Abstract
Glycogen synthase kinase-3β (GSK-3β), a serine/threonine protein kinase, is a complex regulator of numerous cellular functions. GSK-3β is a unique kinase which is constitutively active in resting and non-stimulated cells. GSK-3β has been implicated in a wide range of diseases including neurodegeneration, inflammation and fibrosis, noninsulin-dependent diabetes mellitus, and cancer. It is a regulator of NF-κB-mediated survival of cancer cells, which provided a rationale for the development of GSK-3 inhibitors targeting malignant tumors. Recent studies, many of them reported over the past decade, have identified GSK-3β as a potential therapeutic target in at over 15 different types of cancer. Whereas only active GSK-3β is expressed in cancer cell nucleus, aberrant nuclear accumulation of GSK-3β has been identified as a hallmark of cancer cells in malignant tumors of different origin. This review focuses on the preclinical and clinical development of GSK-3 inhibitors, and the potential therapeutic impact of targeting GSK-3β in human cancer.
Keywords: Glycogen Synthase Kinase-3, GSK-3, chemoresistance, metastasis, drug development, combination treatment
Background
Glycogen synthase kinase-3 (GSK-3), a serine/threonine protein kinase, was initially described as a key enzyme involved in glycogen metabolism (1). However, it has now been shown to regulate a variety of different cellular functions, including the regulation of many metabolic and signaling pathways as well as the modification of structural proteins (2). There are two highly homologous forms of GSK-3 in mammals, GSK-3α and GSK-3β, that have different tissue-specific functions and substrates (1, 2). Despite their homology, one isoform cannot compensate for the loss of the other (2). Historically, GSK-3β has been thought of as a potential tumor suppressor due to its ability to phosphorylate pro-oncogenic molecules e.g. c-Jun (3), c-Myc (4), cyclin D1 (5) and β-catenin (6), targeting them for proteosomal degradation. However, a large and ever increasing body of published data has emerged over the past decade demonstrating that GSK-3β is a positive regulator of cancer cell proliferation and survival in advanced cancer (7–35). This suggests that GSK-3β mediates different signaling pathways in early compared to advanced cancer and has led to the credentialing of GSK-3β as a therapeutic target for advanced disease. This has stimulated significant interest in discovering and developing inhibitors of this enzyme (7–35). One of the pioneering studies identified GSK-3β as a potential therapeutic target in human pancreatic cancer (8). Using a number of toolkit inhibitors of GSK-3β, translational studies credentialed GSK-3β as a therapeutic target in multiple tumor types including pancreatic cancer (8, 9), chronic lymphocytic leukemia (10), colon (11), renal (12) and bladder (13) cancer. More recent studies have also shown that the inhibition of GSK-3β suppressed cancer cell viability in models of glioblastoma (14–16), leukemia (17–20), neuroblastoma (21, 22), osteosarcoma (23), melanoma (24), ovarian (25, 26), thyroid (27), prostate (28–30), breast (31–33) and lung cancer (34, 35). Thus, GSK-3β has emerged as a viable therapeutic target for the treatment of a broad spectrum of different cancer types.
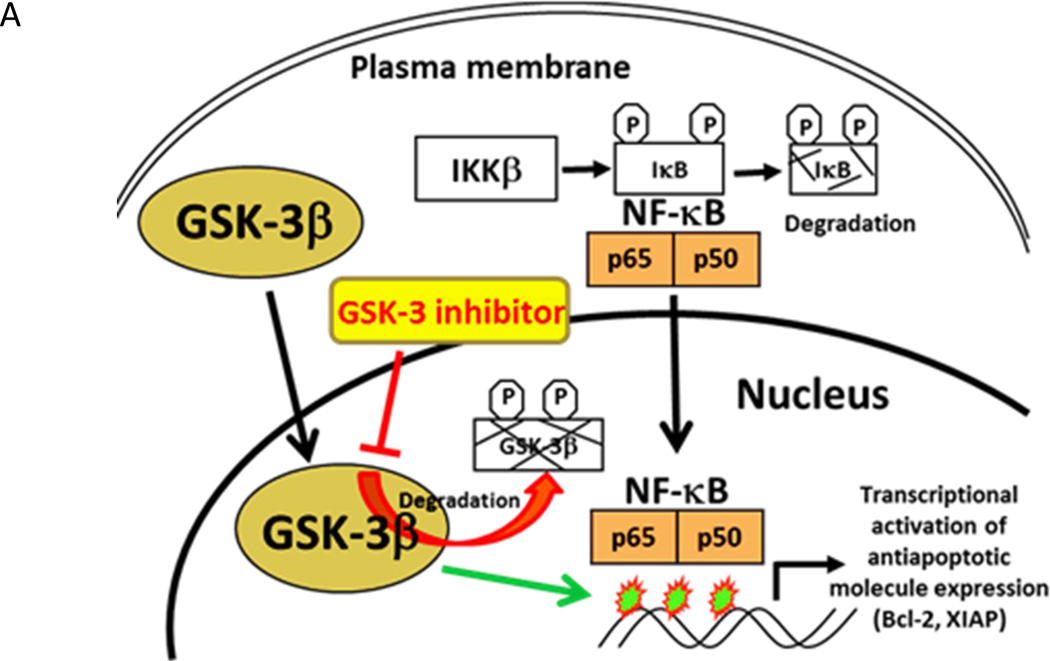
NF-κB activation is known to promote human cancer progression, metastasis and chemoresistance (36, 37). Disruption of the GSK-3β gene in mice leads to embryonic lethality due to hepatocyte apoptosis and massive liver degeneration, a phenotype that is similar to the disruption of the NF-κB p65 or IKKβ genes (38). These findings suggested a link between GSK-3β and the activation of the NF-κB pathway (Fig. 1A). The induction of apoptosis observed when the GSK-3β gene was disrupted raised the possibility that GSK-3β may represent a therapeutic target for the treatment of cancer. Using GSK-3β-deficient mouse embryonic fibroblasts, it was shown that the early steps leading to NF-κB activation following TNF-α treatment (degradation of IκBα and translocation of NF-κB to the nucleus) were unaffected by the loss of GSK- 3β, suggesting that NF-κB is regulated by GSK-3β at the level of the transcriptional complex in the nucleus (38). GSK-3β nuclear accumulation has been previously described occurring selectively in pancreatic, bladder and renal cancer cells, as well as malignant B cells, but not in benign cells or tissues (9, 10, 12, 13). Only the active form of GSK-3β accumulates in the nucleus of pancreatic cancer cells as the inhibition of GSK-3β enzymatic activity depletes its nuclear accumulation via proteosomal degradation (9). In contrast, only weak expression of cytoplasmic GSK-3β was observed in benign pancreatic ducts and pancreatic intraepithelial neoplasia (PanIN) lesions (9). Aberrant nuclear accumulation of GSK-3β was not observed in any of the PanIN lesions but was found in well, moderately, and poorly differentiated pancreatic adenocarcinomas in 2 of 22 (9%), 23 of 59 (39%), and 37 of 41 (90%) cases, respectively (9). These results demonstrate that aberrant nuclear accumulation of GSK-3β is significantly correlated with poorly differentiated pancreatic adenocarcinoma (9). Further studies also demonstrated that nuclear GSK-3β plays an important role in regulating histone modifications, which may contribute to NF-κB p65/p50 binding to its promoters and activation of target genes in cancer cells, leading to increased cancer cell survival (10). These studies led to a hypothesis that targeting nuclear GSK-3β in cancer cells would lead to the subsequent inhibition of NF-κB mediated transcription and decreased cell survival, providing a rationale for discovering and translating novel GSK-3β inhibitors to the clinic for the treatment of cancer.
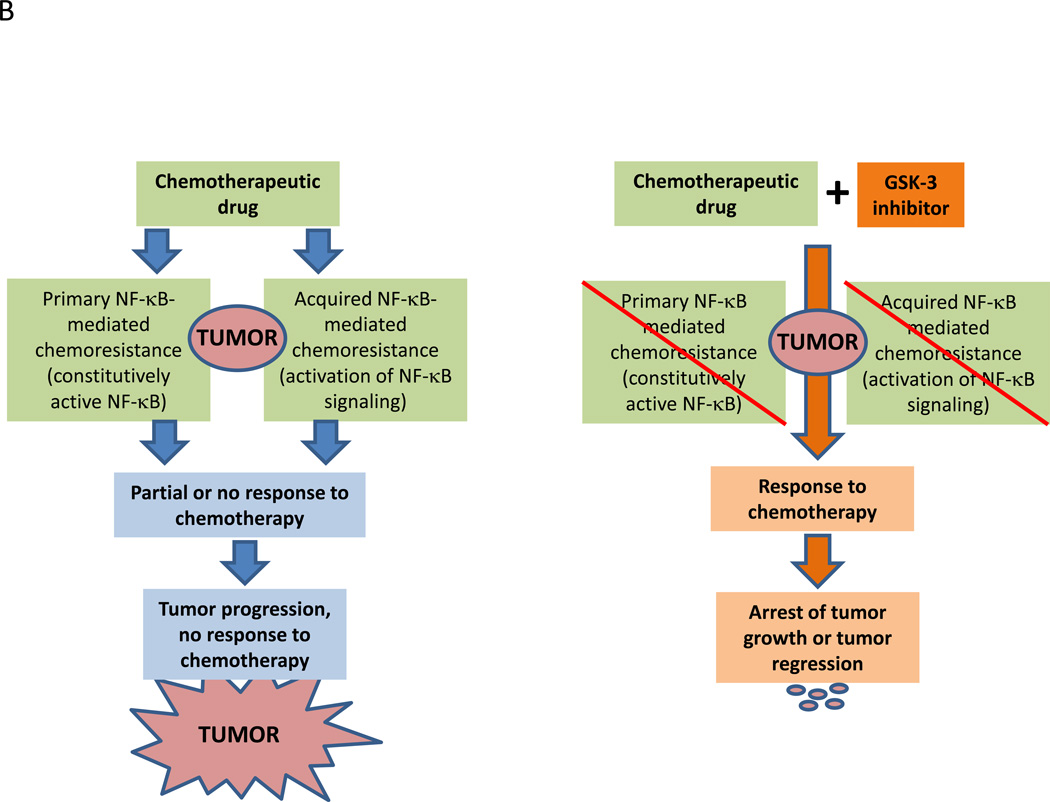
Figure 1.
A. The role of aberrant nuclear accumulation of GSK-3β in human cancer. Aberrant nuclear accumulation of GSK-3β in leukemia, pancreatic, renal and bladder cancer cells (9, 10, 12, 13). Nuclear GSK-3β positively regulates NF-κB binding to its target gene promoters and NF-κB transcriptional activity in cancer cells and contributes to maintenance of active chromatin at NF-κB target gene promoters, allowing p65 binding and transcriptional activation (10). The inhibition of GSK-3β resulted in rapid ubiquitin-proteosomal degradation of its nuclear pool in cancer cell (9).
B. Schematic of the hypothesis that inhibition of GSK-3β overcomes NF-κB-mediated chemoresistance to chemotherapeutic drugs in human cancer.
Clinical-Translational Advances
Rationale for therapeutic targeting of GSK-3β in cancer
GSK-3β represents a signaling node at the intersection of multiple pathways implicated in cancer progression (7). Based on data describing the role of NF-κB in mediating radiation and chemoresistance in cancer (36, 37) and the regulation of NF-κB-mediated gene expression by GSK-3β (7, 38), combining radiation or chemotherapy with GSK-3 inhibitors to re-sensitize resistant tumors to these treatments could be a rational approach to developing new GSK-3 inhibitors for the treatment of cancer (Fig. 1A, B). Indeed, several GSK-3 inhibitors in early development are focusing on this approach. Recent data has emerged that combining GSK-3 inhibitors will enhance the antitumor activity of targeted tyrosine kinase inhibitors (39) and immune checkpoint modulators (40). Thus, there will be sufficient opportunity to explore many novel drug combinations with inhibitors of GSK-3 as these agents enter into clinical development. In this section, we briefly summarize progress to date on the development of GSK-3 inhibitors for clinical use.
Alzheimer’s disease (AD): insights into safety and tolerability of targeting GSK-3
GSK-3 plays a key role in AD other neurodegenerative processes, including inflammation (41), apoptosis (42), impaired axonal transport (43), reduced synaptic plasticity (44), and regulation of long-term potentiation (45). Inhibition of GSK-3 reduces both tau phosphorylation (46, 47) and amyloid production (48, 49) in vitro and in vivo. Several recent early clinical studies have evaluated the safety and efficacy of the irreversible GSK-3 inhibitor tideglusib in the treatment of patients with AD. Tideglusib is a thiadiazolidinone (50) that reduces tau phosphorylation in murine primary neurons (51). In a pilot, double-blind, placebo-controlled, randomized, escalating dose trial, thirty mild to moderate AD patients were enrolled to receive either tideglusib (400–1000 mg/day) or placebo administered orally at escalating doses for a total of 20 weeks. Overall, tideglusib was well-tolerated with 65% of the patients treated with tideglusib experiencing adverse events attributable to study drug (versus 30% in the placebo group) that were not dose-dependent (52). Adverse events occurred equally in the treatment and placebo arms (52). A subsequent ARGO double-blind, randomized, placebo controlled phase II trial in patients with AD enrolled 306 patients aged 50 to 85 years old with mild to moderate disease (53). Patients were randomized to active (1000 mg QD: n = 86, 1000 mg QOD: n = 90, and 500 mg QD: n = 50) or placebo (n=85) treatment arms. There were no deaths during the trial and the most common adverse events were: diarrhea in 14–18% of tideglusib patients versus 11% in the placebo arm and a dose-dependent, mild to moderate, reversible increase in transaminases in 9–16% of tideglusib patients versus 3.5% in the placebo arms (53). The ARGO sponsored trial concluded that tideglusib could be given safely over a 26 week treatment period, providing substantial clinical safety data for targeting GSK-3.
Development of GSK-3 inhibitors for the treatment of cancer
Tideglusib
Recently published study demonstrated that tideglusib induces apoptosis in human neuroblastoma cells (54). Moreover, another recent study has demonstrated that disruption of the GSK-3β-USP22-KDM1A axis by tideglusib therapy suppresses glioma tumorigenesis and sensitizes intracranial GBM xenografts to TMZ leading to an improved mouse survival suggesting an inhibition of GSK-3 as a potential therapeutic approach for the treatment of human GBM (55).
LY2090314
LY2090314 is a potent and selective inhibitor of GSK-3 (56). Initial safety studies of LY2090314 (LY) were conducted in a first-in-human, phase I, dose-escalation study evaluating IV administration of LY in patients with advanced solid tumors (56). This study enrolled 41 patients with enrichment for patients with non-small cell lung cancer (24%) and mesothelioma (22%) (56). Forty-one patients received single-dose LY monotherapy lead-in and thirty seven patients received LY in combination with pemetrexed and carboplatin (56). The MTD of LY in combination with pemetrexed and carboplatin was 40 mg (56). Overall, LY monotherapy and combination therapy with pemetrexed and carboplatin were well-tolerated at the MTD with treatment related adverse events being similar across the dose cohorts (56). During the LY lead-in portion of the study, treatment-related adverse events were observed in 26 patients (63%), whereas 12% of patients had at least one drug-related grade 3–4 adverse with 7% of patients stopping treatment secondary to the side effects (56). In the LY combination with pemetrexed and carboplatin patients, 97% experienced treatment-related adverse events. 60% of patients had at least one drug-related grade 3–4 adverse event (mostly hematologic in nature), with 20% of patients stopping treatment secondary to side effects (56). For the entire study, there were 11 dose-limiting toxicities (DLTs) in 10 patients; in the LY monotherapy patients, the DLTs occurred at doses of ≥40 mg in 5 patients (56). Overall, 85% of patients who received combination treatment were evaluable for efficacy with 5 partial responses (3 patients with non-small cell lung cancer, one with mesothelioma, and one with breast cancer), 19 stable disease, and 11 progressive disease (56). Clinical benefit was durable in a subset of patients but the contribution of LY to the clinical benefit will require further evaluation.
In a recent open-label, phase II study, Rizzieri et al. evaluated the safety of LY in patients with acute myelogenous leukemia (AML) (57). Inhibition of GSK-3 in pre-clinical murine models of mixed lineage leukemia provided evidence for the antitumor activity of GSK-3 inhibitors in hematologic malignancies and a rationale for this phase II study (58). Twenty patients were enrolled in the phase II AML study with confirmed diagnosis of de novo (65%) or secondary AML (35%) that was refractory or relapsed or untreated AML for which the standard induction therapy would not be appropriate (57). LY was administered intravenously at a dose of 40 mg following pre-medication with ranitidine (57). The primary objective of this study was to determine the safety of LY in patients with AML and there were no DLTs reported in this study (57). The majority of the adverse events were of grade 1 severity, with only seven grade 3 and one grade 4 event (57). Twenty six percent of patients showed a reduced blast count, 32% had stable blast counts, and 42% had progressive disease (57). This study concluded that LY has an acceptable safety profile, but showed limited clinical benefits when administered as a monotherapy to patients with AML.
9-ING-41
The overexpression of GSK-3β correlates with poor prognosis in breast cancer patients (59). Patients with the highest GSK-3β expression had a 2.7 and 1.7-fold increased risk of distant relapse 5 and 10 years post-resection, respectively (59). We found aberrant nuclear accumulation of GSK-3β in five human breast cancer cell lines and in 89 of 128 (70%) human breast carcinomas, whereas no detectable expression of GSK-3β was found in benign breast tissue (AU and APM, unpublished results). These results suggest that detection of aberrant nuclear accumulation of GSK-3β in needle biopsy specimens may be a useful method for the pathological diagnosis of breast cancer and for patient identification for GSK-3-targeted therapy. To follow up on these observations in clinical breast cancer cases, a recent study showed that pharmacological inhibition of GSK-3 by two novel small molecule ATP-competitive GSK-3 inhibitors, 9-ING-41 and 9-ING-87 (60), suppressed the viability of breast cancer cells in vitro (61), consistent with the results of Shin et al. who demonstrated that the knockdown of GSK-3β expression significantly inhibited breast cancer cell proliferation (62). Compounds 9-ING-41 and 9-ING-87 are selective for GSK-3 over ~320 other related kinases by at least one order of magnitude, including closely related serine/threonine kinases such as CDKs, PDKs, PKA, Akt, and PKCs (60). In vitro results demonstrated that 9-ING-41 is a more potent inhibitor of breast cancer cell growth than other clinically tractable as well as toolkit GSK-3 inhibitors including LY2090314 (61). The treatment with 9-ING-41 enhanced the antitumor effect of CPT-11 (irinotecan) in breast cancer cells in vitro (61). Using breast patient-derived xenograft (PDX) tumor models established from metastatic pleural effusions obtained from patients with progressive, chemorefractory breast cancer, it has been demonstrated that 9-ING-41 potentiated the antitumor effect of CPT-11, leading to regression of established breast PDX tumors in vivo (61). These results support the hypothesis that targeting GSK-3 can overcome chemoresistance in human breast cancer, and credentialed 9-ING-41 as a novel GSK-3 targeted agent for the treatment of metastatic breast cancer. Consistent with the results in breast carcinoma models, 9-ING-41 antitumor activity has been demonstrated in ovarian, pancreatic and renal cancer models in vitro and in vivo and initial DMPK and toxicology studies support advancing this molecule into clinical translation (26, 60, 63).
It has been previously demonstrated that GSK-3β is a positive regulator of NF-κB-mediated survival in cancer cells, and that inhibition of GSK-3 decreases cancer cell survival via suppression of NF-κB-mediated Bcl-2 and XIAP expression, in leukemia, pancreatic and renal cancer cells (9, 10, 12). Constitutive activation of NF-κB has been reported in human GBM tumors and promotes GBM invasion and resistance to alkylating agents (64–66). It leads to a hypothesis that targeting NF-κB mediated expression by inhibiting GSK-3β represents a therapeutic strategy to overcome GBM chemoresistance and recent studies have independently credentialed GSK-3β as a therapeutic target for the treatment of human GBM (14–16). Using IVIS imaging of live mice, it has been shown that NF-κB is constitutively active in orthotopic GBM PDX tumors expressing an NF-κB luciferase reporter, and that a single intravenous injection of 9-ING-41 significantly reduced NF-κB transcriptional activity in intracranial GBM tumors (67). Then, it has been demonstrated that 9-ING-41 enhanced the antitumor effect of CCNU (lomustine) leading to complete regression of intracranial GBM PDX tumors (68). GSK-3 inhibitor 9-ING-41 significantly increased CCNU antitumor activity in two different orthotopic PDX models: GBM12, which is completely resistant to CCNU, and GBM6, which shows a partial response to CCNU (68). These in vivo studies are the first to our knowledge that demonstrate cures in orthotopic intracranial GBM PDX models with distinct chemoresistant phenotypes (68). Moreover, CCNU+9-ING-41 combination treatment also led to a complete recovery of mouse brain structures affected by intracranial GBM growth, as indicated by histopathological evaluation of serial H&E sections of mouse brain (68). Additional studies are now underway to test whether treatment with 9-ING-41 can also overcome radioresistance in orthotopic GBM PDX tumor models.
In fact, monotherapy with 9-ING-41 did not significantly affect GBM PDX tumor progression (68). These results are consistent with previously published studies showing that monotherapy with drugs having activity against GSK-3 are not effective in treating patients with GBM (69, 70). Enzastaurin, a small molecule inhibitor of GSK-3 (IC50~24 nM) and PKCβ (14, 71), failed to improve GBM patient survival despite some radiographic evidence of antitumor activity (69, 70). These results support a hypothesis that a GSK-3 inhibitor should be combined with chemotherapy for the potential curative treatment of GBM. However, the lack of activity in GBM PDX models observed when 9-ING-41 was combined with temozolomide suggests that it is not a universal enhancer of chemotherapy (68). Additional studies will be required with 9-ING-41 and other GSK-3 inhibitors to understand the molecular basis for combination treatments and to identify molecular profiles and biomarkers that can be used to identify and enrich clinical trials for patients most likely to benefit from combination treatments that include 9-ING-41 or other GSK-3 inhibitors.
Conclusions and future direction
A number of small molecule GSK-3 inhibitors (CHIR-99021&98014, SB216763 & 415286, AR-A011418, CG701338 & CG202796, other compounds described in the patent literature) have been used in cell and animal models to study the role of GSK-3 in cancer. However, the majority of these compounds are considered to be toolkit compounds and do not have the necessary ADMET properties to be advanced as drug candidates into the clinic. Of the GSK-3 inhibitors studied in humans to date, tideglusib and LY2090314 were well-tolerated suggesting that some of the concerns in the field that targeting GSK-3 would lead to broad acute metabolic toxicities were unfounded although the pharmacokinetic profile of LY2090314 is poor so the lack of toxicity could be due to insufficient systemic exposures (72). Nevertheless, the clinical data suggests that toxicities of GSK-3 targeted drugs will be manageable and the hypothesis that targeting GSK-3 will sensitize resistant tumors to radiation and chemotherapy remains to be tested in patients. However, off target effects due to differences in kinase selectivity may lead to toxicities and other pharmacological effects that go beyond GSK-3 inhibition. There is now sufficient evidence to show that targeting GSK-3β is a rational clinical strategy for the treatment of cancer and that the role of GSK-3β in tumor progression is contextual and dependent on the clinical setting. The evaluation of GSK-3 expression profile and localization in subcellular compartments remains to be conducted in many types of cancer. This data combined with molecular profiling data of signatures associated with radiation and chemoresistance may help select cancer patients likely to benefit from treatment with novel GSK-3 inhibitors.
Acknowledgments
Financial Support: This work was supported by generous donations from the Baskes family to the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, by the Woman’s Board of Northwestern Memorial Hospital to the Developmental Therapeutics Program, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University and by Cancer Center Support Grant 2 P30 CA060553-19 (APM, AU) to the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
A. Ugolkov is a consultant/advisory board member for and has ownership interest in Actuate Therapeutics, Inc. S. Chandra is a consultant/advisory board member for Bristol Myers Squibb and EMD Serono. A. Kozikowski is co-founder and co-owner of Actuate Therapeutics, Inc., is a co-inventor on the patent that covers 9-ING-41 by Actuate Therapeutics, Inc., and is a consultant/advisory board member for Actuate Therapeutics, Inc. B. A. Carneiro is a consultant/advisory board member for Bayer and Bristol Myers Squibb. T. V. O’Halloran holds ownership interest in Actuate Therapeutics, Inc. A. P. Mazar holds ownership interest in and is a consultant/advisory board member for Actuate Therapeutics, Inc.
Footnotes
Financial disclosures. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot R, Auwerx J, Bourouis M, Sassone-Corsi P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene. 1993;8:841–847. [PubMed] [Google Scholar]
- 4.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl J, Cheng M, Roussel M, Sherr C. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinfeld B, Albert I, Porfir E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 7.Ougolkov A, Billadeau DD. Targeting GSK-3: a promising approach for cancer therapy? Future Oncology. 2006;2:91–100. doi: 10.2217/14796694.2.1.91. [DOI] [PubMed] [Google Scholar]
- 8.Ougolkov A, Fernandez-Zapico M, Savoy D, Urrutia R, Billadeau D. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 9.Ougolkov A, Fernandez-Zapico M, Bilim V, Smyrk T, Chari S, Billadeau D. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12:5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ougolkov A, Bone N, Fernandez-Zapico M, Kay N, Billadeau D. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110:735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakoori A, Ougolkov A, Zhang B, Modarressi M, Billadeau D, Mai M, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334:1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Bilim V, Ougolkov A, Yuuki K, Naito S, Kawazoe H, Muto A, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009;101:2005–2014. doi: 10.1038/sj.bjc.6605437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, et al. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010;16:5124–5132. doi: 10.1158/1078-0432.CCR-10-0275. [DOI] [PubMed] [Google Scholar]
- 14.Kotliarova S, Pastorino S, Kovell L, Kotliarov Y, Song H, Zhang W, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, NF-kappaB, and glucose regulation. Cancer Res. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15:887–897. doi: 10.1158/1078-0432.CCR-08-0760. [DOI] [PubMed] [Google Scholar]
- 16.Korur S, Huber RM, Sivasankaran B, Petrich M, Morin P, Jr, Hemmings BA, et al. GSK3beta regulates differentiation and growth arrest in glioblastoma. PLoS One. 2009;4:e7443. doi: 10.1371/journal.pone.0007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song EY, Palladinetti P, Klamer G, Ko KH, Lindeman R, O'Brien TA, et al. Glycogen synthase kinase–3beta inhibitors suppress leukemia cell growth. Exp Hematol. 2010;38:908–921. doi: 10.1016/j.exphem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Si J, Mueller L, Collins SJ. GSK3 inhibitors enhance retinoic acid receptor activity and induce the differentiation of retinoic acid-sensitive myeloid leukemia cells. Leukemia. 2011;25:1914–1918. doi: 10.1038/leu.2011.171. [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Ueda M, Stetson L, Ignatz-Hoover J, Moreton S, Chakrabarti A, et al. A novel Glycogen Synthase Kinase-3 inhibitor optimized for acute myeloid leukemia differentiation activity. Mol Cancer Ther. 2016;15:1485–1494. doi: 10.1158/1535-7163.MCT-15-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta K, Stefan T, Ignatz-Hoover J, Moreton S, Parizher G, Saunthararajah Y, et al. GSK-3 inhibition sensitizes acute myeloid leukemia cells to 1,25D-mediated differentiation. Cancer Res. 2016;76:2743–2753. doi: 10.1158/0008-5472.CAN-15-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickey A, Schleicher S, Leahy K, Hu R, Hallahan D, Thotala DK. GSK-3β inhibition promotes cell death, apoptosis, and in vivo tumor growth delay in neuroblastoma Neuro-2A cell line. J Neurooncol. 2011;104:145–153. doi: 10.1007/s11060-010-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter YM, Kunnimalaiyaan S, Chen H, Gamblin TC, Kunnimalaiyaan M. Specific glycogen synthase kinase-3 inhibition reduces neuroendocrine markers and suppresses neuroblastoma cell growth. Cancer Biol Ther. 2014;15:510–515. doi: 10.4161/cbt.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura H, Nakamura O, Yamagami Y, Mori M, Horie R, Fukuoka N, et al. GSK-3 inhibitor inhibits cell proliferation and induces apoptosis in human osteosarcoma cells. Oncol Rep. 2016;35:2348–2354. doi: 10.3892/or.2016.4565. [DOI] [PubMed] [Google Scholar]
- 24.Kubic JD, Mascarenhas JB, Iizuka T, Wolfgeher D, Lang D. GSK-3 promotes cell survival, growth, and PAX3 levels in human melanoma cells. Mol Cancer Res. 2012;10:1065–1076. doi: 10.1158/1541-7786.MCR-11-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Research. 2006;16:671–677. doi: 10.1038/sj.cr.7310078. [DOI] [PubMed] [Google Scholar]
- 26.Hilliard T, Gaisina I, Muehlbauer A, Gaisin A, Gallier F, Burdette J. Glycogen synthase kinase 3beta inhibitors induce apoptosis in ovarian cancer cells and inhibit in-vivo tumor growth. Anticancer Drugs. 2011;22:978–985. doi: 10.1097/CAD.0b013e32834ac8fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunnimalaiyaan M, Vaccaro A, Ndiaye M, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 28.Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene. 2004;23:7882–7892. doi: 10.1038/sj.onc.1208068. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, Yang J, Han S, Liu J, Holzbeierlein J, Thrashe J, et al. Suppression of glycogen synthase kinase 3 activity reduces tumor growth of prostate cancer in vivo. Prostate. 2011;71:835–845. doi: 10.1002/pros.21300. [DOI] [PubMed] [Google Scholar]
- 30.Kroon J, in't Veld LS, Buijs JT, Cheung H, van der Horst G, van der Pluijm G. Glycogen synthase kinase-3β inhibition depletes the population of prostate cancer stem/progenitor-like cells and attenuates metastatic growth. Oncotarget. 2014;5:8986–8994. doi: 10.18632/oncotarget.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HM, Kim CS, Lee JH, Jang SJ, Hwang JJ, Ro S, et al. CG0009, a novel glycogen synthase kinase 3 inhibitor, induces cell death through cyclin D1 depletion in breast cancer cells. PLoS One. 2013;8:e60383. doi: 10.1371/journal.pone.0060383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin S, Wolgamott L, Tcherkezian J, Vallabhapurapu S, Yu Y, Roux PP, et al. Glycogen synthase kinase-3β positively regulates protein synthesis and cell proliferation through the regulation of translation initiation factor 4E-binding protein 1. Oncogene. 2014;33:1690–1699. doi: 10.1038/onc.2013.113. [DOI] [PubMed] [Google Scholar]
- 33.Ugolkov A, Gaisina I, Zhang JS, Billadeau DD, White K, Kozikowski A, et al. GSK-3 inhibition overcomes chemoresistance in human breast cancer. Cancer Lett. 2016;380:384–392. doi: 10.1016/j.canlet.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent EE, Elder DJ, O'Flaherty L, Pardo OE, Dzien P, Phillips L, et al. Glycogen synthase kinase 3 protein kinase activity is frequently elevated in human non-small cell lung carcinoma and supports tumour cell proliferation. PLoS One. 2014;9:e114725. doi: 10.1371/journal.pone.0114725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng J, Liu D, Qiu Z, Huang Y, Chen B, Wang L, et al. GSK3β overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS One. 2014;9:e91231. doi: 10.1371/journal.pone.0091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tas S, Vervoordeldonk M, Tak P. Gene therapy targeting nuclear factor-kB: towards clinical application in inflammatory diseases and cancer. Curr Gene Ther. 2009;9:160–170. doi: 10.2174/156652309788488569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal B. Nuclear factor-kB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Hoeflich K, Luo J, Rubie E, Tsao M, Jin O, Woodgett J. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 39.Thorne C, Wichaidit C, Coster A, Posner B, Wu L, Altschuler S. GSK-3 modulates cellular responses to a broad spectrum of kinase inhibitors. Nat Chem Biol. 2015;11:58–63. doi: 10.1038/nchembio.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor A, Harker J, Chanthong K, Stevenson P, Zuniga E, Rudd C. Glycogen Synthase Kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity. 2016;44:274–286. doi: 10.1016/j.immuni.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beurel E, Jope R. Glycogen synthase kinase-3 regulates inflammatory tolerance in astrocytes. Neuroscience. 2010;169:1063–1070. doi: 10.1016/j.neuroscience.2010.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, She H, Mao Z. Phosphorylation of neuronal survival factor MEF2D by glycogen synthase kinase 3beta in neuronal apoptosis. J Biol Chem. 2009;284:32619–32626. doi: 10.1074/jbc.M109.067785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 2001;7:1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 46.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina M, Castro A. Glycogen synthase kinase-3 (GSK-3) inhibitors reach the clinic. Curr Opin Drug Discov Devel. 2008;11:533–543. [PubMed] [Google Scholar]
- 48.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 49.Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 50.Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 51.Dominguez JM, Fuertes A, Orozco L, del Monte-Millan M, Deldago E, Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3beta by tideglusib. J Biol Chem. 2012;287:893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Ser T, Steinwachs KC, Gertz HJ, Andres MV, Gomez-Carillo B, Medina M, et al. Treatment of Alzheimer's disease with the GSK-3 inhibitor tideglusib: a pilot study. J Alzheimers Dis. 2013;33:205–215. doi: 10.3233/JAD-2012-120805. [DOI] [PubMed] [Google Scholar]
- 53.Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer's disease. J Alzheimers Dis. 2015;45:75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 54.Mathuram TL, Ravikumar V, Reece LM, Karthik S, Sasikumar CS, Cherian KM. Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G0/G1 accumulation and ROS generation. Environ Toxicol Pharmacol. 2016;46:194–205. doi: 10.1016/j.etap.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Zhou A, Lin K, Zhang S, Chen Y, Zhang N, Xue J, et al. Nuclear GSK3β promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol. 2016;18:954–966. doi: 10.1038/ncb3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray JE, Infante JR, Brail LH, Simon GR, Cooksey JF, Jones SF, et al. A first-in-human phase I dose-escalation, pharmacokinetic, and pharmacodynamic evaluation of intravenous LY2090314, a glycogen synthase kinase 3 inhibitor, administered in combination with pemetrexed and carboplatin. Invest New Drugs. 2015;33:1187–1196. doi: 10.1007/s10637-015-0278-7. [DOI] [PubMed] [Google Scholar]
- 57.Rizzieri DA, Cooley S, Odenike O, Moonan L, Chow KH, Jackson K, et al. An open-label phase 2 study of glycogen synthase kinase-3 inhibitor LY2090314 in patients with acute leukemia. Leuk Lymphoma. 2016;57:1800–1806. doi: 10.3109/10428194.2015.1122781. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455:1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintayo MA, Munro AF, Thomas J, Kunkler IH, Jack W, Kerr GR, et al. GSK3β and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Res Treat. 2012;136:161–168. doi: 10.1007/s10549-012-2229-8. [DOI] [PubMed] [Google Scholar]
- 60.Gaisina I, Gallier F, Ougolkov A, Kim K, Kurome T, Guo S, et al. From a natural product lead to the identification of potent and selective benzofuran-3-yl-(indol-3-yl)maleimides as glycogen synthase kinase 3beta inhibitors that suppress proliferation and survival of pancreatic cancer cells. J Med Chem. 2009;52:1853–1863. doi: 10.1021/jm801317h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ugolkov A, Gaisina I, Zhang J, Billadeau D, White K, Kozikowski A, et al. GSK-3 inhibition overcomes chemoresistance in human breast cancer. Cancer Letters. 2016;380:384–392. doi: 10.1016/j.canlet.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin S, Wolgamott L, Tcherkezian J, Vallabhapurapu S, Yu Y, Roux PP, et al. Glycogen synthase kinase-3β positively regulates protein synthesis and cell proliferation through the regulation of translation initiation factor 4E-binding protein 1. Oncogene. 2014;33:1690–1699. doi: 10.1038/onc.2013.113. [DOI] [PubMed] [Google Scholar]
- 63.Pal K, Cao Y, Gaisina IN, Bhattacharya S, Dutta SK, Wang E, et al. Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol Cancer Ther. 2014;13:285–296. doi: 10.1158/1535-7163.MCT-13-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raychaudhuri B, Han Y, Lu T, Vogelbaum MA. Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J Neurooncol. 2007;85:39–47. doi: 10.1007/s11060-007-9390-7. [DOI] [PubMed] [Google Scholar]
- 65.Brown RE, Law A. Morphoproteomic demonstration of constitutive nuclear factor-kappaB activation in glioblastoma multiforme with genomic correlates and therapeutic implications. Ann Clin Lab Sci. 2006;36:421–426. [PubMed] [Google Scholar]
- 66.Bredel M, Bredel C, Juric D, Duran GE, Yu RX, Harsh GR, et al. Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol. 2006;24:274–287. doi: 10.1200/JCO.2005.02.9405. [DOI] [PubMed] [Google Scholar]
- 67.Ugolkov A, Dubrovskyi O, Gaisina I, Yemelyanov A, Bondarenko G, James C, et al. Targeting GSK-3: a novel approach to enhance glioblastoma chemosensitivity. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. 2015;75(15 Suppl) [Google Scholar]
- 68.Ugolkov A, Qiang W, Bondarenko G, Procissi D, Gaisina I, James CD, et al. Combination treatment with the GSK-3 inhibitor 9-ING-41 and CCNU cures orthotopic chemoresistant glioblastoma in patient-derived xenograft models. Neuro-Oncology. 2016 doi: 10.1016/j.tranon.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:168–174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreisl TN, Kotliarova S, Butman JA, Albert PS, Kim L, Musib L, et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12:181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim G, Billadeau D. GSK-3β inhibition in pancreatic cancer. In: Lowy AM, Leach SD, Philip PA, editors. Pancreatic Cancer. New York: Springer Science+Business Media, LLC; 2008. pp. 635–646. [Google Scholar]
- 72.Zamek-Gliszczynski M, Abraham T, Alberts J, Kulanthaivel P, Jackson K, Chow K, et al. Pharmacokinetics, metabolism, and excretion of the glycogen synthase kinase-3 inhibitor LY2090314 in rats, dogs, and humans: a case study in rapid clearance by extensive metabolism with low circulating metabolite exposure. Drug Metab Dispos. 2013;41:714–726. doi: 10.1124/dmd.112.048488. [DOI] [PubMed] [Google Scholar]