Abstract
Detection and evaluation of the mismatch between the intended and actually obtained result of an action (reward prediction error) is an integral component of adaptive self-regulation of behavior. Extensive human and animal research has shown that evaluation of action outcome is supported by a distributed network of brain regions in which the anterior cingulate cortex (ACC) plays a central role, and the integration of distant brain regions into a unified feedback-processing network is enabled by long-range phase synchronization of cortical oscillations in the theta band. Neural correlates of feedback processing are associated with individual differences in normal and abnormal behavior, however, little is known about the role of genetic factors in the cerebral mechanisms of feedback processing. Here we examined genetic influences on functional cortical connectivity related to prediction error in young adult twins (age 18, n=399) using event-related EEG phase coherence analysis in a monetary gambling task. To identify prediction error-specific connectivity pattern, we compared responses to loss and gain feedback. Monetary loss produced a significant increase of theta-band synchronization between the frontal midline region and widespread areas of the scalp, particularly parietal areas, whereas gain resulted in increased synchrony primarily within the posterior regions. Genetic analyses showed significant heritability of frontoparietal theta phase synchronization (24 to 46%), suggesting that individual differences in large-scale network dynamics are under substantial genetic control. We conclude that theta-band synchronization of brain oscillations related to negative feedback reflects genetically transmitted differences in the neural mechanisms of feedback processing. To our knowledge, this is the first evidence for genetic influences on task-related functional brain connectivity assessed using direct real-time measures of neuronal synchronization.
Keywords: feedback, EEG, prediction error, genetics, brain oscillations, neural synchrony, connectivity, phase coherence
1. Introduction
Evaluation of action outcome plays a key role in the organization of adaptive goal-directed behavior (Anokhin, 1974), such that a discrepancy between the predicted and actual outcome (prediction error, or negative feedback) leads to updating of action-outcome associations and enables subsequent behavioral adaptations. The present study is focused on one important component of these processes, namely processing of negative versus positive feedback (monetary loss and gain outcomes in a gambling task).
Studies using event-related brain potentials (ERPs) have identified a number of electrophysiological signatures of feedback processing. In the first study of this kind, Haschke et al. (1987) investigated neural correlates of the mismatch between the intended and actually achieved result of an action using ERP responses to external feedback stimulus informing the participants about the correctness of their choice. They found striking differences between ERPs elicited by positive and negative feedback, with error feedback producing both negative-going and positive-going potential shifts, while positive feedback elicited a positive-going wave only. Subsequent studies have further characterized the difference between ERP responses to negative and positive feedback stimuli using a variety of paradigms such as time estimation (Miltner et al., 1997) and gambling tasks (Gehring and Willoughby, 2002). Converging evidence from these and many other ERP studies suggests that a discrepancy between the negative and positive outcomes producing a net negative ERP deflection that was variably termed as feedback-ERN, medial frontal negativity (MFN), or feedback-related negativity (FRN; see Figure 1). Studies using reinforcement learning paradigms have also shown that FRN reflects outcome expectation failure, rather than subsequent behavioral adjustment; the latter was associated with the P3 component immediately following FRN (Chase et al., 2011).
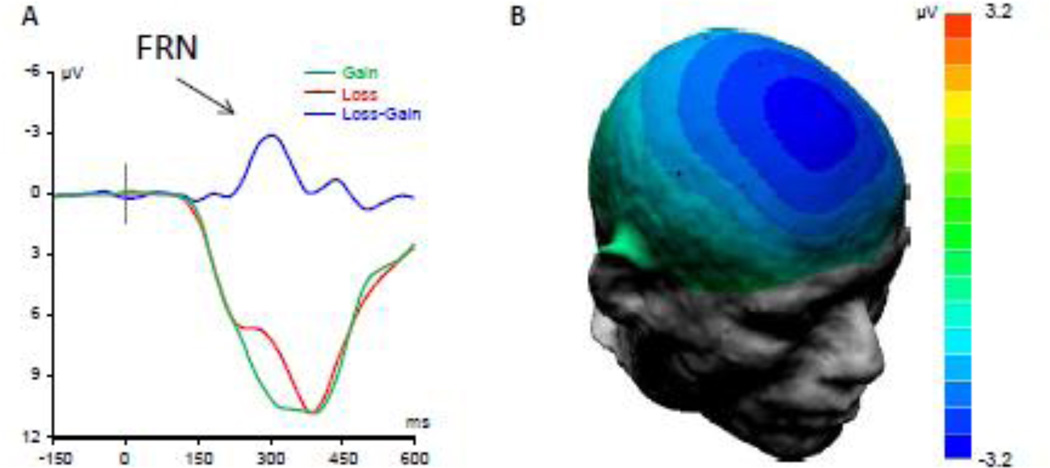
Fig. 1.
Event-related brain potentials (ERPs) elicited by gains and losses in the monetary gambling task. A. Grand-averaged waveforms for loss trials (red), gain trials (green), and the difference wave (blue) showing the Feedback-Related Negativity (FRN). B. Scalp potential map showing topographical distribution of the FRN. The ERP signal is bi-mastoid referenced, and −200ms–0ms baselined.
Extensive human and animal research has shown that evaluation of action outcome is supported by a distributed network of brain regions, in which the anterior cingulate cortex (ACC), and the dopamine system play a central role (Anokhin, 1974; Bush et al., 2000; Rushworth and Behrens, 2008; Schultz and Dickinson, 2000; Ullsperger et al., 2014). Studies using source localization (reviewed in Walsh and Anderson, 2012) and multimodal imaging with simultaneous EEG and fMRI registration (Hauser et al., 2014) have localized the FRN source in the anterior cingulate cortex (ACC), although some evidence also suggests the involvement of basal ganglia (Foti et al., 2014), but see (Cohen et al., 2011).
According to the functional systems perspective (Anokhin, 1974), goal-directed behavior is subserved by a dynamic integration of neural activity in diverse brain regions involved in action planning, execution, and evaluation of the result. How can such a large-scale and rapid communication between spatially segregated brain regions be achieved? The theory of spatiotemporal organization of brain processes (summarized in Livanov, 1977) posits that synchronized (coherent) neural oscillations constitute a neurobiological basis of dynamic functional connectivity, and enable the integration of disparate brain regions into a unified functional network. According to Livanov’s theory, an important condition for the transmission of excitation in the cerebral cortex is the temporal coordination of the functional state (excitability) of interacting but spatially distributed neuronal groups, which is achieved by phase coupling of their excitability cycles reflected in neural oscillations (Livanov, 1977). Numerous studies in humans and animals provided a strong support for this perspective and showed that synchronization of neural oscillations represents a fundamental neurophysiological mechanism of integrative brain activity underlying cognition and behavior (Buzsaki and Draguhn, 2004; Fries, 2005; Jacobs et al., 2007; Livanov, 1977; Palva et al., 2005; Varela et al., 2001). Human studies have consistently shown connectivity between the prefrontal regions with widespread cortical areas during complex cognitive tasks, although patterns of connectivity showed some task specificity (Livanov, 1977; Livanov et al., 1964).
Of particular importance, theta-band oscillations play an important role in long-range communication among distant brain regions. Increased spatial coherence in the theta band was observed during a variety of cognitive and behavioral paradigms in animal and human experiments, leading to a conclusion that theta-band synchrony serves as a basic mechanism of long-range neuronal communication (Benchenane et al., 2011; Cavanagh et al., 2012; Cohen, 2011; Livanov, 1977; Livanov et al., 1964; Nigbur et al., 2012; Womelsdorf et al., 2010b). For instance, studies using EEG spectral power analysis and time-frequency decomposition found increased activity in the theta-band (3–7 Hz) power in response to negative feedback and performance errors compared to positive feedback (Cavanagh et al., 2010; Cohen et al., 2007; Luu et al., 2004; Marco-Pallares et al., 2008; Womelsdorf et al., 2010a).
In addition, a number of recent studies investigating spatial synchronization of neural oscillations related to action outcome processing found that negative feedback leads to a transient increase in theta band synchronization between the medial prefrontal cortex, dorsolateral prefrontal cortex, central, and parietal areas (Cavanagh et al., 2010; Luft et al., 2013; van de Vijver et al., 2011). A study using arrays of surgically implanted microelectrodes in humans has shown that theta oscillations, which are related to error processing, are generated in the area 24 of the dorsal ACC (Wang et al., 2005). Importantly, this study also provided evidence for ACC-neocortical interaction as indicated by a transient increase in the phase locking of their synaptic activity in the theta range, suggesting that the ACC theta forms part of a larger network involving widespread cortical locations in other cortical areas (Wang et al., 2005). Another study using implanted electrodes showed interaction between the medial prefrontal cortex and the nucleus accumbens during feedback processing (Cohen et al., 2009). These data are consistent with fMRI studies indicating co-activation of ACC with widespread cortical regions during cognitive control (Liddle et al., 2001). Furthermore, the strength of theta-band synchronization was related to white matter connectivity between the ACC and other regions (Cohen, 2011). In summary, these studies suggest that FRN triggers a formation of a widespread frontoparietal cognitive control network where the dynamic connectivity is achieved by a transient phase synchronization of theta oscillations.
There is increasing evidence that individual differences in neural synchrony are associated with individual differences in cognition and behavior, both normal and abnormal. In our previous study, the strength of frontoparietal coherence in the theta band during the performance of both verbal and non-verbal cognitive tasks predicted individual differences in general cognitive abilities (Anokhin et al., 1999). A recent study has shown that stronger theta synchronization during feedback processing in a learning task is associated with better learning performance revealing individual differences in feedback processing (Luft et al., 2013). Furthermore, the theory of spatial organization of brain processes (Livanov, 1977) proposed that abnormal local and spatial synchronization of neuronal oscillations may result in disruptions in neuronal communication and coordinated activity of brain regions supporting complex cognition and behavior and thus play an important role in the etiology of neuropsychiatric disorders. This hypothesis was supported by many clinical studies showing abnormalities in neural synchrony in psychopathology (Barry et al., 2009; Livanov, 1977; Mathalon and Sohal, 2015; Stephan et al., 2009; Uhlhaas et al., 2008; Voytek and Knight, 2015).
Predisposition to psychopathology is strongly heritable, but little is known about specific neural processes and mechanisms mediating genetic influences on disordered cognition and behavior. Given the associations between abnormal neural synchrony and psychopathology, we hypothesize that genetic risk for psychopathology is mediated in part by genetic influences on neural synchrony. The first step in testing this hypothesis is establishing significant genetic influences on measures of neural synchrony underlying important aspects of cognitive processing such as FRN, where positive and negative outcomes are contrasted.
Several previous studies have shown genetic influences on spatial synchronization of the resting EEG oscillations (Ibatoullina et al., 1994; Smit et al., 2010; van Baal et al., 2001; van Beijsterveldt et al., 1998), but little is known about genetic influences on task-related synchronization reflecting the dynamics of functional networks supporting specific cognitive processes.
The goal of the present study was to assess genetic and environmental influences on functional brain connectivity related to prediction error using theta band inter-regional phase coherence during the performance of a monetary gambling task in twins. To this end, we first identified specific patterns of inter-regional synchronization associated with prediction error by comparing phase coupling measures between the loss and gain conditions across all possible electrode pairs and selecting connections with the largest loss>gain difference (i.e. providing the best discrimination between conditions). Furthermore, to minimize the influence of volume conduction effects, we focused on long-range connections. Next, we assessed heritability of these selected connectivity measures using structural equation modeling of twin data.
2. Method
2.1. Participants
Participants were twins, n=399, 201 female, age (Mean±S.D.): 18.7±.35, including 87 complete monozygotic (MZ) and 101 dyzogotic (DZ) pairs. All participants were recruited from the local population using the state birth records database. Since exclusion criteria were minimal (see below), the sample is largely representative of the general population with respect to a broad range of variables, including demographic characteristics, psychiatric symptoms, substance use, etc. Zygosity was determined using a set of >1400 DNA markers and, in 4 pairs no genomic data available, using an interview administered to the twins and their parents, as well as research assistants' ratings of twins' physical similarity. Participants with a history of serious head trauma or health conditions precluding a laboratory visit or the ability to perform the experimental tasks (e.g. severe visual impairment or mental retardation) were excluded The study was approved by Washington University Institutional Review Board, and written informed consent was obtained from all participants.
2.2. Procedure
We used an adaptation of a previously described gambling task (Gehring and Willoughby, 2002) that has been widely used in ERP research on feedback processing (Supplementary Figure 1). In this task, participants made a "bet" on one of the two options (10 or 50 cents) presented side by side by pressing a corresponding button (left or right) using the index or the middle finger of their right hand, respectively. Response time was not limited. Following a 2-second interval, feedback was presented showing which of the two options has won and which has lost (the winning number turned green and the losing number turned red). Gains and losses were random and equiprobable. A total of 160 test trials were presented. The subjects were told that this is a “guessing” game and that they would be paid any balance they accrue in cash after the end of the game, but they will owe nothing, should the balance be negative (in fact, all participants were paid an amount of 5 or 7 dollars, selected randomly). A short training series (at least 10 trials) was administered before the test trials to ensure understanding of the instruction. The total duration of the task was 26 min on average, ranging from 18 to 34 min.
2.3. EEG recording
EEG signals were recorded from 30 scalp locations according to the 10–20 system using an elastic cap with Ag/AgCl electrodes and a ground electrode on the forehead, with high- and low-pass filters set at 0.05 and 70 Hz, respectively. The left mastoid served as a reference, and an averaged mastoid reference was digitally computed off-line using the right mastoid recording as a separate channel. The ground electrode was placed in the midline frontal area. The impedance was kept under 5 KΩ.
2.3.1. EEG Analysis
We followed a two-step approach in the EEG pre-processing; i) artefact correction, and ii) manual check of the epochs. In the first step vertical electro-oculogram recording was used for eye-blink artifact correction using a regression-based procedure in the continuous recording (Semlitsch et al., 1986). In the second step, three-second epochs, time-locked to the onset of feedback stimuli (from −1 to +2 s), were extracted from the continuous recording. A broad time window was selected to prevent edge artefacts in time-frequency analysis. Epochs contaminated with other ocular, muscular or any other type of artifacts (falling between −500 to 1000ms within the epoch covering pre- and post-stimulus presentation) were identified by visual inspection and were rejected. The total number of trials used in the coherence analysis was as follows: Win trials: mean=70.9, std=13.62, min=25, max=98; Loss trials: mean=56.8, std=11.96, min=15, max=91. Then, Current Source Density Transform (CSD) was applied to the epoched dataset (Kayser and Logothetis, 2009; Kayser and Tenke, 2015) which implements a high-pass spatial filter that is useful for i) obtaining reference-independent data, ii) increasing topographical localization, iii) facilitating electrode-level connectivity analyses, and iv) attenuating volume-conduction effects. This method has been previously applied with data collected from participants with neuropsychiatric disorders (Kamarajan et al., 2015).
2.3.2. Event-Related Phase Coherence (Phase Locking Value, PLV) calculation
The phase stability and consistency between electrodes across trials were assessed by the function ‘newcrossf’ implemented in EEGLAB software with parameter ‘phasecoh’ for the coherence analysis, yielding event-related phase locking (PLV) between electrodes (Delorme and Makeig, 2004). This function initially finds time-frequency measures on a single trial for each channel for a number of time points via convolving that signal with a complex Gaussian Morlet’s wavelet (Kronland-Martinet et al., 1987), where the complex wavelet is described as:
Here σt stands for temporal standard deviation, and it has an inverse relationship with the standard deviation in frequency domain represented as σf, such that σt ~ 1 /σf. In order to increase the time resolution as well as the phase precision for the PLV calculation (Herrmann et al., 2014; Roach and Mathalon, 2008), we used wavelets with the number of cycles increasing from 1 to 16 as frequency linearly increases from 1 to 20 Hz,. Temporal width of the wavelet window was always set to 7σt, so that the number of cycles was equal to 7σt f0 (where f0 is the value of the frequency of interest used for the estimation of the spectral components of the oscillations around that frequency value). This resulted in 2.4 cycles for 3Hz, and 5.6 cycles for 7Hz, where relatively constant temporal resolution of σt=114ms and frequency resolution with a standard deviation σf=1.4Hz were provided for the whole theta band. Complex wavelet (w) above was then convolved with the time-series signal to yield the spectral measure (W) containing phase and amplitude information of the theta band signals (see below). Then, the phase-locking value (PLV) was computed between each pair of electrodes for each time point across trials, with resulting values ranging from 0 to 1, where 0 indicated no coherence (i.e. signal phase varied independently at each of the two electrodes), and 1 indicated a perfect phase coupling between the two electrodes across trials. The PLV was computed according to the following formula:
where N is the number of trials, and W(t,f)k,a and W(t,f)k,b are the spectral estimates of electrodes (a and b), calculated for trial k, at frequency f and time t. Following the calculation of event-related phase locking for each channel with other channels (30×29/2 = 435 pairs), to approximate the normal distributions of the PLV values, we applied Fisher’s z-transform on these values before the parametric statistical tests.
2.4. Selection of the time-frequency window of interest
To define the window of interest for the pair-wise twin correlation and the genetic analyses, the time-frequency distribution of the PLV responses to gains versus losses was used. The examination of the responses revealed a highly significant increase of synchronization peaking in the time window of 250–450ms confined in the theta band (3–7 Hz). The timing of the oscillatory theta power changes was consistent with previous studies examining local and/or distant theta synchronization during positive and negative feedback processing (Cavanagh et al., 2010; Cohen et al., 2007; Luft et al., 2013; Luu et al., 2004; Marco-Pallares et al., 2008; van de Vijver et al., 2011; Womelsdorf et al., 2010a). Thus, to quantify the genetic effects on the FRN related theta PLV activity, we selected a region of interest defined by the time window 250–450ms and the frequency range 3–7 Hz (theta band). The Z-scores of the PLV values for each time point were averaged within this time window, and entered into a paired t-test for pairwise channel-condition contrasts. Those p values were then entered into an algorithm to adjust for false discovery rate (FDR) by using the 'mafdr' function in MATLAB Bioinformatics Toolbox (www.mathworks.com) with parameter ['BHFDR', 1]. This algorithm uses the Benjamini-Hochberg false discovery approach (Benjamini and Hochberg, 1995). The Benjamini-Hochberg method was used because it does not need large number of tests (i.e., number of p values) in order to be consistent and reliable. The results of the Benjamini-Hochberg are comparable to those of the Storey false discovery approach (Storey, 2002), if a bit more conservative (Krzywinski and Altman, 2014).
2.5. Selection of the phenotypes (coherence measures) for genetic analysis
The analysis of inter-electrode phase coherence using all possible pairings among 30 electrodes yielded 435 variables in each of the two outcome conditions (a total of 970 variables). For subsequent genetic analysis, we selected most representative and condition-specific synchronization pairs based on theoretical, empirical, and methodological considerations.
First, we identified connections that provided the best discrimination between loss and gain conditions by running a paired t-test for the loss-gain differences for each of the 435 synchronization pairs. We selected connections with the effect size of d>0.33, i.e. the magnitude of loss-gain difference of at least one-third of a standard deviation. We reasoned that connections with a small loss-gain difference, i.e. d≤0.3 according to Cohen’s convention, would have little condition-specificity. The range of t-values for connections meeting this criterion was 6.84 to 12.85. To account for non-independence of observations in twin pairs, we reduced the degrees of freedom by half (from the original df=398 to df=199) for the computation of p-values. Even under this highly conservative approach, all p-values were < 10−5, indicating that the selected variables provide a robust discrimination between loss and gain conditions.
Second, we restricted the final set of variables to long-distance connections because they are less confounded by volume conduction compared to connections between spatially close electrodes. Furthermore, there is strong evidence for the key role of the fronto-parietal cognitive control network in the organization of complex goal-directed behavior, including error-monitoring and action outcome processing (Naghavi and Nyberg, 2005; Neta et al., 2015; Palva et al., 2010; Peters et al., 2014; Rangaswamy et al., 2004; Velanova et al., 2008). The same procedure was applied to select gain-specific connection (i.e. showing gain>loss synchronization differences with an effect size of d> .35). This focused and rigorous approach resulted in the selection of 9 long-range connections for the loss condition and 6 connections for the gain condition.
To establish whether the limited number of selected connectivity pairs was representative of the broader connectivity pattern, we conducted a set of additional exploratory analyses by setting substantially lower significance thresholds for the loss-gain difference. This was expected to result in a larger number of connections and a dense connectivity map that could be compared with the sparse connectivity pattern based on selected connections only.
2.6. Data reduction: Principal Component Analysis (PCA) of coherence measures
In the next step, we computed Pearson correlations among the nine coherence measures in the loss condition across all participants (i.e., we calculated r values pairwise between coherence measures, leading to 9 × (8/2) = 36 values). All correlations were positive and significant, ranging from .4 to .8 (mean .57) (See Supplementary Table 1). Since this pattern of correlations suggested the existence of a common factor influencing the overall strength of event-related connectivity, we conducted a Principal Components Analysis (PCA) and extracted the score on the first principal component that accounted for 58.9% of the total variance of the original coherence variables. This score was used as a generalized theta connectivity index in the genetic analysis, serving as another coherence measure.
2.7. Twin correlations, and the assessment of heritability
Next, to characterize twin resemblance with respect to connectivity measures, intra-pair Pearson correlations were computed for each of the selected coherence measures within each of the twin groups.
To estimate heritability, i.e. the relative contribution of genetic and environmental sources to the total phenotypic variance, we fit linear structural equation models using the Mx package (Neale et al., 2002), a standard approach in twin genetic research (Neale and Cardon, 1992; Rijsdijk and Sham, 2002). These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), non-additive genetic influences (D) including within-locus allelic interaction (dominance) and between-locus interaction (epistasis), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). The non-additive component (D) is additional variance resulting from the deviation from the additive effects. It can only be present, if additive effects are present (Falconer and Mackay, 1998). Importantly, A, D, and C increase, whereas E decreases, intra-pair twin similarity. When using only data from twin pairs reared together, it is only possible to test three of these four components simultaneously, and a decision regarding whether to test an ADE or an ACE model is made based upon the observed twin correlations (see Anokhin, 2014; Rijsdijk and Sham, 2002). Specifically, if the MZ correlation is equal to twice the DZ correlation, then AE is the most parsimonious and the best fitting model. An MZ correlation which is smaller than twice the DZ correlation indicates the possibility of shared environmental effects, warranting consideration of ACE and CE models. Conversely, if the MZ correlation is greater than twice the DZ correlation, then the contribution of non-additive genetic effects is possible and the ADE model should be considered because a DE model is biologically implausible (see Table 1, and also Supplementary Table 2.)
Table 1.
Twin correlations and heritability of functional connectivity measures related to prediction error and gain.
| Theta phase coherence |
rmz (n=87) |
rdz (n=101) |
Model | a2(95%CI) (Heritability) |
c2(95%CI) | e2(95%CI) | |
|---|---|---|---|---|---|---|---|
| Prediction Error related pairs | F3-TP7 | .46** | .21* | AE | .47 (.30–.61) | .53 (.39–.70) | |
| F4-TP7 | .44** | .26** | AE | .49 (.32–.62) | .51 (.38–.68) | ||
| F3-TP8 | .42** | .30** | AE | .45 (.29–.58) | .54 (.42–.71) | ||
| F4-TP8 | .29** | .20* | AE | .33 (.14–.49) | .67 (.51–.86) | ||
| Fz-T7 | .23* | .19* | CE | .21 (.07–.34) | .79 (.66–.93) | ||
| T8-Fz | .40** | .29** | CE | .34 (.21–.46) | .66 (.54–.80) | ||
| Fz-TP7 | .44** | .15 | AE | .43 (.25–.57) | .57 (.43–.75) | ||
| Fz-TP8 | .27** | .23* | CE | .24 (.10–.37) | .76 (.63–.90) | ||
| Fz-CPz | .27** | .15 | AE | .28 (.09–.44) | .72 (.56–.91) | ||
|
Connectivity factor |
.50** | .28** | AE | .52 (.36–.64) | .48 (.36–.64) | ||
| Gain related pairs | C3-CP4 | .27** | .23* | CE | .26 (.12–.39) | .74 (.61–.88) | |
| C4-CP4 | .21* | .22* | CE | .21 (.07–.35) | .79 (.65–.93) | ||
| CP3-CP4 | .46** | .23* | AE | .46 (.29–.59) | .54 (.41–.71) | ||
| P3-CP4 | .39** | .11 | AE | .32 (.15–.47) | .68 (.52–.84) | ||
| P4-CP3 | .36** | .29** | CE | .29 (.15–.41) | .71 (.59–.85) | ||
| P4-P3 | .32** | .20** | AE | .30 (.13–.46) | .7 (.54–.86) | ||
The leftmost column shows electrode pairs used to compute theta-band coherence in loss and gain trials; Connectivity Factor is the score on the first principal component derived from individual connectivity measures from the prediction error related pairs ; AE and CE are alternative models that attribute twin resemblance to additive genetic or shared environmental factors, respectively; results are presented for the best fitting model only (see Supplementary Table 2); rmz and rdz are intra-pair twin correlations showing the degree of twins’ resemblance with respect to connectivity measures (numbers of twin pairs are shown in brackets); a2, c2, and e2 are variance components estimates based on the best-fitting models showing the proportions of total phenotypic variance attributable to additive genetic (heritability), shared environmental, and non-shared environmental factors, respectively (95% confidence intervals for the parameter estimates are shown in brackets). Significance of twin correlations:
p<.05;
p<.01 (one-sided).
As described elsewhere (Neale and Cardon, 1992; Sham, 1998), the fit of nested submodels was tested by dropping individual paths from the full model, with the significance of individual paths tested by comparing the fit of the restricted submodel with the fit of the more general model using a χ2 test with degrees of freedom corresponding to the difference in the degrees of freedom between two models (e.g., df=1 if only one parameter is dropped in the restricted model). If dropping a path significantly reduced the goodness of fit (the change in χ2 was significant), the path was retained in the model, otherwise the more parsimonious model was chosen (i.e. the one that accounted for the variance equally well, but with a fewer number of parameters). To choose between non-nested models (AE and CE), we used Akaike’s Information Criterion (AIC), where AIC=χ2 − 2df (Neale and Cardon, 1992). Lower AIC values indicate better fit (see Supplementary Table 2). Path coefficients for the best-fitting models were estimated using the method of maximum likelihood, and the goodness of model fit was indicated by −2 times the log likelihood (−2LL). Heritability was estimated as the percentage of the total variance of the trait attributable to genetic factors (Table 1).
3. Results
3.1. Patterns of functional cortical connectivity associated with gains and losses
Analysis of averaged ERPs showed that, consistent with previous studies (reviewed in Walsh and Anderson, 2012; Weinberg et al., 2015), loss feedback resulted in a negative potential shift that occurred in the time window of 220–400ms, and peaked in the frontocentral midline scalp area (Figure 1). The difference between the mean amplitude in the loss and gain conditions within this time window was highly significant (paired t-test: t=11.7, df=398, p<.0001).
Time-frequency analysis of event-related oscillations showed that monetary gains and losses produced highly significant changes in cross-channel phase coherence of cortical oscillations (Figure 2; all depicted Loss-Gain and Gain-Loss differences are highly significant as indicated by a paired t-test: all t-values exceed 6.0, df=398, p<.0001). However, loss and gain conditions were characterized by topographically distinct connectivity patterns. Most notably, loss feedback resulted in a significant increase in phase coupling for theta-band (3–7 Hz) oscillations, both within the frontal regions and long-range fronto-parietal and fronto-temporal connections, particularly in the right hemisphere. In contrast, gain feedback was associated with increased coherence among widespread posterior regions (temporal-parietal), with relatively few changes in anterior regions.
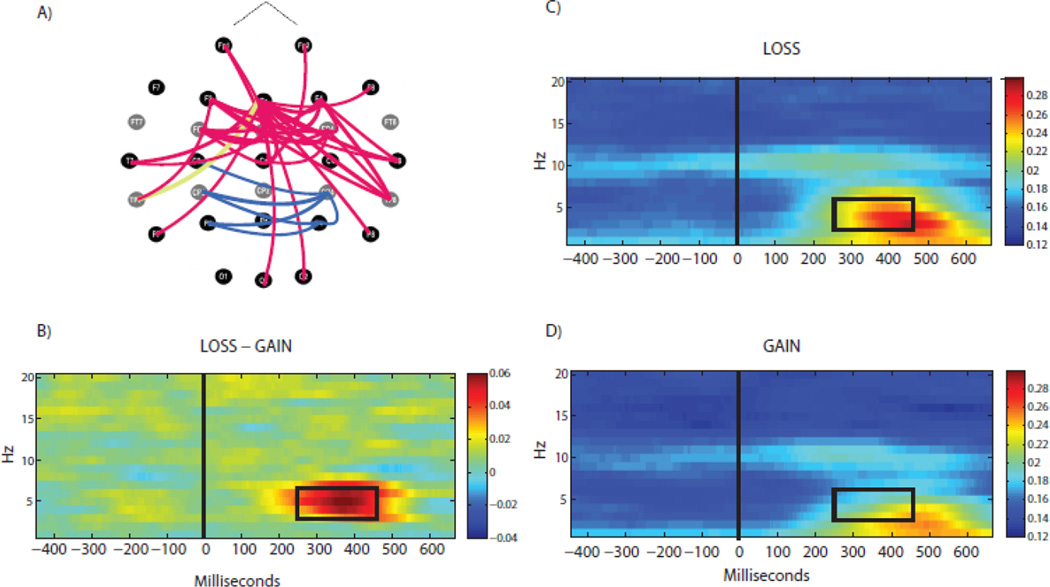
Fig. 2.
Large-scale synchronization of neuronal oscillations during feedback processing. (A) Topographical plot of inter-site phase coherence in the theta band (top view of the scalp, nose up) shows connections discriminating between Loss and Gain feedback (Red: Loss>Gain, Blue: Gain>Loss; Yellow: Loss>Gain for a representative Fz-TP7 pair). (B) Time-frequency plot of the Loss – Gain contrast (shown for a representative connection, Fz-TP7 pair) reveals a strong theta-band (3–7 Hz) response in the 250–450ms time window associated with prediction error. Each colored pixel represents inter-site phase coherence at specific time (X axis) and oscillation frequency (Y axis). Panels C and D show time-frequency coherence plots of responses to Gain and Loss feedback, respectively for the same electrode pair. Taken together, these plots show that a transient theta-response to loss feedback is a dominant feature of the large-scale network dynamics associated with action outcome processing, and negative outcomes are associated with an increase in connectivity between the frontal and parietal regions, with the mid-frontal site (Fz) being a “hub” showing the largest number of significant connections.
Lowering the threshold for the loss-gain difference in additional exploratory analyses resulted, as expected, in a denser connectivity map but did not lead to any noticeable change in the overall pattern, i.e. predominantly prefrontal connections in the loss condition and predominantly parietal connections in the gain condition. Thus, the connections selected for genetic analyses using stringent criteria described above were well representative of the overall connectivity pattern. Topographical plots of connectivity patterns obtained using relaxed criteria are shown in Supplementary Figure 2 (left image with p<10−6 and the right image with p<10−5 thresholds.)
Phase synchronization in gain and loss conditions correlated significantly, with correlations ranging from .39 to .67, suggesting that there is also a general, valence-independent increase in spatial synchronization during feedback processing.
Next, we tested for gender differences with respect to synchronization measures. Despite the large sample size of the present study, only one phase coherence measure in the loss condition (Fz-CPz) reached significance, with somewhat higher values in males, however, the effect size was very small (d<.3). None of the gain feedback measures showed a significant sex difference.
3.2. Twin correlations and heritability of cross-channel phase coherence
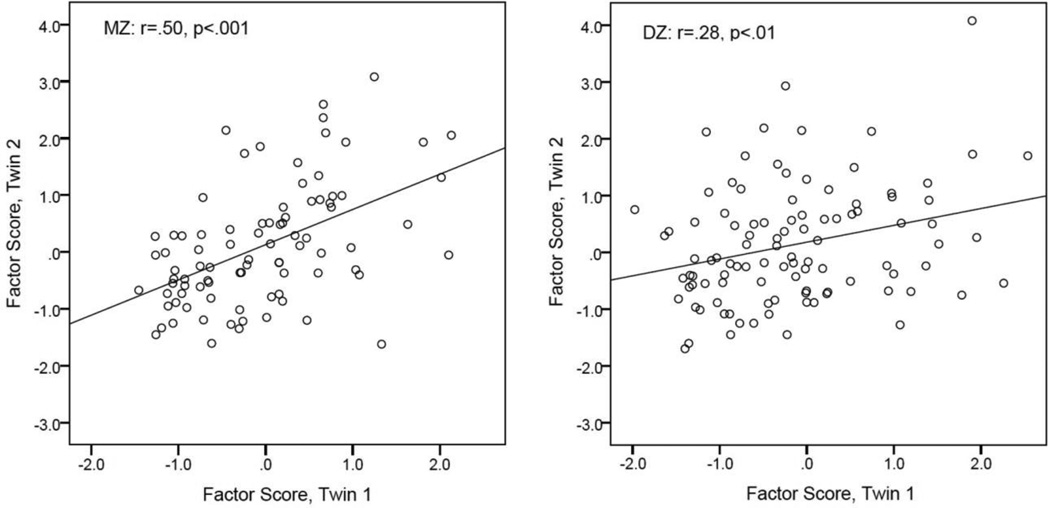
Table 1 presents intra-pair twin correlations with respect to phase coherence measures and summarizes the results of genetic model parameter estimates for the nine electrode pairs selected in line with the criteria described above. In addition, the generalized theta connectivity factor calculated via PCA is shown. All MZ intra-twin correlations were significant and ranged from .23 to .50, indicating significant familial influences on the coherence measures. DZ correlations were generally smaller (.15–.30), suggesting that familial influences may be genetic (see Figure 3 for the correlation plots of the correlation factor scores). However, for some variables, the MZ correlation was less than twice of the DZ correlation (rMZ<2*rDZ), suggesting a contribution of shared environmental influences.
Fig. 3.
Scatterplots of twin correlations for the theta synchronization factor (the first principal component derived from the most significant long-range connections) illustrates greater intra-pair resemblance of MZ twins relative to DZ twins.
The number of connections with significant intrapair correlations was larger in MZ compared with DZ twins, and the overall magnitude of correlations tended to be higher in MZ twins, a pattern consistent with genetic influences on phase coherence measures (Supplementary Figure 3).
Next, to test for significant genetic and environmental effects on connectivity measures, we fit linear structural equation models to the observed twin data. Based on the pattern of twin correlations (rMZ>2*rDZ), we fit the ADE model for two variables (F3-TP7 and Fz-TP7). In both cases, the D path could be dropped without a significant decrement in the goodness of fit, and AE was selected as the best-fitting model. For all other variables, the pattern of correlations (rMZ<2*rDZ) suggested the ACE model, which showed a good fit, except for the Connectivity Factor, where the shared environmental (C) path, but not the additive genetic (A) path, could be dropped without a significant decrement in model fit (indicating that for this phenotype the AE model was the best fitting, while the CE model could be rejected.) For the remaining variables, either A or C could be dropped from the ACE model in favor of more parsimonious models AE or CE. However, neither AE, nor CE could be rejected, therefore the best fitting model was chosen based on Akaike’s Information Criterion (AIC, Supplementary Table 2). In summary, the AE model was the best-fitting for seven variables, and the CE model was chosen for the remaining three variables (Table 1, see also Supplementary Figure 4 showing connectivity measures produced from the best fitting models.)
Heritability estimates under the AE model (i.e. the proportion of the total phenotypic variance explained by additive genetic factors A) ranged from .28 to .52 and were all significant, suggesting that measures of event-related cross-channel coherence are moderately heritable. The proportion of variance accounted for by shared environmental factors under the CE model was smaller and ranged from .21 to .34 (Table 1).
4. Discussion
The present study provides the first evidence for genetic influences on task-related functional brain connectivity assessed using direct measures of cortical neuronal synchronization. Furthermore, the findings indicate significant genetic influences on brain activity related to feedback processing, one of the fundamental processes underlying self-regulation of adaptive goal-directed behavior.
There was a striking dissociation between connectivity patterns produced by signals of feedback with respect to topography. Loss feedback led to early increase in theta synchrony between the frontal channels and other regions of the scalp, whereas gain feedback produced theta-synchronization in posterior regions. FRN related phase synchrony, measured as the difference between the loos and gain conditions, induced increases in frontoparietal connectivity, which is consistent with the evidence from animal studies implicating both frontal and posterior parietal cortices in feedback processing (Schiffino et al., 2014) and human studies showing the activation of frontoparietal cognitive control network during feedback-based control processes including error monitoring (Endrass et al., 2012), external feedback processing (Peters et al., 2014), and trial-and-error learning (Koch et al., 2008). A recent imaging study has shown that error processing in adults, but not children, is characterized by a greater reliance on posterior attentional systems, in addition to the task-general frontal networks (Velanova et al., 2008). Another fMRI study (Peters et al., 2014) showed significantly higher activation of both frontal and parietal regions to negative feedback compared to positive feedback in young adults, suggesting a recruitment of the frontoparietal network.
The present findings are also consistent with the notion that conflict, errors, or negative feedback activate a common control mechanism mediated by theta oscillations (Cavanagh and Frank, 2014). A question arises whether frontoparietal theta connectivity related to negative feedback is specific for reward prediction error, or it is a manifestation of a more ubiquitous frontoparietal cognitive control network that has been described in many previous studies (for review see Niendam et al., 2012). In our previous work, we have shown that frontoparietal coherence in the theta band during the performance of both verbal and spatial cognitive tasks predicts general cognitive abilities (Anokhin et al., 1999), suggesting that the strength of frontoparietal functional connections may represent a universal trait affecting performance in a broad variety of cognitive tasks. More recent studies using both sensor-level and reconstructed source-level connectivity provide convergent evidence for the emergence of a transient frontoparietal network in tasks involving conflict and the need for cognitive control (Cohen and Ridderinkhof, 2013). This issue can be addressed in future studies by using connectivity data from tasks differentially relying on cognitive control, cognitive update, and negative feedback processing.
Twin correlations and genetic model-fitting indicate significant familial effects on large-scale theta-band synchronization related to prediction error. Although for nearly half of the individual connections we were not able to discriminate between genetic (AE) and shared environmental (CE) models of twin resemblance, the overall pattern of results favors the genetic model. First, AE was found to be the best fitting model in seven out of ten measures based on the AIC criterion. Second, for three of the variables (the Connectivity Factor, F3-TP7, and Fz-TP7) the pattern of twin correlations (rMZ>2*rDZ) suggested that shared environmental factors do not contribute to twin resemblance, which was confirmed by subsequent model fitting analyses. Third, even though the best fitting model in three coherence measures (Fz-T7, T8-Fz, and Fz-TP8) was CE, AE model fitting values (i.e., AIC) for those measures were very close to the CE model (Supplementary Table 2). Finally, the broader pattern of twin correlations including all of the prediction-error related coherence measures (Fig. 2A) reveals a stronger resemblance of MZ twins relative to DZ twins (Fig. 3) both with respect to the number of connections showing significant twin correlations and the strength of these correlations. Taken together, these findings strongly suggest that individual differences in the long-range theta-band synchronization related to prediction-error processing are strongly influenced by genetic factors accounting for up to 50% of the total variance of the connectivity measures. However, the contribution of shared environmental factors cannot be ruled out. The present sample is representative of the general population and is therefore quite diverse with respect to socioeconomic background, quality of schools, and other factors that potentially can affect neurocognitive development. These hypotheses will be tested in future analyses of the present sample using available data about the participants’ environmental variables.
It is important to note that heritability of functional connectivity measures in gain and loss conditions is approximately in the same range (Table 1). Thus, despite the fact that prediction error and reward feedback show distinct patterns of connectivity, the extent of genetic influences on these measures is of similar magnitude.
The pattern of twin correlations (Figure 3) suggests that there is a certain trend toward hemispheric differences in the role of genetic versus shared environmental influences on long-range frontoparietal and frontotemporal connections. In the left hemisphere, coherence measures were influenced primarily by genetic factors (higher MZ than DZ correlations; the CE model could be rejected for some connections), whereas in the right hemisphere shared environmental factors could equally play an important role. In this connection, it is important that a recent meta-analysis of fMRI studies of error monitoring has revealed hemispheric differences in the time course of functional activation of the frontoparietal control network: prolonged response profiles in the right hemisphere, and fast profiles in the left hemisphere (Neta et al., 2015), suggesting that the left hemisphere frontopariatal system may be primarily involved in trial-level outcome processing, while in the right hemisphere, it may be involved in a more sustained, tonic monitoring of outcomes over the course of the task. Thus, our study contributes to the current approaches examining the interhemispheric differences in action outcome processing by shedding light on its potential underlying genetic sources.
Specific genetic variants involved in the determination of these individual differences in functional connectivity and neurobiological mechanisms mediating their action remain to be determined in future studies. In early studies of spatiotemporal oscillatory dynamics, Livanov et al. (1964) noted that the topography and strength of oscillatory synchronization during mental activity showed a good correspondence with well-known maps of structural cortical connectivity, including both commissural and association fibers. Thus, one possibility is that individual differences in the strength of oscillatory synchrony may be mediated by individual variation in morphological characteristics such as the thickness of fiber tracts and/or the degree of the myelination. Genes affecting white matter development and myelin synthesis can be potential candidates.
The finding of significant genetic influences on functional connectivity has important implications for the understanding of the mechanisms mediating genetic risk for psychopathology. Disturbances in brain connectivity including abnormalities in inter-regional synchronization of brain oscillations have been implicated in a variety of neuropsychiatric disorders (Livanov, 1977). Psychiatric disorders, most notably schizophrenia, autism, and ADHD, are highly heritable, however, little is known about the neurobiological pathways and mechanisms that mediate genetically transmitted risk. Since adaptive goal-directed behavior is subserved by communication among neuronal groups in spatially distributed brain regions by means of coherent neuronal oscillations, one such mechanism could be a disturbance of neuronal communication, particularly its timing. Dynamic functional networks underlying behavior require a precise temporal orchestration of activity in distant brain regions. According to the neural synchronization theory (Livanov, 1977), transmission of excitation across different cortical regions critically depends on the temporal coordination of the functional state of those regions, which is reflected in phase coupling and the coherence of neural oscillations across regions. Abnormalities in neural synchrony have been reported in many EEG and ERP studies of psychiatric disorders (reviewed in (Mathalon and Sohal, 2015; Stephan et al., 2009; Uhlhaas and Singer, 2012). Importantly, abnormalities in frontoparietal connectivity as revealed using MRI have been associated with familial risk for alcoholism (Wetherill et al., 2012), suggesting that frotoparietal connectivity measures can serve as a neurobiological marker of familial and, possibly, genetic risk for substance use disorders.
In view of this evidence and the current findings, we propose that genetically determined variation in neuronal synchronization as revealed by EEG phase coupling can serve as an intermediate phenotype (endophenotype) for psychiatric disorders characterized by impaired feedback processing. There are different ways in which such endophenotypes may be useful (Anokhin, 2014). First, investigation of connectivity phenotypes related to specific neurocognitive deficits can help to resolve the problems of heterogeneity and comorbidity of psychiatric disorders, and perhaps develop a new nosology based on the underlying genetics and neurobiology, which is the long-term goal of the recently launched Research Domain Criteria (RDoC) initiative (Cuthbert, 2014; Insel et al., 2010). Second, endophenotypes can provide a functional characterization of genetic variants for psychiatric disorders being identified by genome-wide association studies. In most cases, the mechanisms by which these variants confer increased risk are unknown. Testing for association between such candidate variants and heritable measures of neuronal connectivity can potentially establish an important pathway from genes to clinical behavioral phenotype.
Several limitations of the present study must be acknowledged. First, a relatively sparse electrode array (n=30) complicated the use of more fine-grained connectivity analyses such as source-level connectivity. To avoid potential problems with the interpretation of source data derived from a limited number of electrodes, in the present study we elected to conduct sensor-level analyses only. Second, due to sparse electrode array, surface Laplacian derivation may not have fully eliminated volume conduction effects that are most pronounced for close cortical areas and decline exponentially with increasing inter-electrode distance. To minimize the residual effects of volume conduction, we focused our analyses on long-range connections. However, some meaningful changes in connectivity have also been described for shorter-range connectivity, e.g. between medial and lateral prefrontal cortices (Luft et al., 2013).
Finally, the gambling task used in the present study has certain limitations. Since its first introduction (Gehring and Willoughby, 2002) this task with various modifications has been widely used in previous EEG research on feedback processing and reward prediction error, however, its “guessing” nature limits the investigation of the dynamics of reinforcement learning processes. Although “guessing” tasks of this type capture a key component of reinforcement learning, namely evaluation of action outcome by its matching to the representation of the desired outcome (Anokhin, 1974), they fall short of addressing the learning itself, namely, adaptive modification of subsequent behavior triggered by the prediction error. Therefore, future studies should investigate dynamic connectivity related to feedback utilization for adaptive changes in behavior.
These limitations notwithstanding, the present study provides important information about the large scale brain network dynamics associated with prediction error and genetic influences on individual differences in these processes. In a broader context, this study is the first to demonstrate genetic influences on task-related brain connectivity using a powerful twin design.
Supplementary Material
Highlights.
We examined genetic influences on functional cortical connectivity related to feedback processing in young adult twins (age 18, n=399) using a monetary gambling task.
Monetary loss produced a significant increase of theta-band synchronization between the frontal midline region and widespread frontoparietal areas of the scalp.
Gain resulted in increased synchrony primarily over posterior regions.
Genetic analyses showed significant heritability of frontoparietal theta phase synchronization (24 to 46%), suggesting that individual differences in large-scale network dynamics are under substantial genetic control.
To our knowledge, this is the first evidence for genetic influences on task-related functional brain connectivity assessed by using direct measures of cortical neuronal synchronization.
Acknowledgments
This work was supported by grants DA018899, DA027096, DA00421, and AA016812 to A.A. from the National Institutes of Health (NIH). The authors acknowledge organizational and technical assistance by Tara Tinnin, MSW, Olga Novak, and other project staff. The authors also acknowledge the generous giving of time and effort by the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anokhin AP. Genetic psychophysiology: advances, problems, and future directions. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2014;93:173–197. doi: 10.1016/j.ijpsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Lutzenberger W, Birbaumer N. Spatiotemporal organization of brain dynamics and intelligence: an EEG study in adolescents. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 1999;33:259–273. doi: 10.1016/s0167-8760(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Anokhin PK. Biology and Neurophysiology of the Conditioned Reflex and its Role in Adaptive Behavior. Oxford, New York, Toronto, Sydney, Braunschweig: Prgamon Press; 1974. [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M. EEG coherence in children with attention-deficit/hyperactivity disorder and comorbid reading disabilities. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2009;71:205–210. doi: 10.1016/j.ijpsycho.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Current opinion in neurobiology. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Swainson R, Durham L, Benham L, Cools R. Feedback-related negativity codes prediction error but not behavioral adjustment during probabilistic reversal learning. J Cogn Neurosci. 2011;23:936–946. doi: 10.1162/jocn.2010.21456. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage. 2011;55:1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Nuclei accumbens phase synchrony predicts decision-making reversals following negative feedback. J Neurosci. 2009;29:7591–7598. doi: 10.1523/JNEUROSCI.5335-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF, Slagter HA. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity: commentary. Human brain mapping. 2011;32:2270–2271. doi: 10.1002/hbm.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR. EEG source reconstruction reveals frontal-parietal dynamics of spatial conflict processing. PloS one. 2013;8:e57293. doi: 10.1371/journal.pone.0057293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN. Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology. 2014;51:1205–1206. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Gruetzmann R, Ischebeck M, Kathmann N. Response-related negativities following correct and incorrect responses: evidence from a temporospatial principal component analysis. Psychophysiology. 2012;49:733–743. doi: 10.1111/j.1469-8986.2012.01365.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th. Harlow: Pearson Education Limited; 1998. [Google Scholar]
- Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2014 doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Haschke R, Dormann WU, Schwind J, Haschke W. ERP analysis of reafferent information processing during the performance of an arithmetical task. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 1987;5:25–31. doi: 10.1016/0167-8760(87)90069-9. [DOI] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stampfli P, Drechsler R, Brandeis D, Walitza S, Brem S. The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. NeuroImage. 2014;84:159–168. doi: 10.1016/j.neuroimage.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Vosskuhl J, Struber D. Time-frequency analysis of event-related potentials: a brief tutorial. Brain topography. 2014;27:438–450. doi: 10.1007/s10548-013-0327-5. [DOI] [PubMed] [Google Scholar]
- Ibatoullina AA, Vardaris RM, Thompson L. Genetic and Environmental-Influences on the Coherence of Background and Orienting Response Eeg in Children. Intelligence. 1994;19:65–78. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27:3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Porjesz B. The use of current source density as electrophysiological correlates in neuropsychiatric disorders: A review of human studies. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2015;97:310–322. doi: 10.1016/j.ijpsycho.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK. Directed Interactions Between Auditory and Superior Temporal Cortices and their Role in Sensory Integration. Front Integr Neurosci. 2009;3:7. doi: 10.3389/neuro.07.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2015;97:189–209. doi: 10.1016/j.ijpsycho.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Schachtzabel C, Wagner G, Reichenbach JR, Sauer H, Schlosser R. The neural correlates of reward-related trial-and-error learning: an fMRI study with a probabilistic learning task. Learning & memory (Cold Spring Harbor, N.Y.) 2008;15:728–732. doi: 10.1101/lm.1106408. [DOI] [PubMed] [Google Scholar]
- Kronland-Martinet R, Morlet J, Grossmann A. Analysis of sound patterns through wavelet transforms. Int. Journal of Pattern Recognition and Artificial Intelligence. 1987;1:273–302. [Google Scholar]
- Krzywinski M, Altman N. Points of significance: Comparing samples-part I. Nature methods. 2014;11:215–216. doi: 10.1038/nmeth.2858. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human brain mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livanov MN. Spatial Organization of Cerebral Processes. New York, Toronto: John Wiley & Sons; 1977. [Google Scholar]
- Livanov MN, Gavrilova NA, Aslanov AS. Intercorrelations between Different Cortical Regions of Human-Brain during Mental Activity. Neuropsychologia. 1964;2:281-&. [Google Scholar]
- Luft CD, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. J Neurosci. 2013;33:2029–2038. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, Munte TF, Rodriguez-Fornells A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sohal VS. Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders: It’s about time. JAMA Psychiatry. 2015;72:840–844. doi: 10.1001/jamapsychiatry.2015.0483. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a "generic" neural system for error detection. J Cognitive Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Consciousness and cognition. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. 6th. Richmond, VA: Department of Psychiatry; 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NU, Schlaggar BL, Petersen SE. Spatial and temporal characteristics of error-related activity in the human brain. J Neurosci. 2015;35:253–266. doi: 10.1523/JNEUROSCI.1313-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, affective & behavioral neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigbur R, Cohen MX, Ridderinkhof KR, Sturmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J Cogn Neurosci. 2012;24:1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25:3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Braams BR, Raijmakers ME, Koolschijn PC, Crone EA. The neural coding of feedback learning across child and adolescent development. J Cogn Neurosci. 2014;26:1705–1720. doi: 10.1162/jocn_a_00594. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. NeuroImage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia bulletin. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature neuroscience. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Zhou V, Holland PC. Posterior parietal cortex is critical for the encoding, consolidation, and retrieval of a memory that guides attention for learning. The European journal of neuroscience. 2014;39:640–649. doi: 10.1111/ejn.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual review of neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sham P. Statistics in Human genetics. New York: Oxford University Press; 1998. [Google Scholar]
- Smit DJ, Boersma M, van Beijsterveldt CE, Posthuma D, Boomsma DI, Stam CJ, de Geus EJ. Endophenotypes in a dynamically connected brain. Behav Genet. 2010;40:167–177. doi: 10.1007/s10519-009-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia bulletin. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B. 2002;64:479–498. [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophrenia bulletin. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiological reviews. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- van Baal GC, Boomsma DI, de Geus EJ. Longitudinal genetic analysis of EEG coherence in young twins. Behav Genet. 2001;31:637–651. doi: 10.1023/a:1013357714500. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Genetic and environmental influences on EEG coherence. Behav Genet. 1998;28:443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal oscillatory dynamics predict feedback learning and action adjustment. J Cogn Neurosci. 2011;23:4106–4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature reviews. Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral cortex (New York, N.Y. : 1991) 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biological psychiatry. 2015;77:1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MM, Anderson JR. Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neuroscience and biobehavioral reviews. 2012;36:1870–1884. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: A review of the literature. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain research. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Frontiers in human neuroscience. 2010b;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.