Abstract
Advances in cardiac imaging have resulted in greater recognition of cardiac amyloidosis (CA) in everyday clinical practice, but the diagnosis continues to be made in patients with late stage disease, suggesting that more needs to be done to improve awareness of its clinical manifestations and the potential of therapeutic intervention to improve prognosis. Light chain CA (AL-CA) in particular, if recognized early and treated with targeted plasma cell therapy, can be managed very effectively. For patients with transthyretin amyloidosis, there are numerous therapies that are currently in late phase clinical trials. In this review we address common questions encountered in clinical practice regarding etiology, clinical presentation, diagnosis and management of cardiac amyloidosis, focusing on recent important developments in cardiac imaging and biochemical diagnosis. The aim is to show how a systematic approach to the evaluation of suspected CA can impact the prognosis of patients in the modern era.
Keywords: cardiac amyloidosis, transthyretin, light chain
Introduction
What is amyloidosis?
Amyloidosis is a localized or systemic deposition disease in which proteins with unstable tertiary structures misfold, aggregate and form amyloid fibrils that deposit with a range of chaperone proteins in the heart, kidneys, liver, gastrointestinal tract, lungs and soft tissues1. Individual amyloid fibrils are non-branching, 7 to 10 nm in width and of variable length. They are insoluble and notably resistant to proteolysis2. The term “lardaceous disease” was first used to describe such deposits at autopsy but was replaced by the botanical term “amyloid” because of their starch-like affinity for iodine3. In polarized light under the microscope, the deposits, when stained with the dye Congo red, display apple-green birefringence, changing from a hyaline pink appearance in normal light4. Under electron microscopy amyloid fibrils appear as needle-like structures with multiple filaments that have a unique highly organized cross β-pleated-sheet configuration5. Among the signature chaperone proteins found in all amyloid deposits are the pentraxin serum amyloid P component (SAP), clusterin, vimentin, vitronectin, glycosaminoglycans and apolipoproteins6.
What forms of amyloidosis affect the heart?
There are more than thirty proteins that can form amyloid fibrils, five of which frequently infiltrate the heart and cause CA: immunoglobulin light chain (AL or primary amyloidosis), immunoglobulin heavy chain (AH), transthyretin (ATTR), serum amyloid A (AA), and apolipoprotein A I (AApoA1). The overwhelming majority of patients with CA are affected by either AL or ATTR CA. While the general mechanism of extracellular deposition of fibrillary proteins leading to tissue and organ dysfunction is common to all forms of CA, there are three major precursor proteins causing CA, each with differing natural histories and therapies including AL and ATTR due to a mutation (ATTRm) or wild type (ATTRwt).7
Epidemiology and Delays in Diagnosis
Is cardiac amyloidosis rare?
AL-CA is a rare condition with an estimated prevalence of 8 to 12 per million8–10. There are ~3,000 newly diagnosed cases of AL amyloid per year in the United States (US) of which 30–50% of which have symptomatic cardiac involvement and 10–15% occur in association with multiple myeloma11. There are currently ~10,000 individuals affected with AL in the US, consistent with the definition of an orphan disease as one that affects less than 200,000 persons. The population prevalence of ATTR-CA is less certain, but recent data suggest that it is overlooked as a cause of common cardiovascular conditions in older people including heart failure with preserved ejection fraction (HFpEF), low flow aortic stenosis and atrial fibrillation12, 13. Autopsy data has demonstrated that among adults more than 80 years of age, 25% have transthyretin amyloid deposits in the myocardium14. Among patients with HFpEF, autopsy data reveal amyloid deposits in 32% of those over 75 years of age compared with 8% of those aged <75 years (8%)15, 16. Emerging data using nuclear scintigraphy has suggested that 13% (95% CI 7.2–19.5%) of patients hospitalized with HFpEF may have ATTRwt-CA17. Some patients, however, have disease caused by mutations in the TTR gene and although these are relatively rare, some genetic forms are endemic in particular geographic regions. The Val122Ile mutation, which almost exclusively affects individuals of African or Afro-Caribbean descent, has a population prevalence of 3–4%18 and while clinical penetrance of this variant is incomplete in subjects up to 80 years of age, it is almost certainly under-recognized as a cause of heart failure19. Emerging data suggest that ATTR-CA, especially due to ATTRwt, is not uncommon and will become the most commonly diagnosed form of CA.
How often is the diagnosis missed or delayed?
CA is often misdiagnosed and the delayed diagnosis has significant consequences for patients. In a survey of more than 500 patients with AL (37% of whom had cardiac involvement) the average time from initial symptoms to diagnosis was 2 years. A substantial proportion of patients (31.8%) reported seeing at least 5 physicians before receiving a diagnosis of amyloidosis. Cardiologists were consulted more often than hematologists, oncologists or nephrologists, but were responsible for making the diagnosis in only 18.7% of cases. Similar data for ATTR-CA, albeit in much smaller populations, reveals that less than half of subjects were diagnosed within 6 months, mainly by cardiologists.20
What are the impediments to early and accurate diagnosis?
CA is a “great pretender”, often misdiagnosed as another condition or delayed in its recognition as a result of both physician and disease related factors. Optimum diagnosis and care requires multidisciplinary management (cardiology, neurology, nephrology and hematology) but amyloidosis teams are relatively few. Additionally, the absence of disease modifying therapies for ATTR and the late presentation of AL-CA patients has contributed to a nihilistic attitude about the condition.
The main disease-related factor that hinders a correct and timely diagnosis is heterogeneity with respect to the cardiac phenotype as well as systemic involvement. The need for a histological demonstration of target organ amyloid infiltration has also delayed the diagnosis as the technique is restricted to referral centers with expertise in the performance of endomyocardial biopsy and requires skilled pathologic analysis of obtained samples. Phenotypic variability of genetic forms of ATTR is also influenced by genetic heterogeneity, geography, endemic vs. non-endemic aggregation, age, sex of the patient and transmitting parent, and probably amyloid fibril composition21, 22,23. Patients also rarely report a family history because of the late presentation and incomplete penetrance.
In whom should one consider cardiac amyloid?
The diagnosis of CA requires a high index of clinical suspicion. In the past, the presence of multi-organ involvement was over-emphasized, resulting in consideration of the diagnosis only in the presence of extreme extra-cardiac findings such as macroglossia and periorbital purpura that while specific, are present only in a minority of AL cases and absent in ATTR-CA. A number of diagnostic red flags (Table 1) in a patient with otherwise unexplained left ventricular hypertrophy or restrictive cardiomyopathy can foster the correct suspicion. A history of carpal tunnel syndrome in ATTR (particularly if bilateral in a male), atraumatic rupture of biceps tendon, unexplained neuropathic pain, orthostatic hypotension and a diagnosis of “hypertrophic cardiomyopathy” after the 6th decade of life are useful clues.
Table 1.
Red Flags and caveats in cardiac amyloidosis
|
Key point.
Increased awareness of cardiac amyloidosis is essential to reduce under-diagnosis and misdiagnosis, which is critical because prognosis worsens rapidly with advancing organ dysfunction.
Pathophysiology and Natural History
Why is the heart affected in some cases of amyloidosis and not others?
The heterogeneity of systemic amyloidosis remains puzzling. Patients with the same type of amyloidosis, whether AL or ATTR, can have different patterns of organ involvement and different amounts of amyloid in the involved organs. Patients with systemic AL amyloidosis without heart involvement at diagnosis are likely to develop cardiac involvement if their culprit light-chain proteins are not eliminated and if they live long enough24. The basis for the organ tropism of AL is not known. Studies of the immunoglobulin light-chain genetic clones underlying AL indicate that the repertoire of light-chain germline genes used in AL is restricted; four light-chain variable region germline genes (IGKV1-16, IGLV6-57, IGLV2-14, IGLV3-1) account for two thirds of cases, and there is tropism for the heart with IGLV2-14 and IGLV3-125–29 although the mechanism is unknown30. The explanation for the differential involvement of the heart and peripheral nervous system in patients with ATTR is also unexplained.
What is the natural history of ATTR cardiac amyloidosis?
ATTR-CA may be due to specific mutations that have a predominant involvement of the heart (Val122Ile, Leu111Met, Ile68Leu) or ATTRwt (Table 2). Ile68Leu and Leu111Met are mutations reported almost exclusively in Italy and Denmark, respectively, causing a severe cardiomyopathy at an early age with a malignant course22, 31–33. In the US, ATTRwt followed by Val122Ile and Thr60Ala mutation forms are most common34. Original reports7, 35, 36 suggested that ATTRwt-CA had a median survival of >5 years. However, recent reports37–39 have demonstrated that outcomes are worse with median survivals of ~3.5 years after initial evaluation40. These discrepancies are in part related to difficulty in defining when the disease begins. Initial manifestations of TTR deposits often include bilateral carpal tunnel syndrome41, which is found in ~70% of individuals with ATTRwt-CA on average 5–7 years prior to the cardiac manifestations. Other manifestations include atrial arrhythmias and a progressive decline in effort tolerance, which are difficult to definitively attribute to amyloidosis as they occur commonly with advancing age. However, it is clear that ATTRwt-CA can manifest as early as the fifth decade of life. Cohort studies among subjects with ATTR-CA are predominately single center reports37, 39, 42. One, multicenter, prospective cohort study (Transthyretin Amyloid Cohort Study – TRACS)38, demonstrated in a small (n=29) population that mortality was high (29%) and differed between ATTRwt (22%) and Val122Ile (79%). Larger single center studies have not confirmed this difference in survival and multivariate analyses of TRACS and the THAOS registry data suggests that cardiac function and not the presence of a mutation was independently associated with mortality34, 42, 43. Progressive left ventricular dysfunction in ATTR-CA is mediated by physiologic derangements that include reduction in chamber capacitance, declines in chamber contractility and increases in arterial elastance.43. Patients with ATTRwt-CA have impairments in peak VO2 and in a large series from the UK National Amyloidosis Center, a positive troponin T, presence of a pacemaker, and NYHA class IV symptoms were associated at baseline with a worse outcome39. Clinically, as ATTR-CA progresses, blood pressure falls due to reduction in cardiac output and heart rate increases to compensate for a reduced stroke volume. Every 6 months, the mean 6-minute walk distance declined by 25.8 meters, NT-pro-BNP increased by 1,816 pg/mL, and left ventricular ejection fraction fell by 3.2%38. Cardiac cachexia is particularly common with ATTR-CA, potentially mediated in part by right heart failure, liver congestion, and possibly alterations in bowel flora.
Table 2.
Phenotypic findings in AL-CA and in the most frequent types of ATTR-CA
| AL39, 47 | ATTRwt34, 37, 39 | Val122Ile34 | Ile 68Leu31 | Thr60Ala33 | |
|---|---|---|---|---|---|
| Median age at diagnosis (yr) | 62 | 76 | 70 | 70 | 62 |
| Males (%) | 66% | 95% | 75% | 75% | 70% |
| Common ethnicity | Variable | Caucasian | Afro-American Caribbean | Caucasian (Italy) | Caucasian (USA Ireland) |
| Cardiac referral route (%) | 65 | >80 | >80 | >80 | 30 |
| IVS/PW (median values) | 15/14 | 18/17 | 17/17 | 17/16 | 17/17 |
| LVEF -% | 56 | 50 | 50 | 50 | 53 |
| Low QRS voltages (%) | 45 | 33 | 45 | 30 | 16 |
| Peripheral sensory-motor neuropathy (%) | 10–20 | <10 | 15 | 25% | 54 |
| History of carpal tunnel syndrome (%) | <10 | 30–45 | 30 | 37 | Unknown |
| Autonomic symptoms (%) | 24 | 12–20 | 10 | <10 | 75 |
| Median survival (yr) | Depends on stage | 3.5 | 2–3 | 4–5 | 3.5 |
| NTproBNP pg/L (median) | ↑↑↑ | ↑↑ | ↑ | ↑ | ↑ |
Key point.
ATTR has been considered a neurologic disease but data from worldwide registries suggest that the heart is the main affected organ in at least half of the cases. ATTR cardiac amyloidosis is a progressive disorder associated with significantly morbidity and mortality.
Diagnosing Cardiac Amyloidosis
Is the ECG useful for identifying CA?
Classically, CA is characterized by low QRS voltage in spite of an increase LV wall thickness observed with non-invasive imaging. In contemporary series, however, the prevalence of low voltage varies with etiology, ranging from 60% in AL to 20% in ATTR44, 45. The absence of low QRS voltages does not, therefore, preclude CA, especially in patients with ATTRwt in whom left ventricular hypertrophy or left bundle branch block is present in up to 30% of patients. The hallmark of CA is the disproportion between left ventricular wall thickness and QRS voltages. While this relationship can be expressed in a relatively complex way46, it can be assessed more simply using left ventricular wall thickness/total QRS voltage ratio47. Pseudo-infarction patterns, present in up to 70% of cases, can lead to an initial misdiagnosis of coronary heart disease. A significant number of patients develop conduction disease and the presence of AV block in an older patient with left ventricular hypertrophy should always prompt consideration of CA.
Is there a lab test to diagnose CA?
As yet, no blood test can identify TTR oligomers and diagnose ATTR-CA. In contrast, in AL, serum and urine immunofixation and quantification of serum free light chains in combination have a 99% sensitivity for identifying the underlying substrate for AL-CA. Importantly, an abnormal free light chain ratio alone is not specific for AL amyloidosis as up to 5% of the population over 65 years of age have a monoclonal gammopathy of undetermined significance (MGUS)48. This can lead to a misdiagnosis of AL-CA in elderly patients who actually have TTR-related CA and MGUS (up to 10% of misdiagnosis even in referral centers)49. In addition, free light chains are filtered by the glomeruli, with renal dysfunction resulting in increased serum concentrations. Renal dysfunction affects kappa and lambda free chains differently and a wider reference range for FLC κ/λ ratio has been proposed for use in patients with advanced renal failure50. The broader κ/λ ratio normal ranges in patients with low GFR makes the diagnosis of AL-CA more challenging in patients with renal failure. While many patients with renal failure have increased left ventricular wall thickness and serum β2-microglobulin can be associated with increased wall thickness51, beta-2 microglobulin amyloid deposition rarely causes meaningful cardiac dysfunction52. Natriuretic peptides tend to be particularly high in CA, often out of proportion from the hemodynamic burden53. Circulating light chains exert a direct toxic effect by modulating p38 mitogen-activated protein kinase, which can directly promote NT-pro-BNP expression.54–56 Thus, for the same range of hemodynamic abnormalities, plasma levels of NT-pro-BNP are higher in AL compared to ATTRwt and ATTRm53. An apoptotic effect can cause an elevation of troponins, sometimes leading to false diagnoses of acute coronary syndrome in patients with CA hospitalized for heart failure. Accordingly, the use of new blood tests such as the free light chain assay, NT-pro-BNP and troponin in patients with new onset dyspnea and no obvious etiology may enable the earlier diagnosis of AL-CA. This approach is particularly relevant to patients with pre-existing clonal plasma cell diseases such as MGUS or smoldering myeloma or in patients with unexplained increased wall thickness on echocardiography.
Should everyone with TTR cardiac amyloidosis have gene sequencing?
TTR gene sequencing is recommended in all cases of TTR-related amyloidosis as hereditary types of amyloidosis cannot be distinguished from acquired types on clinical grounds alone and a family history indicating autosomal dominant inheritance is often absent due to incomplete and late disease penetrance. Thus sequencing the transthyretin gene in conjunction with genetic counseling is recommended in cases in which hereditary TTR is proven by mass spectroscopy or suspected even with a negative profile on mass spectroscopy.
Do laboratory tests predict prognosis in CA?
There are well established prognostic models in AL amyloidosis. Current staging systems for AL-CA are based on serum levels of N-terminal pro-brain natriuretic peptide (NT-pro-BNP), cardiac troponin T, and the concentration of circulating free light chains. The combination of the three biomarkers constitutes the most powerful prognostic tool available in AL-CA and is the basis for the staging systems elaborated by the Mayo Clinic57. The scoring system assigns one point for each of the following: NT-pro-BNP ≥ 1800 pg/mL, Troponin T ≥0.025 ng/mL, and difference between the kappa and lambda free light chains ≥ 18 mg/dL. Median survival of stage III patients was only 3.5–4.1 months. Patients with pronounced elevations of both NT-pro-BNP and troponin have a particularly poor prognosis58. Within stage III, NT-pro-BNP > 8500 pg/ml combined with a systolic blood pressure less than 100 mmHg identifies patients with particularly high mortality (Stage III B). NT-pro-BNP has also been shown to be a biomarker of clinical response and progression, and the change in its level after therapy is a surrogate for survival59, with a decrease associated with improved and an increase with worse overall survival60 (Figure 1). Thus, even patients with advanced cardiac amyloid can achieve meaningful improvements in survival if the light chains are reduced and accompanied by a cardiac response reflected by a decline in NT-pro-BNP response, defined as a decrease in NT-pro-BNP of >30% and >300 ng/L assuming a baseline value were ≥650 ng/L. Such responses predict clinical outcome and survival independent of therapy type, treatment class, or regimen61. Emerging data, suggest cardiac biomarkers are also a useful prognostic marker of cardiac function in ATTR62
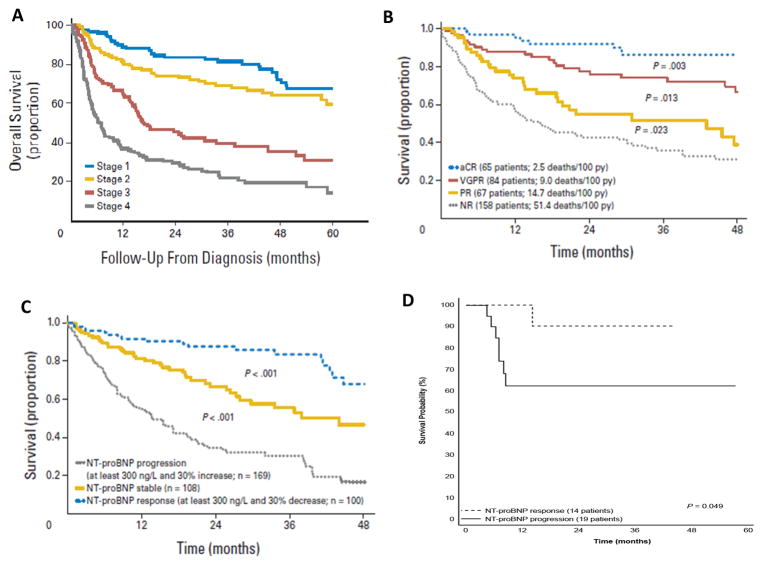
Figure 1.
Mayo staging system for risk stratifying subjects with AL-CA in which one point is assigned for each of the following: NT-pro-BNP ≥ 1800 pg/mL, Troponin T ≥0.025 ng/mL, and difference in serum free light chains ≥ 18 mg/dL. Those with highest score have the worst prognosis57 (Panel A).
Survival from the 3-month landmark of 300 patients with AL amyloidosis based on hematologic response. The proportion of stage III patients was not significantly different among the four hematologic response groups. CR, complete response; NR, no response; PR, partial response; VGPR, very good partial response and py, person-year; 60 (Panel B).
Prognostic relevance of cardiac response and progression criteria showing survival from the 6-month landmark of 377 patients with immunoglobulin light chain (AL) amyloidosis and baseline N-terminal natriuretic peptide type B (NT-proBNP)>650 ng/L according to NT-proBNP response and progression60 (Panel C).
Survival according to NT-proBNP response in an ongoing phase 3 trial comparing melphalan-dexamethasone with melphalan-bortezomib-dexamethasone (NCT01277016)61 (Panel D).
Key point.
In AL cardiac amyloid, light chains can directly induce secretion of high levels of natriuretic peptides. NT-pro-BNP is a biomarker of clinical response as well as progression of illness; with changes in its level after therapy is a surrogate for survival.
What is the role of imaging modalities in diagnosing CA?
The diagnosis of cardiac amyloidosis should be systematic and directed towards the cause as well as the presence of amyloid protein in the myocardium. Laboratory tests, bone-tracer scintigraphy, genetic analysis and tissue biopsy all have a place in achieving these goals. In clinical practice, cardiac imaging is relevant to CA in three scenarios: (1) differential diagnosis of hypertensive hypertrophic and restrictive cardiomyopathy (most common); (2) the evaluation of patients known or suspected to have systemic AL or familial TTR amyloidosis; and (3) in the presence of definite CA, to stage the disease and monitor the response to therapy. Cardiac imaging must be interpreted alongside other clinical findings and different imaging techniques should be considered complementary. The question is not what the “best” examination is, but which is most useful and appropriate for each step of the diagnostic and therapeutic pathway (Table 3).
Table 3.
Diagnostic Tests and Their Role in the Various Phases of Evaluation and Management of Cardiac Amyloidosis
| Imaging and biomarkers of Cardiac Amyloidosis | ||||
|---|---|---|---|---|
|
| ||||
| Phase of work-up | ECHO | MRI* | “Bone tracers” scintigraphy | BNP |
| Suspicion | +++ | ++ | +/− (TTR) | ++ |
| Early diagnosis | +/− | ++ | ++ (TTR) | +/− |
| Definite diagnosis | +/− | +/− | +++ | +/− |
| Etiologic diagnosis | +/− | +/− | +++ (TTR) | - |
| Functional evaluation | +++ | ++ | + (MIBG) | +/− |
| Prognostic stratification | ++ | + | + | +++ |
| Amyloid burden | - | ++ | +/− | - |
| Response to therapy | +/ | +/− | +/− | +++ (AL) |
T1 native, LGE ECV, TTR – useful for TTR amyloid, MIBG - iodine-131-meta-iodobenzylguanidine.
+++ = very useful, recommended, ++= useful, to be considered, += possibly useful
+/− = role uncertain, − = not useful.
What are the findings on cardiac ultrasound that raise suspicion of CA?
The echocardiographic findings of CA have been extensively reviewed elsewhere and are summarised in Figures 2 and 363–65. Nearly all are non-specific, but in context they can be highly suggestive of CA. The major morphological abnormalities in patients with advanced disease are symmetrical thickening of the left ventricle, thickening of the free wall of the right ventricle and a small pericardial effusion. Classical signs also include thickening of the atrioventricular valves and inter-atrial septum and abnormal myocardial “texture” characterized as a speckled appearance, although the latter is less reliable when using harmonic imaging. Amyloidosis is the archetype for diastolic LV dysfunction, with findings dependent on the stage of disease66. Early cardiac involvement is often associated with impairment of relaxation which progresses to typical restrictive LV pathophysiology in advanced symptomatic disease. Analogous changes in RV diastolic function occur in the RV inflow, superior vena cava, and hepatic vein flow velocities67. CA is often listed as a cause of HFpEF, but this underplays its impact on ventricular systolic performance 7, 39, 68–71. While LVEF may be normal until the advanced stage of disease, reduction in peak systolic wall motion velocities, especially in the longitudinal axis, are present even in early disease72. In general, CA alters strain parameters to a much greater degree than other causes of LV hypertrophy and is characterized by a base-to-apex strain gradient (Figure 3, upper right panel)70, 71, 73. This is readily appreciated in parametric polar maps that show relative apical sparing of longitudinal strain, representing an important diagnostic clue differentiating CA from other cardiomyopathies70. In general, differentiating AL from ATTR-CA with echocardiography is not possible but on average patients with AL-CA tend to be more restrictive while those with ATTR-CA tend to have greater LV wall thickness7, 39, 47.
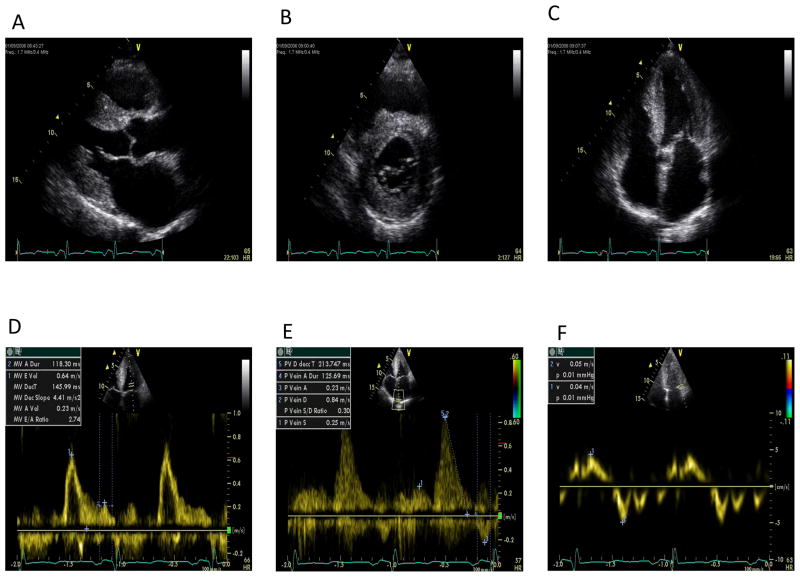
Figure 2.
Sequence of still images showing typical echocardiographic features of cardiac amyloidosis. A: 2-D Parasternal long axis showing concentric left ventricular hypertrophy, bright myocardium and left atrial dilatation. B: 2-D Parasternal short axis showing concentric left ventricular hypertrophy and bright myocardium. C: 2-D Apical four chamber view showing concentric left ventricular hypertrophy and biatrial dilatation D: Pulsed wave Doppler of mitral inflow showing an increase in E/A ratio, normal E wave deceleration time but a marked reduction in transmitral A wave velocity. E: Pulsed wave Doppler of pulmonary vein inflow showing marked diastolic prominence and increased duration and peak velocity of atrial reversal compared to the transmitral signal. F: Pulsed tissue Doppler of the lateral mitral annulus showing marked reduction in apical systolic and diastolic velocities (normal velocities being: >6 cm/sec and >8 cm/sec, respectively). Images courtesy of Professor Elliott, University College London, UK
Figure 3.
Upper left panel: Subcostal 2D echocardiogram showing some the typical findings in advanced cardiac amyloidosis. A: Biatrial enlargement, B: Thickened interatrial septum (and valves), C: Thickening of RV free wall and D: Concentric LVH and hyperechoic myocardial texture; Upper right panel: Apical 4-chamber peak systolic strain image illustrating relatively well-preserved apical strain with significant basal impairment. This is seen in the series of curves and the bull’s-eye color-coded strain image, Lower left panel: Patient with atrial fibrillation and moderate to severely impaired LV function. Echocardiogram showed increased myocardial density and concentric LVH with moderate aortic stenosis. Cardiac MRI showed biventricular hypertrophy, moderate aortic stenosis, and diffuse fibrosis, in excess of that expected for LVH and aortic valve disease. A) Early whole body planar DPD image. B) 3h Delayed whole body planar image showing cardiac retention of racer and reduced bony uptake; Perugini Grade 2. C), D), and E) 2 chamber, short axis, and 4 chamber PSIR LGE cardiac MR images showing diffuse fibrosis. Images courtesy of Professor Elliott, University College London, UK
Lower right panel: Fused Florbetapir PET/MR imaging. A) Native T1 map shows high (>1400) values pre-contrast. B) ECV map, the ECV/interstitial space expansion is as high as blood. C) LGE image- There is differential fibrosis, between the ‘core’ of the interventricular septum and the epi- and endocardial borders (white vs red arrows) and also in the lateral wall, which implies a non-uniform amyloid distribution. D) Fused Florbetapir PET/MR uptake with a LV basal septal predominance. Images courtesy of Dr Leon Menezes, University College London, UK
What is the role of cardiac magnetic resonance imaging in diagnosis?
Much of the structural information derived from CMR is similar to that obtained using echocardiography, but the ability to interrogate tissue composition with gadolinium based contrast agents has led to an increase in the frequency of diagnosis of CA74–79. Gadolinium is a purely extracellular agent and does not enter intact cardiomyocytes74. In some cases of CA, there is a virtually pathognomonic appearance of global sub-endocardial late gadolinium enhancement in a non-coronary artery territory distribution with a dark blood pool. However, enhancement can also be diffuse and transmural or more localized and patchy74, 76, 80. False negative studies can also arise for technical reasons when scans are nulled using abnormal myocardium 75. This latter problem is reduced by using phase-sensitive inversion recovery (PSIR) sequence which reduces the need for an optimal null point setting and makes scans less operator dependent74, 81. T1 mapping is a new magnetic resonance technique in which a quantitative signal from the myocardium is measured, before (native T1) or after contrast administration74, 77, 78. CA substantially increases native T1 and in AL and in ATTR-CA (Figure 3, lower right panel). T1 values are higher in AL-CA compared with ATTR-CA, while the extracellular volume is higher in ATTR-CA than in AL-CA79. As native T1 requires no contrast administration, it can be used in patients with renal impairment and may be abnormal before the left ventricular wall thickens. Combined, native T1 mapping and measures of extracellular volume (ECV) post contrast can delineate three aspects of CA including amyloid burden or infiltration via measure of ECV, edema via measure of native T1 and myocyte response via measure of intracellular volume. Such an approach can provide a richer understanding of the pathophysiological processes that may be used to monitor disease progression and response to therapy. Current limitations of the T1 imaging are the effect of confounding pathologies such as myocardial edema and platform dependent variation in normal ranges for T1.
What is the role of myocardial scintigraphy in making a specific diagnosis?
The 99mTc-phosphate derivatives, originally developed for bone imaging, were observed to accumulate in the early healing phase after acute myocardial infarction. 99mTc-PYP imaging was first described as a potential diagnostic test for CA in the early 1980s following reports describing increased cardiac uptake in patients with amyloid heart disease 82–84. More recently in Europe, studies have evaluated the role of the tracer 99mTc-3,3-diphosphono-1,2-propanodicarboxylic (DPD) in diagnosing CA (Figure 3, lower left panel) 85–87. Collectively, the data show that ATTR-CA is particularly avid for bone tracers, whereas uptake in AL-CA is either absent or only minimal. The explanation for this differential uptake is unknown, but it has been suggested that the preferential binding to ATTR may be a result of higher calcium content. Bone tracers seem to be useful for early identification of ATTR-CA and may more closely correlate with amyloid load than estimates from CMR 88 Additionally, there is also uptake in skeletal muscle, which may appear to suppress bone activity due to masking by extensive soft-tissue uptake. In selected patients the specificity of “bone tracer scintigraphy for ATTR CA is so high that this method can be considered a “diagnostic standard” (see below).
Can PET be used to image and quantify amyloid?
18F-florbetapir is approved for imaging beta amyloid protein in the brain. Recent studies have shown that it is also taken up in the heart in patients with ATTR-CA and AL-CA (Figure 3, lower right panel). Similar findings have been reported for another beta amyloid imaging agent 11C-PiB 89. These agents hold promise for absolute quantification of amyloid burden.
How can one integrate the various imaging modalities into a diagnostic algorithm?
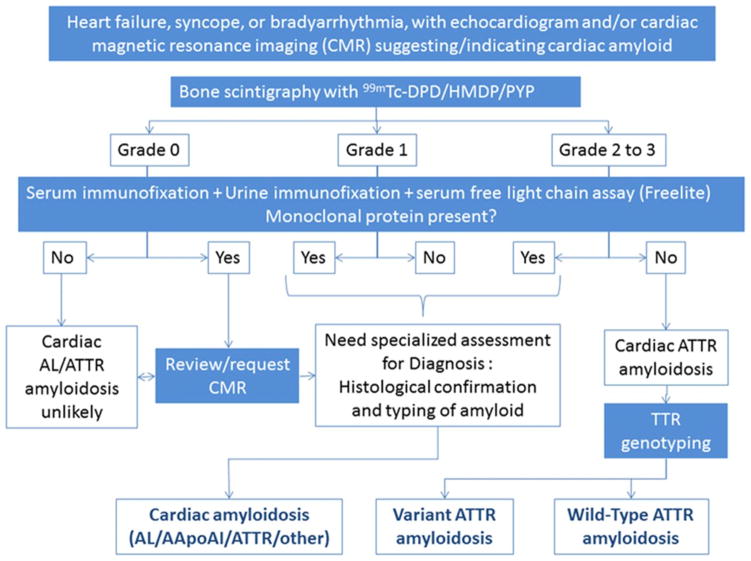
A consensus on the role of bone scintigraphy for non-invasive diagnosis of CA has emerged (Figure 4). If CA is suspected, blood and urine should be analysed for evidence of a plasma cell dyscrasia and imaging with bone tracer considered if ATTR-CA is a possibility. If these tests are negative, then current evidence suggests that CA is very unlikely90. CMR scanning may be used prior to nuclear scintigraphy as an aid to diagnosis, but a 10–20% false negative (particularly in-CA) and false-positive rate79 (possibly more so in people of Afro-Caribbean ancestry) for conventional contrast enhanced scans means that it does not substitute for other tests. Until very recently, a tissue diagnosis was considered essential in all cases of suspected CA. However, in the setting of a positive 99mTc-phosphate scan without evidence for plasma clone on blood and urine testing, a diagnosis of ATTR-CA can be made without a biopsy. For those patients with evidence for a plasma cell dyscrasia, a histologic diagnosis is still required as the presence of uptake on a 99mTc-phosphate scan is not 100% specific for ATTR-CA.
Figure 4.
Diagnostic algorithm for patients with suspected amyloid cardiomyopathy. The grading system for bone scintigraphy is Grade 0 – absent cardiac uptake; Grade 1 – mild uptake less than bone; Grade 2 - moderate uptake equal to bone; Grade 3 - high uptake greater than bone. AApoA1 indicates apolipoprotein A-I; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid; HDMP, hydroxymethylene diphosphonate; and PYP, pyrophosphate (from Gilmore, Circulation 201690)
When and how should tissue biopsy be undertaken?
The diagnostic accuracy of an extra-cardiac biopsy depends on the type of amyloidosis and on the examined tissue. In general, the yield of an extra-cardiac biopsy (abdominal fat pad, gingiva, skin, salivary gland, or gastrointestinal tract) is higher in AL than in ATTRm; the yield is low in ATTRwt. In AL-CA, the yield of a fat pad biopsy is >70% and is strongly associated with whole body amyloid load. In a series of 131 patients with ATTR-CA and a positive endomyocardial biopsy (EMB), fat aspiration was positive in 67% of ATTRm and only 14% of ATTRwt CA.91. Thus, while a fat pad biopsy is a preferred initial site, a negative result is insufficient to exclude the diagnosis and an endomyocardial biopsy should be performed.
How does one confirm the precursor protein causing amyloidosis?
The ultimate goal of a biopsy, in addition to documenting amyloid infiltration, is to provide a definite etiological classification. Immunohistochemistry remains the most widely available method for fibril typing. Its diagnostic value is high in most cases of ATTR amyloidosis, but the results are not always definitive in patients with AL amyloidosis. The most frequent pitfall is the coexistence of positivity for more than one type of antisera, typically those for TTR and lambda or kappa-chains, which are the result of the antibody binding to circulating proteins present in the pathological specimen. In a recent series, 8/15 patients with monoclonal gammopathy, showed strong TTR staining in the histological samples whereas mass spectrometry demonstrated light chain amyloid in 5 of these 8 patients92. Mass spectrometric analysis of amyloid is the new gold standard for fibril typing6. This method involves laser micro-dissection and laser capture of amyloid using a microscope followed by tryptic digestion and tandem mass spectrometry. Computer algorithms match the peptides to a reference database, where amyloid fibril subtype can be identified. Frequently, mass spectroscopy can identify a mutant TTR genotype, though when a mutation is not identified, gene sequencing may be necessary.
Key point.
Tissue diagnosis is required for AL amyloid and endomyocardial biopsy should be pursued if index of suspicion is high despite a negative fat pad. Nuclear scintigraphy agents combined with assessment for monoclonal proteins are reducing the need for tissue confirmation in ATTR; CMR characterization of myocardial tissue can be used to monitor disease progression and response to therapy.
Standard and Emerging Therapies for Cardiac Amyloidosis
Are there specific treatments for CA?
Diagnosis and clinical care have been held back by the erroneous belief that there are limited effective treatments for AL-CA and by the absence of disease modifying therapy for ATTR-CA. It is now recognized that a reduction in the precursor proteins that forms amyloid fibrils is the key to improving prognosis in CA. Combination chemotherapy is effective in lowering free light chains in AL-CA and orthotropic liver transplantation to remove the hepatic source of genetically variant amyloid-forming proteins either alone or in combination with cardiac transplant has a role in selected patients with ATTRm-CA. TTR stabilizers and silencers hold promise for managing all forms of ATTR-CA and newer anti-fibrillar therapies aimed at reversing cardiac dysfunction are in clinical trials. The key determinant of the efficacy of therapy is the severity of disease at diagnosis. In advanced disease, therapy is poorly tolerated and often ineffective93. Multidisciplinary team management and supportive care is critical in controlling symptoms, optimizing nutritional status, maintaining cardiac function and managing the toxicities of treatments. Collectively, therapeutic advances have begun to shift CA from a universally fatal disease to a manageable chronic condition.
AL Cardiac Amyloidosis
What are the goals of therapy for AL cardiac amyloidosis?
The toxic amyloid-forming light chains that cause AL are produced in the bone marrow and the clonal plasma cells in AL possess many of the same genetic abnormalities found in other plasma cell dyscrasias 94–97. The major difference between AL and myeloma is that in AL organ damage occurs because of the monoclonal light chains while in myeloma the organ damage is due largely to cell mass98. Anti-plasma cell treatments for AL have evolved from those used in myeloma, including steroids, high-dose melphalan, proteasome inhibitors and immunomodulatory agents 99. The goals of therapy in AL-CA are to eliminate the clonal plasma cells and provide optimal supportive care to address and control side effects of therapy. Patients with advanced cardiac involvement cannot tolerate high-dose therapy with autologous stem cell transplantation (SCT) and therefore patient selection for SCT has become restrictive100, 101. Despite excellent hematologic response rates, patients with elevations of cardiac biomarkers who are poor candidates for SCT are also at significant risk even with standard bortezomib-based therapy with 40% dead within 2 years of diagnosis and only 20% of responders experiencing cardiac improvement by biomarker criteria102. However, when a response is achieved survival can be meaningfully improved.
Is halting deposition of amyloid the only treatment or can removal of amyloid and reversal of organ dysfunction be achieved?
Hematologic response and degree of cardiac involvement are the deciding factors regarding overall survival in systemic AL amyloidosis4. The categories of hematologic response with initial therapy in newly diagnosed patients are complete (CR, normal FLC ratio and negative serum and urine immunofixation), very good partial response (VGPR, difference between involved and uninvolved free light chains < 40 mg/L or <4 mg/dl), partial response (PR, difference between uninvolved and involved free light chains decreased > 50%), and no response (NR)60. The frequency of cardiac responses defined as a decline in NT-pro-BNP to <30% of baseline and of at least 300 pg/ml in 374 newly diagnosed treated AL-CA patients were 57% with a CR, 37% with a VGPR, 18% with a PR and 4% with NR60 (Figure 1). At 6 months after starting anti-plasma cell chemotherapy, cardiac responses occurred in 26% and progression in 45% of patients, with 85% of responders and 30% of progressors still alive after 2 years60. Such data indicate that anti-plasma cell chemotherapy, including the use of high-dose chemotherapy with SCT, does not enable reliable cardiac improvement for all patients especially those with advanced cardiac involvement. Many patients with hematologic responses continue to have persistent cardiac dysfunction despite improved overall survival. After initial therapy, the median hematologic progression-free survival (PFS) is 1 to 4 years, depending on the type of initial therapy. The PFS for the 25% of newly diagnosed patients who were candidates for SCT appears to be higher than that of patients receiving standard therapy with bortezomib-based combinations. Indeed, about 80% of the long-term post-SCT survivors with CR have organ responses.101. At hematologic relapse, the majority of patients have stage 2 or 3 chronic renal disease and stage 2 cardiac disease (see Figure 1)24.
The unmet need for patients is therapy that reverses organ damage and dysfunction30. Several approaches are in clinical trials at this time, including three monoclonal antibody based therapies that may enhance phagocytic clearance of amyloid deposits. The anti-amyloid 11–1F4 monoclonal was used in a phase I trial (NCT02245867) in AL patients with relapsed refractory disease, and cardiac and GI organ responses were reported in 3 of the first 6 patients treated103. Employing a different approach in their phase I trial (NCT01777243), based on the fact that amyloid fibril deposits always contain the non-fibrillar normal plasma protein, serum amyloid P component (SAP), investigators from the National Amyloidosis Center in the UK used a combination of two agents. The drug, R-1-[6-[R-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl] pyrrolidine-2-carboxylic acid (CPHPC), efficiently depletes SAP from the plasma but leaves some SAP in amyloid deposits that can be specifically targeted by therapeutic IgG anti-SAP antibodies, inciting an inflammatory multinucleated giant cell response104. In this phase I trial, whole-body scans using iodinated SAP demonstrated depletion of hepatic amyloid in 50% of patients with liver involvement. There were also reductions in in C3 and spikes in CRP, indicating ongoing inflammation. Patients with cardiac involvement were excluded from the trial105. The third, and furthest along in clinical development, is the anti-amyloid monoclonal NEOD001 that binds to an epitope in amyloid fibrils. In a phase 1/2 trial (NCT01707264), previously treated AL patients who were in hematologic remission with biomarker evidence of on-going organ damage, received NEOD001 monthly106. There were no toxicities and organ responses were seen in majorities of both cardiac (57%) and renal (60%) patients. NEOD001 is now in a phase 3 trial for newly diagnosed patients with cardiac involvement (NCT02312206), in which patients receive bortezomib-based therapy and are randomized to receive either NEOD001 or saline monthly.
What are the mechanisms of therapeutic efficacy (reduction in precursor protein/amyloid production versus amyloid clearance)?
Therapeutic efficacy in AL has two dimensions, survival and improvement in organ function. Hematologic response to therapy is measured in terms of the reduction or elimination of the clonal plasma cells that produce the culprit light-chain protein. Patients who achieve a hematologic CR or VGPR live longer than those who do not60. Improvement in organ function is measured with cardiac and renal biomarkers and, for patients with other organs involved, with a mix of exam and laboratory findings such as normalization of orthostatic symptoms and vital signs for patients with autonomic neuropathy or of liver span and alkaline phosphatase for those with hepatic involvement107. Patients who achieve cardiac biomarker responses live longer than those who do not 59, 60, 108.
Hematologic responses based on changes in the serum free light chain assay are validated surrogates for the impact of therapy on the clonal marrow cells. With elimination or near elimination of the culprit light-chain protein, patients usually experience improvement in organ function and in their QOL. However, while hematologic response is necessary for organ response, it is not sufficient30. There is general consensus that both early cardiac death and survival with persistent organ damage are critical unmet clinical challenges.
What factors influence treatment decisions (rapidity of response, toxicity and physiologic reserve)?
The initial evaluation of newly diagnosed patients with AL is focused on the extent of both cardiac and extra-cardiac organ involvement, physiologic reserve and the character of the clonal marrow plasma cells109. In a cohort of over 800 patients from 6 countries, 26% were NYHA class 2 or 3, 25% CKD Stage 3, and 15% CKD Stage 4 or 5; 50% were Mayo cardiac stage II with either Troponin T or NTpro-BNP above the threshold and 34% were cardiac stage III with both elevated; 19% had liver and 16% peripheral nervous system involvement60.
Who is eligible for SCT?
Eligibility criteria for high-dose therapy with SCT include age less than 65, Karnofsky performance status of 80% or higher, adequate cardiac function (systolic blood pressure > 90 mm Hg, ejection fraction >45%, troponin T <0.06 ng/ml, NT-proBNP < 8,500 pg/ml or pulmonary artery saturation of >55%) and compensated or controlled orthostatic changes in blood pressure110. Patients who are Mayo cardiac stage III or CKD 4 are rarely eligible; young patients on hemodialysis, however, who are cardiac stage I or II are often eligible for SCT. Most patients are not eligible for SCT and receive bortezomib-based therapy with doses adjusted for frailty, impaired liver function and NYHA class111, 112. Bortezomib may not be the first choice of proteasome inhibitors for patients with peripheral neuropathy because of the risk of worsened neuropathy due to the drug; alternatives exist113. After SCT, consolidation is recommended for AL patients who do not achieve a CR (in myeloma post-SCT consolidation is recommended for all)4, 114. For patients treated with bortezomib-based therapy, the lack of a hematologic response after 2 cycles is usually a signal to change therapy and, in most cases, bortezomib and dexamethasone will be retained but other agents such as melphalan or immune-modulatory drugs are added109.
What is the data on efficacy of therapies?
The mix of therapies used to treat newly diagnosed patients with AL and the reported outcomes are recounted in Supplementary Table 1. High-dose therapy with SCT and bortezomib-based combination chemotherapy are the two options used most frequently in the USA100. In the UK and EU, however, SCT is less often employed and other regimens such as oral melphalan and dexamethasone or oral cyclophosphamide, thalidomide and dexamethasone are commonly used99, 115, 116. Doses of agents in all of these regimens are often adjusted for age, frailty, renal and hepatic function, and NYHA class.
Key point.
New targeted plasma cell therapies have rapidly expanded the treatment option for patients with AL cardiac amyloid, improving outcomes for patients with earlier stage disease.
ATTR Cardiac Amyloidosis
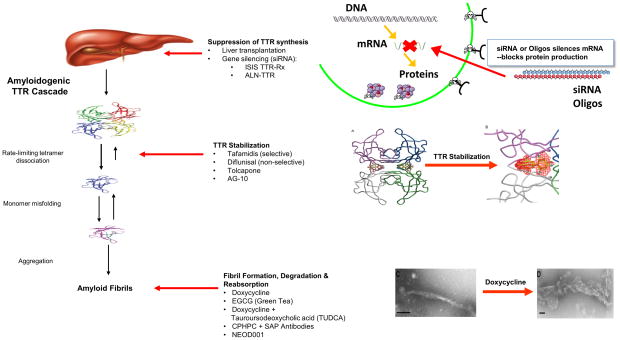
What are the targets for therapy in TTR?
Current treatment for ATTR-CA is focused on various stages in the TTR amyloid cascade including silencing of TTR production, TTR stabilization and amyloid clearance from tissues (Figure 5)117–119. Because 95% of TTR protein is produced by the liver, orthotopic liver transplantation (OLT) has been employed in ATTRm to replace amyloid forming mutant with wild type TTR and theoretically arrest amyloid formation. The majority of OLTs for ATTRm amyloid are performed in patients with a primary neuropathic phenotype, most commonly the Val30Met variant120. OLT is considered a preventative measure to forestall the development of the sensorimotor neuropathy or multiple organ involvement. Survival after liver transplantation in Val30Met patients is > 50% at 20 years120. Since the liver function of TTR liver transplant recipients is otherwise normal, their livers have been transplanted into high risk recipients (“domino liver transplant”), causing TTR amyloidosis in recipients several years later121. While survival is prolonged by liver transplantation120, there is slowing but not arrest of cardiac dysfunction. This is attributed to deposition of wild type TTR amyloid in the heart from the transplanted liver causing progressive cardiac dysfunction. Patients with fragmented ATTR fibrils (type A) developed HF after OLT while those who had intact ATTR fibrils (type B) did not deteriorate to the same degree122. The scarcity of organ availability, the need for lifelong immunosuppression and the relatively older age of affected subjects make transplantation a suboptimal option for treating ATTR-CA.
Figure 5.
Disease modifying targets in TTR cardiac amyloidosis
Both RNA interference (RNAi) and antisense oligonucleotides (ASOs) have been used to inhibit hepatic expression of amyloid forming TTR. These therapies capitalize on endogenous cellular mechanism for controlling gene expression in which siRNAs or ASOs, mediates the destruction of target messenger RNA (mRNA) essential for TTR protein production. These agents cause a robust and durable reduction in genetic expression (knockdown) of TTR and retinol binding protein123–125. Both methods of silencing have completed recruitment for phase III trials in ATTR peripheral neuropathy with results expected in 2017 but a phase III trial in ATTR cardiomyopathy was recently suspended with unexpected adverse impact on those receiving active treatment (Supplementary Table 2).
TTR stabilizers have demonstrated efficacy in FAP126, 127 but their role in ATTR-CA is unknown. Diflunisal, a nonsteroidal anti-inflammatory drug, binds and stabilizes common familial TTR variants against acid-mediated fibril formation in vitro and has been tested in human clinical trials126. Use of diflunisal is controversial given the known consequences of chronic inhibition of cyclooxygenase enzymes including gastrointestinal bleeding, renal dysfunction, fluid retention, and hypertension that may precipitate HF in vulnerable individuals. Tafamidis is a novel compound that binds to the thyroxine-binding sites of the TTR tetramer, inhibiting its dissociation into monomers128 and blocking the rate-limiting step in the TTR amyloid forming cascade (Figure 5).
Doxycycline disrupts fibril formation129 and when combined with the bile salt, tauroursodeoxycholic acid, demonstrated a synergistic effect on removal of tissue TTR deposits in an animal model, leading to open label trials in humans130. Epigallocatechin gallate (EGCG), the most abundant flavonoid in green tea, inhibits amyloid fibril formation in vitro, leading to open label trials in ATTR-CA131, 132.
What are the results of recent trials in TTR?
A majority of the trials completed to date have been small (<100 subjects), phase II, open label, intermediate duration (1–2 years) with primary endpoints being the NIS-LL and QOL for ATTR peripheral neuropathy and echocardiographic, biomarkers and six minute hall walk for ATTR-CA (Supplementary Table 2). Trials for neuropathy are further along than those for cardiomyopathy. Trials to date have demonstrated short term efficacy limited to either a surrogate maker (TTR stabilization) and/or the absence or slowing of disease progression, albeit with no control group.
The only phase III trials completed to date involved administration of diflunisal126 and tafamidis127, 133, both in FAP. In the diflunisal trial, echocardiography demonstrated a prevalence of presumptive CA in >50% of these FAP patients but analysis of echocardiographic variables including strain did not show significant differences with treatment, though statistical power was low134. Tafamidis, in patients with early-stage ATTRm due to Val30Met127, slowed progression of neurological symptoms and early treatment was associated with greater preservation of neurologic function133, which was not confirmed in late phase FAP135. Thus, early administration of tafamidis seems to be important for its efficacy. Additionally, all previous trials employed a dose of 20 mg but an 80 mg dose is being tested in an ongoing phase III trial, with biochemical data suggesting that a higher dose may be required for TTR stabilization136. Five year safety data in a small cohort has been reassuring in that tafamidis was generally well tolerated at 20 mg, though efficacy data showed few patients with ATTR-CA were clinically stabilized at 3.5 years.
What therapies are in late phase development?
Currently there is 1 clinical trial for ATTR-CA in Phase III involving tafamidis. A second trial with revusiran, a siRNA, was recently stopped and the development of the drug suspended. Both of these trials were placebo controlled with allocation to active therapy of ~2:1 and a fixed study duration that encourages participation by subjects who have a progressive, life threatening condition. Differences in study populations include a focus on ATTRm in the case of revusiran while the other (ATTR-ACT) enrolled both ATTRm and ATTRwt. The ATTR-ACT trial of tafamidis is expected to conclude in 2018.
Key point.
The biologic process underlying ATTR-CA has led to the development of numerous therapies that are currently in late phase clinical trials. A recent trial of a siRNA for transthyretin in CA was stopped prematurely due to an unexpected worsening of subject’s clinical status.
Cardiac Replacement therapy – Transplant and VAD
Is AL amyloidosis a contraindication to cardiac transplantation?
AL-CA was previously considered a contraindication to orthotopic heart transplant (OHT) because of concerns regarding worse long term outcomes due to the amyloid involvement of other organs or the risk of recurrent amyloid in the graft137. However, the development of multiple therapies targeted at the monoclonal protein including high dose chemotherapy with SCT has made long term control of the plasma cell dyscrasia possible. Accordingly, series in selected patients for whom heart transplant was performed at experienced centers with established multi-disciplinary collaborations followed by either stem cell transplant or ongoing chemotherapy have reported outcomes comparable to other subjects with restrictive cardiomyopathies.138–141 Additionally, an analysis of UNOS data shows improved post-OHT survival in patients with CA, in part due to improved patient selection including an increase in those with ATTR-CA.141. The one year survival post OHT in UNOS for CA (including both AL-CA and ATTR-CA) from 2010 to 2012 was 81.6%. Accordingly, current guidelines endorse consideration of selected patients with either AL and ATTR-CA142.
What additional testing is required for heart transplant evaluation?
The degree of organs involved is an important prognostic variable influencing the outcomes of patients with AL undergoing SCT. Accordingly, multiple other organ systems must be thoroughly assessed for the functional effect of amyloid and if necessary for the anatomic/histologic degree of amyloid infiltration. Specific guidelines by the ISHLT have been published (see Table 4).142
Table 4.
Recommendation for Evaluation of Extra-cardiac Organs in AL Amyloid Prior to OHT according to ISHLT Guidelines
| Organ System | Screening Tests |
|---|---|
|
| |
| Pulmonary | Pulmonary function testing including arterial oximetry, diffusion capacity CXR and CT to assess for interstitial disease, effusions Thoracentesis may be necessary to differentiate amyloidosis from heart failure |
|
|
|
| Gastrointestinal | Nutritional assessment including pre-albumin, albumin. Assessment for bleeding by esophagogastroduodenoscopy, colonoscopy Assessment of amyloid deposition on random biopsy Assessment of intestinal motility with gastric-emptying studies |
|
|
|
| Hepatic | Serum alkaline phosphatase and bilirubin.
|
|
|
|
| Renal | Measured creatinine clearance or eGFR and 24-hour urinary protein excretion.
|
|
|
|
| Coagulation | Factor X and thrombin time
|
|
|
|
Adopted from J Heart Lung Transplant. 2016 Jan; 35(1):1–23).
Do outcomes differ in subjects with AL vs. TTR cardiac amyloidosis undergoing OHT?
Data from Columbia University Medical Center, has shown better survival for ATTR-CA subjects (n=10) than AL-CA (n=16) undergoing OHT with transplants performed from 1999–2008. However, a more contemporary series from Stanford has shown better survival in AL (n=10) than TTR (n=9) CA140. Multicenter data obtained as part of the International Consortium in Cardiac Amyloid Transplantation (iCCAT) has demonstrated that outcomes of patients with CA do not differ statistically from those with AL compared to TTR143.
How should the plasma clone be managed in patients with AL cardiac amyloid undergoing OHT?
Control of the underlying plasma cell dyscrasia is critical to successful outcomes in AL cardiac amyloid. Data from iCCAT demonstrates that survival without recurrent amyloid post OHT is higher in those who receive chemotherapy prior to OHT than those who did not receive chemotherapy144. Accordingly, upfront plasma cell therapy to achieve a complete or very good hematologic response while waiting for OHT is increasingly being advised. While high or dose adjusted melphalan with SCT remains the most cytotoxic therapy for AL amyloid, combination therapy using proteasome inhibitors has been shown to be effective in AL-CA patients post OHT140.
How can I bridge my patient with advanced cardiac amyloid to an OHT?
The options for mechanical circulatory support as a bridge to OHT for CA patients is confounded by the small ventricular chamber size and increased wall thickness that increase the risk for suction events, right heart failure and overall worse outcomes in very small series reported to date.145 Survival with a VAD is strongly associated with left ventricular end diastolic diameter146. Other forms of circulatory support including intra-aortic balloon pump, BiVAD or total artificial heart (the latter employed given the need for biventricular support) have been employed though survival at one year is reduced if circulatory support is required147. A theoretical analysis using a cardiovascular simulation of a novel micro-VAD for CA has shown that sourcing of blood from the left atrium was not associated with suction, as assessed by declines in left ventricular end systolic volumes, but did lower left atrial pressure and increase cardiac output and blood pressure in subjects148. Whether such an approach can be effective in-vivo in CA requires additional study.
Key point.
While the overall goal is to eliminate the need for OHT in CA, advances in patient selection and available therapies have made intermediate term outcomes of OHT in CA comparable to non-amyloid patients.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Maurer is supported by a Mid-career Mentoring Award from the NIA (AG036778-06).
Footnotes
Disclosures: Dr. Maurer’s institution, receives funding for research and serving on advisory boards and DSMBs from Pfizer Inc., Alnylam Pharmaceuticals Inc., ISIS Pharmaceuticals and Prothena Inc. Dr. Elliott reports Consultancies with Pfizer, Sanofi, Shire and Amicus. Dr. Comenzo reports being a scientific advisor to Takeda, Prothena, Janssen and Unum. Dr. Semigran has received research funding from Alnylam. Dr. Rapezzi has received research grants from Pfizer.
References
- 1.Glenner GG, Ein D, Eanes ED, Bladen HA, Terry W, Page DL. Creation of “amyloid” fibrils from Bence Jones proteins in vitro. Science. 1971;174:712–4. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- 2.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529–38. doi: 10.1046/j.1365-2141.2001.02999.x. [DOI] [PubMed] [Google Scholar]
- 4.Merlini G, Comenzo RL, Seldin DC, Wechalekar A, Gertz MA. Immunoglobulin light chain amyloidosis. Expert Rev Hematol. 2014;7:143–56. doi: 10.1586/17474086.2014.858594. [DOI] [PubMed] [Google Scholar]
- 5.Cohen AS, Calkins E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959;183:1202–3. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- 6.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 7.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM, Kurland LT. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–22. [PubMed] [Google Scholar]
- 9.Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, Dungu J, Banypersad SM, Wechalekar AD, Whelan CJ, Hawkins PN, Gillmore JD. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161:525–32. doi: 10.1111/bjh.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemminki K, Li X, Forsti A, Sundquist J, Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12:974. doi: 10.1186/1471-2458-12-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muchtar E, Buadi FK, Dispenzieri A, Gertz MA. Immunoglobulin Light-Chain Amyloidosis: From Basics to New Developments in Diagnosis, Prognosis and Therapy. Acta Haematol. 2016;135:172–90. doi: 10.1159/000443200. [DOI] [PubMed] [Google Scholar]
- 12.Galat A, Guellich A, Bodez D, Slama M, Dijos M, Zeitoun DM, Milleron O, Attias D, Dubois-Rande JL, Mohty D, Audureau E, Teiger E, Rosso J, Monin JL, Damy T. Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Eur Heart J. 2016;37:3525–3531. doi: 10.1093/eurheartj/ehw033. [DOI] [PubMed] [Google Scholar]
- 13.Maurer MS. Noninvasive Identification of ATTRwt Cardiac Amyloid: The Re-emergence of Nuclear Cardiology. Am J Med. 2015;128:1275–80. doi: 10.1016/j.amjmed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–9. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–22. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–54. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22:171–4. doi: 10.3109/13506129.2015.1051219. [DOI] [PubMed] [Google Scholar]
- 19.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E, Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372:21–9. doi: 10.1056/NEJMoa1404852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light Chain Amyloidosis: Patient Experience Survey from the Amyloidosis Research Consortium. Adv Ther. 2015;32:920–8. doi: 10.1007/s12325-015-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C Investigators T. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey) J Am Coll Cardiol. 2016;68:161–72. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson S, Pilebro B, Westermark P, Lindqvist P, Suhr OB. Amyloid Cardiomyopathy in Hereditary Transthyretin V30M Amyloidosis - Impact of Sex and Amyloid Fibril Composition. PLoS One. 2015;10:e0143456. doi: 10.1371/journal.pone.0143456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Blade J, Fermand JP, Hassoun H, Heffner L, Kukreti V, Vescio RA, Pei L, Enny C, Esseltine DL, van de Velde H, Cakana A, Comenzo RL. Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis. Blood. 2014;124:2498–506. doi: 10.1182/blood-2014-04-568329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comenzo RL, Wally J, Kica G, Murray J, Ericsson T, Skinner M, Zhang Y. Clonal immunoglobulin light chain variable region germline gene use in AL amyloidosis: association with dominant amyloid-related organ involvement and survival after stem cell transplantation. Br J Haematol. 1999;106:744–51. doi: 10.1046/j.1365-2141.1999.01591.x. [DOI] [PubMed] [Google Scholar]
- 26.Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–20. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 27.Abraham RS, Geyer SM, Price-Troska TL, Allmer C, Kyle RA, Gertz MA, Fonseca R. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL) Blood. 2003;101:3801–8. doi: 10.1182/blood-2002-09-2707. [DOI] [PubMed] [Google Scholar]
- 28.Bodi K, Prokaeva T, Spencer B, Eberhard M, Connors LH, Seldin DC. AL-Base: a visual platform analysis tool for the study of amyloidogenic immunoglobulin light chain sequences. Amyloid. 2009;16:1–8. doi: 10.1080/13506120802676781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perfetti V, Palladini G, Casarini S, Navazza V, Rognoni P, Obici L, Invernizzi R, Perlini S, Klersy C, Merlini G. The repertoire of lambda light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1–44. Blood. 2012;119:144–50. doi: 10.1182/blood-2011-05-355784. [DOI] [PubMed] [Google Scholar]
- 30.Weiss BM, Wong SW, Comenzo RL. Beyond the plasma cell: emerging therapies for immunoglobulin light chain amyloidosis. Blood. 2016;127:2275–80. doi: 10.1182/blood-2015-11-681650. [DOI] [PubMed] [Google Scholar]
- 31.Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, Biagini E, Lorenzini M, Grigioni F, Leone O, Cappelli F, Palladini G, Rimessi P, Ferlini A, Arpesella G, Pinna AD, Merlini G, Perlini S. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–8. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 32.Svendsen IH, Steensgaard-Hansen F, Nordvag BY. A clinical, echocardiographic and genetic characterization of a Danish kindred with familial amyloid transthyretin methionine 111 linked cardiomyopathy. Eur Heart J. 1998;19:782–9. doi: 10.1053/euhj.1997.0841. [DOI] [PubMed] [Google Scholar]
- 33.Sattianayagam PT, Hahn AF, Whelan CJ, Gibbs SD, Pinney JH, Stangou AJ, Rowczenio D, Pflugfelder PW, Fox Z, Lachmann HJ, Wechalekar AD, Hawkins PN, Gillmore JD. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33:1120–7. doi: 10.1093/eurheartj/ehr383. [DOI] [PubMed] [Google Scholar]
- 34.Maurer MSHM, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Planté-Bordeneuve V, Coelho R, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C on behalf of THAOS Investigators. Genotype and Phenotype of Transthyretin-Related Cardiac Amyloidosis in the United States: A Report from the Transthyretin Amyloid Outcome Survey (THAOS) JACC. 2016;68:161–72. doi: 10.1016/j.jacc.2016.03.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyle RA, Spittell PC, Gertz MA, Li CY, Edwards WD, Olson LJ, Thibodeau SN. The premortem recognition of systemic senile amyloidosis with cardiac involvement. Am J Med. 1996;101:395–400. doi: 10.1016/S0002-9343(96)00229-X. [DOI] [PubMed] [Google Scholar]
- 36.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–9. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 37.Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC. Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study. Circulation. 2016;133:282–90. doi: 10.1161/CIRCULATIONAHA.115.018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, Falk RH, Cheung KN, Patel AR, Pano A, Packman J, Grogan DR. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS) Am Heart J. 2012;164:222–228. e1. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N, McCarthy CA, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2:e000098. doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016;68:1014–20. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, Koyama J, Yanagisawa S, Ikeda S. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23:58–63. doi: 10.3109/13506129.2015.1135792. [DOI] [PubMed] [Google Scholar]
- 42.Givens RC, Russo C, Green P, Maurer MS. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging health. 2013;9:229–235. doi: 10.2217/ahe.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhuiyan T, Helmke S, Patel AR, Ruberg FL, Packman J, Cheung K, Grogan D, Maurer MS. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS) Circ Heart Fail. 2011;4:121–8. doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114:1089–93. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Mussinelli R, Salinaro F, Alogna A, Boldrini M, Raimondi A, Musca F, Palladini G, Merlini G, Perlini S. Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol. 2013;18:271–80. doi: 10.1111/anec.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9–13. doi: 10.1016/0002-9149(82)90270-3. [DOI] [PubMed] [Google Scholar]
- 47.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C, Falk RH. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 48.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, 3rd, Colby CL, Therneau TM, Clark R, Kumar SK, Bradwell A, Fonseca R, Jelinek DF, Rajkumar SV. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, Pepys MB, Hawkins PN. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786–91. doi: 10.1056/NEJMoa013354. [DOI] [PubMed] [Google Scholar]
- 50.Hutchison CA, Harding S, Hewins P, Mead GP, Townsend J, Bradwell AR, Cockwell P. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–90. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masuda M, Ishimura E, Ochi A, Tsujimoto Y, Tahahra H, Okuno S, Tabata T, Nishizawa Y, Inaba M. Serum beta2-microglobulin correlates positively with left ventricular hypertrophy in long-term hemodialysis patients. Nephron Clin Pract. 2014;128:101–6. doi: 10.1159/000365447. [DOI] [PubMed] [Google Scholar]
- 52.Noel LH, Zingraff J, Bardin T, Atienza C, Kuntz D, Drueke T. Tissue distribution of dialysis amyloidosis. Clin Nephrol. 1987;27:175–8. [PubMed] [Google Scholar]
- 53.Perfetto F, Bergesio F, Grifoni E, Fabbri A, Ciuti G, Frusconi S, Angelotti P, Spini V, Cappelli F. Different NT-proBNP circulating levels for different types of cardiac amyloidosis. J Cardiovasc Med (Hagerstown) 2016;17:810–7. doi: 10.2459/JCM.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 54.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107:4188–93. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–10. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 56.Liao R, Jain M, Teller P, Connors LH, Ngoy S, Skinner M, Falk RH, Apstein CS. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104:1594–7. [PubMed] [Google Scholar]
- 57.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–95. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dietrich S, Schonland SO, Benner A, Bochtler T, Kristen AV, Beimler J, Hund E, Zorn M, Goldschmidt H, Ho AD, Hegenbart U. Treatment with intravenous melphalan and dexamethasone is not able to overcome the poor prognosis of patients with newly diagnosed systemic light chain amyloidosis and severe cardiac involvement. Blood. 2010;116:522–8. doi: 10.1182/blood-2009-11-253237. [DOI] [PubMed] [Google Scholar]
- 59.Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, Falk R, Wells K, Solomon A, Wechalekar A, Zonder J, Dispenzieri A, Gertz M, Streicher H, Skinner M, Kyle RA, Merlini G. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26:2317–25. doi: 10.1038/leu.2012.100. [DOI] [PubMed] [Google Scholar]
- 60.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schonland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C, Merlini G. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–9. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 61.Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, Maurer MS, Sanchorawala V, Wechalekar A, Palladini G, Comenzo RL. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia. 2016;30:1979–1986. doi: 10.1038/leu.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grogan MSC, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural History of Wild Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016;68:1014–20. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 63.Damy T, Maurer MS, Rapezzi C, Plante-Bordeneuve V, Karayal ON, Mundayat R, Suhr OB, Kristen AV. Clinical, ECG and echocardiographic clues to the diagnosis of TTR-related cardiomyopathy. Open Heart. 2016;3:e000289. doi: 10.1136/openhrt-2015-000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapezzi C, Lorenzini M, Longhi S, Milandri A, Gagliardi C, Bartolomei I, Salvi F, Maurer MS. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–24. doi: 10.1007/s10741-015-9480-0. [DOI] [PubMed] [Google Scholar]
- 65.Koyama J, Ikeda S, Ikeda U. Echocardiographic assessment of the cardiac amyloidoses. Circ J. 2015;79:721–34. doi: 10.1253/circj.CJ-14-1425. [DOI] [PubMed] [Google Scholar]
- 66.Klein AL, Hatle LK, Burstow DJ, Seward JB, Kyle RA, Bailey KR, Luscher TF, Gertz MA, Tajik AJ. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989;13:1017–26. doi: 10.1016/0735-1097(89)90254-4. [DOI] [PubMed] [Google Scholar]
- 67.Klein AL, Hatle LK, Burstow DJ, Taliercio CP, Seward JB, Kyle RA, Bailey KR, Gertz MA, Tajik AJ. Comprehensive Doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;15:99–108. doi: 10.1016/0735-1097(90)90183-p. [DOI] [PubMed] [Google Scholar]
- 68.Liu D, Niemann M, Hu K, Herrmann S, Stork S, Knop S, Ertl G, Weidemann F. Echocardiographic evaluation of systolic and diastolic function in patients with cardiac amyloidosis. Am J Cardiol. 2011;108:591–8. doi: 10.1016/j.amjcard.2011.03.092. [DOI] [PubMed] [Google Scholar]
- 69.Di Bella G, Minutoli F, Pingitore A, Zito C, Mazzeo A, Aquaro GD, Di Leo R, Recupero A, Stancanelli C, Baldari S, Vita G, Carerj S. Endocardial and epicardial deformations in cardiac amyloidosis and hypertrophic cardiomyopathy. Circ J. 2011;75:1200–8. doi: 10.1253/circj.cj-10-0844. [DOI] [PubMed] [Google Scholar]
- 70.Phelan D, Thavendiranathan P, Popovic Z, Collier P, Griffin B, Thomas JD, Marwick TH. Application of a parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J Am Soc Echocardiogr. 2014;27:888–95. doi: 10.1016/j.echo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, Radu C, Guendouz S, Couetil JP, Benhaiem N, Hittinger L, Dubois-Rande JL, Plante-Bordeneuve V, Mohty D, Deux JF, Damy T. Causes and Consequences of Longitudinal LV Dysfunction Assessed by 2D Strain Echocardiography in Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2016;9:126–38. doi: 10.1016/j.jcmg.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–52. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 73.Senapati A, Sperry BW, Grodin JL, Kusunose K, Thavendiranathan P, Jaber W, Collier P, Hanna M, Popovic ZB, Phelan D. Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart. 2016;102:748–54. doi: 10.1136/heartjnl-2015-308657. [DOI] [PubMed] [Google Scholar]
- 74.Fontana M, Chung R, Hawkins PN, Moon JC. Cardiovascular magnetic resonance for amyloidosis. Heart Fail Rev. 2015;20:133–44. doi: 10.1007/s10741-014-9470-7. [DOI] [PubMed] [Google Scholar]
- 75.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 76.Ruberg FL, Appelbaum E, Davidoff R, Ozonoff A, Kissinger KV, Harrigan C, Skinner M, Manning WJ. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544–9. doi: 10.1016/j.amjcard.2008.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488–97. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 78.Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, Pica S, Castelletti S, Piechnik SK, Robson MD, Gilbertson JA, Rowczenio D, Hutt DF, Lachmann HJ, Wechalekar AD, Whelan CJ, Gillmore JD, Hawkins PN, Moon JC. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–65. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Whelan CJ, Hawkins PN, Anderson LJ. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–42. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, Gertz MA, Dispenzieri A, Oh JK, Bellavia D, Tajik AJ, Grogan M. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3:155–64. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 81.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–83. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wizenberg TA, Muz J, Sohn YH, Samlowski W, Weissler AM. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J. 1982;103:468–73. doi: 10.1016/0002-8703(82)90331-3. [DOI] [PubMed] [Google Scholar]
- 83.Falk RH, Lee VW, Rubinow A, Hood WB, Jr, Cohen AS. Sensitivity of technetium-99m-pyrophosphate scintigraphy in diagnosing cardiac amyloidosis. Am J Cardiol. 1983;51:826–30. doi: 10.1016/s0002-9149(83)80140-4. [DOI] [PubMed] [Google Scholar]
- 84.Gertz MA, Brown ML, Hauser MF, Kyle RA. Utility of technetium Tc 99m pyrophosphate bone scanning in cardiac amyloidosis. Arch Intern Med. 1987;147:1039–44. [PubMed] [Google Scholar]
- 85.Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi-Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–84. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 86.Rapezzi C, Guidalotti P, Salvi F, Riva L, Perugini E. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. J Am Coll Cardiol. 2008;51:1509–10. doi: 10.1016/j.jacc.2007.12.038. author reply 1510. [DOI] [PubMed] [Google Scholar]
- 87.Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, Ferlini A, Salvi F, Gallo P, Gagliardi C, Branzi A. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470–8. doi: 10.1007/s00259-010-1642-7. [DOI] [PubMed] [Google Scholar]
- 88.Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, Lane T, Whelan CJ, Lachmann HJ, Gillmore JD, Hawkins PN, Wechalekar AD. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15:1289–98. doi: 10.1093/ehjci/jeu107. [DOI] [PubMed] [Google Scholar]
- 89.Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V, Falk RH, Di Carli MF, Dorbala S. 18F-Florbetapir Binds Specifically to Myocardial Light Chain and Transthyretin Amyloid Deposits: Autoradiography Study. Circ Cardiovasc Imaging. 2015;8:e002954. doi: 10.1161/CIRCIMAGING.114.002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016;133:2404–12. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 91.Fine NM, Arruda-Olson AM, Dispenzieri A, Zeldenrust SR, Gertz MA, Kyle RA, Swiecicki PL, Scott CG, Grogan M. Yield of noncardiac biopsy for the diagnosis of transthyretin cardiac amyloidosis. Am J Cardiol. 2014;113:1723–7. doi: 10.1016/j.amjcard.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Satoskar AA, Efebera Y, Hasan A, Brodsky S, Nadasdy G, Dogan A, Nadasdy T. Strong transthyretin immunostaining: potential pitfall in cardiac amyloid typing. Am J Surg Pathol. 2011;35:1685–90. doi: 10.1097/PAS.0b013e3182263d74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–54. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 94.Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, Kimmich C, Goldschmidt H, Ho AD, Hose D, Jauch A, Schonland SO. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33:1371–8. doi: 10.1200/JCO.2014.57.4947. [DOI] [PubMed] [Google Scholar]
- 95.Bochtler T, Hegenbart U, Kunz C, Benner A, Seckinger A, Dietrich S, Granzow M, Neben K, Goldschmidt H, Ho AD, Hose D, Jauch A, Schonland SO. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid. 2015;21:9–17. doi: 10.3109/13506129.2013.854766. [DOI] [PubMed] [Google Scholar]
- 96.Bochtler T, Hegenbart U, Kunz C, Benner A, Schonland SO. Reply to R. Warsame et al. J Clin Oncol. 2015;33:3976–7. doi: 10.1200/JCO.2015.63.2141. [DOI] [PubMed] [Google Scholar]
- 97.Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, Leung N, Dingli D, Lust JA, Ketterling RP, Lin Y, Russell S, Hwa L, Kapoor P, Go RS, Zeldenrust SR, Kyle RA, Rajkumar SV, Dispenzieri A. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015;5:e310. doi: 10.1038/bcj.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520–30. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 99.Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29:1924–33. doi: 10.1200/JCO.2010.32.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26:137–54. doi: 10.1016/j.blre.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 101.Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, Segal A, Ruberg F, Meier-Ewert H, Andrea NT, Sloan JM, Finn KT, Doros G, Blade J, Skinner M. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–52. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaccard A, Comenzo RL, Hari P, Hawkins PN, Roussel M, Morel P, Macro M, Pellegrin JL, Lazaro E, Mohty D, Mercie P, Decaux O, Gillmore J, Lavergne D, Bridoux F, Wechalekar AD, Venner CP. Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naive patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III) Haematologica. 2014;99:1479–85. doi: 10.3324/haematol.2014.104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Langer AL, Miao S, Mapara M, Radhakrishnan J, Maurer MS, Raza S, Mears JG, Solomon A, Lentzsch S. Results of Phase I Study of Chimeric Fibril-Reactive Monoclonal Antibody 11–1F4 in Patients with AL Amyloidosis. Blood. 2015;126:188–188. [Google Scholar]
- 104.Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106–14. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 105.Comenzo RL. Out, Out--Making Amyloid’s Candle Briefer. N Engl J Med. 2015;373:1167–9. doi: 10.1056/NEJMe1508746. [DOI] [PubMed] [Google Scholar]
- 106.Gertz MALH, Comenzo RL, et al. Cardiac and renal biomarker responses in a phase 1/2 study of NEOD001 in patients with AL amyloidosis and persistent organ dysfunction. Journal of Clinical Oncology. 2015;33:8514a. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–28. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 108.Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, Vidus Rosin M, Albertini R, Moratti R, Merlini G, Schonland S. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–32. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 109.Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121:5124–30. doi: 10.1182/blood-2013-01-453001. [DOI] [PubMed] [Google Scholar]
- 110.Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287–94. doi: 10.1182/asheducation-2010.1.287. [DOI] [PubMed] [Google Scholar]
- 111.Shah G, Kaul E, Fallo S, Cossor F, Smith H, Sprague K, Klein A, Miller K, Comenzo R. Bortezomib subcutaneous injection in combination regimens for myeloma or systemic light-chain amyloidosis: a retrospective chart review of response rates and toxicity in newly diagnosed patients. Clin Ther. 2013;35:1614–20. doi: 10.1016/j.clinthera.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 112.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, Basset M, Hawkins P, Merlini G, Wechalekar AD. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–5. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 113.Kuhn DJ, Orlowski RZ, Bjorklund CC. Second Generation Proteasome Inhibitors: Carfilzomib and Immunoproteasome-Specific Inhibitors (IPSIs) Current Cancer Drug Targets. 2011;11:285–295. doi: 10.2174/156800911794519725. [DOI] [PubMed] [Google Scholar]
- 114.Landau H, Hassoun H, Rosenzweig MA, Maurer M, Liu J, Flombaum C, Bello C, Hoover E, Riedel E, Giralt S, Comenzo RL. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia. 2013;27:823–8. doi: 10.1038/leu.2012.274. [DOI] [PubMed] [Google Scholar]
- 115.Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007;109:457–64. doi: 10.1182/blood-2006-07-035352. [DOI] [PubMed] [Google Scholar]
- 116.Palladini G, Russo P, Nuvolone M, Lavatelli F, Perfetti V, Obici L, Merlini G. Treatment with oral melphalan plus dexamethasone produces long-term remissions in AL amyloidosis. Blood. 2007;110:787–8. doi: 10.1182/blood-2007-02-076034. [DOI] [PubMed] [Google Scholar]
- 117.Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163–78. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47:625–38. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dubrey S, Ackermann E, Gillmore J. The transthyretin amyloidoses: advances in therapy. Postgrad Med J. 2015;91:439–48. doi: 10.1136/postgradmedj-2014-133224. [DOI] [PubMed] [Google Scholar]
- 120.Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, Furtado E, Barroso E, Daniel J, Samuel D, Adam R, Karam V, Poterucha J, Lewis D, Ferraz-Neto BH, Cruz MW, Munar-Ques M, Fabregat J, Ikeda S, Ando Y, Heaton N, Otto G, Suhr O. Liver Transplantation for Hereditary Transthyretin Amyloidosis: After 20 Years Still the Best Therapeutic Alternative? Transplantation. 2015;99:1847–54. doi: 10.1097/TP.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 121.Stangou AJ, Heaton ND, Hawkins PN. Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med. 2005;352:2356. doi: 10.1056/NEJM200506023522219. [DOI] [PubMed] [Google Scholar]
- 122.Gustafsson S, Ihse E, Henein MY, Westermark P, Lindqvist P, Suhr OB. Amyloid fibril composition as a predictor of development of cardiomyopathy after liver transplantation for hereditary transthyretin amyloidosis. Transplantation. 2012;93:1017–23. doi: 10.1097/TP.0b013e31824b3749. [DOI] [PubMed] [Google Scholar]
- 123.Niemietz C, Chandhok G, Schmidt H. Therapeutic Oligonucleotides Targeting Liver Disease: TTR Amyloidosis. Molecules. 2015;20:17944–75. doi: 10.3390/molecules201017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 125.Ackermann EJ, Guo S, Booten S, Alvarado L, Benson M, Hughes S, Monia BP. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19(Suppl 1):43–4. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
- 126.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, Heneghan MA, Gorevic PD, Litchy WJ, Wiesman JF, Nordh E, Corato M, Lozza A, Cortese A, Robinson-Papp J, Colton T, Rybin DV, Bisbee AB, Ando Y, Ikeda S, Seldin DC, Merlini G, Skinner M, Kelly JW, Dyck PJ, Diflunisal Trial C. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceicao IM, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Wilson A, Grogan DR. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, Packman J, Powers ET, Wiseman RL, Foss TR, Wilson IA, Kelly JW, Labaudiniere R. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:9629–34. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ward JE, Ren R, Toraldo G, Soohoo P, Guan J, O’Hara C, Jasuja R, Trinkaus-Randall V, Liao R, Connors LH, Seldin DC. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118:6610–7. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Obici LCA, Lozza A, Palladini G, Perlini S, Gobbi M, et al. A phase II study of doxycycline plus tauroursodeoxycholic acid in transthyretin amyloidois. XIVth International Symposium on Amyloidosis (ISA); 27 April – 1 May 2014; [DOI] [PubMed] [Google Scholar]
- 131.Kristen AV, Lehrke S, Buss S, Mereles D, Steen H, Ehlermann P, Hardt S, Giannitsis E, Schreiner R, Haberkorn U, Schnabel PA, Linke RP, Rocken C, Wanker EE, Dengler TJ, Altland K, Katus HA. Green tea halts progression of cardiac transthyretin amyloidosis: an observational report. Clin Res Cardiol. 2012;101:805–13. doi: 10.1007/s00392-012-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.aus dem Siepen F, Bauer R, Aurich M, Buss SJ, Steen H, Altland K, Katus HA, Kristen AV. Green tea extract as a treatment for patients with wild-type transthyretin amyloidosis: an observational study. Drug Des Devel Ther. 2015;9:6319–25. doi: 10.2147/DDDT.S96893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coelho T, Maia LF, da Silva AM, Cruz MW, Plante-Bordeneuve V, Suhr OB, Conceicao I, Schmidt HH, Trigo P, Kelly JW, Labaudiniere R, Chan J, Packman J, Grogan DR. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–14. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quarta CCF, Solomon RHSD, Suhr OB, Obici L, Perlini S, Lindqvist P, Koyama J, Sekijima Y, Zeldenrust SR, Yamashita T, Horibata Y, Miller F, Gorevic P, Merlini G, Ando Y, Ikeda S, Ruberg F, Berk JL. The prevalence of cardiac amyloidosis in familial amyloidotic polyneuropathy with predominant neuropathy: The Diflunisal Trial. International Symposium on Amyloidosis. 2014:88–89. [Google Scholar]
- 135.Lozeron P, Theaudin M, Mincheva Z, Ducot B, Lacroix C, Adams D French Network for FAP. Effect on disability and safety of Tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20:1539–45. doi: 10.1111/ene.12225. [DOI] [PubMed] [Google Scholar]
- 136.Cho Y, Baranczak A, Helmke S, Teruya S, Horn EM, Maurer MS, Kelly JW. Personalized medicine approach for optimizing the dose of tafamidis to potentially ameliorate wild-type transthyretin amyloidosis (cardiomyopathy) Amyloid. 2015;22:175–80. doi: 10.3109/13506129.2015.1063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hosenpud JD, DeMarco T, Frazier OH, Griffith BP, Uretsky BF, Menkis AH, O’Connell JB, Olivari MT, Valantine HA. Progression of systemic disease and reduced long-term survival in patients with cardiac amyloidosis undergoing heart transplantation. Follow-up results of a multicenter survey. Circulation. 1991;84:III338–43. [PubMed] [Google Scholar]
- 138.Maurer MS, Raina A, Hesdorffer C, Bijou R, Colombo P, Deng M, Drusin R, Haythe J, Horn E, Lee SH, Marboe C, Naka Y, Schulman L, Scully B, Shapiro P, Prager K, Radhakrishnan J, Restaino S, Mancini D. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplantation. 2007;83:539–45. doi: 10.1097/01.tp.0000255567.80203.bd. [DOI] [PubMed] [Google Scholar]
- 139.Dey BR, Chung SS, Spitzer TR, Zheng H, Macgillivray TE, Seldin DC, McAfee S, Ballen K, Attar E, Wang T, Shin J, Newton-Cheh C, Moore S, Sanchorawala V, Skinner M, Madsen JC, Semigran MJ. Cardiac transplantation followed by dose-intensive melphalan and autologous stem-cell transplantation for light chain amyloidosis and heart failure. Transplantation. 2010;90:905–11. doi: 10.1097/TP.0b013e3181f10edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davis MK, Kale P, Liedtke M, Schrier S, Arai S, Wheeler M, Lafayette R, Coakley T, Witteles RM. Outcomes after heart transplantation for amyloid cardiomyopathy in the modern era. Am J Transplant. 2015;15:650–8. doi: 10.1111/ajt.13025. [DOI] [PubMed] [Google Scholar]
- 141.Davis MK, Lee PH, Witteles RM. Changing outcomes after heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant. 2015;34:658–66. doi: 10.1016/j.healun.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 142.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A International Society for Heart Lung Transplantation Infectious Diseases C, International Society for Heart Lung Transplantation Pediatric Transplantation C, International Society for Heart Lung Transplantation Heart F, Transplantation C. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 143.Gilstrap LNE, Maurer M, Feltrin G, Estep J, Witteles R, Zucker M, Baran D, Kushwaha S, Seldin D, Semigran M. Multi-Center, International Registry of Cardiac Transplantation for Light Chain (AL) and Transthyreitin (TTR) Amyloidosis, A [abstract] Am J Transplant. 2013;13(suppl 5) [Google Scholar]
- 144.Niehaus EGL, Maurer MS, Witteles R, Zucker M, Baran D, Estep J, Feltrin G, Stone JR, Patel J, Seldin D, Semigran M. Outcomes from an international registry of cardiac transplantation for light chain (AL) and transthyreitin (TTR) amyloidosis. Amyloid: Insoluble, but Solvable; XIVth International Symposium on Amyloidosis. 2014 [Google Scholar]
- 145.Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS, Joyce LD, Park SJ. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011;4:266–75. doi: 10.1161/CIRCHEARTFAILURE.110.959288. [DOI] [PubMed] [Google Scholar]
- 146.Grupper A, Park SJ, Pereira NL, Schettle SD, Gerber Y, Topilsky Y, Edwards BS, Daly RC, Stulak JM, Joyce LD, Kushwaha SS. Role of ventricular assist therapy for patients with heart failure and restrictive physiology: Improving outcomes for a lethal disease. J Heart Lung Transplant. 2015;34:1042–9. doi: 10.1016/j.healun.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 147.Tabtabai S, Steiner J, Vaduganathan M, Stone J, Estep J, Witteles R, Giuseppe F, Zucker M, Baran D, Seldin D, Patel J, Hanna M, Cordero-Reyes A, Selby V, Maurer M, Semigran MJ. The Use of Circulatory Support While Awaiting Heart Transplant in Patients With AL and TTR: Amyloidosis: A Report From iCCAT, the International Consortium for Cardiac Amyloid Transplant. The Journal of Heart and Lung Transplantation. 34:S95–S96. [Google Scholar]
- 148.Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, Shah SJ. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3:275–82. doi: 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.