Abstract
Infants with short bowel syndrome (SBS) are at high risk for malabsorption, malnutrition, and failure to thrive. The objective of this study was to evaluate in a porcine model of SBS, the systemic absorption of a novel enteral Docosahexaenoic acid (DHA) formulation that forms micelles independent of bile salts (DHA-ALT®). We hypothesized that enteral delivery of DHA-ALT® would result in higher blood levels of DHA compared to a control DHA preparation due to improved intestinal absorption. SBS was induced in term piglets through a 75% mid-jejunoileal resection and the piglets randomized to either DHA-ALT® or control DHA formulation (N=5 per group) for 4 postoperative days. The median ± IQR difference in final versus starting weight was 696 ± 425g in the DHA-ALT® group compared to 132 ± 278g in the controls (p=.08). Within 12 hours, median ± IQR DHA and eicosapentaenoic acid plasma levels (mol%) were significantly higher in the DHA-ALT® vs. control group (4.1 ± 0.3 vs 2.5 ± 0.5, p=0.009; 0.7 ± 0.3 vs 0.2 ± 0.005, p=0.009, respectively). There were lower fecal losses of DHA and greater ileal tissue incorporation with DHA-ALT® versus the control. Morphometric analyses demonstrated an increase in proximal jejunum and distal ileum villus height in the DHA-ALT® group compared to controls (p=0.01). In a neonatal porcine model of SBS, enteral administration of a novel DHA preparation that forms micelles independent of bile salts resulted in increased fatty acid absorption, increased ileal tissue incorporation, and increased systemic levels of DHA.
Keywords: short bowel syndrome, intestinal adaptation, docosahexaenoic acid, fatty acids, fat, piglet
1. INTRODUCTION
Congenital gastrointestinal anomalies and acquired intestinal diseases in the infant may require significant resection of bowel length as a life-saving measure. Although many infants postoperatively will be able to transition to enteral feeding volumes to sustain hydration and growth, a significant proportion of these infants may experience insufficient post-resection intestinal adaptation resulting in the failure to establish full enteral feedings leading to a prolonged dependence on parenteral nutrition (PN). The inability for the remaining bowel to serve its full absorptive and nutritive roles in supporting adequate growth is known as short bowel syndrome (SBS). Infants with SBS are at high risk for severe malabsorption, malnutrition, failure to thrive, parenteral nutrition associated liver disease, catheter-related sepsis and a mortality rate approaching 40% [1].
Prior rodent models of SBS have shown a positive effect on intestinal adaptation with high fat diets, especially those with enhanced long chain polyunsaturated fatty acids including Docosahexaenoic acid (DHA) and Arachidonic acid (ARA) [2–4]. Interestingly, although parenteral fish oil has also demonstrated the ability to induce intestinal regrowth in this animal model, this effect was not observed with enteral administration of a fish oil emulsion [5].
One potential mitigating factor in the success of fat and fish oil enteral supplements to promote intestinal adaptation is the capacity of the remnant bowel to effectively hydrolyze and absorb these fatty acids allowing for their bioavailability. An essential step in effective absorption after lipolysis of dietary fats is micelle formation aided by bile salts. The micelle allows transport of free fatty acids and monoglycerides to the intestinal epithelium where it can be absorbed and repackaged in chylomicrons for transport into the portal circulation. The presence of bile salts is developmentally regulated and may be further dampened by impaired signaling due to compromised nutrition, liver failure, and intestinal dysfunction induced by the underlying condition and/or the intestinal resection [6–8].
The goal of this study was to evaluate in an established porcine model of SBS, the systemic absorption and intestinal adaptation capacity of an ethyl ester form of DHA with Advanced Lipid Technology® (DHA-ALT®; Sancilio & Company, Inc., Riviera, FL), which allows for micelle formation independent of the presence of bile acids compared to a standard ethyl ester form of DHA without ALT® (Control). We hypothesized that enteral delivery of DHA-ALT® would result in higher improve intestinal absorption and blood levels of DHA compared to a control DHA preparation due to improved intestinal absorption. Plasma levels of DHA were measured by GC-MS. Intestinal absorption of DHA was analyzed using two parallel parameters, fecal losses of DHA and DHA incorporation in the phospholipid fraction of the intestinal epithelium. Finally, to assess safety and the potential for intestinal adaptation of both DHA formulations, small bowel morphologic assessment and the cellular balance between apoptosis and proliferation were determined.
2. METHODS AND MATERIALS
2.1. Animal Care and Surgical Induction of SBS
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals: Eight Edition [National Research Council. Washington, DC: The National Academies Press, 2011].
Newborn (6-day-old), crossbred (Hampshire × Landrace × Duroc × Yorkshire) piglets (n = 10), were obtained from an approved local swine farm and were transported to the animal facility at the Children’s Nutrition Research Center (Houston, TX). Upon arrival, piglets were weighed and placed in cages in a heated room (~30°C) and were fed with a sow milk replacer (Litterlife; Merrick, Middleton, WI). On the following 2 days, after an overnight fast, piglets underwent surgery under isoflurane anesthesia and sterile conditions. Surgical procedures included placement of jugular and intragastric catheters for postoperative parenteral and enteral nutrition, respectively, and small bowel resection. After placement of a 20-gauge silastic catheter in the left external jugular vein lactated Ringer’s solution (10mL/kg*h) was infused intravenously for the remainder of the surgical procedure. Following a 4-cm midline incision an 80% mid-jejunoileal resection was performed. The total amount of bowel resection was approximated using the following equation: total intestinal length (cm) = 280 × body weight^0.60. A 20% remnant (~60 cm total per kg BW) remained and was represented equally between proximal jejunum and ileum, representing ~ 30 cm per remnant segment per kg BW. Once the amount of bowel to be preserved was determined, half of that amount was measured out in the jejunum distal to the ligament of Treitz and the other half in the ileum proximal to the ileocecal junction using sterile silk ribbon placed along the antimesenteric border of the gently stretched small intestine. Intestine not included in the measurement was removed by dividing the bowel and mesentery with electrocautery. Bowel continuity was restored using an end-to-end jejunoileal anastomosis with interrupted 5-0 PDS sutures. The anastomoses were tested for patency and absence of a leak using the luminal contents. Finally, an intragastric, 18-gauge silastic catheter was implanted in the greater curvature of the stomach before the abdomen was closed in 2 layers with absorbable 2-0 Vicryl and non-absorbable 3-0 Surgilene sutures. Preoperatively, pigs received one dose of slow release (SR) formulation of buprenorphine (0.12mg/kg SC) to ensure analgesia for 72h, and one dose of antibiotic enrofloxacin (10mg/kg SC) which was continued at the same dose postoperatively every 12 hours until the end of the study. Postoperatively, piglets were housed in individual cages. Rooms were maintained at 30° C, with a 12-hour light/dark cycle.
At the end of the experimental protocol, the animals were euthanized with IV injection of pentobarbital sodium (50 mg/kg) and phenytoin sodium (5 mg/kg, Beuthanasia-D, Schering-Plough Animal Health, Kenilworth, NJ). Blood, fecal and tissue samples were collected and frozen immediately in liquid nitrogen. All samples were stored at −80°C until final analysis.
2.2. Postoperative Nutrition
PN was provided within 3 h after the operation. All piglets received PN at 50% of full intake providing (in g/(kg*d)) 12.5 dextrose, 6.5 L-amino acids, 2.5 lipid (Intralipid®, Fresenius Kabi, Bad Homburg, Germany), and 412 kJ/(kg*d) at a volume of 120 mL/(kg*d). Continuous enteral feeding with a sow milk replacer formula (Litterlife®; Merrick, Middleton, WI) also lacking DHA was slowly introduced 24 h after intestinal resection at 3 mL/kg*h via the intragastric catheter. The volume of enteral feedings was then advanced every 24 h by 1 ml/kg*h until a final enteral volume of 5 mL/kg*h was achieved on postoperative day 3 which represents approximately 50% of full enteral feeding volume and nutrient intake.
2.3. Provision of oral DHA – Treatment & Control Groups
After the surgical procedure, piglets were randomly allocated into two groups (N=5 per group). The control group received a standard DHA ethyl ester preparation and the treatment group received a DHA ethyl ester preparation with Advanced Lipid Technology® (DHA-ALT®). Both the control and treatment DHA preparations were provided by Sancilio & Company, Inc. (Riviera, FL). DHA was provided at a dose of 1 g/kg*day. The dose was determined from published clinical experience using fish-oil based lipid emulsions to treat parenteral nutrition associated liver disease in children with severe short bowel syndrome. (Ref: Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management [9]. The DHA was mixed in the enteral feeding at the respective dose and infused via the intragastric catheter accordingly for a total of 4 days after which time, animals were sacrificed.
2.4. Growth Data and Sample Collection
2.4.1. Growth
Piglets were weighed every other day. In addition to calculating a change in weight by subtracting the starting weight at the time of randomization from the final weight, percent weight change and growth velocity (g/kg*d) were also calculated. Percent weight change was calculated by: [((final weight-starting weight)/starting weight) × 100]. Growth velocity was calculated by: [(weight change/starting weight)/4 days of treatment].
2.4.2. Longitudinal plasma fatty acid levels
Blood samples for systemic fatty acid profiles were obtained on the day of surgery and then twice daily until sacrifice on fourth postoperative day. The blood samples were fractionated and the plasma was stored at −80°C until analysis. Plasma fatty acids were isolated and methylated using a modified Folch method as described previously [10,11]. Briefly, 100 μl of plasma was added to 0.4 ml of phosphate buffered saline solution. After the addition of an internal standard (30 μg of heptadecanoic acid), the sample was mixed with 3 ml of chloroform: methanol (2:1 v/v) and vortexed. The sample was then incubated on ice for 10 min, vortexed and centrifuged at 2500 rpm for 10 min. The bottom infranatant was removed and completely dried under nitrogen gas vapors. The dried sample was then methylated by addition of 0.5 ml 0.0.5M methanolic NaOH and incubated for 3 min at 100°C. Once cooled, 0.5 ml of BF3-methanol was added, and the sample was incubated at 100°C for an additional minute. After cooling, the sample was mixed with 1 ml of hexane, followed by 6.5 mL saturated NaCl solution. The sample was vortexed and centrifuged at 1700 rpm for 4 min and the upper hexane phase was transferred to a fresh vial and quantified by gas chromatography-mass spectroscopy (GC-MS). Peak identification was based on comparisons of retention time and mass spectra to known standards within the GC-MS database. Fatty acid methyl ester mass was determined by comparing areas of unknown fatty acid methyl esters to that of a fixed concentration of 17:0 internal standard. Individual fatty acids are expressed as a percent of the total fatty acid mass (mol%).
2.4.3. Intestinal samples and morphometry measurements
Samples of intestinal tissue resected at the time of surgery and remnants at sacrifice were collected from proximal jejunum (proximal to anastomosis) and distal ileum (distal to anastomosis). The intestinal samples were cut into 0.5 cm segments and put into plastic cassettes. The cassettes were put into 10% buffered formalin for 24 h and then transferred to 70% ethanol at room temperature. Tissue was embedded in paraffin and 5 um sections were stained with hematoxylin eosin solution, dehydrated, cleared and mounted on slides. The average villus height and crypt depth were measured in at least 10–20 fields from three sections per sample with Scion Image software (Alpha 4.0.3.2 release, Scion Corporation 2000–2001, Maryland, USA) and an Axiophot microscope (Carl Zeiss, Werk Gottingen, Germany) at 5X magnification. Only intact villi and crypts were measured. All measurements were made by the same, single, blinded observer.
2.4.4. Fecal losses of fatty acids and thin layer chromatography of intestinal enterocytes
Fecal losses of fatty acids
Colon contents were removed and placed in a cryovial for long-term storage at −80°C at the time of tissue collection. Frozen samples of colon contents were thawed, vortexed thoroughly, and 250 uL was removed for fatty acid extraction. To account for the varying water content of each sample, the aliquot was placed in a pre-weighed glass conical tube, and dried in a 70°C heat block apparatus under a stream of nitrogen vapors for 2 hours. The conical was then re-weighed and the dry weight determined for each sample. Phosphate buffered saline (0.5 mL) was then added to each tube and fatty acids were extracted, methylated and quantitated by GC-MS.
Thin layer chromatography
Approximately 50 mg segments of distal ileum were homogenized in 1.0 mL of Phosphate- Buffered Saline solution using a Fisher PowerGen 125 homogenizer. Five hundred microliters of the aliquot was removed and extracted using a modified Folch method [10]. The extracted sample was dried under N2 vapors, and reconstituted in dichloromethane-methanol (2:1 v/v). Samples were then applied to a Whatman Silica Gel G thin layer chromatography (TLC) plate and exposed to a two solvent system, Solvent 1: diethyl ether: petroleum ether: glacial acetic acid (65:35:0.5ml) followed by Solvent 2: diethyl ether; petroleum ether (6:94ml). A TLC reference standard (Nu-Chek Prep 18-5A; Elysian, MN) was simultaneously run with samples in order to properly identify lipid classes. After exposure to iodine vapors, the appropriate area corresponding to the phospholipid class was scraped, extracted, methylated, and fatty acids were identified and quantitated by GC-MS.
2.4.5 Cellular apoptosis and proliferation
Apoptosis
Approximately 100mg of distal ileum tissue was homogenized thoroughly using 500μl RIPA buffer (Boston BioProducts; Ashland, MA) supplemented with 1mM phenylmethylsulfonyl fluoride, 0.2mM sodium orthovanadate and protease inhibitor (cOmplete™ Mini EDTA-free, Roche; Basel, Switzerland). Homogenized samples were kept on ice for 30 minutes and then sonicated with an ultrasonic probe (Sonifier cell disruptor model w185, heat systems ultrasonics Inc., Plainview, NY) for 2–5 min (in rounds of 10 seconds sonication/10 seconds rest for each cycle) on ice. The lysates were centrifuged at 17000xg for 20 min at 4°C. The resulting supernatants were collected and protein concentrations were determined using the colorimetric BCA assay (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific; Waltham, MA). Samples were reduced and denatured at 95°C in Laemmli buffer (Bio-Rad; Hercules, CA) supplemented with 10mM DTT. Samples were run on SDS-PAGE gel and transferred to nitrocellulose membranes. To block non-specific binding sites, membranes were incubated for one hour at room temperature in Tris-buffered saline-Tween 20 (TBS-T) containing 5% of nonfat milk. Membranes were next immunoblotted with a primary antibody against both total and cleaved caspase-3 (Cell Signaling, Danvers, MA, catalog #9662) at 4°C overnight diluted in 5% BSA/TBS-T. Next day, membranes were washed three times using TBS-T for 10 min each on a shaker at room temperature (RT). Subsequently, membranes were incubated with HRP conjugated secondary antibody for 1h at RT on a shaker and then washed three times as above. The blots were developed by Clarity™ Western ECL Blotting Substrate (Bio-Rad) according to the manufacturer’s instructions. Membranes were next stripped using a stripping buffer (Bio-Rad) for 15 min at RT, washed as above and blocked using 5% milk/TBS-T for 1h. Membranes were immunoblotted with primary antibody anti-GAPDH (Ambion, AM4300, Carlsbad, CA) diluted in 5% milk/TBS-T for normalization and developed as described above. Protein bands of interest were visualized using a FluorChem R system (ProteinSimple; San Jose, CA). Densitometric quantification of the bands and normalization to GAPDH was performed using AlphaViewSA software (ProteinSimple).
Proliferation
Immunofluorescence staining for cellular proliferation using paraffin embedded distal ileum segments was performed with Ki-67. The sections on a slide were deparaffinized and hydrated. Antigen retrieval was performed by boiling the slides for 10 minutes in 10mM sodium citrate pH 6.0 with a pressure cooker. The sections were then incubated with 1mg/ml sodium borohdyride (MP-Biomedicals; Santa Ana, CA) for 5 minutes at room temperature. After three washes with TBS, the sections were incubated with 5% normal donkey serum (Jackson ImmunoResearch Lab Inc.; West Grove, PA) for an hour at room temperature. Slides were then incubated with rabbit anti-Ki-67 (1:200, Abcam Cat# 15580). The slides were washed three times with TBS and incubated with 1:200 Alexa 647 conjugated Donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch Lab; West Grove, PA). Samples were then washed three times with TBS and the slides were mounted with Prolong Gold anti-fade mounting media containing DAPI (Invitrogen). The fluorescent images were taken using a Zeiss AxioImager wide field fluorescent microscope using Zeiss AxioVision image acquisition software. The images were taken using a 20x/0.8 Plan-Apochromat objective. Images were analyzed using Volocity visualization and quantitation software (Perkin Elmer; Waltham, MA). At least three crypts per image and five images per piglet were analyzed. Both Ki67 and DAPI were quantified using the same parameters to determine the number of proliferating nuclei compared to non-proliferating nuclei.
2.5. Statistical Analyses
The primary outcome measure was a change in plasma DHA level of 2-fold or greater in the DHA-ALT® group versus control group. Prior to the study it was determined that a total sample of 10 (5 piglets per group) would give 80% power to detect a 2-fold difference between the two groups.
Given the number of piglets per group, assumptions of normality are difficult to assess and thus more robust nonparametric analytical tools were used to evaluate differences in outcome variables between groups. Variable summary statistics are expressed as median ± interquartile range (IQR). The Wilcoxon rank sum test was used to compare outcome measures between groups; while the Wilcoxon signed-rank test was used to compare paired, before and after surgery outcome measures within groups. Finally, differences in growth measurements and fatty acid levels over time between groups were evaluated using generalized linear mixed models (GLMM) to account for repeated measures and non-Gaussian, non-normal data. All analyses were performed using STATA statistical software, version 13 (StataCorp) and GraphPad Prism version 6.00 for Windows, GraphPad Software (San Diego, CA, www.graphpad.com).
3. RESULTS
3.1. Piglet Characteristics and Weight Trends
Four of the 5 piglets were male in the control group while 3 of the 5 were male in the DHA-ALT® group (Table 1). There was no difference in enteral feeding advancement nor tolerance between the two groups. The median starting weight was similar in both groups. At the end of the protocol, the median ± IQR difference in final weight from starting final weight was 696 ± 425g in the DHA-ALT® group compared to 132 ± 278g in the control group. In concordance with the weight difference the percent weight change and growth velocity was greater in the DHA-ALT® group compared to the control group. However, due to the short duration of this model as well as the sample size, these differences in growth parameters did not reach statistical significance.
Table 1.
Cohort characteristics and weight parameters (median ± IQR)
| Control (n=5) | DHA-ALT (n=5) | p | |
|---|---|---|---|
|
|
|||
| Male, n(%) | 4(80) | 3(60) | 0.51 |
| Weight at start, g | 2490 ± 114 | 2474 ± 325 | 0.75 |
| Weight at finish, g | 2594 ± 170 | 3170 ± 800 | 0.35 |
| Change in weight, g | 132 ± 278 | 696 ± 475 | 0.08 |
| Growth velocity, g/kg/d | 13.5 ± 30.1 | 69.9 ± 41.5 | 0.08 |
| Weight change, % | 5.4 ± 12.1 | 28.0 ± 16.6 | 0.08 |
IQR: Interquartile range; DHA-ALT: Treatment group with Docosahexaenoic acid with Advanced Lipid Technology
3.2. Small Bowel Resection and Final Organ Weights
There were no differences in the total length or weight of resected bowel per kg weight of the piglet (Table 2). A median ± IQR total of 138 ± 34 cm per kg of small bowel was removed from piglets in the DHA-ALT® group compared to 119 ± 7 cm per kg in the control group. Similarly, there were no differences in the final length or weight of the jejunum and ileum segments of the small bowel per kg weight. Finally, the liver and brain weights per kg at sacrifice were comparable between the two groups.
Table 2.
Small intestine resection and final organ weights (median ± IQR)
| Control | DHA-ALT | p | |
|---|---|---|---|
|
|
|||
| Resected SI, g/kg | 29.5 ± 2.2 | 28.7 ± 4.9 | 0.92 |
| Resected SI, cm/kg | 119 ± 7.0 | 138 ± 3.4 | 0.17 |
| Jejunum, g/kg | 5.2 ± 1.4 | 5.1 ± 0.5 | 0.92 |
| Jejunum, cm/kg | 21.2 ± 6.7 | 16.5 ± 3.8 | 0.60 |
| Ileum, g/kg | 5.3 ± 1.2 | 7 ± 1.4 | 0.05 |
| Ileum, cm/kg | 17.5 ± 4.7 | 19.2 ± 4.4 | 0.33 |
| Liver, g/kg | 36.3 ± 2.5 | 37.0 ± 4.2 | 0.71 |
| Brain, g/kg | 14.0 ± 1.6 | 11.6 ± 2.3 | 0.09 |
IQR: Interquartile range; SI: Small Intestine; DHA-ALT: Treatment group with Docosahexaenoic acid with Advanced Lipid Technology
3.3. Plasma Fatty Acid Levels
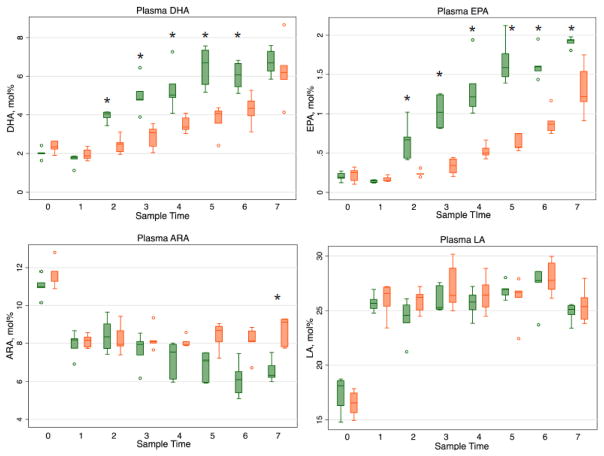
Initial median ± IQR plasma fatty acid levels in mol% (Time 0) of DHA, Eicosapentaenoic acid (EPA), ARA, and Linoleic acid (LA) obtained at the time of surgery were similar between groups (2.0 ± 0.04 vs 2.3 ± 0.4; 0.2 ± 0.06 vs 0.3 ± 0.1; 11.0 ± 0.3 vs 11.8 ± 0.5; 18.1 ± 2.3 vs 16.6 ± 1.8, respectively) (Figure 1). Within 12 hours (after the first enteral dose of DHA), the median ± IQR DHA and EPA levels were significantly greater in the DHA-ALT® group versus control group (4.1 ± 0.3 vs 2.5 ± 0.5, p=0.009; 0.7 ± 0.3 vs 0.2 ± 0.005, p=0.009, respectively). For DHA these differences persisted until the final measurement; while the EPA plasma level differences persisted throughout the remaining duration of the protocol. On the third post-operative day, median ± IQR plasma ARA levels began to diverge between the groups, with lower values in the DHA-ALT® group versus the control group (7.1 ± 1.5 vs 8.7 ± 0.8, p=0.03). The difference persisted throughout the remaining duration of the protocol. There were no differences throughout the study protocol in plasma LA levels.
Figure 1.
Plasma fatty acid profiles (mol%) in DHA-ALT® (green) versus Control (orange) groups. Data represented as longitudinal box and whisker plots with line at the median value. DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic acid; ARA: Arachidonic acid, LA, Linoleic acid. Sample time 0= day of surgery; 1= postoperative day (POD) 1/AM; 2=POD 1/PM; 3=POD 2/AM; 4= POD 2/PM; 5=POD 3/AM; 6= POD 3/PM; 7= POD 4/AM. *p< 0.05.
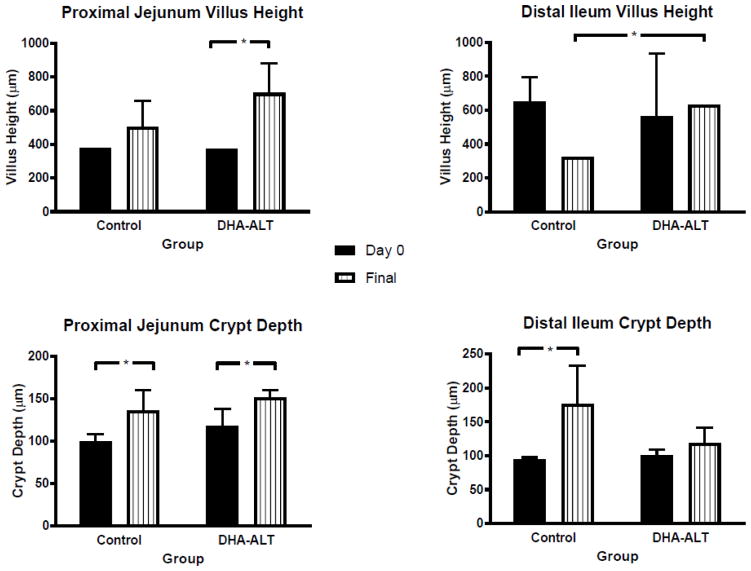
3.4. Small Bowel Morphometry
At the time of surgery (Day 0), villus height measurements of the proximal jejunum were the same comparing across the two groups (Figure 2). At sacrifice (Final, Figure 2), in the DHA-ALT® group, the villus height was significantly increased compared to Day 0 (p=0.04). Both groups showed an increase in crypt depth in the proximal jejunum comparing Day 0 with Final (p=0.04).
Figure 2.
Intestinal morphology before and after surgical induction of SBS and DHA treatment. SBS: short bowel syndrome; DHA: Docosahexaenoic acid; Data represents median ± IQR. Black bars represent tissue morphometry from samples obtained at the time of surgery (Day 0); Striped white bars represent tissue morphometry from samples obtained at the end of the study protocol (Final). *p< 0.05.
In the distal ileum, the villus height was no different at Day 0 across the two groups. The villus height was maintained in the DHA-ALT® group. In the control group, although the median villus height decreased from Day 0 to Final, this was not statistically different (p=0.07). The ileal villus height on Final was significantly increased in the DHA-ALT® group compared to the control group (p= 0.01). Distal ileum crypt depth was increased comparing Day 0 with Final in both groups although it was statistically significant only for the control group (p=0.04).
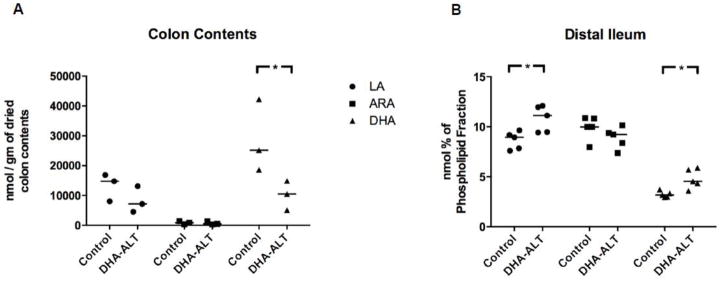
3.5. Fecal losses of fatty acids and thin layer chromatography of intestinal enterocytes
Colon contents from three piglets from each group contributed to the analysis of the presence of fatty acids left unabsorbed in the intestinal lumen expressed as nanomols per gram of dried colon contents (nmol/gm). There was an approximately two-fold increase in the median ± IQR concentration of LA (14,763 ± 8840 vs. 7139 ± 8573 nmol/gm) and ARA (840 ± 1031 vs. 466 ± 1025 nmol/gm) remaining in the lumen of the colon of the control group compared to the DHA-ALT® group (Figure 3, Panel A) but neither reached statistical difference (p=.13 and .51, respectively). Piglets receiving the control DHA had a 2.4-fold increase in the intestinal loss of DHA compared to piglets receiving DHA-ALT® (median ± IQR of 25,168 ± 23,645 vs. 10,522 ± 9,822 nmol/gm. This difference was marginally statistically significant at p=.0495.
Figure 3.
Fatty acid losses as measured in colon contents (n=3, Panel A) and fatty incorporation in the phospholipid fraction of distal ileum tissue (n=5, Panel B). Data represented as scatter dot plots. Each symbol represents an individual piglet. Bar represents the median value. *p< 0.05.
To examine whether these differences in fatty acid absorptions were linked to higher or lower incorporation of these specific fatty acids into the ileal tissue, we quantified the fatty acid composition of the phospholipid fraction in the distal ileum expressed as mol%. Compared to control samples, the enterocytes of the distal ileum of piglets receiving DHA-ALT® had a greater median mol% (IQR) of the phospholipid class as LA (11.1 (2.5) versus 8.9 (1.3) mol%; p=.03) and DHA (4.5 (1.3) vs. 3.2 (0.3) mol%; p=.02) compared to the control piglets (Figure 3, Panel B). The mol% for ARA was not different between the groups (p=0.2).
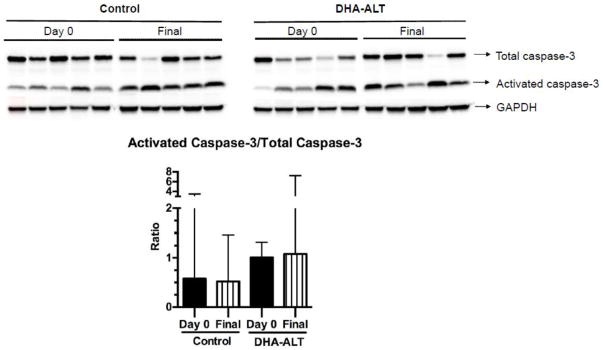
3.4. Cellular apoptosis and proliferation
Apoptosis, as measured by the ratio of activated caspase-3 to total caspase-3, was not significantly different between Day 0 and Final samples within each of the two groups (Figure 4).
Figure 4.
Comparison of activated caspase-3/Total caspase-3 ratios in Day 0 and Final samples in the two treatment groups. The western blot is shown for Day 0 and Final time points in each treatment arm (n=5 in each group). Densitometric quantification (median ± IQR) of the western blot data is shown. Black bars represent Day 0 samples; Striped white bars represent Final samples.
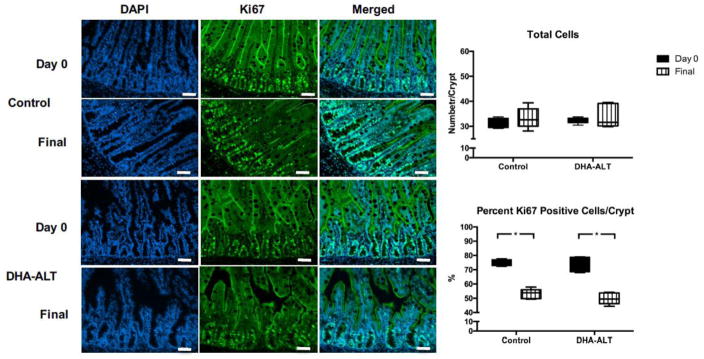
For assessment of proliferation, total cell counts in crypts were quantified and expressed as number of cells/crypt (cells/crypt). The total nuclei count of the distal ileum did not change collectively over time from Day 0 to Final within each group. (Figure 5). All piglets regardless of group demonstrated a significant decrease of the median (IQR) percent Ki67 positive cells in the crypt from Day 0 to Final specimens (75.7 (5.6) vs. 51.6 (4.8) %, p=.0002). The degree of negative change was equal comparing the control and DHA-ALT® groups.
Figure 5.
Ki67 immunofluorescence with quantification. Immunofluorescence for DAPI (total nuclear staining), Ki67, and the merged images are shown. White bar in lower right corner of images = 50 μM. In the right panel, total nuclei cell count per crypt and percent ki67 positive cells per crypt are shown as box and whisker plots with a line at the median value. Black bars represent Day 0 samples; striped white bars represent Final samples. *p< 0.05.
4. DISCUSSION
The data support the hypothesis that enteral delivery of DHA-ALT® results in higher blood levels of DHA compared to a control DHA preparation due to improved intestinal absorption. Piglets with surgically induced SBS who were given a novel, pre-emulsified preparation of DHA bypassing the need for endogenous micelle formation demonstrated reduced fecal losses of DHA, greater systemic and ileal tissue levels of DHA, and a trend towards improved growth compared to piglets given a standard preparation of DHA. Within 12 hours, the plasma levels of both DHA and EPA in the DHA-ALT® group began to significantly diverge from the control group. The increase in EPA is likely due to retro-conversion of DHA to EPA as neither DHA formulation contained an EPA supplement. The observed reduction in ARA with increasing DHA levels in the DHA-ALT® group is a pattern observed and documented by other investigators [12]. As DHA levels increase with DHA-only dietary supplementation, ARA levels decline likely as a result of the competition for enzyme activity, the sn-2 position of phospholipids in membranes, as well as the body’s ability within the plasma compartment to maintain a constant number of double bonds [13,14]. This is not a generalized phenomenon as LA levels were unchanged throughout the experimental period. This study adds to the current literature demonstrating increased systemic and tissue DHA levels through modification of the carrier system and/or fatty acid structure to facilitate absorption and optimize tissue incorporation [15].
The ability to perform a coefficient of fat absorption that traditionally requires a 3-day block collection of intakes and fecal outputs in humans as an additional measure of intestinal absorption was not feasible. Thus, we elected to incorporate several different measurements that together strongly suggested improved absorption – a onetime measurement of fecal DHA losses, phospholipid incorporation of DHA by the intestinal epithelium, and plasma levels of DHA. A onetime assessment of fecal DHA is likely comparable across pigs as they were all maintained on the same diet. Actual phospholipid bound DHA in the cell membrane is the gold standard of absorption as it requires bioavailability of DHA to make this tissue level change. Finally, blood levels would represent the most vigorous analysis of fatty acid bioavailability as it represents the fidelity of triglyceride hydrolysis as well as intestinal absorption and ultimate delivery to the blood.
An increase in villus height and crypt depth is a known observation in intestinal adaptation after significant bowel resection in both animal models and in humans [16]. Seven of the eight paired measurements of villus height and crypt depth demonstrated no change or an increase in these quantitative measures comparing Day 0 with Final across both groups suggesting that in this short duration study, our model of SBS with DHA treatment did not demonstrate adverse intestinal adaptation. Additionally, the morphologic data did not clearly demonstrate that the treatment group influenced intestinal adaptation beyond what was observed in the control group. Although we did show an increase in the final villus height in the DHA-ALT® group compared to control DHA, our measures of apoptosis and proliferation did not explain this difference. Whether the improved ileal incorporation seen in the DHA-ALT® group is modulating intestinal cell growth and differentiation through other mechanisms such as peroxisome proliferator-activated receptor (PPAR) or other growth factors in this model of SBS remains to be determined. The etiology for both the jejunal and ileal changes may reflect the short term nature of this model whereby over the long term, more significant changes in the ileum may occur.
The increase in growth velocity demonstrated by the pigs in the DHA-ALT® was not statistically significant, but may be clinically relevant. A full assessment in growth parameters was limited due to the small numbers of pigs per group as the study was powered to demonstrate a difference in DHA absorption not in growth. A longer term study is being planned. Lastly, there were no concerning observations regarding safety comparing the two groups.
Current strategies for nutritional induced intestinal adaptation after bowel resection are limited. High fat diets and diets rich in long chain polyunsaturated fatty acids have shown benefit in rodent models of SBS [2–5] and have been shown to be effective in human infant clinical trials [17,18]. We have shown that DHA-ALT®, a pre-emulsified enteral DHA formulation, improved intestinal absorption and increased systemic and intestinal fatty acid levels compared to a non-emulsified, control preparation. This study represents the first to use DHA in a porcine model of SBS and substantiates the benefit of a more bioavailable form of DHA.
Acknowledgments
This study was supported by a research grant from Sancilio and Company, Inc. (Riviera Beach, FL). In addition, CRM was in part supported by NIDDK, R01DK104346; DB and BS was supported in part by federal funds from the U.S. Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, NIH Grant DK-094616 (DGB), and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas. The content is solely the responsibility of the authors and does not necessarily represent the official views or policies of Harvard University and its affiliated academic health care centers, the National Center for Research Resources, the National Institutes of Health, or the U.S. Department of Agriculture. The mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. None of the funding bodies had any role in the study design or conduct; data collection, management, analysis or interpretation; or manuscript preparation, review, or approval. CRM, KMG MP, and SDF serve on the Scientific Advisory Board at Sancilio & Company (Riviera, FL).
ABBREVIATIONS
- ALT®
Advanced Lipid Technology®
- ARA
Arachidonic acid
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- LA
Linoleic acid
- PN
parenteral nutrition
- SBS
short bowel syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goulet O, Olieman J, Ksiazyk J, Spolidoro J, Tibboe D, Köhler H, et al. Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clin Nutr. 2013;32:162–71. doi: 10.1016/j.clnu.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr. 1999;28:41–5. doi: 10.1097/00005176-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Lan T, Chen Y, Dawson PA. Dietary fish oil increases fat absorption and fecal bile acid content without altering bile acid synthesis in 20-d-old weanling rats following massive ileocecal resection. Pediatric Research. 2012;72:38–42. doi: 10.1038/pr.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi PM, Sun RC, Guo J, Erwin CR, Warner BW. High-fat diet enhances villus growth during the adaptation response to massive proximal small bowel resection. J Gastrointest Surg. 2014;18:286–94. doi: 10.1007/s11605-013-2338-7. discussion 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhotnik I, Shany A, Bashenko Y, Hayari L, Chemodanov E, Mogilner J, et al. Parenteral but not enteral omega-3 fatty acids (Omegaven) modulate intestinal regrowth after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2010;34:503–12. doi: 10.1177/0148607110362586. [DOI] [PubMed] [Google Scholar]
- 6.Pereira-Fantini PM, Lapthorne S, Joyce SA, Dellios NL, Wilson G, Fouhy F, et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J Hepatol. 2014;61:1115–25. doi: 10.1016/j.jhep.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Bongaerts GP, Severijnen RS, Tangerman A, Verrips A, Tolboom JJ. Bile acid deconjugation by Lactobacilli and its effects in patients with a short small bowel. J Gastroenterol. 2000;35:801–4. doi: 10.1007/s005350070016. [DOI] [PubMed] [Google Scholar]
- 8.Ohkohchi N, Andoh T, Izumi U, Igarashi Y, Ohi R. Disorder of bile acid metabolism in children with short bowel syndrome. J Gastroenterol. 1997;32:472–9. doi: 10.1007/BF02934085. [DOI] [PubMed] [Google Scholar]
- 9.Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159:743–749. e1–2. doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin CR. Fatty acid requirements in preterm infants and their role in health and disease. Clin Perinatol. 2014;41:363–82. doi: 10.1016/j.clp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Tu WC, Cook-Johnson RJ, James MJ, Mühlhäusler BS, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;83:61–8. doi: 10.1016/j.plefa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Bojesen IN, Bojesen E. Nature of the elements transporting long-chain fatty acids through the red cell membrane. J Membr Biol. 1998;163:169–81. doi: 10.1007/s002329900381. [DOI] [PubMed] [Google Scholar]
- 15.Subbaiah PV, Dammanahalli KJ, Yang P, Bi J, O’Donnell JM. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim Biophys Acta. 2016;1861:723–9. doi: 10.1016/j.bbalip.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. JPEN J Parenter Enteral Nutr. 2014;38:427–37. doi: 10.1177/0148607113512678. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Welch CD, Ayers K, Turner C, Pranikoff T. Early enteral fat supplementation with microlipid® and fish oil in the treatment of two premature infants with short bowel. Neonatology. 2010;98:348–53. doi: 10.1159/000316067. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Ayers K, Welch CD, O’Shea TM. Randomized controlled trial of early enteral fat supplement and fish oil to promote intestinal adaptation in premature infants with an enterostomy. J Pediatr. 2014;165:274–279. e1. doi: 10.1016/j.jpeds.2014.02.002. [DOI] [PubMed] [Google Scholar]