Summary
Many studies of eosinophilic esophagitis (EoE) utilize expert pathology review, but it is unknown whether less experienced pathologists can reliably assess EoE histology. We aimed to determine whether trainee pathologists can accurately quantify esophageal eosinophil counts and identify associated histologic features of EoE, as compared to expert pathologists. We used a set of 40 digitized slides from patients with varying degrees of esophageal eosinophilia. Each of six trainee pathologists underwent a teaching session and used our validated protocol to determine eosinophil counts and associated EoE findings. The same slides had previously been evaluated by expert pathologists, and these results comprised the gold standard. Eosinophil counts were correlated, and agreement was calculated for the diagnostic threshold of 15 eosinophils per high-power field (eos/hpf) as well as for associated EoE findings. Peak eosinophil counts were highly correlated between the trainees and the gold standard (Rho ranged from 0.87–0.92; p<0.001 for all). Peak counts were also highly correlated between trainees (0.75–0.91; p<0.001), and results were similar for mean counts. Agreement was excellent for determining if a count exceeded the diagnostic threshold (kappa ranged from 0.83 to 0.89; p<0.001). Agreement was very good for eosinophil degranulation (kappa 0.54 to 0.83; p<0.01) and spongiosis (kappa 0.44–0.87; p<0.01), but was lower for eosinophil microabscesses (kappa 0.37–0.64; p<0.01). In conclusion, using a teaching session, digitized slide set, and validated protocol, the agreement between pathology trainees and expert pathologists for determining eosinophil counts was excellent. Agreement was very good for eosinophil degranulation and spongiosis, but less so for microabscesses.
Keywords: Eosinophilic esophagitis, histology, training, agreement
Introduction
Eosinophilic esophagitis (EoE) is an allergy/immune-mediated condition defined clinically by symptoms of esophageal dysfunction and histologically by eosinophilic infiltration of the esophageal mucosa [1, 2]. The clinical presentation consists of a spectrum of symptoms depending upon age, with children having feeding difficulties, failure to thrive, vomiting, heartburn, and abdominal pain, and adolescents and adults complaining of dysphagia and food impaction [3]. A subsequent esophagogastroduodenoscopy (EGD) is required to obtain esophageal biopsy samples. The hallmark pathologic feature of EoE is a peak eosinophil count ≥ 15 eosinophils per high-power field (eos/hpf), which persists after high-dose proton-pump inhibitor and after other potential causes of secondary esophageal and systemic eosinophilia have been excluded [1, 2, 4, 5]. In addition to the presence of eosinophils, there are a number of other associated histologic findings. Though these are not specific for EoE, they include epithelial spongiosis, basal layer hyperplasia, lamina propria fibrosis, eosinophilic microabscesses, eosinophilic degranulation, and superficial infiltration of the epithelium [4, 5]. However, all findings may not be noted in a single biopsy [6–9].
The histopathologic findings of EoE are well described, and recently a new summary score has been developed and validated for use in EoE that may be more accurate than the eosinophil count alone [10]. However, much of the research on EoE has been performed at referral centers in conjunction with expert pathology review [11–20]. There are few studies of the reproducibility of determining eosinophil counts and assessing the other associated findings outside of this setting [21–23]. For example, we have previously validated a protocol for determining eosinophil counts with excellent inter reliability, but these results were limited to expert gastrointestinal (GI) pathologists [21]. It is currently unknown how well this protocol would perform outside of an expert setting. However, it is important to understand how diagnosis could translate to other settings.
The aim of this study was to determine whether non-expert trainee pathologists could accurately quantify esophageal eosinophil counts and identify associated histologic features of EoE, as compared to expert pathologists.
Materials and Methods
Patients and pathology samples
This study utilized a previously constructed set of esophageal biopsy samples from 40 patients in the University of North Carolina (UNC) EoE Clinicopathologic Database. These were selected specifically to represent a wide spectrum of eosinophilia (ranging from 0–400 eos/hpf), and the methodology for selecting these patients has been described previously [21, 24–27]. Archived slides were de-identified (and there was no linked reference to clinical or endoscopic findings) and scanned using the Aperio ScanScope® CS slide scanner (Aperio Technologies, Vista, CA). The digitized slides were evaluated by six second and third year pathology residents after a training session as detailed below. The images were viewed using the Aperio ImageScope software. This study was approved by the UNC Institutional Review Board.
Training curriculum
Prior to analysis of the 40 patient slide set, each trainee pathologist reviewed our previously published protocol for determination of eosinophilic counts and associated histologic findings of EoE [21]. Additionally, each pathologist participated in a teaching session to acclimate to the Aperio software, quantify eosinophil counts, characterize the eosinophil infiltrate, and identify microabscesses, eosinophil degranulation, spongiosis, and lamina propria fibrosis. Questions could be addressed to the senior study pathologist (JTW) or the senior author (ESD) during this time, but not during the evaluation of the study slide set. The pathology residents were either in their second or early in their third year of pathology training at the time of participation.
Histologic analysis
Our previously validated protocol, which also showed excellent agreement between scanned and glass slides, was used for analysis [8, 21]. In brief, after reviewing the entire biopsy sample, five high-power fields on the digitized slides were evaluated for maximum eosinophil density (eosinophils/mm2 (eos/mm2). Trainees were instructed to quantify eosinophils in the most highly inflamed field, the second most highly inflamed field, and in three additional fields that were representative of the biopsy overall. The field was selected on the computer screen using the ImageScope software, and this provided an area (mm2). The eosinophil density (eos/mm2) was then converted to an eosinophil count (eos/hpf) for an assumed hpf size of 0.24mm2, which is the most commonly reported hpf size in the literature [28].
Next, each hpf was evaluated for the other histologic features of EoE. These included the presence of eosinophilic microabscesses (defined as clusters of ≥ 4eosinophils), eosinophilic degranulation (defined as release of eosinophilic granules from eosinophils into the surrounding epithelium), basal layer hyperplasia (expansion of the basal zone by >25% of epithelial height; evaluated in properly oriented specimens only), spongiosis (also termed dilated intercellular spaces), and lamina propria fibrosis (increased deposition of collagen in the lamina propria, if sufficient subepithelial stroma was present for evaluation).
Statistical analysis
We calculated the peak eosinophil count (highest count of all of the 5 hpfs examined) as well as the mean count of the 5 hpfs for each sample as recorded by each of the participating pathologists. Additionally, it was noted whether a sample had eosinophil degranulation, eosinophil microabscesses, or spongiosis in any hpf examined. Of note, lamina propria fibrosis was not seen commonly enough to be included in this analysis, and not enough of the samples were properly oriented to accurately assess basal layer hyperplasia.
To create a gold standard for comparison, we utilized the data previously determined in our original pathology protocol validation study, as the identical 40 patient samples were used in that analysis [21]. Specifically, the data from three expert pathologists were averaged to generate reference standards for peak and mean eosinophil counts, and for the presence of eosinophil degranulation, eosinophil microabscesses, and spongiosis.
Correlations between the trainee and gold standard peak eosinophil counts, and the trainee and gold standard mean eosinophil counts were calculated using Pearson’s Rho (R). We also assessed correlations amongst all pairwise comparisons of the trainees. We then used the kappa statistic to determine agreement for determining a count above the diagnostic threshold of ≥ 15 eos/hpf, both comparing trainees to the gold standard, and comparing trainees amongst themselves. Finally, we assessed agreement, again with kappa, between the trainees and the gold standard for the associated histologic findings of EoE. Statistical analysis was performed with Stata version 9 (College Station, TX).
Results
Correlation for peak and mean eosinophil counts
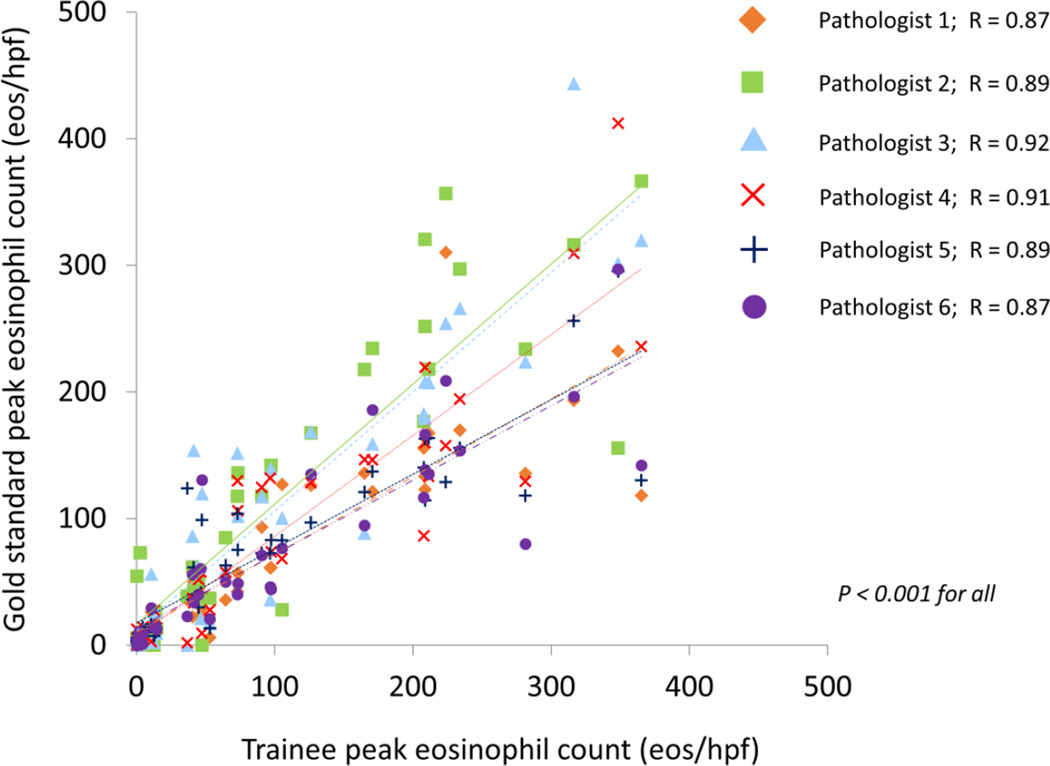
In the training set of 40 slides, the gold standard peak eosinophil count was 101 ± 107 eos/hpf, and the trainee counts ranged from 72 to 113 eos/hpf (Table 1). The correlation for peak eosinophil counts between the trainees and gold standard was excellent, with R values ranging from 0.87 to 0.92 (p < 0.001 for all) (Figure 1). Additionally, correlations between trainees for peak counts were similarly good, ranging from 0.75 to 0.91 (p < 0.001 for all) (Table 2).
Table 1.
Summary data for histologic findings (n=40 in the training slide set)
| Gold standard |
Path 1 | Path 2 | Path 3 | Path 4 | Path 5 | Path 6 | |
|---|---|---|---|---|---|---|---|
| Max eosinophil count (mean eos/hpf ± SD) |
101 ± 107 | 72 ± 75 | 113 ± 115 | 111 ± 109 | 87 ± 94 | 77 ± 71 | 72 ± 72 |
| Mean eosinophil count (mean eos/hpf ± SD) |
53 ± 60 | 44 ± 51 | 72 ± 78 | 46 ± 46 | 41 ± 47 | 42 ± 45 | 38 ± 40 |
| Diagnostic threshold assessment (n, %) |
|||||||
| Above ≥15 eos/hpf | 27 (68) | 28 (70) | 26 (65) | 29 (73) | 26 (65) | 27 (68) | 29 (73) |
| Overdiagnosisa | -- | 2 (5) | 0 (0) | 2 (5) | 1 (3) | 1 (3) | 2 (5) |
| Underdiagnosisb | -- | 1 (3) | 1 (3) | 0 (0) | 2 (5) | 1 (3) | 0 (0) |
| Associated findings (n, %) | |||||||
| Degranulation | 27 (68) | 34 (85) | 26 (65) | 23 (58) | 28 (70) | 32 (80) | 24 (60) |
| Microabscesses | 17 (43) | 18 (45) | 19 (48) | 12 (30) | 13 (33) | 28 (70) | 26 (65) |
| Spongiosis | 29 (73) | 28 (70) | 24 (60) | 20 (50) | 32 (80) | 31 (78) | 29 (73) |
indicates a slide was assessed to have ≥15 eos/hpf when the gold standard did not
indicated a slides was assessed to have <15 eos/hpf when the gold standard had ≥15 eos/hpf
Figure 1.
Correlation, as measured by Pearson’s R, between the gold standard peak eosinophil count, and the peak eosinophil counts determined by each of the trainees.
Table 2.
Correlation of peak and mean eosinophil counts between pathology trainees
| Path 1 | Path 2 | Path 3 | Path 4 | Path 5 | Path 6 | |
|---|---|---|---|---|---|---|
| For peak eosinophil countsa | ||||||
| Path 1 | -- | 0.83 | 0.84 | 0.81 | 0.84 | 0.89 |
| Path 2 | -- | -- | 0.86 | 0.79 | 0.75 | 0.78 |
| Path 3 | -- | -- | -- | 0.86 | 0.91 | 0.86 |
| Path 4 | -- | -- | -- | -- | 0.89 | 0.87 |
| Path 5 | -- | -- | -- | -- | -- | 0.90 |
| Path 6 | -- | -- | -- | -- | -- | -- |
| For mean eosinophil countsb | ||||||
| Path 1 | -- | 0.90 | 0.88 | 0.91 | 0.91 | 0.92 |
| Path 2 | -- | -- | 0.91 | 0.83 | 0.79 | 0.79 |
| Path 3 | -- | -- | -- | 0.84 | 0.86 | 0.83 |
| Path 4 | -- | -- | -- | -- | 0.94 | 0.93 |
| Path 5 | -- | -- | -- | -- | -- | 0.96 |
| Path 6 | -- | -- | -- | -- | -- | -- |
Values represent Pearson’s R
all p < 0.001;
all p < 0.001
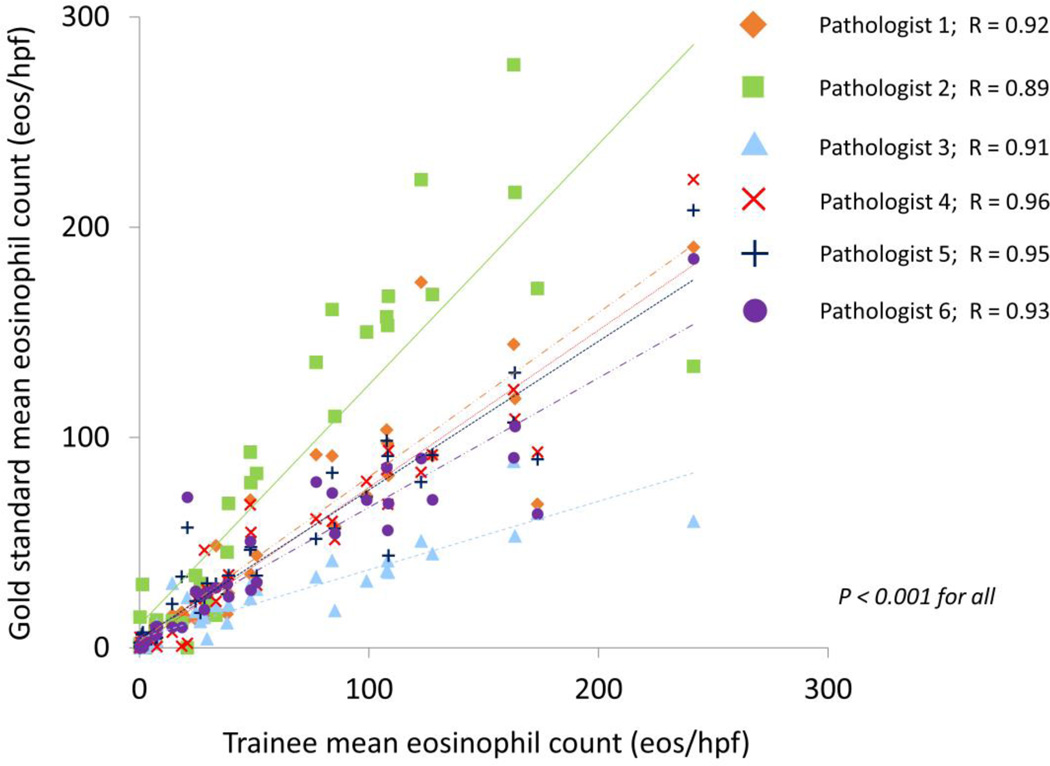
The gold standard mean eosinophil count was 53 ± 60 eos/hpf, and the trainee counts ranged from 38 to 72 eos/hpf (Table 1). The correlation for mean eosinophil counts between the trainees and the gold standard was also excellent, with R values ranging from 0.89 to 0.96 (p < 0.001 for all) (Figure 2). Similarly, the correlation among trainees ranged from 0.79 to 0.96 (p<0.001 for all) (Table 2).
Figure 2.
Correlation, as measured by Pearson’s R, between the gold standard mean eosinophil count, and the mean eosinophil counts determined by each of the trainees.
Agreement for the diagnostic threshold of 15 eos/hpf
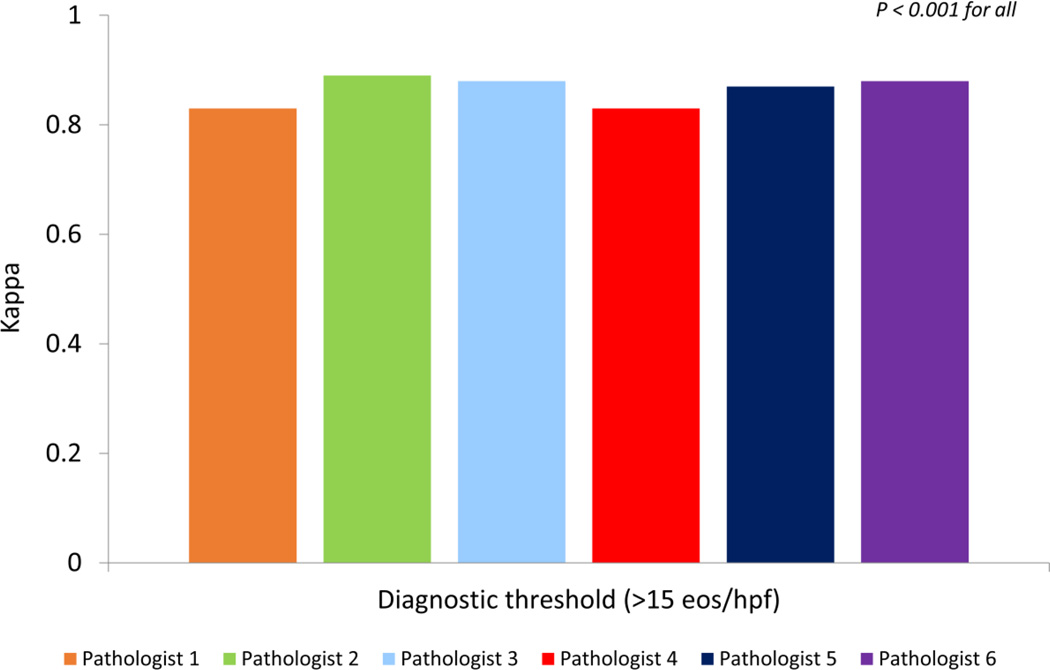
A total of 27 (68%) of the gold standard slides were above the diagnostic threshold, and trainees only classified a range of 0–2 cases as above the threshold when the gold standard was below it, and a similar number were classified below the threshold when the gold standard was above it (Table 1). The overall agreement between the trainees and the gold standard for determining an eosinophil count above the diagnostic threshold of 15 eos/hpf was excellent; kappa values ranged from 0.83 to 0.89 (p < 0.001 for all) (Figure 3). Agreement amongst the trainees was also very good to excellent (Table 3). Fourteen of the fifteen comparisons fell within the range of 0.71 to 1.00 (p < 0.001 for all) with a single correlation that was lower at 0.66 (p < 0.001).
Figure 3.
Agreement, as measured by the kappa statistic, between the gold standard and trainee-determined eosinophil count, for a count ≥ 15 eos/hpf.
Table 3.
Agreement for determining a peak eosinophil count ≥ 15 eos/hpf among pathology trainees*
| Path 1 | Path 2 | Path 3 | Path 4 | Path 5 | Path 6 | |
|---|---|---|---|---|---|---|
| Path 1 | -- | 0.66 | 0.82 | 0.77 | 0.94 | 0.82 |
| Path 2 | -- | -- | 0.89 | 0.89 | 0.78 | 0.89 |
| Path 3 | -- | -- | -- | 0.76 | 0.88 | 1.00 |
| Path 4 | -- | -- | -- | -- | 0.72 | 0.71 |
| Path 5 | -- | -- | -- | -- | -- | 0.88 |
| Path 6 | -- | -- | -- | -- | -- | -- |
Values represent kappa statistics; all p < 0.001
Agreement for associated EoE histologic findings
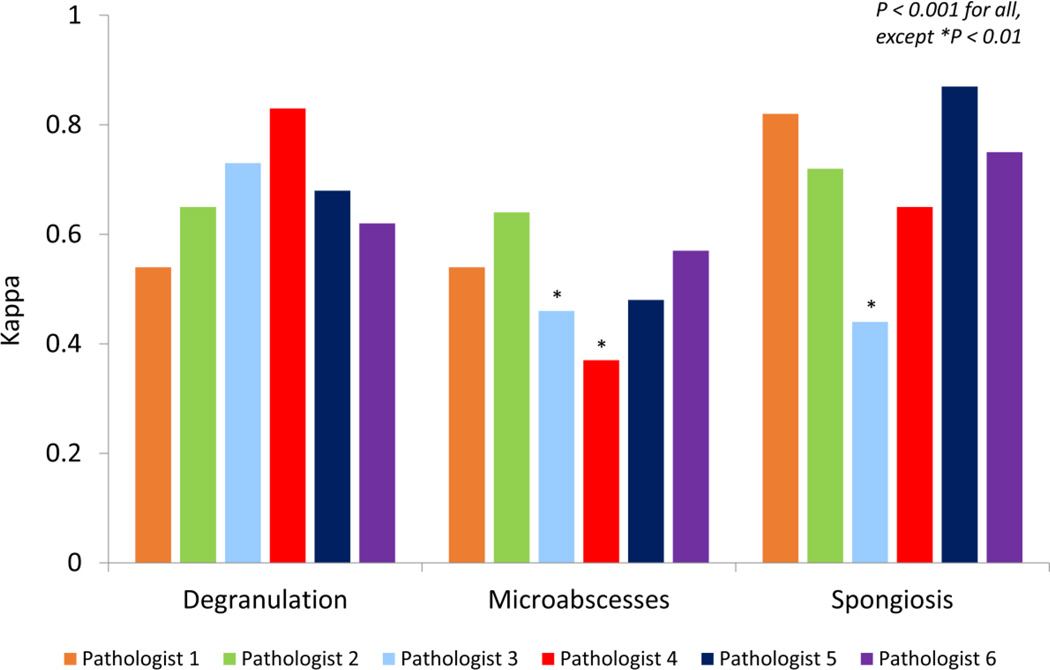
A total of 27 (68%) of slides had eosinophil degranulation on the gold standard analysis, with trainee ranges from 58–85% (Table 1). There was very good agreement between the trainees and the gold standard, with four of the six residents having kappa values between 0.62 and 0.73 (p < 0.001 for all) (Figure 4). The two values outside of this range were 0.54 and 0.83 (p<0.001 for both).
Figure 4.
Agreement, as measured by the kappa statistic, between the gold standard and trainee pathologists, for determining the presence of eosinophil degranulation, eosinophil microabscesses, and spongiosis.
A total of 29 (73%) of slides had spongiosis on the gold standard analysis, with trainee ranges from 50–80% (Table 1). The evaluation of epithelial spongiosis also showed very good agreement (Figure 4). The range of kappa values was 0.65 to 0.87 (p < 0.001 for all) for five of the trainees, with one value of 0.44 (p = 0.001) out of this range.
A total of 17 (43%) of slides had microabscesses on the gold standard analysis, with trainee ranges from 58–85% (Table 1). Agreement on the presence of microabscesses was not as high as the other microscopic findings (Figure 4). While the kappa values for four of the trainees ranged from 0.48–0.64 (p < 0.001 for all), values of 0.46 (p = 0.001) and 0.37 (p = 0.009) were also noted.
Discussion
EoE is a clinicopathologic diagnosis, and pathologic features must be interpreted in the context of clinical symptoms. However, diagnosis does require accurate determination of the peak esophageal eosinophil count as well as associated histopathologic findings [1, 2, 4, 5]. To date, most studies of EoE have involved expert pathologists reviewing biopsy samples. With the rapidly increasing incidence and prevalence of EoE [29, 30], however, many patients are seen outside of centers with this expert pathology review available, and the accuracy of assessing histologic features of EoE in this setting is poorly understood. Our study, which was performed to determine the accuracy of non-expert trainee pathologists quantifying esophageal eosinophil counts and identifying associated histologic features of EoE, had a number of interesting findings. As compared to a composite gold standard from expert pathologists, trainees had excellent correlation for both peak and mean eosinophil counts, and excellent agreement for identifying specimens with counts above the diagnostic threshold. While there was very good agreement for the associated findings of eosinophil degranulation and spongiosis, eosinophilic microabscesses were less reliably determined. Overall, however, our results suggest that non-expert pathologist can accurately characterize esophageal biopsies with a range of eosinophil counts and EoE-associated findings.
In our previous study that focused only on expert pathologists, we found that correlations for peak and mean counts were similarly excellent, and that agreement for the diagnostic threshold was also outstanding [21]. However, there are few other studies in the literature that have assessed this topic, and much of the literature on histologic findings in EoE focuses on describing the features and characterizing the variability and patchiness of findings throughout the esophagus [5–10, 12, 13, 31–34]. A study by Stucke et al assessed accuracy of esophageal biopsy interpretation by re-reviewing a 106 biopsies with eosinophil counts in the 1–14 eos/hpf range [22]. The authors found 23 of these samples (22%) met the criteria for EoE on a second review by a research assistant who was trained by an expert pathologist, and they further calculated that re-review of biopsies may yield additional EoE diagnoses in 5% of all biopsies. While we are not able to directly investigate their hypothesis that under diagnosis could be due to non-specialized pathologist review, our results would indicate that non-expert pathologists can provide reliable results compared with experts. In one of the few published studies that we could identify addressing this topic, agreement between pathologists was 95% with a kappa of 0.89, in range with what our results show [23]. In a recent study validating a histologic scoring system for EoE, there was strong-to-moderate agreement for the score among expert pathologists [10], and in a prior autopsy study interobserver correlation for eosinophils in the gastrointestinal tract (with the exception of the esophagus) was 0.96 [35].
There are several limitations of this study to consider when interpreting the results. First, we assessed a relatively small and limited number of trainees, and because of this could not comment on differences that may be present by length of training. We also did not assess medical students or other training levels. Second, this work was conducted at a tertiary referral center, so results may not be generalizable. Third, the training for this protocol was largely self-directed, but even with this limitation the results were still excellent overall. It is possible, however, that additional training may be required, particularly with the associated histologic findings of EoE. Finally, we did not have enough samples with lamina propria fibrosis or that were oriented well enough to assess basal zone hyperplasia. However, these limitations are balanced by the strengths of the study which included use of a previously validated pathologic interpretation protocol, use of digitized slides, standardized training, and a standardized set of patient slides specifically selected to demonstrate a range of esophageal eosinophilia and associated EoE findings.
In conclusion, we found that after undergoing a training session and using a standardized digitized slide set and a validated counting protocol, non-expert trainee pathologists could determine eosinophil counts with excellent agreement and correlation compared to gold standard values derived from expert pathologists. Though agreement was not as good for the identification of eosinophil microabscesses, it was still in the very good range for eosinophil degranulation, and spongiosis. These results show that biopsy slide analysis techniques with a validated protocol are generalizable to non-specialized pathologists when assessing esophageal biopsy samples for features of EoE, and that pathology trainees can be important collaborators in EoE research.
Highlights.
The study assessed non-expert trainee pathologists compared to expert pathologists.
Correlation was excellent between trainees and experts for total eosinophil counts.
Agreement was excellent for eosinophil counts above the diagnostic threshold.
Agreement was very good for degranulation and spongiosis, but not for microabscesses.
Acknowledgments
Funding: This research was supported, in part, but NIH grants K23 DK90073 (ESD) and R01 DK101856 (ESD). It also utilized the Histology Core of the UNC Center for Gastrointestinal Biology and Disease which is funded by NIH P30 DK034987, and the UNC Translational Pathology lab which is funded by NIH P30 CA016086.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None of the authors have potential conflicts of interest related to this manuscript.
Author contributions:
Rusin: Data collection and interpretation; drafting of manuscript; critical revision of manuscript; approval of final version.
Covey: Data collection and interpretation; critical revision of manuscript; approval of final version.
Perjar: Data collection and interpretation; critical revision of manuscript; approval of final version.
Hollyfield: Data collection and interpretation; critical revision of manuscript; approval of final version.
Speck: Data collection and interpretation; critical revision of manuscript; approval of final version.
Woodward: Data collection and interpretation; critical revision of manuscript; approval of final version.
Woosley: Pathology supervision; data interpretation; critical revision of manuscript; approval of final version.
Dellon: Project conception; data analysis and interpretation; drafting of manuscript; critical revision of manuscript; approval of final version.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3.e6–20.e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras C, Katzka DA. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014;147:1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43:257–268. doi: 10.1016/j.gtc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol. 2009;104:485–490. doi: 10.1038/ajg.2008.40. [DOI] [PubMed] [Google Scholar]
- 6.Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF., 3rd Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130:798–800. doi: 10.1016/j.jaci.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen JA, Lager DJ, Lewin M, Rendon G, Roberts CA. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol. 2014;109:515–520. doi: 10.1038/ajg.2013.463. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Shaheen NJ, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28:383–390. doi: 10.1038/modpathol.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salek J, Clayton F, Vinson L, Saffari H, Pease LF, 3rd, Boynton K, et al. Endoscopic appearance and location dictate diagnostic yield of biopsies in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41:1288–1295. doi: 10.1111/apt.13201. [DOI] [PubMed] [Google Scholar]
- 10.Collins MH, Martin LJ, Alexander ES, Boyd JT, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2016 doi: 10.1111/dote.12470. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, Dose Reduction, and Resistance to High-dose Fluticasone in Patients with Eosinophilic Esophagitis. Gastroenterology. 2014;147:324.e5–333.e5. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–319. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716–721. doi: 10.1038/ajg.2008.117. [DOI] [PubMed] [Google Scholar]
- 14.DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210–218. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 15.DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa'ad AH, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol. 2010;126:112–119. doi: 10.1016/j.jaci.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellon ES, Rusin S, Gebhart JH, Covey S, Speck O, Woodward K, et al. A clinical prediction tool identifies cases of eosinophilic esophagitis without endoscopic biopsy: A prospective study. Am J Gastroenterol. 2015;110:1347–1354. doi: 10.1038/ajg.2015.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and Endoscopic Characteristics do Not Reliably Differentiate PPI-Responsive Esophageal Eosinophilia and Eosinophilic Esophagitis in Patients Undergoing Upper Endoscopy: A Prospective Cohort Study. Am J Gastroenterol. 2013;108:1854–1860. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–1405. doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capocelli KE, Fernando SD, Menard-Katcher C, Furuta GT, Masterson JC, Wartchow EP. Ultrastructural features of eosinophilic oesophagitis: impact of treatment on desmosomes. J Clin Pathol. 2015;68:51–56. doi: 10.1136/jclinpath-2014-202586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzka DA, Geno DM, Ravi A, Smyrk TC, Lao-Sirieix P, Miramedi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:77–83. e2. doi: 10.1016/j.cgh.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Fritchie KJ, Rubinas TC, Woosley JT, Shaheen NJ. Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci. 2010;55:1940–1949. doi: 10.1007/s10620-009-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stucke EM, Clarridge KE, Collins MH, Henderson CJ, Martin LJ, Rothenberg ME. Value of an Additional Review for Eosinophil Quantification in Esophageal Biopsies. J Pediatr Gastroenterol Nutr. 2015;61:65–68. doi: 10.1097/MPG.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel N, Busler JF, Geisinger K, Hill I. Are Pathologists Accurately Diagnosing Eosinophilic Esophagitis in Children? A 9-Year Single Academic Institutional Experience With Interobserver Observations. Int J Surg Pathol. 2011;19:260–266. doi: 10.1177/1066896910363707. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577.e4–585.e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ, Dellon ES. Dietary Elimination Therapy Is an Effective Option for Adults With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2014;12:1272–1279. doi: 10.1016/j.cgh.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eluri S, Runge TM, Cotton CC, Burk CM, Wolf WA, Woosley JT, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc. 2016;83:1142–1148. doi: 10.1016/j.gie.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runge TM, Eluri S, Cotton CC, Burk CM, Woosley JT, Shaheen NJ, et al. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am J Gastroenterol. 2016;111:206–213. doi: 10.1038/ajg.2015.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102:2300–2313. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 29.Dellon ES, Erichsen R, Baron JA, Shaheen NJ, Vyberg M, Sorensen HT, et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment Pharmacol Ther. 2015;41:662–670. doi: 10.1111/apt.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90–96. doi: 10.1038/modpathol.3800498. [DOI] [PubMed] [Google Scholar]
- 32.Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol. 2011;18:335–348. doi: 10.1097/PAP.0b013e318229bfe2. [DOI] [PubMed] [Google Scholar]
- 33.Genta RM, Spechler SJ, Souza RF. The twentieth eosinophil. Adv Anat Pathol. 2007;14:340–343. [Google Scholar]
- 34.Genevay M, Rubbia-Brandt L, Rougemont AL. Do eosinophil numbers differentiate eosinophilic esophagitis from gastroesophageal reflux disease? Arch Pathol Lab Med. 2010;134:815–825. doi: 10.5858/134.6.815. [DOI] [PubMed] [Google Scholar]
- 35.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110–114. [PubMed] [Google Scholar]