6% of the sample) while an additional 68 children (26.8% of the sample) remained stunted from baseline to end-line. Of the 203 children who were stunted (n = 116) or who were marginally-stunted (n = 87) at baseline, only 37 (18%) had attained not-stunted HAZ by age 11. We found a strong relation between baseline and end-line height (Kendall’s tau-b coefficient of 0.6); 62% of children at end-line retained the same height category they had at baseline. In spite of this, there was movement among height categories (Figure 4); a significant proportion of stunted or marginally-stunted children moved to a category of higher height.
Figure 4.
Changes in height category among Tsimane’ children 2–7 years at baseline (2002) and end-line (2010) or age 11, whichever came first
In Table II we explore year-to-year changes in height categories for the same child over the period of study. Because a child could move in and out of the categories of stunted, marginally-stunted, and non-stunted over the nine years of the panel, comparing height for children 2 ≤ age ≤ 7 years at baseline with their height at age 11, as in Figure 4, glosses over year-to-year changes in their height categories that might have taken place before reaching their height at age 11. Table II and Figure 5 contain summaries of all the changes in height categories between surveys for a child, and show two results.
Table II.
Intra-subject changes in height categories between annual surveys for children 2 ≤ age ≤ 7 at baseline in 2002 and no more than 11 years old by 2010.
| Stunting status. During two surveys, child height changes: | Girls (n=174) | Boy (n=179) | Total (n=353) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| From | To | N | % | N | % | N | % |
| Not-stunted | Not-stunted | 124 | 15.8 | 147 | 17.7 | 271 | 16.8 |
|
| |||||||
| Marginally-stunted | 36 | 4.6 | 21 | 2.5 | 57 | 3.5 | |
|
| |||||||
| Stunted | 4 | 0.5 | 1 | 0.1 | 5 | 0.3 | |
|
| |||||||
| (Subtotal) | (164) | (169) | (333) | ||||
|
| |||||||
| Marginally-stunted | Not-stunted | 16 | 2.0 | 18 | 2.2 | 34 | 2.1 |
|
| |||||||
| Marginally-stunted | 260 | 33.1 | 258 | 31.0 | 518 | 32.1 | |
|
| |||||||
| Stunted | 42 | 5.4 | 48 | 5.8 | 90 | 5.6 | |
|
| |||||||
| (Subtotal) | (318) | (324) | (642) | ||||
|
| |||||||
| Stunted | Not-stunted | 3 | 0.4 | 2 | 0.2 | 5 | 0.3 |
|
| |||||||
| Marginally-stunted | 25 | 3.2 | 25 | 3.0 | 50 | 3.1 | |
|
| |||||||
| Stunted | 275 | 35.0 | 311 | 37.4 | 586 | 36.3 | |
|
| |||||||
| (Subtotal) | (303) | (338) | (641) | ||||
|
| |||||||
| Total | 785 | 100 | 831 | 100 | 1616 | 100 | |
Notes: Numbers in cells are episodes of year-to-year change in height category.
Figure 5.
Intra-subject changes in height categories between annual surveys for children 2–7 years at baseline (2002) and no more than 11 years of age by 2010
First, children seemed fairly locked into their height categories from one year to the next. For instance, the column with totals in Table II suggests that of the 333 episodes of children who had normal HAZ at some point during 2002–2009, 81% (n = 271) continued to have normal HAZ in the next annual survey, while 57 (17%) became marginally-stunted and an additional 5 children (1.5%) became stunted. The same finding appears when we consider marginally-stunted children. Of the 642 episodes of children who had been marginally-stunted during one of the annual measures, 124 episodes (19.3%) were for children who either attained normal HAZ (34 / 642 = 5.3%) or became stunted (90 / 642 = 14.0%) in the next annual survey; most (80.7%; 518 / 642) of the children who had been marginally-stunted during one annual survey continued to be marginally-stunted in the next annual survey. Last, of the 641 episodes of children with stunting at one point during the panel, 55 episodes (55 / 641=8.6%) were for children who attained normal HAZ (5 / 641 = 0.8%) or who became marginally-stunted (50 / 641=7.1%) in the next survey. Of all 1616 episodes of annual change for all the children, 1375 episodes (85.1%) were for children who remained in their height category from one annual survey to the next annual survey, 89 episodes (5.5%) captured children who improved their HAZ status, and 152 episodes (9.4%) were for children who experienced a worsening of their HAZ status from one survey to the next. Even though there were changes in HAZ from one year to the next, as shown earlier with the annual growth rates, most of these changes had to do with children changing height within a category (e.g., a stunted children becoming taller, but still remaining stunted), instead of moving across categories (e.g., a stunted child attaining normal height). The second noteworthy finding of Table II is that both girls and boys were likely to experience about the same mobility between height categories.
These results then raise two questions: to what extent do baseline biological and socioeconomic conditions during early childhood lock children into their height at age 11? And to what extent do baseline conditions affect change? Table III contains regression results to estimate the share of variation of height by age 11 predicted by antecedent child heights. The results suggest that early childhood height predicts 50 – 70% of the variation in height by age 11. If at least half of the variation in the height of children by age 11 can be predicted by biological and socioeconomic factors during early childhood, what could explain the remaining variation?
Table III.
Share of variation in child height by age 11 explained by height during early childhood
| Explanatory variable is child height in cm at age: | Dependent variable is child height in cm at age 11 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| [A] Girls | [B] Boys | |||||
|
|
|
|||||
| Coef. (st. error) | R2 | N | Coef. (st. error) | R2 | N | |
| 3 | 0.4 (0.2)* | 0.17 | 18 | 0.9 (0.1)*** | 0.64 | 22 |
|
| ||||||
| 4 | 1.2 (0.2)*** | 0.70 | 36 | 0.9 (0.1)*** | 0.55 | 39 |
|
| ||||||
| 5 | 1.1 (0.1)*** | 0.67 | 55 | 0.8 (0.1)*** | 0.55 | 56 |
|
| ||||||
| 6 | 1.1 (0.1)*** | 0.55 | 73 | 1.0 (0.1)*** | 0.74 | 81 |
|
| ||||||
| 7 | 1.1 (0.1)*** | 0.54 | 102 | 1.0 (0.1)*** | 0.71 | 110 |
|
| ||||||
| 8 | 1.1 (0.1)*** | 0.72 | 117 | 1.1 (0.04)*** | 0.81 | 127 |
|
| ||||||
| 9 | 1.1 (0.1)*** | 0.77 | 138 | 1.0 (0.1)*** | 0.78 | 158 |
|
| ||||||
| 10 | 0.8 (0.1)*** | 0.67 | 157 | 1.1 (0.03)*** | 0.84 | 182 |
Notes: Regressions are OLS with robust standard errors (in parenthesis), clustering by child, age when child first enters the panel, and a constant.
Table IV contains regression results for the average annual change in HAZ from 2002 through 2010 with respect to baseline stunting to attempt to identify the covariates associated to child growth rates (Eq. 2). Table IV supports some of the findings presented earlier, suggesting that stunted and marginally-stunted children grew at faster rates than their age and sex peers of normal height. The coefficients of Table IV suggest that the HAZ of stunted girls at baseline grew by 0.1 HAZ units / year more than the HAZ of not-stunted girls, while the HAZ of stunted boys at baseline grew by 0.2 HAZ units / year more than the HAZ of not-stunted boys. Marginally-stunted girls and boys also had higher growth rates than their age and sex not-stunted peers, but the growth rates for marginally-stunted children were half as large as the growth rates of stunted children, and results were only significant for boys. Marginally-stunted boys had 0.1 HAZ unit higher annual HAZ growth rate than not-stunted boys. Beyond showing that the HAZ of stunted and marginally-stunted girls or boys grew at higher annual rates than the HAZ of not-stunted children, Table IV shows one striking result. Except for boys born during the dry season, none of the other covariates was associated with child growth. Boys born during the dry season had 0.05 HAZ units / year higher growth rates than boys born during other seasons.
Table IV.
Random-effect panel linear regression results for annual change in HAZ during 2002–2010 in relation to baseline (2002) stunting, controlling for child, mother, household, and community fixed effects among Tsimane’ children 2 ≤ age ≤ 7 at baseline but no more than 11 years old by 2010.
| Explanatory variables | Dependent variable: year-to-year change in height z score (HAZ) | |
|---|---|---|
|
| ||
| Girls (standard error) | Boys (standard error) | |
| I. Baseline stunting | ||
|
| ||
| Stunted | 0.14 (0.06)* | 0.21 (0.03)** |
|
| ||
| Marginally-stunted | 0.08 (0.04) | 0.11 (0.04)** |
|
| ||
| II. Child | ||
|
| ||
| Birth order | 0.01 (0.02) | 0.03 (0.02) |
|
| ||
| Lagged weight | −0.003 (0.01) | −0.003 (0.01) |
|
| ||
| Baseline Age | 0.003 (0.01) | −0.01 (0.01) |
|
| ||
| Dry-season birth | 0.009 (0.02) | 0.06 (002)* |
|
| ||
| Survival | 0.001 (0.01) | 0.009 (0.02) |
|
| ||
| III. Mother: | ||
|
| ||
| Age | −0.001 (0.002) | 0.004 (0.002) |
|
| ||
| Schooling | 0.01 (0.01) | −0.01 (0.01) |
|
| ||
| Current height | 0.006 (0.004) | 0.003 (0.002) |
|
| ||
| Current weight | −0.002 (0.002) | −0.002 (0.002) |
|
| ||
| IV. Household: | ||
|
| ||
| No. of children | −0.01 (0.02) | −0.01 (0.01) |
|
| ||
| Current income | −0.001 (0.01) | −0.01 (0.01) |
|
| ||
| Current wealth | −0.02 (0.04) | −0.003 (0.03) |
|
| ||
| Forest clearance | 0.04 (0.04) | −0.02 (0.02) |
|
| ||
| V. Constant | −0.65 (0.63) | −0.42 (0.43) |
|
| ||
| R2 overall | 0.03 | 0.05 |
|
| ||
| R2 between | 0.17 | 0.14 |
|
| ||
| R2 within | 0.01 | 0.01 |
|
| ||
| N | 769 | 789 |
Notes: For definition of variables see Appendix E. The table shows the results for the average annual change in HAZ from 2002 through 2010 with respect to baseline stunting. Regressions include clustering by child, village fixed-effects, and robust standard errors, and also includes a variable for the number of times the child was measured (survival). In section I, the excluded category is children with HAZ>−1 (not-stunted height).
p≤0.05,
≤0.01
We did additional analysis to assess the robustness of results shown in Table IV and to explore new topics. The results of these additional regressions are not shown. We included lagged child weight-for-age z scores instead of raw weight measures in kg, and found much weaker results for the rates of growth of stunted and marginally-stunted children. We added morbidity as perceived by the caretaker or the number of days the child had been confined to bed owing to illness, and found essentially the same results as those shown in Table IV. Last, we ran separate OLS regressions for girls and boys with village fixed-effects and a binary outcome: one if the child was stunted at baseline but become marginally-stunted or not-stunted by end-line, and zero if the child remained stunted at both baseline and end-line. We used all the same covariates as in Table IV. None of the covariates was significantly associated with the probability of changing height status except baseline age. Each additional year of age at baseline was associated with a 10 percentage point decrease in the probability of improving a child’s height status, with about the same magnitudes for girls and boys.
Discussion
The results of this study show some evidence of catch-up growth, but persistent height deficits. Children who were stunted and marginally stunted at baseline saw higher annual growth rates than not-stunted children. However, about 80% of marginally-stunted children remained so by age 11. We found increased height deficits from age 2 to 11 and modest movement between height categories. Most height variation at age 11 was predicted by height in early childhood. In this section we discuss three topics. First, how much catch-up growth and growth deficit do we see by age 11? Second, what might explain the mobility of children among height categories (beyond the child’s age at baseline)? Last, we discuss some of the main limitations of our study.
Catch-up growth and growth deficit by age 11
We found some evidence of partial catch-up growth. Each cm shorter height at baseline was associated with 0.02 cm / year higher growth rate. Stunted children at baseline grew by 0.02 cm / year more than not-stunted children (Table I). 41% of children stunted at baseline became marginally-stunted or attained normal height by the time they had reached age 11 (Figure 4 and Appendix F). Stunted children had higher HAZ growth rates than not-stunted children, and children who had been marginally-stunted at baseline saw small improvement of HAZ by the time they reached age 11. These results are in broad agreement with growing evidence from current low-income and middle-income nations showing evidence of catch-up growth (Coly et al., 2006, Crookston et al., 2010, Crookston et al., 2013, Hirvonen, 2014, Outes and Porter, 2013, Said-Mohamed et al., 2015, Schott et al., 2013, Victora et al., 2008). However, the comparison of the growth rate of Tsimane’ children and the USA hints at more divergence in height rather than catch-up at the end of the study.
The absence of convergence in height might be traceable to the absence of economic transformations in this remote rural economy. The mean monetary daily earnings per person toward the end of the panel reached only $0.9, about half the global poverty line (US$1.9 Purchasing Power Parity) used by the World Bank (2015). Low income and the absence of public-health interventions geared to redressing growth faltering could explain the deficit. That said, the share of stunted Tsimane’ children has fallen. In 2000 43% of Tsimane’ girls and 52% of Tsimane’ boys <age 9 were stunted. The last survey of the panel (2010) suggests that, compared with 2000, for children <age 9, the share of girls who were stunted had dropped from 43% to 30%, and the share of boys who were stunted had dropped from 52% to 34%. Thus, although living conditions might be improving for the Tsimane’, as reflected in the declining share of stunted children, the improvements in living conditions are not large enough to reduce or eliminate the height deficits.
Mobility of children among height categories
Changes in household socioeconomic conditions might not be large and permanent enough to move children into higher height categories. For instance, we found that socioeconomic attributes of the household or human-capital attributes of the mother bore no statistically significant association with child growth rates. Possible reasons for the negligible role of household socioeconomic attributes or maternal human-capital attributes could relate to one or more of the following: (1) the widespread practice of sharing resources and reciprocity in this strongly endogamous society, (2) low levels and little variation in socioeconomic and human-capital variables related to maternal education, and (3) endogeneity, including random errors in the measure of these variables (see below). When combined, the three factors might attenuate the effect of socioeconomic and human-capital attributes on child growth.
Our results about mobility among height categories resemble results from other studies. In rural DR Congo, Kismul et al. (2014) reported low mobility among childhood height categories. In rural Malawi, Teivaanmäki et al. (2015) found a large drop in the share of stunted children from age two to age 10 (~80% to ~39%) and an increase in the share of non-stunted children from about ~15% to ~45%, but they did not find much change in the share of children who were marginally stunted. In a sample of 143 shantytown children in Lima, Peru, half the sample remained stunted from 6 months of age until age nine (Berkman et al., 2002). The Young Lives international comparison of four nations (Vietnam, Peru, India, Ethiopia) with a total sample size of 7266 children estimated changes in HAZ between ages one and eight (Crookston et al., 2013). They found that, depending on the country, 69–82% of the children did not change their height category. In addition, 18% to 32% of the sample was not-stunted at age one but then became stunted at age eight, or was stunted at age one and then became not-stunted at age eight. The estimates from the Tsimane’ are broadly consistent with these results, though they show more mobility than the results of the Young Lives Study. For example, Figure 4 (Appendix F) suggest that 62% of the Tsimane’ sample did not change their height category compared with 68–82% from the Young Lives Study, while 38% of Tsimane’ children changed their height categories compared with 18–32% from the Young Lives Study. In general, we find a fair amount of mobility as compared with the assertions of “irreversibility” and a critical window that closes at age two in prominent studies such as Victora et al. (2008, 2010)
The pattern of mobility among height categories with Tsimane’ children raises the question of why children fall into the stunted, marginally-stunted, or not-stunted category. If socio-economic conditions of households or maternal human-capital attributes do not explain much of the variation in Tsimane’ childhood growth rates between ages two and 11, then what makes children fall into one of the three categories? We have no satisfactory answer. One possibility has to do with environmental events. In an earlier publication we showed that environmental perturbations (rainfall amount and variability) during gestation and infancy was negatively associated with adult height among females (Godoy et al, 2008b). If there is an association between maternal and child height or child growth rate, then this might explain why children fall into a height category. The problem with this interpretation is that in the sample studied, maternal height bore no strong association with children growth rate. Another environmental factor could be birth season. A boy’s birth during the dry season was associated with a 0.05 HAZ units higher annual growth rate. One problem with this interpretation is why season of birth would have no effect on the growth rate of girls.
Limitations
The study rests on a small sample size and too short a duration to permit examination of adolescence, though the study contains more repeated annual observations than most similar studies. It is possible that Tsimane’ have a longer growth period than children in other settings; if so, then the amount of time to catch-up and reduce the growth deficit would be longer than the age span considered in this study. Previous research among indigenous populations of highland Peru (Frisancho and Baker, 1970) and nomadic pastoralists in Kenya (Little and Johnson Jr, 1987) have documented extended growth periods under conditions of chronic nutritional stress. However, we doubt a longer growth period would yield stronger evidence for catch-up growth and for eliminating the growth deficit because adult Tsimane’ are short (females = 151 cm, 4.8 SD, males=162.9 cm, 4.8 SD), and have shown no secular change in height during the 20th century (Godoy et al., 2006). Also, the metrics to estimate catch-up growth during late childhood and adolescence remain imperfectly developed because of the onset of puberty, although depending on the age of menarche, the sample could have included some of the changes of puberty for some females. However, based on evidence for the Tsimane’ probably most females in our sample were at least a year younger. Using data from 603 Tsimane’ girls, Hochberg et al. (2011) found that the average age of menarche was about 14 years of age. Another study (Byron, 2003), based on a smaller sample of 87 Tsimane’ girls, estimated the average age of menarche falls around 12–13 years of age. Even if we had data for a longer growth period it is unclear how we could estimate meaningful growth rates for cross-cultural comparisons (Leroy et al., 2015). We used three height categories in our analysis, to achieve a more nuanced analysis of movement among categories. The trade-off was a smaller sample size in each category, which may have resulted in less precise estimates. Also, WHO z-scores are based on the growth of healthy children from 6 countries (Brazil, Ghana, India, Norway, Oman, USA), in an effort to make the charts representative of a global population. It is possible that they do not represent growth of healthy native Amazonian children (Urlacher et al. 2016). Last, our height data possibly includes some measurement errors. For example, time intervals between measurements were approximately one year, but were not exactly the same for all participants as data gathering depended on weather conditions, accessibility to the communities, and the participant being present on the day of the survey. If so, this may have affected estimates of height at given ages and, more so, annual changes in height.
Conclusion
The fact that 50–70% of the height variation by age 11 was predicted by height in early childhood lends credence to the idea that in low-resource settings at least half of the blame or half of the credit for growth and faltering might be traceable to events during gestation and the first two years of life. We do not try to answer the question of how much of child height by age two is shaped by biological or by socioeconomic factors, but since at least half of the variation in height by age 11 remains unaccounted for by height during early childhood one might reasonably conclude that there is room after age two to redress growth faltering, though the long-term health consequences of promoting linear growth after age two remain contested (Victora et al., 2014).
Supplementary Material
Acknowledgments
The views expressed in this article do not necessarily represent the views of the Federal Reserve Board or the United States. We thank the following institutions for grant support: (1) Cultural Anthropology Program of the USA National Science Foundation (BCS: 0650378, 0552296, 0200767, 0111905), (2) Bill & Melinda Gates Foundation (Global Health Grant OPP1032713), (3) Eunice Shriver Kennedy National Institute of Child Health and Development (Grant R01 HD070993), and (4) Grand Challenges Canada (Grant 0072-03). The study received IRB approval from Northwestern University (Study 00007), and the Consejo Tsimane’ (governing body of the Tsimane’). We would also like to thank the Consejo Tsimane’ for continuous support and two AHB referees for helpful comments on earlier drafts.
Footnotes
Declaration of interest statement
The authors declare they have no conflict of interest.
References
- Adair LS. Filipino children exhibit catch-up growth from age 2 to 12 years. J Nutr. 1999;129:1140–1148. doi: 10.1093/jn/129.6.1140. [DOI] [PubMed] [Google Scholar]
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, Mathers C, Rivera J, Maternal &, Group CUS. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Byron EM. PhD Dissertation. Department of Anthropology, University of Florida; 2003. Market Integration and Health: The Impact of Markets on the Nutritional Status, Morbidity, and Diet of the Tsimane’ Amerindians of Lowland Bolivia. [Google Scholar]
- Cameron N, Preece MA, Cole TJ. Catch-up growth or regression to the mean? Recovery from stunting revisited. Am J Hum Biol. 2005;17:412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]
- Cole T. Growth monitoring with the British 1990 growth reference. Arch Dis Child. 1997;76:47–49. doi: 10.1136/adc.76.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coly AN, Milet J, Diallo A, Ndiaye T, Bénéfice E, Simondon F, Wade S, Simondon KB. Preschool stunting, adolescent migration, catch-up growth, and adult height in young Senegalese men and women of rural origin. J Nutr. 2006;136:2412–2420. doi: 10.1093/jn/136.9.2412. [DOI] [PubMed] [Google Scholar]
- Crookston BT, Penny ME, Alder SC, Dickerson TT, Merrill RM, Stanford JB, Porucznik CA, Dearden KA. Children who recover from early stunting and children who are not stunted demonstrate similar levels of cognition. J Nutr. 2010;140:1996–2001. doi: 10.3945/jn.109.118927. [DOI] [PubMed] [Google Scholar]
- Crookston BT, Schott W, Cueto S, Dearden KA, Engle P, Georgiadis A, Lundeen EA, Penny ME, Stein AD, Behrman JR. Postinfancy growth, schooling, and cognitive achievement: Young Lives. The American journal of clinical nutrition. 2013;98:1555–1563. doi: 10.3945/ajcn.113.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25:S15–26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7:5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster Z, Byron E, Reyes-García V, Huanca T, Vadez V, Apaza L, Pérez E, Tanner S, Gutierrez Y, Sandstrom B. Physical growth and nutritional status of Tsimane’Amerindian children of lowland Bolivia. Am J Phys Anthropol. 2005;126:343–351. doi: 10.1002/ajpa.20098. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Reduced rate of fat oxidation: a metabolic pathway to obesity in the developing nations. Am J Hum Biol. 2003;15:522–532. doi: 10.1002/ajhb.10191. [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Baker PT. Altitude and growth: a study of the patterns of physical growth of a high altitude Peruvian Quechua population. Am J Phys Anthropol. 1970;32:279–292. doi: 10.1002/ajpa.1330320217. [DOI] [PubMed] [Google Scholar]
- Georgiadis A, Benny L, Crookston BT, Hermida P, Mani S, Woldehanna T, Stein AD, Behrman JR. Growth trajectories from conception through middle childhood and cognitive achievement at age 8 years: Evidence from four low-and middle-income countries. SSM-Population Health. 2016;2:43–54. doi: 10.1016/j.ssmph.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy RA, Reyes-Garcia V, Byron E, Leonard WR, Vadez V. The effect of market economies on the well-being of indigenous peoples and on their use of renewable natural resources. Annu Rev Anthropol. 2005;34:121–138. [Google Scholar]
- Godoy RA, Leonard WR, Reyes-Garcia V, Goodman E, McDade TW, Huanca T, Tanner S, Vadez V. Physical stature of adult Tsimane’ Amerindians, Bolivian Amazon in the 20th century. Econ Hum Biol. 2006;4:184–205. doi: 10.1016/j.ehb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Godoy RA, Reyes-Garcia V, Tanner S, Leonard WR, McDade TW, Huanca T. Can we trust an adult’s estimate of parental school attainment? Disentangling social desirability bias and random measurement error. Field Methods. 2008a;20:26–45. [Google Scholar]
- Godoy RA, Tanner S, Reyes-Garcia V, Leonard WR, McDae TW, Vento M, Broesch J, Fitzpatrick IC, Giovannini P, Huanca T, Jha N, Team BTS. The effect of rainfall during gestation and early childhood on adult height in a foraging and horticultural society of the Bolivian Amazon. Am J Hum Biol. 2008b;20:23–34. doi: 10.1002/ajhb.20679. [DOI] [PubMed] [Google Scholar]
- Godoy RA, Nyberg C, Eisenberg DTA, Magvanjav O, Shinnar E, Leonard WR, Gravlee C, Reyes-Garcia V, McDade TW, Huanca T, Tanner S TAPS Bolivia Study Team. Short but catching up: statural growth among native Amazonian Bolivian children. Am J Hum Biol. 2010;22:336–347. doi: 10.1002/ajhb.20996. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, Group ICDS. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanussen M, Grasedyck L, Kromeyer-Hauschild K, Prokopec M, Chrzastek-Spruch H. Growth tracks in pre-pubertal children. Ann Hum Biol. 2002;29:667–676. doi: 10.1080/03014460210160750. [DOI] [PubMed] [Google Scholar]
- Hirvonen K. Measuring catch-up growth in malnourished populations. Ann Hum Biol. 2014;41:67–75. doi: 10.3109/03014460.2013.827239. [DOI] [PubMed] [Google Scholar]
- Hochberg Ze, Gawlik A, Walker RS. Evolutionary fitness as a function of pubertal age in 22 subsistence-based traditional societies. Int J Pediatr Endocrinol. 2011;2011:2. doi: 10.1186/1687-9856-2011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371:411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013:064584. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000;72:702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- Kismul H, Schwinger C, Chhagan M, Mapatano M, Van den Broeck J. Incidence and course of child malnutrition according to clinical or anthropometrical assessment: a longitudinal study from rural DR Congo. BMC pediatrics. 2014;14:1. doi: 10.1186/1471-2431-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WR, Reyes-García V, Tanner S, Rosinger A, Schultz A, Vadez V, Zhang R, Godoy R. The Tsimane’Amazonian Panel Study (TAPS): nine years (2002–2010) of annual data available to the public. Econ Hum Biol. 2015;19:51–61. doi: 10.1016/j.ehb.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy JL, Ruel M, Habicht J-P, Frongillo EA. Using height-for-age differences (HAD) instead of height-for-age z-scores (HAZ) for the meaningful measurement of population-level catch-up in linear growth in children less than 5 years of age. BMC pediatrics. 2015;15:145. doi: 10.1186/s12887-015-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MA, Johnson BR., Jr Mixed-longitudinal growth of nomadic Turkana pastoralists. Hum Biol. 1987:695–707. [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Illinois: Human Kinetics Books; 1988. [Google Scholar]
- Lundeen EA, Behrman JR, Crookston BT, Dearden KA, Engle P, Georgiadis A, Penny ME, Stein AD. Growth faltering and recovery in children aged 1–8 years in four low-and middle-income countries: Young Lives. Public health nutrition. 2014a;17:2131–2137. doi: 10.1017/S1368980013003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen EA, Stein AD, Adair LS, Behrman JR, Bhargava SK, Dearden KA, Gigante D, Norris SA, Richter LM, Fall CH. Height-for-age z scores increase despite increasing height deficits among children in 5 developing countries. Am J Clin Nutr. 2014b;100:821–825. doi: 10.3945/ajcn.114.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Reyes-Garcia V, Tanner S, Huanca T, Leonard WR. Maintenance versus growth: Investigating the costs of immune activation among children in lowland Bolivia. Am J Phys Anthropol. 2008;136:478–484. doi: 10.1002/ajpa.20831. [DOI] [PubMed] [Google Scholar]
- Orr CM, Dufour DL, Patton JQ. A comparison of anthropometric indices of nutritional status in Tukanoan and Achuar Amerindians. Am J Hum Biol. 2001;13:301–309. doi: 10.1002/ajhb.1053. [DOI] [PubMed] [Google Scholar]
- Outes I, Porter C. Catching up from early nutritional deficits? Evidence from rural Ethiopia. Econ Hum Biol. 2013;11:148–163. doi: 10.1016/j.ehb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Popkin B, Richards M, Montiero C. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr. 1996;126:3009–3016. doi: 10.1093/jn/126.12.3009. [DOI] [PubMed] [Google Scholar]
- Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–265. doi: 10.1179/2046905514Y.0000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, Prentice A. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97:911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said-Mohamed R, Micklesfield LK, Pettifor JM, Norris SA. Has the prevalence of stunting in South African children changed in 40 years? A systematic review. BMC Public Health. 2015;15:534. doi: 10.1186/s12889-015-1844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott WB, Crookston BT, Lundeen EA, Stein AD, Behrman JR. Periods of child growth up to age 8 years in Ethiopia, India, Peru and Vietnam: key distal household and community factors. Soc Sci Med. 2013;97:278–287. doi: 10.1016/j.socscimed.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinger C, Fadnes LT, Van den Broeck J. Using growth velocity to predict child mortality. Am J Clin Nutr. 2016;103:801–807. doi: 10.3945/ajcn.115.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- Tanner S, Leonard WR, McDade TW, Reyes-Garcia V, Godoy R, Huanca T. Influence of Helminth Infections on Childhood Nutritional Status in Lowland Bolivia. Am J Hum Biol. 2009;21:651–656. doi: 10.1002/ajhb.20944. [DOI] [PubMed] [Google Scholar]
- Tanner S, Leonard WR, Reyes-García V. The consequences of linear growth stunting: influence on body composition among youth in the Bolivian Amazon. Am J Phys Anthropol. 2014;153:92–102. doi: 10.1002/ajpa.22413. [DOI] [PubMed] [Google Scholar]
- Teivaanmäki T, Cheung YB, Kortekangas E, Maleta K, Ashorn P. Transition between stunted and nonstunted status: both occur from birth to 15 years of age in Malawi children. Acta Paediatrica. 2015;104:1278–1285. doi: 10.1111/apa.13060. [DOI] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS, Maternal &, Group CUS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- Victora CG, de Onis M, Shrimpton R. Linear growth faltering should be assessed in absolute and relative terms. J Nutr. 2014;144:2092–2093. doi: 10.3945/jn.114.200543. [DOI] [PubMed] [Google Scholar]
- World Bank. [Accessed 5 January 2016];FAQs: Global Poverty Line Update [Online] 2015 Available: http://www.worldbank.org/en/topic/poverty/brief/global-poverty-line-faq.
- World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Methods and development. Geneva, Switzerland: Multicentre Growth Reference Study Group, World Health Organization; 2006. [Google Scholar]
- World Health Organization. WHO AnthroPlus software [Online] Geneva, Switzerland: Department of Nutrition, WHO; 2009. [Accessed May 2015]. Available: http://www.who.int/growthref/tools/en/ [Google Scholar]
- World Health Organization. UNICEF-WHO-World Bank 2012 Joint child malnutrition estimates. Levels and trends. [Online] Geneva: 2015. [Accessed May 2015]. Available: http://www.who.int/nutgrowthdb/estimates2012/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.